Abstract
Objective
To identify and validate an fMRI-based neural marker for migraine without aura (MwoA) and to examine its association with treatment response.
Methods
We conducted cross-sectional studies with resting-state fMRI data from 230 participants and machine learning analyses. In studies 1 through 3, we identified, cross-validated, independently validated, and cross-sectionally validated an fMRI-based neural marker for MwoA. In study 4, we assessed the relationship between the neural marker and treatment responses in migraineurs who received a 4-week real or sham acupuncture treatment, or were waitlisted, in a registered clinical trial.
Results
In study 1 (n = 116), we identified a neural marker with abnormal functional connectivity within the visual, default mode, sensorimotor, and frontal-parietal networks that could discriminate migraineurs from healthy controls (HCs) with 93% sensitivity and 89% specificity. In study 2 (n = 38), we investigated the generalizability of the marker by applying it to an independent cohort of migraineurs and HCs and achieved 84% sensitivity and specificity. In study 3 (n = 76), we verified the specificity of the marker with new datasets of migraineurs and patients with other chronic pain disorders (chronic low back pain and fibromyalgia) and demonstrated 78% sensitivity and 76% specificity for discriminating migraineurs from nonmigraineurs. In study 4 (n = 116), we found that the changes in the marker responses showed significant correlation with the changes in headache frequency in response to real acupuncture.
Conclusion
We identified an fMRI-based neural marker that captures distinct characteristics of MwoA and can link disease pattern changes to brain changes.
Despite migraine being one of the most prevalent and disabling disorders worldwide, its pathophysiologic mechanisms remain poorly understood.1,2 Neuroimaging studies have provided evidence of widespread structural and functional alterations of brain regions in migraineurs, as well as broad reorganization of brain networks that might influence pain experience and multisensory integration.3,4 However, a critical gap remains between characterizing abnormalities in migraine and identifying a sensitive and specific marker for migraine-related CNS dysfunction. Because developing new interventions or optimizing treatments for migraine headaches may benefit from targeting brain networks or systems rather than single structures, identifying connectome-based markers for migraine is necessary in linking treatment response to changes in the brain.4–8
To narrow this gap, we conducted a multilevel study using a machine learning approach and whole-brain resting-state functional connectivity (rsFC) to identify and validate a connectome-based marker that has the potential to diagnosis patients with migraine. In study 1, we applied an advanced feature selection approach9 to select the most discriminative rsFC patterns and to discriminate migraine without aura (MwoA) from healthy controls (HCs). In study 2, we investigated the validity of the identified connectome-based marker using an independent migraine cohort. In study 3, we tested the specificity of the identified marker for migraine by examining 2 chronic pain disorders, chronic low back pain (cLBP) and fibromyalgia. In study 4, we examined the association between the identified connectome-based marker and treatment response.
Methods
Standard protocol approvals, registrations, and consents
The studies included a total of 230 participants who were specifically recruited for research purposes. All patients with migraine were scanned only if they were migraine-free (interictal) for at least 72 hours at the time of their MRI scan. Otherwise, the study visit was rescheduled. The Institutional Review Board (IRB) of Chengdu University of Traditional Chinese Medicine approved studies 1, 2, and 4 (MwoA and HCs), and study 4 was registered at ClinicalTrials.gov (NCT01152632, June 27, 2010). Study 3 was approved by the Ethics Committee of Guangdong Provincial Hospital of Chinese Medicine (patients with MwoA) and the IRB of Massachusetts General Hospital (patients with cLBP, those with fibromyalgia, and HCs). All experiments were performed in accordance with the guidelines set forth by the IRB for ethics and protection of human participants. All patients and HCs gave their written informed consent.
Participants
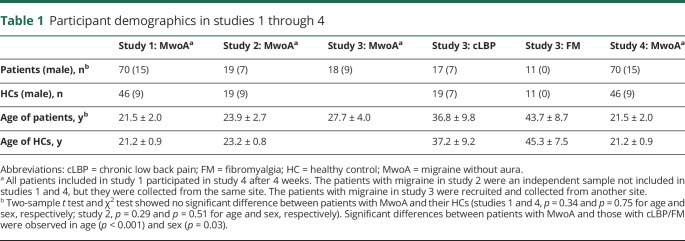
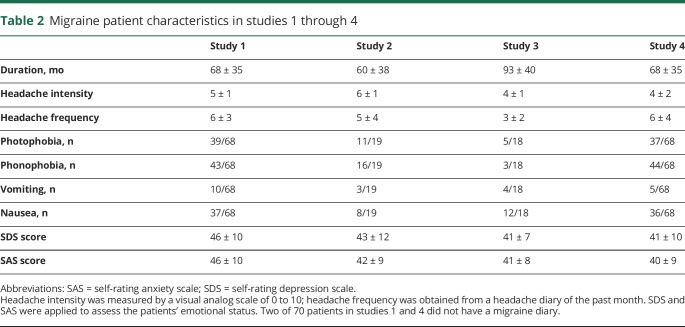
Study 1 included 81 patients diagnosed with MwoA on the basis of the International Classification of Headache Disorders, 2nd Edition (ICHD-II) MwoA criteria10 by the consensus of 3 neurologists and 46 demographically matched HCs. Of the 81 patients, 70 were included in the final data analysis. Nine were excluded due to incomplete scans (lack of resting-state fMRI scan or T1 anatomic scan), and 2 patients were excluded due to excessive head movement (>3 mm). Per the inclusion/exclusion criteria of the study, no participants had comorbid substance abuse disorders, and patients with migraine had no or low levels of depression and anxiety (as determined by self-rating depression11 and anxiety12 scales). Study 2 included a separate sample of 19 patients with MwoA and 19 HCs. Study 3 included 18 patients with MwoA collected from another site and 2 nonmigraine pain datasets (cLBP and fibromyalgia) with a total of 76 participants. The cLBP data were obtained from 17 patients and 19 matched HC participants. The fibromyalgia data were obtained from 11 female patients and 11 matched HC participants. Study 4 included 70 patients from study 1 who were rescanned after 4 weeks. All patients with cLBP and fibromyalgia were free of migraine comorbidity.
Participant demographics for studies 1 through 4 are provided in table 1. The characteristics of patients with migraine are summarized in table 2. Details of the inclusion/exclusion criteria for patients and HCs can be found in data available from Dryad (appendix e-1, doi.org/10.5061/dryad.2f82381).
Table 1.
Participant demographics in studies 1 through 4
Table 2.
Migraine patient characteristics in studies 1 through 4
Medications and treatments
Each patient underwent a medical history evaluation, and none of the included participants reported medication overuse before or during the study period per the study requirements. Patients in studies 1 through 4 were instructed and agreed not to take any prophylactic medications for migraine 1 month before the MRI scan. In cases of severe pain, ibuprofen (300 mg each capsule with sustained release) was permitted. None of the patients in the 4 studies took any medications 12 hours before the MRI scan. In study 4, 41 participants received real acupuncture, 12 received sham acupuncture, and 17 were on the wait-list.6 The detailed protocol for treatment is available in our previous studies13 and in data available from Dryad (appendix e-2, doi.org/10.5061/dryad.2f82381). We have provided tables (tables e-1–e-4 available from Dryad) to present medications, treatments, and other clinical symptoms for every patient with migraine.
MRI data acquisition
In all 4 studies, we acquired resting-state fMRI and structural MRI scans. Participants were asked to keep their heads still, and all included participants reported that they remained awake during the scan. In studies 1, 2, and 4, we used a 3T Siemens (Munich, Germany) scanner, and both migraineurs and HCs kept their eyes closed to avoid unexpected activation of the occipital region. In study 3, we also used 3T Siemens scanners for patients with migraine, cLBP, and fibromyalgia from different sites. The patients and their matched HCs had the same imaging parameters to minimize the systematic differences. Details of the MRI acquisition parameters for different datasets can be found in data available from Dryad (table e-5, doi.org/10.5061/dryad.2f82381).
Study design
Study 1: Identifying a dysfunctional connectome of migraine
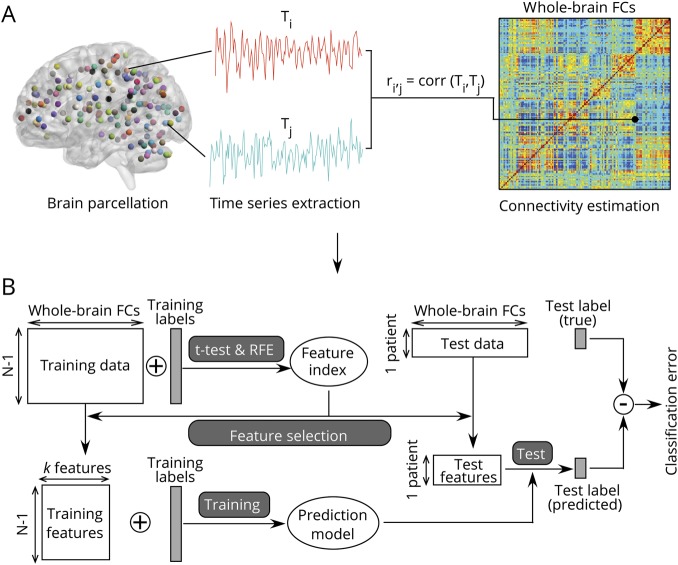
The aim of study 1 was to identify a dysfunctional connectome of migraine that could discriminate patients with migraine from HCs. As shown in figure 1A, after a preprocessing routine (as described in the fMRI Preprocessing section below), resting-state fMRI data were divided into 160 regions and 6 networks (data available from Dryad, table e-6, doi.org/10.5061/dryad.2f82381) according to the Dosenbach functional atlas.14 All region of interest (ROI) masks were generated with WFU_PickAtlas,15 had peak activation of gray matter, and showed overlap with known brain regions. Regional mean time series were obtained for each participant by averaging the fMRI time series over all voxels in each of the 160 regions. Functional connectivity (FC) for each participant was measured by the pairwise Pearson correlation coefficient between all possible [(160 × 159/2) = 12,720] ROI pairs. A symmetric connectivity matrix was constructed to represent these connections (figure 1A). Correlation coefficients were Fisher z transformed to increase normality for statistical analyses.
Figure 1. Overview of the proposed classification approach.
(A) Preprocessed resting-state data were decomposed by the Dosenbach atlas into 160 regions of interest (ROIs). The resting-state functional connectivities (FCs) between ROIs were estimated as the Pearson correlations between the average fMRI time series extracted from the ROIs. (B) Whole-brain resting-state FC matrices were fed to a feature selection and classification framework. Feature selection and classification were performed on the basis of a leave-one-out cross-validation strategy. Discriminative features and SVM parameters were obtained from a training set and applied to a testing set. This process was repeatedly performed 116 times to get the final classification results. RFE = recursive feature elimination; SVM = support vector machine.
Feature selection
To reduce computational complexity and to diminish noise, we applied a feature selection procedure to all FCs that were used as features using a combination of univariate (t test) and multivariate wrapper techniques (recursive feature elimination [RFE]) to construct the most discriminative feature set for classification of MwoA and HCs (figure 1B).9,14,16,17 To avoid the risk of overfitting, all analyses were based on a leave-one-out cross-validation (LOOCV) technique. Thus, the analyses were unbiased in the sense that the training features were selected independently of each test case.
In the first step, we analyzed group-level differences of rsFC between patients with MwoA and HCs. Significant differences for each pair of ROIs were assessed with a mass univariate 2-sample t test with a threshold of p < 0.05 and false discovery rate (FDR) correction. Features showing significant differences were retained for the remaining analyses.
In the second step, we used RFE combined with support vector machine (SVM) to select the features with the most discriminative power for the classifier itself. SVM-RFE was used to train the classification model and to obtain weights for each feature. The features were ranked according to the absolute values of weight, and the lowest ranking feature was discarded. The classification model was then trained using the new feature set (i.e., without the lowest ranking features that were discarded). This procedure was performed repeatedly until the feature set was empty. Two hundred FCs with the most discriminative power, as tested by a 2-sample t test in the first step, were fed to the classifier, and all features included in the feature sets passed pFDR < 0.05. To further select the features with the highest classification accuracy, we conducted a full backward elimination procedure the above feature set.9 This process was performed 116 times (i.e., LOOCV for all 116 participants in study 1) to get the final classification results.
Because we used an LOOCV strategy to estimate the performance of the classifiers and feature ranking and because each iteration was based on a slightly different dataset, the selected feature sets differed slightly from iteration to iteration. To determine the most discriminative features, a consensus discrimination map that aggregated features selected in all LOOCV iterations was used.14 Regional weight, which represents the contribution of each brain area for discriminating patients with migraine and HCs, was denoted by the number of ROI occurrences in the consensus discrimination map. The discrimination power of each feature was denoted by the average of its classification weights across all iterations.
Classification and performance evaluation
Features with the most discriminative power were fed to a linear SVM, which was implemented with the use of LIBSVM.18 We used default parameters in LIBSVM for all classification analyses. The classification was also based on an LOOCV strategy, and the performance of a classifier was evaluated by accuracy, sensitivity, and specificity. Here, sensitivity and specificity represent the proportion of patients correctly classified and the proportion of HCs correctly classified, respectively. We also calculated the area under the receiver operating characteristics curve to illustrate the performance of the classification. Nonparametric permutation tests (10,000 times) were used to estimate the statistical significance of the observed classification accuracy.
Study 2: Investigating the validity/generalizability of the connectome-based marker
The aim of study 2 was to validate the migraine classifier and to determine whether the identified connectome was generalizable across different patients. Therefore, this study was conducted in a new cohort of patients with migraine and HCs from the same site as in study 1. The data were processed with the same pipeline as in study 1. We extracted the rsFCs on the basis of the identified migraine connectome and used the classifier trained in study 1 to discriminate patients with MwoA from HCs. The labels of the new cohort were blinded, and the migraine classifier was applied to the feature set without any model fitting procedure (e.g., model training, parameter optimization).
Study 3: Testing the specificity of the connectome-based marker
In this study, we used an independent dataset of patients with migraine and 2 datasets of patients with cLBP and fibromyalgia (see the Participants section for details) from different sites to test the specificity of the connectome-based marker for migraine. These datasets were preprocessed with the same pipeline as in study 1. We performed the following analyses. We extracted FCs from patients with cLBP and fibromyalgia on the basis of the migraine connectome and tested whether the migraine classifier could be used to discriminate other chronic pain disorders. We also combined all datasets (migraine, cLBP, fibromyalgia, and HCs), extracted rsFC features according to the migraine connectome, and tested the performance of the migraine classifier for discriminating patients with migraine from patients without migraine.
Study 4: Linking the connectome-based marker to treatment response
To examine the clinical utility of the identified connectome-based marker and its efficacy in linking changes in clinical outcome measures to changes in the brain, we took advantage of patients from study 1 (n = 70) who attended a second scan 4 weeks after their first scan. During the 4 weeks between the 2 scans, they received real acupuncture treatment (n = 41), sham acupuncture treatment (n = 12), or were wait-listed (n = 17). We processed the data with the same pipeline as in study 1 and tested the hypothesis using the following analyses. First, we extracted the identified connectome-based marker and used the classifier trained in study 1 to discriminate patients with migraine from HCs. Then, using an approach similar to that applied in a previous study,19 we computed the strength of expression of the marker by taking the dot product of the vectorized classification weights with the connectivity patterns in the connectome, yielding a connectome-based response for each patient before and after treatment (studies 1 and 4, respectively). We calculated the correlation between connectome-based response changes and headache frequency changes (primary clinical outcome measure20).
fMRI preprocessing
The fMRI data were preprocessed and analyzed with SPM12 (Wellcome Trust Center for Neuroimaging, London, UK). The first 5 volumes were discarded to allow time for signal equilibration. Images were corrected for slice timing and head motion. The resulting images were normalized to the Montreal Neurologic Institute space,21 spatially smoothed with a gaussian kernel of 8-mm full width at half-maximum, and temporally filtered with a bandpass filter (0.01–0.15 Hz).
Additional analysis
Head motion
To minimize the effect of head motion on the estimation of FC, we followed the strategy suggested by a recent benchmark study22 by combining the 6 motion estimates and 2 physiologic time series (CSF and the white matter signals) with global signal regression.
To address the potential effects of head motion on the MwoA classification results, we computed the maximal and mean frame-wise displacement (FD) per participant for each dataset.23 We first compared the between-group (patients with MwoA vs HCs) differences in FD using 2-sample t tests and found no significant differences between patients with migraine and HCs in all 4 studies (p = 0.51 for study 1, p = 0.80 for study 2, p = 0.28 for study 3, and p = 0.31 for study 4). Second, we included single-participant FD values as independent variables in the classification model and verified whether motion contributed significant variance in discriminating patients with MwoA from HCs. We found that FD values could not provide significant predictive power for the classifier (p > 0.05, permutation test). Details of analysis and results are provided in data available from Dryad (appendix e-3, doi.org/10.5061/dryad.2f82381).
Drowsiness
Participants' relative levels of drowsiness during the fMRI scan could be an important confounding parameter for the strength of rsFC. To exclude the contribution of drowsiness in discriminating patients with MwoA from HCs, we extracted FC features using the well-established rsFC neural markers of drowsiness24 and tested whether these features could significantly classify patients with MwoA and HCs. Results showed no systematic differences between patients with MwoA and HCs and demonstrated that our migraine connectome-based marker was not confounded by drowsiness. Details of analysis and results are provided in data available from Dryad (appendix e-4, doi.org/10.5061/dryad.2f82381).
Statistical analysis
Permutation testing
In permutation testing, we randomly permuted the class labels of the data before training. LOOCV was then performed on the permuted dataset, and the procedure was repeated 10,000 times. If the classifier trained on real class labels had an accuracy exceeding the 95% confidence interval generated from the accuracies of the classifiers trained on randomly relabeled class labels, this classifier was considered to be well performing.
Data availability
All MATLAB (MathWorks, Inc, Natick, MA) codes in this study can be requested from the authors. Additional data related to this article may be provided on reasonable request.
Results
Identifying a connectome-based marker for migraine
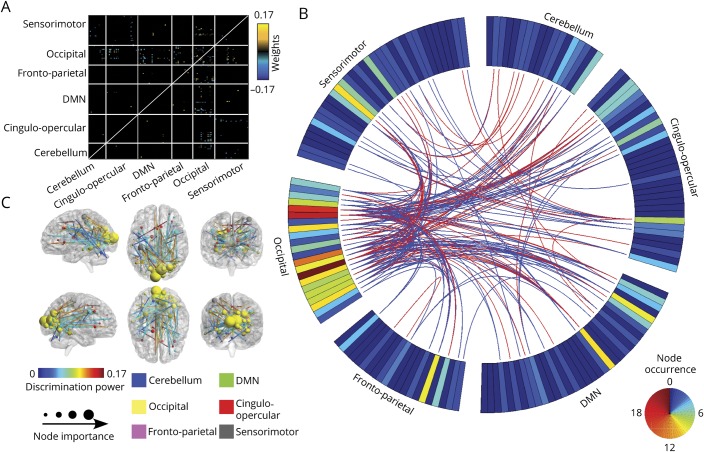
In study 1, we found 192 of 12,720 rsFCs that were retained after feature selection (features retained after 2-sample test and RFE are compared in figure e-3 available from Dryad, doi.org/10.5061/dryad.2f82381) and used those as features for the classifier to represent differences between patients with MwoA and HCs. The differences were located primarily within (1) the occipital lobe, including both occipital and postoccipital areas (i.e., middle occipital gyrus and calcarine); (2) the sensorimotor network, including the parietal and postparietal (i.e., inferior parietal lobule) cortices; (3) part of the medial-cerebellum; (4) the cingulo-opercular network, including the anterior-insula, dorsal anterior cingulate cortex, medial frontal cortex, and thalamus; (5) the default mode network (DMN), including the angular gyrus, fusiform gyrus, and occipital gyrus; and (6) the frontal parietal network, including the anterior frontal cortex and ventral lateral prefrontal cortex (figure 2, A and B and data available from Dryad, table e-7, doi.org/10.5061/dryad.2f82381). Figure 2C shows the top 100 consensus connections (evaluated by their weights) of altered rsFC. Details of the connections can be found in data available from Dryad (table e-7). With rsFC, the classifier achieved an accuracy of 91.4% (89.1% for HCs and 92.9% for patients with MwoA; p < 0.001), and the area under the curve was 0.97 (p < 0.001) for the receiver operating characteristics curve (figure 3A).
Figure 2. Identified FC patterns using the proposed feature selection approach.
(A) Heat map depicting patterns of abnormal resting-state (rs) functional connectivity (FC) in patients with migraine without aura (MwoA) relative to healthy controls (HCs). Warm colors represent an increase and cool colors represent a decrease in rsFC in patients with MwoA compared to controls. (B) Consensus map of discriminative rsFCs. The ring shows node weights reflecting the number of occurrences for each region of interest in the consensus map. Red connections indicate significantly higher and blue connections indicate significantly lower rsFC patterns in patients with MwoA compared to HCs. (C) Full view of the most discriminative features formed by the top 100 consensus FCs. The discriminative power of each FC was determined by the average of its absolute classification weights across all iterations of CV. DMN = default mode network.
Figure 3. Classification performance of the proposed approach.
(A) An overall prediction accuracy (PA) of 91.4% (p < 0.001, permutation test) was obtained with the use of identified resting-state functional connectivity (FC) patterns to classify patients with migraine without aura (MwoA) and healthy controls (HCs). The area under the receiver operating characteristics (ROC) curve was 0.97, and the confusion matrix shows the absolute numbers of classification made for patients with MwoA and HCs. (B) An overall accuracy of 84.1% (p < 0.001, permutation test) and area under the curve (AUC) of 0.91 were obtained using identified FC patterns to classify an independent dataset of patients with MwoA and HCs in study 2. (C) The migraine classifier obtained a sensitivity of 77.8% (p < 0.001) and a specificity of 75.9% (p = 0.006) for distinguishing participants with and without migraine in study 3.
Validating the identified connectome-based marker in independent dataset
We further tested the generalizability of the connectome-based marker and classifier in study 2 using an independent dataset of 19 patients with MwoA and 19 HCs. Figure 3B shows that the classifier achieved 84.2% accuracy (84.2% sensitivity and 84.2% specificity; p < 0.001) and an area under the curve of 0.91 (p < 0.001), indicating good generalizability in an independent dataset.
Specificity of connectome-based marker for migraine vs other chronic pain disorders
In study 3, we tested the specificity of the connectome-based marker by testing it on other chronic pain disorders. First, the migraine classifier was not able to discriminate between patients with 2 chronic pain disorders and their matched HCs, with accuracies of only 52.8% for cLBP (p = 0.31) and 54.5% for fibromyalgia (p = 0.32). Second, the migraine classifier could discriminate 18 patients with MwoA (from a different site, with the same inclusion criteria as studies 1 and 2) from 58 nonmigraineurs (including patients with cLBP, those with fibromyalgia, and HCs). As shown in figure 3C, we obtained a sensitivity of 77.8% (14 of 18; p < 0.001) and a specificity of 75.9% (44 of 58; p = 0.006). The accuracy for discriminating MwoA from the other chronic pain disorders was 73.1%, and the specificity was 71.4% (5 of 17 patients with cLBP and 3 of 11 patients with fibromyalgia were wrongly classified; p = 0.010). The classification results were not affected if we included age, sex, and site as covariates (i.e., we regressed out these covariates from connectivity features for classification).
Association between connectome-based marker and treatment response
In the first approach, we tested the reliability of the connectome-based marker in identifying patients with migraine who were rescanned. The classifier was trained on the data from study 1 and directly applied to the MwoA data from study 4. Results showed that 67 of 70 (sensitivity 95.7%) patients with migraine were correctly classified as migraineurs after 4 weeks, indicating a good test-retest reliability despite the acupuncture treatments they had received.
In addition, we investigated the relationship between changes in headache frequency (primary clinical outcome measure) and changes in the connectome-based marker. Repeated analysis of variance showed a main effect of treatment (F3,65 = 21.8, p < 0.001). Both real (before: 6.36 ± 1.02 attacks; after: 4.74 ± 0.76 attacks; p < 0.001; figure 4A) and sham (before: 6.67 ± 1.92 attacks; after: 5.00 ± 1.44 attacks; p < 0.001) acupuncture treatments significantly reduced headache frequency, with no significant difference between the 2 treatments. These results are consistent with our previous clinical trial findings suggesting that real acupuncture may yield significantly stronger long-term headache reduction than sham acupuncture (>16 weeks).20 We linked the changes in the connectome-based response to changes in headache frequency and found a significant correlation in patients who received real acupuncture (r = 0.32, p = 0.04; figure 4B) but not in patients who received sham acupuncture (r = 0.24, p = 0.45) or patients who were on the wait-list (r = −0.02, p = 0.95).
Figure 4. Link between changes in the connectome-based response and treatment response.
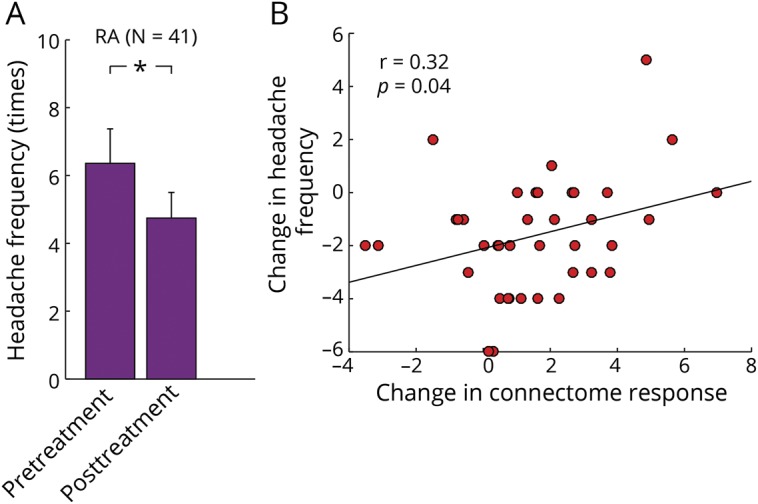
(A) Migraineurs who received 4 weeks of real acupuncture (RA) had a significant reduction in their headache frequency. (B) Changes in the connectome-based responses were significantly correlated with treatment response as indicated by changes in headache frequency in patients who received real acupuncture.
Discussion
In this study, we identified a distinct pattern of abnormal brain connections that can serve as a connectome-based marker for MwoA to discriminate a migraine brain from a healthy brain. In 4 studies, we investigated the accuracy, reliability, generalizability, and specificity of the identified connectome-based marker using 6 additional datasets (4 migraine datasets and 2 other chronic pain datasets). We found that the identified connectome-based marker in this study (1) achieved an accuracy, sensitivity, and specificity of >90% in discriminating patients with migraine from HCs; (2) obtained an accuracy, sensitivity, and specificity of >84% in an independent migraine cohort; (3) showed a specificity of 75.9% to migraine when discriminating migraine from other chronic pain disorders; and (4) showed an association with changes in headache frequency after acupuncture treatment. Taken together, these results show that this fMRI-based brain connectome marker has the potential to capture distinct characteristics of migraine and can be used to link changes in migraine disease patterns to changes in the brain.
We performed a comprehensive investigation using machine learning techniques and identified differences in network-based connectivity between patients with MwoA and HCs that could serve as features for classifying the migraine brain based solely on FC patterns. About 89.0% of the connections were located between the 6 resting-state networks.
We demonstrated that rsFC was decreased mainly between the occipital lobe and other cortical areas. This expands on previous findings demonstrating reduced connectivity between the visual cortex and cortical areas in salience networks using a seed-based approach.25 The visual cortex is hyperexcitable during the interictal state of migraine with aura and MwoA.26,27 Our results indicate that the occipital cortex contributed most to discriminating migraineurs from HCs. Because all the patients participating in our study had MwoA, our findings may implicate the significance of this region in relation to the pathophysiology of migraine independent of visual auras.
Although still under debate,28 researchers have found reduced FC in the prefrontal and temporal cortices of the DMN in patients with MwoA in the interictal stage.7 Disruption of DMN connectivity has also been linked to differences in pain catastrophizing and disease severity.29 In the present study, we did not observe disrupted rsFC within the DMN in patients with migraine but found increased connectivity between the DMN and the sensorimotor network. This result extends previous findings of increased connectivity between the DMN and premotor/somatosensory cortices for patients with MwoA during migraine attacks and suggests a disrupted system-level control of pain circuits for patients with migraine in both ictal and interictal states.30
Consistent with previous studies, our results also suggest that atypical rsFC in migraine involves other resting-state networks. One important network is the sensorimotor network, which has been implicated in the pathophysiology of migraine31 and other pain conditions.32 We observed abnormal rsFC between the sensorimotor and occipital, DMN, and cingulo-opercular networks, which may indicate disrupted multisensory integration in migraineurs. Another network is the frontal parietal network, a network encompassing the prefrontal, cingulate, and parietal cortices that is strongly associated with executive functions. Functional connections within affective pain regions such as the anterior insula, thalamus, and dorsal ACC differed in patients with MwoA compared to HCs. These regions are involved in sensory-discriminative, cognitive, and integrative domains of the pain experience, and atypical rsFCs may relate to aberrant pain processing in migraine.33 In particular, several previous studies have suggested that migraine is a disorder of multiple sensory processing and have highlighted that migraines interfere with the flow of information between the thalamus and cortex with consequent disturbances in sensory, cognitive, and motor neural processes.34–36
While recent translational neuroimaging studies have provided a basis for identifying neuropathologic features of MwoA, the present study has achieved a significantly increased level of classification accuracy from 82.0% (without feature selection) to 91.4% compared to previous studies using machine learning to discriminate migraine from healthy brains.5,37 We believe this was made possible by our feature selection approach, which is key for extracting an essential set of meaningful features to diagnose neurologic diseases. Moreover, the classifier achieved an 84.2% accuracy and diagnostic rate for discriminating migraine in an independent cohort of participants. In addition, by testing the classifier on other chronic pain disorders, we confirmed its specificity for migraine. It is worth mentioning that patients with migraine in the study were diagnosed by the consensus of 3 neurologists. This ensured that the trained classifier was not confounded by the validity of the labels (e.g., MwoA or other). Thus, we believe the identified functional connectome-based marker is reliable, generalizable, and specific for MwoA.
The ICHD criteria have been widely used in migraine clinical diagnosis. Some studies have reported that the sensitivity and specificity can be higher than 85%,38,39 while others have reported that the sensitivity was less well established (perhaps as poor as 53%).40 Nevertheless, it is believed that migraine diagnosis using ICHD criteria can be reliable if determined by 2 different neurologists.41 However, this inevitably increases the resources and manpower required for the diagnosis. Our identified fMRI-based objective neural marker shows potential as a supplement for the clinical diagnosis of MwoA. Moreover, our neural marker could be a useful diagnostic tool in situations in which patients are unable to communicate or when self-reports are otherwise unreliable.
We found an association between changes in the connectome-based marker and headache frequency in patients who received real acupuncture (but not sham acupuncture), linking changes in the brain to changes in migraine disease patterns. This result is consistent with previous findings that real acupuncture and sham acupuncture modulate different brain regions/networks in patients with chronic pain. More specifically, real acupuncture can target pain processing systems, while sham acupuncture/placebo may lower pain ratings by reducing negative emotions42–44 or report bias.45,46 Our results are also consistent with a previous study indicating that, unlike pharmacologic treatment, placebo interventions (placebo pills and sham acupuncture) can change only subjective measurements, not objective measurements.47 Our findings suggest that real treatment (acupuncture), but not sham treatment, may modulate the identified pathologic pathways associated with migraine.
Several methodologic limitations must be considered when performing FC analyses and interpreting results. In this study, we demonstrated the feasibility and reliability of using rsFCs to detect a clinically useful neural marker on 3 cohorts of patients with migraine from different sites that were collected with varying acquisition protocols. However, the reproducibility of this approach could be further investigated to account for more sources of variability such as differences in MRI acquisition protocols, instructions to participants, and recruitment strategies.48
Second, we did not include other types of headache (i.e., tension-type headache) in this study. A recent study found that migraine and tension-type headaches are separate headache disorders with different characteristics in relation to gray matter changes.49 Future studies are needed to directly compare functional brain activity and connectivity between migraineurs and patients with other types of headaches. In addition, the patients with MwoA in study 3 had relatively low headache frequency, and patients with more headache attacks may provide additional information in the assessment of the specificity of the neural marker toward other chronic pain disorders.
Another limitation of this study was the small sample size of the other pain conditions we assessed for comparison. With a larger sample size, we likely could have assessed similarities and differences between different chronic pain disorders rather than just showing specificity of the marker to migraine, for which the sample sizes were sufficient.
Finally, we found a significant association between changes of connectome-based markers and symptom changes only after real acupuncture, not after sham acupuncture. This may be due to the limited number of patients who received sham treatment, and further studies are needed to validate this finding.
We identified an fMRI-based brain functional connectome marker for migraine that has the ability to capture distinct characteristics of the disease and may be used to link changes in migraine disease patterns to changes in the brain. Our study also provides a potential framework for identifying and validating the performance of fMRI-based neural markers that is generalizable and could be applied to other neuropsychiatric diseases.
Study funding
J.K. is supported by R01 AT008563, R33 AT009310, R33AT009341, R34DA046635, and R21AT008707 from the NIH/National Center for Complementary and Integrative Health. Z.Z. is supported by the National Natural Science Foundation of China (No. 81871443).
Disclosure
The authors report no disclosures relevant to the manuscript. Go to https://n.neurology.org/lookup/doi/10.1212/WNL.0000000000008962 for full disclosures.
Glossary
- cLBP
chronic low back pain
- DMN
default mode network
- FC
functional connectivity
- FD
frame-wise displacement
- FDR
false discovery rate
- HC
healthy control
- ICHD-II
International Classification of Headache Disorders, 2nd edition
- IRB
Institutional Review Board
- LOOCV
leave-one-out cross-validation
- MwoA
migraine without aura
- RFE
recursive feature elimination
- ROI
region of interest
- rsFC
resting-state functional connectivity
- SVM
support vector machine
Appendix. Authors


Footnotes
Editorial, page 291
References
- 1.Goadsby PJ, Holland PR, Martins-Oliveira M, Hoffmann J, Schankin C, Akerman S. Pathophysiology of migraine: a disorder of sensory processing. Physiol Rev 2017;97:553–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pietrobon D, Moskowitz MA. Pathophysiology of migraine. Annu Rev Physiol 2013;75:365–391. [DOI] [PubMed] [Google Scholar]
- 3.Messina R, Filippi M, Goadsby PJ. Recent advances in headache neuroimaging. Curr Opin Neurol 2018;31:379–385. [DOI] [PubMed] [Google Scholar]
- 4.Maleki N, Gollub RL. What have we learned from brain functional connectivity studies in migraine headache? Headache 2016;56:453–461. [DOI] [PubMed] [Google Scholar]
- 5.Chong CD, Gaw N, Fu Y, Li J, Wu T, Schwedt TJ. Migraine classification using magnetic resonance imaging resting-state functional connectivity data. Cephalalgia 2016;37:828–844. [DOI] [PubMed] [Google Scholar]
- 6.Li Z, Liu M, Lan L, et al. Altered periaqueductal gray resting state functional connectivity in migraine and the modulation effect of treatment. Sci Rep 2016;6:20298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tessitore A, Russo A, Giordano A, et al. Disrupted default mode network connectivity in migraine without aura. J Headache Pain 2013;14:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tu Y, Fu Z, Zeng F, et al. Abnormal thalamo-cortical network dynamics in migraine. Neurology 2019;92:e2706–e2716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.De Martino F, Valente G, Staeren N, Ashburner J, Goebel R, Formisano E. Combining multivariate voxel selection and support vector machines for mapping and classification of fMRI spatial patterns. Neuroimage 2008;43:44–58. [DOI] [PubMed] [Google Scholar]
- 10.Headache Classification Subcommittee of the International Headache Society. The International Classification of Headache Disorders: 2nd edition. Cephalalgia 2004;24(suppl 1):9–160. [DOI] [PubMed] [Google Scholar]
- 11.Zung WWK. A self-rating depression scale. Arch Gen Psychiatry 1965;12:63–70. [DOI] [PubMed] [Google Scholar]
- 12.Zung WWK. A rating instrument for anxiety disorders. Psychosomatics 1971;12:371–379. [DOI] [PubMed] [Google Scholar]
- 13.Li Z, Lan L, Zeng F, et al. The altered right frontoparietal network functional connectivity in migraine and the modulation effect of treatment. Cephalalgia 2017;37:161–176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dosenbach N, Nardos B, Cohen A, Fair D. Prediction of individual brain maturity using fMRI. Science 2010;329:1358–1361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Maldjian JA, Laurienti PJ, Kraft RA, Burdette JH. An automated method for neuroanatomic and cytoarchitectonic atlas-based interrogation of fMRI data sets. Neuroimage 2003;19:1233–1239. [DOI] [PubMed] [Google Scholar]
- 16.Gysels E, Renevey P, Celka P. SVM-based recursive feature elimination to compare phase synchronization computed from broadband and narrowband EEG signals in brain-computer interfaces. Signal Process 2005;85:2178–2189. [Google Scholar]
- 17.Jung M, Tu Y, Park J, et al. Surface-based shared and distinct resting functional connectivity in attention-deficit hyperactivity disorder and autism spectrum disorder. Br J Psychiatry 2019;214:339–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chang C, Lin C. LIBSVM: a library for support vector machines. ACM Trans Intell Syst Technol 2011;2:27. [Google Scholar]
- 19.Wager TD, Atlas LY, Lindquist MA, Roy M, Woo CW, Kross E. An fMRI-based neurologic signature of physical pain. N Engl J Med 2013;368:1388–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhao L, Chen J, Li Y, et al. The long-term effect of acupuncture for migraine prophylaxis. JAMA Intern Med 2017;177:508–515. [DOI] [PubMed] [Google Scholar]
- 21.Ashburner J, Friston K. Unified segmentation. Neuroimage 2005;26:839–851. [DOI] [PubMed] [Google Scholar]
- 22.Ciric R, Wolf DH, Power JD, et al. Benchmarking of participant-level confound regression strategies for the control of motion artifact in studies of functional connectivity. Neuroimage 2017;154:174–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tu Y, Jung M, Gollub RL, et al. Abnormal medial prefrontal cortex functional connectivity and its association with clinical symptoms in chronic low back pain. Pain 2019;160:1308–1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tagliazucchi E, von Wegner F, Morzelewski A, Borisov S, Jahnke K, Laufs H. Automatic sleep staging using fMRI functional connectivity data. Neuroimage 2012;63:63–72. [DOI] [PubMed] [Google Scholar]
- 25.Niddam DM, Lai KL, Fuh JL, Chuang CYN, Chen WT, Wang SJ. Reduced functional connectivity between salience and visual networks in migraine with aura. Cephalalgia 2016;36:53–66. [DOI] [PubMed] [Google Scholar]
- 26.Mulleners WM, Chronicle EP, Palmer JE, Koehler PJ, Vredeveld JW. Visual cortex excitability in migraine with and without aura. Headache 2001;41:565–572. [DOI] [PubMed] [Google Scholar]
- 27.Aurora S, Cao Y, Bowyer S, Welch KMA. The occipital cortex is hyperexcitable in migraine: experimental evidence. Headache 1999;39:469–476. [DOI] [PubMed] [Google Scholar]
- 28.Xue T, Yuan K, Zhao L, et al. Intrinsic brain network abnormalities in migraines without aura revealed in resting-state fMRI. PLoS One 2012;7:e52927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubbard CS, Khan SA, Keaser ML, Mathur VA, Goyal M, Seminowicz DA. Altered brain structure and function correlate with disease severity and pain catastrophizing in migraine patients. eNeuro 2014;1:e20.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Amin FM, Hougaard A, Magon S, et al. Change in brain network connectivity during PACAP38-induced migraine attacks: a resting-state functional MRI study. Neurology 2016;86:180–187. [DOI] [PubMed] [Google Scholar]
- 31.Schwedt TJ, Chong CD, Chiang CC, Baxter L, Schlaggar BL, Dodick DW. Enhanced pain-induced activity of pain-processing regions in a case-control study of episodic migraine. Cephalalgia 2014;34:947–958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kong J, Spaeth RB, Wey HY, et al. S1 is associated with chronic low back pain: a functional and structural MRI study. Mol Pain 2013;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Schwedt TJ, Schlaggar BL, Mar S, et al. Atypical resting-state functional connectivity of affective pain regions in chronic migraine. Headache 2013;53:737–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Llinás RR, Ribary U, Jeanmonod D, Kronberg E, Mitra PP. Thalamocortical dysrhythmia: a neurological and neuropsychiatric syndrome characterized by magnetoencephalography. Proc Natl Acad Sci USA 1999;96:15222–15227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hodkinson DJ, Wilcox SL, Veggeberg R, et al. Increased amplitude of thalamocortical low-frequency oscillations in patients with migraine. J Neurosci 2016;36:8026–8036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Coppola G, Bracaglia M, Di Lenola D, et al. Lateral inhibition in the somatosensory cortex during and between migraine without aura attacks: correlations with thalamocortical activity and clinical features. Cephalalgia 2016;36:568–578. [DOI] [PubMed] [Google Scholar]
- 37.Zhang Q, Wu Q, Zhang J, et al. Discriminative analysis of migraine without aura: using functional and structural MRI with a multi-feature classification approach. PLoS One 2016;11:1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eriksen MK, Thomsen LL, Olesen J. Sensitivity and specificity of the new international diagnostic criteria for migraine with aura. J Neurol Neurosurg Psychiatry 2005;76:212–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hershey AD, Winner P, Kabbouche MA, et al. Use of the ICHD-II criteria in the diagnosis of pediatric migraine. Headache 2005;45:1288–1297. [DOI] [PubMed] [Google Scholar]
- 40.Lima M, Padula N, Santos L, Oliveira L, Agapejev S, Padovani C. Critical analysis of the International Classification of Headache Disorders diagnostic criteria (ICHD I-1988) and (ICHD II-2004), for migraine in children and adolescents. Cephalalgia 2005;25:1042–1047. [DOI] [PubMed] [Google Scholar]
- 41.Olesen J. The International Classification of Headache Disorders. Headache 2008;48:691–693. [DOI] [PubMed] [Google Scholar]
- 42.Tu Y, Ortiz A, Gollub RL, et al. Multivariate resting-state functional connectivity predicts responses to real and sham acupuncture treatment in chronic low back pain. Neuroimage Clin Elsevier 2019;23:101885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Egorova N, Gollub RL, Kong J. Repeated verum but not placebo acupuncture normalizes connectivity in brain regions dysregulated in chronic pain. Neuroimage Clin 2015;9:430–435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Harris RE, Zubieta J-K, Scott DJ, Napadow V, Gracely RH, Clauw DJ. Traditional Chinese acupuncture and placebo (sham) acupuncture are differentiated by their effects on mu-opioid receptors (MORs). Neuroimage 2009;47:1077–1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kong J, Kaptchuk TJ, Polich G, Kirsch I, Gollub RL. Placebo analgesia: findings from brain imaging studies and emerging hypotheses. Rev Neurosci 2007;18:173–190. [DOI] [PubMed] [Google Scholar]
- 46.Amanzio M, Benedetti F, Porro CA, Palermo S, Cauda F. Activation likelihood estimation meta-analysis of brain correlates of placebo analgesia in human experimental pain. Hum Brain Mapp 2012;34:738–752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wechsler ME, Kelley JM, Boyd IOE, et al. Active albuterol or placebo, sham acupuncture, or no intervention in asthma. N Engl J Med 2011;365:119–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Abraham A, Milham MP, Di Martino A, et al. Deriving reproducible biomarkers from multi-site resting-state data: an autism-based example. Neuroimage 2017;147:736–745. [DOI] [PubMed] [Google Scholar]
- 49.Chen WT, Chou KH, Lee PL, et al. Comparison of gray matter volume between 579 migraine and “strict-criteria” tension-type headache. J Headache Pain 2018;19:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All MATLAB (MathWorks, Inc, Natick, MA) codes in this study can be requested from the authors. Additional data related to this article may be provided on reasonable request.