Abstract
Background
Awareness of the importance of shared decision making (SDM) is widespread; however, little research has focused on discussions surrounding investigations, despite increasing laboratory testing in primary care.
Aim
To explore the discussion of blood tests in routine primary care consultations.
Design and setting
A secondary analysis of 50 video-recorded routine primary care consultations, linked surveys, and records data (all from the One in a Million [OiaM] archive). The consultations were taken by 22 GPs across 12 practices.
Method
A coding scheme was developed, using qualitative content analysis, to explore discussion of blood tests in transcripts of recorded consultations. Codes focused on instigating testing, the extent of SDM, and how results were explained. Survey data were used to compare patients’ pre-visit expectations with consultation content. Medical records were reviewed to compare tests discussed with those ordered.
Results
In 36 out of 50 consultations that discussed ordering blood tests, 11 patients (31%) hinted that they wanted a blood test; however, none asked explicitly. Only four patients (11%) were offered alternative options. In 29 cases (81%) the GP gave some explanation of the indication, but only in six cases (17%) were the limitations of testing explained. Only 10 out of 31 patients (32%) were informed about all blood tests ordered. Of the 23 out of 50 consultations in which results were conveyed, the GP gave no explanation of the results in six cases (26%). Thirteen patients (57%) were only informed of an assessment of the results (for example, ‘normal’), rather than the actual results.
Conclusion
A lack of information dissemination and SDM exists around ordering tests and conveying results. Promoting SDM could reduce unnecessary testing and improve patient-centred care.
Keywords: consultation, decision making, general practice, information dissemination, qualitative research
INTRODUCTION
The ‘active engagement of patients when fateful health care decisions must be made’ has been described as the pinnacle of patient-centred care.1 Shared decision making (SDM) is a process in which clinicians and patients work together to make decisions based on clinical evidence and the patients’ informed preferences, and is appropriate in any healthcare setting in which more than one option is available, including the option to do nothing.2 SDM should provide patients with information about their options, including benefits, limitations, and risks, support patients to articulate what they hope to achieve and what they perceive as harm, and ensure there is mutual understanding before agreeing any action.3,4 As with treatment, tests have the potential to cause harm, not least through false positives and negatives. As such, the importance of providing accurate information about testing should not be overlooked.
A systematic review of patients’ expectations of investigations and interventions found that patients tend to overestimate benefit and underestimate harm.5 Patients have been found to regard reassuring results as proof of good health.6 Perhaps partly because of this, rates of testing continue to increase, with an 8.7% annual increase in laboratory testing in UK primary care between 2000 and 2015, and an estimated £1.8 billion of spending on laboratory tests in primary care in 2015/2016.7 Seizing the opportunity to educate patients about the risks and benefits of tests in order to promote realistic expectations and SDM, as suggested by Hoffmann et al,5 may aid in reducing unnecessary testing. This could lighten the workloads of overstretched GPs8 who are under increasing pressure to reduce costs and improve efficiency.9
Sparse research has used naturalistic data to examine discussion of investigations in primary care. Existing studies have used audio- and video-recordings to examine negotiations surrounding testing and how results are conveyed; however, these did not focus on blood tests and were based in the US.10,11 One UK-based observational study examined video-recorded primary care consultations for the degree to which doctors met their patients’ preferences for involvement in decisions; however, they used a simple standardised tool to rate patient involvement in decision making and did not focus on decisions around testing.12
Although there is a growing body of evidence examining the benefits, limitations, and implementation of SDM, much of this research focuses on treatment decisions, rather than on investigations. Prior research examining the implementation of SDM has used tools, such as the observing patient involvement (OPTION) scale,13 or surveys relying on patient recall.4,14 Despite research exploring motivations for investigations15–17 and the logistics of conveying results to patients,18 there is little evidence about what actually occurs during consultations, including the discussion that precedes the decision to test and how subsequent results are discussed, particularly in UK general practice. In the context of rising rates of blood testing in primary care, growing awareness of the importance of SDM,4 and the lack of research into SDM around testing, it is pertinent to examine the content of consultations in which decisions are made about blood tests. This observational study used inductive and deductive qualitative content analysis to examine the discussion of blood tests in primary care, using an existing archive of video-recorded UK primary care consultations.
How this fits in
| There is increasing awareness of the importance of shared decision making (SDM), but most research focuses on treatment decisions rather than investigations. This study analysed transcripts of video-recorded primary care consultations, identifying a lack of information sharing and SDM around blood testing. Improvement in this area may reduce unnecessary investigations and promote patient engagement. |
METHOD
This study used data previously collected for the One in a Million (OiaM) study,19 archived at the University of Bristol, in accordance with the university’s research data access agreement. OiaM was a prospective observational study that created a repository of primary care consultations. Twenty-three GPs from 12 practices, situated in areas of high and low deprivation, across three clinical commissioning groups in the West of England, were recruited to have routine consultations recorded between July 2014 and April 2015.
Of 421 eligible adult patients, 334 consented to participate. Three-hundred-and-twenty-seven consultations were successfully video-recorded, anonymised, and transcribed verbatim. Pre- and post-visit survey data and medical record entries linked to the index recordings were also collected. All consultations were coded for problems and issues discussed. Three hundred patients consented for their data to be reused by other researchers subject to further NHS Research Ethics Committee approval.20
Sampling
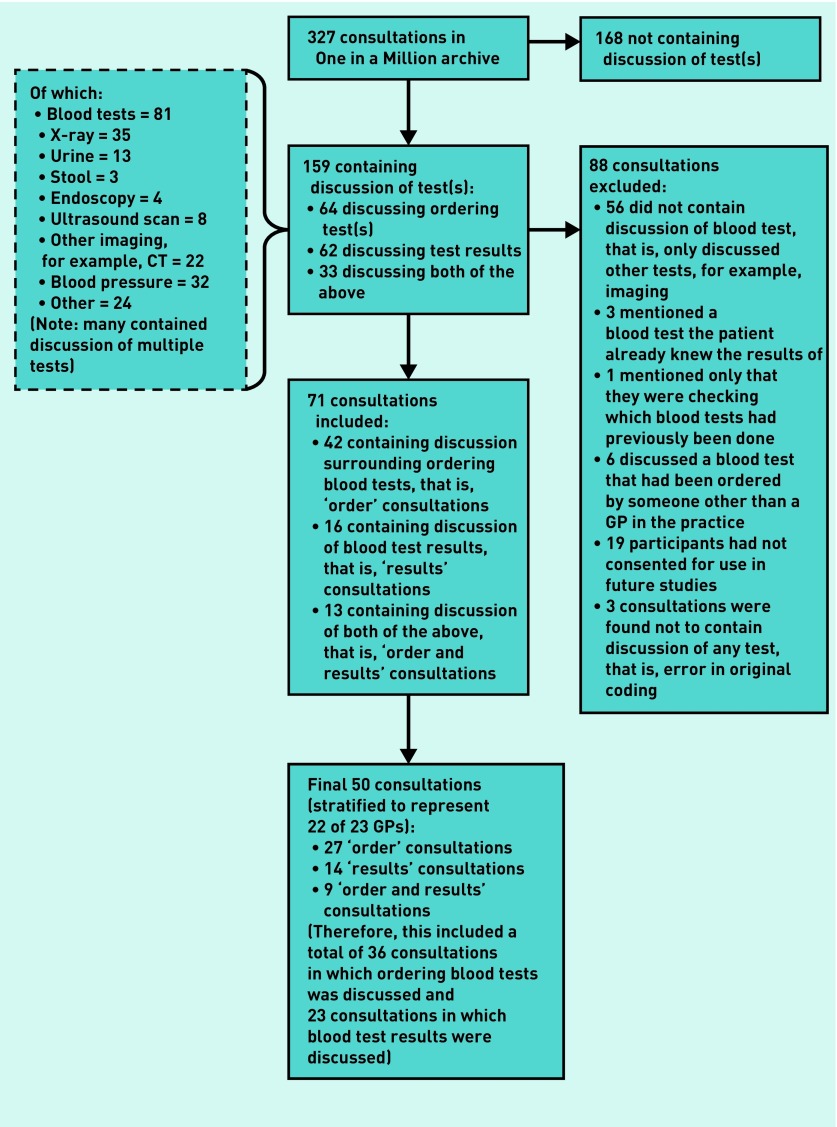
Of 327 consultations in the archive, 159 consultations had already been coded as containing discussion surrounding tests. There were 168 consultations with no discussion regarding tests that were excluded, as were 19 consultations where participants had not consented for their data to be used in future studies.
These 159 consultations were screened for type of test discussed, and whether the discussion was focused around test ordering or test results (Figure 1). Consultations were excluded if they did not discuss blood tests, if the patient was already aware of the test result, or the blood test was not ordered by a GP in the practice (for example, secondary care tests). Sixteen consultations (10%) were coded independently by one of the authors, and discrepancies were highlighted in three cases; these were discussed with another author to reach a consensus.
Figure 1.
Flowchart showing inclusion and exclusion process.
Seventy-one consultations were identified as suitable for inclusion in this study (Figure 1). Fifty consultations were selected for further coding, using stratified sampling to ensure a range of GPs. Of the 50 consultations analysed, 27 contained discussion surrounding ordering blood tests (‘order’ consultations), 14 contained discussion of blood test results (‘results’ consultations), and nine contained both (‘order and results’ consultations) (Figure 1). All 12 practices, and 22 of 23 GPs, in the archive were represented within these 50 consultations. Table 1 summarises GP and patient demographics.
Table 1.
Sample characteristics
| GPs (N = 22) | n | % |
|---|---|---|
| Consultations per GP, n | ||
| 1 | 3 | 14 |
| 2 | 10 | 45 |
| 3 | 9 | 41 |
|
| ||
| GP sex | ||
| Male | 9 | 41 |
| Female | 13 | 59 |
|
| ||
| Years since GP qualification | ||
| ≤5 | 4 | 18 |
| 6–15 | 5 | 23 |
| 16–25 | 9 | 41 |
| ≥26 | 4 | 18 |
|
| ||
| GP employment status | ||
| Salaried GP | 4 | 18 |
| GP partner | 18 | 82 |
|
| ||
| Patients (N = 50) | n | % |
|
| ||
| Patient sex | ||
| Male | 20 | 40 |
| Female | 30 | 60 |
|
| ||
| Patient age, years | ||
| 18–35 | 12 | 24 |
| 36–60 | 16 | 32 |
| >60 | 19 | 38 |
| No age data available | 3 | 6 |
|
| ||
| Patient deprivation quintile | ||
| 1st (least deprived) | 16 | 32 |
| 2nd | 8 | 16 |
| 3rd | 6 | 12 |
| 4th | 4 | 8 |
| 5th (most deprived) | 16 | 32 |
|
| ||
| Patient ethnicity | ||
| Asian/Asian British | 2 | 4 |
| Black/African/ | 4 | 8 |
| Caribbean/black British | ||
| Mixed/multiple ethnic groups | 2 | 4 |
| Other ethnic group | 1 | 2 |
| Unknown | 1 | 2 |
| White/white British/white other | 40 | 80 |
Patient survey data were used to compare patients’ pre-visit expectations for testing with consultation content. Data on pre-visit expectations of ‘tests or investigations’ were available for 23 (64%) of 36 consultations from the final sample in which ordering blood tests were discussed. Medical record entries were available for 31 (86%) of the 36 consultations in which ordering blood tests were discussed; these were reviewed to allow comparison between tests discussed and those ordered by GPs.
Inductive and deductive content analysis
Qualitative content analysis was chosen as the analytic approach to allow objective and content-sensitive analysis of the consultations.21 Using NVivo 11, the content of 10 consultations was initially examined, and the themes generated were grouped into categories. Based on these content categories and previous literature, a coding scheme was developed to allow a combination of inductive analysis, with categories derived from the data, and deductive analysis, with categories derived from existing literature.21
Codes for ‘order’ consultations focused on who instigated blood testing, information given to patients, and the degree of SDM surrounding blood tests. Codes for ‘results’ consultations focused on information shared with patients about blood test results. The codes were developed in response to the content of the data, so were not restricted to using pre-existing scales.
Fifty consultations were coded, with five (10%) double-coded by another author; discrepancies were discussed with another author, and the coding scheme was adapted as necessary. The coding scheme was reviewed by an independent GP, with a view to identifying any obvious omissions. Descriptive statistics were used to summarise the data.
RESULTS
The results focus, first, on ‘order’ consultations, specifically who instigates blood testing, information sharing, and SDM. How GPs inform patients about blood test results, and how they promote patient understanding, were then explored.
Who instigates blood testing?
In the 36 ‘order’ consultations (including ‘order’ and ‘order and results’ consultations; hereafter referred to as ‘order’ consultations), no patients explicitly requested blood tests; however, 11 patients (31%) ‘hinted’ at wanting a blood test. Of these 11 examples, five hints came in the form of the patient reporting another doctor had suggested that a blood test might be beneficial, for example:
‘With my GP in London, I was talking about getting, maybe, tests, to see if there’s something wrong, kind of, hormonally …’
(Practice 4, GP4, female, partner; Patient 17d, female, aged 18–35 years)
Of the 23 out of 36 ‘order’ consultations, for which patient pre-visit survey data on expectations were available, 11 patients (48%) had been expecting one or more ‘tests or investigations’ to be ordered, 10 (43%) were unsure, and two (9%) did not expect any to be ordered. This compares with 62 (32%) of the 193 patients expecting ‘tests or investigations’ in the OiaM archive, for whom pre-visit expectations data were available. Of 11 patients expecting one or more ‘tests or investigations’ to be ordered, five (45%) went on to ‘hint’ at wanting a blood test.
Information sharing
In five (14%) ‘order’ consultations, the GP used only generic terms, such as ‘blood tests’, to describe the tests to the patient, and gave no further detail. Table 2 describes the number of consultations in which there was any use of different referencing practices by the GP. GPs favoured naming specific tests, such as ‘cholesterol’, over naming groups of tests, such as ‘full blood count’; however, this was often at the expense of naming all included tests.
Table 2.
Types of description of blood tests in ‘order’ consultationsa
| Type of description of blood test | nb | % | Common examples |
|---|---|---|---|
| Generic term | 34 | 94 | ‘Bloods’, ‘blood test’ |
| Names by group | 17 | 47 | ‘Kidney function’, ‘your kidneys’, ‘liver function’, ‘thyroid test’, ‘full blood count’ |
| Specific name | 24 | 67 | ‘Cholesterol’, ‘sugar level’, ‘PSA’, ‘vitamin D’ |
| Specific by diagnosis | 9 | 25 | ‘Diabetic check’, ‘coeliac blood test’ |
The percentage of consultations that used each type of description totals more than 100 because many GPs used multiple types of description, for example, a generic statement about ‘blood tests’, followed by more specifically naming some of the included tests.
N = 36.
GPs gave some explanation as to why the test was indicated in 29 (81%) of the 36 ‘order’ consultations. However, some explanations were very brief, such as:
GP: ‘Is there anything else you wanted to talk about today? ’
Patient: ‘Just too much headache.’
GP: ‘OK, and that can often be linked to low vitamin D, as well.’
(Practice 6, GP8, female, partner; Patient 15h, female, aged 18–35 years)
Other explanations were more thorough:
‘… we might do some bloods, like an MOT. I shouldn’t use the term MOT, but, for example, checking cholesterol level, doing a diabetic check, maybe some simple blood counts, kidney, liver. That will give us a good idea of what your cardiovascular risk is, so the risk of a problem in the future to your heart or a stroke, and whether we need to do anything about it.’
(Practice 7, GP 10, male, partner; Patient 12j, male, aged 36–60 years)
In only six (17%) ‘order’ consultations did the GP give some explanation of the risks or limitations of the tests, such as the risk of false positives or negatives. Examples included the GP informing the patient there can be variation between laboratory results, that results of blood tests may be skewed by a cold (in reference to inflammatory markers), and explaining to a patient that inflammatory markers are non-specific and cannot determine whether inflammation is in the joints. The most extensive discussions about limitations were regarding prostate-specific antigen (PSA) testing. For example:
‘It can lead to more problems than less problems because, as you rightly say, sometimes the PSA can be raised and you can have nothing wrong with you. Sometimes, it can be not raised and you can still have something wrong with you, in terms of what we’re looking for, which is an actual tumour of the prostate […] If it comes back as moderately high, the moderate ones are the ones that are the most problematic. It probably means you’re OK, but you end up having a biopsy because it’s raised. If it’s sky-high, in the hundreds, then we know you’ve got something pretty much wrong with you. So, it’s the ones in the middle that are so problematic and what we do with it.’
(Practice 3, GP5, male, partner; Patient 10e, male, aged >60 years)
Shared decision making about blood testing
In only four (11%) ‘order’ consultations, did the GP explicitly offer any alternative option(s), including the option of no blood test, or an alternative test, for example, imaging. Of the consultations where more than one option was presented, half were regarding testing PSA, the other half were regarding screening for arthritis, and all were tests that the patient ‘hinted’ at:
‘It’s up to you. You’re more than welcome to book it, and maybe you choose to wait and do it in the spring with your other blood test.’
(Practice 5, GP7, female partner; Patient 11g, female, aged >60 years)
‘… I will give you a leaflet, just regarding the PSA, because I did rush through that, just so you can think about it. If, when you come for your bloods, you decide you don’t want it, then fine; they can take the request off, but it’s on there at the moment.’
(Practice 7, GP10, male, partner; Patient 13j, male, aged 36–60 years)
In 13 (36%) ‘order’ consultations, the patient did not ask any questions about the blood test, nor were they offered the opportunity to do so by the GP. In 18 (50%) consultations, the GP offered the patient the opportunity to ask questions. However, only twice was this explicit, for example, ‘Is there anything else you want to ask me? ’ The remainder were more subtle, for example, ‘If you are happy doing that? ’ Five patients (14%) asked questions about the blood test without being prompted by the GP.
During 10 (32%) of the 31 ‘order’ consultations in which data were available from the medical record regarding which tests were ordered, patients were informed of all tests ordered, at least to the level of test group or organ. In 16 consultations (52%) additional tests were ordered, beyond those about which the patient was informed, and in five consultations (16%) the patient was never told more detail than just ‘blood tests’ would be ordered.
Informing patients about blood test results
Of the 23 consultations in which the results of blood tests were discussed (both ‘results’ and ‘order and results’ consultations, hereafter referred to as ‘results’ consultations), eight (35%) were regarding entirely normal results, 11 (48%) contained discussion of a new abnormal result, and four (17%) contained discussion regarding borderline results, those of unclear significance, or an abnormal result that was anticipated due to a previously known diagnosis.
Table 3 summarises how results were conveyed to patients and examples are given below. In 13 (57%) ‘results’ consultations, only an assessment of the result was conveyed to the patient (for example, ‘high’, ‘low’, ‘normal’), with no numerical detail of the results shared:
‘Well, your blood count has dropped quite low. You’ve never been as low as this before.’
(Practice 8, GP16, male, partner; Patient 8p, female, aged >60 years)
Table 3.
How results are conveyed to patients in consultations
| Code | Consultation (N = 23) | |
|---|---|---|
| n | % | |
| Assessment only | 13 | 57 |
| Numerical result only | 1 | 4 |
| Assessment plus numerical result | 5 | 22 |
| Multiple results given, using a combination of the above | 4 | 17 |
In one (4%) consultation a numerical result only was given:
Patient: ‘I’ve had cholesterol recently, because I had it done a year ago and it was slightly high, so they asked me to come back in a year’s time.’
GP: ‘It was 6.4.’
Patient: ‘Yes, so it’s gone down.’
GP: ‘… which is the absolute figure, having come down from 7.1.’
(Practice 5, GP7, female, partner; Patient 5g, female, aged 36–60 years)
Assessment plus numerical result was given in five (22%) consultations:
GP: ‘So that was normal, it was 2.7.’
Patient: ‘Right.’
(Practice 2, GP1, female, salaried; Patient 9a, female, aged 36–60 years)
Multiple results, using a combination of the above, were given in four (17%) consultations:
‘Thyroid blood test is normal. Your haemoglobin is a little low but it has improved compared to the last blood test. It’s now 112, the last time that it was taken was actually 9.4 so was a lot lower. The important thing is your iron, your iron is quite low. Your iron is 10, in a normal individual iron is 30 ideally 40.’
(Practice 2, GP2, female, salaried; Patient 15b, female, aged 18–35 years)
Imparting understanding about results
In 13 (57%) of the 23 ‘results’ consultations, the GP gave no explanation as to why the test was done and in no consultation did the GP explain any risks or limitations of the test.
Table 4 summarises the explanation of results. In six (26%) ‘results’ consultations, the GP gave no explanation of what the result meant for the patient (for example, aetiology or diagnosis) beyond a simple assessment (for example, ‘high’, ‘low’, ‘normal’):
GP: ‘Because you’ve had the blood test done that I asked for, haven’t you?’
Patient: ‘I did, not long ago.’
GP: ‘Which were all normal, and they were going to do the flu jab at the same time …’
(Practice 5, GP7, female, partner; Patient 1g, female, aged >60 years)
Table 4.
Explanation of results in consultations
| Code | Consultation (N = 23) | |
|---|---|---|
| n | % | |
| GP gives no explanation of what the result meant for the patient (for example, aetiology or diagnosis), beyond a simple assessment (for example, ’high’, ‘low’, ‘normal’) | 6 | 26 |
| GP gives an explanation of some of the results conveyed, but not others | 10 | 44 |
| GP gives some explanation of all results mentioned, either individually or as a collectivea | 7 | 30 |
This does not account for results that may not have been mentioned at all.
In 10 (44%) ‘results’ consultations, the GP gave an explanation of some of the results conveyed, but not others:
GP: ‘[…] liver test was normal, and your kidney test was good. Your blood sugar is fine and your thyroid is all right, so you’re on the right dose of thyroxine.’
Patient: ‘Right, that’s good.’
GP: ‘[…] your haemoglobin was just slightly lower than it has been. […] Well, I think the dilemma always is, is your anaemia due to the fact that you just don’t absorb iron very well? Which some people don’t, and it sounds like that’s how you’ve always been, doesn’t it?’
Patient: ‘Yes.’
GP: ‘Or is it that you’re losing blood from somewhere else? So what we sometimes do when people have an unexplained anaemia is we investigate your bowels.’
(Practice 3, GP3, female, partner; Patient 1c, female, aged 36–60 years)
In 7 (30%) ‘results’ consultations, the GP gave some explanation of all results mentioned either individually or as a collective:
‘So the blood tests all came back as normal. So […] we checked your blood count. We checked your kidney and liver function. We checked you for gluten intolerance and any signs of infection and that was all normal. […] So we get to the situation where we haven’t found an obvious cause for it.’
(Practice 5, GP6, male partner; Patient 12f, female, >60 years)
Table 5 describes the questions asked or offered about results. Examples are given below. In 10 (43%) ‘results’ consultations, the patient did not ask questions about the result, nor did the GP offer the opportunity for the patient to do so.
Table 5.
Questions asked or offered about results in consultations
| Code | Consultation (N = 23) | |
|---|---|---|
| n | % | |
| No questions offered by GP or asked by patient | 10 | 43 |
| GP explicitly offers patient opportunity to ask questions about blood test | 1 | 4 |
| GP offers the opportunity to ask questions about blood test less explicitly | 2 | 9 |
| GP offers general opportunity to ask questions at later time | 1 | 4 |
| Patient asks questions without being prompted | 9 | 39 |
In one (4%) consultation the GP explicitly offered the patient the opportunity to ask questions about the blood test:
‘Your inflammatory marker was really raised and then it came down … It showed that things were starting to settle but because it’s not back to normal it’s important that we repeat that blood test to make sure it goes back to baseline and that hasn’t been persistently up … I will add that to the bloods as well. Is there anything else you want to ask me?’
(Practice 2, GP 2, female, salaried; Patient 10b, male, aged 36–60 years)
In two (9%) consultations the GP offered the opportunity to ask questions about the blood test less explicitly:
GP: ‘We checked the vitamin D level. Technically, this is slightly low, but it’s certainly an adequate level of vitamin D. It doesn’t explain your symptoms, OK?’
Patient: ‘Right.’
(Practice 9, GP15, male, partner; Patient 1o, female, aged >60 years)
In one (4%) consultation the GP offered general opportunity to ask questions at a later time:
‘Any further questions?’
(Practice 2, GP2, female, salaried; Patient 17b, female, age unknown)
Nine (39%) patients asked questions about the result of the blood test without being prompted by the GP:
GP: ‘Well, the one we were looking at is this one here, called the ALT, it’s a type of enzyme which is in your liver, and when we looked at it before, in November, it was 249, and it came down to 103. Now, we’re trying to get it down to 40.
Patient: ‘Yes. What has caused it to come down?’
(Practice 7, GP12, male, partner; Patient 5l, female, aged >60 years)
DISCUSSION
Summary
Perhaps unsurprisingly, GPs initiate the majority of blood testing, and there is a lack of information giving and SDM surrounding ordering tests and conveying results. There were no examples of patients explicitly requesting blood tests. However, patients often hinted that they wanted a blood test, which reflects previous literature suggesting that patients may preferentially use implicit or indirect requests to prompt the doctor to offer an action, rather than explicitly asking, and that negotiations between doctors and patients are complex and subtle.22,23
SDM requires the patient to be given options, yet rarely were patients explicitly offered more than one option, including the option to not have a blood test. Arguably, every time a blood test is offered, the patient should at least be explicitly offered the opportunity to decline. Where options exist, information giving is crucial within SDM, as well as mandated by the General Medical Council.24 Only a minority of patients were informed about all tests ordered, at least to the level of test group or organ; this has implications, not only for SDM but also, more fundamentally, for informed consent. It is impossible to create a universal standard for how much information is enough, given variable patient health literacy, willingness to engage, and logistical factors, such as time; however, although there was usually some explanation of the indications for testing, explanations were inarguably sparse.
Blood tests are not without risk, namely the possibility of false positives, leading to further investigations and associated iatrogenic harms, and false negatives, propagating unjustified reassurance. It has been suggested that informing patients about the risks and limitations of tests could reduce rates of tests that are unlikely to confer any benefit.5 Despite this, GPs rarely touched on the limitations of tests before they were ordered, and never mentioned limitations when conveying results. Consultations in which PSA testing was discussed exhibited more thorough explanations of limitations and explicit offers of options than other consultations, perhaps due to the National Screening Committee’s Prostate Cancer Risk Management Programme promoting informed choices about PSA testing, supported by a decision aid.25 It could be argued that PSA is a ‘special case’ as it confers a sizeable risk of future unnecessary, invasive investigations, so discussion is more important, and, by nature of it often being a standalone test, more time is available for this. However, any spurious abnormal blood test result could trigger a cascade of invasive investigations, and these examples indicate that more thorough explanation of blood test limitations is possible in primary care.
Frequently, patients did not ask questions about tests being ordered or results being discussed, nor did the GP offer them the opportunity to do so; this not only indicates to the patient that their participation is not important in the decision making process but also limits patient education. Positively, some patients felt comfortable asking questions without being prompted, indicating that patients are keen to understand and expected more information than was provided. It is likely that there are more patients with questions who are not confident enough to ask.
It has been suggested that giving patients only a simple assessment of a result (for example, ‘high’, ‘low’), rather than the result itself, is an example of paternalism.11 Despite this, the majority of patients were given only an assessment, in some cases accompanied by no explanation of what the result meant. Giving patients raw results, accompanied by an assessment and explanation, along with context and limitations of the test, could allow patients to be more proactive in interpreting results and promote patient engagement in monitoring their health.11 Arguably, SDM should be employed not only in the decision to test but also in the interpretation of the results. For example, where difficulties arise in how to proceed with borderline results, patients may express a strong preference if assisted to understand results themselves.
Strengths and limitations
This is the first UK study to use naturalistic data to examine discussions of blood tests in primary care. It did not rely on doctor or patient recall of consultation content. Where previous studies were limited to assessing SDM according to specific scales or surveys, such as the OPTION scale,4 this study was able to adapt the analysis to what was observed in the consultations.
Data were limited to that collected for the OiaM archive; the patient pre-visit expectations data recorded whether patients expected tests or investigations, not blood tests specifically. Access to laboratory reports of the results being discussed were not available. Therefore, the authors were unable to identify discrepancies between actual results and what the patient was told. It was not possible to identify occasions where results were not conveyed to the patient at all, or were conveyed by an alternative means; a significant proportion of ‘normal’ results are conveyed over the phone by non-medical staff or by text message, and this was not recorded. Although GPs and patients were aware they were being filmed at the time of data collection, neither party knew the aims of this particular study, so were therefore less likely to have altered their behaviour in relation to discussion around testing. It was not possible to assess reported patient understanding or preferences for SDM in blood testing, specifically, as these data were not collected as part of the original study.
There was an element of subjectivity in some of the codes, such as whether the patient ‘hinted’; however, the coding scheme minimised this with thorough rules and double-coding to improve reliability. Analysis was carried out by a junior doctor, an academic GP, and a qualitative researcher, who had their own prior experiences of blood-testing discussions from both clinician and patient perspectives, which could influence their reflexivity.
Comparison with existing literature
Keitz et al used 200 audio-recordings of primary care consultations in the US to examine modes of negotiation between patients, with expectations for tests, medications, or referrals, and their primary care physicians.10 In contrast with the findings of this study that no patients explicitly requested a blood test, they found nearly half of patient expectations were expressed by direct patient request, and patient requests altered the outcome of nearly half of consultations in which they were made.10 This may reflect cultural differences between UK and US doctor–patient relationships. Pomerantz et al used a sample of 33 video-recordings of consultations in ambulatory clinics in the US to examine how test results were conveyed to patients, and whether this was paternalistic or promoted the patient as an independent expert.11 They identified only four consultations discussing results, of which none were blood tests, although they noted a spectrum of doctors’ reporting practices, ranging from offering patients assessments of results (for example, ‘normal’) only, to sharing numerical results only. They suggest that the former is an example of paternalism, and the latter proposes the patient as an independent expert. Results of this study reflect this range of reporting practices; however, the study identified that the majority were informed of an assessment only. This supports the findings of Kurhila et al, who used 7.5 hours of video-recorded interactions in a Finnish hospital to examine how nurses adapt their talk about numerical results depending on the recipient and activity.26 They found nurses tend to provide patients with qualitative assessments of numerical results, yet they provide doctors with numerical information about results. A study of 212 video-recorded primary care consultations in England examined the degree to which doctors met their patients’ preferences for involvement in decisions. It identified that 91% of decisions about investigations were doctor led, compared with 62% of decisions about referrals or procedures, and doctors showed variable ability in adapting the decision making process to their patients’ preferences.12 There is evidence that providing patients with thorough information about treatment options and a structured opportunity to discuss their preferences leads to higher patient satisfaction, reduced rates of intervention, and lower costs.27 Despite this, research suggests that SDM is not widely implemented,3 particularly for decisions about investigations.12 This supports the results that identify a lack of SDM and information sharing regarding decisions around blood testing.
Implications for research and practice
This research has identified room for improvement in information giving and SDM in blood testing in primary care. Discussions around PSA testing emerged as an example of more thorough information giving, and highlighted that SDM for blood tests is possible in primary care. However, implementing SDM is not without barriers; in one study, GP consultations lasted 50% longer following interventions to improve SDM and risk communication.28 Debate exists about whether SDM is appropriate for all decisions, with some arguing it is only appropriate where there are multiple genuine options, and some patients may decline to be involved regardless.29 Despite this, when the opportunity for blood testing arises, it is appropriate to at least offer the patient information, an opportunity to ask questions, and multiple options, even if those options are whether to test or not. Evidence suggests when patients are presented with comprehensive information about risks and benefits of treatments they are more inclined to opt for conservative management than doctors.3 A Cochrane review found that use of decision aids reduced the number of patients choosing PSA screening.30 This effect was not seen for most other testing and screening choices; however, it suggests that promoting SDM does not uniformly increase time and spending.
There is growing awareness of the importance of SDM in treatment decisions; however, less research exists about SDM for investigations. A study using semi-structured interviews with GPs identified that GPs considered ordering investigations to be a biomedical decision, which allowed the clinician to display their medical authority and were not appropriate for SDM.31 Future research should focus on attitudes towards SDM in testing between both patients and doctors, as well as exploring time and monetary implications of SDM in testing, and the role for SDM aids.
Funding
No specific funding was obtained for this study.
Ethical approval
Ethical approval for this study was given by the West Midlands — Coventry & Warwickshire Research Ethics Committee (Ref: 18/WM/0241).
Provenance
Freely submitted; externally peer reviewed.
Competing interests
The authors have declared no competing interests.
Discuss this article
Contribute and read comments about this article: bjgp.org/letters
REFERENCES
- 1.Barry MJ, Edgman-Levitan S. Shared decision making — pinnacle of patient-centered care. N Engl J Med. 2012 doi: 10.1056/NEJMp1109283.. [DOI] [PubMed] [Google Scholar]
- 2.King E, Taylor J, Williams R, Vanson T. The MAGIC programme: evaluation. London: Health Foundation; 2013. https://www.health.org.uk/sites/default/files/TheMagicProgrammeEvaluation.pdf (accessed 26 Mar 2020). [Google Scholar]
- 3.Coulter A, Collins A. Making shared decision-making a reality: no decision about me, without me. London: King’s Fund; 2011. https://www.kingsfund.org.uk/sites/default/files/Making-shared-decision-making-a-reality-paper-Angela-Coulter-Alf-Collins-July-2011_0.pdf (accessed 26 Feb 2020). [Google Scholar]
- 4.Da Silva D. Evidence: helping people share decision making. London: Health Foundation; 2012. https://www.health.org.uk/sites/default/files/HelpingPeopleShareDecisionMaking.pdf (accessed 26 Mar 2020). [Google Scholar]
- 5.Hoffmann TC, Del Mar C. Patients’ expectations of the benefits and harms of treatments, screening, and tests: a systematic review. JAMA Intern Med. 2015 doi: 10.1001/jamainternmed.2014.6016.. [DOI] [PubMed] [Google Scholar]
- 6.van Bokhoven MA, Pleunis-van Empel MC, Koch H, et al. Why do patients want to have their blood tested? A qualitative study of patient expectations in general practice. BMC Fam Pract. 2006;7:75. doi: 10.1186/1471-2296-7-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.O’Sullivan JW, Stevens S, Hobbs FDR, et al. Temporal trends in use of tests in UK primary care, 2000–15: retrospective analysis of 250 million tests. BMJ. 2018 doi: 10.1136/bmj.k4666.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hobbs FDR, Bankhead C, Mukhtar T, et al. Clinical workload in UK primary care: a retrospective analysis of 100 million consultations in England, 2007–14. Lancet. 2016;387(10035):2323–2330. doi: 10.1016/S0140-6736(16)00620-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.NHS England . Five year forward view. London: NHS England; 2014. https://www.england.nhs.uk/wp-content/uploads/2014/10/5yfv-web.pdf (accessed 26 Mar 2020). [Google Scholar]
- 10.Keitz SA, Stechuchak KM, Grambow SC, et al. Behind closed doors: management of patient expectations in primary care practices. Arch Intern Med. 2007;167(5):445–452. doi: 10.1001/archinte.167.5.445. [DOI] [PubMed] [Google Scholar]
- 11.Pomerantz A, Rintel ES. Practices for reporting and responding to test results during medical consultations: enacting the roles of paternalism and independent expertise. Discourse Stud. 2004 doi: 10.1177/1461445604039437. [DOI]
- 12.Ford S, Schofield T, Hope T. Observing decision-making in the general practice consultation: who makes which decisions? Health Expect. 2006;9(2):130–137. doi: 10.1111/j.1369-7625.2006.00382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Couet N, Desroches S, Robitaille H, et al. Assessments of the extent to which healthcare providers involve patients in decision making: a systematic review of studies using the OPTION instrument. Health Expect. 2015 doi: 10.1111/hex.12054.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zikmund-Fisher BJ, Couper MP, Singer E, et al. Deficits and variations in patients’ experience with making 9 common medical decisions: the DECISIONS survey. Med Decis Making. 2010 doi: 10.1177/0272989X10380466.. [DOI] [PubMed] [Google Scholar]
- 15.Michiels-Corsten M, Donner-Banzhoff N. Beyond accuracy: hidden motives in diagnostic testing. Fam Pract. 2017 doi: 10.1093/fampra/cmx089.. [DOI] [PubMed] [Google Scholar]
- 16.Watson J, de Salis I, Banks J, Salisbury C. What do tests do for doctors? A qualitative study of blood testing in UK primary care. Fam Pract. 2017;34(6):735–739. doi: 10.1093/fampra/cmx051. [DOI] [PubMed] [Google Scholar]
- 17.Little P, Dorward M, Warner G, et al. Importance of patient pressure and perceived pressure and perceived medical need for investigations, referral, and prescribing in primary care: nested observational study. BMJ. 2004;328(7437):444. doi: 10.1136/bmj.38013.644086.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Litchfield I, Bentham L, Lilford R, et al. Test result communication in primary care: a survey of current practice. BMJ Qual Saf. 2015 doi: 10.1136/bmjqs-2014-003712.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barnes RK. One in a Million: a study of primary care consultations. University of Bristol. 2017 doi: 10.5523/bris.l3sq4s0w66ln1x20sye7s47wv.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jepson M, Salisbury C, Ridd MJ, et al. The ‘One in a Million’ study: creating a database of UK primary care consultations. Br J Gen Pract. 2017 doi: 10.3399/bjgp17X690521. [DOI] [PMC free article] [PubMed]
- 21.Elo S, Kyngäs H. The qualitative content analysis process. J Adv Nurs. 2008 doi: 10.1111/j.1365-2648.2007.04569.x.. [DOI] [PubMed] [Google Scholar]
- 22.Gill VT, Halkowski T, Roberts F. Accomplishing a request without making one: a single case analysis of a primary care visit. Interdisciplinary J Stud Discourse. 2001;21(1–2):55–81. [Google Scholar]
- 23.Pomerantz A, Heritage J. Preference. In: Sindell J, Stivers T, editors. The handbook of conversation analysis. Oxford: Wiley-Blackwell; 2012. pp. 210–228. [DOI] [Google Scholar]
- 24.General Medical Council . Consent: patients and doctors making decisions together. London: GMC; 2008. https://www.gmc-uk.org/ethical-guidance/ethical-guidance-for-doctors/consent (accessed 26 Mar 2020). [Google Scholar]
- 25.Public Health England . Prostate cancer risk management programme (PCRMP): benefits and risks of PSA testing. London: PHE; 2016. https://www.gov.uk/government/publications/prostate-cancer-risk-management-programme-psa-test-benefits-and-risks/prostate-cancer-risk-management-programme-pcrmp-benefits-and-risks-of-psa-testing (accessed 26 Mar 2020). [Google Scholar]
- 26.Kurhila S, Lehtimaja I. Dealing with numbers: nurses informing doctors and patients about test results. Discourse Stud. 2018 doi: 10.1177/1461445618802662. [DOI]
- 27.Kennedy AD, Sculpher MJ, Coulter A, et al. Effects of decision aids for menorrhagia on treatment choices, health outcomes, and costs: a randomized controlled trial. JAMA. 2002;288(21):2701–2708. doi: 10.1001/jama.288.21.2701. [DOI] [PubMed] [Google Scholar]
- 28.Elwyn G, Edwards A, Hood K, et al. Achieving involvement: process outcomes from a cluster randomized trial of shared decision making skill development and use of risk communication aids in general practice. Fam Pract. 2004;21(4):337–346. doi: 10.1093/fampra/cmh401. [DOI] [PubMed] [Google Scholar]
- 29.Elwyn G, Hutchings H, Edwards A, et al. The OPTION scale: measuring the extent that clinicians involve patients in decision-making tasks. Health Expect. 2005;8(1):34–42. doi: 10.1111/j.1369-7625.2004.00311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stacey D, Légaré F, Lewis K, et al. Decision aids for people facing health treatment or screening decisions. Cochrane Database Syst Rev. 2017;(4):CD001431. doi: 10.1002/14651858.CD001431.pub5.. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.O’Flynn N, Britten N. Does the achievement of medical identity limit the ability of primary care practitioners to be patient-centred? A qualitative study. Patient Educ Couns. 2006;60(1):49–56. doi: 10.1016/j.pec.2004.12.002. [DOI] [PubMed] [Google Scholar]