Abstract
Studies of occupational solvent exposures and amyotrophic lateral sclerosis (ALS) have been conflicting. We conducted a population-based case-control study of mixed occupational solvent exposures and ALS. Using the Danish National Patient Registry, we identified ALS cases in Denmark from 1982 to 2013, and matched them to 100 controls based on sex and birth year. We estimated cumulative exposures to solvents (benzene, methylene chloride, toluene, trichloroethylene, perchloroethylene, and 1,1,1-trichloroethane) via job exposure matrices and applied them to occupational history from the Danish Pension Fund. Sex-stratified conditional logistic regression analyses revealed higher adjusted odds of ALS for men with exposure to benzene (aOR=1.20; 95% Confidence interval (CI): 1.02, 1.41) and methylene chloride (aOR=1.23; 95% CI: 1.07, 1.42). We used weighted quantile sum regression to explore combined solvent exposures and risk of ALS in exposed subjects and found increased odds of 26 to 28% for all exposure lag periods in odds of ALS for every one-unit increase in the mixture index in men. Weights of methylene chloride predominated the mixture index for all lag periods. Our study suggests an increased risk of ALS in men exposed to multiple solvents, with the greatest influence being from methylene chloride. These findings highlight the need to utilize mixtures analysis when considering co-occurring exposures.
Keywords: Amyotrophic lateral sclerosis, ALS, motor neuron disease, mixtures, solvents, occupational exposures
INTRODUCTION
Amyotrophic lateral sclerosis (ALS) is a motor neuron disease characterized by increasing loss of motor function [1]. Although death from ALS complications typically occurs within 3 to 5 years of diagnosis, 4% of cases survive over 10 years [2]. Only 5 to 10% of ALS cases can be attributed to familial genetic susceptibility, but little is known about the factors influencing risk of sporadic ALS [3]. Some hypothesize that certain environmental and occupational exposures may be associated with increased risk of ALS [4, 1, 5].
Organic solvents are volatile liquid chemicals ubiquitous in society due to their incorporation into various household and industrial solutions, including fuels, paints [6, 7], degreasers [7, 6], household cleaners [8], and adhesives [6]. These lipophilic toxicants can then potentially cross the blood-brain barrier, and acute exposure at high doses has been shown to be neurotoxic while chronic exposure has also been associated with encephalopathy and cognitive deficit [9–11].
Solvents have been suspected to play a role in ALS with many studies exploring occupational exposures. Some studies have reported significantly positive associations between overall occupational solvent exposure and ALS [12–16], while others have reported positive, but not significant, associations [17, 18]. However, many of these previous studies were in small samples, and did not distinguish between specific solvents within their analysis. Additionally, no previous study of solvent exposures and ALS has used job exposure matrices (JEMs) to objectively estimate exposure to a variety of solvents based on the entire work history. In our population-based study using Danish registry data, we utilize JEMs to estimate cumulative exposure to various solvents and their association with ALS, then further investigate the impact of mixed exposures to these toxicants.
METHODS
Study participants
Using the extended Danish version of International Classification of Diseases and Related Health Problems, Eighth Revision (ICD-8) codes from 1977 to 1994 and Tenth Revision (ICD-10) codes from 1994 to 2013, we were able to identify all ALS cases in Denmark based on patient records included in the Danish National Patient Registry diagnosed between 1977 and 2013 [19]. We classified ALS diagnosis as any records with a primary discharge ICD-8 code of 348.0 (amyotrophic lateral sclerosis) or ICD-10 code of G12.2 (motor neuron disease), with a previous analysis indicating 93% concordance with this strategy and medical record review [20]. The first recorded date of ALS diagnosis was defined as the “index date”, and each ALS case was matched to 100 controls selected from the Danish Central Person Registry based on birth year and sex who were alive at the time of ALS diagnosis, and assigned the same index date [21]. We excluded subjects with an index date within five years of the Danish National Patient Registry initiation January 1, 1977 to ensure that our analysis captured ALS incidence instead of prevalence [19], limiting our diagnosis inclusion window to 1982–2013, which left a total of 4599 ALS cases and 459,900 matched controls. Our exposure data was based on the Danish Pension Fund, which started in 1964 [22]. Therefore, to diminish potential exposure misclassification from jobs held before the start of the Pension Fund we excluded 2773 ALS cases and 277,300 matched controls born before 1940 (≥25 years of age in 1964). We also removed 187 cases with <5 years of work experience from our analysis to diminish healthy-worker hire bias, along with 30,625 controls with <5 years of work experience or no matched case. In doing this, we have attempted to diminish this particular type of selection bias under the expectation that generally unhealthy individuals are less likely to be employed for five years, and simultaneously more likely to develop disease.
Exposure assessment
We used unique Danish Central Person numbers to link employment history from the Danish Pension Fund to the previously mentioned demographic and diagnosis data. Cumulative occupational exposure to solvents, including benzene, methylene chloride, toluene, trichloroethylene, perchloroethylene, and 1,1,1-trichloroethane were estimated based on JEMs created for the Nordic Occupational Cancer Study (NOCCA) for Denmark [23]. These JEMs were developed by a team of experts using templates of previously developed Finnish JEMs with estimated annual mean levels of exposure (ppm), based on workplace-based biomonitoring and ambient environmental measures [23, 24]. Modifications for relevance to Denmark were based on industrial measurements recorded in Denmark and expert evaluations [23]. Exposure level and probability estimates are time-specific with a priori cut points of 1945, 1960, 1975, 1985, and 1995 [23]. For each identified solvent-exposed occupation, a probability of exposure and estimated level of exposure is assigned for each solvent at designated timepoints for those who are exposed. For example, a person employed in lithography between 1975 and 1984 would be designated a time-specific (i.e. 1975–1984) perchloroethylene exposure probability of 0.05 with an estimated daily exposure level of 5 ppm for those who are exposed. Commonly exposed occupations presented in these solvent JEMs include service station attendants, upholsterers, refinery workers, painters, distillers, furnacemen, cobblers, and laundry workers. However, each JEM is solvents specific, and though some occupations overlap between JEMs, others do not.
Estimated exposures to each solvent were calculated for each subject by multiplying the time-specific level of exposure by the probability of exposure for each recorded occupation, then further multiplying the solution by the time (years) employed in that job based on Pension Fund data [23]. Cumulative exposure up to the index date was calculated by summing these estimated exposure measures for each case and matched control. Additionally, to reduce healthy-work survivor bias and assess potential differences in ALS risk for exposures experienced at distinct times prior to ALS, we lagged exposures. Specifically, we investigated 5-year lagged exposures by excluding estimated occupational solvent exposures experienced within 5 years prior to the diagnosis/index date, then explored the impact of 10-year lagged exposures by excluding any estimated solvent exposure experienced within the 10 years prior to the diagnosis/index date from our analysis.
Statistical analysis
Due to expected differences in jobs and job tasks between males and females, analyses were stratified a priori by sex. We used conditional logistic regression to obtain odds ratios and 95% confidence intervals, with cumulative organic solvent exposures categorized into quartiles, and the unexposed serving as the reference group. Cut points for quartiles were based on the sex-specific distributions for 10-year lagged exposures of controls for comparability across exposure periods. All analysis was adjusted for the higher of the subject’s or subject’s spouse’s socioeconomic status based on tax-recorded occupation title (i.e. unskilled workers, skilled workers, low-salary positions, high-salary positions, and academics and corporate managers) and residential location (Copenhagen, Copenhagen suburbs, Aarhus/Odense, provincial towns, rural areas, and Greenland) at the index date.
Considering the single-point sources of co-occurring solvent exposures, we used weighted quantile sum (WQS) regression to further explore associations between combined estimated cumulative solvent exposures and risk of ALS in exposed subjects. WQS is an analytical method used to reduce issues with collinearity in combinations of correlated exposures for epidemiological investigations.[25] WQS analysis is based on additive cumulative effects, and chemical exposures are assumed to be normally distributed. Thus, because a large portion of study subjects had no occupational solvent exposures (61%), only subjects with any JEM-based solvent exposure (45,712 males and 16,782 females) are included in the WQS analysis. Among these, each solvent is categorized into quartiles and a summary exposure score is derived by empirically weighting individual solvents assuming positive effects of each individual organic solvent exposure in risk of ALS. The solvent index score is calculated using the following equation where we include 6 solvents with weights wj between 0 and 1estimated by an optimization algorithm for each jth solvent in quartiles (4 categories), and the total of the weights summing to 1:
To maximize the smaller sample size, we used the entire reduced dataset for validation and testing. Through bootstrap sampling (n=100), the weighted quantile score estimates the mean of all estimated independent solvent weights for each subject [25]. In this way, the toxicity of each individual solvent is taken into account for the weighted solvent index score based on the number of significant (p<0.05) estimates observed in the bootstrapping procedure. The solvent index scores for each subject were then entered into a multivariable logistic regression model adjusting for age, residential location, and socioeconomic status (SES) at the time of the index date. All statistical analyses were conducted with a 5% level of significance using SAS 9.4 statistical software [26].
Because this secondary analysis was conducted using pre-existing data, this study was determined to be exempt by the Harvard T.H. Chan School of Public Health Institutional Review Board and approved by the Danish Data Protection Agency. Therefore, informed consent was not required.
RESULTS
After our study exclusion criteria, we had a total of 1639 ALS cases and 151,974 controls. Table 1 shows, as expected, a greater portion of solvent-exposed subjects were male (73%). A large portion of subjects, both solvent-exposed and unexposed, were from provincial towns (41%) or Copenhagen suburbs (24%). Additionally, although the greatest portion of study participants were classified as “skilled workers” (i.e. people with specialized occupational training), the portion of skilled workers in the solvent-exposed group was slightly higher than that in the unexposed group (35% vs 28%, respectively).
Table 1.
Demographic Characteristics at the Index Date by Solvent Exposure
| Characteristic | No solvents exposure (N=93,773) | Any solvent exposure (N=60,080) | ||
|---|---|---|---|---|
| n | % | n | % | |
| Sex | ||||
| Male | 47,796 | 52.5% | 45,712 | 73.2% |
| Female | 43,323 | 47.5% | 16,782 | 26.8% |
| Age (years) | ||||
| <45 | 10,565 | 11.6% | 7,874 | 12.6% |
| 45–54 | 22,990 | 25.2% | 16,426 | 26.3% |
| 55–64 | 37,199 | 40.8% | 25,579 | 40.9% |
| 65–74 | 20,365 | 22.4% | 12,615 | 20.2% |
| Socioeconomic status | ||||
| Academics and managers | 12,448 | 13.7% | 5,065 | 8.1% |
| High-s alary positions | 15,003 | 16.5% | 7,610 | 12.2% |
| Low-salary positions | 17,418 | 13.1% | 10,221 | 16.4% |
| Skilled workers | 25,827 | 28.3% | 22,090 | 35.3% |
| Unskilled workers | 12,450 | 13.7% | 11,578 | 18.5% |
| Unknown | 7,973 | 8.8% | 5,930 | 9.5% |
| Residential location | ||||
| Copenhagen | 9,416 | 10.3% | 5,855 | 9.4% |
| Copenhagen suburbs | 22,124 | 24.3% | 14,963 | 23.9% |
| Aarhus/Odense | 8,993 | 9.9% | 6,077 | 9.7% |
| Provincial towns | 37,082 | 40.7% | 25,535 | 40.9% |
| Rural areas | 13,183 | 14.5% | 9,854 | 15.8% |
| Greenland | 89 | 0.1% | 49 | 0.1% |
| Unknown | 232 | 0.2% | 161 | 0.2% |
| Marital status | ||||
| Married | 63,156 | 69.3% | 40,547 | 64.9% |
| Unmarried | 11,119 | 12.2% | 9,006 | 14.4% |
| Divorced | 11,898 | 13.1% | 10,279 | 16.5% |
| Widowed | 4,772 | 5.2% | 2,582 | 4.1% |
| Unknown | 174 | 0.2% | 80 | 0.1% |
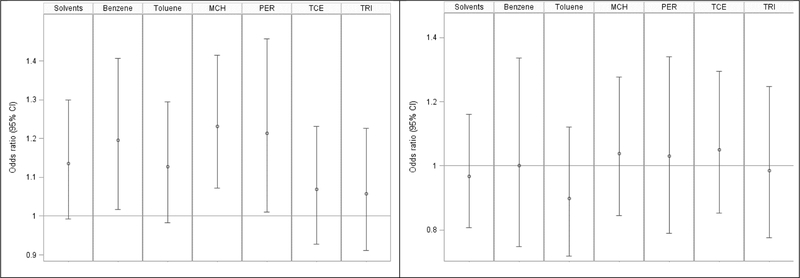
Solvent-specific results for our analysis of any exposure to individual solvents up to 5-years prior to the index date are displayed in Figure 1. Our analysis revealed higher adjusted odds of ALS for men with exposure to benzene (aOR=1.20; 95% CI 1.02–1.41, p=0.03), methylene chloride (aOR=1.23; 95% CI 1.07–1.42, p=0.003), and perchloroethylene (aOR=1.21; 95% CI 1.01–1.46, p=0.04). Ever exposure to any organic solvent up to 5 years prior to the index date increased odds of ALS by 14% (p=0.06). For women there were no significant associations between any exposures to individual solvents and ALS risk.
Figure 1. Analysis of any 5-year lagged exposure to solvents and ALS cases status in Denmark, 1982–2013.
Data are the odds ratios (95% confidence intervals) of ALS by individual solvent exposures. All models were adjusted for socioeconomic status and residential location at the index date. Abbreviations are as follows: MCH = Methylene chloride, PER = Perchloroethylene, TCE = trichloroethylene, TRI = 1,1,1- Trichloroethane
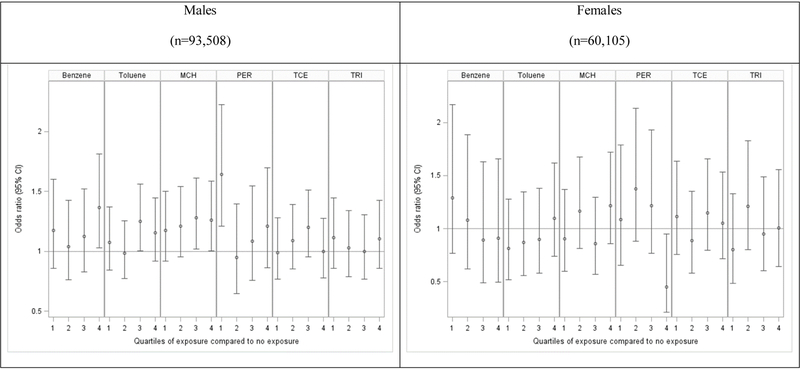
As shown in Figure 2, when examining 5-year lagged cumulative estimated exposures of specific organic solvents, odds ratios were generally elevated for highest quartile levels of exposure to benzene (aOR=1.37; 95% CI 1.03–1.81, p=0.03) and methylene chloride (aOR=1.26; 1.00–1.59, p<0.05) compared to those with no exposure. However, patterns of association were not consistent across quartiles, and tests for trends were not statistically significant. Results of analyses with no lag or a 10-year lag were similar (Supplemental Table 1). There was less of an association seen among women, although there was a significant inverse association seen for ALS in women with the highest quartile exposure to perchloroethylene compared to those with no exposure (aOR=0.45; 95% CI 0.21–0.95, p=0.02), which could be attributed to multiple comparisons of so many statistical tests increasing likelihood significant results due to chance. Thus, under a more constrained Bonferroni corrected p-value of 0.008 these results would not be considered significant; however, under the more liberal p<0.05 the results are significant. All other quartile exposures exhibited higher, but not significant, aORs, indicating no trend in this association. There were no consistent patterns seen for other solvent exposures and ALS in women (Supplemental Table 2).
Figure 2: Analyses of 5-year lagged cumulative exposure to solvents and ALS case status in Denmark, 1982–2013.
Data are the odds ratios (95% confidence intervals) of ALS for quartile categories of individual solvent exposures compared to those with no exposures. All models were adjusted for SES and residential location at the index date. Results are stratified by sex. Abbreviations are as follows: MCH = Methylene chloride, PER = Perchloroethylene, TCE = Trichloroethylene, TRI = 1,1,1-Trichloroethane
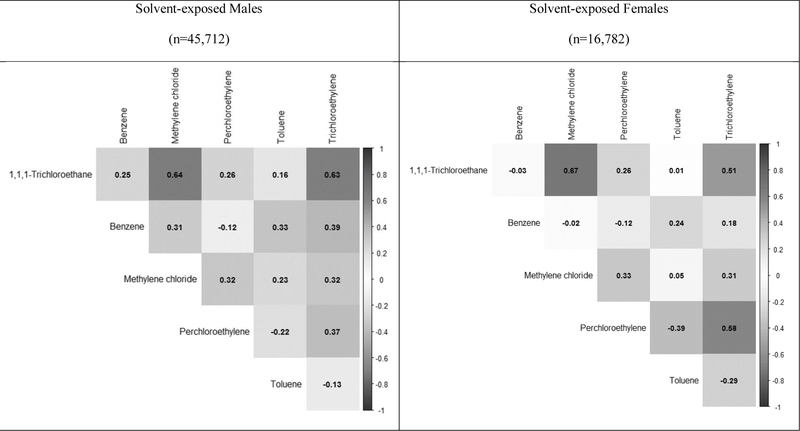
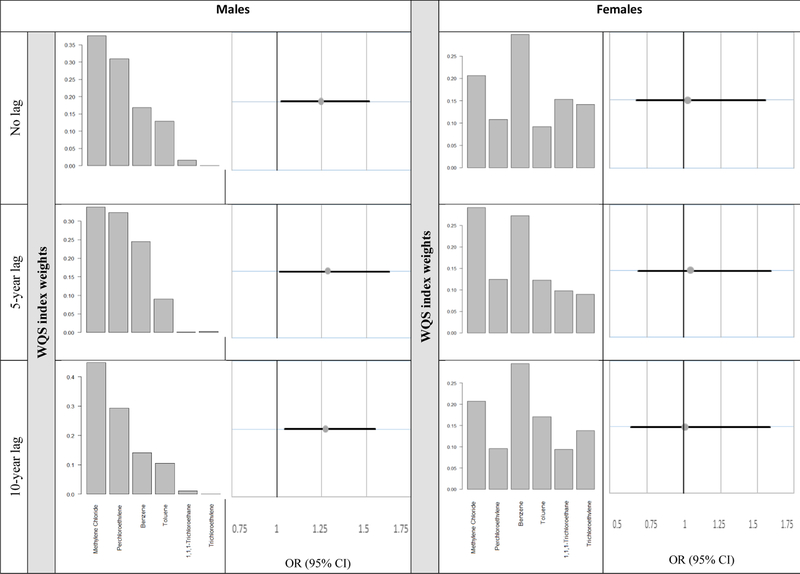
Exposures to the different solvents are often co-occurring, yet have a complicated correlation structure (Figure 3). Because of this, we used WQS to analyze the relation between exposure to the mixture of solvents and ALS (Figure 4). Among men with any solvent exposure, we observed a 25% increase in odds of ALS (aOR=1.25; 95% CI 1.02–1.52, p=0.04) for every one-unit increase in the derived solvent mixture index for exposures prior to the index date. For 5year and 10-year lagged exposures, odds were also significantly higher for increased mixed solvent exposure index score (aOR=1.28, p=0.03 and aOR=1.27, p=0.02, respectively). Methylene chloride dominated the weights of the solvent mixture index for all lag periods (no lag = 37.6%, 5-year lag = 33.8%, and 10year lag = 44.8%). This was followed by the weight of perchloroethylene (no lag = 30.9%, 5-year lag = 32.3%, and 10-year lag = 29.3%), indicating the importance of these solvents in driving the observed association seen between combined organic solvent exposures and ALS among men. Among women, benzene dominated the weights of the solvent mixture index in all lag periods (no lag = 52.9%, 5-year lag = 43.8%, and 10-year lag = 44.7%), followed by perchloroethylene. However, associations between solvent index scores and ALS were not statistically significant, with aOR ranging from 1.03 to 1.05 and p>0.75.
Figure 3. Heat map of correlations between solvents.
Data are correlation coefficients for correlations between individual cumulative solvent exposures in subjects with any solvent exposure. Results are stratified by sex.
Figure 4. Association between solvent levels and risk of ALS based on weighted quantile sum (WQS) regression analysis.
Bar graphs indicate the magnitude of WQS weight for each solvent. Data are the odds ratios (95% confidence intervals) associations between ALS and derived solvent mixture index scores by exposure lag periods. Results are stratified by sex. All models were adjusted for age, SES, and residential location at the index date.
DISCUSSION
In our large prospective study of occupational organic solvent exposures and ALS in Denmark, the analysis of individual solvents showed generally small, but positive associations with ALS in men. In women, there was a significant inverse association seen for perchoroethylene, which may have been due to multiple comparisons. Although multiple comparison could also play a role in some of the results seen for men, this is less likely as evident by the consistency of positive associations for specific solvents. When we analyzed all solvents together as a mixture using WQS regression, we found a statistically significant association between combined organic solvent exposures and ALS among men, with a large portion of the association being contributed by methylene chloride and perchloroethylene. Results were similar when exposures were lagged by 10 years, 5 years, or when we included all jobs up to the index date (no lag). Contrary to one previous study of 179 matched pairs which saw 2.57 times greater risk of ALS in females self-reporting occupational exposure to solvents between 2000 and 2005 [14], we found no notable patterns of association in females and no associations with mixed solvent exposures. Our JEMs are not sex specific, and sex differences seen in our results may not reflect true biological differences of solvent exposure by sex, but rather are more likely due to variances in industries of employment, job tasks, and lower frequency of exposure in women.
Solvents are known neurotoxicants,[9] and exposures have been previously linked to cognitive impairment,[10] as well as neurodegenerative disorders like Alzheimer’s disease [27] and Parkinson’s disease [28, 29]. Some previous studies investigating risk of ALS in subjects ever exposed to unspecified solvents showed higher risk [30, 13, 14], while others did not [18, 31, 32, 12]. One study of JEM-estimated solvent exposures reported a statistically significant increase in odds of death from ALS; however, this was based on occupations reported on death certificates, which are often inaccurate [15]. Additionally, a study in the New England region of the US investigating exposures to individual solvents, including benzene, methylene chloride, perchloroethylene, toluene, and trichloroethylene, found no significant associations with ALS [17]. However, the sample size in that study was only 109 ALS cases and 253 controls, and though exposure duration was assessed, there was no assessment of probability and level of exposure, nor were the multiple exposures analyzed with mixtures approaches [17].
Industrial solvent exposures occur most frequently through use of cleaning agents, adhesives, sealants, paints, and some rubber and plastic products [6]. Exposure to specific solvents can occur concurrently because many of the common exposure sources are composed of multiple solvents. For example, toluene and benzene were both used as additives in gasoline [33, 34]; toluene, benzene, and xylene were used in paints [34, 6], while methylene chloride was used as paint stripper [35]; and methylene chloride, toluene, benzene, perchloroethylene, and trichloroethylene are all used in cleaning products [33, 36, 34, 37, 35]. Furthermore, certain solvents serve as reactants for manufacture or byproducts of other solvents. Specifically, perchloroethylene is a substantial byproduct of reactions for the production of trichloroethylene [38] and benzene is often produced via hydrodemethylation of toluene [39]. As such, co-exposure to certain solvents may induce or inhibit metabolism and excretion of other solvents, potentially increasing potency of exposures [40]. As seen in our analysis, methylene chloride, perchloroethylene, and benzene were each individually associated with risk of ALS. However, when there were evaluated in combination through our WQS analysis, we could see that the greatest weight of risk for ALS was contributed to methylene chloride. Therefore, multi-exposure approaches, such as the WQS used in our study, should be used in conjunction with single toxicant models to better evaluate associations between common-source occupational exposures and ALS.
Although our use of advanced statistical methodology for evaluation of mixtures, along with our utilization of JEMs for individualized estimates of cumulative exposure in a large-scale, prospective data source considerably strengthens our study, we acknowledge that there may be some limitations. Due to the 1964 establishment of the Danish Pension Fund, we were not able to determine exposures for any jobs held before that time point, which may have introduced some exposure misclassification. However, any such misclassification would not likely differ by case status, and thus be expected to bias our results towards the null. We attempted to mitigate this potential bias by excluding subjects from our analysis who were over 25 years of age at the start of the Pension Fund. Through use of a JEM for organic solvent estimates, some exposure measurement error may also be present. The JEMs used, specific to the Danish population, were not directly validated; however, creation of these tools was based on biological, samples of workers and environmental samples from various industries.
Furthermore, all JEMs for the Nordic Occupational Cancer Study are consistently re-evaluated by numerous experts and edited as needed [24]. These JEMs may also have been indirectly validated through the use of the original Finnish JEM and derived Norwegian and Swedish JEMS in studies of cancer risk [41–43]. Specifically, in using average non-sex-specific level and probabilities of exposures based on industry-level measurements, and having no information on individual personal protection, there is likely misclassification of individual exposures, which would also tend to result in attenuation of effect estimates. Also, because the purpose of this study was specifically focusing on occupational exposures, we do not evaluate any additional risk from home-based solvent exposures, which are common. The WQS analysis also has some limitations, in that it assumes only one direction of effect, and cannot account for positive and negative individual effects in the same model [25]; however, results from our initial analysis in men suggested that results were overwhelmingly positive, though this is not true for women. Additionally, WQS cannot account for multiplicative interactions [25]. Despite this, the model does successfully identify which individual solvents contribute most to observed associations.
In conclusion, this study is the first, to our knowledge, to estimate long-term occupational exposures to organic solvents, both alone and in combination, in a large sample. Considering the hypothesized gene-environment interaction mechanism behind ALS [44, 5], with genetic susceptibility and environmental triggers, the results of our study are plausible. We observed an increased risk of ALS in men exposed to multiple solvents, with the greatest exposure of influence being methylene chloride. Additionally, methylene chloride, a known carcinogen with primary occupational exposures from paint strippers and degreasers [35, 45], was individually associated with increased risk of ALS in men. Suspected mechanisms for this exposure-disease relationship include oxidative stress [31, 5] and genotoxicity [46], indicating that the long-term neurological impacts of these exposures should be investigated further. Although the use of personal protective equipment may be required or advised during occupational use of solvents, many solvent-containing products are available commercially [6, 9]. Given the widespread use of these toxicants, more attention should also be given to organic solvent exposures in the general population.
Supplementary Material
Acknowledgements
This work was supported by the National Institute of Environmental Health Sciences grants R01 ES019188 and P30 ES000002 to MGW. ASD was supported in part by a National Institutes of Health training grant T32 ES007069. We also appreciate the assistance of summer intern, [Blinded for review] for his assistance compiling and organizing literature in preparation for this study.
This work was supported by the National Institute of Environmental Health Sciences (grants R01 ES019188 and P30 ES000002 to MW). Aisha Dickerson was supported in part by a National Institutes of Health (NIH) training grant (grant T32 ES007069). We appreciate the assistance of summer intern, Mr. William Crystal for his assistance compiling and organizing literature in preparation for this study.
Footnotes
Competing financial interests: No authors have any competing financial interests to declare.
None of the authors reported any conflicts of interest.
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
REFERENCES
- 1.Brown RH, Al-Chalabi A. Amyotrophic Lateral Sclerosis. N Engl J Med. 2017;377(2):162–72. 10.1056/NEJMra1603471. [DOI] [PubMed] [Google Scholar]
- 2.Mitchell JD, Borasio GD. Amyotrophic lateral sclerosis. Lancet. 2007;369(9578):2031–41. 10.1016/s0140-6736(07)60944-1. [DOI] [PubMed] [Google Scholar]
- 3.Hardiman O, Al-Chalabi A, Chio A, Corr EM, Logroscino G, Robberecht W et al. Amyotrophic lateral sclerosis. Nat Rev Dis Primers. 2017;3:17071 10.1038/nrdp.2017.71. [DOI] [PubMed] [Google Scholar]
- 4.Dickerson AS, Hansen J, Kioumourtzoglou MA, Specht AJ, Gredal O, Weisskopf MG. Study of occupation and amyotrophic lateral sclerosis in a Danishcohort. Occup Environ Med. 2018. 10.1136/oemed-2018-105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Oskarsson B, Horton DK, Mitsumoto H. Potential Environmental Factors in Amyotrophic Lateral Sclerosis. Neurol Clin. 2015;33(4):877–88. 10.1016/j.ncl.2015.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boyes WK. Chapter 18 - Chemicals: Solvents In: Wexler P, Gilbert SG, Hakkinen PJ, Mohapatra A, editors. Information Resources in Toxicology (FourthEdition). San Diego: Academic Press; 2009. p. 167–73. [Google Scholar]
- 7.Miller RK, Peters P, McElhatton PR. 2.23 - Occupational, industrial, and environmental agents In: Schaefer C, Peters P, Miller RK, editors. Drugs During Pregnancy and Lactation (Second Edition). Oxford: Academic Press; 2007. p. 561–608. [Google Scholar]
- 8.Steber J. 3 - The Ecotoxicity of Cleaning Product Ingredients In: Johansson I, Somasundaran P, editors. Handbook for Cleaning/Decontamination of Surfaces. Amsterdam: Elsevier Science B.V.; 2007. p. 721–46. [Google Scholar]
- 9.Sainio MA, Sr. Neurotoxicity of solvents. Handb Clin Neurol. 2015;131:93–110. 10.1016/b978-0-444-62627-1.00007-x. [DOI] [PubMed] [Google Scholar]
- 10.Sabbath EL, Gutierrez LA, Okechukwu CA, Singh-Manoux A, Amieva H, Goldberg M et al. Time may not fully attenuate solvent-associated cognitive deficits in highly exposed workers. Neurology. 2014;82(19):1716–23. 10.1212/wnl.0000000000000413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dick FD. Solvent neurotoxicity. Occup Environ Med. 2006;63(3):221–6, 10.1136/oem.2005.022400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McGuire V, Longstreth WT Jr., Nelson LM, Koepsell TD, Checkoway H, Morgan MS et al. Occupational exposures and amyotrophic lateral sclerosis. A population-based case-control study. Am J Epidemiol. 1997;145(12):1076–88. [DOI] [PubMed] [Google Scholar]
- 13.Chancellor AM, Slattery JM, Fraser H, Warlow CP. Risk factors for motor neuron disease: a case-control study based on patients from the Scottish Motor Neuron Disease Register. J Neurol Neurosurg Psychiatry. 1993;56(11):1200–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Morahan JM, Pamphlett R. Amyotrophic lateral sclerosis and exposure to environmental toxins: an Australian case-control study. Neuroepidemiology. 2006;27(3):130–5. 10.1159/000095552. [DOI] [PubMed] [Google Scholar]
- 15.Park RM, Schulte PA, Bowman JD, Walker JT, Bondy SC, Yost MG et al. Potential occupational risks for neurodegenerative diseases. Am J Ind Med. 2005;48(1):63–77. 10.1002/ajim.20178. [DOI] [PubMed] [Google Scholar]
- 16.Wang MD, Little J, Gomes J, Cashman NR, Krewski D. Identification of risk factors associated with onset and progression of amyotrophic lateral sclerosis using systematic review and meta-analysis. Neurotoxicology. 2017;61:101–30. 10.1016/j.neuro.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 17.Fang F, Quinlan P, Ye W, Barber MK, Umbach DM, Sandler DP et al. Workplace exposures and the risk of amyotrophic lateral sclerosis. Environ Health Perspect. 2009;117(9):1387–92. 10.1289/ehp.0900580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Koeman T, Slottje P, Schouten LJ, Peters S, Huss A, Veldink JH et al. Occupational exposure and amyotrophic lateral sclerosis in a prospective cohort. Occup Environ Med. 2017;74(8):578–85. 10.1136/oemed-2016-103780. [DOI] [PubMed] [Google Scholar]
- 19.Schmidt M, Schmidt SA, Sandegaard JL, Ehrenstein V, Pedersen L, Sorensen HT. The Danish National Patient Registry: a review of content, data quality, and research potential. Clin Epidemiol. 2015;7:449–90. 10.2147/clep.s91125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kioumourtzoglou MA, Seals RM, Himmerslev L, Gredal O, Hansen J, Weisskopf MG. Comparison of diagnoses of amyotrophic lateral sclerosis by use of death certificates and hospital discharge data in the Danish population. Amyotroph Lateral Scler Frontotemporal Degener. 2015;16(3–4):224–9. 10.3109/21678421.2014.988161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pedersen CB, Gotzsche H, Moller JO, Mortensen PB. The Danish Civil Registration System. A cohort of eight million persons. Dan Med Bull. 2006;53(4):441–9. [PubMed] [Google Scholar]
- 22.Hansen J, Lassen CF. The Supplementary Pension Fund Register. Scand J Public Health. 2011;39(7 Suppl):99–102. 10.1177/1403494810394716. [DOI] [PubMed] [Google Scholar]
- 23.Kauppinen T, Heikkila P, Plato N, Woldbaek T, Lenvik K, Hansen J et al. Construction of job-exposure matrices for the Nordic Occupational Cancer Study (NOCCA). Acta Oncol. 2009;48(5):791–800. 10.1080/02841860902718747. [DOI] [PubMed] [Google Scholar]
- 24.Kauppinen T, Uuksulainen S, Saalo A, Makinen I, Pukkala E. Use of the Finnish Information System on Occupational Exposure (FINJEM) in epidemiologic,surveillance, and other applications. Ann Occup Hyg. 2014;58(3):380–96. 10.1093/annhyg/met074. [DOI] [PubMed] [Google Scholar]
- 25.Carrico C, Gennings C, Wheeler DC, Factor-Litvak P. Characterization of Weighted Quantile Sum Regression for Highly Correlated Data in a Risk Analysis Setting. Journal of Agricultural, Biological, and Environmental Statistics. 2015;20(1):100–20. 10.1007/s13253-014-0180-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Inc. SI. SAS Insititute Inc. 9.4 ed. Cary, NC: SAS Institute Inc; 2013.
- 27.Kukull WA, Larson EB, Bowen JD, McCormick WC, Teri L, Pfanschmidt ML et al. Solvent exposure as a risk factor for Alzheimer’s disease: a case-controlstudy. Am J Epidemiol. 1995;141(11):1059–71; discussion 72–9. [DOI] [PubMed] [Google Scholar]
- 28.Goldman SM, Quinlan PJ, Ross GW, Marras C, Meng C, Bhudhikanok GS et al. Solvent exposures and Parkinson disease risk in twins. Ann Neurol. 2012;71(6):776–84. 10.1002/ana.22629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Seidler A, Hellenbrand W, Robra BP, Vieregge P, Nischan P, Joerg J et al. Possible environmental, occupational, and other etiologic factors for Parkinson’s disease: a case-control study in Germany. Neurology. 1996;46(5):1275–84. [DOI] [PubMed] [Google Scholar]
- 30.Andrew AS, Caller TA, Tandan R, Duell EJ, Henegan PL, Field NC et al. Environmental and Occupational Exposures and Amyotrophic Lateral Sclerosis in New England. Neurodegener Dis. 2017;17(2–3):110–6. 10.1159/000453359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gunnarsson LG, Bodin L, Soderfeldt B, Axelson O. A case-control study of motor neurone disease: its relation to heritability, and occupational exposures, particularly to solvents. Br J Ind Med. 1992;49(11):791–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gait R, Maginnis C, Lewis S, Pickering N, Antoniak M, Hubbard R et al. Occupational exposure to metals and solvents and the risk of motor neuron disease. A case-control study. Neuroepidemiology. 2003;22(6):353–6. 10.1159/000072925. [DOI] [PubMed] [Google Scholar]
- 33.USEPA USEPA-. Integrated Risk Information System (IRIS) on Toluene. Washington, DC: 2005. [Google Scholar]
- 34.ATSDR AfTSaDR-. Toxicological Profile for Benzene. Atlanta, GA: Centers for Disease Control and Prevention - CDC; 2007. [Google Scholar]
- 35.ATSDR AfTSaDR-. Toxicological Profile for Methylene Chloride In: Branch DoTTI, editor. Atlanta Georgia: USEPA, U.S. Environmental Protection Agency; -; 2000. [Google Scholar]
- 36.ATSDR AfTSaDR-. Public Health Statment: Trichloroethylene. Atlanta, GA: Centers for Disease Control and Prevention; 2016. [Google Scholar]
- 37.ATSDR AfTSaDR-. Public Health Statement - Tetrachloroethylene. Atlanta, GA: Cneters for Disease Control and Prevention, Division of Toxicology and Human Health Sciences PHS;2014. October 2014. [Google Scholar]
- 38.USEPA USEPA-. Problem Formulation of the Risk Evaluation for Perchloroethylene (Ethene, 1,1,2,2-Tetrachloro). Washington, DC: Environmental Protection Agency; 2018. Contract No.: CASRN: 127–18-4. [Google Scholar]
- 39.NLM USNLoM-. Hazardous Substances Data Bank - Benzene. Bethesda, MD: U.S. National Library of Medicine; 2014. Report No.: CASRN: 71–43-2 Contract No.: CASRN: 71–43-2 [Google Scholar]
- 40.Lof A, Johanson G. Toxicokinetics of organic solvents: a review of modifying factors. Crit Rev Toxicol. 1998;28(6):571–650. 10.1080/10408449891344272. [DOI] [PubMed] [Google Scholar]
- 41.Hadkhale K, Martinsen JI, Weiderpass E, Kjaerheim K, Sparen P, Tryggvadottir L et al. Occupational exposure to solvents and bladder cancer: A populationbased case control study in Nordic countries. Int J Cancer. 2017;140(8):1736–46. 10.1002/ijc.30593. [DOI] [PubMed] [Google Scholar]
- 42.Vlaanderen J, Straif K, Pukkala E, Kauppinen T, Kyyronen P, Martinsen JI et al. Occupational exposure to trichloroethylene and perchloroethylene and the risk of lymphoma, liver, and kidney cancer in four Nordic countries. Occup Environ Med. 2013;70(6):393–401. 10.1136/oemed-2012-101188. [DOI] [PubMed] [Google Scholar]
- 43.Talibov M, Auvinen A, Weiderpass E, Hansen J, Martinsen JI, Kjaerheim K et al. Occupational solvent exposure and adult chronic lymphocytic leukemia: No risk in a population-based case-control study in four Nordic countries. Int J Cancer. 2017;141(6):1140–7. 10.1002/ijc.30814. [DOI] [PubMed] [Google Scholar]
- 44.Bradley WG, Andrew AS, Traynor BJ, Chio A, Butt TH, Stommel EW. Gene-Environment-Time Interactions in Neurodegenerative Diseases: Hypotheses and Research Approaches. Ann Neurosci. 2018;25(4):261–7. 10.1159/000495321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schlosser PM, Bale AS, Gibbons CF, Wilkins A, Cooper GS. Human health effects of dichloromethane: key findings and scientific issues. Environ Health Perspect. 2015;123(2):114–9. 10.1289/ehp.1308030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Spencer PS, Kisby G. Chemicals, somatic mutations and neurodegeneration: evidence from Western Pacific amyotrophic lateral sclerosis-parkinsonism-dementia complex (ALS-PDC): Commentary on: Leija-Salazar M, Piette C, Proukakis C. Review: Somatic mutations in neurodegeneration. Neuropathol Appl Neurobiol 2018; 44: 267–85. Neuropathol Appl Neurobiol. 2018. 10.1111/nan.12533. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.