Abstract
With the development of modern solid-phase assays to detect anti-HLA antibodies and a more precise histological classification, the diagnosis of antibody-mediated rejection (AMR) has become more common and is a major cause of kidney graft loss. Currently, there are no approved therapies and treatment guidelines are based on low-level evidence. The number of prospective randomized trials for the treatment of AMR is small, and the lack of an accepted common standard for care has been an impediment to the development of new therapies. To help alleviate this, The Transplantation Society convened a meeting of international experts to develop a consensus as to what is appropriate treatment for active and chronic active AMR. The aim was to reach a consensus for standard of care treatment against which new therapies could be evaluated. At the meeting, the underlying biology of AMR, the criteria for diagnosis, the clinical phenotypes, and outcomes were discussed. The evidence for different treatments was reviewed, and a consensus for what is acceptable standard of care for the treatment of active and chronic active AMR was presented. While it was agreed that the aims of treatment are to preserve renal function, reduce histological injury, and reduce the titer of donor-specific antibody, there was no conclusive evidence to support any specific therapy. As a result, the treatment recommendations are largely based on expert opinion. It is acknowledged that properly conducted and powered clinical trials of biologically plausible agents are urgently needed to improve patient outcomes.
INTRODUCTION
Despite modern immunosuppression, ongoing kidney injury and graft loss due to alloantibody-induced immunity remains an important issue.1–4 Driving this response are polymorphic HLA antigens. While the impact of antibodies to HLA on kidney allograft survival has been known for some time, only recently, with the advent of sensitive solid-phase assays to detect donor-specific anti-HLA antibodies (DSA) and the development of the Banff diagnostic criteria for antibody-mediated rejection (AMR), has the size of the problem been realized. By 10 years, after kidney transplant, up to 25% have developed de novo DSA (dnDSA).5 Thus, it is not surprising that AMR was the most common cause of allograft failure in a cohort of renal transplant recipients with indication biopsies before graft failure.3 Moreover, in a multicenter cohort study, antibody-mediated damage caused allograft dysfunction late posttransplant in nearly 60% of renal transplant recipients.4
Given the scope and severity of the problem, it is unfortunate that there are no commonly accepted guidelines for treatment. To date, clinical trials of AMR have been small or inconclusive, and there are no Federal Drug Administration (FDA)-approved therapies for the prevention and treatment of the condition.6 The lack of an accepted common standard for the treatment of AMR has been an impediment to the development of new therapies because it is difficult for industry to initiate phase 2 and 3 clinical trials for novel treatments or prevention of AMR. To overcome this lack of evidence-based guidelines, The Transplantation Society brought together a group of experts from around the globe for a 1.5-day meeting, with the aim of producing a consensus document that outlined recommended treatments for active and chronic active AMR, based on the best available evidence. This publication is a summary of that meeting and includes up-to-date information about the pathogenesis of the condition, the criteria for diagnosis, prognosis, and long-term outcome.
BIOLOGY OF THE ALLOIMMUNE RESPONSE
A general appreciation of the complex immunologic processes underlying antibody production in immunologically naive and presensitized individuals is central to understanding the varied presentations of AMR and potential treatment options (Figure 1). In alloimmune naive individuals, the generation of antibody-secreting cells follows a scripted series of checkpoint events, starting with the initial encounter of alloantigen with B cells expressing the appropriate B-cell antigen receptor. This event activates B-cell migration to the T- and B-cell interface in the lymph node, where it receives help from alloreactive T cells that encountered alloantigen presented indirectly on recipient dendritic cells. Some of B cells differentiate into memory B cells or short-lived plasmablasts, while the rest enter into germinal centers to emerge as high-affinity and class-switched memory B cells, plasmablasts, and long-lived plasma cells.7,8 In the context of transplantation, presensitized individuals have a robust long-lived plasma cells constitutively secreting anti-HLA antibodies and resting memory B cells primed to secrete large amounts of antibody upon antigen reexposure leading to a rapid anamnestic antibody response.
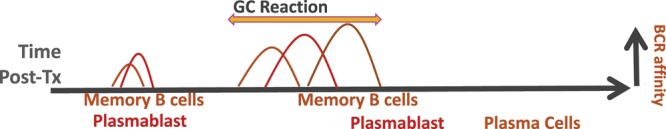
FIGURE 1. Kinetics of memory B cells and plasma cell generation relative to the germinal center (GC) reaction following transplantation. Following encounter with alloantigen, activated B cells migrate to the T- and B-cell interface and receive T-cell help. Some of the helped B cells differentiate into memory B cells or plasma cells, while the rest enter into a germinal center to emerge as high-affinity and class-switched memory B cells and plasma cells. Memory B cells tend to have low levels of somatic hypermutations and lower B-cell receptor (BCR) affinity compared with plasma cells, and cells generated pre-GC tend to be of lower affinity than cells generated post-GC.
Some features of the alloimmune response complicate our understanding of DSA production, limiting our ability to predict and develop therapeutic approaches for AMR. In general, memory B cells are derived from B cells with receptors that are less mutated and of lower affinity than those that are destined to become plasma cells.9–11 As a result, the repertoire of plasma cells and memory B cells are not identical. Furthermore, the repertoire of plasma cells and the antibodies they produce are up to 100-fold more restricted compared with the repertoire of memory B cells.12 These differences between memory B cells and plasma cell generation predict that treatment aiming to prevent plasma cell generation and subsequent DSA production may not stop the generation of memory B cells. Likewise, the absence of DSA does not imply the lack of memory B cells and the potential for an anamnestic response. Thus, the ability to quantify donor-specific memory B cells may aid in risk stratification and treatment of presensitized recipients susceptible for an active AMR early posttransplant.
The diversity at the level of antibodies presents an additional challenge. Antibodies have different Fc regions corresponding to their isotype and subclasses, each with nonoverlapping functions including their ability to bind to Fc receptors and activate complement. Less appreciated is heterogeneity in the anti-HLA antibody repertoire, which comprise antibodies that bind to private specificities on HLA molecules and thus are highly donor specific. Alternatively, cross-reactive alloantibodies may be donor reactive but not donor specific, and some may bind multiple HLA molecules. As a result, the breadth of the circulating antibody with HLA reactivity may not be a direct readout of the plasma cell repertoire.
DIAGNOSTIC CRITERIA AND HISTOLOGICAL FEATURES OF AMR
AMR is a clinicopathological diagnosis that was first formally described in a 2003 addition to the 1997 International Banff Classification of kidney allograft rejection13 but has continually evolved with our increased understanding of AMR particularly with regards to the relevance of C4d-negative AMR and the utility of molecular diagnostics. The salient features of active AMR based on the Banff 2017 classification14 are (1) histological evidence of graft injury via microvascular inflammation (MVI), intimal or transmural arteritis (v > 0), acute thrombotic microangiopathy in the absence of any other cause, or acute tubular injury in the absence of any other apparent cause; (2) histological evidence of antibody-endothelial interactions either by C4d deposition or at least moderate MVI; and (3) the presence of circulating DSA, predominantly anti-HLA antibody (Table 1). Clearly, the main histological manifestation of active AMR in renal allografts is MVI in the form of glomerulitis (g) and peritubular capillaritis (ptc). The presence of either (g + ptc > 0) satisfies criterion 1, and a (g + ptc) sum score of ≥2 also satisfies criterion 2. The exception is that peritubular capillaritis alone is insufficient for diagnosis in the presence of T-cell–mediated rejection (TCMR), including borderline rejection. Recurrent or de novo glomerulonephritis must be considered as a differential diagnosis, especially in the context of glomerulitis and thrombotic microangiopathy. To diagnose chronic active AMR, morphological features of chronic tissue injury are present in addition to criteria 2 and 3 for active AMR. Signs of chronic tissue injury include transplant glomerulopathy (Banff chronic glomerulitis [cg] score > 0), severe peritubular capillary basement membrane multilayering on electron microscopy, or new arterial intimal fibrosis without another obvious cause.
TABLE 1.
Banff 2017 classification of AMR in renal allografts14

CLINICAL PHENOTYPES OF AMR
The Banff classification has been a major advancement in the field of transplantation to increase the awareness of AMR and standardize definitions. However, a classification schema based on histological features oversimplifies the complexity of AMR. The Banff classification has 3 AMR diagnostic categories (including chronic AMR with transplant glomerulopathy and current or prior DSA but no MVI or C4d): the clinical reality is that AMR is frequently a chronic progressive disease process. This chronic disease process starts with the formation of DSA. The DSA may or may not lead to active AMR with histological features that often include but are not limited to MVI. Moreover, not all active AMR will progress to chronic active AMR. Over time, chronic histological features such as transplant glomerulopathy become evident, and eventually, the patient develops allograft dysfunction, proteinuria, and probable allograft loss. Thus, finding an active versus chronic active AMR on the biopsy may be more reflective of the timing of the biopsy rather than the underlying pathological process itself.
Further complicating the diagnosis and management of AMR are various clinical phenotypes. AMR can present with abrupt allograft dysfunction early posttransplant but can also have an insidious or subclinical onset, presenting later posttransplant. Anti-HLA antibody can also be present before transplant (preexisting DSA) or develop after transplant (dnDSA) in the setting of under-immunosuppression. In some circumstances, the histological features suggestive of AMR are present, but anti-HLA antibody is not detected. Incorporating these clinical features of AMR into the current Banff classification while considering the likely underlying immunologic mechanisms is critical to appropriately guide therapeutic decisions and ultimately design efficient and effective therapeutic clinical trials. Therefore, we recommend considering the timing of presentation, and type of DSA (preexisting or de novo), in relation to the histological classification as discussed below (Table 2).
TABLE 2.
Antibody-mediated rejection phenotypes

Early Posttransplant (<30 Days) Active AMR
In patients who have measurable DSA at the time of kidney transplant or who have an immunologic amnestic response due to previous exposure to allo-HLA, active AMR can occur within the first 30 days posttransplant. The risk of early posttransplant AMR increases with growing DSA strength or breadth at the time of transplant as determined by DSA mean fluorescence intensity (MFI), the degree of flow cytometric crossmatch positivity, and the number or breadth of cross-reactive DSA specificities.15,16 In general, this form of AMR is uncommon, as it is common practice to avoid allocating kidneys to patients with known preformed DSA, as early posttransplant AMR occurs in up to 40% of patients with preformed DSA and a positive flow cytometric crossmatch.38,39 This aggressive form of active AMR typically presents with an abrupt increase in DSA accompanied by allograft dysfunction (increased creatinine and oliguria with or without proteinuria). If not recognized and treated quickly, it can lead to cortical necrosis and allograft loss within days. From a histological perspective, the criteria for Banff active AMR are met and C4d is usually positive.40 There is often interstitial hemorrhage, glomerular fibrin thrombi, and microvascular coagulative necrosis. With prompt diagnosis and treatment, patients can recover allograft function and histological features of active AMR frequently resolve completely.40,41 In other cases, the histological features of active AMR persist and chronic active AMR, allograft dysfunction, and ultimate allograft failure ensues.
Late (>30 Days) Posttransplant AMR With Preexisting DSA
While many patients with preexisting DSA do not develop an aggressive early AMR as described above, they can develop an indolent and progressive form of AMR that is usually initially detected on a surveillance biopsy (in the setting of stable function) or on a for-cause biopsy for mild allograft dysfunction.42,43 Histological findings are dependent on the timing of the biopsy. When detected early, MVI in glomeruli and peritubular capillaries is the predominant finding and C4d staining may or may not be present. MVI tends to persist and is later accompanied by chronic histological features including transplant glomerulopathy and peritubular basement membrane multilayering.17,44,45 At diagnosis, there is often minimal if any reduction in glomerular filtration rate (GFR) or proteinuria even when mild chronic features are present. Overtime, however, the GFR declines and the patient becomes proteinuric39 with graft failure often occurring several years after transplant.18,21 In an observational prospective cohort study of >100 renal transplant recipients who underwent surveillance biopsy at 1 year, patients with AMR were the most likely to experience allograft failure.21 Allograft survival was only 56% at 8 years posttransplant compared with 88% if subclinical TCMR was present, and 90% if the biopsy was normal.21
Late (>30 Days) AMR Associated With dnDSA
In the current era of sensitive DSA testing and a general avoidance of preexisting DSA, the most common form of AMR is associated with dnDSA. In general, dnDSA is a new DSA detected after >3 months posttransplant in the context of inadequate immunosuppression which is either due to patient nonadherence, physician directed, or genetically determined variability in metabolism of immunosuppressive drugs. This form of AMR often presents with allograft dysfunction and concomitant or preexisting TCMR.3,46,47 In patients who have routine surveillance DSA testing or surveillance biopsies, the presentation can be more indolent and is similar to that of late posttransplant AMR in patients with preexisting DSA (subclinical AMR associated with dnDSA).46
Results from 2 recent studies have suggested that AMR with dnDSA is associated with inferior allograft survival when compared with AMR from preexisting DSA after adjusting for clinical, histological, and immunologic characteristics.19,23 Allograft survival was 63% in patients with preexisting DSA and only 34% in patients with dnDSA 8 years after the rejection diagnosis.19 Despite these findings, it remains unclear whether it is the dnDSA itself that is associated with inferior allograft survival or a delay in AMR diagnosis. Compared with patients with preexisting DSA, those with dnDSA tend to have increased proteinuria and increased expression of interferon-γ–inducible, natural killer cell, and T-cell transcripts at presentation.19
FEATURES ASSOCIATED WITH REDUCED ALLOGRAFT SURVIVAL IN LATE AMR (PREEXISTING OR dnDSA)
Although there are differences in the initial presentation of AMR and pace of clinical deterioration depending on whether the DSA is formed before the transplant, or is dnDSA, the histological, clinical, and alloantibody features associated with reduced allograft survival are similar (Table 2). Allograft histology is key to document the chronicity and extent of injury. Chronic histological features such as the presence of transplant glomerulopathy (Banff cg score >0)19,21,22 and the degree of interstitial fibrosis and tubular atrophy23–25 are predictive of allograft failure. Other histological features associated with inferior allograft survival include concomitant TCMR,22–24,26,27 C4d positivity,28–30,48 and vascular lesions (Banff cv score >0).24 Not surprisingly, clinical factors are also predictive of outcome including allograft dysfunction at diagnosis,19,22,24,25,31,49 proteinuria,19,25,31,32 and time of diagnosis posttransplant.24,25 To illustrate the relevance of having allograft dysfunction at presentation: time to 50% graft failure was 3.3 years in patients with allograft dysfunction versus 8.3 years in patients without allograft dysfunction among 47 patients with dnDSA.22 Although it is clear that under-immunosuppression is a major risk factor for dnDSA, prior studies have also shown that a history of medication nonadherence is independently associated with inferior allograft survival among patients with dnDSA.22,25,50,51
Lastly, several alloantibody characteristics have been associated with outcome including the presence of C1q-positive DSA34,35,52 and anticlass II DSA.17,36 Additionally, the level or strength of DSA correlates with graft failure as determined by DSA titer or flow cytometric crossmatch positivity. Notably, several studies have correlated DSA titer and MFI with C1q positivity53; thus, it is unclear whether complement binding characteristics or levels of alloantibody determine outcomes.
CONSENSUS FOR MEASURING AND MONITORING OF DSA
Initial Assessment for Anti-HLA DSA
The initial assessment of a renal transplant candidate involves donor and recipient HLA typing, anti-HLA antibody screening, and obtaining a history of allosensitizing events (previous transplant, blood transfusion, and pregnancy) (strength of recommendations and level of evidence 1A).54–56 Molecular HLA typing ideally includes A; B; C; DRB1; DRB3, 4, 5; DQA1/DQB1; and DPA1/DPB1 (2B). For anti-HLA–sensitized recipients, a high-resolution level of typing, approaching or even reaching the allelic level (ie, the so-called “4-digit” typing), should be undertaken as often as possible on the potential donor, to match the resolution of the alloantibody identification assays. The first-line screening for alloantibody would be with single-antigen bead (SAB) solid-phase assays (LABScreen [one Lambda] or LifeScreen [LifeCodes-Immucor]), but multiantigen beads can also be used (1A). Patients with no history of allosensitizing events and with negative anti-HLA antibody testing using single-antigen or multiantigen bead solid-phase assays are at low risk for AMR.
Monitoring for De Novo DSA
Immunosuppression reduction either as a result of nonadherence or under physician direction is associated with development of dnDSA.46,47,51 Monitoring for dnDSA is recommended in the following settings: immunosuppression reduction by physician for any reason, known patient medication nonadherence, or at the time of rejection episode (T cell or antibody mediated) (2B). The presence of dnDSA is a general indicator of under-immunosuppression and signals the need to reevaluate maintenance immunosuppression. Based on the strong relationship between dnDSA, AMR, and graft loss, transplant patients with dnDSA should undergo close monitoring of allograft function19,22,47 (1B). A kidney biopsy is also recommended to detect T-cell or AMR (clinically evident or subclinical).6
Interpreting Positive DSA Results
The SAB test detecting DSA has been an important advancement to the field; however, the test has limitations that must be identified for correct interpretation. First, the SAB test has a high coefficient of variation, and thus, the positive cutoff varies among and within laboratories. In general, a positive cutoff MFI of 1000–1500 is associated with the detection of specific anti-HLA antibodies.57 SAB tests are also prone to interference from external substances, bead saturation, and “shared-epitope” phenomenon, which can lead to a falsely low MFI.53,58,59 Methods to identify interference and bead saturation include performing serum dilution or using ethylenediaminetetraacetic acid. We recommend the routine use of these methods in the following situations: transplant candidates/recipients who are not immunologically naive, unexpected positive crossmatch, or AMR with unexpectedly low DSA MFI (2B).
Additional DSA Testing for Risk Stratification
All patients with DSA are at some risk for AMR. Crossmatch testing can be used with SAB testing for AMR risk stratification. The risks of AMR from highest to lowest based on crossmatch and SAB-positive testing are the following: positive complement-dependent cytotoxicity (CDC) crossmatch, positive flow cytometric crossmatch, and negative crossmatch.60 Importantly, hyperacute AMR is also associated with having a positive CDC crossmatch.
Testing to assess the complement binding ability of DSA (C1q or C3d) is commercially available, and positive results are associated with AMR and allograft loss.34,52 However, C1q and C3d binding positivity is associated with a high DSA titer.53 It remains unclear whether complement binding assays outperform antibody titers for AMR risk stratification. For this reason, we do not recommend routine use of complement binding assays unless it is used as a means of predicting high strength DSA. Lastly, DSA IgG subclass testing has been used for research purposes. This testing has not been thoroughly validated for clinical use and at the moment cannot be recommended.
AVAILABLE EVIDENCE FOR THE TREATMENT OF ACTIVE AND CHRONIC ACTIVE AMR
Most reports on the treatment of AMR are small and include heterogeneous patient populations. These studies frequently include mixed antibody and TCMRs, do not differentiate responses based on the timing of AMR detection, and make no distinction between dnDSA and preformed DSA, although all these factors have an impact on outcome.61 The heterogeneity of available studies makes it difficult to draw meaningful conclusions about treatment effects. As recommended by guidelines,56 most studies describe the use of a variable mix of interventions (eg, variable intensity of plasmapheresis, different doses of intravenous immune globulins [IVIG], variable use of steroid pulses together with or without different T-cell–depleting and B-cell–depleting antibodies). Obviously, these different interventions create a challenge in the interpretation of treatment effects. As a consequence, treatment studies for AMR are rarely comparable, and the available evidence is generally of low quality.55,62
Plasma Exchange and IVIG
The primary aims of nearly all therapeutic approaches for AMR are removing circulating DSA and reducing DSA production. In this sense, the strongholds for contemporary treatment of AMR are represented by plasma exchange (PLEX) and IVIG, although neither of these have FDA approval. This treatment regimen is most commonly used to treat active AMR, although frequency, modality, and dosing may vary14,55,56,61,62 (Table 3). On those grounds, the expert consensus at the FDA Antibody-Mediated Rejection Workshop in 201767 as well as Kidney Disease: Improving Global Outcomes (KDIGO) in 201068 was that PLEX and IVIG could be regarded as a standard of care for acute active AMR, despite the weakness of evidence in support of efficacy. In particular, their ability to improve short-term outcomes has been demonstrated by several studies,65,66,70,71 while their results on long-term effects remain variable, emphasizing the need for new alternatives or adjunctive therapy for the treatment of AMR. In addition, there is a need to better define the amount of PLEX and dosing of IVIG.
TABLE 3.
Evidence for use of plasma exchange and intravenous immune globulins as SOC in active AMR

The rationale for using PLEX and IVIG is to combine removal of circulating DSA with immunomodulation of the antigraft immune response and in particular modulation of the B-cell response. In experimental models, IVIG has been shown to inhibit B-cell responses by the Fc portion of the Ig binding the Fc fragment of IgG2b receptor on B cells, and sialylated IVIG binds CD22, inducing apoptosis of mature B cells.72 It also functions as a scavenger of activated complement.72 While PLEX and IVIG have formed the mainstay of treatment for acute active AMR, the evidence consists largely of case series and poorly controlled randomized trials. Well-designed clinical trials in this area have proven difficult. One of the best-designed trials recruited only 10 patients (5 in each arm) and consisted of immunoabsorption without IVIG. While all of the patients receiving immunoabsorption responded to treatment, the trial was ceased at the first interim analysis because of 80% graft loss in the control arm, which suggests that immunoabsorption was beneficial in this setting.71
Complement Inhibitors
Over the last decade, the complement system has attracted increasing attention as an important contributor to AMR. Hence, several studies have been undertaken to evaluate the ability of various complement inhibitors to prevent and treat AMR. The main goal of using complement inhibitors is to avoid the downstream damage to the allograft from DSA.
Eculizumab results in terminal complement blockade as a monoclonal antibody targeting C5. A single-center study showed that among patients who received positive crossmatch HLA-incompatible transplants, the incidence of early active AMR was decreased from approximately 40% in historical controls to 7% among treated patients. Furthermore, 2 multicenter randomized phase 2 trials confirmed the protective effect of eculizumab for preventing early active AMR in positive crossmatch HLA-incompatible living73 and deceased74 donor populations. A single-center small case series has also shown that eculizumab has effectiveness in treating early active AMR that occurs within the first month posttransplant.40 Despite these promising results, long-term follow-up of eculizumab-treated positive crossmatch patients in a single-center study has shown that despite prevention of early active AMR, the long-term incidence of chronic AMR and allograft survival is comparable to historical controls.18,45
Proximal complement inhibition has also been studied as a therapeutic target. The plasma C1 esterase inhibitors Berinert (CSL Behring) and Cinryze (Takeda/Shire/ViroPharma) have been tested in 2 pilot studies and indicate a possible improvement in allograft function in kidney recipients with AMR.75,76 An additional clinical trial evaluating a C1 esterase inhibitor for the treatment of AMR that is resistant to PLEX and IVIG (NCT03221842) in renal transplant recipients is ongoing.
Rituximab
Rituximab, a B-cell–depleting agent, was suggested as a treatment option by KDIGO guidelines.56 Despite its frequent use,69 the evidence is low and 3 small randomized trials have investigated its utility without demonstrating a clear benefit.55,62,77 A small study in 20 children investigated the effect of Rituximab compared with standard of care (pulse steroids) in B-cell–rich rejections (of whom 40% in the control group and 80% in treatment group had DSA).78 There were no major differences in outcome, and Rituximab had a reasonable safety profile. However, small numbers, demographic, and baseline differences as well as an unclear AMR definition preclude meaningful conclusions. The second trial was a French prospective, double-blind, multicenter, randomized study investigating 38 patients with active AMR in the first year after transplantation. All patients received treatment with steroids, IVIG, and PLEX and were randomized to either rituximab or placebo. There was no difference in any outcome parameter, except side effects.79 More recently, there was a Spanish prospective, randomized, placebo-controlled, double-blinded clinical trial where patients were randomized to receive IVIG plus Rituximab or IVIG plus saline infusion. Only 50% enrollment was achieved (25 patients), and at 12 months, there were no differences between treatment and control groups in estimated glomerular filtration rate decline, level of proteinuria, Banff score on biopsy, or MFI of the immunodominant DSA.80 In contrast to these prospective RCTs, several retrospective analyses have suggested some positive effects of rituximab in multimodal treatment regimens together with steroids, plasmapheresis, and high-dose IVIG, especially on patients with vascular AMR.55,62,81 A recent study developed a prognostic score on the basis of a treatment response to a regimen with Rituximab in the context of multimodal therapy. Moreover, a single-center nonrandomized study suggests that Rituximab as an add-on therapy may prevent DSA rebound as part of a desensitization protocol in highly sensitized patients.82 However, optimal doses, number of treatment cycles, and the effect on patients without a vascular component remain unclear, as is the need for Rituximab within a multimodal regimen.83
Imlifidase
Imlifidase (Hansa Biopharma AB), an IgG-degrading enzyme of Streptococcus pyogenes (IdeS), can rapidly reduce or even eliminate anti-HLA DSA and is undergoing clinical trials in AMR.84 IdeS cleaves human IgG at a highly specific amino acid sequence within the hinge region producing Fc and F(ab)2 fragments and effectively blocking CDC and antibody-dependent cellular cytotoxicity.85 Although data are lacking for using IdeS in AMR, this agent has been used safely in highly sensitized individuals for desensitization. After administration of IdeS, all previously positive crossmatches became negative and all studied patients received a transplant.86 Unfortunately within 7–10 days of administration, patients often experience a rebound in DSA and anti-IdeS antibodies develop after 1 or 2 doses, thereby preventing repeated administrations. Thus, IdeS will unlikely be an isolated treatment for active or chronic active AMR, but rather an adjunct to other therapies aiming to reduce DSA in the long term. The unique feature of this drug is that it permits any highly sensitized patient to undergo transplantation within hours of a donor being identified regardless of the crossmatch status.
Antithymocyte Globulin
Since its introduction, antithymocyte globulin (ATG) or other T-cell–depleting antibodies have been used for treatment of refractory rejection, vascular rejection, mixed rejections, and AMR.69,87 Although depleting antibodies were proposed by KDIGO guidelines as potential treatment options,56 no benefit has been demonstrated for treatment of pure AMR with T-cell–depleting therapy.55,62,87 No prospective trial with ATG for AMR had been performed, and a large retrospective series suggests that T-cell depletion in combination with steroids has no effect on the outcome in vascular AMR.81 Side effects are well described with a higher risk of infectious-associated death, particularly when ATG was combined with B-cell depletion.88
Splenectomy
There are several case series of surgical splenectomy, splenic embolization, and splenic radiation being used as a salvage procedure for severe early AMR.89,90 It must be performed rapidly after the onset of early AMR to be effective. Designing a proper study would be challenging and patients who have undergone splenectomy are known to be sensitized, have preformed antibody, or have undergone desensitization therapy. Most of these AMR cases occur in the first week after transplantation and result in profound graft dysfunction and a sudden rise in DSA strength, usually from an anamnestic response. Some patients who recover develop transplant glomerulopathy and premature graft loss.
Proteasome Inhibitor: Bortezomib
Bortezomib is a proteasome inhibitor approved for the treatment of multiple myeloma that directly targets antibody-producing plasma cells making it an attractive candidate for the treatment of active AMR.91 Data supporting its use are limited to case series suggesting a positive effect within a multimodal treatment regimen of PLEX, IVIG, steroids, and depleting antibodies.55,62,91 The only prospective randomized, double-blind, placebo-controlled trial was in “late” AMR and did not demonstrate any beneficial effect of bortezomib alone.92 The drug has well-documented side effects, and at the present time, there are no trial data to support its use.93
Cyclophosphamide
Cyclophosphamide is used for the treatment of antibody-mediated diseases such as anti-neutrophil cytoplasmic antibody vasculitis or lupus nephritis. Previous anecdotal reports describe its use within a multimodal treatment regimen for the treatment of refractory rejections.62,94 While it is relatively inexpensive, there are no trial data to support its use.
Interleukin-6 Inhibitors
A single-center, nonrandomized trial of tocilizumab (anti-interleukin-6 receptor monoclonal antibody) was undertaken in 36 patients with chronic active AMR that had failed IVIG plus rituximab. Patient and graft survival at 6 years (91% and 80%, respectively) were found to be superior to historical controls, with significant reductions in DSA and stabilization of renal function.95 Partly based on these encouraging results, a small investigator-initiated randomized control trial has begun recruitment and a large multicenter randomized control trial has been initiated to evaluate Clazakizumab, an anti-interleukin-6 monoclonal antibody, for the treatment of chronic active AMR.96
CONSENSUS FOR TREATMENT OF EARLY ACTIVE AMR (≤30 DAYS POSTTRANSPLANT)
The current evidence for treatment options in active AMR is of limited quality. The consensus view was that the combination of PLEX, IVIG with corticosteroids could be regarded as standard of care, consistent with the conclusions of the FDA workshop and KDIGO guidelines (Table 4).6,56 However, in some centers, the use of corticosteroids is reserved for patients with concompetant TCMR. While adjunctive therapy with other agents has been used in specific settings, there have been only 3 (underpowered) prospective randomized trials for treatment of active AMR.78,79,92 These trials had many limitations, and most evidence comes from small retrospective studies with different combination therapies using different AMR definitions in different populations.40,55,62,83 Thus, the available evidence supporting the use of any adjunctive agents is of low quality with the best evidence relating to drug toxicity and costs. Nevertheless, these rejections are relatively rare with a high incidence of graft loss and a randomized clinical trial would be difficult to achieve. Hence, in the absence of trial data, the consensus was that adjunctive therapy may be warranted especially when the risk of graft loss is considered high. The recommended adjunctive therapies include complement inhibitors, rituximab, or splenectomy depending on availability (Table 4). Where concomitant TCMR is present, it should be treated.
TABLE 4.
Consensus treatment recommendations based on available evidence and expert opinion

CONSENSUS FOR TREATMENT OF LATE ACTIVE AND CHRONIC ACTIVE AMR (≥30 DAYS POSTTRANSPLANT)
Preexisting DSA
As described above, the transition from active to chronic active AMR should be considered a continuum, and the DSA may have been present at the time of transplant or appear de novo. Among patients with known preexisting DSA and active AMR without chronic features, the consensus treatment recommendations include PLEX, IVIG, and corticosteroids.
In cases of chronic active AMR or chronic transplant vasculopathy, goals of therapy should be to stabilize or reduce the rate of decline in GFR, proteinuria, histological injury score, and titer of DSA while minimizing drug toxicity. The use of IVIG and PLEX, with or without Rituximab, has not been shown to improve outcomes in patients with chronic active AMR (as distinct from acute active AMR) and has to be balanced against increased risk of adverse events such as infection and cost. The consensus opinion was that treatment should focus on optimizing immunosuppression and supportive care, with reintroduction of steroids (if on a steroid-free regimen), maintaining trough tacrolimus levels >5 ng/mL, and optimizing medical management with focus on blood pressure, blood glucose, and lipid control.
De Novo DSA
dnDSA generally occurs in the context of reduced immunosuppression whether from patient nonadherence or a physician-directed change in immunosuppression. AMR in this setting is also often initially detected with concomitant TCMR. Therefore, the standard for managing AMR in this setting (active or chronic active) is to optimize baseline immunosuppression and manage potential medication nonadherence. Treatment of concomitant TCMR is recommended in all cases of AMR but is particularly relevant in these cases. Similar to patients with chronic active AMR in the context of preexisting antibodies, treatment with PLEX, IVIG, and Rituximab is used in some centers, although the evidence level (3C) is low.
CONCLUSIONS
Despite the severity of the problem and poor outcomes for patients who develop AMR, there is very little high-level evidence to support the use of any therapy. Most trials in this area have been small investigator-initiated studies with small numbers of participants, lacking appropriate controls. As a result, there were no clear treatment regimens to recommend and there are no approved treatments. The consensus opinion of those present at the meeting was based largely on observational studies, low-level evidence, and expert opinion. Despite the clear lack of evidence, it was considered important to define a standard of care for AMR, which could be used as a benchmark for future studies and prospective trials. It is obvious that new agents and clinical trials are needed urgently. Future directions in this field will require new trial designs and large transnational trial consortia to undertake these studies.97 In addition, better characterization of the different forms of AMR based on pathophysiology, histology, as well as clinical and genetic phenotypes is needed.
Footnotes
C.A.S. and R.B.M. are equal first author.
This meeting was organized by The Transplantation Society with the assistance of an unconditional education grant from CSL Behring. CSL Behring had no involvement in the development of the program, the choice of speakers, nor in the writing of the manuscript. The manuscript was written by the authors.
P.J.O. is an advisory board member for CSL Behring, eGenesis, Qihan Biotech, and Renalytix AI and received research funding from CSL Behring. C.A.S. involved in current contracts with CSL Bering and negotiating research contracts with Vitaeris. R.B.M. has received research grants from CSL Behring, Alexion, and Mallinckrodt and honoraria from Novartis and Hansa. He serves on the Medical Steering Committee for Vitaeris Imagine Trial. K.B. has received research funds and honoraria from Abbvie, Alexion, Astellas, Bristol-Meyer Squibb, Chiesi, CSL, Behring, Fresenius, Genetech, Hexal, Novartis, Otsuka, Pfizer, Roche, Shire, Siemens, Veloxis, and Vitaeris. M.H. serves as a paid consultant on pathology adjudication committees for industry-sponsored clinical trials by Shire ViroPharma and AstraZeneca. He received honoraria for serving as a speaker and advisor for CareDx and Novartis. M.M. has research funding from Roche Diagnostics and advisory board membership from Novartis and Vitaeris. C.L. has a research grant from CSL Behring. R.A.M. served on advisory boards for Genentech Scientific/ROCHE, Alexion, Novartis, CSL Behring, eGenesis, Sanofi, Viela Bio, Vitaeris Bio, and Hansa Medical. He received consulting fees or travel expenses from Alexion, Hansa Medical, CLS Behring, Viela Bio, Vitaeris Bio, and Shire/Takeda. He received research grants from ViroPharma/Shire, Hansa Medical, United Therapeutics, and Alexion. P.N. is a consultant for Vitaeris Inc., Astellas Pharma, Vielo Bio, Paladin, and Renalytix AI Inc. and received honoraria from Astellas and Thermo Fisher Scientific.
M.A. is an advisory board member for Immucor. S.J.C. has received travel support, speakers fees, or advisory board payments from Novartis, Astellas, Vitaeris, Alexion, and AstraZeneca. S.F. is an advisory board member for Novartis. S.C.J. is a consultant for CSL Behring, Vitaeris, and Hansa Medical and has received research grants from CSL Behring, Vitaeris, and Hansa Medical. I.O. works for the Critical Path Institute that received funding from the FDA and member companies. D.S. received grants from Astellas, TEVA, and Chiesi and is an advisory board member for Astellas, Novartis, CSL Behring, and Vitaeris. A.R.T. is an advisory board member for Astellas and Viela Bio and is the central lab for HLA testing for an Astellas study. The other authors declare no conflicts of interest.
P.J.O. proposed and organized the meeting and was primarily responsible for the development of the program and overall editing of the manuscript. C.A.S. and R.B.M. delivered presentations at the meeting, were involved in discussion, and undertook a major role in writing the manuscript. K.B., A.S.C., M.H., S.K., C.L., R.A.M., P.N., and S.G.T. were responsible for writing sections of the manuscript. All other authors either delivered presentations or chaired sessions, were involved in discussions, and participated in the development of the consensus. All authors reviewed and edited the manuscript and agreed with the final document.
REFERENCES
- 1.Meier-Kriesche HU, Ojo AO, Hanson JA, et al. Increased impact of acute rejection on chronic allograft failure in recent era. Transplantation 2000701098–1100 [DOI] [PubMed] [Google Scholar]
- 2.El-Zoghby ZM, Stegall MD, Lager DJ, et al. Identifying specific causes of kidney allograft loss. Am J Transplant 20099527–535 [DOI] [PubMed] [Google Scholar]
- 3.Sellarés J, de Freitas DG, Mengel M, et al. Understanding the causes of kidney transplant failure: the dominant role of antibody-mediated rejection and nonadherence. Am J Transplant 201212388–399 [DOI] [PubMed] [Google Scholar]
- 4.Gaston RS, Cecka JM, Kasiske BL, et al. Evidence for antibody-mediated injury as a major determinant of late kidney allograft failure. Transplantation 20109068–74 [DOI] [PubMed] [Google Scholar]
- 5.Everly MJ, Rebellato LM, Haisch CE, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation 201395410–417 [DOI] [PubMed] [Google Scholar]
- 6.Archdeacon P, Chan M, Neuland C, et al. Summary of FDA Antibody-Mediated Rejection Workshop. Am J Transplant 201111896–906 [DOI] [PubMed] [Google Scholar]
- 7.Kurosaki T, Kometani K, Ise W. Memory B cells. Nat Rev Immunol 201515149–159 [DOI] [PubMed] [Google Scholar]
- 8.Nutt SL, Hodgkin PD, Tarlinton DM, et al. The generation of antibody-secreting plasma cells. Nat Rev Immunol 201515160–171 [DOI] [PubMed] [Google Scholar]
- 9.Sciammas R, Shaffer AL, Schatz JH, et al. Graded expression of interferon regulatory factor-4 coordinates isotype switching with plasma cell differentiation. Immunity 200625225–236 [DOI] [PubMed] [Google Scholar]
- 10.Ochiai K, Maienschein-Cline M, Simonetti G, et al. Transcriptional regulation of germinal center B and plasma cell fates by dynamical control of IRF4. Immunity 201338918–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shinnakasu R, Inoue T, Kometani K, et al. Regulated selection of germinal-center cells into the memory B cell compartment. Nat Immunol 201617861–869 [DOI] [PubMed] [Google Scholar]
- 12.Lavinder JJ, Horton AP, Georgiou G, et al. Next-generation sequencing and protein mass spectrometry for the comprehensive analysis of human cellular and serum antibody repertoires. Curr Opin Chem Biol 201524112–120 [DOI] [PubMed] [Google Scholar]
- 13.Racusen LC, Solez K, Colvin RB, et al. The Banff 97 working classification of renal allograft pathology. Kidney Int 199955713–723 [DOI] [PubMed] [Google Scholar]
- 14.Haas M, Loupy A, Lefaucheur C, et al. The Banff 2017 Kidney Meeting Report: revised diagnostic criteria for chronic active T cell-mediated rejection, antibody-mediated rejection, and prospects for integrative endpoints for next-generation clinical trials. Am J Transplant 201818293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gloor JM, Winters JL, Cornell LD, et al. Baseline donor-specific antibody levels and outcomes in positive crossmatch kidney transplantation. Am J Transplant 201010582–589 [DOI] [PubMed] [Google Scholar]
- 16.Schinstock CA, Gandhi M, Cheungpasitporn W, et al. Kidney transplant with low levels of DSA or low positive B-flow crossmatch: an underappreciated option for highly sensitized transplant candidates. Transplantation 20171012429–2439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bentall A, Cornell LD, Gloor JM, et al. Five-year outcomes in living donor kidney transplants with a positive crossmatch. Am J Transplant 20131376–85 [DOI] [PubMed] [Google Scholar]
- 18.Schinstock CA, Bentall AJ, Smith BH, et al. Long-term outcomes of eculizumab-treated positive crossmatch recipients: allograft survival, histologic findings, and natural history of the donor-specific antibodies. Am J Transplant 2019191671–1683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Aubert O, Loupy A, Hidalgo L, et al. Antibody-mediated rejection due to preexisting versus de novo donor-specific antibodies in kidney allograft recipients. J Am Soc Nephrol 2017281912–1923 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Amrouche L, Aubert O, Suberbielle C, et al. Long-term outcomes of kidney transplantation in patients with high levels of preformed DSA: the Necker high-risk transplant program. Transplantation 20171012440–2448 [DOI] [PubMed] [Google Scholar]
- 21.Loupy A, Vernerey D, Tinel C, et al. Subclinical rejection phenotypes at 1 year post-transplant and outcome of kidney allografts. J Am Soc Nephrol 2015261721–1731 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Rates and determinants of progression to graft failure in kidney allograft recipients with de novo donor-specific antibody. Am J Transplant 2015152921–2930 [DOI] [PubMed] [Google Scholar]
- 23.Haas M, Mirocha J, Reinsmoen NL, et al. Differences in pathologic features and graft outcomes in antibody-mediated rejection of renal allografts due to persistent/recurrent versus de novo donor-specific antibodies. Kidney Int 201791729–737 [DOI] [PubMed] [Google Scholar]
- 24.Viglietti D, Loupy A, Aubert O, et al. Dynamic prognostic score to predict kidney allograft survival in patients with antibody-mediated rejection. J Am Soc Nephrol 201829606–619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Malheiro J, Santos S, Tafulo S, et al. Correlations between donor-specific antibodies and non-adherence with chronic active antibody-mediated rejection phenotypes and their impact on kidney graft survival. Hum Immunol 201879413–423 [DOI] [PubMed] [Google Scholar]
- 26.Krisl JC, Alloway RR, Shield AR, et al. Acute rejection clinically defined phenotypes correlate with long-term renal allograft survival. Transplantation 2015992167–2173 [DOI] [PubMed] [Google Scholar]
- 27.Matignon M, Muthukumar T, Seshan SV, et al. Concurrent acute cellular rejection is an independent risk factor for renal allograft failure in patients with c4d-positive antibody-mediated rejection. Transplantation 201294603–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Loupy A, Hill GS, Suberbielle C, et al. Significance of C4D Banff scores in early protocol biopsies of kidney transplant recipients with preformed donor-specific antibodies (DSA). Am J Transplant 20111156–65 [DOI] [PubMed] [Google Scholar]
- 29.Moktefi A, Parisot J, Desvaux D, et al. C1Q binding is not an independent risk factor for kidney allograft loss after an acute antibody-mediated rejection episode: a retrospective cohort study. Transpl Int 201730277–287 [DOI] [PubMed] [Google Scholar]
- 30.Issa N, Cosio FG, Gloor JM, et al. Transplant glomerulopathy: risk and prognosis related to anti-human leukocyte antigen class II antibody levels. Transplantation 200886681–685 [DOI] [PubMed] [Google Scholar]
- 31.Redfield RR, Ellis TM, Zhong W, et al. Current outcomes of chronic active antibody mediated rejection - a large single center retrospective review using the updated BANFF 2013 criteria. Hum Immunol 201677346–352 [DOI] [PubMed] [Google Scholar]
- 32.Naesens M, Lerut E, Emonds MP, et al. Proteinuria as a noninvasive marker for renal allograft histology and failure: an observational cohort study. J Am Soc Nephrol 201627281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orandi BJ, Chow EH, Hsu A, et al. Quantifying renal allograft loss following early antibody-mediated rejection. Am J Transplant 201515489–498 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Loupy A, Lefaucheur C, Vernerey D, et al. Complement-binding anti-HLA antibodies and kidney-allograft survival. N Engl J Med 20133691215–1226 [DOI] [PubMed] [Google Scholar]
- 35.Bailly E, Anglicheau D, Blancho G, et al. Prognostic value of the persistence of c1q-binding anti-HLA antibodies in acute antibody-mediated rejection in kidney transplantation. Transplantation 2018102688–698 [DOI] [PubMed] [Google Scholar]
- 36.Willicombe M, Brookes P, Santos-Nunez E, et al. Outcome of patients with preformed donor-specific antibodies following alemtuzumab induction and tacrolimus monotherapy. Am J Transplant 201111470–477 [DOI] [PubMed] [Google Scholar]
- 37.Lefaucheur C, Loupy A, Hill GS, et al. Preexisting donor-specific HLA antibodies predict outcome in kidney transplantation. J Am Soc Nephrol 2010211398–1406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vo AA, Peng A, Toyoda M, et al. Use of intravenous immune globulin and rituximab for desensitization of highly HLA-sensitized patients awaiting kidney transplantation. Transplantation 2010891095–1102 [DOI] [PubMed] [Google Scholar]
- 39.Stegall MD, Diwan T, Raghavaiah S, et al. Terminal complement inhibition decreases antibody-mediated rejection in sensitized renal transplant recipients. Am J Transplant 2011112405–2413 [DOI] [PubMed] [Google Scholar]
- 40.Tan EK, Bentall A, Dean PG, et al. Use of eculizumab for active antibody-mediated rejection that occurs early post-kidney transplantation: a consecutive series of 15 cases. Transplantation 20191032397–2404 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Orandi BJ, Zachary AA, Dagher NN, et al. Eculizumab and splenectomy as salvage therapy for severe antibody-mediated rejection after HLA-incompatible kidney transplantation. Transplantation 201498857–863 [DOI] [PubMed] [Google Scholar]
- 42.Loupy A, Hill GS, Jordan SC. The impact of donor-specific anti-HLA antibodies on late kidney allograft failure. Nat Rev Nephrol 20128348–357 [DOI] [PubMed] [Google Scholar]
- 43.Gloor JM, Cosio FG, Rea DJ, et al. Histologic findings one year after positive crossmatch or ABO blood group incompatible living donor kidney transplantation. Am J Transplant 200661841–1847 [DOI] [PubMed] [Google Scholar]
- 44.Wavamunno MD, O’Connell PJ, Vitalone M, et al. Transplant glomerulopathy: ultrastructural abnormalities occur early in longitudinal analysis of protocol biopsies. Am J Transplant 200772757–2768 [DOI] [PubMed] [Google Scholar]
- 45.Cornell LD, Schinstock CA, Gandhi MJ, et al. Positive crossmatch kidney transplant recipients treated with eculizumab: outcomes beyond 1 year. Am J Transplant 2015151293–1302 [DOI] [PubMed] [Google Scholar]
- 46.Wiebe C, Gibson IW, Blydt-Hansen TD, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant 2012121157–1167 [DOI] [PubMed] [Google Scholar]
- 47.Schinstock CA, Cosio F, Cheungpasitporn W, et al. The value of protocol biopsies to identify patients with de novo donor-specific antibody at high risk for allograft loss. Am J Transplant 2017171574–1584 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Orandi BJ, Alachkar N, Kraus ES, et al. Presentation and outcomes of c4d-negative antibody-mediated rejection after kidney transplantation. Am J Transplant 201616213–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orandi BJ, Garonzik-Wang JM, Massie AB, et al. Quantifying the risk of incompatible kidney transplantation: a multicenter study. Am J Transplant 2014141573–1580 [DOI] [PubMed] [Google Scholar]
- 50.Wiebe C, Nevins TE, Robiner WN, et al. The synergistic effect of class II HLA epitope-mismatch and nonadherence on acute rejection and graft survival. Am J Transplant 2015152197–2202 [DOI] [PubMed] [Google Scholar]
- 51.Schinstock CA, Dadhania DM, Everly MJ, et al. Factors at de novo donor-specific antibody initial detection associated with allograft loss: a multicenter study. Transpl Int 201932502–515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bouquegneau A, Loheac C, Aubert O, et al. Complement-activating donor-specific anti-HLA antibodies and solid organ transplant survival: a systematic review and meta-analysis. Plos Med. 2018;15:e1002572. doi: 10.1371/journal.pmed.1002572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tambur AR, Herrera ND, Haarberg KM, et al. Assessing antibody strength: comparison of MFI, c1q, and titer information. Am J Transplant 2015152421–2430 [DOI] [PubMed] [Google Scholar]
- 54.Uhlig K, Macleod A, Craig J, et al. Grading evidence and recommendations for clinical practice guidelines in nephrology. A position statement from kidney disease: improving global outcomes (KDIGO). Kidney Int 2006702058–2065 [DOI] [PubMed] [Google Scholar]
- 55.Roberts DM, Jiang SH, Chadban SJ. The treatment of acute antibody-mediated rejection in kidney transplant recipients-a systematic review. Transplantation 201294775–783 [DOI] [PubMed] [Google Scholar]
- 56.Kidney Disease: Improving Global Outcomes (KDIGO) Transplant Work Group KDIGO clinical practice guideline for the care of kidney transplant recipients. Am J Transplant 20099Suppl 3S1–S155 [DOI] [PubMed] [Google Scholar]
- 57.Reed EF, Rao P, Zhang Z, et al. Comprehensive assessment and standardization of solid phase multiplex-bead arrays for the detection of antibodies to HLA. Am J Transplant 2013131859–1870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Visentin J, Vigata M, Daburon S, et al. Deciphering complement interference in anti-human leukocyte antigen antibody detection with flow beads assays. Transplantation 201498625–631 [DOI] [PubMed] [Google Scholar]
- 59.Tambur AR, Campbell P, Claas FH, et al. Sensitization in transplantation: assessment of risk (STAR) 2017 working group meeting report. Am J Transplant 2018181604–1614 [DOI] [PubMed] [Google Scholar]
- 60.Montgomery RA, Warren DS, Segev DL, et al. HLA incompatible renal transplantation. Curr Opin Organ Transplant 201217386–392 [DOI] [PubMed] [Google Scholar]
- 61.Loupy A, Lefaucheur C. Antibody-mediated rejection of solid-organ allografts. N Engl J Med 20183791150–1160 [DOI] [PubMed] [Google Scholar]
- 62.Wan SS, Ying TD, Wyburn K, et al. The treatment of antibody-mediated rejection in kidney transplantation: an updated systematic review and meta-analysis. Transplantation 2018102557–568 [DOI] [PubMed] [Google Scholar]
- 63.Akiyoshi T, Hirohashi T, Alessandrini A, et al. Role of complement and NK cells in antibody mediated rejection. Hum Immunol 2012731226–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gelfand EW. Intravenous immune globulin in autoimmune and inflammatory diseases. N Engl J Med 20123672015–2025 [DOI] [PubMed] [Google Scholar]
- 65.Rocha PN, Butterly DW, Greenberg A, et al. Beneficial effect of plasmapheresis and intravenous immunoglobulin on renal allograft survival of patients with acute humoral rejection. Transplantation 2003751490–1495 [DOI] [PubMed] [Google Scholar]
- 66.Lefaucheur C, Nochy D, Andrade J, et al. Comparison of combination plasmapheresis/IVIG/anti-CD20 versus high-dose IVIG in the treatment of antibody-mediated rejection. Am J Transplant 200991099–1107 [DOI] [PubMed] [Google Scholar]
- 67.Velidedeoglu E, Cavaillé-Coll MW, Bala S, et al. Summary of 2017 FDA public workshop: antibody-mediated rejection in kidney transplantation. Transplantation 2018102e257–e264 [DOI] [PubMed] [Google Scholar]
- 68.Kasiske BL, Zeier MG, Chapman JR, et al. ; Kidney Disease: Improving Global Outcomes KDIGO clinical practice guideline for the care of kidney transplant recipients: a summary. Kidney Int 201077299–311 [DOI] [PubMed] [Google Scholar]
- 69.Burton SA, Amir N, Asbury A, et al. Treatment of antibody-mediated rejection in renal transplant patients: a clinical practice survey. Clin Transplant 201529118–123 [DOI] [PubMed] [Google Scholar]
- 70.Montgomery RA, Zachary AA, Racusen LC, et al. Plasmapheresis and intravenous immune globulin provides effective rescue therapy for refractory humoral rejection and allows kidneys to be successfully transplanted into cross-match-positive recipients. Transplantation 200070887–895 [DOI] [PubMed] [Google Scholar]
- 71.Böhmig GA, Wahrmann M, Regele H, et al. Immunoadsorption in severe c4d-positive acute kidney allograft rejection: a randomized controlled trial. Am J Transplant 20077117–121 [DOI] [PubMed] [Google Scholar]
- 72.Fehr T, Gaspert A. Antibody-mediated kidney allograft rejection: therapeutic options and their experimental rationale. Transpl Int 201225623–632 [DOI] [PubMed] [Google Scholar]
- 73.Marks WH, Mamode N, Montgomery RA, et al. ; C10-001 Study Group Safety and efficacy of eculizumab in the prevention of antibody-mediated rejection in living-donor kidney transplant recipients requiring desensitization therapy: a randomized trial. Am J Transplant 2019192876–2888 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Glotz D, Russ G, Rostaing L, et al. ; C10-002 Study Group Safety and efficacy of eculizumab for the prevention of antibody-mediated rejection after deceased-donor kidney transplantation in patients with preformed donor-specific antibodies. Am J Transplant 2019192865–2875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Viglietti D, Gosset C, Loupy A, et al. C1 inhibitor in acute antibody-mediated rejection nonresponsive to conventional therapy in kidney transplant recipients: a pilot study. Am J Transplant 2016161596–1603 [DOI] [PubMed] [Google Scholar]
- 76.Montgomery RA, Orandi BJ, Racusen L, et al. Plasma-derived C1 esterase inhibitor for acute antibody-mediated rejection following kidney transplantation: results of a randomized double-blind placebo-controlled pilot study. Am J Transplant 2016163468–3478 [DOI] [PubMed] [Google Scholar]
- 77.Macklin PS, Morris PJ, Knight SR. A systematic review of the use of rituximab for the treatment of antibody-mediated renal transplant rejection. Transplant Rev (Orlando) 20173187–95 [DOI] [PubMed] [Google Scholar]
- 78.Zarkhin V, Li L, Kambham N, et al. A randomized, prospective trial of rituximab for acute rejection in pediatric renal transplantation. Am J Transplant 200882607–2617 [DOI] [PubMed] [Google Scholar]
- 79.Sautenet B, Blancho G, Büchler M, et al. One-year results of the effects of rituximab on acute antibody-mediated rejection in renal transplantation: RITUX ERAH, a multicenter double-blind randomized placebo-controlled trial. Transplantation 2016100391–399 [DOI] [PubMed] [Google Scholar]
- 80.Moreso F, Crespo M, Ruiz JC, et al. Treatment of chronic antibody mediated rejection with intravenous immunoglobulins and rituximab: a multicenter, prospective, randomized, double-blind clinical trial. Am J Transplant 201818927–935 [DOI] [PubMed] [Google Scholar]
- 81.Lefaucheur C, Loupy A, Vernerey D, et al. Antibody-mediated vascular rejection of kidney allografts: a population-based study. Lancet 2013381313–319 [DOI] [PubMed] [Google Scholar]
- 82.Vo AA, Lukovsky M, Toyoda M, et al. Rituximab and intravenous immune globulin for desensitization during renal transplantation. N Engl J Med 2008359242–251 [DOI] [PubMed] [Google Scholar]
- 83.Budde K, Dürr M. Any progress in the treatment of antibody-mediated rejection? J Am Soc Nephrol 201829350–352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jordan SC, Lorant T, Choi J, et al. IgG endopeptidase in highly sensitized patients undergoing transplantation. N Engl J Med 2017377442–453 [DOI] [PubMed] [Google Scholar]
- 85.Winstedt L, Järnum S, Nordahl EA, et al. Complete removal of extracellular IgG antibodies in a randomized dose-escalation phase I study with the bacterial enzyme ides–a novel therapeutic opportunity. PLoS One. 2015;10:e0132011. doi: 10.1371/journal.pone.0132011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lonze BE, Tatapudi VS, Weldon EP, et al. Ides (imlifidase): a novel agent that cleaves human IgG and permits successful kidney transplantation across high-strength donor-specific antibody. Ann Surg 2018268488–496 [DOI] [PubMed] [Google Scholar]
- 87.Bamoulid J, Staeck O, Crépin T, et al. Anti-thymocyte globulins in kidney transplantation: focus on current indications and long-term immunological side effects. Nephrol Dial Transplant 2017321601–1608 [DOI] [PubMed] [Google Scholar]
- 88.Kamar N, Milioto O, Puissant-Lubrano B, et al. Incidence and predictive factors for infectious disease after rituximab therapy in kidney-transplant patients. Am J Transplant 20101089–98 [DOI] [PubMed] [Google Scholar]
- 89.Locke JE, Zachary AA, Haas M, et al. The utility of splenectomy as rescue treatment for severe acute antibody mediated rejection. Am J Transplant 20077842–846 [DOI] [PubMed] [Google Scholar]
- 90.Kaplan B, Gangemi A, Thielke J, et al. Successful rescue of refractory, severe antibody mediated rejection with splenectomy. Transplantation 20078399–100 [DOI] [PubMed] [Google Scholar]
- 91.Ejaz NS, Alloway RR, Halleck F, et al. Review of bortezomib treatment of antibody-mediated rejection in renal transplantation. Antioxid Redox Signal 2014212401–2418 [DOI] [PubMed] [Google Scholar]
- 92.Eskandary F, Regele H, Baumann L, et al. A randomized trial of bortezomib in late antibody-mediated kidney transplant rejection. J Am Soc Nephrol 201829591–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Moreno Gonzales MA, Gandhi MJ, Schinstock CA, et al. 32 doses of bortezomib for desensitization is not well tolerated and is associated with only modest reductions in anti-HLA antibody. Transplantation 20171011222–1227 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Waiser J, Duerr M, Budde K, et al. Treatment of acute antibody-mediated renal allograft rejection with cyclophosphamide. Transplantation 20171012545–2552 [DOI] [PubMed] [Google Scholar]
- 95.Choi J, Aubert O, Vo A, et al. Assessment of tocilizumab (anti-interleukin-6 receptor monoclonal) as a potential treatment for chronic antibody-mediated rejection and transplant glomerulopathy in HLA-sensitized renal allograft recipients. Am J Transplant 2017172381–2389 [DOI] [PubMed] [Google Scholar]
- 96.Eskandary F, Dürr M, Budde K, et al. Clazakizumab in late antibody-mediated rejection: study protocol of a randomized controlled pilot trial. Trials. 2019;20:37. doi: 10.1186/s13063-018-3158-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.O’Connell PJ, Kuypers DR, Mannon RB, et al. Clinical trials for immunosuppression in transplantation: the case for reform and change in direction. Transplantation 20171011527–1534 [DOI] [PubMed] [Google Scholar]


