Abstract
Cytoskeletal networks are foundational examples of active matter and central to self-organized structures in the cell. In vivo, these networks are active and densely crosslinked. Relating their large-scale dynamics to the properties of their constituents remains an unsolved problem. Here, we study an in vitro active gel made from aligned microtubules and XCTK2 kinesin motors. Using photobleaching, we demonstrate that the gel’s aligned microtubules, driven by motors, continually slide past each other at a speed independent of the local microtubule polarity and motor concentration. This phenomenon is also observed, and remains unexplained, in spindles. We derive a general framework for coarse graining microtubule gels crosslinked by molecular motors from microscopic considerations. Using microtubule-microtubule coupling through a force-velocity relationship for kinesin, this theory naturally explains the experimental results: motors generate an active strain rate in regions of changing polarity, which allows microtubules of opposite polarities to slide past each other without stressing the material.
Active materials are made from energy-consuming constituents whose activity keeps the system out of equilibrium. Their study has deepened our understanding of self-organizing processes, both in vivo and in vitro1,2. Its foundational examples include suspensions of microswimmers, in vitro assemblies of purified cellular components and the cell cytoskeleton3. Although notable progress has been made in deciphering the non-equilibrium physics of active materials by using experiments and symmetry-based phenomenological theories4–6, there is far less understanding of how the large-scale dynamics actually devolves from microscopic activity. Such a theoretical framework would allow for rational design of new active materials and quantitative understanding of the cell cytoskeleton. We address this challenge for systems made of cytoskeletal polymers that are fully percolated by moving molecular motors. First, we describe the microscopic dynamics of a dense nematically aligned active gel made from purified microtubules and XCTK2 kinesin motors. Using photobleaching and second harmonic generation (SHG) microscopy, we show that the microtubule sliding speeds are independent of the local gel polarity and motor concentration, suggesting a robust internal coupling of the filamentous material. To investigate this phenomenon, we introduce a framework for systematically deriving continuum theories of densely crosslinked active gels from prescribed microscopic considerations. We use this framework to obtain a theory for the X CTK 2-microtu-bule system, which explains our experimental findings without adjustable parameters.
Many aspects of cell biology, including cell shape, motility and division, are driven by the cytoskeleton7. The cytoskeleton consists of polar filaments (mainly actin and microtubules) and the proteins that crosslink them and organize their behaviour. Molecular motors are active crosslinkers that use chemical energy to move filaments relative to each other. They play a central role in determining the architecture and dynamics of cytoskeletal structures such as the cell cortex and the spindle3. How large-scale behaviours of actively crosslinked networks emerge from the properties of their constituents is important for quantitative understanding of the cellular cytoskeleton8–10. The in vitro XCTK2-microtubule system that we study recapitulates the previously unexplained polarity-independent sliding motion of microtubules that was observed using speckle microscopy in Xenopus meiotic spindles11–13 and allows us to study how polarity-independent filament motion can emerge in actively crosslinked networks.
Phenomenological theories for actively crosslinked networks have been derived from conservation laws and symmetry considerations1,4–6,14. They can reproduce the flow patterns observed in artificial15 and biological systems, such as the cell cortex8,16–18, and quantitatively explain some aspects of spindle structure and dynamics19. However, such theories do not address how the large-scale behaviours of actively crosslinked networks emerge from the properties of their constituents. For this, an approach that derives macroscopic material laws from the properties of motors and filaments is needed.
Previous efforts to derive continuum theories for active gels from microscopic interactions have considered sparsely crosslinked systems, in which individual motor-filament clusters are thought of as disconnected objects20–30. In contrast, actively crosslinked networks, such as the cytoskeleton3, are tightly coupled over length scales comparable to the system size and display behaviours different from those predicted for sparsely crosslinked active fluids. The rheologi-cal properties of highly crosslinked gels have been studied using combinations of numerical and analytical techniques31–35. Here, we present an analytic framework for deriving continuum equations that capture their dynamics.
Experiments
To study the behaviour of heavily crosslinked active gels, we created one from purified microtubules and 0.4 μM of XCTK2, a minus-end directed kinesin-14 molecular motor capable of crosslinking and sliding aligned microtubules36. Fluorescently labelled tubulin and fluorescently labelled XCTK2 were mixed together, and then paclitaxel was added, which nucleated and stabilized the microtubules. The sample was rapidly loaded into a 0.1 × 1 × 20mm3 rectangular microfluidic chamber (see Supplementary Information). The microtubule-motor mixture contracted within the first ~10 min and formed a macroscopic gel, with its microtubules nematically aligned parallel to the long axis of the microfluidic chamber. The sample remained in this macroscopically aligned state for ~1 h, after which the gel buckled and displayed complex dynamics for hours longer. Along the long axis, the boundaries of the material remained frictionally pinned at the chamber’s ends throughout the experiment. In this study, we characterize the motions of microtubules in the macroscopically aligned state of the gel, before buckling occurred.
We used a femtosecond laser to photobleach microtubules in the aligned gels, near the centre of the chamber, and investigate their dynamics. We generated multiple bleach lines orthogonal to the nematic director (Fig. 1a). Each of the bleached lines split into two parallel lines, which moved apart along the direction of alignment (Fig. 1b, left), indicating that microtubules in the gel are continually sliding relative to each other. Control experiments confirmed that the laser bleached, but did not ablate, the microtubules (see Supplementary Information).
Fig. 1 |. Bleaching of aligned active gels reveals microtubule sliding speed.
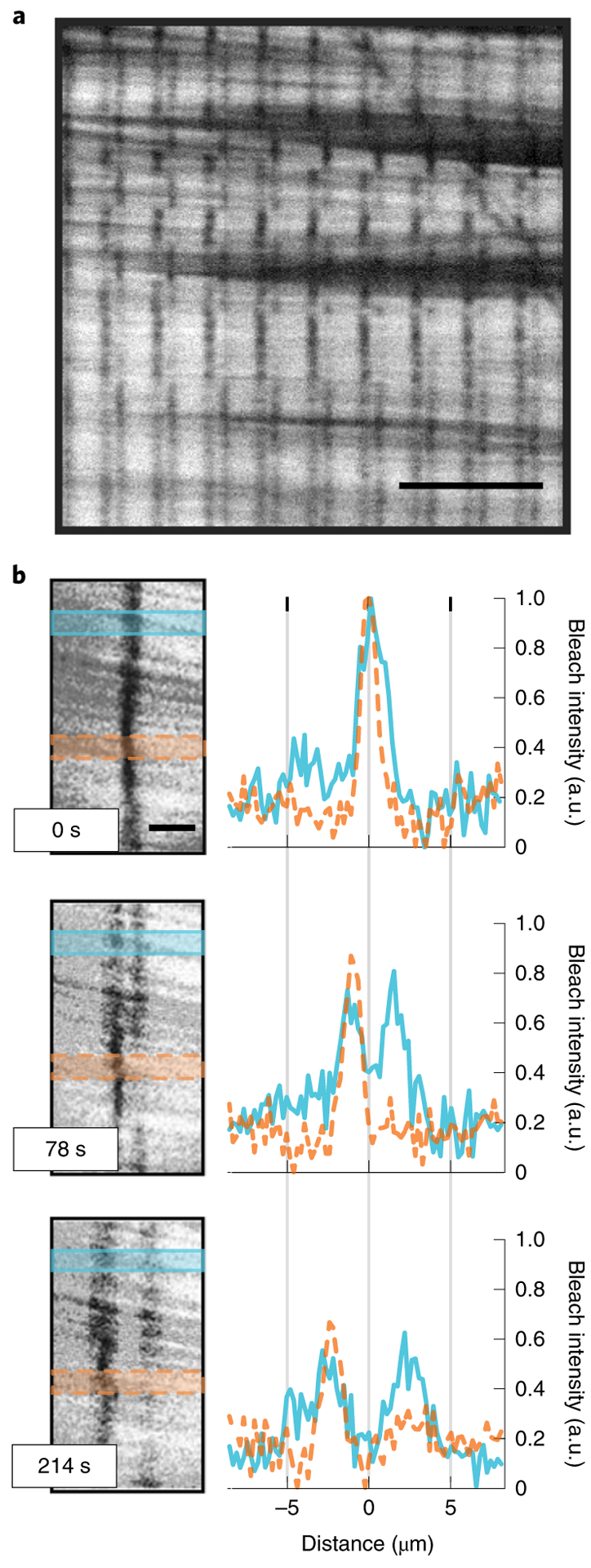
a, Aligned active gel after multi-line photobleaching. Scale bar, 20 μm. b, Left: higher magnification images of a bleached line taken at three different times. The bleached line with spatially varying polarity splits into two parallel lines as the microtubules slide apart. Solid blue and dashed orange shaded areas highlight regions of low and high polarity respectively. Right: intensity profiles of the high polarity (dashed orange, Pbleach = 0.63 ±0.12 standard error) and low polarity (solid blue, Pbleach = 0.08 ± 0.17 standard error) regions. Despite the difference in polarity, the peaks move apart at nearly identical speeds. Scale bar, 5 μm. XCTK2 concentration is 0.4 μM.
When a bleached line splits into two, the relative fluorescent intensity of the two new lines reflects the relative number of left-moving and right-moving microtubules. Hence, the relative bleach intensity provides a measure of the polarity at the initial location of the bleach. We define the bleach polarity, , where I1 and I2 are the bleach intensities of the two lines (see Supplementary Information). The speed at which the two lines move apart provides a measure of the speed of microtubule sliding. Along the same bleach line, some regions have very high polarity (Fig. 1b, orange, Pbleach = 0.63 ± 0.12), while others have very low polarity (Fig. 1b, blue, Pbleach = 0.08 ± 0.17). Despite this difference in polarity, the speed of sliding is very similar (Fig. 1b, right, compare orange and blue). We next performed experiments with varying motor concentrations. We measured the polarity and sliding speeds for four different motor concentrations (0.3, 0.4, 0.5 and 0.75 μM), each at many different locations, and found that the speed of microtubule sliding was independent of both polarity and motor concentration (Fig. 2). For motor concentrations below 0.3 μM, the microtubules did not contract and did not spontaneously align. For motor concentrations above 0.75 μM the gels buckled before settling down into an aligned state. The average speed that microtubules slid apart in these XCTK2 active gels was 18.6 ± 0.9 nm s−1, which is very close to the observed speed of 20 nm s−1 at which XCTK2 slides apart isolated pairs of microtubules36.
Fig. 2 |. Microtubule sliding speed is independent of polarity and motor concentration.
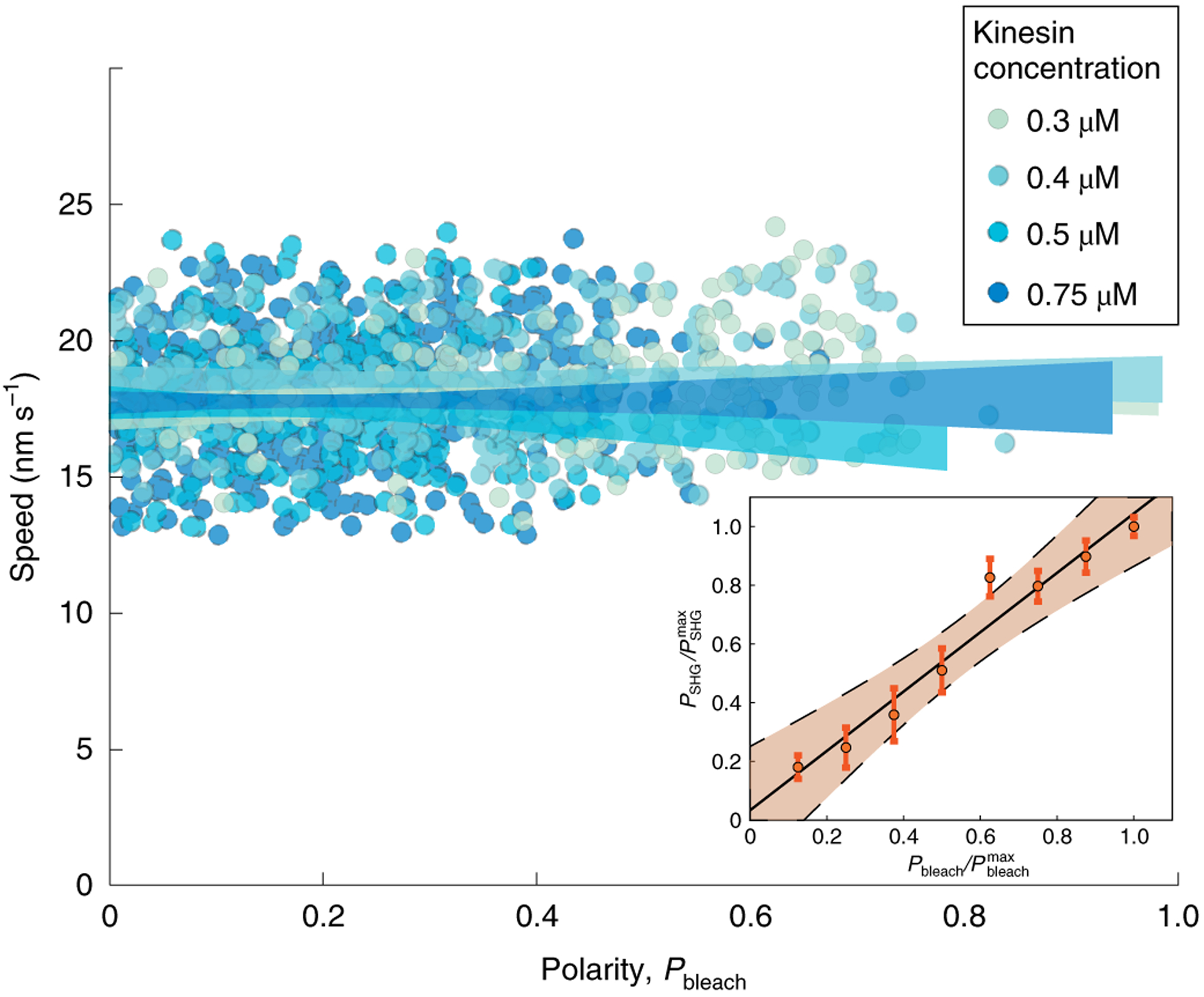
Local measurements (2μm bins) from photobleaching of microtubule sliding speed and polarity, Pbleach, for different XCTK2 concentrations. The shaded regions indicate the 95% confidence intervals of a linear regression for each motor concentration. Inset: correlation between local polarity Pbleach of microtubule motion measured by bleaching and local physical polarity PSHG of microtubules measured by SHG. The black line is a linear regression and the shaded region indicates the corresponding 99% confidence interval.
Pbleach provides a local measure of the polarity of microtubule motion, that is, of the relative number of left-moving and right-moving microtubules. We next sought to determine to what extent this polarity of microtubule motion is related to the physical polarity of microtubules in the gel, that is, of the relative number of left-facing and right-facing microtubules. The physical polarity of microtubule arrays can be measured by simultaneous SHG, a nonlinear optical technique in which a signal can be produced from non-centrosym metric materials, and two-photon microscopy of fluorescent microtubules. The local physical polarity is proportional to , where ISHG is the intensity measure from SHG and Iρ is the intensity measure from two-photon microscopy37. We simultaneously measured PSHG and Pbleach at multiple locations, and found that they are highly correlated (Fig. 2, inset). Therefore, in this gel, the local dynamic polarity of microtubule motion reflects the local physical polarity of microtubules, and the microtubule sliding speed is independent of the local polarity. This is reminiscent of the independence of microtubule sliding speed and polarity observed in spindles11–13.
The polarity-independent sliding speed of microtubules is difficult to reconcile with existing kinetic theories, which apply in the limit of dilute and sparsely crosslinked networks where the length scale over which filaments are interconnected is small compared to the system size. To illustrate the issue we describe previously presented arguments20,28. In the absence of external driving forces, the balance of forces yields ρ+f+ + ρ−f− = 0, where ρ± are the densities of microtubules pointing along the nematic axis of the system in the positive and negative directions, respectively, and f± are the forces acting on microtubules from their interactions with ∓ microtubules, respectively. In a sparsely crosslinked system the force exerted by a motor on a microtubule is balanced only by the drag between the microtubule and the surrounding medium. Assuming that the medium is locally at rest, the velocity of microtubules, v±, will be given by v±=μf±, where μ is the microtubule mobility. A molecular motor crosslinking two microtubules moving at a speed V imposes v+−v−= 2V. Taken together, this leads to v±=∓V(1∓P), where is the local microtubule polarity.
The argument described above predicts that the microtubule sliding velocity changes linearly with polarity, in conflict with our experimental measurements of reconstituted gels and spindle. Fundamentally, this prediction is a consequence of force balance in sparsely crosslinked systems: the mass fluxes of left moving and right moving microtubules locally balance because the momentum transfer in the system is dominated by fluid-mediated interactions. Polarity-dependent sliding generalizes to more sophisticated versions of dilute theories that include effects such as long-range hydrodynamics (see Supplementary Information). The disagreement between this theory and experiment suggests that microtu-bule-XCTK2 gels may not be in a sparsely crosslinked regime. To examine this possibility, we quantified the microtubule density in our gel by measuring the initial molarity of components in our mixture and then determined the percentage of components that were incorporated into the final gel via fluorescence microscopy. We estimate a 5% volume fraction of polymerized microtubules in the gels, with 17 microtubules per μm3 (see Supplementary Information) and ~25 XCTK2 dimers bound to each microtubule. Given that microtubules are significantly longer than their average spacing, this result argues that these networks are heavily crosslinked by molecular motors—far from the dilute theory described above. Motivated by such considerations, we developed a model of heavily crosslinked microtubule networks.
Theory for densely crosslinked microtubule gels
In our theory, microtubules are characterized by their length L, velocities vi and orientations pi. The distribution of microtubule positions and orientations obeys the Smoluchowski equation . The microtubules’ translational and rotational fluxes, , and , respectively, can be determined from the conditions Fi = 0 and Ti= 0, which state that the total force Ft and torque Tt on each microtubule are zero.
The forces and torques applied to microtubules are generated by the molecular motors that actively crosslink them. Here, motors are characterized by their force-velocity relation, the distance R (the motor size) over which they can crosslink filaments, and the crosslinking torque that they apply. The force that microtubule j exerts on microtubule i, via active crosslinks between xt + stpt and xj + sjpj (Fig. 3a), is given by Fijdsidsj and the total force on microtubule i is
| (1) |
where Ω(x) denotes a sphere of radius R centred at x (Fig. 3b). The force density can be written as the divergence of the network stress tensor Σ according to Kirkwood’s formula since Fij = −Fji (see Supplementary Information). The force balance of the network reads
| (2) |
In a dense and highly crosslinked system, the motion of the fluid in which microtubules are suspended follows the microtubule motion and can be ignored (see Supplementary Information). The torque that crosslinkers between filaments i and j exert on filament i is given by Tijdsidsj with Tij= sipi×Fij+Γij, where Γij is the contribution stemming from an explicit crosslinker torque. The total torque Ti on microtubule i obeys an equation analogous to equation (1) (see Supplementary Information).
Fig. 3 |. Sketch of the microscopic model.
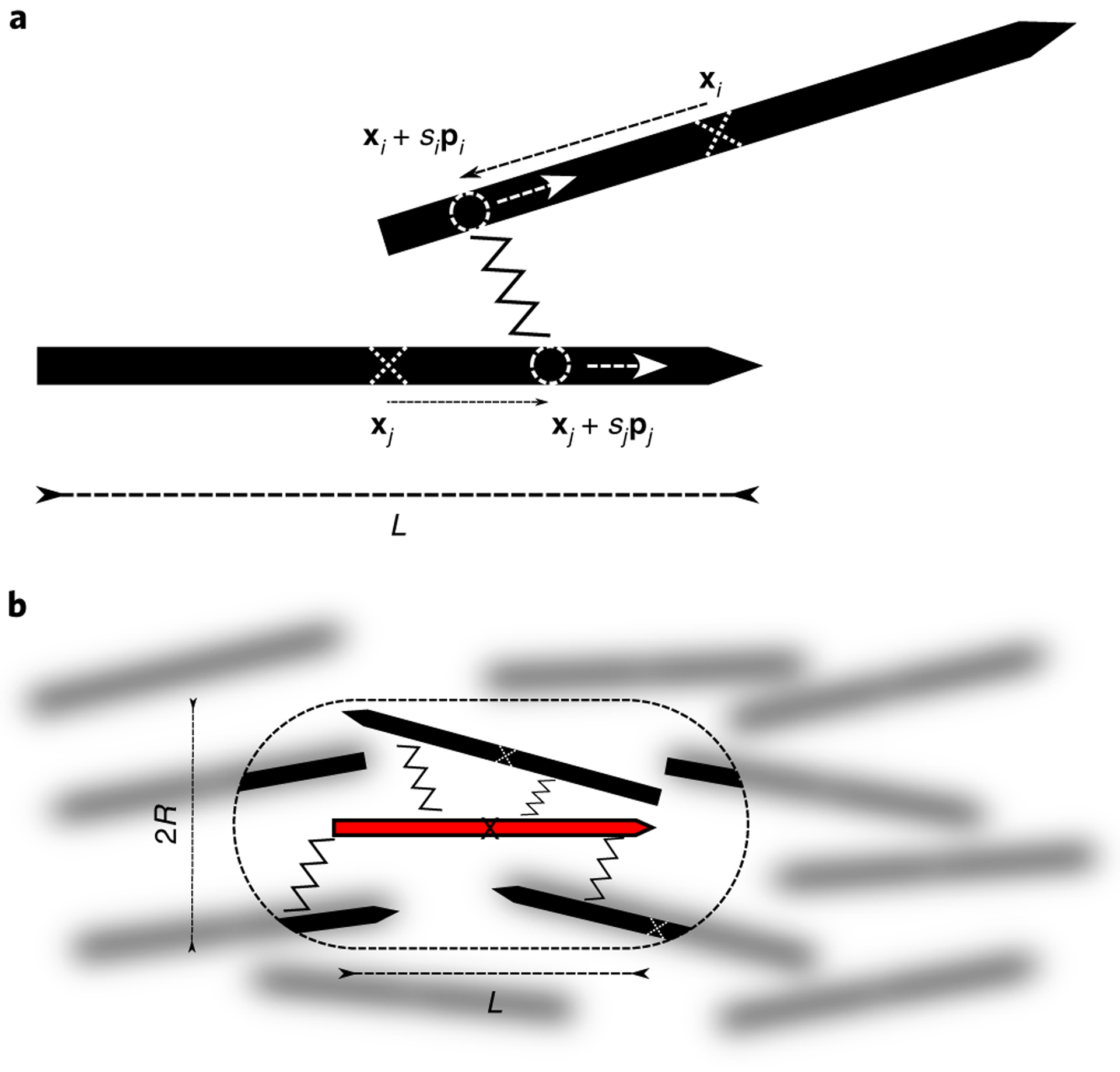
a, Each microtubule (black solid arrow) is characterized by its centre of mass position x (white crosses) and its orientation p. Microtubules interact via motor molecules (zigzag line), which connect them at arclength positions si, sj (white circles) and walk towards their plus ends (white arrows). b, In the gel, crosslinkers of a test microtubule (red) explore all possible connections to other microtubules within reach (within the dashed capsule), that is, within a sphere of radius R around any position along the test microtubule.
We model the XCTK2 motors by a force-velocity relation and a crosslinking torque density. The force density Fij(Δvij.) exerted by motors depends on the velocity difference Δvij between their two motor heads. The crosslinking torque density Γij(pi, pj) depends on their orientations. For simplicity, we ignore the effects of non-uniform distributions of motors along microtubules. Furthermore, we do not account for the buildup of elastic strain in the motors, which is a good approximation on timescales that are large compared to motor binding/unbinding times. To linear order in Δvij
| (3) |
where Vpi is the velocity of the motor relative to the microtubule it is bound to. This choice renders the force between two microtubules dependent on their relative polarities. The linear response coefficient G is the motor friction and is in general a second rank tensor. In the following we choose G = γI for simplicity. Note that our results also hold for force velocity relations that are nonlinear in Δvij (see Supplementary Information). For the crosslinking torque density we choose Γij = vpi×pj(pi·pj), which aligns microtubules, as would for example a torsional stiffness of the crosslinker. The coefficient v characterizes the magnitude of this effect. Note that, dimensionally, Fij and Tij are forces and torques per area, respectively. It is straightforward to generalize our formulation to include motor activity that varies along the length of a filament, say by pausing at microtubule ends. This effect has been linked to network contractions20,27,35.
We next derive a continuum theory for our system. We start by expanding equation (1) around the centre-of-mass positions xi and xj:
| (4) |
Here we have assumed that the length scale a of gradients in the system is large compared to the microtubule length L and dropped terms with more than two spatial derivative (see Supplementary Information). We also introduced density ρ(x) = ∫dpψ(x, p), the polarity P(x) = 〈pi〉, the rotation rate tensor and the velocity field v(x) = 〈vi〉, where the angled brackets denote local averaging. The force balance equation (2) becomes
| (5) |
Now, using R/a≪1, we expand the integrand in equation (5) around x and perform the integration. In this way, for the gel stress tensor we obtain
| (6) |
The coefficients and have dimensions of viscosity by density squared. The first term of the stress tensor in equation (6) is viscous-like (that is, it depends on ∇v) and captures long-ranged coupling between microtubules. In contrast to a dilute suspension theory, in which a viscous coupling would be induced by the fluid, here it is induced by the crosslinkers. For active crosslinkers (V≠ 0) the stress-free state is self-straining and the gel’s spontaneous strain rate is ∇v = −V∇P. The second term of equation (6) is generated by microtubules reorienting in the gel and is analogous to a nematic alignment stress in liquid crystal theory. (We note that the ordering stress recovers the form derived for kinetic theories that approximate microtubule alignment by the Maier-Saupe free-energy26 when using equation (8).) From equation (4), using ∇ · Σ= 0, we obtain the translational flux of microtubules:
| (7) |
An analogous calculation for the torque balance (see Supplementary Information) yields
| (8) |
which is reminiscent of Maier-Saupe theory, where and where is the nematic tensor order parameter. The force balance ∇ · Σ = 0 and equations (6), (7) and (8) fully specify the system’s dynamics and can be used to evolve the distribution of microtubule positions and orientations.
Comparison and interpretation
We next use the above described theory to investigate microtubule sliding in actively crosslinked networks. First we show that vi = −Vpi is the solution to an aligned network, which is the experimentally observed behaviour. Consider a fully aligned gel with all , for some unit orientation vector . With this, equation (8) yields . We make the ansatz vi = −Vpi+ C0 + xi × C1, where C0, C1 denote constant rates of translation and rotation, respectively. After evaluating v = 〈vi〉 and p = 〈pi〉, this micro-scale ansatz obeys equation (7) and yields a macro-scale spatially uniform stress tensor satisfying Σ ≡ 0. It thus solves the force balance ∇ · Σ = 0 with stress free boundary conditions for any system geometry. To determine the constants C0 and C1 we impose that the gel be at rest in the lab frame, such that and , where we choose a coordinate system for which x = 0 is the system’s centre of mass. In our experiments, to good approximation and , that is the total number of left and right pointing microtubules are approximately equal and polar domains are distributed homogeneously throughout the system. With this, we find vi =−Vpi.
This means that all microtubules translate with the motor velocity in the direction of their plus end. The microtubule velocities are independent of the local polarity and only depend on the speed of the kinesin motors. This phenomenon is explained by motors generating an active strain rate in regions of varying polarity. Thus, microtubules can slide past each other without stressing the material because ∇v= −V∇P; see equation (6). This surprising behaviour is a collective effect, as can easily be seen when considering the case of two isolated microtubules: two parallel microtubules linked by motors will be at rest in the lab frame, while two anti-parallel ones will move. Ultimately, the system consists of two inter-penetrating gels of microtubules of opposite polarity that push against each other, with each gel held together by viscous coupling. In contrast, for sparsely crosslinked networks, the mass fluxes of left-moving and right-moving microtubules locally balance, leading to a strong dependence of sliding speed on the local polarity.
Thus our theory makes a series of quantitative predictions. First, the microtubules’ sliding speed should be independent of the local network polarity. Second, the sliding velocity should be independent of the motor concentration. We find both of these predictions to be accurate in our experiments (Fig. 2). Third, we predict the sliding speed is set by the speed of the motor itself. We measured speeds of 18.6 ± 0.9 nm s−1, which are comparable to the single-molecule motor speeds of XCTK2 (~20 nms−1)36. Importantly, our predictions do not depend on adjustable parameters and arise directly from the form of equations (1) and (3). In turn, the form of equation (3) results from imposing that molecular motors act uniformly along the length of the microtubules, which is a good approximation for the XCTK2 motor at high enough densities36.
Polarity-independent sliding velocities are also observed in spindles formed in Xenopus egg extracts. These spindles consist of an array of microtubules that are anti-parallel near the spindle centre and highly polar at the spindle poles37–38. Microtubules in these spindles continually slide toward spindle poles, a motion believed to be driven by the molecular motor kinesin-511. Due to the symmetries observed in spindles38, and , as in our in vitro experiments. Because motors in spindles are abundant, we speculate that spindles might be self-straining, like the XCTK2-micro-tubule gel presented here. If this were the case, the speed of sliding should be the speed of kinesin-5 itself and constant throughout the spindle. Very suggestively, this is exactly what has been observed. In spindles, and particularly so when dynein is inhibited, microtubules slide at a constant speed12,13 that is approximately equal to the speed at which kinesin-5 slides apart pairs of anti-parallel microtubules in vitro39. This suggests that, like our experimental system, spindles might consist of two inter-penetrating gels of microtubules of opposite polarity. Mechanically, this puts them in a regime between polymer suspensions and solid-like structures.
Our analytical framework can be used to investigate microtubule networks crosslinked by different motors. For example, dyneins pause and accumulate at microtubule minus ends40. This motor dynamics can be implemented within our theory by modifying equation (3), which gives rise to and ρ dependent active stresses (the calculation that predicts stresses for a generalized force-velocity curve will be presented in a subsequent publication). Such active stresses explain dynein-driven contractions of microtubule networks27,35,40. Thus, the described theory generalizes previous models for network contractions27 by providing a rigorous framework to predict how different motor properties give rise to different active stresses.
The self-straining state depends only on the balance between activity and viscous material response. Elastic deformations of either microtubules or motors do not play a role in this behaviour. Elastic, active networks can behave quite differently from the purely viscous systems31–33. For example, in elastic, active polymer gels, passive crosslinkers facilitate the buildup of contractile stresses34, which do not arise in the purely viscous limit. In our viscous theory, adding passive crosslinkers turns out to be exactly equivalent to slowing the motor. The interplay between viscous and elastic effects can be studied in our framework by augmenting equation (3) with elastic, history-dependent terms.
Many cytoskeletal networks are densely crosslinked. For example, the contractility of acto-myosin networks has been explained as emerging from its heavily crosslinked nature33,34. The work presented here is an important step towards predicting the material properties of actively crosslinked materials from the properties of their constituents and will enable the design of novel dynamics from first principles.
Supplementary Material
Acknowledgements
C.E.W. acknowledges support by NIH R35GM122482. D.J.N. acknowledges the Kavli Institute for Bionano Science and Technology at Harvard University and National Science Foundation grants PHY-1305254, PHY-0847188, DMR-0820484 and DBI-0959721. P.J.F. acknowledges support from the Gordon and Betty Moore Foundation for support as a Physics of Living Systems Fellow through grant no. GBMF4513. M.J.S. acknowledges support from National Science Foundation grants DMR-0820341 (NYU MRSEC), DMS-1463962 and DMS-1620331. Z.D. acknowledges support from NSF MRSEC DMR-1420382.
Footnotes
Reporting Summary. Further information on research design is available in the Nature Research Reporting Summary linked to this article.
Online content
Any methods, additional references, Nature Research reporting summaries, source data, statements of code and data availability and associated accession codes are available at https://doi.org/10.1038/s41567-019-0642-1.
Data availability
Figures 1 and 2 are based on microscopy data. The raw data are available from the authors upon reasonable request.
Competing interests
The authors declare no competing interests.
Supplementary information is available for this paper at https://doi.org/10.1038/s41567-019-0642-1.
Reprints and permissions information is available at www.nature.com/reprints.
Peer review information: Nature Physics thanks Karin John, Gijsje Koenderink and the other, anonymous, reviewer(s) for their contribution to the peer review of this work.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Marchetti MC et al. Hydrodynamics of soft active matter. Rev. Mod. Phys 85, 1143–1189 (2013). [Google Scholar]
- 2.Needleman D & Dogic Z Active matter at the interface between materials science and cell biology. Nat. Rev. Mater 2, 17048 (2017). [Google Scholar]
- 3.Alberts B et al. Molecular Biology of the Cell 4th edn (Garland, 2002). [Google Scholar]
- 4.Kruse K, Joanny J-F, Jülicher F, Prost J & Sekimoto K Generic theory of active polar gels: a paradigm for cytoskeletal dynamics. Eur. Phys. J. E 16, 5–16 (2005). [DOI] [PubMed] [Google Scholar]
- 5.Joanny JF, Jülicher F, Kruse K & Prost J Hydrodynamic theory for multi-component active polar gels. New J. Phys 9, 422 (2007). [Google Scholar]
- 6.Jülicher F, Grill SW & Salbreux G Hydrodynamic theory of active matter. Rep. Prog. Phys 81, 076601 (2018). [DOI] [PubMed] [Google Scholar]
- 7.Bray D Cell Movements: From Molecules to Motility 2nd edn (Garland, 2001). [Google Scholar]
- 8.Naganathan SR et al. Morphogenetic degeneracies in the actomyosin cortex. elife 7, e37677 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roostalu J, Rickman J, Thomas C, Nédélec F & Surrey T Determinants of polar versus nematic organization in networks of dynamic microtubules and mitotic motors. Cell 175, 796–808 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Foster PJ, Fürthauer S, Shelley MJ & Needleman DJ From cytoskeletal assemblies to living materials. Curr. Opin. Cell Biol 56, 109–114 (2019). [DOI] [PubMed] [Google Scholar]
- 11.Mitchison TJ Mechanism and function of poleward flux in Xenopus extract meiotic spindles. Phil. Trans. R. Soc. Lond. B 360, 623–629 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burbank KS, Mitchison TJ & Fisher DS Slide-and-cluster models for spindle assembly. Curr. Biol 17, 1373–1383 (2007). [DOI] [PubMed] [Google Scholar]
- 13.Yang G, Cameron LA, Maddox PS, Salmon ED & Danuser G Regional variation of microtubule flux reveals microtubule organization in the metaphase meiotic spindle. J. Cell Biol 182, 631–639 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fürthauer S, Strempel M, Grill SW & Jülicher F Active chiral fluids. Eur. Phys. J. E 35, 1–13 (2012). [DOI] [PubMed] [Google Scholar]
- 15.Thampi SP, Golestanian R & Yeomans JM Velocity correlations in an active nematic. Phys. Rev. Lett 111, 118101 (2013). [DOI] [PubMed] [Google Scholar]
- 16.Salbreux G, Prost J & Joanny J-F Hydrodynamics of cellular cortical flows and the formation of contractile rings. Phys. Rev. Lett 103, 058102 (2009). [DOI] [PubMed] [Google Scholar]
- 17.Mayer M, Depken M, Bois JS, Jülicher F & Grill SW Anisotropies in cortical tension reveal the physical basis of polarizing cortical flows. Nature 467, 617–621 (2010). [DOI] [PubMed] [Google Scholar]
- 18.Naganathan SR, Fürthauer S, Nishikawa M, Jülicher F & Grill SW Active torque generation by the actomyosin cell cortex drives left-right symmetry breaking. elife 3, e04165 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brugués J & Needleman D Physical basis of spindle self-organization. Proc. Natl Acad. Sci. USA 111, 18496–18500 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kruse K & Jülicher F Actively contracting bundles of polar filaments. Phys. Rev. Lett 85, 1778–1781 (2000). [DOI] [PubMed] [Google Scholar]
- 21.Aranson IS & Tsimring LS Pattern formation of microtubules and motors: inelastic interaction of polar rods. Phys. Rev. E 71, 050901 (2005). [DOI] [PubMed] [Google Scholar]
- 22.Liverpool TB & Marchetti MC Bridging the microscopic and the hydrodynamic in active filament solutions. Europhys. Lett 69, 846–852 (2005). [Google Scholar]
- 23.Liverpool TB & Marchetti MC in Cell Motility (ed. Lenz P) 177–206 (Springer, 2008). [Google Scholar]
- 24.Saintillan D & Shelley MJ Instabilities and pattern formation in active particle suspensions: kinetic theory and continuum simulations. Phys. Rev. Lett 100, 178103 (2008). [DOI] [PubMed] [Google Scholar]
- 25.Saintillan D & Shelley MJ Instabilities, pattern formation and mixing in active suspensions. Phys. Fluids 20, 123304 (2008). [DOI] [PubMed] [Google Scholar]
- 26.Saintillan D & Shelley MJ Active suspensions and their nonlinear models. C. R. Phys 14, 497–517 (2013). [Google Scholar]
- 27.Foster PJ, Fürthauer S, Shelley MJ & Needleman DJ Active contraction of microtubule networks. eLife 4, e10837 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gao T, Blackwell R, Glaser MA, Betterton MD & Shelley MJ Multiscale polar theory of microtubule and motor-protein assemblies. Phys. Rev. Lett 114, 048101 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heidenreich S, Dunkel J, Klapp SHL & Bär M Hydrodynamic length-scale selection in microswimmer suspensions. Phys. Rev. E 94, 020601 (2016). [DOI] [PubMed] [Google Scholar]
- 30.Maryshev I, Marenduzzo D, Goryachev AB & Morozov A Kinetic theory of pattern formation in mixtures of microtubules and molecular motors. Phys. Rev. E 97, 022412 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Broedersz CP & MacKintosh FC Modeling semiflexible polymer networks. Rev. Mod. Phys 86, 995 (2014). [Google Scholar]
- 32.Ronceray P & Lenz M Connecting local active forces to macroscopic stress in elastic media. Soft Matter 11, 1597–1605 (2015). [DOI] [PubMed] [Google Scholar]
- 33.Ronceray P, Broedersz CP & Lenz M Fiber networks amplify active stress. Proc. Natl Acad. Sci. USA 113, 2827–2832 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Belmonte JM, Leptin M & Nédélec F A theory that predicts behaviors of disordered cytoskeletal networks. Mol. Syst. Biol 13, 941 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Foster PJ, Yan W, Fürthauer S, Shelley MJ & Needleman DJ Connecting macroscopic dynamics with microscopic properties in active microtubule network contraction. New J. Phys 19, 125011 (2017). [Google Scholar]
- 36.Hentrich C & Surrey T Microtubule organization by the antagonistic mitotic motors kinesin-5 and kinesin-14. J. Cell Biol 189, 465–480 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yu C-H et al. Measuring microtubule polarity in spindles with second-harmonic generation. Biophys. J 106, 1578–1587 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Brugues J, Nuzzo V, Mazur E & Needleman DJ Nucleation and transport organize microtubules in metaphase spindles. Cell 149, 554–564 (2012). [DOI] [PubMed] [Google Scholar]
- 39.Kapitein LC et al. The bipolar mitotic kinesin Eg5 moves on both microtubules that it crosslinks. Nature 435, 114–118 (2005). [DOI] [PubMed] [Google Scholar]
- 40.Tan R, Foster PJ, Needleman DJ & McKenney RJ Cooperative accumulation of dynein-dynactin at microtubule minus-ends drives microtubule network reorganization. Dev. Cell 44, 233–247 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


