Abstract
Objective:
To evaluate pregnancy risk following copper (CuT380A) intrauterine device (IUD) placement 6 – 14 days after unprotected intercourse.
Study design:
We used a combined dataset from four protocols in which participants had received a CuT380A IUD regardless of recent unprotected intercourse. At entry, participants had negative point of care urine pregnancy testing and reported all acts of unprotected intercourse in the two weeks prior to IUD placement. We identified a subset of women who had placement 6 – 14 days after unprotected intercourse and provided follow-up information on pregnancy status 2–4 weeks after IUD insertion. This follow-up within the four protocols included self -administered home urine pregnancy test (UPT) results 2–4 weeks after IUD placement or continued contact for up to 6 months.
Results:
We identified 134 women who had a CuT380A IUD placed 6–14 days after unprotected intercourse and provided follow-up information on pregnancy status. Ninety-five (71%) participants reported UPT results 2–4 weeks after placement and the other 39 women were followed for 6 months after IUD placement to assess pregnancy status. Zero (97.5% CI 0–2.7%) participants reported a pregnancy within four weeks of CuT380A IUD placement.
Conclusion:
In these collected data, no women with recent unprotected intercourse became pregnant within 1 month of CuT380A IUD placement.
Implication:
These data indicate a low likelihood of pregnancy among women who reported unprotected intercourse 6– 14 days preceding IUD insertion. For many women and their providers, these data may be sufficient to support same-day placement of a copper IUD rather than delaying IUD placement until the next menses.
Keywords: Copper IUD, unprotected intercourse, pregnancy rate
1. Introduction
Same-day contraception start increases contraception use [1,2]. The American College of Obstetricians and Gynecologists recommends same-day intrauterine device (IUD) placement as safe, appropriate, and necessary to reduce delays in care [3,4]. Although prior studies examining copper IUD placement six or more days following unprotected intercourse report no pregnancies [5,6], the Centers for Disease Control and Prevention’s (CDC) Selected Practice Recommendations (SPR) limits copper IUD placement to within five days after unprotected intercourse [7].
We previously reported that 40 of 176 (23%) participants seeking an IUD for emergency contraception (EC) who reported unprotected intercourse in the previous 5 days also reported unprotected intercourse in the prior 6 – 14 days [8]. As women reporting multiple episodes of unprotected intercourse in a single menstrual cycle are at increased risk of unintended pregnancy compared to those with a single episode of unprotected intercourse [9], such women may have a particular need for highly effective emergency contraception. At the same time, clinicians may be reluctant to insert an IUD in such women.
We performed this analysis to evaluate pregnancy risk with copper IUD placement 6 – 14 days after unprotected intercourse for women with a negative urine pregnancy test (UPT) on the placement date.
2. Materials and methods
We combined data from four similar studies to evaluate the primary outcome. The first study enrolled 49 women at 7 family planning clinics in Minnesota, Pennsylvania, and Utah using a shared dedicated protocol [10]. The three other studies were conducted at the University of Utah and include two published trials [11][12], and one ongoing study [13]. In all four trials, women requesting a CuT380A (Paragard®, CooperSurgical, Trumbull, CT) IUD were asked about unprotected intercourse and an IUD was placed regardless of the response as long as the pregnancy test was negative. All participants signed informed consent prior to study participation. The institutional review boards (IRB) at the University of Utah, University of Pittsburgh, and University of Minnesota approved the shared research protocol. The University of Utah IRB approved the protocols for the three other studies.
Data collection began in June 2013 and concluded in October 2018. Eligible women included those 15 – 45 years old, requesting CuT380A IUD placement, reporting unprotected intercourse 6 – 14 days prior to IUD placement, with a negative urine pregnancy test (human chorionic gonadotropin ≤20 mIU/mL) on the IUD placement date, and English or Spanish fluency. For this analysis, we excluded participants with cycle lengths that were irregular or likely to be misreported (<20 and >35 days). Exclusion criteria included contraindications to copper IUD use (identified as category 4 in the CDC Medical Eligibility Criteria or known allergy), history of sterilization, and hysterectomy.
After providing informed consent, participants enrolled in the shared protocol completed point-of-care urine pregnancy testing. Participants with a negative pregnancy test completed a menstrual and sexual history and had a CuT380A IUD placed by a clinician. Prior to discharge, staff provided participants with a home pregnancy test and instructed them to complete testing two weeks post-IUD placement. Two to four weeks after IUD placement, the study team prompted participants to complete a follow-up survey by phone call, text message, or email. Follow-up surveys were hosted on Research Electronic Data Capture (REDCap), a secure web-based application [14]. Participants received $15 after completing the follow-up survey.
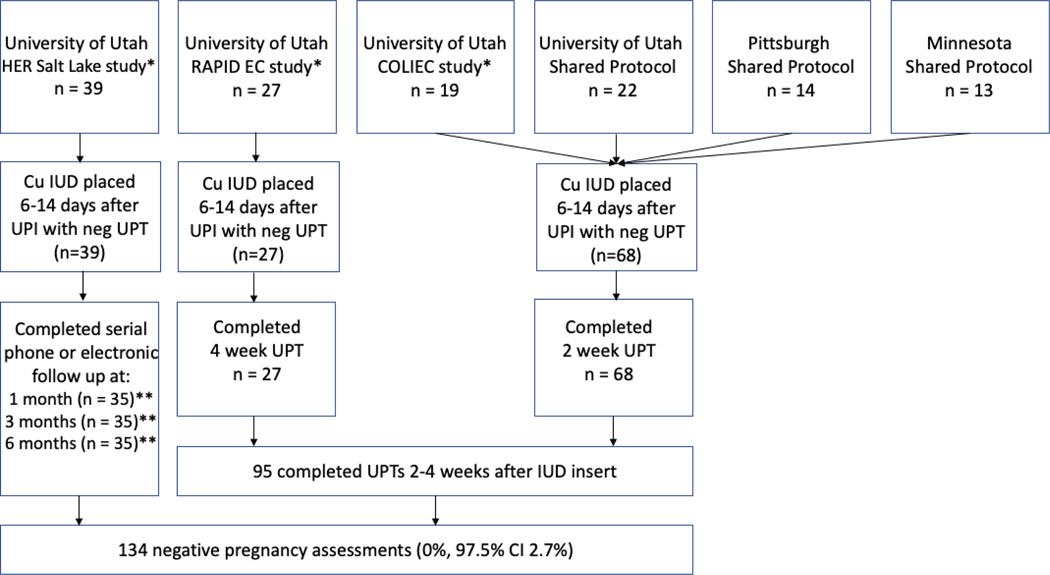
Follow-up varied across the other contributing protocols and is detailed in Figure 1. Of note, HER Salt Lake study[12] participants self-reported any concerns of pregnancy and home or clinic pregnancy test results 1 month after IUD placement. Participants continued to report pregnancy status at subsequent data collection points 3 and 6 months after IUD placement.
Figure 1:
Study flow (N=134)
* HER Salt Lake [12], RAPID EC [13], and COLIEC [11] are University of Utah research efforts that contributed data to this analysis.
** 33 participants report follow up data at 1, 3, and 6 months. 6 participants report follow at only one time point (2 participants at 1 month, 2 participants at 3 months, and 2 participants at 6 months).
We report the overall incidence of pregnancy within four weeks of IUD placement among women contributing data through these 4 study protocols. We further examined rates of pregnancy among the subgroup of participants who had unprotected intercourse during their fertile window and those who reported multiple episodes of unprotected intercourse in the two weeks prior to IUD placement. We estimated ovulation dates for each cycle in which an IUD was placed by adding the reported cycle length to the participant’s last menstrual period and then subtracting 14 days [6]. Consistent with prior studies, we defined the fertile window as 5 days before and one day after the estimated date of ovulation [6]. We calculated 97.5% confidence intervals using the exact binomial method [15]. We conducted data analysis with Stata 15 statistical software (StataCorp LP, College Station, TX USA).
3. Results
One hundred thirty-four women from the four studies met inclusion criteria. Figure 1 illustrates participant flow. Table 1 details participant characteristics; 93% of the participants were less than 25 years of age. Follow-up determination from the combined dataset included urine pregnancy testing and follow-up 2–4 weeks after IUD placement in 95 (71%) women and reporting of concern for pregnancy 1 month after IUD placement in 39 (29%) women. These two groups total 134 women determined eligible for analysis who were not pregnant 1 month after IUD placement.
Table 1:
Participant characteristics among women initiating a copper intrauterine device 6–14 days after unprotected sex (N=134)
| Age (years) | |
| 18–19 | 111 (83%) |
| 20–24 | 13 (10%) |
| 25+ | 10 (7%) |
| Race/Ethnicity | |
| White | 81 (60%) |
| Hispanic | 27 (20%) |
| Other | 26 (19%) |
| Previously pregnant | 83 (62%) |
| Relationship status* | |
| Single | 63 (48%) |
| Married/Cohabitating | 69 (52%) |
| Education | |
| High school or less | 57 (43%) |
| College, vocational training | 75 (57%) |
| BMI (kg/m2)* | |
| Underweight (<18.5) | 2 (3%) |
| Normal (18.5–24.9) | 26 (39%) |
| Overweight (25–29.9) | 17 (26%) |
| Obese (≥30) | 21 (32%) |
| Cycle length 25–35 days | 109 (81%) |
| Menstrual cycle day | 18.5 (9–25) |
| UPI in fertile window** | 59 (44%) |
| UPI episodes 6–14 days | 2.4 ±2.0 |
Data are presented as n(%), mean ± standard deviation, or median (interquartile range)
Variables with missing data
Fertile window is the set of 5 days prior to and one day after calculated ovulation date [6]
BMI – body mass index
UPI – unprotected intercourse
None of the 134 women experienced a pregnancy within 1 month of IUD placement (0%, 97.5% CI 0–2.7%) (Figure 1). Nearly half (n=59, 44%) of participants had reported unprotected intercourse during their fertile window; when the sample is limited to these women, the 97.5% CI ranges from 0–6%. Approximately two thirds of study participants (n=85, 63%) reported unprotected intercourse on multiple days during the two weeks prior to IUD placement, with a median of 4 days with unprotected intercourse events. When we limited the sample to individuals reporting multiple episodes of unprotected intercourse, risk of pregnancy following IUD placement remained low (97.5% CI 0–4.2%).
4. Discussion
In this dataset that included prospectively collected data from four similar study protocols that included 134 women desiring placement of a CuT380A IUD 6–14 days after unprotected intercourse, none reported a pregnancy within 1 month of IUD placement. The dataset included women who reported multiple acts of unprotected intercourse as well as unprotected intercourse in the fertile window. Two prior studies also reported no pregnancies within one month among women who had CuT380A IUD placement more than 5 days after unprotected intercourse [5,6]. Wu et al [6] reported on 52 women who reported unprotected intercourse 6–14 days prior to IUD insertion and Goldstuck et al [5] reported on 64 women with IUD placement on or after the 6th day following unprotected intercourse. Combining their data with our current study provides a total of 250 women with no pregnancies (0%, 97.5% CI 0–1.5%); this calculation provides even greater reassurance that pregnancy is unlikely when an IUD is placed for a woman reporting unprotected intercourse in the prior 6–14 days.
Previously, the CDC’s SPR has recommended that providers be “reasonably certain that a woman is not pregnant” prior to IUD insertion [7] and avoid IUD placement for those who report unprotected intercourse 6–14 days prior to a clinic visit [7,16]. The combined results presented here indicate a high likelihood that less than 1.5% of women are expected to experience a pregnancy following copper IUD placement, even if they report unprotected intercourse in the prior 6–14 days. For many women and their clinicians, these data may be sufficient to support same-day placement of a copper IUD rather than rescheduling IUD placement. Larger clinical trials designed to provide more precise estimates of the rate of pregnancy following Cu-IUD placement for women with recent unprotected intercourse would be ideal to answer this question more precisely. However, we believe such trials would be challenging to execute as it has taken this multi-site collaboration five years to accumulate the data presented here.
Strengths of this analysis include using multiple prospective studies, multisite recruitment and a high rate of follow up. In addition, 93% of study participants were less than 25 years of age, a time of peak fecundability [17]; still, the pregnancy rate was zero. Participant recruitment posed more of a challenge than anticipated. Aggregation of data from several studies required use of different measures to assess pregnancy status, and HER Salt Lake participants were not required to perform a urine pregnancy test unless they were concerned about pregnancy. However, the availability of 6 months of follow up data from these participants, strengthens our ability to conclude that none of these 39 women became pregnant after IUD placement.
In conclusion, this evidence supports placement of a Cu-IUD for women reporting unprotected intercourse in the prior 6–14 days if patients and providers are willing to accept a low risk of experiencing an undesired pregnancy after IUD placement.
Acknowledgements:
Special thanks to Kathy Burke, Penny Davies, and the staff at Planned Parenthood Association of Utah and Planned Parenthood of Minnesota.
Funding: This work was supported by the Society of Family Planning Research Fund Grant # SFPRF15–2 and the William and Flora Hewlett Foundation. We collected and managed study data using REDCap (Research Electronic Data Capture) software hosted at the University of Utah; this service is supported by Center for Clinical and Translational Sciences grant 8UL1TR000105 (formerly UL1RR025764, NCATS/NIH). All authors played a crucial role in the combined effort of researching and writing this paper. Team members receive support from the Eunice Kennedy Shriver National Institute of Child Health & Human Development and the Office of Research on Women’s Health of the National Institute of Health, JNS via Award Number K12HD085852 and DKT via K24HD087436 and 1R01HD083340. The content is solely the responsibility of the authors and does not necessarily represent the official view of any of the funding agencies or participating institutions, including the National Institutes of Health, the University of Utah or Planned Parenthood Federation of America, Inc.
Conflict of Interest: The University of Utah Department of Obstetrics and Gynecology Program in Family Planning receives research funding from Bayer Women’s Health Care, Merck & Co. Inc., Cooper Surgical, Bioceptive, Sebela Pharmaceuticals, and Medicines 360. The other authors have no conflicts of interest to report.
Key:
- CI
Confidence interval
- Cu IUD
Copper T380A intrauterine device
- Neg
negative
- UPI
Unprotected intercourse
- UPT
Urine pregnancy test
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Westhoff C, Heartwell S, Edwards S, Zieman M, Cushman L, Robilotto C, et al. Initiation of oral contraceptives using a quick start compared with a conventional start: a randomized controlled trial. Obstet Gynecol 2007;109:1270–6. [DOI] [PubMed] [Google Scholar]
- [2].Murthy AS, Creinin MD, Harwood B, Schreiber CA. Same-day initiation of the transdermal hormonal delivery system (contraceptive patch) versus traditional initiation methods. Contraception 2005;72:333–6. [DOI] [PubMed] [Google Scholar]
- [3].Committee on Adolescent Health Care Long-Acting Reversible Contraception Working Group, The American College of Obstetricians and Gynecologists. Committee Opinion No. 539. Obstet Gynecol 2012;120:983–8. [DOI] [PubMed] [Google Scholar]
- [4].Committee on Gynecologic Practice Long-Acting Reversible Contraception Working Group. Committee Opinion No. 642. Obstet Gynecol 2015;126:e44–8.26393458 [Google Scholar]
- [5].Goldstuck ND. Delayed postcoital IUD insertion. Contracept Deliv Syst 1983;4:293–6. [PubMed] [Google Scholar]
- [6].Turok DK, Godfrey EM, Wojdyla D, Dermish A, Torres L, Wu SC. Copper T380 intrauterine device for emergency contraception: highly effective at any time in the menstrual cycle. Hum Reprod 2013;28:2672–6. [DOI] [PubMed] [Google Scholar]
- [7].Curtis KM, Jatlaoui TC, Tepper NK, Zapata LB, Horton LG, Jamieson DJ, et al. U.S. Selected Practice Recommendations for Contraceptive Use, 2016. MMWR Recomm Reports 2016;65:1–66. [DOI] [PubMed] [Google Scholar]
- [8].Sanders JN, Howell L, Saltzman HM, Schwarz EB, Thompson IS, Turok DK. Unprotected intercourse in the 2 weeks prior to requesting emergency intrauterine contraception. Am J Obstet Gynecol 2016;215. [DOI] [PubMed] [Google Scholar]
- [9].Glasier A, Cameron ST, Blithe D, Scherrer B, Mathe H, Levy D, et al. Can we identify women at risk of pregnancy despite using emergency contraception? Data from randomized trials of ulipristal acetate and levonorgestrel. Contraception 2011;84:363–7. [DOI] [PubMed] [Google Scholar]
- [10].Turok DK, Schwarz EB. Quick Start of Highly Effective Contraception. ClinicalTrialsGov 2014. https://clinicaltrials.gov/ct2/show/NCT02076217. [Google Scholar]
- [11].Turok DK, Sanders JN, Thompson IS, Royer PA, Eggebroten J, Gawron LM. Preference for and efficacy of oral levonorgestrel for emergency contraception with concomitant placement of a levonorgestrel IUD: a prospective cohort study. Contraception 2016;93:526–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Sanders JN, Myers K, Gawron LM, Simmons RG, Turok DK. Contraceptive method use during the community-wide HER salt lake contraceptive initiative. Am J Public Health 2018;108:550–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Turok D RAPID EC - Rct Assessing Pregnancy With Intrauterine Devices for EC. ClinicalTrialsGov 2014. https://clinicaltrials.gov/ct2/show/NCT02175030 (accessed August 7, 2018). [Google Scholar]
- [14].Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform 2009;42:377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Jovanovic BD, Zalenski RJ. Safety evaluation and confidence intervals when the number of observed events is small or zero. Ann Emerg Med 1997;30:301–6. [DOI] [PubMed] [Google Scholar]
- [16].Labbok MH, Perez A, Valdes V, Sevilla F, Wade K, Laukaran VH, et al. The Lactational Amenorrhea Method (LAM): a postpartum introductory family planning method with policy and program implications. Adv Contracept 1994;10:93–109. [DOI] [PubMed] [Google Scholar]
- [17].Schwartz D, Mayaux MJ, Mayaux MJ. Female Fecundity as a Function of Age. N Engl J Med 1982;306:404–6. [DOI] [PubMed] [Google Scholar]