Supplemental Digital Content is available in the text.
Keywords: athletes, computed tomography angiography, coronary atherosclerosis, exercise
Abstract
Physical activity and exercise training are effective strategies for reducing the risk of cardiovascular events, but multiple studies have reported an increased prevalence of coronary atherosclerosis, usually measured as coronary artery calcification, among athletes who are middle-aged and older. Our review of the medical literature demonstrates that the prevalence of coronary artery calcification and atherosclerotic plaques, which are strong predictors for future cardiovascular morbidity and mortality, was higher in athletes compared with controls, and was higher in the most active athletes compared with less active athletes. However, analysis of plaque morphology revealed fewer mixed plaques and more often only calcified plaques among athletes, suggesting a more benign composition of atherosclerotic plaques. This review describes the effects of physical activity and exercise training on coronary atherosclerosis in athletes who are middle-aged and older and aims to contribute to the understanding of the potential adverse effects of the highest doses of exercise training on the coronary arteries. For this purpose, we will review the association between exercise and coronary atherosclerosis measured using computed tomography, discuss the potential underlying mechanisms for exercise-induced coronary atherosclerosis, determine the clinical relevance of coronary atherosclerosis in middle-aged athletes and describe strategies for the clinical management of athletes with coronary atherosclerosis to guide physicians in clinical decision making and treatment of athletes with elevated coronary artery calcification scores.
Cardiovascular diseases (CVDs) are the dominant cause of death worldwide, accounting for approximately 18 million deaths per year (31% of total mortality).1 Atherosclerotic coronary heart disease is the leading cause of deaths attributable to CVD and accounts for almost 45% of all cases. There is clear evidence that chronic physical activity and exercise training significantly reduce the risk for cardiovascular events.2 However, several recent studies have suggested that high-volume, high-intensity exercise training may actually increase the prevalence and severity of coronary atherosclerosis.3–5 Of note, analysis of plaque morphology has shown fewer mixed plaques and more often only calcified plaques in the athletes, suggesting a more stable atherosclerotic pattern. The mechanisms leading to increased coronary atherosclerosis in athletes are largely unknown. Furthermore, the clinical relevance of these findings and how to manage athletes with coronary atherosclerosis are unclear. In this review we describe the short-term and long-term effects of exercise training on coronary atherosclerosis in athletes who are middle-aged and older. This review will summarize the association between exercise and coronary atherosclerosis measured using computed tomography (CT), discuss the potential underlying mechanisms, determine the clinical relevance of atherosclerosis in middle-aged athletes, and describe strategies for managing athletes with coronary atherosclerosis.
Methods to Assess Coronary Atherosclerosis Characteristics
Two different CT-scan protocols can be used for the assessment of coronary atherosclerosis. A noncontrast CT-scan demonstrates the amount of coronary artery calcification (CAC), which is expressed as a CAC score (CACS) in Agatston units.6 The CACS is predictive of future CVD events.7,8 The CACS is the product of CAC volume and CAC density, and although CAC volume has a positive association with cardiovascular events, CAC density is inversely associated with cardiovascular events,9 suggesting that not all CACS have the same risk. Coronary CT angiography (CCTA) uses contrast to assess luminal stenoses, plaque characteristics, and plaque volume. Luminal stenoses are visually graded and significant stenoses (>50%) are strongly associated with cardiovascular events.10 The number of segments affected can also be summed to produce a segment involvement score, which is a strong predictor of events.11 Both CACS and CCTA derived scores predict events, but the addition of the number, location, and severity of stenoses from CCTA does not appear to improve event prediction more than standard risk factors and CACS in asymptomatic patients,12 suggesting that CACS is as good a predictor of cardiovascular events in such patients.
Plaques can be characterized as calcified, noncalcified, or mixed (containing both calcified and noncalcified material) plaques, with a distinct difference in prognosis. Mixed plaques are associated with the worst prognosis, whereas calcified plaques are associated with the best event-free survival, and noncalcified plaques have an associated risk in between the other 2 types.13 CCTA also allows the identification of potentially high-risk plaque features such as the napkin ring sign (a ring of high attenuation around a low-attenuation plaque suggesting atheroma with a thin fibrous cap), vessel expansion or positive remodeling, low (<30) Hounsfield unit plaque suggesting lipid enrichment, and spotty calcification.14 CCTA plaque characteristics and high-risk plaque features are good predictors of CVD risk,13,15 although the spatial resolution of CT-scans is too low to reliably identify the most vulnerable (thin-cap) plaques.16 New software allows quantification of plaque volume which will likely improve understanding and risk prediction of coronary atherosclerosis.17
Exercise and Coronary Atherosclerosis
Findings in the General Population
Physical activity is defined as any bodily movement that results in energy expenditure beyond resting levels,18 and is often quantified as Metabolic Equivalent of Task (MET) minutes or hours per week. Physical activity can include activities at work, during commuting, or during recreation.
Regular physical activity and exercise improves cardiovascular risk factors including blood pressure, serum lipid profile, glucose control, and cardiovascular function, but studies examining the relationship between physical activity and CAC have reported an inverse relationship (n=8 studies),19–26 positive relationship (n=2 studies),24,27 U/J-shaped relationship (n=3 studies),23,25,26 or no relationship (n=7 studies, Table I in the Data Supplement).19,20,28–32 Population cohorts have demonstrated a wide prevalence of CAC (CACS>0) ranging from 29%27 to 93%20. This variation in CAC prevalence is partly attributable to differences in age, sex, and cardiovascular risk factors. For example, women aged 74±4 years had a prevalence of CAC of 74%,20 whereas women aged 62±4 years had a CAC prevalence of 40%.20 CAC prevalence differs among physical activity categories (Figure 1A), but not after adjustment for potential confounders (Figure 1B). No clear sex differences were observed in the association between physical activity and CAC.
Figure 1.
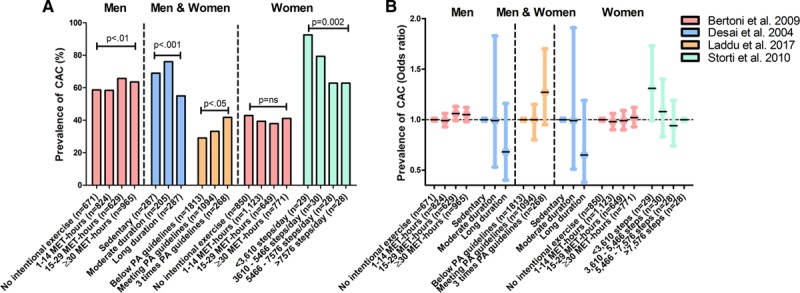
Prevalence of coronary artery calcification in the general population. Studies reporting coronary artery calcification (CAC) prevalence and adjusted odds ratios for the association between physical activity and CAC are shown.20,22,27,30 A, Percentage of CAC prevalence (CAC Score [CACS]>0) across physical activity/exercise groups. B, Adjusted odds ratios for CAC prevalence across physical activity/exercise groups. In B, Desai et al (blue color) included 520 men and 259 women, but the sample size per physical activity categories was not reported. In summary, there is no clear net effect toward either a positive or inverse association between physical activity volumes and CAC prevalence in general population studies. MET indicates metabolic equivalent of task; and PA, physical activity.
The variability in outcomes, in studies of the relationship between physical activity and CAC, may also be a result of the methods used to measure physical activity. For example, a study showed that physical activity measured by accelerometer was inversely associated with CACS, but there was no association when levels of physical activity were determined with subjective questionnaires.19 The quality of questionnaires, whether past or current activity is measured, and if total activity or only intentional exercise is measured could also impact findings. The spectrum of physical activity levels in the study population may also affect results because an inverse relationship was found at low levels of physical activity22 and a positive relationship was found at the highest physical activity levels,27 suggesting a difference in the relationship between physical activity and CAC at different activity levels. Finally, differences in age of the participants may also contribute to the conflicting findings, as an inverse association was found between physical activity and CACS among older (74±4 years) postmenopausal women where there was no significant association in younger (57±3 years) postmenopausal women.20 This may be because of the age- and sex-dependence of CAC with a low prevalence in middle-aged women.33
Similar associations were observed between CAC and cardiorespiratory fitness. Cardiorespiratory fitness comprises a set of attributes that individuals inherit or achieve through exercise training that is measured by their ability to perform physical activity.18 Several Korean studies found an inverse association between cardiorespiratory fitness and CAC among mainly middle-aged men.34–36 Similar results were found in middle-aged women from the Cooper Clinic,37 however the observed modest inverse relationship between fitness and CAC was no longer significant after adjustment for traditional risk factors. In contrast, the CARDIA study (Coronary Artery Risk Development in Young Adults) found a positive association between cardiorespiratory fitness and CAC in young adults followed for 27 years,38 which disappeared after multivariate adjustment. Kermott et al found a reverse J-shaped association between cardiorespiratory fitness and CAC prevalence in middle-aged men, with an increased CAC prevalence in the fittest group.39 This effect remained significant after adjustment for some variables (age, body mass index, and family history), but did not include a full adjustment for potential confounders. Adjustment for confounding factors plays an important role in the association between physical activity, cardiorespiratory fitness, and CAC. Therefore, adequate and clearly described adjustment of confounding factors is important when presenting and interpreting the results. Taken together, studies assessing the relationship between physical activity, cardiorespiratory fitness, and CAC have shown mixed results, potentially because of differences in study population and methods, with no clear net effect toward either a positive or inverse association.
Endurance Exercise Training
Exercise is a subset of physical activity that is planned, structured, repetitive, and intended to improve or maintain physical fitness.18 Endurance exercise training refers to repetitively performing aerobic physical activity, such as running or cycling, to obtain a training adaptation.
Lin et al studied the acute effects of endurance exercise on coronary atherosclerosis characteristics in 8 participants of the Race Across the USA (a 140-day foot race).40 Four runners had at least 1 cardiovascular risk factor and coronary atherosclerosis at baseline, and showed increases in noncalcified plaque volume after the race.40 Runners with no baseline coronary atherosclerosis remained free of coronary atherosclerosis after the race. Although the numbers are small, these data raise the possibility that high-volume endurance exercise may accelerate coronary atherosclerosis in vulnerable individuals.
Most studies have assessed the long-term effect of endurance exercise training on coronary atherosclerosis (Table II in the Data Supplement). Figure 2 summarizes the findings of studies that compared the prevalence of CAC between athletes and less active individuals. CAC is present in 34% to 71% of athletic cohorts (Figure 2A) and 11% to 36% have CACS≥100, a value often used to signify increased risk (Figure 2B). Differences in age and cardiovascular risk factors contribute to this variability. For example, CAC was present in 71% of 108 male marathon runners (57±6 years old), of whom 12% had a history of hypertension, and 57% were current (4.6%) or former (52%) smokers,3 whereas CAC was present in only 48% of 106 male athletes (54±9 years old) without cardiovascular risk factors.5
Figure 2.
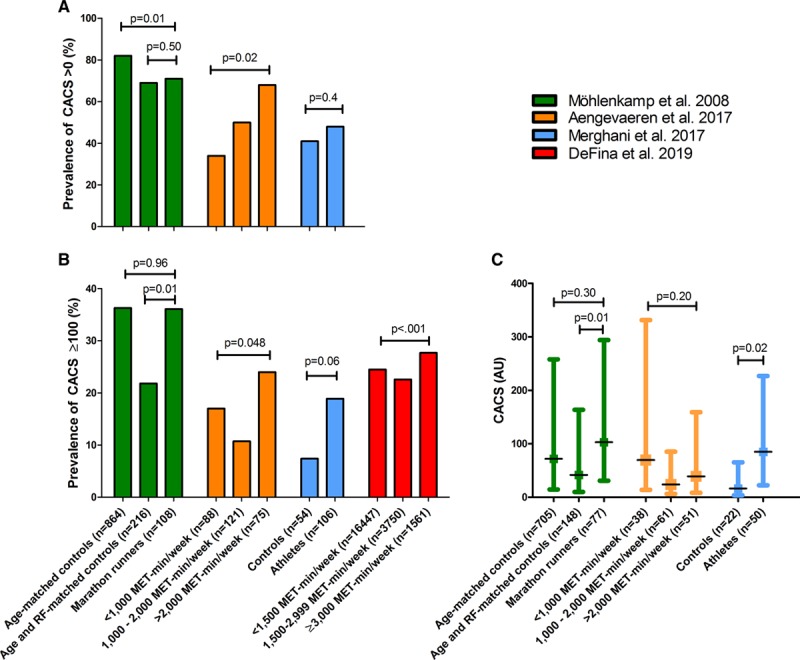
Prevalence of coronary artery calcification and coronary artery calcification scores in studies comparing male athletes with controls. A, Prevalence of coronary artery calcification (CAC) scores (CACS) >0 within athletic and control subjects.3–5 B, Prevalence of CACS >100.3-5, 41 C, CACS within those individuals with prevalent CAC.3–5 These data illustrate increased CAC in the most active athletes. AU indicates arbitrary units; MET, metabolic equivalent of task; and RF, risk factors.
Only 1 of 3 studies found a higher prevalence of CAC among the most active athletes (Figure 2A). Aengevaeren et al divided 284 male athletes into 3 groups on the basis of their lifelong exercise volume.4 CAC prevalence was higher in the most active athletes (>2000 MET-min/week; adjusted odds ratio [ORadjusted] 3.2 [95% CI, 1.6–6.6]) compared with the least active athletes (<1000 MET-min/week), but there was no difference in CAC area, density, and number of lesions among exercise volume groups in those with CAC.4 Möhlenkamp et al compared 108 male marathon runners with 864 age-matched controls and 216 age and risk factor–matched controls.3 CAC prevalence was lower in the marathon runners versus age-matched controls but did not differ when matched for age and cardiovascular risk factors. Merghani et al found no difference in the prevalence of CAC between 106 male athletes (≥10 miles of running or ≥30 miles of cycling per week for ≥10 years) and 54 male controls (median of 1.5 hours of exercise per week), all without cardiovascular risk factors.5 In contrast to Aengevaeren et al, Möhlenkamp et al, and Merghani et al did report higher CACS in athletes with CAC compared with controls with CAC (Figure 2C).
Three of 4 studies revealed a greater prevalence of CACS≥100 in more active subjects, and the fourth study showed a trend approaching significance (P=0.06, Figure 2B). For example, among 21 758 men divided into 3 groups on the basis of their physical activity volumes (<1500 MET-min/week, 1500–2999 MET-min/week, and ≥3000 MET-min/week),41 the most active individuals had an 11% greater risk for CACS ≥100 compared with those accumulating <3000 MET-min/week (relative risk [RR] 1.11 [95%CI, 1.03–1.20]). Despite the higher risk of CACS ≥100 for the most active individuals, CAC volume, density, and number of lesions did not differ between physical activity groups within each category of CAC (CACS ≥100 and <100).
Using CCTA, an American study compared 50 marathon runners with 23 sedentary controls who underwent CCTA for clinical indications and found significantly higher plaque volume, both calcified (83.8±67.7 mm3 versus 44.0±36.8 mm, P<0.0001)3 and noncalcified (116.1±95.7 mm3 versus 81.5±58.1 mm3, P=0.04), in the runners,42 but the runners were older (59±7 versus 55±10 years, P=0.051) than the controls. The British study found an increased prevalence of atherosclerotic plaques in male athletes (44%) versus controls (22%, P=0.01),5 but found no differences among female study participants. The Dutch study found an increased plaque prevalence in the most active (77%; ORadjusted 3.3 [95% CI, 1.6–7.1]) versus the least active (56%) male middle-aged athletes. In both the British and Dutch studies, the most active individuals had a more benign atherosclerotic plaque composition, with fewer mixed plaques and a higher prevalence of only having calcified plaques (Figure 3).4,5 Exercise intensity and sporting discipline may also affect the results. Only very vigorous exercise (≥9 MET) was associated with atherosclerotic plaque (OR 1.56 per hour/week [95% CI, 1.17–2.08]) and CAC prevalence (OR 1.47 per hour/week [95% CI, 1.14–1.91]) in the Dutch study.4 Moreover, cyclists appeared to have a lower risk of atherosclerotic plaque (OR 0.41 [95% CI, 0.19–0.87]) and CAC (OR 0.55 [95% CI, 0.26–1.16]) compared with runners and individuals performing other sports (eg, soccer, hockey, water polo).43
Figure 3.
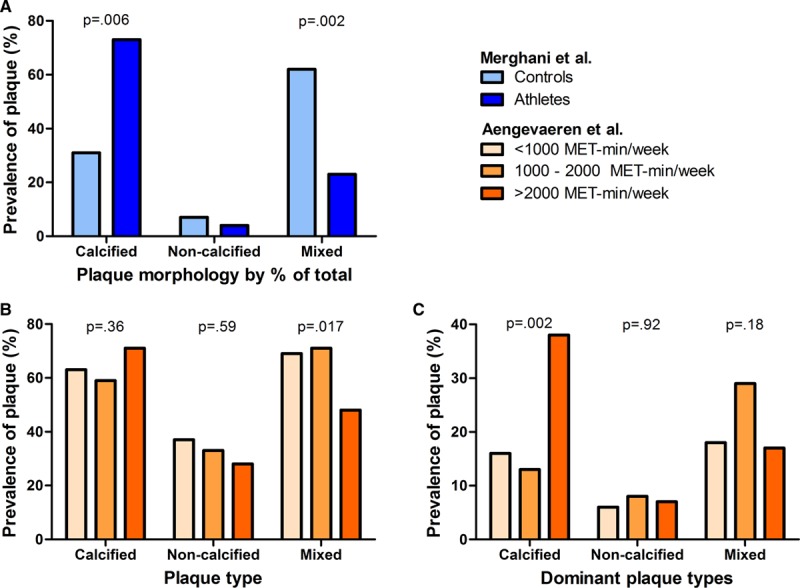
Coronary plaque morphology in athletes. Panel A illustrates the percentages of different coronary plaque morphologies of the 99 plaques in athletes and 26 plaques in the control subjects (total equals 100%).5 Panel B illustrates the percentages of different coronary plaque morphologies in athletes with plaques, presented for lifelong exercise volume groups,4 whereas Panel C illustrates the percentage of athletes with plaques who had only calcified, only noncalcified or only mixed plaque morphology.4 Adapted and reprinted with permission from Merghani et al5 (A) and Aengevaeren et al4 (B and C). MET indicates metabolic equivalent of task.
Future longitudinal studies are required to investigate whether CAC progression is accelerated in athletes. Although the studies in endurance athletes are relatively small and include mostly men, the results suggest more coronary atherosclerosis in (the most active) athletes whereas plaque morphology appears possibly more benign.
Influence of Sex and Race
Limited data are available in female athletes, but small studies (n=46 and n=26)5,44 do not suggest increased coronary atherosclerosis,5 and possibly suggest a lower prevalence of coronary atherosclerosis in female athletes compared with controls.44 Supplemental data from DeFina et al showed no association between physical activity and CACS ≥100 in 9501 women (P=0.91)41. The lower prevalence of coronary atherosclerosis in some studies of female marathon runners may be a result of selection of control subjects, who in one study44 were referred for CCTA to evaluate coronary artery disease and had significantly higher body mass index, hypertension, hyperlipidemia, smoking history, and family history for coronary artery disease. There is insufficient data on the association between exercise volumes and coronary atherosclerosis characteristics among female athletes and studies in male athletes cannot be extrapolated to females. Therefore, the influence of female sex is not specifically addressed in the following sections.
Race is known to impact CAC.45 However, most studies have evaluated only white individuals. Laddu et al performed race-specific analyses and found different associations between physical activity and CAC in black and white nonathletic subjects,27 suggesting that race may affect this association. Consequently, we have not addressed the influence of race in the following sections.
Strength Training
Strength training aims to increase skeletal muscle strength and size by repetitively performing anaerobic physical activity such as weightlifting. No differences were found in the prevalence of CAC and distribution of CACS categories between retired American football players (n=150, 51±10 years old) and age-matched community controls (n=150, 51±10 years old).46 Linemen, typically the largest players who are often classified as overweight or obese, had similar CAC prevalence compared with nonlinemen.46 However, another study comparing linemen with nonlinemen among 931 retired professional American football players (≈54 years old) found that the linemen had a higher prevalence of CAC and severity of CACS compared with nonlinemen.47 CACS>100 (OR 1.59 [95% CI, 1.01–2.49]) remained more common in the linemen after full adjustment for cardiovascular risk factors and ethnicity.47
The use of performance enhancing drugs may accelerate coronary atherosclerosis. Anabolic-androgenic steroid use is prevalent among strength-trained athletes, with an estimated ≈3 million users in the United States.48 Steroids are mainly used to increase muscle mass, performance, and personal appearance.48 However, they are also popular among endurance athletes to aid in recovery and strength.49 A pilot study found higher CACS than expected, on the basis of reference values from the Cooper Clinic, in 14 professional body builders with a long history of steroid use.50 Seven of 14 (50%) had CAC compared with an expected value of 3 (21%). Of those with CAC, 6 of 7 had CACS >90th percentile. Baggish et al compared coronary atherosclerosis between anabolic steroid using and nonusing weightlifters, and nonweightlifting controls.51 Anabolic steroid use was associated with increased coronary plaque volume (users, 3 [0–174] mm3, versus nonusers, 0 [0–69] mm3; P=0.012) as was cumulative lifetime duration of use.51 Widespread use of anabolic steroids did not appear until the 1980s and 1990s, so the long-term atherosclerotic effects are likely to become more apparent in the near future, when (ex)users reach middle age and beyond.48,51
Potential Explanations for Increased Coronary Atherosclerosis in Athletes
The mechanisms underlying increased coronary atherosclerosis in athletes are largely unknown, but there are several potential pathways, although speculative, that may link exercise training to CAC and plaque development (Figure 4).
Figure 4.
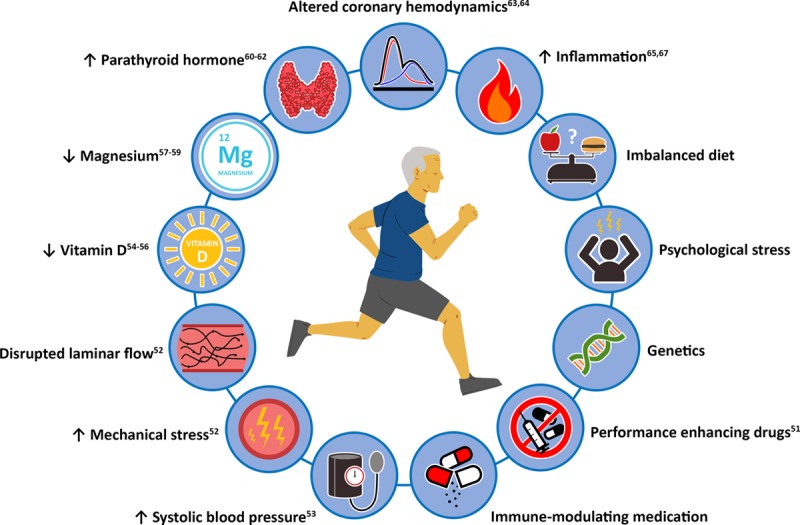
Potential explanations for increased coronary atherosclerosis in athletes.
Catecholamines increase heart rate and cardiac contractility during exercise. The exercise-induced increase in cardiac output may increase mechanical stress on the coronary vessel wall and disrupt laminar blood flow patterns, leading to vessel wall injury and accelerated atherosclerosis.52 High blood pressure may accelerate coronary atherosclerosis,53 and because systolic blood pressure increases during exercise, this may contribute to accelerated atherosclerosis. The finding that very vigorous exercise was associated with atherosclerotic plaque and CAC prevalence fits with this hypothesis because the most intense exercise is associated with the greatest increases in both heart rate and systolic blood pressure.4
The effects of exercise on vitamins, minerals, and hormones, may also influence the association between exercise and coronary atherosclerosis. Serum vitamin D concentrations are inversely related to CAC,54,55 and could accelerate atherosclerosis in athletes who are often deficient in vitamin D.56 Similarly, magnesium can prevent vascular calcification via multiple mechanisms,57 and serum magnesium concentrations are inversely associated with CAC,58 whereas athletes may have low magnesium concentrations.59 Parathyroid hormone increases during exercise.60 The increase in parathyroid hormone likely follows a decrease in ionized calcium concentration during exercise. The reason and fate of the reduced serum concentration of calcium are unknown.61 However, higher levels of parathyroid hormone are associated with greater atherosclerotic disease burden.62 Repeated exposure to higher levels of parathyroid levels after exercise may therefore accelerate coronary atherosclerosis in athletes.
Running induces a large pressure concussion wave during foot strike, which alters coronary hemodynamics and could accelerate coronary atherosclerosis.63,64 This effect is also dependent on the timing of steps during running with reference to the cardiac cycle.63,64
Inflammation has a major role in the development of coronary atherosclerosis and exercise modulates inflammation.65 Chronic exercise lowers inflammation,66 but acute exercise can increase inflammation.67 Although there is far more evidence supporting a suppression of inflammation in athletes, high-intensity, frequent, and prolonged exercise could potentially produce an inflammatory effect, thereby accelerating coronary atherosclerosis.
Other potential explanations for increased coronary atherosclerosis that have not been sufficiently adjusted for in previous studies include dietary intake, psychological stress, and genetics. It is also possible that performance enhancing drugs or immune-modulating medication could contribute to the higher prevalence of CAC and plaque among athletes.68
Clinical Relevance
Prevalence and severity of CAC and atherosclerotic plaques are strongly associated with the 5- and 10-year risk of cardiovascular events in the general and patient populations,7,8,10,13 yet there is strong evidence that elite and amateur athletes live longer than the general population.69,70 Exercise training increases longevity by approximately 3 to 6 years with the most benefit for athletes performing endurance sports.70,71 The increase in cardiorespiratory fitness after aerobic exercise training is also positively associated with increased longevity.72
Möhlenkamp et al were the first to examine the prognosis of higher CACS in athletes. They followed 108 marathon runners for 6±1 years and found a higher event rate in higher CAC categories (1 of 69 [1%] in CAC<100; 3 of 25 [12%] in CAC 100–399; and 3 of 14 [21%] in CAC ≥400; P=0.002), similar to the observed event rates in their control cohort.73 However, this study was limited by the small sample size (n=108) and few events (n=7). A more recent study showed that the amount of self-reported exercise impacts the relationship between CACS and mortality among asymptomatic patients.74 Among individuals with similar CACS, performing “no exercise” had a hazard ratio (HR) of 2.35 (95% CI, 1.49–3.70) for all-cause mortality, whereas “low exercise” had an HR of 1.56 (95% CI, 1.06–2.30), and “moderate exercise” had an HR of 1.29 (95% CI, 0.86–1.95) compared with highly active patients (reference group). Similarly, higher cardiorespiratory fitness significantly reduces the risk of cardiovascular events. Lamonte et al showed that individuals with a fitness ≥10 METs had a 73% reduction in cardiovascular events compared with those with a fitness <10 METs when adjusting for CACS.75 More recent data from the Cooper Clinic Longitudinal study revealed a reduction of 11% for each additional MET of fitness (HR 0.89 [95% CI, 0.84–0.94]) when adjusting for CACS categories (scores of 0, 1–99, 100–399, and ≥400).76 In a subsequent publication, DeFina et al demonstrated that among individuals with CACS<100, those in the highest physical activity category (≥3000 MET-min/week) had a lower risk of all-cause mortality compared with those in the lowest physical activity category (<1500 MET-min/week).41 The beneficial association between physical activity and all-cause mortality was attenuated for individuals with CACS ≥100 (HR 0.77 [95% CI, 0.52–1.15] for the highest versus the lowest physical activity category), whereas CACS≥100 was more prevalent among the most active individuals compared with the less active individuals (RR 1.11 [95% CI, 1.03–1.20]).
Increases in exercise and cardiorespiratory fitness thus seem to lower the cardiovascular risk of CAC. This risk reduction may follow from a large coronary flow reserve because of a combination of increases in epicardial coronary artery diameter, coronary vasodilatory capacity, capillary density, and vasomotor reactivity produced by exercise training.77–79 Similarly, high-volume athletes also have a biological age of their large blood vessels that is ≈30 years younger than their chronological age,80,81 with substantially improved ventriculo-arterial coupling.82
Coronary atherosclerotic plaques associated with increasing exercise volume may also be more stable and less likely to rupture. We found that the most active athletes had fewer mixed plaques and more often only calcified plaques,4,5 which are associated with a lower risk of cardiovascular events.13,15 Similarly, high-intensity statin therapy increases CAC, but decreases coronary atheroma volume and cardiovascular risk.83 Thus, an increase in CACS may not necessarily reflect an increase in cardiovascular risk. Exercise may increase calcification similar to the increase observed with statin therapy, without an associated increase in cardiovascular risk.
Intimal and medial vascular calcification differ by their causal pathways and risk for cardiovascular diseases.84,85 Calcification in an atherosclerotic plaque occurs primarily in the intimal layer of the vessel wall and is associated with luminal stenosis and potential plaque rupture.84 Medial calcification is associated with vessel wall stiffening, specifically with aging, chronic kidney disease, and metabolic diseases such as disorders of calcium and phosphate metabolism.85,86 Athletes may be more prone to developing medial rather than intimal calcification through smooth muscle damage in the vascular wall or exercise-induced metabolic changes. However, the differentiation between intimal and medial calcification cannot be performed reliably using CT, and ex vivo histological analysis is the standard.86
Overall, current evidence suggests that higher CACS in athletes are similarly associated with an increased cardiovascular risk as in a nonathletic cohort. However, the absolute risk of CAC is likely lower in athletes as a result of several beneficial adaptations (Figure 5). The relevance of increased CAC in athletes deserves careful attention and additional longitudinal studies are required to study the influence of exercise on coronary atherosclerosis.
Figure 5.
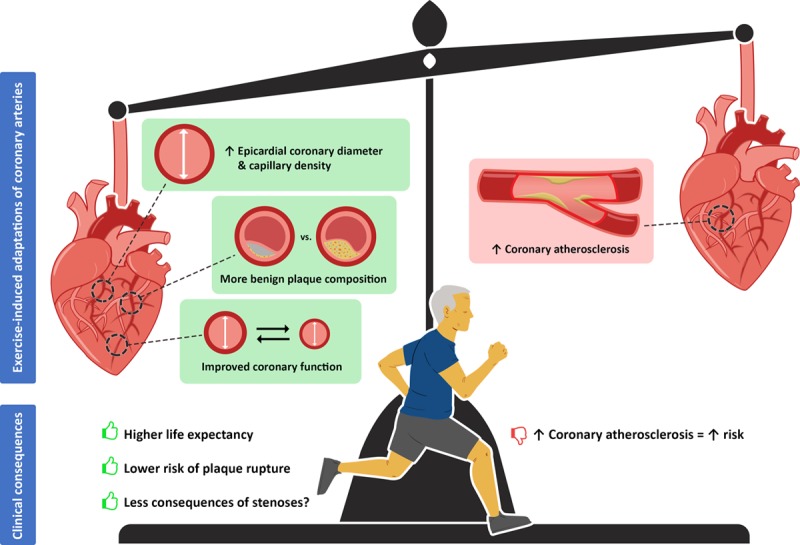
The benefits and risks of long-term exercise training on coronary function, morphology, and atherosclerosis.
Clinical Management
On the basis of our findings, and until the significance of coronary calcification in athletes is better defined, we do not recommend the routine assessment of CACS in athletes based purely on their training history. CAC scoring could be considered in asymptomatic individuals aged 40 to 75 years with a 10-year atherosclerotic CVD risk of 5% to 20% and should be considered only in selected individuals with risk below 5%.87 Our general guidance is not to repeat CAC scoring, particularly in athletes treated with statins, because CACS may increase with statin therapy and continued exercise training, and CAC does not reverse with aggressive lipid therapy.88 Repeated CACS assessment after approximately 5 to 10 years may provide additional information for risk prediction of major cardiovascular events, with the most recent CACS providing the best risk estimate,89 however this strategy appears only reasonable for those in whom follow-up results may influence treatment.87,90
Treatment should be individualized depending on the athlete’s overall risk for cardiovascular disease. All athletes should be questioned about symptoms of myocardial ischemia, family history of atherosclerotic coronary artery disease, and current and previous risk factors. It is important to note that well-trained individuals may present with atypical symptoms of coronary artery disease such as a decline in exercise performance, shortness of breath, or fatigue. Symptomatic athletes should be investigated and managed in the same fashion as the general population. Asymptomatic athletes, including those with high CACS, should be informed that the significance of CAC in middle-aged and older athletes is currently unclear.
In athletes with low-density lipoprotein cholesterol levels ≥70 mg/dL or ≥1.8 mmol/L, and CACS ≥100 or ≥75th percentile compared with their age and sex matched, nonathletic peers, statin therapy should be considered after atherosclerotic CVD risk calculation and clinician-patient risk discussion.90,91 Risk enhancers such as extensive (noncalcified) atherosclerotic plaque on CCTA or a strong family history for premature CVD support initiating statin therapy. The American guidelines for lipid management favor statin therapy when CACS>0 if the 10-year atherosclerotic CVD risk is ≥7.5%.90 However, this cut point is very sensitive to the scoring system used. Given that the CACS is known in these individuals, the ASTRO-CHARM (Astronaut Cardiovascular Health and Risk Modification) risk calculator can be used,92 which incorporates CACS and calculates 10-year risk of fatal or nonfatal myocardial infarction or stroke. Athletes with CACS ≥400 should be advised to commence high-intensity statin therapy and other atherosclerotic risk factors should be strictly managed. Aspirin may be considered for individuals not at increased bleeding risk.93 Episodic use of aspirin (eg, prerace) has been suggested to prevent exercise-related sudden cardiac arrests,94 although evidence for this approach is lacking.
Because CAC scoring is not routinely recommended, the current guidelines do not clearly indicate how to proceed with additional testing in asymptomatic athletes with high CACS.95,96 The following options can be considered when evaluating an asymptomatic athlete. Management strategies differ per region in the world (eg, United States versus Europe), country, and even per physician. Additional testing strongly depends on hospital logistics, costs, and availability of tests. CCTA may be considered in athletes with CAC to assess the number and nature of coronary plaques and to quantify the degree of luminal narrowing. In some hospitals, all individuals with a CACS>0 proceed to CCTA. Exercise or pharmacological stress testing may also be considered to check for inducible myocardial ischemia. In individuals with CACS ≥400 and/or luminal stenoses >50% an exercise stress test or stress imaging tests should be considered to detect evidence of ischemia. Evidence of ischemia could prompt coronary intervention or provide guidance for setting exercise heart rate limits, suggest modification of training, and prompt consideration of additional preventive therapy such as beta blockers. Some physicians may proceed to invasive coronary angiography with fractional flow reserve measurements. In the future, CCTA-based fractional flow reserve measurements may lower the need for invasive coronary angiography. At present, there are insufficient data to provide definitive recommendations for additional testing in asymptomatic athletes with CAC.
American97 and European98 guidelines are available for exercise recommendations in athletes with subclinical coronary artery disease and many of such athletes are detected by CAC scoring. These recommendations generally allow participation in all sports, even in athletes with high CACS, if the athlete is asymptomatic, has no evidence of ischemia or electric instability, and has a normal ejection fraction. As such, athletes can continue their exercise training despite a high CACS. Although the most active individuals with CACS≥100 did not have a higher risk for (cardiovascular) mortality compared with less active individuals with similar CACS,41 presence of CAC is strongly associated with clinical outcomes, in athletes as well as less active individuals. Because exercise does appear to increase CAC, future longitudinal studies are required to confirm this recommendation.
Future Research
Future longitudinal studies are necessary to further investigate the association between exercise training and the development and progression of coronary atherosclerosis among athletes and its clinical relevance. Also, more insight is needed into the mechanisms responsible for increased coronary atherosclerosis in athletes. In this regard, the emerging ability of CT to identify coronary inflammation through measuring the perivascular fat attenuation index may provide additional information regarding the link between exercise and coronary atherosclerosis.99 Most studies included primarily male white runners so little is known on how exercise affects coronary atherosclerosis in females, different ethnicities, and across sporting disciplines. Future ultralow dose CT-scanning will make assessment of CACS more feasible by lowering the radiation exposure in healthy subjects.100 Furthermore, the current CAC scoring using the Agatston score may be refined in the future because it has been shown that the volume and density components have different associations with cardiovascular events. Other potentially meaningful characteristics of calcifications such as number of lesions, location, or distribution may be added in such a new CAC scoring method.6
Conclusions
Studies investigating the relationship between physical activity/exercise and coronary atherosclerosis in the general population have revealed mixed results that show no clear net effect. However, studies in athletes have demonstrated a higher prevalence of CACS≥100 compared with less active controls. Increased coronary atherosclerosis in athletes may be mediated via several mechanisms. The clinical relevance of increased coronary atherosclerosis in athletes is unclear, but the absence of CAC or plaque is better than the presence of any atherosclerosis. Higher CACS among athletes may not necessarily reflect an increased risk for cardiovascular events similar to the general population because exercise promotes beneficial coronary adaptations and increased calcification may be associated with plaque stabilization, which likely explains some of the significant reduction in cardiovascular events because of exercise training. Statin therapy and intensive risk factor management are recommended for athletes with CAC, depending on their CACS and estimated 10-year atherosclerotic cardiovascular disease risk, to stabilize plaques and prevent coronary events. Future longitudinal studies are anticipated to further investigate the role of exercise in coronary atherosclerosis.
Sources of Funding
Drs Aengevaeren and Eijsvogels are financially supported by grants from the Dutch Heart Foundation (#2017T088 and #2017T051, respectively).
Disclosures
None.
Supplementary Material
Footnotes
Sources of Funding, see page 1347
The Data Supplement is available with this article at https://www.ahajournals.org/doi/suppl/10.1161/circulationaha.119.044467.
References
- 1.Benjamin EJ, Virani SS, Callaway CW, Chamberlain AM, Chang AR, Cheng S, Chiuve SE, Cushman M, Delling FN, Deo R, et al. American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2018 update: a report from the American Heart Association. Circulation. 2018;137:e67–e492. doi: 10.1161/CIR.0000000000000558. doi: 10.1161/CIR.0000000000000558. [DOI] [PubMed] [Google Scholar]
- 2.Eijsvogels TM, Molossi S, Lee DC, Emery MS, Thompson PD. Exercise at the extremes: the amount of exercise to reduce cardiovascular events. J Am Coll Cardiol. 2016;67:316–329. doi: 10.1016/j.jacc.2015.11.034. doi: 10.1016/j.jacc.2015.11.034. [DOI] [PubMed] [Google Scholar]
- 3.Möhlenkamp S, Lehmann N, Breuckmann F, Bröcker-Preuss M, Nassenstein K, Halle M, Budde T, Mann K, Barkhausen J, Heusch G, et al. Marathon Study Investigators; Heinz Nixdorf Recall Study Investigators. Running: the risk of coronary events: prevalence and prognostic relevance of coronary atherosclerosis in marathon runners. Eur Heart J. 2008;29:1903–1910. doi: 10.1093/eurheartj/ehn163. doi: 10.1093/eurheartj/ehn163. [DOI] [PubMed] [Google Scholar]
- 4.Aengevaeren VL, Mosterd A, Braber TL, Prakken NHJ, Doevendans PA, Grobbee DE, Thompson PD, Eijsvogels TMH, Velthuis BK. Relationship between lifelong exercise volume and coronary atherosclerosis in athletes. Circulation. 2017;136:138–148. doi: 10.1161/CIRCULATIONAHA.117.027834. doi: 10.1161/CIRCULATIONAHA.117.027834. [DOI] [PubMed] [Google Scholar]
- 5.Merghani A, Maestrini V, Rosmini S, Cox AT, Dhutia H, Bastiaenan R, David S, Yeo TJ, Narain R, Malhotra A, et al. Prevalence of subclinical coronary artery disease in masters endurance athletes with a low atherosclerotic risk profile. Circulation. 2017;136:126–137. doi: 10.1161/CIRCULATIONAHA.116.026964. doi: 10.1161/CIRCULATIONAHA.116.026964. [DOI] [PubMed] [Google Scholar]
- 6.Blaha MJ, Mortensen MB, Kianoush S, Tota-Maharaj R, Cainzos-Achirica M. Coronary artery calcium scoring: is it time for a change in methodology? J Am Coll Cardiol. Cardiovasc Imaging. 2017;10:923–937. doi: 10.1016/j.jcmg.2017.05.007. doi: 10.1016/j.jcmg.2017.05.007. [DOI] [PubMed] [Google Scholar]
- 7.Budoff MJ, Achenbach S, Blumenthal RS, Carr JJ, Goldin JG, Greenland P, Guerci AD, Lima JA, Rader DJ, Rubin GD, et al. American Heart Association Committee on Cardiovascular Imaging and Intervention; American Heart Association Council on Cardiovascular Radiology and Intervention; American Heart Association Committee on Cardiac Imaging, Council on Clinical Cardiology. Assessment of coronary artery disease by cardiac computed tomography: a scientific statement from the American Heart Association Committee on Cardiovascular Imaging and Intervention, Council on Cardiovascular Radiology and Intervention, and Committee on Cardiac Imaging, Council on Clinical Cardiology. Circulation. 2006;114:1761–1791. doi: 10.1161/CIRCULATIONAHA.106.178458. doi: 10.1161/CIRCULATIONAHA.106.178458. [DOI] [PubMed] [Google Scholar]
- 8.Hecht HS. Coronary artery calcium scanning: past, present, and future. J Am Coll Cardiol. Cardiovasc Imaging. 2015;8:579–596. doi: 10.1016/j.jcmg.2015.02.006. doi: 10.1016/j.jcmg.2015.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Criqui MH, Knox JB, Denenberg JO, Forbang NI, McClelland RL, Novotny TE, Sandfort V, Waalen J, Blaha MJ, Allison MA. Coronary artery calcium volume and density: potential interactions and overall predictive value: the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. Cardiovasc Imaging. 2017;10:845–854. doi: 10.1016/j.jcmg.2017.04.018. doi: 10.1016/j.jcmg.2017.04.018. [DOI] [PubMed] [Google Scholar]
- 10.Bamberg F, Sommer WH, Hoffmann V, Achenbach S, Nikolaou K, Conen D, Reiser MF, Hoffmann U, Becker CR. Meta-analysis and systematic review of the long-term predictive value of assessment of coronary atherosclerosis by contrast-enhanced coronary computed tomography angiography. J Am Coll Cardiol. 2011;57:2426–2436. doi: 10.1016/j.jacc.2010.12.043. doi: 10.1016/j.jacc.2010.12.043. [DOI] [PubMed] [Google Scholar]
- 11.Ayoub C, Erthal F, Abdelsalam MA, Murad MH, Wang Z, Erwin PJ, Hillis GS, Kritharides L, Chow BJW. Prognostic value of segment involvement score compared to other measures of coronary atherosclerosis by computed tomography: A systematic review and meta-analysis. J Cardiovasc Comput Tomogr. 2017;11:258–267. doi: 10.1016/j.jcct.2017.05.001. doi: 10.1016/j.jcct.2017.05.001. [DOI] [PubMed] [Google Scholar]
- 12.Cho I, Chang HJ, Sung JM, Pencina MJ, Lin FY, Dunning AM, Achenbach S, Al-Mallah M, Berman DS, Budoff MJ, et al. CONFIRM Investigators. Coronary computed tomographic angiography and risk of all-cause mortality and nonfatal myocardial infarction in subjects without chest pain syndrome from the CONFIRM Registry (Coronary CT Angiography Evaluation for Clinical Outcomes: an International Multicenter registry). Circulation. 2012;126:304–313. doi: 10.1161/CIRCULATIONAHA.111.081380. doi: 10.1161/CIRCULATIONAHA.111.081380. [DOI] [PubMed] [Google Scholar]
- 13.Hou ZH, Lu B, Gao Y, Jiang SL, Wang Y, Li W, Budoff MJ. Prognostic value of coronary CT angiography and calcium score for major adverse cardiac events in outpatients. J Am Coll Cardiol. Cardiovasc Imaging. 2012;5:990–999. doi: 10.1016/j.jcmg.2012.06.006. doi: 10.1016/j.jcmg.2012.06.006. [DOI] [PubMed] [Google Scholar]
- 14.Sandfort V, Lima JA, Bluemke DA. Noninvasive imaging of atherosclerotic plaque progression: status of coronary computed tomography angiography. Circ Cardiovasc Imaging. 2015;8:e003316. doi: 10.1161/CIRCIMAGING.115.003316. doi: 10.1161/CIRCIMAGING.115.003316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nerlekar N, Ha FJ, Cheshire C, Rashid H, Cameron JD, Wong DT, Seneviratne S, Brown AJ. Computed tomographic coronary angiography-derived plaque characteristics predict major adverse cardiovascular events: a systematic review and meta-analysis. Circ Cardiovasc Imaging. 2018;11:e006973. doi: 10.1161/CIRCIMAGING.117.006973. doi: 10.1161/CIRCIMAGING.117.006973. [DOI] [PubMed] [Google Scholar]
- 16.Obaid DR, Calvert PA, Gopalan D, Parker RA, Hoole SP, West NE, Goddard M, Rudd JH, Bennett MR. Atherosclerotic plaque composition and classification identified by coronary computed tomography: assessment of computed tomography-generated plaque maps compared with virtual histology intravascular ultrasound and histology. Circ Cardiovasc Imaging. 2013;6:655–664. doi: 10.1161/CIRCIMAGING.112.000250. doi: 10.1161/CIRCIMAGING.112.000250. [DOI] [PubMed] [Google Scholar]
- 17.Hell MM, Motwani M, Otaki Y, Cadet S, Gransar H, Miranda-Peats R, Valk J, Slomka PJ, Cheng VY, Rozanski A, et al. Quantitative global plaque characteristics from coronary computed tomography angiography for the prediction of future cardiac mortality during long-term follow-up. Eur Heart J Cardiovasc Imaging. 2017;18:1331–1339. doi: 10.1093/ehjci/jex183. doi: 10.1093/ehjci/jex183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Thompson PD, Buchner D, Pina IL, Balady GJ, Williams MA, Marcus BH, Berra K, Blair SN, Costa F, Franklin B, et al. American Heart Association Council on Clinical Cardiology Subcommittee on Exercise, Rehabilitation, and Prevention; American Heart Association Council on Nutrition, Physical Activity, and Metabolism Subcommittee on Physical Activity. Exercise and physical activity in the prevention and treatment of atherosclerotic cardiovascular disease: a statement from the Council on Clinical Cardiology (Subcommittee on Exercise, Rehabilitation, and Prevention) and the Council on Nutrition, Physical Activity, and Metabolism (Subcommittee on Physical Activity). Circulation. 2003;107:3109–3116. doi: 10.1161/01.CIR.0000075572.40158.77. doi: 10.1161/01.CIR.0000075572.40158.77. [DOI] [PubMed] [Google Scholar]
- 19.Gabriel KP, Matthews KA, Pérez A, Edmundowicz D, Kohl HW, III, Hawkins MS, Janak JC, Kriska AM, Kuller LH. Self-reported and accelerometer-derived physical activity levels and coronary artery calcification progression in older women: results from the Healthy Women Study. Menopause. 2013;20:152–161. doi: 10.1097/gme.0b013e31826115af. doi: 10.1097/gme.0b013e31826115af. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Storti KL, Pettee Gabriel KK, Underwood DA, Kuller LH, Kriska AM. Physical activity and coronary artery calcification in two cohorts of women representing early and late postmenopause. Menopause. 2010;17:1146–1151. doi: 10.1097/gme.0b013e3181e3a356. doi: 10.1097/gme.0b013e3181e3a356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Delaney JA, Jensky NE, Criqui MH, Whitt-Glover MC, Lima JA, Allison MA. The association between physical activity and both incident coronary artery calcification and ankle brachial index progression: the Multi-Ethnic Study of Atherosclerosis. Atherosclerosis. 2013;230:278–283. doi: 10.1016/j.atherosclerosis.2013.07.045. doi: 10.1016/j.atherosclerosis.2013.07.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Desai MY, Nasir K, Rumberger JA, Braunstein JB, Post WS, Budoff MJ, Blumenthal RS. Relation of degree of physical activity to coronary artery calcium score in asymptomatic individuals with multiple metabolic risk factors. Am J Cardiol. 2004;94:729–732. doi: 10.1016/j.amjcard.2004.06.004. doi: 10.1016/j.amjcard.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 23.Imran TF, Patel Y, Ellison RC, Carr JJ, Arnett DK, Pankow JS, Heiss G, Hunt SC, Gaziano JM, Djoussé L. Walking and calcified atherosclerotic plaque in the coronary arteries: the National Heart, Lung, and Blood Institute Family Heart Study. Arterioscler Thromb Vasc Biol. 2016;36:1272–1277. doi: 10.1161/ATVBAHA.116.307284. doi: 10.1161/ATVBAHA.116.307284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weinberg N, Young A, Hunter CJ, Agrawal N, Mao S, Budoff MJ. Physical activity, hormone replacement therapy, and the presence of coronary calcium in midlife women. Women Health. 2012;52:423–436. doi: 10.1080/03630242.2012.682705. doi: 10.1080/03630242.2012.682705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kwaśniewska M, Jegier A, Kostka T, Dziankowska-Zaborszczyk E, Rębowska E, Kozińska J, Drygas W. Long-term effect of different physical activity levels on subclinical atherosclerosis in middle-aged men: a 25-year prospective study. PLoS One. 2014;9:e85209. doi: 10.1371/journal.pone.0085209. doi: 10.1371/journal.pone.0085209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kwaśniewska M, Kostka T, Jegier A, Dziankowska-Zaborszczyk E, Leszczyńska J, Rębowska E, Orczykowska M, Drygas W. Regular physical activity and cardiovascular biomarkers in prevention of atherosclerosis in men: a 25-year prospective cohort study. BMC Cardiovasc Disord. 2016;16:65. doi: 10.1186/s12872-016-0239-x. doi: 10.1186/s12872-016-0239-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Laddu DR, Rana JS, Murillo R, Sorel ME, Quesenberry CP, Jr, Allen NB, Gabriel KP, Carnethon MR, Liu K, Reis JP, et al. 25-year physical activity trajectories and development of subclinical coronary artery disease as measured by coronary artery calcium: the Coronary Artery Risk Development in Young Adults (CARDIA) study. Mayo Clin Proc. 2017;92:1660–1670. doi: 10.1016/j.mayocp.2017.07.016. doi: 10.1016/j.mayocp.2017.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hamer M, Venuraju SM, Lahiri A, Rossi A, Steptoe A. Objectively assessed physical activity, sedentary time, and coronary artery calcification in healthy older adults. Arterioscler Thromb Vasc Biol. 2012;32:500–505. doi: 10.1161/ATVBAHA.111.236877. doi: 10.1161/ATVBAHA.111.236877. [DOI] [PubMed] [Google Scholar]
- 29.Taylor AJ, Watkins T, Bell D, Carrow J, Bindeman J, Scherr D, Feuerstein I, Wong H, Bhattarai S, Vaitkus M, et al. Physical activity and the presence and extent of calcified coronary atherosclerosis. Med Sci Sports Exerc. 2002;34:228–233. doi: 10.1097/00005768-200202000-00008. doi: 10.1097/00005768-200202000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Bertoni AG, Whitt-Glover MC, Chung H, Le KY, Barr RG, Mahesh M, Jenny NS, Burke GL, Jacobs DR. The association between physical activity and subclinical atherosclerosis: the Multi-Ethnic Study of Atherosclerosis. Am J Epidemiol. 2009;169:444–454. doi: 10.1093/aje/kwn350. doi: 10.1093/aje/kwn350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Whelton SP, Silverman MG, McEvoy JW, Budoff MJ, Blankstein R, Eng J, Blumenthal RS, Szklo M, Nasir K, Blaha MJ. Predictors of long-term healthy arterial aging: coronary artery calcium nondevelopment in the MESA study. J Am Coll Cardiol. Cardiovasc Imaging. 2015;8:1393–1400. doi: 10.1016/j.jcmg.2015.06.019. doi: 10.1016/j.jcmg.2015.06.019. [DOI] [PubMed] [Google Scholar]
- 32.Folsom AR, Evans GW, Carr JJ, Stillman AE Atherosclerosis Risk in Communities Study Investigators. Association of traditional and nontraditional cardiovascular risk factors with coronary artery calcification. Angiology. 2004;55:613–623. doi: 10.1177/00033197040550i602. doi: 10.1177/00033197040550i602. [DOI] [PubMed] [Google Scholar]
- 33.Pletcher MJ, Sibley CT, Pignone M, Vittinghoff E, Greenland P. Interpretation of the coronary artery calcium score in combination with conventional cardiovascular risk factors: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2013;128:1076–1084. doi: 10.1161/CIRCULATIONAHA.113.002598. doi: 10.1161/CIRCULATIONAHA.113.002598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sung J, Cho SJ, Choe YH, Choi YH, Hong KP. Prevalence of coronary atherosclerosis in asymptomatic middle-age men with high aerobic fitness. Am J Cardiol. 2012;109:839–843. doi: 10.1016/j.amjcard.2011.11.009. doi: 10.1016/j.amjcard.2011.11.009. [DOI] [PubMed] [Google Scholar]
- 35.Sung J, Cho SJ, Choe YH, Yoo S, Woo KG, Choi YH, Hong KP. Relationship between aerobic fitness and progression of coronary atherosclerosis. Heart Vessels. 2016;31:1418–1423. doi: 10.1007/s00380-015-0745-2. doi: 10.1007/s00380-015-0745-2. [DOI] [PubMed] [Google Scholar]
- 36.Jae SY, Franklin BA, Schmidt-Trucksass A, Kim DK, Choi YH, Park JB. Relation of cardiorespiratory fitness to risk of subclinical atherosclerosis in men with cardiometabolic syndrome. Am J Cardiol. 2016;118:1282–1286. doi: 10.1016/j.amjcard.2016.07.064. doi: 10.1016/j.amjcard.2016.07.064. [DOI] [PubMed] [Google Scholar]
- 37.DeFina L, Radford N, Leonard D, Gibbons L, Khera A. Cardiorespiratory fitness and coronary artery calcification in women. Atherosclerosis. 2014;233:648–653. doi: 10.1016/j.atherosclerosis.2014.01.016. doi: 10.1016/j.atherosclerosis.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 38.Shah RV, Murthy VL, Colangelo LA, Reis J, Venkatesh BA, Sharma R, Abbasi SA, Goff DC, Jr., Carr JJ, Rana JS, et al. Association of fitness in young adulthood with survival and cardiovascular risk: the Coronary Artery Risk Development in Young Adults (CARDIA) study. JAMA Intern Med. 2016;176:87–95. doi: 10.1001/jamainternmed.2015.6309. doi: 10.1001/jamainternmed.2015.6309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kermott CA, Schroeder DR, Kopecky SL, Behrenbeck TR. Cardiorespiratory fitness and coronary artery calcification in a primary prevention population. Mayo Clin Proc Innov Qual Outcomes. 2019;3:122–130. doi: 10.1016/j.mayocpiqo.2019.04.004. doi: 10.1016/j.mayocpiqo.2019.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lin J, DeLuca JR, Lu MT, Ruehm SG, Dudum R, Choi B, Lieberman DE, Hoffman U, Baggish AL. Extreme endurance exercise and progressive coronary artery disease. J Am Coll Cardiol. 2017;70:293–295. doi: 10.1016/j.jacc.2017.05.016. doi: 10.1016/j.jacc.2017.05.016. [DOI] [PubMed] [Google Scholar]
- 41.DeFina LF, Radford NB, Barlow CE, Willis BL, Leonard D, Haskell WL, Farrell SW, Pavlovic A, Abel K, Berry JD, et al. Association of all-cause and cardiovascular mortality with high levels of physical activity and concurrent coronary artery calcification. JAMA Cardiol. 2019;4:174–181. doi: 10.1001/jamacardio.2018.4628. doi: 10.1001/jamacardio.2018.4628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schwartz RS, Kraus SM, Schwartz JG, Wickstrom KK, Peichel G, Garberich RF, Lesser JR, Oesterle SN, Knickelbine T, Harris KM, et al. Increased coronary artery plaque volume among male marathon runners. Mo Med. 2014;111:89–94. [PMC free article] [PubMed] [Google Scholar]
- 43.Aengevaeren VL, Mosterd A, Sharma S, Braber TL, Thompson PD, Velthuis BK, Eijsvogels TMH. Coronary atherosclerosis in athletes: exploring the role of sporting discipline. J Am Coll Cardiol. Cardiovasc Imaging. 2019;12(8 Pt 1):1587–1589. doi: 10.1016/j.jcmg.2019.01.002. doi: 10.1016/j.jcmg.2019.01.002. [DOI] [PubMed] [Google Scholar]
- 44.Roberts WO, Schwartz RS, Kraus SM, Schwartz JG, Peichel G, Garberich RF, Lesser JR, Oesterle SN, Wickstrom KK, Knickelbine T. Long-term marathon running is associated with low coronary plaque formation in women. Med Sci Sports Exerc. 2017;49:641–645. doi: 10.1249/MSS.0000000000001154. doi: 10.1249/MSS.0000000000001154. [DOI] [PubMed] [Google Scholar]
- 45.McClelland RL, Chung H, Detrano R, Post W, Kronmal RA. Distribution of coronary artery calcium by race, gender, and age: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2006;113:30–37. doi: 10.1161/CIRCULATIONAHA.105.580696. doi: 10.1161/CIRCULATIONAHA.105.580696. [DOI] [PubMed] [Google Scholar]
- 46.Chang AY, FitzGerald SJ, Cannaday J, Zhang S, Patel A, Palmer MD, Reddy GP, Ordovas KG, Stillman AE, Janowitz W, et al. Cardiovascular risk factors and coronary atherosclerosis in retired National Football League players. Am J Cardiol. 2009;104:805–811. doi: 10.1016/j.amjcard.2009.05.008. doi: 10.1016/j.amjcard.2009.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Basra SS, Pokharel Y, Hira RS, Bandeali SJ, Nambi V, Deswal A, Nasir K, Martin SS, Vogel RA, Roberts AJ, et al. Relation between playing position and coronary artery calcium scores in retired National Football League players. Am J Cardiol. 2014;114:1836–1840. doi: 10.1016/j.amjcard.2014.09.021. doi: 10.1016/j.amjcard.2014.09.021. [DOI] [PubMed] [Google Scholar]
- 48.Pope HG, Jr, Wood RI, Rogol A, Nyberg F, Bowers L, Bhasin S. Adverse health consequences of performance-enhancing drugs: an Endocrine Society scientific statement. Endocr Rev. 2014;35:341–375. doi: 10.1210/er.2013-1058. doi: 10.1210/er.2013-1058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.La Gerche A, Brosnan MJ. Cardiovascular effects of performance-enhancing drugs. Circulation. 2017;135:89–99. doi: 10.1161/CIRCULATIONAHA.116.022535. doi: 10.1161/CIRCULATIONAHA.116.022535. [DOI] [PubMed] [Google Scholar]
- 50.Santora LJ, Marin J, Vangrow J, Minegar C, Robinson M, Mora J, Friede G. Coronary calcification in body builders using anabolic steroids. Prev Cardiol. 2006;9:198–201. doi: 10.1111/j.1559-4564.2006.05210.x. doi: 10.1111/j.1559-4564.2006.05210.x. [DOI] [PubMed] [Google Scholar]
- 51.Baggish AL, Weiner RB, Kanayama G, Hudson JI, Lu MT, Hoffmann U, Pope HG., Jr. Cardiovascular toxicity of illicit anabolic-androgenic steroid use. Circulation. 2017;135:1991–2002. doi: 10.1161/CIRCULATIONAHA.116.026945. doi: 10.1161/CIRCULATIONAHA.116.026945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franck G, Even G, Gautier A, Salinas M, Loste A, Procopio E, Gaston AT, Morvan M, Dupont S, Deschildre C, et al. Haemodynamic stress-induced breaches of the arterial intima trigger inflammation and drive atherogenesis. Eur Heart J. 2019;40:928–937. doi: 10.1093/eurheartj/ehy822. doi: 10.1093/eurheartj/ehy822. [DOI] [PubMed] [Google Scholar]
- 53.Kronmal RA, McClelland RL, Detrano R, Shea S, Lima JA, Cushman M, Bild DE, Burke GL. Risk factors for the progression of coronary artery calcification in asymptomatic subjects: results from the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2007;115:2722–2730. doi: 10.1161/CIRCULATIONAHA.106.674143. doi: 10.1161/CIRCULATIONAHA.106.674143. [DOI] [PubMed] [Google Scholar]
- 54.Watson KE, Abrolat ML, Malone LL, Hoeg JM, Doherty T, Detrano R, Demer LL. Active serum vitamin D levels are inversely correlated with coronary calcification. Circulation. 1997;96:1755–1760. doi: 10.1161/01.cir.96.6.1755. doi: 10.1161/01.cir.96.6.1755. [DOI] [PubMed] [Google Scholar]
- 55.Malik R, Aneni EC, Roberson L, Ogunmoroti O, Ali SS, Shaharyar S, Younus A, Jamal O, Aziz MA, Martin SS, et al. Measuring coronary artery calcification: is serum vitamin D relevant? Atherosclerosis. 2014;237:734–738. doi: 10.1016/j.atherosclerosis.2014.10.087. doi: 10.1016/j.atherosclerosis.2014.10.087. [DOI] [PubMed] [Google Scholar]
- 56.Farrokhyar F, Tabasinejad R, Dao D, Peterson D, Ayeni OR, Hadioonzadeh R, Bhandari M. Prevalence of vitamin D inadequacy in athletes: a systematic-review and meta-analysis. Sports Med. 2015;45:365–378. doi: 10.1007/s40279-014-0267-6. doi: 10.1007/s40279-014-0267-6. [DOI] [PubMed] [Google Scholar]
- 57.Ter Braake AD, Shanahan CM, de Baaij JHF. Magnesium counteracts vascular calcification: passive interference or active modulation? Arterioscler Thromb Vasc Biol. 2017;37:1431–1445. doi: 10.1161/ATVBAHA.117.309182. doi: 10.1161/ATVBAHA.117.309182. [DOI] [PubMed] [Google Scholar]
- 58.Lee SY, Hyun YY, Lee KB, Kim H. Low serum magnesium is associated with coronary artery calcification in a Korean population at low risk for cardiovascular disease. Nutr Metab Cardiovasc Dis. 2015;25:1056–1061. doi: 10.1016/j.numecd.2015.07.010. doi: 10.1016/j.numecd.2015.07.010. [DOI] [PubMed] [Google Scholar]
- 59.Nielsen FH, Lukaski HC. Update on the relationship between magnesium and exercise. Magnes Res. 2006;19:180–189. [PubMed] [Google Scholar]
- 60.Bouassida A, Latiri I, Bouassida S, Zalleg D, Zaouali M, Feki Y, Gharbi N, Zbidi A, Tabka Z. Parathyroid hormone and physical exercise: a brief review. J Sports Sci Med. 2006;5:367–374. [PMC free article] [PubMed] [Google Scholar]
- 61.Kohrt WM, Wherry SJ, Wolfe P, Sherk VD, Wellington T, Swanson CM, Weaver CM, Boxer RS. Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J Bone Miner Res. 2018;33:1326–1334. doi: 10.1002/jbmr.3428. doi: 10.1002/jbmr.3428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hagström E, Michaëlsson K, Melhus H, Hansen T, Ahlström H, Johansson L, Ingelsson E, Sundström J, Lind L, Arnlöv J. Plasma-parathyroid hormone is associated with subclinical and clinical atherosclerotic disease in 2 community-based cohorts. Arterioscler Thromb Vasc Biol. 2014;34:1567–1573. doi: 10.1161/ATVBAHA.113.303062. doi: 10.1161/ATVBAHA.113.303062. [DOI] [PubMed] [Google Scholar]
- 63.O’Rourke M, Avolio A, Stelliou V, Young J, Gallagher DE. The rhythm of running: can the heart join in? Aust N Z J Med. 1993;23:708–710. doi: 10.1111/j.1445-5994.1993.tb04732.x. doi: 10.1111/j.1445-5994.1993.tb04732.x. [DOI] [PubMed] [Google Scholar]
- 64.Constantini K, Stickford ASL, Bleich JL, Mannheimer PD, Levine BD, Chapman RF. Synchronizing gait with cardiac cycle phase alters heart rate response during running. Med Sci Sports Exerc. 2018;50:1046–1053. doi: 10.1249/MSS.0000000000001515. doi: 10.1249/MSS.0000000000001515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hansson GK. Inflammation, atherosclerosis, and coronary artery disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 66.Palmefors H, DuttaRoy S, Rundqvist B, Börjesson M. The effect of physical activity or exercise on key biomarkers in atherosclerosis–a systematic review. Atherosclerosis. 2014;235:150–161. doi: 10.1016/j.atherosclerosis.2014.04.026. doi: 10.1016/j.atherosclerosis.2014.04.026. [DOI] [PubMed] [Google Scholar]
- 67.Suzuki K, Nakaji S, Yamada M, Liu Q, Kurakake S, Okamura N, Kumae T, Umeda T, Sugawara K. Impact of a competitive marathon race on systemic cytokine and neutrophil responses. Med Sci Sports Exerc. 2003;35:348–355. doi: 10.1249/01.MSS.0000048861.57899.04. doi: 10.1249/01.MSS.0000048861.57899.04. [DOI] [PubMed] [Google Scholar]
- 68.Baggish AL, Levine BD. Coronary artery calcification among endurance athletes: “hearts of stone”. Circulation. 2017;136:149–151. doi: 10.1161/CIRCULATIONAHA.117.028750. doi: 10.1161/CIRCULATIONAHA.117.028750. [DOI] [PubMed] [Google Scholar]
- 69.Garatachea N, Santos-Lozano A, Sanchis-Gomar F, Fiuza-Luces C, Pareja-Galeano H, Emanuele E, Lucia A. Elite athletes live longer than the general population: a meta-analysis. Mayo Clin Proc. 2014;89:1195–1200. doi: 10.1016/j.mayocp.2014.06.004. doi: 10.1016/j.mayocp.2014.06.004. [DOI] [PubMed] [Google Scholar]
- 70.Kontro TK, Sarna S, Kaprio J, Kujala UM. Mortality and health-related habits in 900 Finnish former elite athletes and their brothers. Br J Sports Med. 2018;52:89–95. doi: 10.1136/bjsports-2017-098206. doi: 10.1136/bjsports-2017-098206. [DOI] [PubMed] [Google Scholar]
- 71.Lee DC, Brellenthin AG, Thompson PD, Sui X, Lee IM, Lavie CJ. Running as a key lifestyle medicine for longevity. Prog Cardiovasc Dis. 2017;60:45–55. doi: 10.1016/j.pcad.2017.03.005. doi: 10.1016/j.pcad.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Clausen JSR, Marott JL, Holtermann A, Gyntelberg F, Jensen MT. Midlife cardiorespiratory fitness and the long-term risk of mortality: 46 years of follow-up. J Am Coll Cardiol. 2018;72:987–995. doi: 10.1016/j.jacc.2018.06.045. doi: 10.1016/j.jacc.2018.06.045. [DOI] [PubMed] [Google Scholar]
- 73.Möhlenkamp S, Leineweber K, Lehmann N, Braun S, Roggenbuck U, Perrey M, Broecker-Preuss M, Budde T, Halle M, Mann K, et al. Coronary atherosclerosis burden, but not transient troponin elevation, predicts long-term outcome in recreational marathon runners. Basic Res Cardiol. 2014;109:391. doi: 10.1007/s00395-013-0391-8. doi: 10.1007/s00395-013-0391-8. [DOI] [PubMed] [Google Scholar]
- 74.Arnson Y, Rozanski A, Gransar H, Hayes SW, Friedman JD, Thomson LEJ, Berman DS. Impact of exercise on the relationship between CAC scores and all-cause mortality. J Am Coll Cardiol. Cardiovasc Imaging. 2017;10:1461–1468. doi: 10.1016/j.jcmg.2016.12.030. doi: 10.1016/j.jcmg.2016.12.030. [DOI] [PubMed] [Google Scholar]
- 75.LaMonte MJ, Fitzgerald SJ, Levine BD, Church TS, Kampert JB, Nichaman MZ, Gibbons LW, Blair SN. Coronary artery calcium, exercise tolerance, and CHD events in asymptomatic men. Atherosclerosis. 2006;189:157–162. doi: 10.1016/j.atherosclerosis.2005.12.014. doi: 10.1016/j.atherosclerosis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 76.Radford NB, DeFina LF, Leonard D, Barlow CE, Willis BL, Gibbons LW, Gilchrist SC, Khera A, Levine BD. Cardiorespiratory fitness, coronary artery calcium, and cardiovascular disease events in a cohort of generally healthy middle-age men: results from the Cooper Center Longitudinal Study. Circulation. 2018;137:1888–1895. doi: 10.1161/CIRCULATIONAHA.117.032708. doi: 10.1161/CIRCULATIONAHA.117.032708. [DOI] [PubMed] [Google Scholar]
- 77.Laughlin MH, Bowles DK, Duncker DJ. The coronary circulation in exercise training. Am J Physiol Heart Circ Physiol. 2012;302:H10–H23. doi: 10.1152/ajpheart.00574.2011. doi: 10.1152/ajpheart.00574.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Haskell WL, Sims C, Myll J, Bortz WM, St Goar FG, Alderman EL. Coronary artery size and dilating capacity in ultradistance runners. Circulation. 1993;87:1076–1082. doi: 10.1161/01.cir.87.4.1076. doi: 10.1161/01.cir.87.4.1076. [DOI] [PubMed] [Google Scholar]
- 79.Nguyen PK, Terashima M, Fair JM, Varady A, Taylor-Piliae RE, Iribarren C, Go AS, Haskell WL, Hlatky MA, Fortmann SP, et al. Physical activity in older subjects is associated with increased coronary vasodilation: the ADVANCE study. J Am Coll Cardiol. Cardiovasc Imaging. 2011;4:622–629. doi: 10.1016/j.jcmg.2011.05.001. doi: 10.1016/j.jcmg.2011.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Shibata S, Levine BD. Biological aortic age derived from the arterial pressure waveform. J Appl Physiol (1985) 2011;110:981–987. doi: 10.1152/japplphysiol.01261.2010. doi: 10.1152/japplphysiol.01261.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Shibata S, Fujimoto N, Hastings JL, Carrick-Ranson G, Bhella PS, Hearon CM, Jr, Levine BD. The effect of lifelong exercise frequency on arterial stiffness. J Physiol. 2018;596:2783–2795. doi: 10.1113/JP275301. doi: 10.1113/JP275301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Shibata S, Hastings JL, Prasad A, Fu Q, Okazaki K, Palmer MD, Zhang R, Levine BD. ‘Dynamic’ starling mechanism: effects of ageing and physical fitness on ventricular-arterial coupling. J Physiol. 2008;586:1951–1962. doi: 10.1113/jphysiol.2007.143651. doi: 10.1113/jphysiol.2007.143651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Puri R, Nicholls SJ, Shao M, Kataoka Y, Uno K, Kapadia SR, Tuzcu EM, Nissen SE. Impact of statins on serial coronary calcification during atheroma progression and regression. J Am Coll Cardiol. 2015;65:1273–1282. doi: 10.1016/j.jacc.2015.01.036. doi: 10.1016/j.jacc.2015.01.036. [DOI] [PubMed] [Google Scholar]
- 84.Doherty TM, Fitzpatrick LA, Inoue D, Qiao JH, Fishbein MC, Detrano RC, Shah PK, Rajavashisth TB. Molecular, endocrine, and genetic mechanisms of arterial calcification. Endocr Rev. 2004;25:629–672. doi: 10.1210/er.2003-0015. doi: 10.1210/er.2003-0015. [DOI] [PubMed] [Google Scholar]
- 85.Johnson RC, Leopold JA, Loscalzo J. Vascular calcification: pathobiological mechanisms and clinical implications. Circ Res. 2006;99:1044–1059. doi: 10.1161/01.RES.0000249379.55535.21. doi: 10.1161/01.RES.0000249379.55535.21. [DOI] [PubMed] [Google Scholar]
- 86.Lanzer P, Boehm M, Sorribas V, Thiriet M, Janzen J, Zeller T, St Hilaire C, Shanahan C. Medial vascular calcification revisited: review and perspectives. Eur Heart J. 2014;35:1515–1525. doi: 10.1093/eurheartj/ehu163. doi: 10.1093/eurheartj/ehu163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hecht H, Blaha MJ, Berman DS, Nasir K, Budoff M, Leipsic J, Blankstein R, Narula J, Rumberger J, Shaw LJ. Clinical indications for coronary artery calcium scoring in asymptomatic patients: expert consensus statement from the Society of Cardiovascular Computed Tomography. J Cardiovasc Comput Tomogr. 2017;11:157–168. doi: 10.1016/j.jcct.2017.02.010. doi: 10.1016/j.jcct.2017.02.010. [DOI] [PubMed] [Google Scholar]
- 88.Noyes AM, Thompson PD. A systematic review of the time course of atherosclerotic plaque regression. Atherosclerosis. 2014;234:75–84. doi: 10.1016/j.atherosclerosis.2014.02.007. doi: 10.1016/j.atherosclerosis.2014.02.007. [DOI] [PubMed] [Google Scholar]
- 89.Radford NB, DeFina LF, Barlow CE, Lakoski SG, Leonard D, Paixao AR, Khera A, Levine BD. Progression of CAC score and risk of incident CVD. J Am Coll Cardiol. Cardiovasc Imaging. 2016;9:1420–1429. doi: 10.1016/j.jcmg.2016.03.010. doi: 10.1016/j.jcmg.2016.03.010. [DOI] [PubMed] [Google Scholar]
- 90.Grundy SM, Stone NJ, Bailey AL, Beam C, Birtcher KK, Blumenthal RS, Braun LT, de Ferranti S, Faiella-Tommasino J, Forman DE, et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation. 2019;139:e1082–e1143. doi: 10.1161/CIR.0000000000000625. doi: 10.1161/CIR.0000000000000625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Budoff MJ, Young R, Burke G, Carr J, Detrano RC, Folsom AR, Kronmal R, Lima JAC, Liu KJ, McClelland RL, et al. Ten-year association of coronary artery calcium with atherosclerotic cardiovascular disease (ASCVD) events: the Multi-Ethnic Study of Atherosclerosis (MESA). Eur Heart J. 2018;39:2401–2408. doi: 10.1093/eurheartj/ehy217. doi: 10.1093/eurheartj/ehy217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Khera A, Budoff MJ, O’Donnell CJ, Ayers CA, Locke J, de Lemos JA, Massaro JM, McClelland RL, Taylor A, Levine BD. Astronaut Cardiovascular Health and Risk Modification (Astro-CHARM) coronary calcium atherosclerotic cardiovascular disease risk calculator. Circulation. 2018;138:1819–1827. doi: 10.1161/CIRCULATIONAHA.118.033505. doi: 10.1161/CIRCULATIONAHA.118.033505. [DOI] [PubMed] [Google Scholar]
- 93.Arnett DK, Blumenthal RS, Albert MA, Buroker AB, Goldberger ZD, Hahn EJ, Himmelfarb CD, Khera A, Lloyd-Jones D, McEvoy JW, et al. 2019 ACC/AHA guideline on the primary prevention of cardiovascular disease: executive summary. Circulation. 2019;140:e563–e595. doi: 10.1161/CIR.0000000000000677. doi: 10.1161/CIR.0000000000000677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Siegel AJ, Noakes TD. Aspirin to prevent sudden cardiac death in athletes with high coronary artery calcium scores. Am J Med. 2019;132:138–141. doi: 10.1016/j.amjmed.2018.09.015. doi: 10.1016/j.amjmed.2018.09.015. [DOI] [PubMed] [Google Scholar]
- 95.Goff DC, Jr, Lloyd-Jones DM, Bennett G, Coady S, D’Agostino RB, Gibbons R, Greenland P, Lackland DT, Levy D, O’Donnell CJ, et al. American College of Cardiology/American Heart Association Task Force on Practice Guidelines. 2013 ACC/AHA guideline on the assessment of cardiovascular risk: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129(25 Suppl 2):S49–S73. doi: 10.1161/01.cir.0000437741.48606.98. doi: 10.1161/01.cir.0000437741.48606.98. [DOI] [PubMed] [Google Scholar]
- 96.Montalescot G, Sechtem U, Achenbach S, Andreotti F, Arden C, Budaj A, Bugiardini R, Crea F, Cuisset T, Di Mario C, et al. Task Force Members; ESC Committee for Practice Guidelines; Document Reviewers. 2013 ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur Heart J. 2013;34:2949–3003. doi: 10.1093/eurheartj/eht296. doi: 10.1093/eurheartj/eht296. [DOI] [PubMed] [Google Scholar]
- 97.Thompson PD, Myerburg RJ, Levine BD, Udelson JE, Kovacs RJ American Heart Association Electrocardiography and Arrhythmias Committee of the Council on Clinical Cardiology, Council on Cardiovascular Disease in the Young, Council on Cardiovascular and Stroke Nursing, Council on Functional Genomics and Translational Biology, and the American College of Cardiology. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 8: coronary artery disease: a scientific statement from the American Heart Association and American College of Cardiology. Circulation. 2015;132:e310–314. doi: 10.1161/CIR.0000000000000244. doi: 10.1161/CIR.0000000000000244. [DOI] [PubMed] [Google Scholar]
- 98.Borjesson M, Dellborg M, Niebauer J, LaGerche A, Schmied C, Solberg EE, Halle M, Adami E, Biffi A, Carré F, et al. Recommendations for participation in leisure time or competitive sports in athletes-patients with coronary artery disease: a position statement from the Sports Cardiology Section of the European Association of Preventive Cardiology (EAPC). Eur Heart J. 2019;40:13–18. doi: 10.1093/eurheartj/ehy408. doi: 10.1093/eurheartj/ehy408. [DOI] [PubMed] [Google Scholar]
- 99.Oikonomou EK, Marwan M, Desai MY, Mancio J, Alashi A, Hutt Centeno E, Thomas S, Herdman L, Kotanidis CP, Thomas KE, et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): a post-hoc analysis of prospective outcome data. Lancet. 2018;392:929–939. doi: 10.1016/S0140-6736(18)31114-0. doi: 10.1016/S0140-6736(18)31114-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gräni C, Vontobel J, Benz DC, Bacanovic S, Giannopoulos AA, Messerli M, Grossmann M, Gebhard C, Pazhenkottil AP, Gaemperli O, et al. Ultra-low-dose coronary artery calcium scoring using novel scoring thresholds for low tube voltage protocols-a pilot study. Eur Heart J Cardiovasc Imaging. 2018;19:1362–1371. doi: 10.1093/ehjci/jey019. doi: 10.1093/ehjci/jey019. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


