Abstract
The recent discovery of lymphatic vessels in the meningeal layers calls into question the known mechanisms of fluid and macromolecule homeostasis and immunoregulation within the central nervous system. These meningeal lymphatic vessels and their potential role in the pathophysiology of neurological disease have become a rapidly expanding area of research, with the hopes that they may provide a novel therapeutic target in the treatment of many devastating conditions. This article reviews the current state of knowledge surrounding the anatomical structure of the vessels, their functions in fluid and solute transport and immune surveillance, as well as their studied developmental biology, relationship with the novel hypothesized “glymphatic” system, and implications in neurodegenerative disease in animal models. Furthermore, this review summarizes findings from the human studies conducted thus far regarding the presence, anatomy, and drainage patterns of meningeal lymphatic vessels and discusses, from a clinical perspective, advancements in both imaging technologies and interventional methodologies used to access ultrafine peripheral lymphatic vessels.
Keywords: Alzheimer, multiple sclerosis, neurodegeneration, neurodegenerative, neuroimmunology
Introduction
Classically, peripheral lymphatics have been implicated in homeostatic mechanisms involving waste and immune cell regulation, mechanisms which have been found to be compromised in a number of neurodegenerative, vascular, traumatic, and inflammatory diseases affecting the central nervous system (CNS). Despite this, lymphatics have been long thought to be absent in the CNS. However, recent findings of lymphatic vessels in the meningeal layers have upended this notion.1,2 A greater understanding of these meningeal lymphatics could play a pivotal role in treatment for many neurological conditions including Alzheimer disease (AD), multiple sclerosis (MS), hydrocephalus, traumatic brain injury, epilepsy, Parkinson disease (PD), and stroke.3-7 Down the line, as knowledge regarding this novel lymphatic network and its implication in disease continues to be uncovered, concepts underlying peripherally used lymphatic imaging modalities and methods of accessing vessels such as these of microscopic scale may be of value for application in the CNS. This article provides a review of the current understanding of lymphatic vessels localized within the meningeal layers to aid in education regarding the state of the field and discusses modern clinical lymphatic imaging and intervention modalities.
Background on Lymphatics
Overview of the lymphatic system
The lymphatic system is a unidirectional transport system of fluid and macromolecules from tissues to venous circulation. Vessels are structured for high permeability and absorption in tissue, and unidirectional propulsion of lymph flow away from tissues by larger vessels. This structure facilitates lymphatic function in immune surveillance, lipid absorption in the intestines, and maintenance of tissue fluid balance.8,9 Lymphatics begin as blind ended capillaries in the interstitial spaces of vascular tissues with a diameter of 10 to 60 µm.10 They absorb fluid, macromolecules, and cells into the lymph, a process driven by interstitial fluid (ISF) pressure gradients produced by muscle contraction and arterial pulsation.5 The lymphatic capillaries drain into larger pre-collecting and subsequently collecting lymphatic vessels, which facilitate unidirectional flow of lymph toward venous circulation. The larger collecting vessels are supported by a basement membrane, contain one-way, semilunar valves demarcated by characteristic integrin α9, and are lined by a smooth muscle cell (SMC) layer for flow propulsion.5,8,10
The lymphatic system is characterized by a number of distinct markers including PROX1, VEGFR3, CCL21, LYVE1, PDPN, and CD31. These markers serve an important role in identifying lymphatic vessels, mapping the architecture and functions of the lymphatic system, and characterizing lymphangiogenesis.1,4 Patterns of VE-cadherin and Claudin-5 expression exhibited by lymphatic vessels are used to characterize the quality of cell-cell junctions and are important for differentiating between initial or collecting lymphatic vessels.11
The “glymphatic system”
The mechanisms by which the brain clears fluid and solute waste have long been subjects of continuous exploration. Prior to 2012, there were no discrete perivascular pathways defined in the brain for ISF and solute clearance. It was known that cerebrospinal fluid (CSF) containing ISF waste drained into the cervical lymphatics, but the pathway was incompletely elucidated.12 In 2012, the hypothesis of a brain-wide, astrocyte-mediated pathway for fluid and solute transport out of the brain parenchyma and into the CSF was proposed. This system was termed the “glymphatic system.”13,14
The “glymphatic system” regulates the flux fluid and macromolecules occur through perivascular spaces called Virchow Robinson spaces. These spaces exist between the vasculature of the brain and the glia limitans layer composed of astrocyte endfeet and are continuations of the CSF-filled subarachnoid space (SAS).4 The high concentration of aquaporin channels (AQP4) contained within the astrocyte endfeet mediates fluid exchange between the brain parenchyma and the CSF. The mechanisms of flow and molecular transport are highly contested and require further exploration, but are theorized to be driven by arterial pulsation.15-17 Regulated by the glymphatic system, CSF is thought to enter the brain parenchyma through the periarterial spaces, mix with the ISF, and re-enter the SAS in the perivenular spaces, carrying parenchymal waste. From there, CNS waste absorption is thought to occur with the CSF into the dural sinuses via arachnoid granulations or into the cervical lymphatics via the cribriform plate.14,18,19
More recently in 2015, meningeal lymphatic vessels were found coursing within murine meninges. These vessels were determined to provide a direct pathway to the deep cervical lymph nodes (dCLNs), suggesting they may provide functional lymphatic capacity to the CNS. Their role in fluid and macromolecule homeostasis and immunoregulation is a rapidly expanding field of study.1,2 The current knowledge surrounding the structure and function of meningeal lymphatics, their role in neurological disease, and therapeutic potential for interventions is reviewed in the following sections.
Methods
A review of the literature was performed to identify current knowledge surrounding the localization and function of meningeal lymphatic vessels, as well as novel findings surrounding their role in the pathophysiology of neurodegenerative disease. The review was performed by author KH using the PubMed database through March 1, 2019. PubMed was searched with the following search terms: (meningeal[All Fields] OR dural[All Fields]) AND (“lymphatic vessels”[MeSH Terms] OR (“lymphatic”[All Fields] AND “vessels”[All Fields]) OR “lymphatic vessels”[All Fields] OR “lymphatics”[All Fields] OR “lymphatic system”[MeSH Terms] OR (“lymphatic”[All Fields] AND “system”[All Fields]) OR “lymphatic system”[All Fields]).
The primary search yielded 226 items. The references of sources found were also reviewed. The search was limited to English manuscripts. The search did not have a specified start date and was conducted through March 1, 2019. References were included based on meningeal lymphatics as the primary focus of the manuscript.
Meningeal Lymphatics
Rodent studies
The meningeal lymphatic vessels were discovered when high concentrations of immune cells were found adjacent to dural sinuses throughout murine meninges, contained within CD31+ structures with a diameter of 20 to 30 µm. These perivascular vessels tested positive for classic lymphatic markers, which led to their hypothesized function in CNS waste drainage and immune cell trafficking.1,2 Structurally, the meningeal lymphatic vessels resemble initial lymphatic capillaries with a spaced pattern of cell junction markers, a noncontinuous basement membrane, no lymphatic valves, and no SMC lining.2
This extensive network of lymphatics provides drainage pathways throughout the cranial and spinal meninges, along arteries, veins, and cranial nerve sheaths, through foramina at the skull base and the cribriform plate to the CLNs.1,2,20 In the anterior cranium, lymphatics were found in the dura covering the frontal bone and overlying the olfactory bulb beside the rostral rhinal veins. Vessels were also associated with the anterior and middle meningeal arteries. In the posterior, inferior cranium, lymphatics were most highly concentrated and complex, with sparse valve containing vessels present. Lymphatics were visualized along the sigmoid sinus tracking superiorly along the retroglenoid vein (a dorsal, murine continuation of the transverse sinus) and the transverse sinus, to the confluence of sinuses, and to the rostral portion of the superior sagittal sinus and continuing anteriorly. Vessels associated with the transverse sinus were found to be larger and more complex when compared with the vessels surrounding the superior sagittal sinus.1,3 Lymphatics were seen in the dural covering of the cerebellum, associated with branches of the transverse sinus.3 Furthermore, initial findings of lymphatic vessels in the meningeal septae penetrating the cerebral cortex were recently reported.21 Vessels seen exiting through foramina at the base of the skull were associated with the meningeal pterygopalatine artery (a murine branch of the internal carotid artery), the sigmoid sinus, the retroglenoid vein, and cranial nerves.1,3
Lymphatics were found to track with the olfactory (CN I), optic (CN II), trigeminal (CN V), glossopharyngeal (CN IX), vagus (CN X), and accessory (CN XI) nerve sheaths.1,3,22 Lymphatic vessels exited the skull through the jugular foramen along with CN IX, X, and XI, toward the dCLNs.3,22 The olfactory nerve associated lymphatics tracked through the cribriform plate, along the olfactory bulb before aligning with the sinuses.2 Lymphatics from the nasal cavity tracked through the palate, to a plexus on the pharynx, and to the dCLNs. Orbit associated lymphatics exited the orbit and flowed into a common collecting vessel from the nasal region that coursed along the anterior facial vein toward the mandibular lymph nodes. Further drainage was seen along the facial nerve (CN VII) through the stylomastoid foramen coursing toward the mandibular and dCLNs.22
Meningeal lymphatics have been further traced to the spinal cord meninges. The spinal lymphatic vessels were found to extend caudally from vessels exiting the foramen magnum. On the anterior spinal cord, they concentrated in meningeal tissues covering the intervertebral disk and did not meet at the midline. However, on the dorsal side, they extended to the midline and concentrated along the interspinous ligament. They demonstrated lateral branches associated with spinal nerves and blood vessels.3
The localization of the lymphatics within the meningeal layers is a subject of continued investigation. The vessels have been found to be contained within the dura mater in rodent, nonhuman primate, and human specimens.1,20 Further anatomical evidence for this localization included their association with the calvaria and the dura-associated vasculature, including the middle meningeal arteries and venous sinuses.1,23 However, this localization remains an area for further research due to the question of CSF access from the dura mater.23,24
Developmental biology
Most of the lymphatic vessels in mice were found to develop postnatally in a VEGF-c-dependent fashion in the first 3 to 4 weeks of life, excluding sparse lymphatic vessels visible at birth surrounding the pterygopalatine artery.3,25 The first lymphatic vessels were seen surrounding vasculature and cranial nerves at the skull base, penetrating the foramen magnum. The vessels developed in the anterior, superior direction along dural blood vessels. The vessels surrounding the jugular veins were the first to develop a direct connection with the dCLNs, and increasing amounts of valves were visible in these vessels throughout the first 3 weeks. Vessels underlying frontal bone and overlying the olfactory bulb associated with the rostral rhinal veins were observed before the anteriorly extending vessels surrounding the superior sagittal sinus reached the rostral area, indicating that the posterior and anterior lymphatic networks developed via separate patterns of growth. The spinal vessels developed simultaneously along spinal nerves and blood vessels, contrary to the posterior-anterior oriented development seen in the cranium. Cervical spinal lymphatics became continuous with lymphatics at the skull base by the end of postnatal week 1, and the pattern of lymphatics along the spine became uniform by the end of postnatal week 2.3
Fluid and solute transport
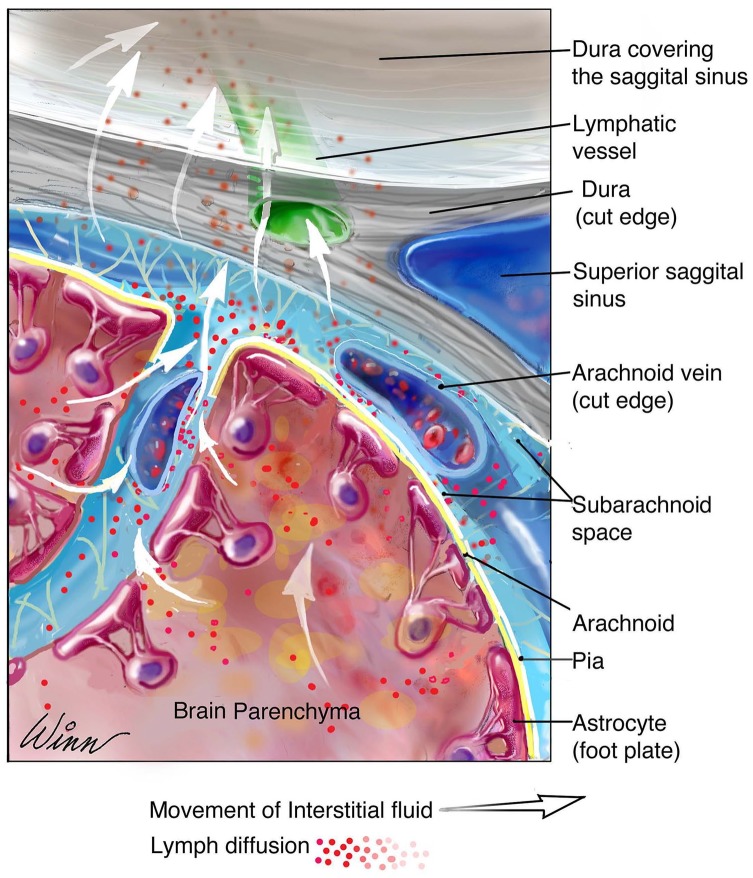
The functions of the meningeal lymphatic vessels have primarily been characterized in rodent models. Given the well-studied function of peripheral lymphatics as regulators of tissue fluid homeostasis and immune surveillance, these were main functions assessed in meningeal lymphatics. Assessment of the overall significance of meningeal lymphatic vessels in ISF clearance demonstrated no appreciable difference in brain fluid content and ISF pressures when vessels were absent. Therefore, it was concluded that these vessels are not the primary modulators of fluid and solute homeostasis in the brain.1 However, further experimentation revealed decreased perfusion of CSF throughout the brain parenchyma in mouse models with ablated lymphatics, a function thought to be regulated by the glymphatic system. From this finding, meningeal lymphatics were theorized to serve as continuations following the glymphatic system in CNS lymphatic drainage.4,26 Examination of CSF drainage pathways into the meningeal lymphatics from the SAS revealed the presence of lymphatic entry extensions, which demonstrated a spaced pattern of VE-cadherin and Claudin-5, concentrated in the areas surrounding the transverse sinus and the olfactory bulb. Although these extensions appeared to provide direct meningeal lymphatic CSF access, whether or not they penetrate the SAS remains to be confirmed.24 Peripheral lymphatic sprouting patterns have been observed similar to this model (Figure 1).11
Figure 1.
The drainage pathway of interstitial fluid from the brain parenchyma to the meningeal lymphatic system. The glymphatic system regulates the flux of interstitial fluid from the brain parenchyma into perivascular spaces, continuous with the CSF containing subarachnoid space. CSF containing interstitial fluid is absorbed into meningeal lymphatic vessels associated with dural blood vessels. CSF indicates cerebrospinal fluid.
The dCLNs and superficial cervical lymph nodes (sCLNs) have been shown to be the primary lymphatic drainage sites for the CNS.12,22,27 The respective contributions of the known major lymphatic outflow pathways, the meningeal lymphatics, and the nasal lymphatics were evaluated. The meningeal lymphatics drained primarily to the dCLNs, with less prominent and rapid contributions to the sCLNs.1,2 Conversely, the nasal lymphatics drained primarily to the sCLNs, and their interruption did not significantly effect overall dCLN drainage.2,4 Murine models lacking meningeal lymphatics demonstrated macromolecule accumulation in the meninges, with no appreciable drainage to the dCLNs, further indicating a significant role in drainage to the dCLNs.1 The time course of macromolecule clearance from the SAS to the dCLNs was evaluated in rats. Clearance to the dCLNs was first observed at 3 hours, followed by a maximum concentration more than 24 hours and a total clearance after 12 days.28 Functional assessment of flow in meningeal lymphatic vessels demonstrated a congruent direction of flow, but slower flow rate of lymph compared to the associated blood vessels, consistent with trends seen in peripheral lymphatics.2,29
Immune surveillance
Immune cells in the meningeal tissues provide immune surveillance in the brain. Meningeal lymphatics and their direct connection with the dCLNs have been shown to impact CNS immune cell homeostasis.24 When initially assessed for their overall capacity to carry immune cells, the meningeal lymphatics were found to contain approximately 24% of all sinusal T cells and approximately 12% of sinusal antigen presenting cells (APCs), indicating a prominent role in CNS immune cell trafficking.2 Both meningeal and intraventricularly injected immune cells have been observed accumulating within meningeal lymphatic vessels and at hypothesized entry extensions around the transverse sinus, suggesting that meningeal lymphatics function in trafficking both meningeal and CSF contained immune cells.2,24 Further evaluation of immune cell migration within meningeal lymphatics demonstrated a pattern of CCR7-CCL21 dependence, similar to peripheral lymphatics.
Interruption of the meningeal lymphatic drainage pathway to the dCLNs has been found to lead to immune cell accumulation in the meninges and resulted in an overall decrease in cellular trafficking into the dCLNs. However, ligation of the lymphatics to the sCLNs did not significantly affect drainage, suggesting an alternate route for immune cells that traffic to these nodes.24
Human studies
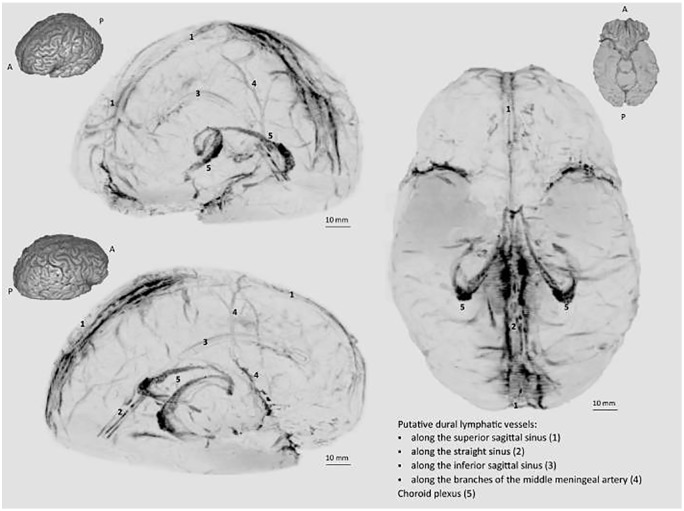
The existence of meningeal lymphatics in vivo has been confirmed in 5 healthy human volunteers and 3 nonhuman primates using magnetic resonance imaging (MRI). The lymphatics were reported to fit the murine distribution findings and were seen in association with the superior sagittal and straight sinuses, as well as with the middle meningeal arteries. The vessels visualized were thought to be ducts as lymphatic capillaries would likely not be revealed by MRI due to size. In autopsy sections of human meninges, 93 lymphatic vessels were identified. These vessels were found to be contained within the dura mater and displayed a wide range of diameters, ranging from 7 to 842 µm (Figure 2).20 This is much larger than the previously determined murine meningeal lymphatic diameter of 20 to 30 µm.1,2
Figure 2.
A schematic of human meningeal lymphatic vessels, adapted from skull-stripped subtraction T1-black-blood imaging.
Source: Reprinted from Absinta et al.20
Vessel location within the dura mater was also observed in a study of autopsy samples from 4 otherwise healthy human samples. They were found to be highly concentrated along the superior sagittal sinus and observed morphology was consistent with that of peripheral lymphatics.30 The dural localization was further confirmed in a study of both the periosteal and meningeal dura mater of human autopsy specimens in 6 of 6 subjects with AD, 9 of 10 subjects with neurological disease or dementia, and 4 of 5 control subjects. This study found >5 lymphatic vessels associated with the superior sagittal sinus, whereas murine models typically demonstrated 2. The diameters of lymphatic vessels seen also demonstrated a large range, from 19 to 470 µm.31
Human CSF drainage to the CLNs has been characterized in vivo through MRI visualization of intrathecally injected tracer in 19 subjects with CSF disorders. Peak CLN enhancement was seen 24 hours after injection in 15 out of 19 individuals and coincided with glymphatic enhancement. This supports the theory that meningeal lymphatic vessels serve as the next step in CNS drainage following the glymphatic system. Peak glymphatic enhancement occurred proximally to the leptomeningeal arterial trunks, supporting arterial pulsation as a driving force behind glymphatic flow between brain parenchyma and perivascular spaces. The findings in this study also indicated a more significant role of meningeal lymphatics than nasal lymphatics in CSF drainage to CLNs due to minimal early enhancement of sCLNs.32
Further functional analysis of lymph flow patterns in meningeal lymphatic vessels was performed using MRI in 6 healthy human volunteers. The study assessed vessels adjacent to the superior sagittal sinus and found that flow tracked countercurrent to venous flow, posterior to anterior, toward the cribriform plate.33 These findings of flow directionality differ from that observed in a murine model and the parallel direction of flow often observed peripherally.2 Although, these data are concurrent with findings of immune cell trafficking pathways between the CNS and the CLNs via the nasal route in rodents.12,34
Impact on Disease
Alzheimer disease
Aging, amyloid-β, and tau protein deposition have been widely recognized as major risk factors for AD.35-38 Furthermore, decreased CSF recirculation throughout the aging brain has been implicated in amyloid-β pathology.14,39,40 As it is also known that peripheral lymphatics demonstrate decreased function with age,41-43 testing to assess the impact of aging on meningeal lymphatics in mouse models has been performed.4,22
Murine meningeal lymphatic vessels exhibited decreased vessel diameter and network dispersion with age. Overall, CSF drainage to the dCLNs was reduced and brain-wide perfusion with CSF was significantly decreased. The effects of aging-associated meningeal lymphatic dysfunction were analyzed for significance in amyloid-β and tau protein deposition in AD models. Mice with ablated meningeal lymphatics showed amyloid-β accumulation in the meninges, increased macrophage recruitment to plaque sites, and an increase in amyloid-β deposition on the hippocampus.4,44 Mice with ablated and ligated meningeal lymphatics also demonstrated decreased extracellular tau protein clearance from brain parenchyma.44-46 The representative pattern of meningeal amyloid deposition following lymphatic ablation in murine meninges was confirmed to match that found in human autopsy specimens of 9 patients with AD.26 However, another study performed on human autopsy sections did not find amyloid-β deposition along the superior sagittal sinus.31 Together, these data indicate that the meningeal lymphatic vessels may play a role in pathological accumulation of extracellular macromolecules implicated in neurological disease.
The behavioral impact of meningeal lymphatic dysfunction was analyzed in AD model mice. Spatial learning and fear memory were found to deteriorate along with lymphatic function.4 However, mice with decreased meningeal lymphatic capacity treated with VEGF-c injection showed an increase in vessel diameter and tracer drainage to dCLNs, as well as improved performance in learning, spatial cognition, and memory testing.26,47 Increased amyloid-β clearance was observed in accordance with VEGF-c administration, indicating that VEGF-c/VEGFR3-induced lymphangiogenesis may serve therapeutic purpose in AD patients.47
Parkinson disease
Another neurodegenerative disease state associated with aging and macromolecule accumulation is PD. Excess levels of α-synuclein in brain tissues have been implicated in the pathogenesis of PD.48 Therefore, it was assessed whether the meningeal lymphatics may provide an extracellular route for macromolecule clearance of α-synuclein. It was found that glymphatic clearance of α-synuclein from brain parenchyma was impaired by the ligation of the dCLNs and excess macromolecule deposition led to an enhanced inflammatory response. This ultimately resulted in increased loss of dopaminergic neurons in mice with ligated dCLNs and an exacerbation of behavioral symptoms associated with PD pathology.7
Multiple sclerosis
Multiple sclerosis is characterized by the abundant inflammation and infiltration of immune cells inciting an autoimmune response throughout the CNS, resulting in a neurodegenerative condition.49 Given the classic role of lymphatics in immune surveillance, the relationship between meningeal lymphatic dysfunction and the immune cell dysregulation characteristic of MS has been assessed in a murine model (experimental autoimmune encephalomyelitis [EAE]). Amid widespread inflammation, there were no observable structural changes or lymphangiogenesis in the meningeal lymphatic vessels; however, there was an observable increase in the density of immune cells within the meningeal lymphatics. Furthermore, the lymphatics located superior to the cribriform plate did demonstrate increased diameter at later time points in disease progression and there was a corresponding detectable increase in VEGF-c levels.24,50 This finding suggests involvement of nasal drainage in the late stages of the disease.
Prior studies indicating that the dCLNs play significant role in MS progression as a T-cell induction site prompted investigation into the effects of meningeal lymphatic drainage to the dCLNs in disease pathology.51-53 Resection of the dCLNs in mice did result in decreased disease severity. Ablation of the meningeal lymphatics resulted in a less severe disease model, but it did not halt the development of EAE.24 Inhibition of VEGFR3 signaling, however, resulted in delayed disease development and lessened severity.50 Interruption of only the nasal lymphatics, obstructing drainage into the sCLNs, did not show an impact on disease progression or severity.24 The findings from these studies demonstrated that increased communication with the dCLNs via meningeal lymphatics may contribute to the pathologic immune cell response of MS.
Stroke
Previous research assessing stroke outcomes in rodent models invited the question of whether or not meningeal lymphatics play a role. One study demonstrated that following photothrombosis-induced stroke, increased levels of VEGF-c, a factor now thought to be linked to the development and dilatation of meningeal lymphatic vessels, was observed in the brain.54 Furthermore, another study demonstrated that ligation of cervical lymphatic vessels following transient middle cerebral artery occlusion (tMCAo)-induced stroke increased cerebral edema and infarct size.55 In a study assessing the potential impact of meningeal lymphatics on stroke outcome, in photothrombosis-induced stroke, lymphangiogenesis was induced from vessels along the sagittal sinuses into zones lacking lymphatics. However, hypoplasia was not found to significantly affect infarct volumes or stroke outcomes in the photothrombotic stroke model. In the tMCAo model, lymphangiogenesis was not stimulated following stroke. In this model, hypoplasia was found to worsen stroke outcome by increasing infarct size and cerebral edema volume.56 The data from this study provided preliminary findings that the impact of cerebral ischemia may negatively impact meningeal lymphatics, and the function of these vessels may play a role in stroke outcome.
Subarachnoid hemorrhage
Subarachnoid hemorrhage may result in numerous, life-threatening complications involving cerebral fluid and macromolecule accumulation and inflammatory response upregulation.57,58 In a mouse model of acute subarachnoid hemorrhage, decreased drainage to the dCLNs was observed, as well as abnormal macromolecular and immune cell accumulation in the brain tissue and meninges.59 These findings suggest that subarachnoid hemorrhage may damage or obstruct the function of meningeal lymphatic vessels, which may contribute to the exacerbation of associated co-morbidities.
Discussion
Thus far, meningeal lymphatic vessels have been identified in murine models that have been confirmed to provide a direct CSF clearance pathway to the dCLNs.1,2,20 In these studies, vessels were localized running with the middle meningeal arteries, dural sinuses, and cranial nerves draining through foramina at the base of the skull and the cribriform plate to the CLNs, as well as with nerves and blood vessels within the spinal canal. They have been further imaged in dura mater penetrating deeper into septae of the cortex.21 The meningeal lymphatics were found to drain primarily to the dCLNs, whereas the lymphatics in the nasal mucosa have been found to demonstrate more significant drainage to the sCLNs. The meningeal lymphatic vessels were found to be largest, valve containing, and most concentrated in the base of the skull.1-3,22,24,28
The meningeal lymphatic vessels have further been confirmed to be present in the human dura mater and to run with dura mater-associated blood vessels, namely the dural venous sinuses and the middle meningeal arteries through analysis of autopsy specimens of human meninges and in vivo models using MRI technology.20,30,31,33 Initial assessment has also demonstrated human in vivo CSF drainage to the CLNs.32
Exploration of the role of meningeal lymphatic vessels in neurological diseases including AD, PD, MS, stroke, and subarachnoid hemorrhage has been performed in rodent models.4,7,22,24,44-47,50,56,59 These studies demonstrated decreased meningeal lymphatic drainage to the dCLNs with age and decreased clearance of macromolecules including amyloid-β, tau protein, and α-synuclein with lymphatic dysfunction, which are implicated in AD and PD models.7,22,26,40,44-47
Although MS models did not reveal widespread meningeal lymphangiogenesis, the density of immune cells within the vessels increased, suggesting a meningeal lymphatic role in immune cell trafficking implicated in MS. Furthermore, removal of the dCLNs lessened disease severity and inhibition of lymphatic development both lessened severity and slowed progression.24,50 In a tMCAo stroke model, lymphatic hypoplasia was found to negatively impact stroke outcome, and damage to meningeal lymphatic vessels observed in a subarachnoid hemorrhage model was thought to worsen lasting effects.56,59 As potential association between meningeal lymphatic dysfunction and neurological conditions continues to be uncovered in animal models, there presents an opportunity to further explore their role in other pervasive neurological conditions involving fluid and macromolecule accumulation or immune cell dysregulation such as amyotrophic lateral sclerosis (ALS).
From a clinical standpoint, treatment of peripheral lymphatic disorders is a rapidly growing field that has stimulated significant advancements in lymphatic imaging modalities, which allow for direct access to these microscopic vessels in the modern day. At the forefront of current interventional techniques in lymphatic surgery is supermicrosurgery, the ability to anastomose microneurovascular bundles ranging 0.3 to 0.8 mm in size. Advancements in real-time, intraoperative lymphatic mapping and visualization technologies have made localization and isolation of these ultrafine lymphatic vessels possible, making them accessible for supermicrosurgical manipulation.60,61 While there remains much to be studied regarding meningeal lymphatic vessels in humans prior to even elucidating their role in human disease processes, the ever-advancing sophistication of lymphatic imaging techniques today may assist in research and education regarding their anatomy and function in humans. Techniques such as near-infrared imaging using indocyanine green (ICG) and nuclear lymphoscintigraphy allow for quantitative assessments lymphatic function, whereas MRI and computed tomography (CT) methods may be used for high-resolution structural visualization of individual lymphatic vessels.61
Indocyanine green lymphangiography uses a subcutaneously injected, near-infrared fluorescent tracer to provide continuous, safe, and minimally invasive stain and radiation-free mode of lymphatic imaging.61-64 Observation of ICG flow rate allows for functional assessment of lymphatic flow and has been used successfully for visualization of vessels as deep as 2 cm from the body surface.61,65 Continuous imaging capability clearly discriminates lymphatic vessels from surrounding vasculature, as rapid dye dispersal ensues if uptake into venous channels occurs.63 Another widely used mode for detection of lymphatic function is nuclear lymphoscintigraphy. This imaging technique detects flow of injected radiotracer, preferentially taken up into lymphatic vessels, and may detect discrete changes to flow even in structurally normal vessels.61,65,66 The drawbacks to this technique include its cost and time intensiveness, as well as decreased depth and spatial resolution, which limits its usefulness in distinguishing individual lymphatic vessels and identifying specific points of static lymph flow.65,67
For highly detailed resolution lymphatic vessels at greater depth, both MRI and CT modalities are used.61,68,69 Magnetic resonance lymphangiography is a noninvasive imaging modality able to elucidate such high structural resolution of individual lymphatic vessels that it is often used for preoperative targeting.61,68 However, as often enhancement of both lymphatic vessels and surrounding superficial veins occurs, a drawback to this imaging technique is its inability to definitively isolate solely lymphatic vessels.61,67,68 Computed tomography modalities used in lymphatic imaging include single-photon emission CT, as well as CT-Lymphography.61 Single-photon emission CT has been shown to provide preoperative value in precise localization of lymphatic vessels and nodes, whereas CT-Lymphography, though it does expose the patient to radiation, provides three-dimensional (3D) imaging of the lymphatic system, allowing for visualization of smaller lymphatic channels than other imaging techniques used, down to 0.7 to 1.2 nm in diameter.61,70 These techniques have greatly assisted in the advancements of research and treatment surrounding peripheral lymphatics, specifically the ability to map defective vessels and augment lymphatic drainage. Further development of lymphatic imaging modalities such as these aforementioned for future application in meningeal lymphatic visualization can help us to achieve greater understanding of the human anatomy and function of these vessels in the CNS.
The amount of knowledge that remains to be uncovered regarding meningeal lymphatics in humans is significant. A major key point, both anatomical and functional, remains to be clarified at the histological level. That is, definitive localization of these vessels within the meningeal layers. Entry extensions of meningeal lymphatics that appear to allow communication directly with the SAS have been identified in rodent models; however, whether or not these vessels completely penetrate the SAS remains to be confirmed.24 Three possible meningeal locations for the vessels have been suggested given current knowledge. They may reside within the dura mater or leaflets, run between the dura and the arachnoid layers, or penetrate into the SAS.23 The association to date of the vessels with the middle meningeal arteries and the dural sinuses is suggestive of a dural location; however, CSF absorption from the SAS into these lymphatic vessels remains a mechanism that draws that localization into question.
Furthermore, while studies mapping lymphatic flow pathways from the meningeal lymphatic vessels to the dCLNs have been performed in rodents, there is a lack of data as of now characterizing these flow paths in humans.1-3,22,24,28 Future steps toward achieving this end may be to investigate lymphatic imaging capabilities sophisticated enough to visualize the in vivo drainage pathways of the meningeal lymphatics between the CNS and the peripheral lymphatic system. Definitive localization of meningeal lymphatics within the human meninges, knowledge of how the vessels access the CSF, and further how they communicate with the peripheral lymphatic system are key points of understanding, essential to building a foundation of education surrounding meningeal lymphatics such that we may begin to uncover the implication of their discovery on human disease.
Conclusion
Exploration within murine models has implicated meningeal lymphatic vessels as potential therapeutic targets for treatment of neurological disease, and findings make further study of these vessels from a clinical perspective the utmost importance. Initial evidence gathered surrounding the anatomy of these vessels in humans provides a basis for future studies mapping their structure, drainage patterns, and function in vivo. Further exploration into the mechanisms by which meningeal lymphatic vessels access the CSF, localizing these vessels definitively within the meningeal layers, and visualizing their communication with the CLNs provide an exciting opportunity for novel understanding of mechanisms of CNS waste drainage and immunoregulation. As knowledge surrounding these vessels continues to grow, their promise as a future novel therapeutic target for neurological disease remains a significant prospect.
Footnotes
Funding:The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests:The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Author Contributions: Each of the authors contributed equally to this manuscript. Review of articles and background research was conducted by KH and OS and the definitive citations were reviewed by DG.
ORCID iD: Daniel J Gould  https://orcid.org/0000-0002-3576-3775
https://orcid.org/0000-0002-3576-3775
References
- 1. Aspelund A, Antila S, Proulx ST, et al. A dural lymphatic vascular system that drains brain interstitial fluid and macromolecules. J Exp Med. 2015;212:919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Louveau A, Smirnov I, Keyes TJ, et al. Structural and functional features of central nervous system lymphatic vessels. Nature. 2015;523:337-341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Antila S, Karaman S, Nurmi H, et al. Development and plasticity of meningeal lymphatic vessels. J Exp Med. 2017;214:3645-3667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Da Mesquita S, Fu Z, Kipnis J. The meningeal lymphatic system: a new player in neurophysiology. Neuron. 2018;100:375-388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Louveau A, Da Mesquita S, Kipnis J. Lymphatics in neurological disorders: a neuro-lympho-vascular component of multiple sclerosis and Alzheimer’s disease? Neuron. 2016;91:957-973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Noe FM, Marchi N. Central nervous system lymphatic unit, immunity, and epilepsy: is there a link? Epilepsia Open. 2019;4:30-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Zou W, Pu T, Feng W, et al. Blocking meningeal lymphatic drainage aggravates Parkinson’s disease-like pathology in mice overexpressing mutated α-synuclein. Transl Neurodegener. 2019;8:7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Schulte-Merker S, Sabine A, Petrova TV. Lymphatic vascular morphogenesis in development, physiology, and disease. J Cell Biol. 2011;193:607-618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Wang Y, Oliver G. Current views on the function of the lymphatic vasculature in health and disease. Genes Dev. 2010;24:2115-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Margaris KN, Black RA. Modelling the lymphatic system: challenges and opportunities. J R Soc Interface. 2012;9:601-612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Baluk P, Fuxe J, Hashizume H, et al. Functionally specialized junctions between endothelial cells of lymphatic vessels. J Exp Med. 2007;204:2349-2362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cserr HF, Harling-Berg CJ, Knopf PM. Drainage of brain extracellular fluid into blood and deep cervical lymph and its immunological significance. Brain Pathol. 1992;2:269-276. [DOI] [PubMed] [Google Scholar]
- 13. Dupont G, Schmidt C, Yilmaz E, et al. Our current understanding of the lymphatics of the brain and spinal cord. Clin Anat. 2019;32:117-121. [DOI] [PubMed] [Google Scholar]
- 14. Iliff JJ, Wang M, Liao Y, et al. A paravascular pathway facilitates CSF flow through the brain parenchyma and the clearance of interstitial solutes, including amyloid β. Sci Transl Med. 2012;4:147ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Abbott NJ, Pizzo ME, Preston JE, Janigro D, Thorne RG. The role of brain barriers in fluid movement in the CNS: is there a “glymphatic” system? Acta Neuropathol. 2018;135:387-407. [DOI] [PubMed] [Google Scholar]
- 16. Iliff JJ, Wang M, Zeppenfeld DM, et al. Cerebral arterial pulsation drives paravascular CSF-interstitial fluid exchange in the murine brain. J Neurosci. 2013;33:18190-18199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Semyachkina-Glushkovskaya O, Postnov D, Kurths J. Blood-brain barrier, lymphatic clearance, and recovery: Ariadne’s thread in labyrinths of hypotheses. Int J Mol Sci. 2018;19:3818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Louveau A, Plog BA, Antila S, Alitalo K, Nedergaard M, Kipnis J. Understanding the functions and relationships of the glymphatic system and meningeal lymphatics. J Clin Invest. 2017;127:3210-3219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mathiisen TM, Lehre KP, Danbolt NC, Ottersen OP. The perivascular astroglial sheath provides a complete covering of the brain microvessels: an electron microscopic 3D reconstruction. Glia. 2010;58:1094-1103. [DOI] [PubMed] [Google Scholar]
- 20. Absinta M, Ha SK, Nair G, et al. Human and nonhuman primate meninges harbor lymphatic vessels that can be visualized noninvasively by MRI. eLife. 2017;6:e29738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Lohrberg M, Wilting J. The lymphatic vascular system of the mouse head. Cell Tissue Res. 2016;366:667-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ma Q, Ineichen BV, Detmar M, Proulx ST. Outflow of cerebrospinal fluid is predominantly through lymphatic vessels and is reduced in aged mice. Nat Commun. 2017;8:1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raper D, Louveau A, Kipnis J. How do meningeal lymphatic vessels drain the CNS? Trends Neurosci. 2016;39:581-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Louveau A, Herz J, Alme MN, et al. CNS lymphatic drainage and neuroinflammation are regulated by meningeal lymphatic vasculature. Nat Neurosci. 2018;21:1380-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Izen RM, Yamazaki T, Nishinaka-Arai Y, Hong YK, Mukouyama YS. Postnatal development of lymphatic vasculature in the brain meninges. Dev Dyn. 2018;247:741-753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Da Mesquita S, Louveau A, Vaccari A, et al. Functional aspects of meningeal lymphatics in ageing and Alzheimer’s disease. Nature. 2018;560:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kida S, Pantazis A, Weller RO. CSF drains directly from the subarachnoid space into nasal lymphatics in the rat. Anatomy, histology and immunological significance. Neuropathol Appl Neurobiol. 1993;19:480-488. [DOI] [PubMed] [Google Scholar]
- 28. Maloveska M, Danko J, Petrovova E, et al. Dynamics of Evans blue clearance from cerebrospinal fluid into meningeal lymphatic vessels and deep cervical lymph nodes. Neurol Res. 2018;40:372-380. [DOI] [PubMed] [Google Scholar]
- 29. Liu NF, Lu Q, Jiang ZH, Wang CG, Zhou JG. Anatomic and functional evaluation of the lymphatics and lymph nodes in diagnosis of lymphatic circulation disorders with contrast magnetic resonance lymphangiography. J Vasc Surg. 2009;49:980-987. [DOI] [PubMed] [Google Scholar]
- 30. Visanji NP, Lang AE, Munoz DG. Lymphatic vasculature in human dural superior sagittal sinus: implications for neurodegenerative proteinopathies. Neurosci Lett. 2018;665:18-21. [DOI] [PubMed] [Google Scholar]
- 31. Goodman JR, Adham ZO, Woltjer RL, Lund AW, Iliff JJ. Characterization of dural sinus-associated lymphatic vasculature in human Alzheimer’s dementia subjects. Brain Behav Immun. 2018;73:34-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Eide PK, Vatnehol SAS, Emblem KE, Ringstad G. Magnetic resonance imaging provides evidence of glymphatic drainage from human brain to cervical lymph nodes. Sci Rep. 2018;8:7194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kuo PH, Stuehm C, Squire S, Johnson K. Meningeal lymphatic vessel flow runs countercurrent to venous flow in the superior sagittal sinus of the human brain. Tomography. 2018;4:99-104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Goldmann J, Kwidzinski E, Brandt C, Mahlo J, Richter D, Bechmann I. T cells traffic from brain to cervical lymph nodes via the cribroid plate and the nasal mucosa. J Leukoc Biol. 2006;80:797-801. [DOI] [PubMed] [Google Scholar]
- 35. Benilova I, Karran E, De Strooper B. The toxic Abeta oligomer and Alzheimer’s disease: an emperor in need of clothes. Nat Neurosci. 2012;15:349-357. [DOI] [PubMed] [Google Scholar]
- 36. Brookmeyer R, Abdalla N, Kawas CH, Corrada MM. Forecasting the prevalence of preclinical and clinical Alzheimer’s disease in the United States. Alzheimer’s Dement. 2018;14:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Erkkinen MG, Kim MO, Geschwind MD. Clinical neurology and epidemiology of the major neurodegenerative diseases. Cold Spring Harb Perspect Biol. 2018;10:a033118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Mandelkow EM, Mandelkow E. Biochemistry and cell biology of tau protein in neurofibrillary degeneration. Cold Spring Harb Perspect Med. 2012;2:a006247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kress BT, Iliff JJ, Xia M, et al. Impairment of paravascular clearance pathways in the aging brain. Ann Neurol. 2014;76:845-861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Xu Z, Xiao N, Chen Y, et al. Deletion of aquaporin-4 in APP/PS1 mice exacerbates brain Aβ accumulation and memory deficits. Mol Neurodegener. 2015;10:58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Chevalier S, Ferland G, Tuchweber B. Lymphatic absorption of retinol in young, mature, and old rats: influence of dietary restriction. FASEB J. 1996;10:1085-1090. [DOI] [PubMed] [Google Scholar]
- 42. Hos D, Bachmann B, Bock F, Onderka J, Cursiefen C. Age-related changes in murine limbal lymphatic vessels and corneal lymphangiogenesis. Exp Eye Res. 2008;87:427-432. [DOI] [PubMed] [Google Scholar]
- 43. Nagai T, Bridenbaugh EA, Gashev AA. Aging-associated alterations in contractility of rat mesenteric lymphatic vessels. Microcirculation. 2011;18:463-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Wang L, Zhang Y, Zhao Y, Marshall C, Wu T, Xiao M. Deep cervical lymph node ligation aggravates AD-like pathology of APP/PS1 mice. Brain Pathol. 2019;29:176-192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Cao X, Xu H, Feng W, Su D, Xiao M. Deletion of aquaporin-4 aggravates brain pathology after blocking of the meningeal lymphatic drainage. Brain Res Bull. 2018;143:83-96. [DOI] [PubMed] [Google Scholar]
- 46. Patel TK, Habimana-Griffin L, Gao X, et al. Dural lymphatics regulate clearance of extracellular tau from the CNS. Mol Neurodegener. 2019;14:11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Wen YR, Yang JH, Wang X, Yao ZB. Induced dural lymphangiogenesis facilities soluble amyloid-beta clearance from brain in a transgenic mouse model of Alzheimer’s disease. Neural Regen Res. 2018;13:709-716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bobela W, Aebischer P, Schneider BL. Alpha-synuclein as a mediator in the interplay between aging and Parkinson’s disease. Biomolecules. 2015;5:2675-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Dendrou CA, Fugger L, Friese MA. Immunopathology of multiple sclerosis. Nat Rev Immunol. 2015;15:545-558. [DOI] [PubMed] [Google Scholar]
- 50. Hsu M, Rayasam A, Kijak JA, et al. Neuroinflammation-induced lymphangiogenesis near the cribriform plate contributes to drainage of CNS-derived antigens and immune cells. Nat Commun. 2019;10:229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Furtado GC, Marcondes MC, Latkowski JA, Tsai J, Wensky A, Lafaille JJ. Swift entry of myelin-specific T lymphocytes into the central nervous system in spontaneous autoimmune encephalomyelitis. J Immunol. 2008;181:4648-4655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Phillips MJ, Needham M, Weller RO. Role of cervical lymph nodes in autoimmune encephalomyelitis in the Lewis rat. J Pathol. 1997;182:457-464. [DOI] [PubMed] [Google Scholar]
- 53. van Zwam M, Huizinga R, Heijmans N, et al. Surgical excision of CNS-draining lymph nodes reduces relapse severity in chronic-relapsing experimental autoimmune encephalomyelitis. J Pathol. 2009;217:543-551. [DOI] [PubMed] [Google Scholar]
- 54. Gu W, Brannstrom T, Jiang W, Bergh A, Wester P. Vascular endothelial growth factor-A and -C protein up-regulation and early angiogenesis in a rat photothrombotic ring stroke model with spontaneous reperfusion. Acta Neuropathol. 2001;102:216-226. [DOI] [PubMed] [Google Scholar]
- 55. Si J, Chen L, Xia Z. Effects of cervical-lymphatic blockade on brain edema and infarction volume in cerebral ischemic rats. Chin J Physiol. 2006;49:258-265. [PubMed] [Google Scholar]
- 56. Yanev P, Poinsatte K, Hominick D, et al. Impaired meningeal lymphatic vessel development worsens stroke outcome [published online ahead of print January 9, 2019]. J Cereb Blood Flow Metab. doi: 10.1177/0271678X18822921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Chen S, Luo J, Reis C, Manaenko A, Zhang J. Hydrocephalus after subarachnoid hemorrhage: pathophysiology, diagnosis, and treatment. Biomed Res Int. 2017;2017:8584753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. El Amki M, Dubois M, Lefevre-Scelles A, et al. Long-lasting cerebral vasospasm, microthrombosis, apoptosis and paravascular alterations associated with neurological deficits in a mouse model of subarachnoid hemorrhage. Mol Neurobiol. 2018;55:2763-2779. [DOI] [PubMed] [Google Scholar]
- 59. Pu T, Zou W, Feng W, et al. Persistent malfunction of glymphatic and meningeal lymphatic drainage in a mouse model of subarachnoid hemorrhage. Exp Neurobiol. 2019;28:104-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Masia J, Olivares L, Koshima I, et al. Barcelona consensus on supermicrosurgery. J Reconstr Microsurg. 2014;30:53-58. [DOI] [PubMed] [Google Scholar]
- 61. Singhal D, Tran BN, Angelo JP, Lee BT, Lin SJ. Technological advances in lymphatic surgery: bringing to light the invisible. Plast Reconstr Surg. 2019;143:283-293. [DOI] [PubMed] [Google Scholar]
- 62. Narushima M, Yamamoto T, Ogata F, Yoshimatsu H, Mihara M, Koshima I. Indocyanine green lymphography findings in limb lymphedema. J Reconstr Microsurg. 2016;32:72-79. [DOI] [PubMed] [Google Scholar]
- 63. Ogata F, Azuma R, Kikuchi M, Koshima I, Morimoto Y. Novel lymphography using indocyanine green dye for near-infrared fluorescence labeling. Ann Plast Surg. 2007;58:652-655. [DOI] [PubMed] [Google Scholar]
- 64. Ogata F, Narushima M, Mihara M, Azuma R, Morimoto Y, Koshima I. Intraoperative lymphography using indocyanine green dye for near-infrared fluorescence labeling in lymphedema. Ann Plast Surg. 2007;59:180-184. [DOI] [PubMed] [Google Scholar]
- 65. Unno N, Nishiyama M, Suzuki M, et al. Quantitative lymph imaging for assessment of lymph function using indocyanine green fluorescence lymphography. Eur J Vasc Endovasc Surg. 2008;36:230-236. [DOI] [PubMed] [Google Scholar]
- 66. Williams WH, Witte CL, Witte MH, McNeill GC. Radionuclide lymphangioscintigraphy in the evaluation of peripheral lymphedema. Clin Nucl Med. 2000;25:451-464. [DOI] [PubMed] [Google Scholar]
- 67. Neligan PC, Kung TA, Maki JH. MR lymphangiography in the treatment of lymphedema. J Surg Oncol. 2017;115:18-22. [DOI] [PubMed] [Google Scholar]
- 68. Mitsumori LM, McDonald ES, Neligan PC, Maki JH. Peripheral magnetic resonance lymphangiography: techniques and applications. Tech Vasc Interv Radiol. 2016;19:262-272. [DOI] [PubMed] [Google Scholar]
- 69. Tangoku A, Yamamoto S, Suga K, et al. Sentinel lymph node biopsy using computed tomography-lymphography in patients with breast cancer. Surgery. 2004;135:258-265. [DOI] [PubMed] [Google Scholar]
- 70. Khafif A, Schneebaum S, Fliss DM, et al. Lymphoscintigraphy for sentinel node mapping using a hybrid single photon emission CT (SPECT)/CT system in oral cavity squamous cell carcinoma. Head Neck. 2006;28:874-879. [DOI] [PubMed] [Google Scholar]