This study shows that optical coherence tomography leakage can identify and locate choroidal neovascularization–related abnormal fluid and its change after anti–vascular endothelial growth factor treatment in neovascular age-related macular degeneration eyes. Low optical reflectivity ratio correlates with functional outcome and may be a marker of early choroidal neovascularization reactivation. Simultaneous optical coherence tomography angiography examination provides a noninvasive analysis of fluid and choroidal neovascularization vascular morphology.
Key words: anti-VEGF therapy, neovascular age-related macular degeneration, OCT-L, OCTA, retinal edema
Abstract
Purpose:
To test optical coherence tomography leakage in the identification and quantification of choroidal neovascularization–related fluid, its change after anti–vascular endothelial growth factor therapy in neovascular age-related macular degeneration eyes and its relation to functional outcome.
Methods:
Prospective analysis of a cohort of neovascular age-related macular degeneration cases treated with 2.0-mg intravitreal aflibercept. Eyes included were analyzed before, 1-week, and 1-month after one injection. Best-corrected visual acuity was assessed using Early Treatment Diabetic Retinopathy Study method. Optical coherence tomography leakage maps depicting low optical reflectivity (LOR) sites were acquired with OCT Cirrus AngioPlex (Zeiss, Dublin, CA). The LOR area ratio was correlated to retinal thickness and best-corrected visual acuity. Optical coherence tomography angiography was simultaneously performed.
Results:
Twenty-two eyes of 18 patients with neovascular age-related macular degeneration were included. The LOR ratio of the full retina scan and retinal pigment epithelium–Bruch layer decreased from baseline to Month 1 (P < 0.05). Changes in retinal thickness and LOR ratio were positively correlated (P < 0.05). Best-corrected visual acuity change correlated with the outer segment layer LOR change (rho = −0.53, P = 0.014), and LOR was inferior in better responders (P = 0.021). Optical coherence tomography leakage identified eyes with recurrent fluid in the external layers.
Conclusion:
Optical coherence tomography leakage identified and quantified the fluid related to choroidal neovascularization activity. Low optical reflectivity change in the outer segment layer correlates with functional outcome and increasing LOR in the external layers may be a marker of early recurrence. Combining optical coherence tomography angiography and optical coherence tomography leakage allows both for choroidal neovascularization morphology and activity analysis.
Age-related macular degeneration (AMD) is the leading cause of irreversible vision loss in the elder population in developed countries, and neovascular AMD (nAMD) affects 10% to 15% of patients with AMD.1–3 Treatment with anti–vascular endothelial growth factor (anti-VEGF) agents in nAMD was a major breakthrough as clinical trials showed for the first time the possibility for disease control and improvement in vision.4–6 However, in regular clinical practice, nAMD treatment strategies are yet to be refined to achieve optimal outcomes.2,4,6,7 Current guidelines base the decision to treat on choroidal neovascularization (CNV) activity, judged by a decrease in best-corrected visual acuity (BCVA) and/or an increase in retinal thickness (RT) obtained with spectral domain OCT (SD-OCT).2,7 Structural improvement assumed by decrease in RT, as a marker of decrease in retinal fluid, however, does not imply a parallel functional gain, as other factors such as development of retinal fibrosis and atrophy also contribute to RT change over time and further compromise visual outcome.4 New structural markers are therefore needed in the retreatment decision in nAMD, to improve functional outcome and to decrease patient and institutional burden related to repeated intravitreal anti-VEGF therapy.4,6,8–10
Optical coherence tomography leakage (OCT-L) is a novel noninvasive imaging technique developed by our group with the aim to identify the location of extracellular retinal fluid and to objectively measure it. It is an indirect way to observe the alteration of the blood–retinal barrier, which results in the accumulation of fluid in the retina. This novel algorithm identifies and displays the sites of lower than normal optical reflectivity (LOR) found between the internal limiting membrane and the retinal pigment epithelium (RPE)—Bruch complex. The software was developed to provide en face fundus images, full retina scan, and layer-by-layer, in which areas of LOR are represented in white and considered to correspond to areas of extracellular fluid. Low optical reflectivity (LOR) ratios can be derived to quantify the fluid in the different retinal layers.11–14
The purpose of this study was to evaluate LOR mapping by OCT-L as a tool to identify, locate, and quantify abnormal retinal fluid in nAMD eyes, better characterizing CNV activity through analysis of the fluid distribution in the different retinal layers and sub-RPE space, where the neovascular complex is mainly located. Correlation with conventional structural parameters such as RT and with functional outcomes was performed, and complementarity with OCT angiography (OCTA) explored.
Methods
Design and Patient Selection
This is a prospective, consecutive case series analysis conducted in patients with nAMD receiving the same interventional treatment following clinical practice guidelines. The tenets of the Declaration of Helsinki were followed and approval from the ethics committee of the clinical site was obtained. Written informed consent was collected from all included patients.
Patients included were being treated and followed according to the standard practice for nAMD in our department with 2.0-mg intravitreal aflibercept (IVA) injections in a pro re nata (PRN) regimen. Eyes were excluded if there were other chorioretinal diseases, dense cataract, or glaucoma, compromising acquisition of OCT scans and/or functional outcomes.
Study Procedures
All patients performed an initial visit before their scheduled IVA injection with the following procedures: medical clinical history, BCVA evaluation using Early Treatment Diabetic Retinopathy Study method, biomicroscopy, and SD-OCT examination with angiography capability (HD-OCT Cirrus 5000 AngioPlex; Zeiss Meditec, Dublin, CA). Included patients repeated the same procedures 1 week (W1) and 1 month (M1) after the IVA injection.
Optical Coherence Tomography Acquisition and Processing
Optical coherence tomography scans of 6 × 6 mm2 were acquired in all patients using HD-OCT Cirrus 5000 AngioPlex (Zeiss Meditec). Raw scan data from the AngioPlex system were exported and processed using the OCT-L software for the full retinal A-scan and for each individual segmented retinal layer. The OCT-L software identifies sites of LOR and depicts them as two-dimensional en face images of the retina by assigning a simple representative value to each A-scan. These representative values register the existence of optical reflectivity values falling below a predefined threshold obtained from the analysis of A-scans gathered from a healthy control population and are believed to correspond to extracellular fluid.11,12 Optical coherence tomography leakage maps were collected for the whole retina and layer-by-layer, after semiautomated segmentation procedure developed in-house. This segmentation algorithm was implemented to identify eight retinal interfaces, namely, the vitreous to inner limiting membrane, retinal nerve fiber layer to ganglion cell layer, inner plexiform layer to inner nuclear layer (INL), INL to outer plexiform layer (OPL), OPL to outer nuclear layer, inner segment to outer segment (OS), OS to RPE, and RPE to Bruch membrane.15 All segmented examinations were reviewed by an experienced ophthalmologist grader, and manual correction was performed whenever necessary. Maps of the LOR sites were obtained for the full retina and layer-by-layer as en face images. The white areas depicted in the LOR maps represent the location of the A-scans having reflectivity values below the predefined threshold, and black areas are above the threshold. The LOR area ratio was calculated as the fraction of the number of LOR A-scans by the total number of A-scans in a given area and represents the extracellular fluid distribution over the defined retinal area. The LOR area ratio was obtained for all segmented layers and for the whole retina scan. Similarly, RT was obtained for the whole retina and layer-by-layer using the same semiautomated segmentation procedure used for OCT-L analysis.
Finally, an Early Treatment Diabetic Retinopathy Study grid was applied over the macular scan in the semiautomated segmentation software after marking the fovea. Low optical reflectivity ratio and RT by subfield were obtained, and the central subfield (CSF) was considered as the 1-mm diameter circle centered in the fovea. The LOR area ratio was also calculated for the total macular area (6-mm circle centered in the fovea) for the external layers: OS layer and RPE–Bruch layer.
Conventional OCTA was performed in all eyes to compare neovascular networks morphology to OCT-L–derived fluid maps and to qualitatively analyze their evolution after treatment.
Data Analysis
Low optical reflectivity ratios in the CSF at baseline, 1 week, and 1 month after treatment were compared with the Wilcoxon matched-pairs test. Full retina and layer-by-layer thickness in the CSF obtained by semiautomated segmentation was also compared between visits, and its change was correlated to LOR ratio change. Central subfield LOR ratio and central RT (CRT) change was correlated to BCVA change during follow-up using the Spearman's correlation coefficient, and the respective statistical significance was computed.
Eyes were divided in two groups—better responders and worse responders—when BCVA change was equal/superior or inferior to the median BCVA change at 1 month. Central RT and CSF LOR ratios at baseline and after 1 week and 1 month of anti-VEGF treatment were compared between treatment response groups by univariate analysis performed with the Wilcoxon test.
All tests were 2 sided, and significance was set at 0.05. Statistical analysis was performed with Stata 12.1 SE (College Station, TX: StataCorp LP).
Results
Twenty-two eyes of 18 consecutive patients with nAMD on intravitreal anti-VEGF treatment with aflibercept were included. Mean age at baseline was 79.8 ± 6.6 years (range, 66–89), and 55.6% were women (n = 10).
Mean BCVA was 50.9 ± 19.4 Early Treatment Diabetic Retinopathy Study letters at baseline, and mean BCVA gain was 3.6 ± 13.7 letters in the 1st week (P = 0.076) and 4.6 ± 12.8 letters in the 1st month (P < 0.001).
Fifteen eyes (68.2%) had Type 1 CNV at baseline. Three eyes (13.6%) had mixed Type 1 and 2 CNV, and 4 eyes (18.2%) had Type 3 CNV. Only 4 eyes (18.2%) were treatment naive at baseline.
Retinal Thickness and Low Optical Reflectivity Ratio Change After Anti–Vascular Endothelial Growth Factor Treatment
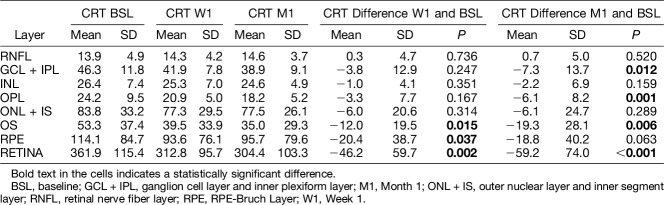
Semiautomated mean CRT change for the full retina and layer-by-layer during follow-up is presented in Table 1. Mean CRT decrease after one IVA injection was more pronounced at the level of the segmented external retinal layers: OPL, OS, and RPE–Bruch layer (P < 0.05).
Table 1.
Central RT Change After 1 Week and 1 Month of anti-VEGF Treatment, Full Retina, and Layer-By-Layer Analysis
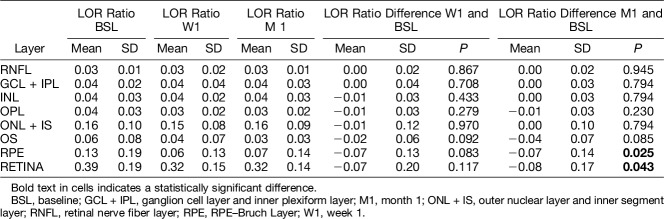
Low optical reflectivity ratio change in the CSF during follow-up is presented in Table 2. Low optical reflectivity ratio decreased mainly in the external layers—OS and RPE–Bruch layer. This was significant for the segmented RPE–Bruch layer (M1: P = 0.025), where the neovascular process was mainly located, and for the full retina scan (M1: P = 0.043).
Table 2.
Central subfield LOR Ratio Change After 1 Week and 1 Month of anti-VEGF Treatment, Full Retina and Layer-By-Layer Analysis
Exemplificative clinical cases of LOR ratio mapping during nAMD treatment with aflibercept and complementarity with OCTA are presented in Figures 1 and 2.
Fig. 1.
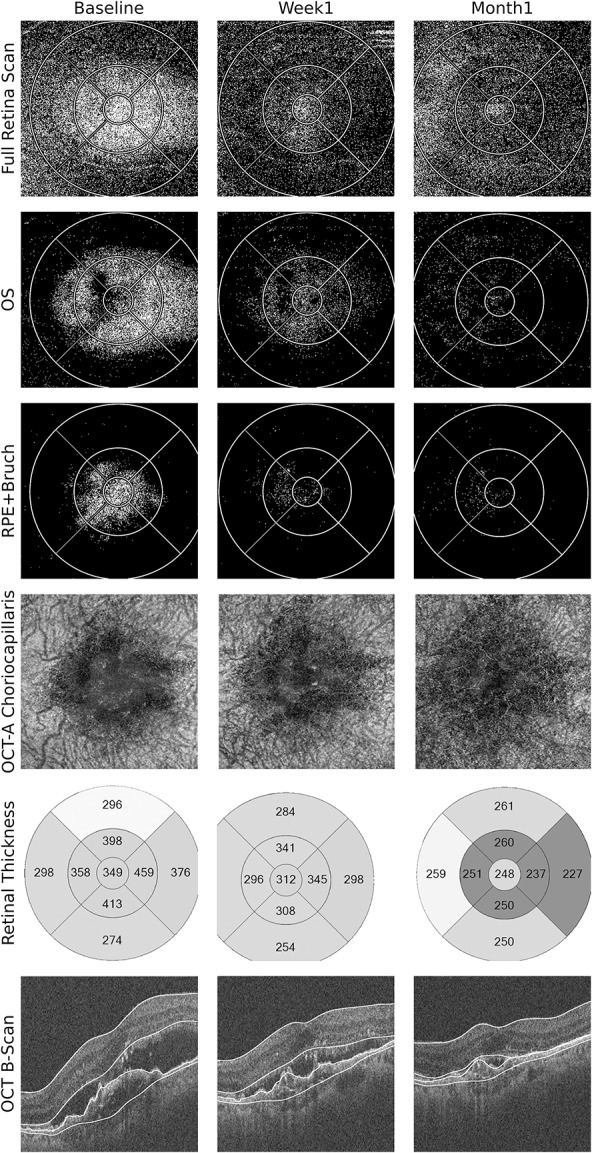
Multimodal OCT-based imaging of the left eye of a patient with exudative AMD immediately before, 1 week, and 1 month after IVA injection. Optical coherence tomography leakage map of the full retina scan clearly shows the extension of abnormal fluid accumulation in the retina, and its decrease during follow-up (1st row). This change was steady and took place mainly in the OS and RPE–Bruch layers (2nd and 3rd rows). The OCTA (4th row) shows reduction in vascularization of the CNV, with rarefaction and disappearance of most vascular channels, being almost imperceptible in the 1st month. The morphology of the CNV in the OCTA resembles the OCT-L fluid distribution in the RPE–Bruch layer in the three visits. The conventional OCT B-scans and RT maps show reduction in subretinal fluid and in the size of the fibrovascular pigment epithelium detachment. Optical coherence tomography leakage segmentation used in the layers depicted is marked in white in the SD-OCT images. Optical coherence tomography angiography segmentation was customized for better visualization of the neovascular network.
Fig. 2.
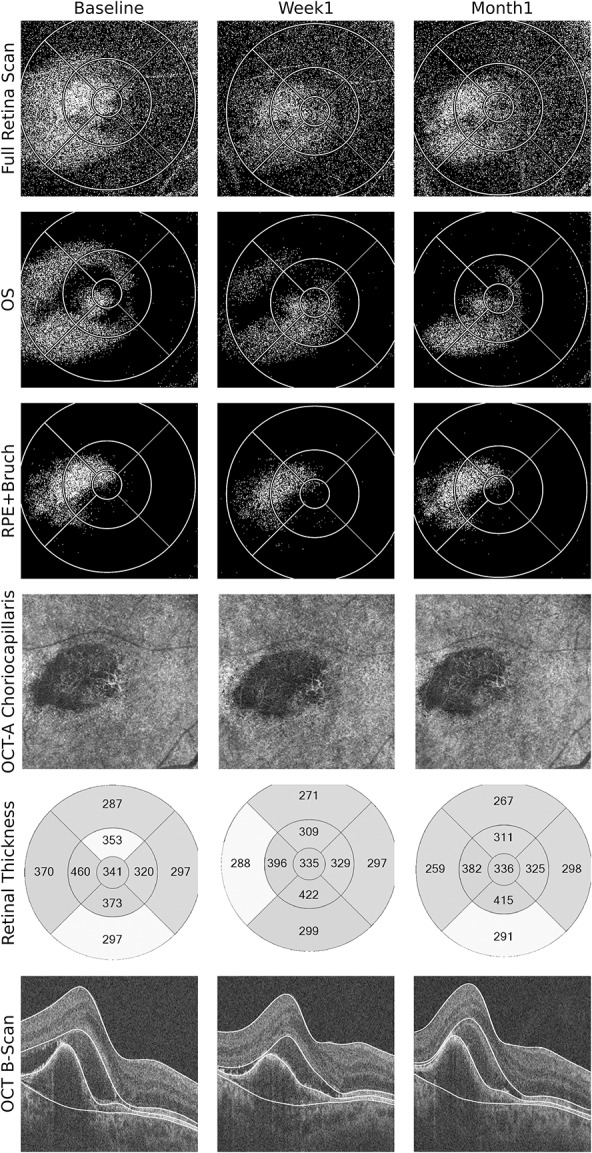
Multimodal OCT-based imaging of the right eye of a patient with exudative age-related macular degeneration immediately before, 1 week, and 1 month after IVA injection. Optical coherence tomography leakage map of the full retina scan in the 1st row shows the extension of fluid accumulation and its change during follow-up. This change took place mainly in the OS and RPE–Bruch layers (2nd and 3rd rows). One can see a steady decrease in fluid distribution in the OS layer; however, in RPE–Bruch layer, there seems to be more fluid in 1st month compared with the 1st week. Accordingly, the conventional B-scans show a decrease in subretinal fluid, but the fibrovascular PED seems to increase again after 1 month (6th row). The OCTA analysis also shows a decrease in vascularization of the CNV after 1 week; however, there is repermeabilization of the vascular network in the Month 1, with a flow signal similar to baseline (4th row). Association of noninvasive OCTA and OCT-L thus show structural changes occurring before significant RT change and provide insight into a case that might need early retreatment to control the exudative membrane. Optical coherence tomography leakage segmentation used in the layers depicted is marked in white in the SD-OCT images. Optical coherence tomography angiography segmentation was customized for better visualization of the neovascular network.
Correlation Between Low Optical Reflectivity Ratio, Best-Corrected Visual Acuity, and Retinal Thickness
Low optical reflectivity change in the CSF and BCVA change were inversely correlated for the OS layer in the 1st month visit (rho = −0.53, P = 0.014). No other correlation was found for any of the remaining layers.
No correlations between RT change in any layer and BCVA change were found.
Central subfield LOR change and CRT change were positively correlated for the whole retina scan in both time points (W1 rho = 0.61, P = 0.004; M1 rho = 0.71, P < 0.001). In the layer-by-layer analysis, a positive correlation was found for several retinal layers: INL (W1 rho = 0.67, P = 0.001); OPL (M1 rho = 0.60, P = 0.004); outer nuclear layer + inner segment layer (W1 rho = 0.59, P = 0.006; M1 rho = 0.53, P = 0.013); OS–RPE layer (M1 rho = 0.69, P = 0.001); and RPE–Bruch layer (W1 rho = 0.90, P < 0.001; M1 rho = 0.85, P < 0.001).
Figure 3 shows the correlation between RT change and LOR ratio change in the 1st month after treatment in the OS layer and RPE–Bruch layer.
Fig. 3.
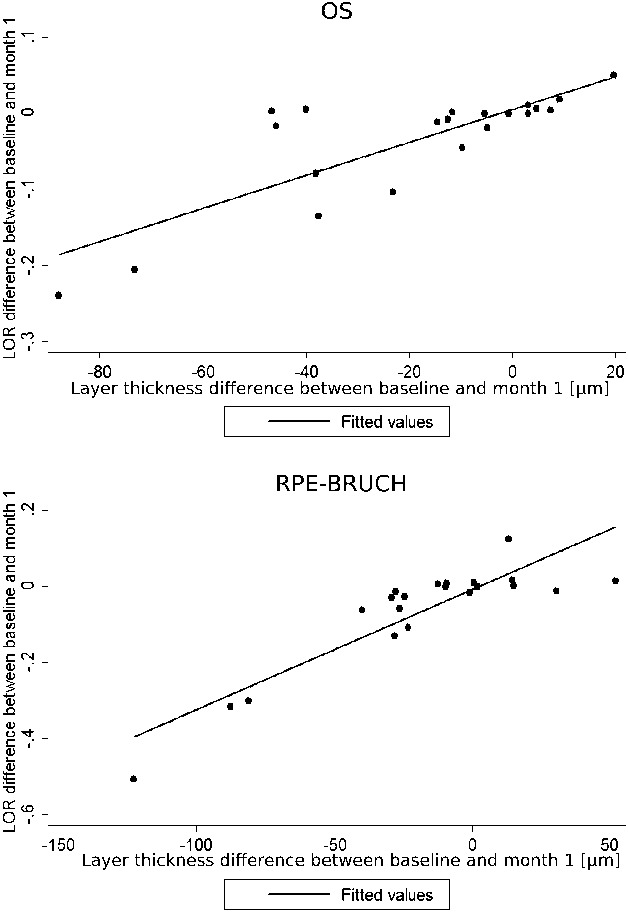
Correlation between layer thickness change and LOR ratio change in the 1st month after treatment in the OS layer and RPE–Bruch layer.
Best-Corrected Visual Acuity Better Versus Worse Responders, Low Optical Reflectivity Ratio, and Retinal Thickness
Eyes were divided in better responders when BCVA change at Month 1 was equal or superior to the median BCVA change for that time point or worse responders if BCVA change was inferior to that threshold. At Month 1, the CSF LOR ratio in the OS layer was significantly different between better and worse responders (0.01 ± 0.01 vs. 0.04 ± 0.04 respectively, P = 0.017), with no difference at baseline between response groups. Therefore, patients with better functional response had lower LOR in the OS–RPE layer compared with poor responders 1 month after treatment. No difference was found for the RT between groups in any layer or for the full retina scan.
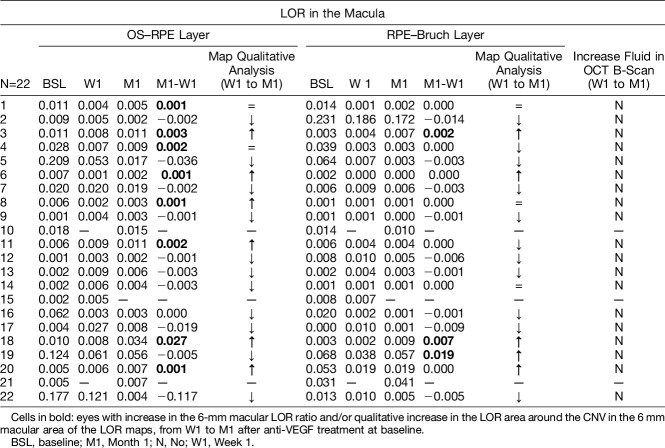
Analysis of Recurrent Fluid From Choroidal Neovascularization at Month 1
A quantitative and qualitative analysis of the LOR maps of the 6-mm macula was performed to investigate for increase in fluid in the OS–RPE layer and RPE–Bruch layer from Week 1 to Month 1, in each patient. Comparisons were made to the presence of intra retinal and subretinal fluid in the conventional OCT B-scans of both visits. The objective was to see whether early CNV reactivation cases could be detected with OCT-L. Results are presented in Table 3. In 5 cases, there was an increase of macular fluid in the RPE–Bruch layer from Week 1 to Month 1, detected by enlarging LOR area in OCT-L maps in the location of the CNV and/or increase in the LOR ratio. In the OS–RPE layer, increasing fluid was detected in six cases in the CNV location in the qualitative analysis and in two more cases using the LOR ratio. By contrast, no fluid increase was clearly seen in the corresponding OCT B-scans in all cases. Figure 2 shows one of these cases, where incipient fluid around the CNV is detected in LOR maps and is accompanied by repermeabilization of the vascular network in OCTA, but no clear fluid change is seen in conventional OCT analysis.
Table 3.
Macular LOR Analysis of the OS–RPE Layer and RPE–Bruch Layer From all Eyes Included. Comparison With Concomitant Fluid Change in SD-OCT B-Scans
Discussion
In this study, we use a novel algorithm developed in our center—OCT-Leakage—to identify the location of LOR sites in the retina of patients with nAMD on anti-VEGF treatment. Low optical reflectivity sites correspond to abnormal accumulating intra retinal and subretinal fluid associated with the neovascular tissue and consequent blood–retinal barrier impairment. Optical coherence tomography leakage is shown to provide an accurate mapping of the retinal extracellular fluid and its change during follow-up. Low optical reflectivity mapping correlated well to both functional and conventional anatomical outcomes and seems to be able to identify early recurrence in CNV activity.
Several studies have reported that management of nAMD is far from optimal in the real-life clinical setting, and that new drugs and/or new biomarkers and treatment strategies must be implemented to improve outcomes and decrease treatment burden.6,8 Optical coherence tomography leakage is a way to map and detect precisely the location of abnormal fluid, and unlike simple RT analysis, focus only on fluid change, and is not influenced by other CNV-related features such as change in pigment epithelial detachment (PED) size or subretinal fibrosis development.
Previously, our group showed that OCT-L was able to detect retinal fluid in several pathologies such as diabetic macular edema, and that fluid areas in OCT-L maps correspond to areas of leakage in fluorescein angiography and of intraretinal fluid in conventional SD-OCT.11–13 Optical coherence tomography leakage was also tested in other retinal pathologies with inner or outer blood–retinal barrier breakdown, showing that OCT-L effectively locates the fluid in the different layers of the retina affected by the underlying pathology.14 This study further validates its use not only in documenting and locating abnormal fluid in the retina but also explores OCT-L ability to evaluate fluid changes in the different layers of the retina after treatment with an anti-VEGF agent in the setting of nAMD, thus inferring about CNV activity.
As expected, a reduction in the amount of CNV-related fluid after treatment with aflibercept could be identified by both LOR ratio and RT decrease during follow-up. This change was more pronounced in the external retinal layers because that is where the exudative CNV is located. A significant positive correlation was found between change in retinal LOR and change in RT, and this correlation was strong for the external retinal layers and very strong for the sub-RPE space, where fluid and thickness changes were more pronounced. However, although LOR ratio and LOR maps represent only the decrease in fluid, RT change includes other confounding morphologic factors, such as fibrovascular tissue volume change. Decrease in LOR area and ratio can also be caused by tissue atrophy; however, its area would not be expected to change after anti-VEGF treatment, as was observed in this study. Change in LOR after treatment further validates that OCT-L effectively monitors fluid changes in patients with nAMD.
Activity of the neovascular complex in nAMD eyes is considered to be associated with the presence of abnormal fluid. Low optical reflectivity ratio and mapping analysis of the RPE–Bruch layer clearly shows fluid changes around and within the CNV after treatment. In some eyes, it increased again at Month 1, while in others continued to decrease, as seen in Table 3 and in both clinical cases presented (Figures 1 and 2). Interestingly, no evident fluid changes were seen in conventional B-scan analysis. This incipient exudative activity observed with OCT-L can be a useful marker of eyes with inferior response to anti-VEGF treatment and early reactivation of the CNV in the maintenance phase of treatment, and thus needing closer follow-up and timely retreatment. Optical coherence tomography leakage seems to be more sensible to fluid changes than RT alone in the evaluation of patients with nAMD.
In our study, there was a negative correlation between CSF LOR change in the OS layer and BCVA change, showing an association between the decrease of fluid around the OS measured with OCT-L and the increase in function. No correlation was found, however, between CRT and BCVA for any layer. Retinal thickness decrease does not imply functional gain, and this is especially true in non-naive nAMD patients, where other factors such as subretinal fibrosis and atrophy development play an important role in limiting functional recovery after treatment.2,5 Fluid analysis with OCT-L correlated better with functional outcome, than simple thickness analysis. Our findings support Santos et al13 who showed that in diabetic macular edema cases treated with anti-VEGF, fluid in the external layers measured by OCT-L was a more robust biomarker of BCVA recovery compared with CRT, disorganization of the inner retinal layers, or ellipsoid zone disruption changes.
To further explore this relationship of fluid measured by OCT-L and functional outcome, we performed a subanalysis considering better and worse BCVA responders. This analysis revealed a significant difference of the LOR at the level of the OS layer, between the two groups. Good responders are those with lower levels of fluid and low LOR values in the OS layer, while poor responders are those with more fluid around the OS 1 month after injection. This too suggests that fluid around the photoreceptors' outer segments may play an important part in functional gain in nAMD, more than fluid in the other layers of the retina.13 Also, the LOR of the OS layer might predict those in need of more frequent injections to achieve a “dry retina” and better functional outcome.
Finally, location and quantification of retinal edema determined by OCT-L complement OCTA, offering the possibility of obtaining valuable information on breakdown of the blood–retinal barrier and fluid accumulation, together with visualization of the neovascular network change during treatment, using only noninvasive OCT-based methodologies. The two cases pictured (Figures 1 and 2) show that the combined use of both technologies gives valuable insight into the changes that occur in the neovascular network and simultaneously of the fluid location and distribution after anti-VEGF treatment. In the second case, OCT-L mapping with OCTA was able to detect early reactivation of the CNV with repermeabilization of the vascular network in OCTA and associated increase in fluid from exudation in the sub-RPE space in OCT-L at Month 1. There was no apparent increase in retinal fluid in conventional OCT B-scans and no change in the central RT map at that time point.
This study has, however, several limitations. The more important is the small number of eyes studied and the fact that most eyes were not naive to treatment, and therefore, gain in vision and correlation to LOR changes might have been compromised by the chronicity of the pathology. Low optical reflectivity ratio can also be influenced by development of atrophy or by other factors that interfere with reflectivity, such as blood or protein exudation, but such changes are readily discriminated with concomitant conventional SD-OCT in multimodal approach. Also, this study has the advantage of being prospective and correlate RT, BCVA, and the new parameter, LOR ratio. We were also able to show the complementarity of OCTA and LOR mapping analysis.
In conclusion, mapping of LOR sites with OCT-L in nAMD eyes demonstrated well the extent and location of fluid in the affected retinal layers and its change after anti-VEGF treatment. Low optical reflectivity ratio change correlated well with RT change and correlated to BCVA change, with better responders having lower LOR ratios in the OS layer 1 month after treatment. Therefore, OCT-L shows potential as a new tool to evaluate both functional and structural response to treatment in nAMD. Optical coherence tomography leakage and OCTA combined evaluate simultaneously in the same examination the morphology and activity of the vascular network.
Footnotes
J. Cunha-Vaz reports grants from Carl Zeiss Meditec, outside the submitted work and is consultant for Alimera Sciences, Allergan, Bayer, Gene Signal, Novartis, Pfizer, Precision Ocular Ltd, Roche, Sanofi-Aventis, Vifor Pharma, and Carl Zeiss Meditec. R. Silva is a consultant of Bayer, Novartis, Alimera Sciences, Alcon, Thea, and Allergan. The remaining authors have no financial/conflicting interests to disclose.
References
- 1.Wong T, Chakravarthy U, Klein R, et al. The natural history and prognosis of neovascular age-related macular degeneration. A systematic review of the literature and meta-analysis. Ophthalmology 2008;115:116–127. [DOI] [PubMed] [Google Scholar]
- 2.Rufai SR, Almuhtaseb H, Paul RM, et al. A systematic review to assess the 'treat-and-extend' dosing regimen for neovascular age-related macular degeneration using ranibizumab. Eye (Lond) 2017;31:1337–1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Friedman DS, O'Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol 2004;122:564–572. [DOI] [PubMed] [Google Scholar]
- 4.Maguire MG, Martin DF, Ying G, et al. Five-Year outcomes with anti–vascular endothelial growth factor treatment of neovascular age-related macular degeneration. Ophthalmology 2016;123:1751–1761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Heier JS, Brown DM, Chong V, et al. Intravitreal aflibercept (VEGF trap-eye) in wet age-related macular degeneration. Ophthalmology 2012;119:2537–2548. [DOI] [PubMed] [Google Scholar]
- 6.Rofagha S, Bhisitkul RB, Boyer DS, et al. Seven-year outcomes in ranibizumab-treated patients in ANCHOR, MARINA, and HORIZON: a multicenter cohort study (SEVEN-UP). Ophthalmology 2013;120:2292–2299. [DOI] [PubMed] [Google Scholar]
- 7.Arnold JJ, Campain A, Barthelmes D, et al. Two-year outcomes of “treat and extend” intravitreal therapy for neovascular age-related macular degeneration. Ophthalmology 2015;122:1212–1219. [DOI] [PubMed] [Google Scholar]
- 8.Schmidt-Erfurth U, Waldstein SM. A paradigm shift in imaging biomarkers in neovascular age-related macular degeneration. Prog Retin Eye Res 2016;50:1–24. [DOI] [PubMed] [Google Scholar]
- 9.Xie P, Zheng X, Yu Y, et al. Vitreomacular adhesion or vitreomacular traction may affect antivascular endothelium growth factor treatment for neovascular age-related macular degeneration. Br J Ophthalmol 2017;101:1003–1010. [DOI] [PubMed] [Google Scholar]
- 10.Dugel PU, Jaffe GJ, Sallstig P, et al. Brolucizumab versus aflibercept in participants with neovascular age-related macular degeneration: a randomized trial. Ophthalmology 2017;124:1296–1304. [DOI] [PubMed] [Google Scholar]
- 11.Cunha-Vaz J, Santos T, Alves D, et al. Agreement between OCT leakage and fluorescein angiography to identify sites of alteration of the blood–retinal barrier in diabetes. Ophthalmol Retin 2017;1:395–403. [DOI] [PubMed] [Google Scholar]
- 12.Cunha-Vaz J, Santos T, Ribeiro L, et al. OCT-leakage: a new method to identify and locate abnormal fluid accumulation in diabetic retinal edema. Investig Ophthalmol Vis Sci 2016;57:6776–6783. [DOI] [PubMed] [Google Scholar]
- 13.Santos A, Alves D, Santos T, et al. Measurements of retinal fluid by OCT-leakage in diabetic macular edema. A biomarker of visual acuity response to treatment. Retina 2019;39:52–60. [DOI] [PubMed] [Google Scholar]
- 14.Farinha C, Santos T, Marques IP, et al. OCT-leakage mapping: a new automated method of OCT data analysis to identify and locate abnormal fluid in retinal edema. Ophthalmol Retin 2017;1:486–496. [DOI] [PubMed] [Google Scholar]
- 15.Staurenghi G, Sadda S, Chakravarthy U, Spaide RF. Proposed lexicon for anatomic landmarks in normal posterior segment spectral-domain optical coherence tomography: the IN OCT consensus. Ophthalmology 2014;121:1572–1578. [DOI] [PubMed] [Google Scholar]