Abstract:
Myopia is a global problem that is increasing at an epidemic rate in the world. Although the refractive error can be corrected easily, myopes, particularly those with high myopia, are susceptible to potentially blinding eye diseases later in life. Despite a plethora of myopia research, the molecular/cellular mechanisms underlying the development of myopia are not well understood, preventing the search for the most effective pharmacological control. Consequently, several approaches to slowing down myopia progression in the actively growing eyes of children have been underway. So far, atropine, an anticholinergic blocking agent, has been most effective and is used by clinicians in off-label ways for myopia control. Although the exact mechanisms of its action remain elusive and debatable, atropine encompasses a complex interplay with receptors on different ocular tissues at multiple levels and, hence, can be categorized as a shotgun approach to myopia treatment. This review will provide a brief overview of the biological mechanisms implicated in mediating the effects of atropine in myopia control.
Key Words: Myopia, Myopia control, Biological mechanisms in myopia, Atropine, Atropine action in myopia
Myopia is the most common refractive disorder worldwide and has become a leading cause of blindness.1 A systemic review and meta-analysis of 145 different studies from around the world involving 2.1 million participants suggests that approximately 23% and 2.7% of the world population had myopia and high myopia in 2000, respectively.2 It has been predicted that myopia prevalence will increase to nearly 50% by 2050 affecting 4.8 billion people in the world, which means that one in every two children will become myopic in approximately 30 years from now. The incidence of myopia varies according to ethnicity and geographical location, affecting approximately 85% of urban population in East Asia, especially in Taiwan3,4 and Singapore,5 and 44% in the United States.6 Interestingly, the prevalence is much lower in underdeveloped areas in the world such as rural Mongolia (5.8%)7 and Pokhara, Nepal (4.05%).8 Myopia has been associated with increased risk of comorbidities such as retinal detachment, macular degeneration, foveoschisis, early-onset glaucoma, cataract,9 and even vision loss,10 such that the risk is greater at higher degrees of myopia. The yearly incidence of retinal detachments increases with the degree of myopia (0.015% in >4.74 D myopia, 0.07% in >5.00 D myopia, and 3.2% in >6.00 D myopia).11 Even the risk of developing macular choroidal neovascularization increases with the level of myopia, with the likelihood being as high as 2-fold between 1.00 to 2.00 D myopia, 4-fold between 3.00 to 4.00 D myopia, and 9-fold for 5.00 to 6.00 D myopia.12 The economic burden of myopic refractive error was estimated to be US$202 billion per annum, with at least $3.8 billion spent as direct costs on myopia correction annually.13,14 These factors pose key challenges in managing myopia, prevention of which has become a major public health issue.3
Myopia is characterized by excessive axial elongation because of an increase in the vitreous chamber depth of the eyes, which causes light from distant objects to focus in front of the retina, leading to formation of blurred images. In other words, there is a mismatch between the focal length and the axial length (AL) of the eye in myopia, with the latter being too long for the refractive power of the lens and cornea. Myopia is a complex multifactorial disorder regulated by interactions between environmental and genetic risk factors15 and occurs as a result of failure to achieve or maintain the normal process of emmetropization, which is essentially endogenous to the eye.16 Myopia generally develops during childhood, during the school years, and its progression gradually stabilizes after adolescence for most individuals. Several epidemiological and animal studies conducted over the past 4 decades have investigated the possible causes underlying the development of myopia. A complexity of conditions including environmental factors such as time spent outdoors,17 near work,5 prolonged intense education,18 and urbanization19 play an important role in the development of myopia in school-going children. Nevertheless, despite a plethora of myopia research, the molecular/cellular mechanisms underlying the development of myopia are not well understood, preventing the search for the most effective pharmacological control. Consequently, there is no established way to prevent the onset of myopia, totally stop the progression, or reverse the progression. Most existing strategies to control the epidemic of myopia, thus, aim for effectively managing the disorder—by delaying its onset or slowing down myopia progression in the actively growing eyes of children through increased time spent outdoors20 and decreased duration of near work,21 by pharmacological interventions22 and other optical strategies (such as bifocal and multifocal lenses, progressive addition lenses, soft bifocal contact lenses, and orthokeratology),23,24 and refractive correction clinically by prescription lenses, contact lenses, or surgery.25
DECREASING PROGRESSION OF MYOPIA
Myopia control using pharmaceutical interventions has been reported to be most effective in comparison with other strategies.26,27 Anticholinergics are a class of drugs which block the action of acetylcholine at the muscarinic receptors (MRs) on structures with parasympathetic innervation and smooth muscles. Acetylcholine plays an important role in the developing retina28 and regulates the growth of the eye.29 Several drugs from this class have displayed variable efficacy to slow myopia progression in the Cochrane database systemic review of 2011.30 However, only atropine sulfate29,31–33 (a nonselective, broad muscarinic acetylcholine receptor antagonist) and pirenzepine34,35 (an antagonist with selectivity for M1R subtype) have shown clinical effectiveness in rigorous trials. Daily topical application of atropine at 1% or lower concentrations reduced myopia progression in children in a dose-dependent manner in several studies, with significant reductions in change in cycloplegic refraction (spherical equivalent [SE]) and AL elongation by as much as 80% and 95%, respectively, at the dose of 1%.29 Likewise, pirenzepine at 2% also demonstrated an efficacy of 50% in slowing myopia progression with the requirement of dosing twice a day instead of once as in the case of atropine.35 Nonetheless, further testing of pirenzepine in clinical trials has been suspended perhaps because of regulatory and economic constraints.36 Thus, atropine is the currently available anticholinergic drug for off-label use in myopia treatment with demonstrated consistent efficacy.26,30 Myopia progression, as determined by change in the cycloplegic refraction, was reduced by 75%, 70%, and 60% at 0.5%, 0.1%, and 0.01% atropine, respectively, in comparison with the historic placebo group after two years of treatment in phase 1 of the ATOM2 study. Axial length elongation, on the other hand, was decreased by 29% and 26% at 0.5% and 0.1% atropine, respectively, with no observed effect of 0.01% atropine on curtailing AL elongation.37 The more recent placebo-controlled low-concentration atropine for myopia progression (LAPMP) study showed that although myopia progression was minimized by 67%, 43%, and 27%, the AL elongation was reduced by 51%, 29%, and 12% in the 0.05%, 0.025%, and 0.01% atropine groups, respectively, after 1 year of treatment.33 Although direct comparisons cannot be made between these studies given the differences in their strategies and trial duration, it must be noted that in both the studies, only the mean change in SE was significantly different between 0.01% atropine-treated and the placebo groups, whereas AL elongation was nonsignificant. Also, AL elongation in the 0.01% atropine group at 2 years was significantly greater in comparison with other groups treated with higher atropine concentrations (0.1% and 0.5%).37 However, the intergroup differences were small and clinically insignificant. This might create some uncertainty over the effectiveness of 0.01% atropine for myopia control, given the importance of axial elongation in myopia progression, but we must be careful about jumping to conclusions and also consider the long-term effects of atropine after stopping the treatment.38 Unfortunately, atropine usage is associated with several ocular side-effects such as mydriasis (pupil dilation), photophobia, glare, local allergic response, loss of accommodation, and near vision (cycloplegia), which wear out eventually with the cessation of atropine treatment. These side-effects are more common at higher concentrations and seem to be dose-dependent.31 In fact, the incidence of photophobia was 100% in children receiving 1% atropine, leading to a high dropout rate of 16% to 58% from a study.39 On the contrary, the percentage of participants experiencing photophobia was reduced to 22% and 7% at lower atropine concentrations of 0.5% and 0.25%, respectively, and none at 0.1% atropine.40 Likewise, only 7% of participants on 0.01% atropine experienced photophobia and requested photochromatic (tinted) glasses in the ATOM2 study.31 Besides the temporary side-effects associated with atropine treatment, there is a more pronounced rebound phenomenon, in which myopia returns at a faster rate in the treated, myopic eyes than in the untreated eyes on cessation of treatment. Even the observed rebound effect was greater at higher concentrations of atropine, with 68% (on 0.5% atropine), 59% (on 0.1% atropine), and only 24% (on 0.01% atropine) of participants experiencing greater than 0.50 D increase in myopia after stopping the treatment (washout phase).31 In reality, during the washout phase, the change in mean SE and AL elongation was the least (P<0.001) in the 0.01% atropine group,38 whereas myopia progression continued at a steady pace in groups previously receiving 0.1% and 0.5% atropine, slowing only when 0.01% atropine was restarted in phase 3. This suggests that atropine, particularly at higher concentrations, could induce complex, long-lasting biochemical changes in the mechanisms regulating eye growth. Gradual tapering of atropine over time could possibly reduce the rebound effect, but this has not been studied in detail. Taken together, over the period of 5 years, the 0.01% atropine group showed the lowest overall myopia progression with least change in SE and AL elongation values and minimal visual side-effects among all the treatment groups. Long-term atropine usage could be associated with increased intraocular pressure and possibly glaucoma. Investigating this, clinical studies have reported no impact of atropine treatment on ocular hypertension in children,41,42 and that the risk of atropine-induced glaucoma is as low as 0.005%.43 Thus, so far, 0.01% atropine has shown the best therapeutic index (appropriate risk:benefit ratio) with overall better effectiveness and more modulated and sustained effect than higher doses.31,38 Although 0.05% atropine has been reported to show improved myopia-suppressive effects than 0.01%, with no adverse effect on vision-related quality of life over the period of 1 year,33 more information is required on the efficacy of 0.05% atropine in the longer term, particularly its effect on myopia rebound, before it can be validated and imbibed in clinical practice regularly. The efficacy of 0.01% atropine in myopia control has also been replicated outside Asia in an ethnically diverse group of children in the United States; however, this was determined from noncycloplegic refraction data.32 In fact, 0.01% atropine formulation has even been commercialized as Myopine. This product is now available in Singapore and Malaysia on an approved, named-patient basis and has been licensed in 15 countries across Europe and Asia to date.44 Yet, the exact mechanisms mediating atropine action in slowing myopia progression are unclear and remain a matter of speculation.
CELLULAR RECEPTORS ARE INVOLVED
Pharmacologically, atropine acts as a reversible competitive antagonist with an affinity for all the five subtypes of acetylcholine MRs (MR1–MR5) and thus has been presumed to exert its myopia-protective effect mainly through the MRs. The MRs belong to the superfamily of G protein–coupled receptors (GPCRs) and have both a neuronal and non-neuronal presence in the eye. Muscarinic receptors are widely distributed in different ocular tissues in mammals. They are found in cornea, iris, ciliary body, and ciliary muscles,45 epithelium of crystalline lens,46 retina (in amacrine cells), retinal pigment epithelium (RPE),47 choroid, and sclera (in scleral fibroblasts [SF]).48,49 However, several studies have reported potential off-target direct and indirect interactions of atropine at other non-MRs such as α2A-adrenergic receptors (αAR),50 ɣ-aminobutyric acid receptors (GABA-R),51 and receptor tyrosine kinases (RTKs)48 in different ocular tissues. Both αAR and GABA-R are well-known members of the GPCR family. Epidermal growth factor receptor (EGFR) is a member of the ErbB family of RTKs and regulates cellular proliferation of primary SF through intracellular signaling pathways involving the classical mitogen-activated protein kinase pathway.48 Atropine has been shown to reduce EGFR activity in mouse primary SF in a dose-dependent manner.48 Thus, atropine is a shotgun approach to myopia treatment. Corroborating this conjecture, a recent study analyzing the ocular pharmacokinetics of 1% topical atropine in rabbits showed that atropine has good bioavailability in most of the ocular tissues with detectable concentrations of twofold greater than its binding affinity (0.4–0.7 nM) at 3-day postdrug instillation.52 Although the penetration of atropine was greater in the anterior tissues (concentration gradient: highest in the conjunctiva, lowest in the lens) at 5 hr after instillation, an increased binding to posterior tissues was observed at 24 hr, with the reversal of the initial concentration gradient (highest in the posterior sclera, followed by the retina). In addition to its antagonistic properties at different biological receptors, atropine can also act as an inverse agonist at MR3, and probably at all the other MR subtypes, since it was shown to reduce the basal receptor activity in vitro in a concentration-dependent manner.53
Atropine was initially used for myopia control because of historical reasons since excessive accommodation by the eye was hypothesized to be responsible for myopia and atropine causes cycloplegia (temporary loss of accommodation/ability to focus on near and distant objects) by paralyzing the smooth ciliary muscle temporarily. However, later animal studies implicated nonaccommodative mechanisms in the cause of myopia and showed that eyes unable to accommodate, either due to lesioning of the Edinger–Westphal nucleus54 (the parasympathetic innervation of the iris sphincter muscle and the ciliary muscle) or sectioning of the optic nerve,55 also compensated to the imposed hyperopic defocus and developed myopia. In addition, atropine was found to reduce experimental form-deprived myopia (FDM) in chick eyes56 that possess acetylcholine nicotinic receptors on their straited intraocular muscles instead of MRs.57 Thus, there has been a paradigm shift of focus on investigating nonaccommodative mechanisms in myopia development. Concordantly, several biological mechanisms such as dysfunction of retinal signaling pathways in response to environmental cues,58–60 role of RPE in relaying ocular growth regulatory signals from the retina to sclera through choroid,61–63 reduced choroidal thickness,64,65 and increased scleral thinning due to remodeling of the extracellular matrix of the sclera66,67 have been thoroughly investigated to comprehend the causes of myopia (Fig. 1). Atropine has been observed to modulate these biological mechanisms and target mainly the retina and sclera, although effects of atropine on the choroid68,69 and RPE70 have also been reported.
FIG. 1.
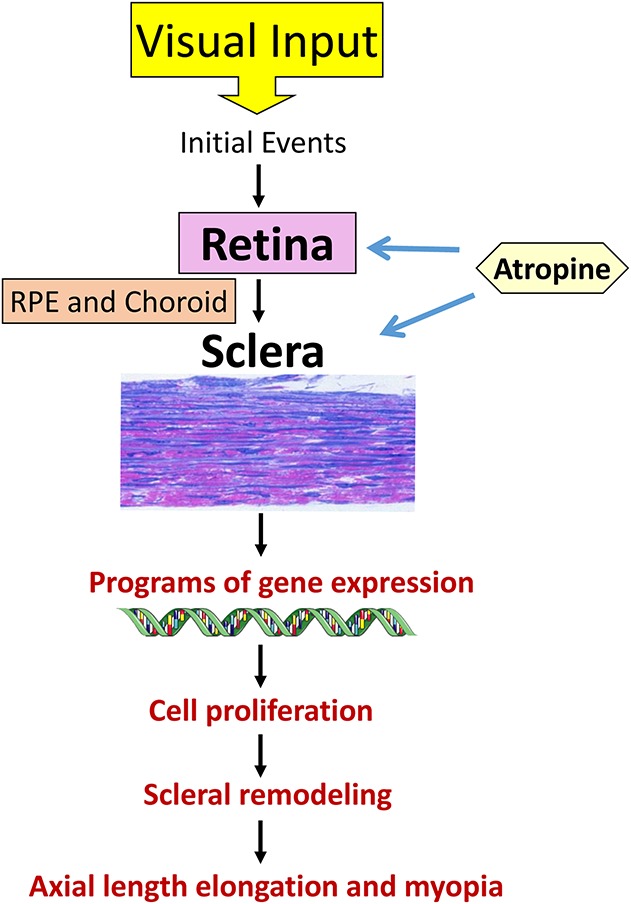
Schematic representation of signal cascade mediating myopia development in animal models. The available evidence points to an interaction between the retina and sclera in the development of myopia. However, this is largely from animal model studies where visual cues such as optical defocus imposed by lens wear predominantly drive axial elongation. These visual inputs initiate certain molecular events in retina. The growth-regulatory signals are then relayed to the sclera through RPE and choroid to facilitate programs of gene expression, cell proliferation, and scleral remodeling, which subsequently lead to axial length elongation causing myopia. We have little idea as to the situation in humans, where genetic and environmental factors may also play a role in myopia induction and development. Moreover, we do not know the nature of intertissue communication that may be quite complex in humans and even change in different stages of myopia development. Atropine targets biological receptors in both the retina and sclera, to curtail myopia progression. RPE, retinal pigment epithelium.
Role of the Retina
Retina is the light-sensitive, sensory layer lining the inner surface of the back of the eye. Intravitreal injection of atropine increased dopamine release and the concentration of its metabolite DOPAC in the chick retina.71 Dopamine is a neurotransmitter that facilitates interneuron signaling and plays a crucial role in ocular growth and myopia development.72,73 Dopamine agonists such as apomorphine inhibit myopia progression in both the myopiagenic animal models of form-deprived74 and lens-induced myopia (LIM).75 Atropine also reduced protein levels of GABA transporter-1 (GAT-1) and to a lesser extent GAT-3 that were elevated in the mouse retina with LIM, thereby suggesting the involvement of GABAergic signaling in the antimyopic effects of atropine in mouse eyes.51 The GABAergic transmission in the neural retina plays a pivotal role in the modulation of eye growth and refractive development in animals.76 GABA transporters, on the other hand, terminate the GABAergic transmission by facilitating its reuptake into presynaptic neurons and surrounding glial cells and removing GABA from the synaptic cleft.77 Interestingly, atropine was shown to restrain FDM in chicks with destroyed cholinergic amacrine cells, MRs, and choline acetyltransferase (ChAT, an enzyme that synthesizes acetylcholine) activity in the retina in a dose-dependent manner.78 This suggests that retinal MRs do not mediate the growth-inhibiting effects of atropine at least in FDM and indicated the involvement of MRs in other ocular tissues.
Role of the Sclera
Sclera is a specialized fibrous connective tissue that forms the protective outer layer of the eye. Excessive axial elongation of the eye causes refractive error and myopia development and can be attributed to scleral thinning due to increased scleral tissue remodeling. Certain molecular and biochemical events take place in the sclera during this remodeling process, which eventually cause structural and biomechanical changes in the tissue, resulting in weakened extracellular matrix and significant scleral tissue thinning (reviewed elsewhere in detail79). Inhibition of myopia progression by atropine can also occur at the scleral site. This conjecture is supported by evidences showing that atropine decreased proliferation of mouse SF in vitro,48 increased the thickness of scleral fibrous layer in the myopic eyes from both chick80 and mouse67 models, reduced extracellular matrix production by decreasing glycosaminoglycan (GAG) synthesis both in the whole sclera and in isolated scleral chondrocytes from form-deprived eyes in chicks,81 and upregulated the mRNA levels of MR1, MR3, and MR4 while downregulating MR2 and MR5 mRNA levels in the sclera from mouse eyes with LIM.67
Role of Retinal Pigment Epithelium and Choroid
Retinal pigment epithelium is a monolayer of highly specialized pigmented cells, which forms a barrier between the neural retina and vascular choroid, and plays a very important role in maintaining retinal homeostasis. Retinal pigment epithelium has been shown to relay the growth regulatory signal from the retina to choroid and sclera and modulate scleral growth and alter scleral GAG content in vitro.61,62 Coculturing of ex vivo RPE (from LIM chick eyes) with primary SF (from nonmyopic eyes) increased the cell proliferation of SF and decreased GAG content in vitro.61 By contrast, there was no effect of ex vivo retina and primary SF coculture on the latter's DNA or GAG content, while choroid and SF coculture increased the GAG content only. This suggests that the growth regulatory defocus signals, although originating in retina, are most likely contained in the RPE and possibly in the choroid to some extent. Choroid is the vascular layer of the eye and supplies oxygen and nourishment to the outer retina. Choroid modulates its thickness mechanistically to adjust the retina to the focal plane of the eye in response to the imposed optic defocus (choroidal accommodation) and hence plays an active role in emmetropization.63 Atropine inhibited the development of myopia and rapidly induced transient choroidal thickening in LIM model of chicks.68 Likewise, atropine eliminated choroidal thinning induced by hyperopic defocus signals in myopic human eyes without changing baseline choroidal thickness.69 Both the RPE (basal surface) and choroid secrete a variety of growth factors including transforming growth factor (TGF-β) and basal fibroblast growth factor (bFGF). Atropine has also been shown to modulate the expression and activity levels of these growth factors (different isoforms) in vitro. Atropine inhibited the expression and secretion of TGF-β2 by blocking MRs in RPE cells.82 Atropine also decreased TGF-β1 activity levels and increased bFGF2 activity levels in primary mouse SF in a dose-dependent manner.48 Transforming growth factor-β1 stimulates collagen synthesis by primary SF in vitro in a dose-dependent manner and thus has been implicated in the regulation of scleral remodeling during myopia development.83 bFGF2, on the other hand, activates Ras/mitogen activated protein kinase-mediated signaling in mouse SF48 and stimulates SF proliferation.84
FUTURE CONSIDERATIONS
Atropine at 0.01% seems to be an effective, low-cost medication to slow myopia progression in the growing eye. Nonetheless, this outcome is mainly based on its sustained impact on restraining changes in refraction, with lesser effect observed on inhibiting AL elongation. The detailed mechanisms and the exact site of action of atropine also remain elusive. Further to this, muscarinic mechanisms have not been per se studied in the development of myopia. Although the side-effects observed at 0.01% atropine are minimal and apparent for short term, in the 0.01% group of ATOM2 study, 24% of participants were subjected to myopia rebound, 7% experienced mild side-effects that were not severe enough to prompt a discontinuation, and 9.3% responded poorly to the treatment since they had greater than 1.5 D myopia progression over 2 years of initial treatment. The percentages of “poor responders” were 6.4% and 4.3% in 0.1% and 0.5% atropine groups, respectively. Similarly, in other studies, 11% of children in the 0.5% atropine group had greater than 0.75 D myopia increase per year,85 whereas 33%, 17%, and 4% of children belonging to 0.1%, 0.25%, and 0.5% atropine groups, respectively, showed greater than 1 D increase of myopia per year in comparison with 44% in the control group.40 Another study found 45% of participants to be “poor responders” to 0.05% atropine with myopia progression of greater than 0.5 D over 6 months.86 However, when switched to 0.1% atropine, only 20% progressed further by greater than 0.5 D per year in comparison with 100% in the control group over 4.5 years of follow-up. Even when treated at 1% atropine, 12% of children at 1 year continued to progress by greater than 0.5 D myopia per year and were likely to be younger, more myopic, and have two myopic parents.87 The treatment strategy for such poorly responding patients remains obscure.
Several studies, including those on the actively growing eyes of children belonging to the similar age group as in the atropine trials (6–12 years), have shown that the distribution of refraction changes with age88,89 and indicated the presence of two different Gaussian subpopulations, with distinct patterns of refraction and AL distribution possibly due to differences in their etiologies, in adult populations.90 Although most population possesses emmetropic eyes during early childhood (6 years) with leptokurtotic distribution of refraction represented by narrow peak, there is a very small subpopulation with myopic refraction that fail to undergo the initial phase of emmetropization (indicating primary homeostatic failure) or show a delay in achieving early emmetropization (poor emmetropizers).88 As the eyes grow over time, by 11 to 12 years of age, a significant proportion of the population remain close to emmetropia (indicating regulated growth), but the spread of refraction data increases, and there is a shift toward negatively skewed distribution, suggesting the presence of another subset that begins to drift in the direction of myopia owing to dysregulated eye growth from the inability to maintain the emmetropic state (secondary homeostatic failure). Interestingly, the distribution of ocular biometry (AL and refraction) becomes bigaussian, with essentially two subpopulations existing in the adult population (20–70 years): an emmetropized subset with a sharp peak at emmetropia and less variability in the data and a dysregulated subgroup with a broader peak/mode, increased data spread/variability, and a myopic mean.90 Thus, myopes begin to become myopic either due to primary (a very small percentage though) or secondary homeostatic failures and therefore warrant a need for intervention to regulate their eye growth. Further to this, not all the eyes will emmetropize to the same extent, and some with low myopic refraction will fall within the tail of emmetropic refraction distribution (poor emmetropizers). It would, then, be expected that interventions such as atropine would have different effects on myopes belonging to these two groups: poor emmetropizers and those with failed homeostatic mechanisms, which can be determined from their individual myopia progression rates. In particular, atropine might have little effect on the poor emmetropizers, that perhaps overlap with the “poor responders” category to a certain extent. Furthermore, the possibility of any intervention exacerbating the extent of myopia in this group also cannot be negated.
There are concerns as well regarding the potential long-term ocular or systemic side-effects of atropine usage. Several anticholinergic drugs and other medications with anticholinergic properties have been associated with central side-effects adversely affecting cognitive function.91 Although this is particularly true for elderly people with high anticholinergic load, prolonged exposure to even mild anticholinergics during midlife can put one at increased risks in old age.92 Given that some clinicians in East Asia advocate continuous treatment of myopic children with low-dose atropine till late adolescent period, after which myopia generally stabilizes, the long-term central effects of extended exposure to atropine, also an anticholinergic, in early life should be investigated thoroughly. Taken together, there is a need to study the mechanisms of action of atropine in preventing myopia development to develop targeted therapies with enhanced efficacy and minimal short-term/long-term adverse effects. There is also a necessity to identify new druggable targets and develop alternative therapeutic strategies for myopia control in the subset of patients who fail to respond to atropine treatment.
Footnotes
R. W. Beuerman received honoraria from Santen Pharmaceutical Co., Ltd, and is a cofounder and chief scientific officer at SinSaLabs. The remaining author has no funding or conflicts of interest to disclose.
R. W. Beuerman received a grant from NMRC Translational and Clinical Research (TCR) Flagship Programme Singapore, grant R1018.
REFERENCES
- 1.Bourne RR, Stevens GA, White RA, et al. Causes of vision loss worldwide, 1990-2010: A systematic analysis. Lancet Glob Health 2013;1:e339–e349. [DOI] [PubMed] [Google Scholar]
- 2.Holden BA, Fricke TR, Wilson DA, et al. Global prevalence of myopia and high myopia and temporal trends from 2000 through 2050. Ophthalmology 2016;123:1036–1042. [DOI] [PubMed] [Google Scholar]
- 3.Wang TJ, Chiang TH, Wang TH, et al. Changes of the ocular refraction among freshmen in National Taiwan University between 1988 and 2005. Eye 2009;23:1168–1169. [DOI] [PubMed] [Google Scholar]
- 4.Lin LKL, Shih YFF, Hsiao CK, et al. Epidemiologic study of the prevalence and severity of myopia among schoolchildren in Taiwan in 2000. J Formos Med Assoc 2001;100:684–691. [PubMed] [Google Scholar]
- 5.Saw SM, Zhang MZ, Hong RZ, et al. Near-work activity, night-lights, and myopia in the Singapore-China study. Evidence-Based Eye Care 2002;3:198–199. [DOI] [PubMed] [Google Scholar]
- 6.Vitale S, Sperduto RD, Ferris FL. Increased prevalence of myopia in the United States between 1971-1972 and 1999-2004. Arch Ophthalmol 2009;127:1632–1639. [DOI] [PubMed] [Google Scholar]
- 7.Morgan A, Young R, Narankhand B, et al. Prevalence rate of myopia in schoolchildren in rural Mongolia. Optom Vis Sci 2006;83:53–56. [DOI] [PubMed] [Google Scholar]
- 8.Niroula DR, Saha CG. Study on the refractive errors of school going children of Pokhara city in Nepal. Kathmandu Univ Med J 2009;7:67–72. [DOI] [PubMed] [Google Scholar]
- 9.Praveen MR, Vasavada AR, Jani UD, et al. Prevalence of cataract type in relation to axial length in subjects with high myopia and mmmetropia in an Indian population. Am J Ophthalmol 2008;145:176–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong TY, Ferreira A, Hughes R, et al. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: An evidence-based systematic review. Am J Ophthalmol 2014;157:9–25. [DOI] [PubMed] [Google Scholar]
- 11.Arevalo JF, Ramirez E, Suarez E, et al. Rhegmatogenous retinal detachment after laser-assisted in situ keratomileusis (LASIK) for the correction of myopia. Retina 2000;20:338–341. [DOI] [PubMed] [Google Scholar]
- 12.Steidl SM, Pruett RC. Macular complications associated with posterior staphyloma. Am J Ophthalmol 1997;123:181–187. [DOI] [PubMed] [Google Scholar]
- 13.Smith TST, Frick KD, Holden BA, et al. Potential lost productivity resulting from the global burden of uncorrected refractive error. Bull World Health Organ 2009;87:431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vitale S, Cotch MF, Sperduto R, et al. Costs of refractive correction of distance vision impairment in the United States, 1999-2002. Ophthalmology 2006;113:2163–2170. [DOI] [PubMed] [Google Scholar]
- 15.Saw SM, Shankar A, Tan SB, et al. A cohort study of incident myopia in Singaporean children. Invest Ophthalmol Vis Sci 2006;47:1839–1844. [DOI] [PubMed] [Google Scholar]
- 16.Troilo D, Gottlieb MD, Wallman J. Visual deprivation causes myopia in chicks with optic nerve section. Curr Eye Res 1987;6:993–999. [DOI] [PubMed] [Google Scholar]
- 17.Wu PC, Tsai CL, Wu HL, et al. Outdoor activity during class recess reduces myopia onset and progression in school children. Ophthalmology 2013;120:1080–1085. [DOI] [PubMed] [Google Scholar]
- 18.Dirani M, Shekar SN, Baird PN. The role of educational attainment in refraction: The genes in myopia (GEM) twin study. Invest Ophthalmol Vis Sci 2008;49:534–538. [DOI] [PubMed] [Google Scholar]
- 19.Morgan I, Rose K. How genetic is school myopia? Prog Retin Eye Res 2005;24:1–38. [DOI] [PubMed] [Google Scholar]
- 20.Rose KA, Morgan IG, Ip J, et al. Outdoor activity reduces the prevalence of myopia in children. Ophthalmology 2008;115:1279–1285. [DOI] [PubMed] [Google Scholar]
- 21.Wu PC, Huang HM, Yu HJ, et al. Epidemiology of myopia. Asia-Pac J Ophthalmol (Phila) 2016;5:386–393. [DOI] [PubMed] [Google Scholar]
- 22.Ganesan P, Wildsoet CF. Pharmaceutical intervention for myopia control. Expert Rev Ophthalmol 2010;5:759–787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walline JJ. Myopia control: A review. Eye Contact Lens 2016;42:3–8. [DOI] [PubMed] [Google Scholar]
- 24.Smith EL. Optical treatment strategies to slow myopia progression: Effects of the visual extent of the optical treatment zone. Exp Eye Res 2013;114:77–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kaimbo DKW. Refractive surgery for myopia. In: Pacheco P, ed. Advances in Eye Surgery. Rijeka, Croatia, InTech, 2016. Ch. 11. [Google Scholar]
- 26.Huang J, Wen D, Wang Q, et al. Efficacy comparison of 16 interventions for myopia control in children: A network meta-analysis. Ophthalmology 2016;123:697–708. [DOI] [PubMed] [Google Scholar]
- 27.Prousali E, Haidich AB, Fontalis A, et al. Efficacy and safety of interventions to control myopia progression in children: An overview of systematic reviews and meta-analyses. BMC Ophthalmol 2019;19:106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ford KJ, Feller MB. Assembly and disassembly of a retinal cholinergic network. Vis Neurosci 2012;29:61–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chua WH, Balakrishnan V, Chan YH, et al. Atropine for the treatment of childhood myopia. Ophthalmology 2006;113:2285–2291. [DOI] [PubMed] [Google Scholar]
- 30.Walline JJ, Lindsley K, Vedula SS, et al. Interventions to slow progression of myopia in children (Review). Cochrane Database Syst Rev 2011;12:CD004916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chia A, Lu QS, Tan D. Five-year clinical trial on atropine for the treatment of myopia 2: Myopia control with atropine 0.01% eyedrops. Ophthalmology 2016;123:391–399. [DOI] [PubMed] [Google Scholar]
- 32.Clark TY, Clark RA. Atropine 0.01% eyedrops significantly reduce the progression of childhood myopia. J Ocul Pharmacol Ther 2015;31:541–545. [DOI] [PubMed] [Google Scholar]
- 33.Yam JC, Jiang Y, Tang SM, et al. Low-concentration atropine for myopia progression (LAMP) study: A randomized, double-blinded, placebo-controlled trial of 0.05%, 0.025%, and 0.01% atropine eye drops in myopia control. Ophthalmology 2019;126:113–124. [DOI] [PubMed] [Google Scholar]
- 34.Bartlett JD, Niemann K, Houde B, et al. A tolerability study of pirenzepine ophthalmic gel in myopic children. J Ocul Pharmacol Ther 2003;19:271–279. [DOI] [PubMed] [Google Scholar]
- 35.Tan DTH, Lam DS, Chua WH, et al. One-year multicenter, double-masked, placebo-controlled, parallel safety and efficacy study of 2% pirenzepine ophthalmic gel in children with myopia. Ophthalmology 2005;112:84–91. [DOI] [PubMed] [Google Scholar]
- 36.Leo SW, Young TL. An evidence-based update on myopia and interventions to retard its progression. J AAPOS 2011;15:181–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chia A, Chua WH, Cheung YB, et al. Atropine for the treatment of childhood myopia: Safety and efficacy of 0.5%, 0.1%, and 0.01% doses (atropine for the treatment of myopia 2). Ophthalmology 2012;119:347–354. [DOI] [PubMed] [Google Scholar]
- 38.Chia A, Chua WH, Wen L, et al. Atropine for the treatment of childhood myopia: Changes after stopping atropine 0.01%, 0.1% and 0.5%. Am J Ophthalmol 2014;157:451–457e1. [DOI] [PubMed] [Google Scholar]
- 39.Yen MY, Liu JH, Kao SC, et al. Comparison of the effect of atropine and cyclopentolate on myopia. Ann Ophthalmol 1989;21:180–187. [PubMed] [Google Scholar]
- 40.Shih YF, Chen CH, Chou AC, et al. Effects of different concentrations of atropine on controlling myopia in myopic children. J Ocul Pharmacol Ther 1999;15:85–90. [DOI] [PubMed] [Google Scholar]
- 41.Wu TE, Yang CC, Chen HS. Does atropine use increase intraocular pressure in myopic children? Optom Vis Sci 2012;89:E161–E167. [DOI] [PubMed] [Google Scholar]
- 42.Lee CY, Sun CC, Lin YF, et al. Effects of topical atropine on intraocular pressure and myopia progression: A prospective comparative study. BMC Ophthalmol 2016;16:114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pandit RJ, Taylor R. Mydriasis and glaucoma: Exploding the myth. A systematic review. Diabet Med 2000;17:693–699. [DOI] [PubMed] [Google Scholar]
- 44.Tan DT. Author's reply. Singapore Med J 2018;59:507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gil DW, Krauss HA, Bogardus AM, et al. Muscarinic receptor subtypes in human iris-ciliary body measured by immunoprecipitation. Invest Ophthalmol Vis Sci 1997;38:1434–1442. [PubMed] [Google Scholar]
- 46.Collison DJ, Coleman RA, James RS, et al. Characterization of muscarinic receptors in human lens cells by pharmacologic and molecular techniques. Invest Ophthalmol Vis Sci 2000;41:2633–2641. [PubMed] [Google Scholar]
- 47.Friedman Z, Hackett SF, Campochiaro PA. Human retinal pigment epithelial cells possess muscarinic receptors coupled to calcium mobilization. Brain Res 1988;446:11–16. [DOI] [PubMed] [Google Scholar]
- 48.Barathi VA, Weon SR, Beuerman RW. Expression of muscarinic receptors in human and mouse sclera and their role in the regulation of scleral fibroblasts proliferation. Mol Vis 2009;15:1277–1293. [PMC free article] [PubMed] [Google Scholar]
- 49.Qu J, Zhou X, Xie R, et al. The presence of m1 to m5 receptors in human sclera: Evidence of the sclera as a potential site of action for muscarinic receptor antagonists. Curr Eye Res 2006;31:587–597. [DOI] [PubMed] [Google Scholar]
- 50.Carr BJ, Mihara K, Ramachandran R, et al. Myopia-inhibiting concentrations of muscarinic receptor antagonists block activation of Alpha2A-Adrenoceptors in vitro. Invest Ophthalmol Vis Sci 2018;59:2778–2791. [DOI] [PubMed] [Google Scholar]
- 51.Barathi VA, Chaurasia SS, Poidinger M, et al. Involvement of GABA transporters in atropine-treated myopic retina as revealed by iTRAQ quantitative proteomics. J Proteome Res 2014;13:4647–4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wang LZ, Syn N, Li S, et al. The penetration and distribution of topical atropine in animal ocular tissues. Acta Ophthalmol 2018;97:238–247. [DOI] [PubMed] [Google Scholar]
- 53.Casarosa P, Kiechle T, Sieger P, et al. The constitutive activity of the human muscarinic M3 receptor unmasks differences in the pharmacology of anticholinergics. J Pharmacol Exp Ther 2010;333:201–209. [DOI] [PubMed] [Google Scholar]
- 54.Schaeffel F, Troilo D, Wallman J, et al. Developing eyes that lack accommodation grow to compensate for imposed defocus. Vis Neurosci 1990;4:177–183. [DOI] [PubMed] [Google Scholar]
- 55.Wildsoet CF. Neural pathways subserving negative lens-induced emmetropization in chicks—Insights from selective lesions of the optic nerve and ciliary nerve. Curr Eye Res 2003;27:371–385. [DOI] [PubMed] [Google Scholar]
- 56.McBrien NA, Moghaddam HO, Reeder AP. Atropine reduces experimental myopia and eye enlargement via a nonaccommodative mechanism. Invest Ophthalmol Vis Sci 1993;34:205–215. [PubMed] [Google Scholar]
- 57.Pilar G, Nuñez R, McLennan IS, et al. Muscarinic and nicotinic synaptic activation of the developing chicken iris. J Neurosci 1987;7:3813–3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Guo SS, Sivak JG, Callender MG, et al. Retinal dopamine and lens-induced refractive errors in chicks. Curr Eye Res 1995;14:385–389. [DOI] [PubMed] [Google Scholar]
- 59.Ohngemach S, Hagel G, Schaeffel F. Concentrations of biogenic amines in fundal layers in chickens with normal visual experience, deprivation, and after reserpine application. Vis Neurosci 1997;14:493–505. [DOI] [PubMed] [Google Scholar]
- 60.Stone RA, Lin T, Laties AM, et al. Retinal dopamine and form-deprivation myopia. Proc Natl Acad Sci USA 1989;86:704–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Christian PG, Harkin DG, Rayner C, et al. Comparative effects of posterior eye cup tissues from myopic and hyperopic chick eyes on cultured scleral fibroblasts. Exp Eye Res 2013;107:11–20. [DOI] [PubMed] [Google Scholar]
- 62.Seko Y, Tanaka Y, Tokoro T. Scleral cell growth is influenced by retinal pigment epithelium in vitro. Graefes Arch Clin Exp Ophthalmol 1994;232:545–552. [DOI] [PubMed] [Google Scholar]
- 63.Marzani D, Wallman J. Growth of the two layers of the chick sclera is modulated reciprocally by visual conditions. Invest Ophthalmol Vis Sci 1997;38:1726–1739. [PubMed] [Google Scholar]
- 64.Williams KM, Verhoeven VJM, Cumberland P, et al. Prevalence of refractive error in Europe: The European Eye Epidemiology (E3) Consortium. Eur J Epidemiol 2015;30:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res 2010;29:144–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.McBrien NA, Gentle A. Role of the sclera in the development and pathological complications of myopia. Prog Retin Eye Res 2003;22:307–338. [DOI] [PubMed] [Google Scholar]
- 67.Barathi VA, Beuerman RW. Molecular mechanisms of muscarinic receptors in mouse scleral fibroblasts: Prior to and after induction of experimental myopia with atropine treatment. Mol Vis 2011;17:680–691. [PMC free article] [PubMed] [Google Scholar]
- 68.Nickla DL, Zhu X, Wallman J. Effects of muscarinic agents on chick choroids in intact eyes and eyecups: Evidence for a muscarinic mechanism in choroidal thinning. Ophthalmic Physiol Opt 2013;33:245–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chiang STH, Phillips JR. Effect of atropine eye drops on choroidal thinning induced by hyperopic retinal defocus. J Ophthalmol 2018;2018:8528315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Schwahn HN, Kaymak H, Schaeffel F. Effects of atropine on refractive development, dopamine release, and slow retinal potentials in the chick. Vis Neurosci 2000;17:165–176. [DOI] [PubMed] [Google Scholar]
- 71.Schaeffel F, Schwahn HN, Diether S, et al. Retinal effects of atropine on the development of experimental myopia, dopamine release and contrast sensitivity in chicks. J Toxicol Cutan Ocul Toxicol 1999;18:216–217. [Google Scholar]
- 72.Feldkaemper M, Schaeffel F. An updated view on the role of dopamine in myopia. Exp Eye Res 2013;114:106–119. [DOI] [PubMed] [Google Scholar]
- 73.Nebbioso M, Plateroti AM, Pucci B, et al. Role of the dopaminergic system in the development of myopia in children and adolescents. J Child Neurol 2014;29:1739–1746. [DOI] [PubMed] [Google Scholar]
- 74.Iuvone PM, Tigges M, Stone RA, et al. Effects of apomorphine, a dopamine receptor agonist, on ocular refraction and axial elongation in a primate model of myopia. Invest Ophthalmol Vis Sci 1991;32:1674–1677. [PubMed] [Google Scholar]
- 75.Nickla DL, Totonelly K, Dhillon B. Dopaminergic agonists that result in ocular growth inhibition also elicit transient increases in choroidal thickness in chicks. Exp Eye Res 2010;91:715–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmid KL, Strasberg G, Rayner CL, et al. The effects and interactions of GABAergic and dopaminergic agents in the prevention of form deprivation myopia by brief periods of normal vision. Exp Eye Res 2013;110:88–95. [DOI] [PubMed] [Google Scholar]
- 77.Wood JD, Sidhu HS. A comparative study and partial characterization of multi-uptake systems for γ-Aminobutyric Acid. J Neurochem 1987;49:1202–1208. [DOI] [PubMed] [Google Scholar]
- 78.Fischer AJ, Miethke P, Morgan IG, et al. Cholinergic amacrine cells are not required for the progression and atropine-mediated suppression of form-deprivation myopia. Brain Res 1998;794:48–60. [DOI] [PubMed] [Google Scholar]
- 79.Metlapally R, Wildsoet CF. Scleral mechanisms underlying ocular growth and myopia. Prog Mol Biol Transl Sci 2015;134:241–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Gallego P, Martínez-García C, Pérez-Merino P, et al. Scleral changes induced by atropine in chicks as an experimental model of myopia. Ophthalmic Physiol Opt 2012;32:478–484. [DOI] [PubMed] [Google Scholar]
- 81.Lind GJ, Chew SJ, Marzani D, et al. Muscarinic acetylcholine receptor antagonists inhibit chick scleral chondrocytes. Invest Ophthalmol Vis Sci 1998;39:2217–2231. [PubMed] [Google Scholar]
- 82.Tan J, Deng ZH, Liu SZ, et al. TGF-beta2 in human retinal pigment epithelial cells: Expression and secretion regulated by cholinergic signals in vitro. Curr Eye Res 2010;35:37–44. [DOI] [PubMed] [Google Scholar]
- 83.Jobling AI, Nguyen M, Gentle A, et al. Isoform-specific changes in scleral transforming growth factor-β expression and the regulation of collagen synthesis during myopia progression. J Biol Chem 2004;279:18121–18126. [DOI] [PubMed] [Google Scholar]
- 84.Seko Y, Tanaka Y, Tokoro T. Influence of bFGF as a potent growth stimulator and TGF-β as a growth regulator on scleral chondrocytes and scleral fibroblasts in vitro. Ophthalmic Res 1995;27:144–152. [DOI] [PubMed] [Google Scholar]
- 85.Shih YF, Kate Hsiao C, Chen CJ, et al. An intervention trial on efficacy of atropine and multi-focal glasses in controlling myopic progression. Acta Ophthalmol Scand 2001;79:233–236. [DOI] [PubMed] [Google Scholar]
- 86.Wu PC, Yang YH, Fang PC. The long-term results of using low-concentration atropine eye drops for controlling myopia progression in schoolchildren. J Ocul Pharmacol Ther 2011;27:461–466. [DOI] [PubMed] [Google Scholar]
- 87.Loh KL, Lu Q, Tan D, et al. Risk factors for progressive myopia in the atropine therapy for myopia study. Am J Ophthalmol 2015;159:945–949. [DOI] [PubMed] [Google Scholar]
- 88.Flitcroft DI. Is myopia a failure of homeostasis? Exp Eye Res 2013;114:16–24. [DOI] [PubMed] [Google Scholar]
- 89.Flitcroft DI. Emmetropisation and the aetiology of refractive errors. Eye (Lond) 2014;28:169–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rozema JJ, Tassignon MJ. The bigaussian nature of ocular biometry. Optom Vis Sci 2014;91:713–722. [DOI] [PubMed] [Google Scholar]
- 91.Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: A systematic review. Age Ageing 2014;43:604–615. [DOI] [PubMed] [Google Scholar]
- 92.Chuang YF, Elango P, Gonzalez CE, et al. Midlife anticholinergic drug use, risk of Alzheimer's disease, and brain atrophy in community-dwelling older adults. Alzheimers Dement (N Y) 2017;3:471–479. [DOI] [PMC free article] [PubMed] [Google Scholar]


