Ultra-widefield fundus autofluorescence imaging reveals abnormalities in degenerative retinoschisis that allow for the identification of an associated neurosensory detachment confirmed by spectral domain optical coherence tomography.
Key words: retinoschisis, autofluorescence, ultra-widefield imaging, retinal detachment, subretinal fluid, optical coherence tomography, hyperautofluorescence, peripheral retinal, outer plexiform layer, neurosensory retina
Abstract
Purpose:
To determine whether neurosensory retinal detachment complicating degenerative retinoschisis (RS) can be reliably detected with ultra-widefield fundus autofluorescence evaluation.
Methods:
Consecutive patients diagnosed with RS who had ultra-widefield fundus autofluorescence imaging were included in this retrospective case series. According to the fundus autofluorescence patterns, we divided the eyes into two groups: 1) eyes with RS and a hyperautofluorescent leading edge and 2) eyes with RS and without hyperautofluorescence. Peripheral spectral domain optical coherence tomography images at the level of RS were obtained.
Results:
Thirty-eight eyes that met eligibility criteria were identified. Review of ultra-widefield fundus autofluorescence demonstrated 21/39 (55%) eyes with distinctive hyperautofluorescence over the area of RS (Group A) and 17/38 (45%) eyes without any form of hyperautofluorescence (Group B). Spectral domain optical coherence tomography images confirmed the presence of full-thickness neurosensory retina separation from the underlying retinal pigment epithelium in the areas of hyperautofluorescence in 10/10 eyes (100%) from Group A. None (0/11; 0%) of the eyes from Group B showed full-thickness neurosensory retina separation on the spectral domain optical coherence tomography imaging of the retina–RS interface.
Conclusion:
Hyperautofluorescent findings suggest the presence of a neurosensory retinal detachment. Retinal detachment associated with RS can be reliably detected on ultra-widefield fundus autofluorescence and may be a useful diagnostic imaging modality.
Degenerative retinoschisis (RS) develops from a splitting of retinal layers.1 In 1973, Straatsma and Foss2 described retinal splitting in the outer plexiform layer, producing a well-circumscribed, transparent dome-shaped elevation of the inner retina that typically extends 2 to 3 mm posterior to the ora serrata. Retinal breaks can develop within inner retinal layers, outer retinal layers, or both, and lead to fluid accumulation in the subretinal space and a full-thickness retinal detachment (RD). A spectrum of full-thickness RD may complicate RS with slow or nonprogressive detachment on one side and progressive rhegmatogenous retinal detachment (RRD) associated with RS on the other.3
Schisis detachment is a clinical entity in which outer layer breaks without inner layer holes permit intraschisis fluid to move into the subretinal space.4 This complication is characterized by a small shallow extension of subretinal fluid (SRF) posterior to the posterior border of the RS, which typically progresses very slowly.5 Accurate diagnosis and understanding of the natural progression of this configuration of schisis detachment allows for optimal management. In comparison, RRDs associated with RS develop when breaks in both the inner and outer retinal layers are present and can result in liquefied vitreous accessing the subretinal space.3 Rhegmatogenous RD associated with RS typically evolve more rapidly, are usually symptomatic, and can progress into very complex configurations warranting surgical intervention.6
Although RS has a classic clinical appearance, the detection of schisis detachment and RRD associated with RS can be a difficult diagnosis, particularly when only based on ophthalmoscopic examination. It is also important to differentiate RS from a chronic, subclinical RD typically associated with small atrophic holes. Different diagnostic tests such as ultrasonography7 and laser photocoagulation8,9 can help differentiate these clinically challenging entities. Cross-sectional spectral domain optical coherence tomography (SD-OCT) images of RS demonstrate a characteristic splitting of the neurosensory retina at the outer plexiform layer, cystic changes in the inner retina, and a thin irregular band of moderately reflective tissue over the retinal pigment epithelium (RPE) band corresponding to the outer retinal layer.10,11 Schisis detachment and RRD associated with RS also exhibit areas of full-thickness RD of the outer layer from the RPE.12 Despite being the gold standard method to assess the presence of SRF, OCT imaging has several limitations, given the peripheral location of RS. Obtaining scans anterior to the equator requires widely dilating pupils and is challenging even with the use of an integrated, slit-lamp SD-OCT and three-mirror contact lens.13 Moreover, peripheral raster scans require a skilled technician and a cooperative patient who is able to maintain a steady, extreme gaze. Therefore, SD-OCT may not be attainable or reproducible for monitoring the progression of these entities. Ho et al14 showed that widefield infrared imaging can help diagnose and monitor the extent of RS, RD, or combined schisis detachment by comparing reflectivity patterns.
Fundus autofluorescence (FAF) imaging is an in vivo method to evaluate the retina, detecting the fluorophores that accumulate in lipofuscin granules in the cytoplasm of RPE cells.15 Ultra-widefield fundus autofluorescence (UWF-FAF) images can be recorded with a widefield scanning laser ophthalmoscope (200Tx; Optos, Dunfermline, United Kingdom) allowing for assessment of the peripheral extent of retinal diseases.16,17
In this study, we describe the findings observed with UWF-FAF and SD-OCT in a consecutive series of patients diagnosed with RS. Our study introduces a novel use of UWF-FAF to aid in the detection and progression of RS, schisis detachment, and RRD associated with RS.
Methods
Consecutive patients from a single center diagnosed with RS and imaged with UWF-FAF were identified and included in this retrospective case series. This study was approved by the institutional review board of the University of California Los Angeles, and the research project adhered to the tenets of the Declaration of Helsinki. Patients with inflammatory conditions, retinal vascular diseases, trauma, and X-linked RS were excluded. Demographic characteristics, complete ophthalmologic examination including best-corrected visual acuity, slit-lamp biomicroscopy, indirect ophthalmoscopy, multimodal imaging, and treatment performed were collected from the patients' health records. Best-corrected visual acuity was reported in Snellen fraction and converted to logarithm of the minimal angle of resolution values for statistical analysis.
After full pharmacologic dilation, UWF-FAF images were obtained using green light (532-nm wavelength) for excitation (Optos P200Tx; Optos), and the emitted signal was detected using a raster scan and detector for light in the wavelengths from 570 to 780 nm (yellow-orange-red range). Peripheral SD-OCT images at the level of RS were obtained with Spectralis OCT (Heidelberg Engineering GmbH, Heidelberg, Germany) and RS-3000 OCT Retina Scan (Nidek Co) through a dilated pupil by an experienced technician. Presence of RS was defined on SD-OCT as derived from the work of Stehouwer et al13 as follows: a splitting of the neurosensory retina into an inner layer and an outer layer, with or without the presence of intraschisis bridging fibers. Subretinal fluid in the setting of RS was defined as full-thickness neurosensory retina separation, associated with splitting of the neurosensory retina with or without an outer retinal layer hole, and a smooth free surface of the RPE.
Two independent and masked observers (N. K. and A. F.) reviewed and analyzed the FAF and SD-OCT images qualitatively. The data were entered into Microsoft Excel 2010 (Microsoft Corporation, Redmond, WA) datasheet for tabulation and descriptive statistics. Cohen's kappa method was used to assess the intergrader reliability, and a value ≥0.81 was considered a significant agreement (SPSS software version 25, Inc, Chicago, IL).
Results
At the end of the review process, 38 eyes of 25 patients with RS, 12 male (48%) and 13 female (52%), were included in the analysis. Bilateral RS was diagnosed in 13 of 25 patients (52%), and the mean age of the study population was 62.4 ± 17.1 years (range 37–95 years). The mean best-corrected visual acuity was 0.09 ± 0.15 logarithm of the minimal angle of resolution (range 0–0.6 logarithm of the minimal angle of resolution, Snellen equivalent 20/25). Thirty-three eyes (86.8%) were phakic and 5 (13.2%) were pseudophakic. Table 1 summarizes the data from the patients included in this study.
Table 1.
Demographics
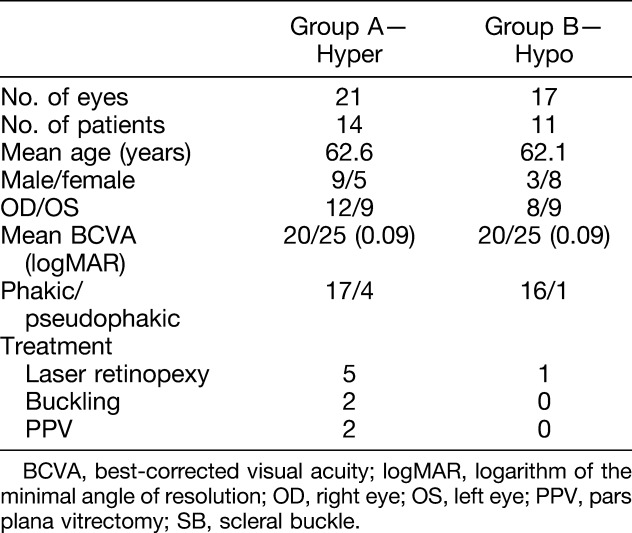
Evidence of hyperautofluorescence was found in 21/38 eyes (55.3%) over the posterior edge and/or beyond the posterior margin of RS (Group A; Figure 1), and no trace of hyperautofluorescence was discovered in the other 17 eyes (44.3%) on fundus imaging (Group B; Figure 2). Isoautofluorescence over the area of RS was demonstrated in 18/21 eyes (85.7%) from Group A and in 15/17 eyes (88.2%) from Group B; the remaining 3 eyes from Group A (14.3%) and 2 eyes from Group B (11.8%) showed hypoautofluorescence at the level of the RS. In addition, a hypoautofluorescence line at the posterior edge of the RS was present in 4/21 eyes (19%) from Group A and 7/17 eyes (41.2%) from Group B. Six eyes from Group A (6/21, 28.6%) exhibited scattered hypoautofluorescence patches corresponding to hyperpigmented RPE. Agreement between the observers was 100% (κ = 1.00).
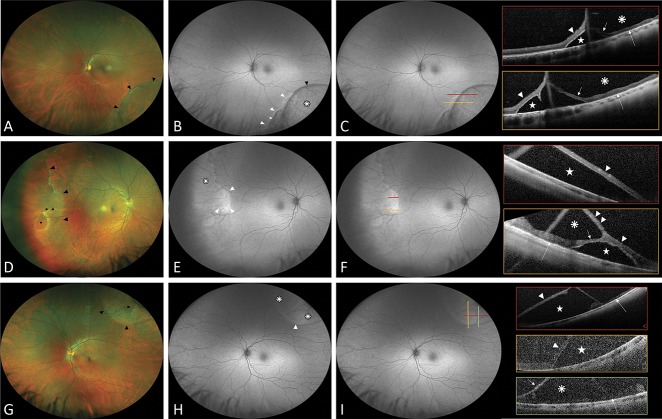
Fig. 1.
Ultra-widefield fundus autofluorescence and spectral domain OCT findings in degenerative RS with underlying neurosensory retinal detachment. Group A: hyperautofluorescence present. A, D, and G. Ultra-widefield color fundus photographs showing a degenerative RS (black arrowheads). B, E, and H. Ultra-widefield fundus autofluorescence demonstrating the area of RS as isoautofluorescence (white asterisk) with a hypoautofluorescence line (black arrowhead) at the posterior edge and highlights the hyperautofluorescence area (white arrowhead) extending under and posterior to the RS limits associated with hypoautofluorescence corresponding to RPE changes (short white arrow). C, F, and I. Colored lines indicate the exact location through which the OCT scans were taken. Scans show the elevation of full-thickness neurosensory retina (white arrowhead) with SRF (white star), the detached outer retinal layer (short white arrow), the inner retinal layer (double white arrowhead), the attached outer retinal layer (long white arrow), and the schisis cavity (white asterisk).
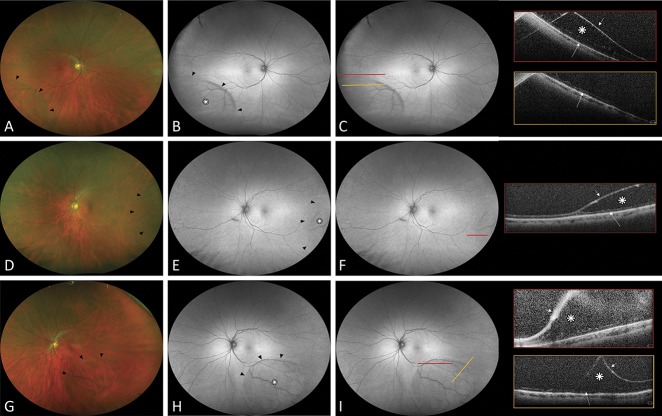
Fig. 2.
Ultra-widefield fundus autofluorescence and spectral domain OCT findings in degenerative RS without neurosensory retinal detachment. Group B: no hyperautofluorescence present. A, D, and G. Ultra-widefield color fundus photographs showing degenerative RS (black arrowheads). B, E, and H. Ultra-widefield fundus autofluorescence demonstrating the area of RS as isoautofluorescence (white asterisk) with a hypoautofluorescence line (black arrowhead) at the posterior edge. C, F, and I. Colored lines indicate the exact location through which the OCT scans were taken. Scans show splitting of neurosensory retina: the schisis cavity (white asterisk), the detached inner retinal layer (short white arrow), and a thin irregular band of moderately reflective tissue over the RPE hyperreflective band corresponding to the outer retinal layer (white large arrow).
Peripheral SD-OCT was obtained in 10 eyes from Group A (10/21, 47.6%); the diagnosis of RS was confirmed by identifying splitting of the neurosensory retina in all eyes (100%). All eyes with UWF-FAF and SD-OCT (100%) revealed that the areas of hyperautofluorescence corresponded to areas of full-thickness neurosensory detachment and SRF associated with RS (Figure 1). Spectral domain OCT also found an outer layer hole matching the field of hyperautofluorescence in two eyes of this group. Spectral domain OCT scans through the areas of isoautofluorescence and hypoautofluorescence confirmed the presence of splitting of the neurosensory retina with no detachment of the neurosensory retina.
Vertical and horizontal SD-OCT scans through the RS in 11 eyes from Group B (11/17, 64.7%) identified splitting of the neurosensory retina in all the patients (11/11, 100%). These SD-OCT findings matched the areas of isoautofluorescence and hypoautofluorescence on the UWF-FAF images. None of these eyes showed areas of full-thickness neurosensory detachment or SRF (Figure 2). Figure 2 also shows that SD-OCT images highlighted intraschisis pillars of variable thickness in six eyes (6/11, 54.5%) and inner/outer schisis cavities separated by the outer plexiform layer in four eyes (4/11, 36.4%).
In 17 eyes from both groups, the border of the RS could not be determined because of a peripheral RS location beyond the limits of the OCT system or poor OCT quality. In 11 eyes from Group A (11/21, 52.4%) without SD-OCT, clinical examination suggested the presence of neurosensory detachment in the areas of hyperautofluorescence. Similarly, in six eyes from Group B (6/17, 35.3%) without SD-OCT, clinical examination revealed a thin and smooth elevation of the peripheral retina consistent with classic RS without the presence of SRF.
Discussion
Ultra-widefield fundus autofluorescence is an in vivo, noncontact, noninvasive imaging method that allows for topographic mapping of fluorophores in the ocular fundus. Fundus autofluorescence imaging of the peripheral retina has important diagnostic and treatment implications for numerous disease states.16–20 The presence of SRF associated with RS is often subtle and difficult to identify. Based on the analysis of FAF and SD-OCT images, our study showed that UWF-FAF may be an important method to detect areas of full-thickness neurosensory retina separation associated with RS. Fundus autofluorescence imaging of patients with presence of SRF and RS revealed characteristic hyperautofluorescence patterns over the area of neurosensory detachment, which was corroborated by SD-OCT scans. Moreover, FAF showed isoautofluorescence and, less frequently, hypoautofluorescence in areas of RS without RD.
Fundus autofluorescence imaging allows for the detection of physiological or pathological levels of fluorophores in the ocular fundus, the primary source of which is lipofuscin.15 Although different patterns of FAF have been reported for several vitreoretinal pathologies with SRF,20–23 a systematic analysis of these FAF alterations in correlation with RS is lacking to date.
The characteristic hyperautofluorescence pattern observed in Group A (RS with RD) was only detected in areas of full-thickness neurosensory retina separation associated with RS. Increased autofluorescence or hyperautofluorescence in this clinical setting may be explained by a number of pathophysiologic possibilities. These include 1) increased fluorophore detection, given the presence of an RD; 2) increased fluorophore production, given the presence of an RD with degraded photoreceptor outer segments shedding into the SRF; and 3) increased fluorophore production due to decreased oxidative stress and metabolic changes as the RPE enters a preapoptotic state.18,24,25
Given that FAF is generated by the visual cycle of the interaction between the photoreceptors and RPE, RS without RD should not produce aberrations in the normal FAF pattern.25 Indeed, the data published here confirm that hypothesis. Specifically, clinical RS cavities were confirmed by SD-OCT imaging in 21 eyes (10 eyes from Group A and 11 eyes from Group B), and isoautofluorescence was found in 18 of these 21 eyes (85.7%). However, in the remaining 3 eyes (3/21, 14.3%), there was attenuation of autofluorescence corresponding to the RS. Interestingly, these cavities were among the most elevated RS cavities studied. Thus, the areas of RS that demonstrate hypoautofluorescence are likely secondary to blocking effect. Previous studies have described a similar phenomenon: areas of bullous RD exhibited hypoautofluorescence due to a blocking effect by the edematous retina and SRF overlying the RPE, although they found a more intense hypoautofluorescence.20,26
Despite the clinical utility of fundus FAF, there is variable reliability in the evaluation of autofluorescence intensities in fundus images. Previous studies have found that fluorescence intensity increased quasilinearly with age that in a healthy cohort aged 5 to 70 years previously revealed.19,27 In addition, FAF values were significantly greater in females and white patients and were lower in Hispanic, black, and Asian patients.19,28 However, the FAF values reported in these studies were obtained in healthy attached retinas. The demographic variability in FAF intensity in the presence of SRF is unknown.
In the setting of RS, detecting the presence of SRF is critical because it may modify the usual monitoring frequency and sometimes require surgical treatment. Indeed, only few of the pure RS may eventually need surgical treatment. However, RD associated with RS can progress rapidly.4 Widefield scanning laser ophthalmoscopy (P200Tx; Optos) allows for noncontact, noninvasive image acquisition in less than 2 seconds, capturing the peripheral extent of retinal diseases such as RS and, based on our data, may detect RD in the setting of RS.18 Therefore, UWF-FAF scanning laser ophthalmoscopy can be a useful technique not only to detect SRF associated with RS, but also to monitor progression.
Although obtaining SD-OCT images of the peripheral retina is challenging and not always possible, in this study, vertical or horizontal SD-OCT scans performed through the area of interest confirmed the diagnosis of RS and identified the presence of outer retinal layer detachment from the RPE.11,12 Our study demonstrated that these OCT findings corresponded to specific patterns of autofluorescence on UWF-FAF. The advantage of using UW-FAF is the capability of capturing all peripheral retinal lesions with less reliance on the technician and patient.
Limitations of this study include the retrospective nature of the study, limited follow-up period, the lack of corroboratory SD-OCT scans from all eyes studied, and a small sample size. Several confounding factors should be considered when comparing FAF intensities between different examinations and different individuals. Fundus autofluorescence intensity may vary depending on age, sex, race (FAF is significantly higher in white patients and lower in black and Asian patients), eye movement, positioning on the chin rest, orientation of the camera, distance between the camera and the cornea, subtle variations in the illumination of the frame, lens and vitreous opacities, and changes in FAF intensities caused by prolonged exposure to the excitation light or previous dark adaptation.
To the best of our knowledge, this is the first report of UWF-FAF imaging for RS. This novel use of UWF-FAF is a quick, objective, and reproducible method to better recognize and monitor neurosensory detachments associated with RS. Larger prospective longitudinal studies should be pursued to confirm our results and to investigate the autofluorescence patterns in RS.
Footnotes
Supported by an unrestricted institutional grant from Research to Prevent Blindness (RPB) New York, NY, and by a donation from the Hess Foundation, New York, NY.
Presented at Retina Society Annual Meeting, San Francisco, CA, September 12, 2018.
S. Schwartz: receives research support from Optos plc (Dunfermline, United Kingdom) and Nidek (Nidek Co, Gamagori, Japan). J. P. Hubschman: consultant for Alcon (Fort Worth, TX) and Allergan (Parsippany-Troy Hills, NJ), and receives research support from The Lowy Medical Research Institute (La Jolla, CA). The remaining authors have no financial/conflicting interests to disclose.
References
- 1.Bartels M. Uber die Entstehung von Netzhautablösungen. Klin Monatsbl Augenheilkd 1933;91:437–450. [Google Scholar]
- 2.Straatsma BR, Foss RY. Typical and reticular degenerative retinoschisis. Am J Ophthalmol 1973;75:551–575. [DOI] [PubMed] [Google Scholar]
- 3.Reed D, Garg SJ. Degenerative Retinoschisis. In: Schachat AP, Sadda SR, editors. Ryan's Retina. 6th ed. Elsevier; 2018. p. 1853. [Google Scholar]
- 4.Byer NE. Long-term natural history study of senile retinoschisis with implications for management. Ophthalmology 1986;93:1127–1137. [DOI] [PubMed] [Google Scholar]
- 5.Ambler JS, Gass JD, Gutman FA. Symptomatic retinoschisis-detachment involving the macula. Am J Ophthalmol 1991;112:8–14. [DOI] [PubMed] [Google Scholar]
- 6.Byer NE. Perspectives on the management of the complications of senile retinoschisis. Eye Lond Engl 2002;16:359–364. [DOI] [PubMed] [Google Scholar]
- 7.Guthoff R, Stachs O, Labriola L. Diagnostic ophthalmic ultrasound. In: Ryan S, eds. Retina. 5th ed: Saunders; 2013:227–284. [Google Scholar]
- 8.Okun E, Cibis PA. The role of photocoagulation in the management of retinoschisis. Arch Ophthalmol 1964;72:309–314. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff H, Kreissig I, Stopa M. A modified laser test for the identification of retinoschisis. Am J Ophthalmol 2003;136:925–926. [DOI] [PubMed] [Google Scholar]
- 10.Landa G, Shirkey BL, Garcia PMT, et al. Acquired senile retinoschisis of the peripheral retina imaged by spectral domain optical coherence tomography: a case report. Eur J Ophthalmol 2010;20:1079–1081. [DOI] [PubMed] [Google Scholar]
- 11.Ip M, Garza-Karren C, Duker JS, et al. Differentiation of degenerative retinoschisis from retinal detachment using optical coherence tomography. Ophthalmology 1999;106:600–605. [DOI] [PubMed] [Google Scholar]
- 12.Yeoh J, Rahman W, Chen FK, da Cruz L. Use of spectral-domain optical coherence tomography to differentiate acquired retinoschisis from retinal detachment in difficult cases. Retina 2012;32:1574–1580. [DOI] [PubMed] [Google Scholar]
- 13.Stehouwer M, Tan SH, van Leeuwen TG, Verbraak FD. Senile retinoschisis versus retinal detachment, the additional value of peripheral retinal OCT scans (SL SCAN-1, Topcon). Acta Ophthalmol (Copenh) 2014;92:221–227. [DOI] [PubMed] [Google Scholar]
- 14.Ho VY, Wehmeier JM, Shah GK. Wide-field infrared imaging: a descriptive review of characteristics of retinoschisis, retinal detachment, and schisis detachments. Retina 2016;36:1439–1445. [DOI] [PubMed] [Google Scholar]
- 15.Delori FC, Dorey CK, Staurenghi G, et al. In vivo fluorescence of the ocular fundus exhibits retinal pigment epithelium lipofuscin characteristics. Invest Ophthalmol Vis Sci 1995;36:718–729. [PubMed] [Google Scholar]
- 16.Oishi M, Oishi A, Ogino K, et al. Wide-field fundus autofluorescence abnormalities and visual function in patients with cone and cone-rod dystrophies. Invest Ophthalmol Vis Sci 2014;55:3572–3577. [DOI] [PubMed] [Google Scholar]
- 17.Nagiel A, Lalane RA, Sadda SR, Schwartz SD. Ultra-widefield fundus imaging: a review of clinical applications and future trends. Retina 2016;36:660–678. [DOI] [PubMed] [Google Scholar]
- 18.Witmer MT, Kozbial A, Daniel S, Kiss S. Peripheral autofluorescence findings in age-related macular degeneration. Acta Ophthalmol (Copenh) 2012;90:e428–e433. [DOI] [PubMed] [Google Scholar]
- 19.Tan CS, Heussen F, Sadda SR. Peripheral autofluorescence and clinical findings in neovascular and non-neovascular age-related macular degeneration. Ophthalmology 2013;120:1271–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Witmer MT, Cho M, Favarone G, et al. Ultra-wide-field autofluorescence imaging in non-traumatic rhegmatogenous retinal detachment. Eye Lond Engl 2012;26:1209–1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai WW, Leung GY, Chan CW, et al. Simultaneous spectral domain OCT and fundus autofluorescence imaging of the macula and microperimetric correspondence after successful repair of rhegmatogenous retinal detachment. Br J Ophthalmol 2010;94:311–318. [DOI] [PubMed] [Google Scholar]
- 22.Shiragami C, Shiraga F, Yamaji H, et al. Unintentional displacement of the retina after standard vitrectomy for rhegmatogenous retinal detachment. Ophthalmology 2010;117:86–92.e1. [DOI] [PubMed] [Google Scholar]
- 23.Spaide RF, Klancnik JM. Fundus autofluorescence and central serous chorioretinopathy. Ophthalmology 2005;112:825–833. [DOI] [PubMed] [Google Scholar]
- 24.Lois N, Forrester J. Fundus Autofluorescence. Philadelphia, PA: Lippincott Williams and Wilkins; 2009. [Google Scholar]
- 25.Schmitz-Valckenberg S, Holz FG, Bird AC, Spaide RF. Fundus autofluorescence imaging: review and perspectives. Retina 2008;28:385–409. [DOI] [PubMed] [Google Scholar]
- 26.Koizumi H, Maruyama K, Kinoshita S. Blue light and near-infrared fundus autofluorescence in acute Vogt-Koyanagi-Harada disease. Br J Ophthalmol 2010;94:1499–1505. [DOI] [PubMed] [Google Scholar]
- 27.Delori FC, Goger DG, Dorey CK. Age-related accumulation and spatial distribution of lipofuscin in RPE of normal subjects. Invest Ophthalmol Vis Sci 2001;42:1855–1866. [PubMed] [Google Scholar]
- 28.Schmitz-Valckenberg S, Fleckenstein M, Scholl HPN, Holz FG. Fundus autofluorescence and progression of age-related macular degeneration. Surv Ophthalmol 2009;54:96–117. [DOI] [PubMed] [Google Scholar]