Abstract
Current inactivated polio vaccine (IPV) products are sensitive to both freezing and elevated temperatures and therefore must be shipped and stored between 2°C and 8°C, a requirement that imposes financial and logistical challenges for global distribution. As such, there is a critical need for a robust, thermally stable IPV to support global polio eradication and post-eradication immunization needs. Here, we present the development of air-dried thin films for temperature stabilization of IPV using the biomaterial silk fibroin. Thin-film product compositions were optimized for physical properties as well as poliovirus D-antigen recovery and were tested under accelerated and real-time stability storage conditions. Silk fibroin IPV films maintained 70% D-antigen potency after storage for nearly three years at room temperature, and greater than 50% potency for IPV-2 and IPV-3 serotypes at 45°C for one year. The immunogenicity of silk fibroin IPV films after 2-week storage at 45°C was assessed in Wistar rats and the stressed films generated equivalent neutralizing antibody responses to commercial vaccine for IPV-1 and IPV-2. However, the absence of IPV-3 responses warrants further investigation into the specificity of ELISA for intact IPV-3 D-antigen. By demonstrating immunogenicity post-storage, we offer the air-dried silk film format as a means to increase IPV vaccine access through innovative delivery systems such as microneedles.
Keywords: Silk, Fibroin, Stabilization, Vaccine, Poliovirus
INTRODUCTION
The development and widespread use of effective vaccines represents one of the most significant steps towards improving global health. Through unprecedented public-private partnership and commitment to global immunization, a remarkable decline in infectious diseases, such as poliomyelitis, has been achieved over the past decades. Having recently arrived at the 30-year anniversary of the World Health Assembly declaration to eradicate polio, affordable and accessible vaccines have reduced new polio cases from more than 1000 per day to a total of 22 cases limited to two countries in 2017 [1, 2]. Much of this progress can be attributed to the widespread use of easy-to-administer oral poliomyelitis vaccine (OPV).
Recently, the World Health Organization initiated a global shift towards the use of inactivated polio vaccine (IPV) from OPV [3]. With the shift from OPV to IPV, the shortcomings of current IPV vaccines have become more apparent. Key amongst those limitations is the thermally induced conversion of poliovirus from the immunogenic D-antigen form to the C-antigen form which fails to elicit a protective immune response [4]. The requirement for vaccine storage at 2°C to 8°C to minimize loss of efficacy imposes challenges on vaccination campaigns from a distribution and logistics standpoint, especially in developing country settings. The creation of a compact, temperature-stable IPV vaccine would reduce barriers to vaccination campaigns in the remaining polio-endemic countries and help with post-eradication immunization efforts. Moreover, if new thermostable formulations were inexpensive and simple to produce, they could be readily integrated into innovative delivery platforms such as microneedles [5] or reconstituted syringes [6], which may serve to fill critical gaps in IPV dosing compliance.
Previous research has investigated stability enhancement of IPV through conversion from liquid to the dried state. Lyophilization, the most commonly used method to dry commercial vaccines, has proved uniquely difficult to utilize with IPV due to the innate sensitivity of poliovirus antigens to freezing. As such, different drying techniques, including encapsulation and vacuum drying as well as controlled evaporation, have demonstrated improved IPV stability over liquid vaccine, especially after reformulation with additional excipients [7, 8, 9]. While these methods offer stability enhancement, maintenance of vaccine potency and efficacy at high temperatures requires further improvement. Of note, published formulations are either accompanied by significant loss of antigen through the drying process [7] or greater than 50% loss of at least one poliovirus serotype antigen after 8 weeks of storage at 37°C to 40°C [8, 9, 10, 11]. As many regions in greatest need for IPV have hot climates, these improvements, although positive, do not substantially diminish the need to refrigerate IPV throughout the distribution chain.
Silk fibroin protein is derived from Bombyx mori silkworm cocoons and can be generated in an aqueous state that is readily miscible with solutions of other biomolecules [12]. Silk fibroin is an amphiphilic copolymer with hydrophobic blocks linked by hydrophilic segments, enabling the material to take mechanically resilient forms such as bone screws or films [13, 14, 15]. Moreover, silk fibroin is biocompatible [16] and has been shown to stabilize a variety of biomacromolecules, including blood proteins, enzymes, and antibodies [17, 18, 19, 20, 21]. While the mechanism underlying silk fibroin stabilization has not been fully characterized, it is believed that the glass dynamics of dried silk fibroin matrices aid in the suppression of biomolecule mobility, particularly β-relaxation, which subsequently improves stability [22]. Moreover, many of the common excipients utilized in vaccine formulation (e.g. proteins, sugars, salts, buffers) are compatible with silk fibroin and can provide additive or synergistic enhancements to stability when combined with silk in dried formats [22]. We hypothesized that glassy silk fibroin protein films could be developed to reduce mobility of IPV antigens and improve stability at elevated temperatures. Such a format could be produced more quickly and at lower cost than a lyophilized product, while being administered with needle and syringe in the same manner as the existing IPV product. In the current work, we build upon prior studies to develop an air-dried IPV product format enabled through silk fibroin protein. We evaluate stability of D-antigen content in films subject to accelerated challenge at high temperature and real-time storage for up to 3 years at room temperature. Furthermore, we evaluate the performance and safety of this product in vivo after a two-week temperature challenge at 45°C to demonstrate the potential for silk fibroin films to improve vaccine access in remote regions of the world.
MATERIALS AND METHODS
Inactivated poliovirus vaccine (IPV)
Trivalent IPV (IPOL®; Sanofi-Pasteur, Swiftwater, PA) contains inactivated type 1 (Mahoney strain), type 2 (MEF-1), and type 3 (Saukett) poliovirus strains in concentrations of 40, 8, and 32 D-antigen units per dose (0.5 mL), respectively.
IPV dialysis for antigen purification
Inactivated polio vaccine was dialyzed to remove commercial excipients (2-phenoxyethanol, formaldehyde) prior to formulation. Vaccine was loaded into dialysis cassettes (Slide-A-Lyzer 3.5 kDa; Thermo-Fisher) and dialyzed against pre-cooled 10 mM citrate-phosphate buffer (pH 7.4). Dialysis was performed at 4°C for 24 hours, with buffer replacement at 2, 4, 6, and 22 hours. The dialyzed vaccine was then recovered from cassettes and refrigerated prior to formulation. It was confirmed that this process did not induce a significant decrease in vaccine antigen content (Supplemental Figure 1).
Silk fibroin preparation
Silk fibroin solution was produced as previously described [23]. Briefly, silk cocoons from Bombyx mori (Tajima Shoji Co. Ltd., Yokohama, Japan) were boiled in 0.02 M sodium carbonate (Sigma-Aldrich S7795) to remove sericin protein. In order to modify the molecular weight of silk fibroin, we modified the duration of boiling as previously described [24]. The raw fibers were then removed, washed with ultrapure water, and allowed to dry completely. After drying, the fibers were dissolved in 9.3 M lithium bromide (Sigma-Aldrich 213225) at 60°C for four hours. The aqueous solution was then dialyzed against ultrapure water in a 12 mL cassette (Slide-A-Lyzer 3.5 kDa; Thermo-Fisher) to remove residual chaotrope. The resultant solution was centrifuged to remove large particulates and protein concentration was determined through residual dry mass. We confirmed the weight average molecular weight of the resultant silk fibroin distribution by size exclusion chromatography, as previously described [25].
Formulation and production of thin silk fibroin films
Formulations for each experiment were prepared by creating concentrated, 0.22 μm sterile-filtered stock solutions of each excipient. Volumes of these sterile excipient stocks (e.g. proteins, sugars, salts, buffers) were mixed before combining with 0.22 μm sterile-filtered silk fibroin solution to achieve a formulation stock at 2X final concentration. This stock was then diluted 1:1 with dialyzed IPV to create the final formulation, which was pipetted onto steam-sterilized polydimethylsiloxane (PDMS; Sylgard 184, Dow Corning) molds and spread evenly (Figure 1). All films were dried for at least 14 hours overnight in a biosafety cabinet at 25–40% relative humidity. Once dry, films were transferred from PDMS molds to sterile 2 mL borosilicate glass serum vials (Wheaton; Fisher Scientific 06-406-37), placed in a shelf lyophilizer (VirTis Genesis 25XL Pilot, SP Scientific), and backfilled with ultra-pure nitrogen. All vials were stoppered at 500 Torr and sealed using 13 mm aluminum caps (Wheaton; VWR 16171–840).
Figure 1.
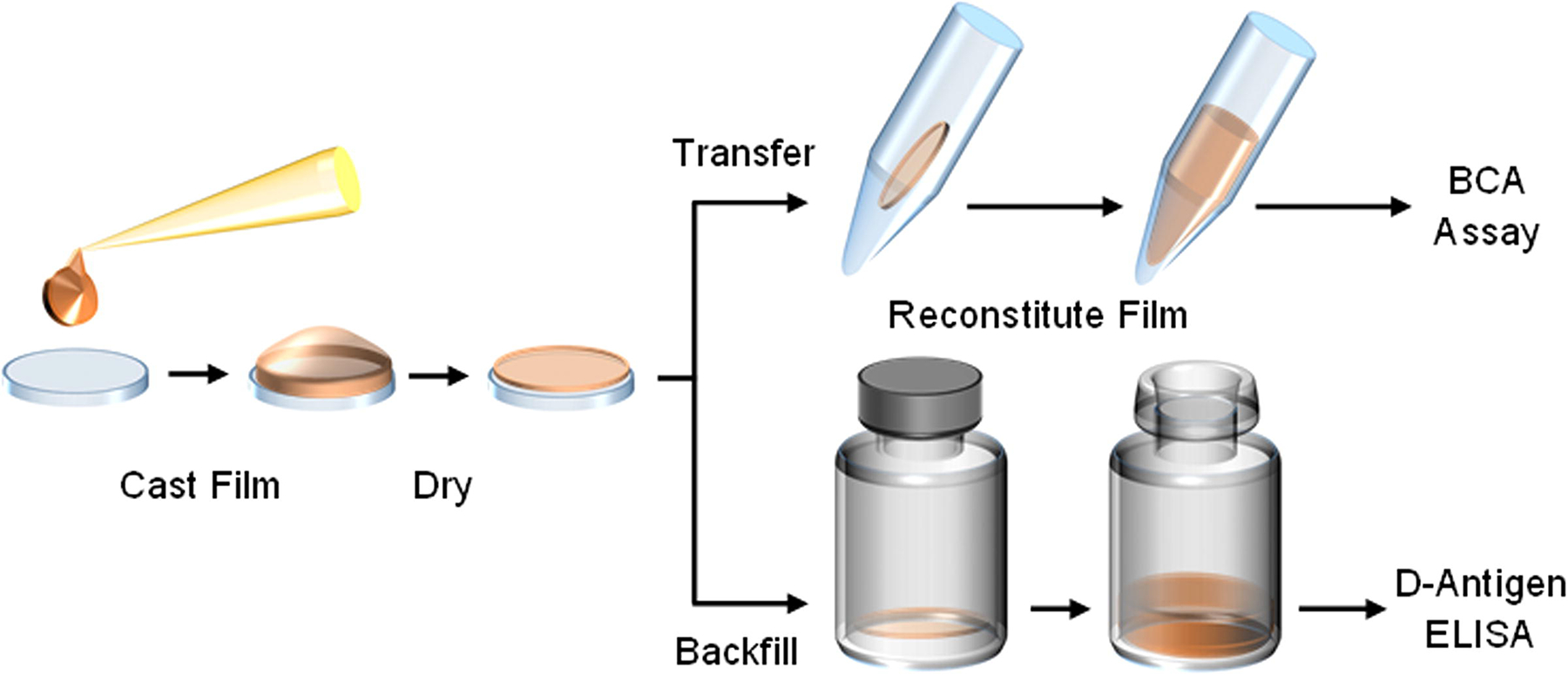
Thin silk fibroin film processing begins by pipetting a volume of neat or IPV-containing silk fibroin solution onto a sterile polydimethylsiloxane (PDMS) chip and spreading evenly across the surface. This volume is air-dried in a biosafety cabinet, and then transferred into an appropriate container for assessment. For baseline solubility assessments of thin silk fibroin films, the material is transferred to a capped tube, reconstituted using sterile buffer, and protein content measured through a bicinchoninic acid (BCA) assay. For stability assessments of IPV, films are stored in sterile glass serum vials and backfilled under inert nitrogen for storage. Upon reaching storage time points, films are reconstituted with buffer and assayed on the D-antigen ELISA.
Protein quantification
Solubility of pure fibroin protein films was examined using a protein quantification assay (Pierce BCA Protein Assay Kit; Thermo Fisher 23225) following reconstitution in buffered saline. Silk fibroin films were completely submerged in Dulbecco’s phosphate-buffered saline (DPBS) and dissolved for 10 minutes with gentle shaking. After dissolution, solutions were centrifuged for 5 minutes at 5000 × g in order to collect undissolved protein fragments into the bottom of the tube. Supernatant was then removed and added to a 96-well plate, with volumes as described in the manufacturer’s protocol. Neat silk fibroin solution was used to generate a standard curve for the assay. Plates were read for absorbance at 562 nm and all samples were run in assay triplicate.
IPV D-Antigen ELISA
A sandwich ELISA protocol developed by the CDC was used to measure IPV D-antigen content, similar to methods previously reported [26]. Briefly, ELISA plates (Immulon 2HB, Thermo Scientific) were coated overnight at 4°C using capture antibodies (anti-polio 1 [14D2 (7C5)], Novus Biologicals; anti-polio 2 [24E2], Enzo Life Sciences; anti-polio 3 [clone 4D5], Fisher Scientific) diluted 1:500 for types 1 and 3 and 1:1000 for type 2 in 50 mM carbonate-bicarbonate buffer (pH 9.6, Sigma Aldrich). Plates were washed with 0.01 M PBS + 0.05% Tween 20. The plates were then blocked in wash buffer with 0.5% gelatin (Difco) and 0.25% Tween 20 for 1 hour. Silk-formulated vaccine samples were reconstituted in blocking buffer and added to assay plates after the plates were washed. After an incubation at 37°C for 1 hour and another wash, HRP-conjugated antibodies (Lightning-Link HRP antibody labeling kit, Novus Biologics) were added to each well and again incubated for 1 hour at 37°C. Plates were washed and TMB substrate (KPL Inc.) was added to each well and allowed to develop for ten minutes before being stopped using TMB BlueSTOP (KPL Inc.). The absorbance of each well was read at 620 nm using a plate reader. Serial dilutions of IPOL® were used as standards on each plate and used to calculate the D-antigen content of other samples.
Immunogenicity assessment
Assessment of immunogenicity in rats was performed under IACUC approval at the CDC and according to NIH guidelines for the care and use of laboratory animals. Female Wistar rats (6–8 weeks-old, Charles River) were immunized with IPV (dose: 8 DU type 1, 1.6 DU type 2, 6.4 DU type 3), either in liquid or reconstituted silk fibroin film format. Refrigerated and 45°C-challenged silk films were reconstituted in sterile 0.9% saline for 15 minutes prior to intramuscular injection. A separate batch of formulated silk fibroin films containing IPV was prepared and temperature-challenged for two weeks for booster immunizations at 28 days. Serum was collected from all animals via tail vein before immunization and at four weeks, and via cardiac puncture as a terminal bleed at eight weeks.
Poliovirus micro-neutralization assay
Poliovirus micro-neutralization assay was conducted at CDC as previously described [27]. Briefly, serial dilutions of rat serum samples were prepared and mixed with 100 CCID50 of each poliovirus serotype; each serum was tested in triplicate against each serotype. After 3 hours incubation at 35°C, approximately 7.5 × 103 HEp-2(C) cells were added to the serum and poliovirus mixture and incubated for 5 days at 35°C with 5% CO2. Crystal violet was used to stain each well of the assay for 40 minutes before being washed three times and dried. Purple wells were counted and the Spearman-Karber method was used to estimate final titers. Seroconversion of immunized rats was defined by the CDC as a titer greater than 3.0 log2.
Characterization of anti-silk fibroin responses
Aliquots of serum samples from the immunogenicity study were characterized for antibodies against silk fibroin protein. A silk-specific ELISA was used, similar to that previously reported [28]. Briefly, assay plates (Immulon 2HB, Thermo Scientific) were coated with the same batch of silk fibroin protein solution used to prepare IPV thin films, at a concentration of 4 μg/ml. Plates were blocked with 0.01 M PBS containing 0.05% Tween 20 and 1% BSA for 2 hours at 37°C. After washing, serum dilutions from film-immunized, neat vaccine-immunized, and pre-immune animals were added and incubated for 1 hour at 37°C. After washing, secondary antibody (HRP-conjugated rabbit anti-rat IgG [H+L] F(ab’)2, Sigma-Aldrich; 1:25,000 dilution) was added and incubated for 30 minutes at 37°C. Development was performed with TMB (Genscript) for 20 minutes and quenched using 1 M HCl. Absorbance was read at 450 nm using a plate reader and endpoint titers were calculated for each serum dilution series as the reciprocal dilution at which the absorbance signal reached twice the baseline signal.
RESULTS
Formulation engineering of thin silk fibroin films
The amphiphilic nature of silk fibroin is hypothesized to lead to stabilization of biomacromolecules. This property is also responsible for the self-assembly of the protein into insoluble, structural forms. However, this self-assembly can be precisely controlled through both processing and formulation approaches to tune the solubility profile of fibroin material for different applications. As part of this work, we sought to characterize the effect of silk fibroin solution casting and drying parameters on film solubility. To accomplish this, we first selected one specific preparation of silk fibroin protein, containing a weight average molecular weight (Mw) of 70 kDa, to use in our assessment of total protein recovery from films cast at different protein concentrations and droplet volumes (Figure 2A). The modification of silk preparation has been shown to influence dry film solubility [24]. For this reason, further assessment expanded the range of analysis to include silk preparations of higher molecular weight, including 235 kDa and 169 kDa, as well as a lower molecular weight, 36 kDa. Films were visually examined for solubility upon reconstitution prior to quantitation. The mechanical strength, defined as the ability of the film to demold from PDMS uniformly, was assessed as well. (Figure 2B).
Figure 2.
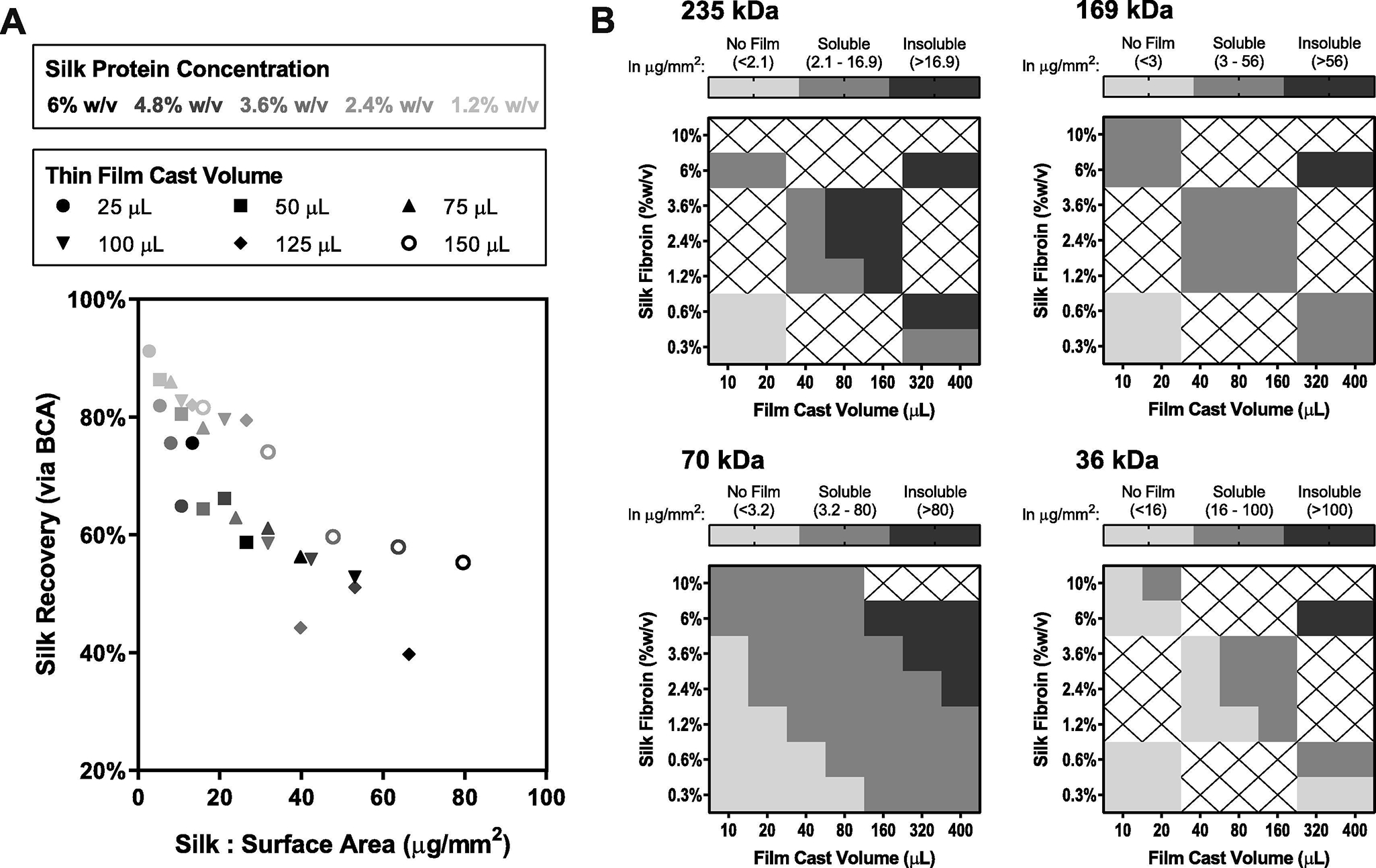
Silk fibroin thin films were engineered for solubility post-drying and for mechanical strength to withstand the manual demolding process. (A) Film casting parameters were evaluated using 70 kDa silk fibroin to determine the relationship between solubility and the film mass-to-surface-area ratio, as measured using a BCA protein quantification assay. (B) Silk fibroin molecular weight was examined as a further mechanism to control film solubility; 235 kDa, 169 kDa, 70 kDa, and 36 kDa material was characterized at various mass-to-surface-area ratios, achieved through combinations of solution casting volume and protein concentration. Light gray indicates that demolding a film was not possible; dark gray indicates films were not soluble upon reconstitution; gray indicates formulations were considered viable for further study in the film format. The ranges of mass-to-surface-area ratios for each outcome are provided in μg/mm2 for each fibroin molecular weight.
We hypothesized that factors contributing to an increased rate of drying would promote better solubility, as it has been shown that slow drying promotes self-assembly of silk fibroin to form an insoluble silk II conformation [20]. Generally, we observed that indeed both smaller casting volumes and lower casting concentrations of protein improved solubility. Films formed from higher MW fibroin (235,169 kDa) were less soluble, though they were generally more resilient to the process of substrate removal. Conversely, films formed from the lowest MW fibroin (36 kDa) were highly soluble but were extremely brittle, and thus difficult to remove from their casting surfaces. On average, optimal 70 kDa silk films required a reconstitution time of only 10 seconds as assessed by a lack of visible particulate, as compared to 13 and 30 seconds for films prepared from 169 kDa and 235 kDa silk respectively. These outcomes are comparable to reconstitution time and appearances for existing lyophilized vaccine products. The 70 kDa silk fibroin films cast at 2.4% w/v with a volume of 100 μL offered the best trade-off between solubility and mechanics, while providing enough volume to include an adequate dose of IPV for stability studies. These casting parameters were advanced into formulation screening studies.
Silk fibroin IPV film formulation development
After identifying a suitable silk casting volume and molecular weight, we began screening the stabilizing potential of silk fibroin and common excipients with IPV. Prior work in our laboratory (Stinson, unpublished data) confirmed that silk fibroin combined with other proteins (BSA, gelatin), sugars (sucrose, trehalose), salts (magnesium chloride, calcium chloride), and buffers (citrate-phosphate, HEPES) does not lead to unwanted aggregation of silk fibroin in solution or insolubility of silk in dried formats. Moreover, published work indicated greater stabilization potential of sucrose over trehalose for IPV antigens [7], which was confirmed in combination with silk fibroin in our laboratory. Combinations of silk fibroin, sucrose, salts, other excipients, and IPV were used to prepare sixteen formulations that were cast as films, dried, and placed on accelerated stability testing (Supplemental Figure 2). IPV recovery from these formulations was assessed via D-antigen ELISA and used to characterize process loss through drying and antigen loss during storage at 45°C (Figure 3).
Figure 3.
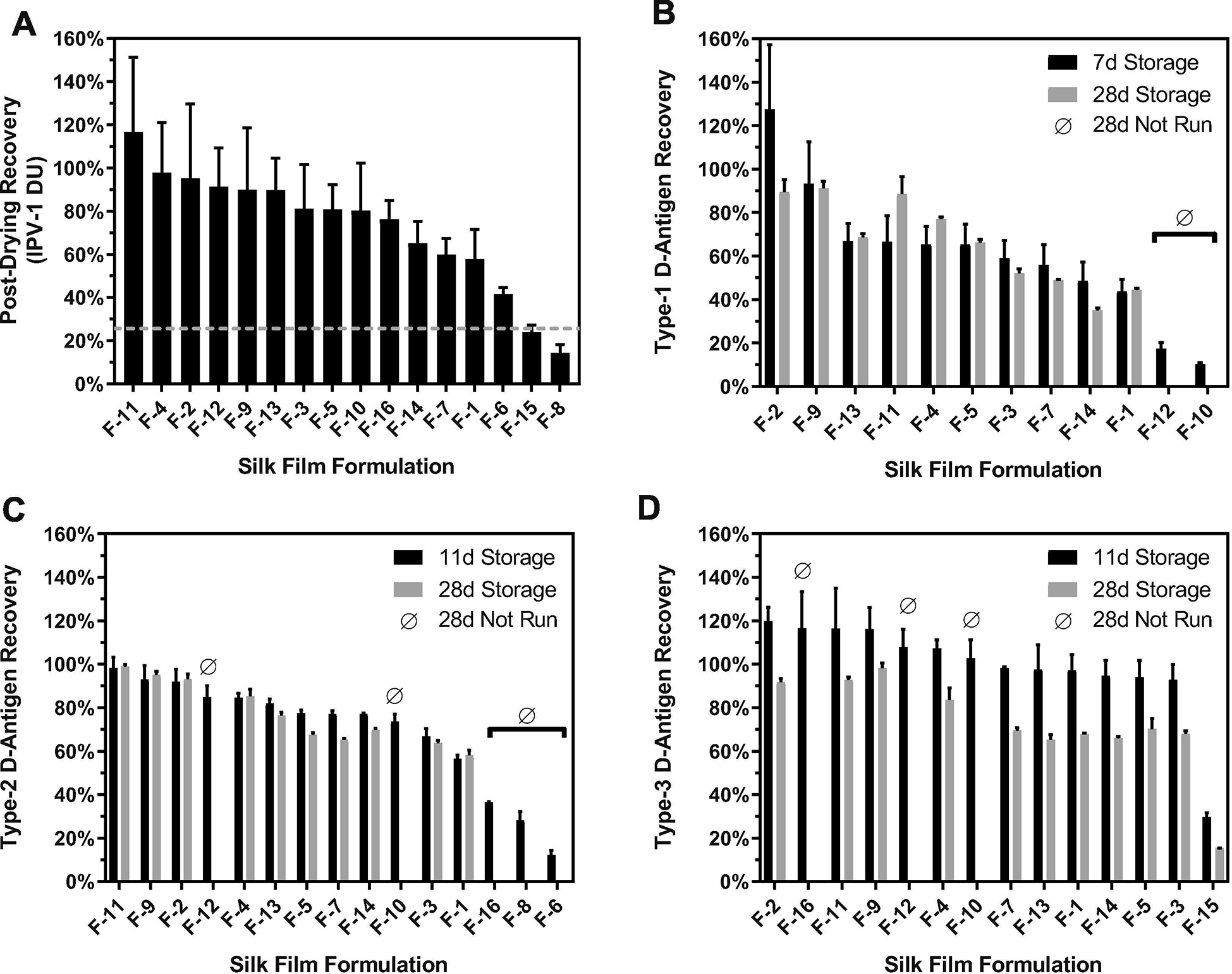
Characterization of process and storage loss of IPV D-antigens in silk fibroin films after storage at 45°C. Formulation development for IPV in silk fibroin films required initial assessment of loss through air-drying (A, type 1 ELISA; dashed line represents recovery from dried IPV alone without silk) and losses during storage at 45°C for one month for (B) type 1, (C) type 2, and (D) type 3 poliovirus strains. After running a mid-point ELISA for recovery, certain groups were abandoned for analysis at day 28. Displayed are average DU recovery values compared to theoretical loading (n=3 films) with standard deviation. Formulations are described in Supplemental Fig. 2.
Assessment of poliovirus type 1 recovery immediately after drying indicated a major role of excipients, as process loss ranged from less than ten percent to more than ninety percent. Formulations that contained both silk fibroin and sucrose (F-2, F-4, F-9, F-11, F-12) appeared to reduce process loss relative to silk-alone formulations (F-1, F-3) (Figure 3A). We found upon accelerated stability testing that the addition of divalent cations improved IPV storage stability in silk fibroin films, with magnesium outperforming calcium, consistent with findings in lyophilized format [7] (F-5, F-7; Figure 3B–D). Certain excipients previously reported to improve IPV stability [7], including sorbitol and monosodium glutamate, were found to cause significant silk insolubility upon drying, and subsequent poor IPV recovery. From this assessment, formulations containing silk fibroin, sucrose, magnesium chloride, and citrate-phosphate buffer offered minimal process loss of IPV antigens while maintaining significant potency after four weeks of storage at elevated temperatures. Further stability screens were performed to modify the concentrations of these excipients to maintain solubility of silk fibroin films through drying and storage (Supplemental Figure 3). As further characterization, we assessed the residual moisture of air-dried silk films through Karl Fischer titration and determined a residual moisture of 6.1%. It is generally accepted that for freeze-dried samples, low residual moisture values, typically less than 3%, are optimal for prolonged storage. However, we hypothesized that the higher residual moisture in silk films may contribute to increased antigen stability by improving the hydration shell around the virus, which in turn may prevent unfolding of surface proteins. We therefore proceeded without further formulation or process efforts to reduce residual moisture content.
Long-term stability of silk fibroin IPV films
To assess the stability profile of IPV-laden silk fibroin films, we conducted a real-time stability study of the air-dried films over nearly three years. Films were prepared with 2.4% (w/v) silk fibroin, 5% (w/v) sucrose, 10 mM magnesium chloride, 10 mM citrate-phosphate buffer, and were loaded with IPV (4 DU type 1, 0.8 DU type 2, and 3.2 DU type 3). These films were stored at 4, 25, 37 and 45°C with liquid IPOL® vaccine controls run alongside at 4°C and 45°C. Samples were removed at 0, 2, 4, 8, 12, 16, 26, and 52 weeks and assayed by ELISA for remaining potency (Figure 4A–C). For all three serotypes of IPV, liquid vaccine lost greater than 70% D-antigen content at 45°C during one week of storage. After one year at 45°C, silk fibroin IPV films had lost less than 50% D-antigen content for two of three poliovirus serotypes. Furthermore, potency of silk-IPV films when stored for over 1000 days at 25°C showed D-antigen levels of 73 ± 1.4%, 85 ± 1.3%, and 94 ± 2.4% for types 1, 2, and 3, respectively (mean ELISA recovery ± standard deviation, n = 8 film samples, Figure 4D). A repeat study with the same formulation was performed at a subset of the time points and temperatures to confirm the long-term results (Supplemental Figure 4).
Figure 4.
Long-term stability profiles of Salk IPV formulated in thin silk fibroin films. Formulated IPV films containing 2.4% (w/v) silk fibroin, 5% (w/v) sucrose, 10 mM MgCl2, 10 mM citrate-phosphate buffer and IPV (4 DU, 0.8 DU, 3.2 DU for types 1, 2, and 3, respectively) were stored in glass vials at 4°C, 25°C, 37°C and 45°C. At pre-determined storage durations, films were removed, reconstituted in buffered saline, and assayed via ELISA to determine residual D-antigen content for (A) type 1, (B) type 2, and (C) type 3 poliovirus strains. IPV vaccine (IPOL®; Sanofi-Pasteur, Swiftwater, PA) was also stored at 45°C for stability comparison. Displayed are mean D-antigen recoveries (n=3) at each storage temperature compared to 4°C recovery. (D) Films (n=3) stored at room temperature had D-antigen recovery of 73%, 85%, and 94% after 144 weeks for type 1, type 2, and type 3, respectively.
Film immunogenicity after accelerated stability testing
To confirm that the IPV product stability profiles observed in vitro translate to in vivo efficacy, we tested the ability of silk fibroin IPV films to induce protective titers in rats. Films of the same formulation as were used for the prolonged stability experiments were prepared and stored for two weeks at either 4°C or 45°C prior to reconstitution and injection intramuscularly to female Wistar rats (6–8 weeks, Charles River). IPOL® was administered at the same dose for immunogenicity comparison. Animals were immunized at day 0 and day 28 with separately prepared batches of silk fibroin IPV films. After two weeks of storage at 45°C, silk-IPV films generated neutralizing antibody responses to type 1 and type 2 polioviruses that were not statistically different than the IPOL® vaccine (Figure 5; unpaired two-tailed Student’s t-test; p=0.0501 type 1, p=0.4104 type 2, analysis in Prism by GraphPad). However, seroconversion was not observed in all animals immunized with silk-IPV films for type 1 and no seroconversion or detectable type 3 responses were observed from animals immunized with air-dried IPV formulations.
Figure 5.
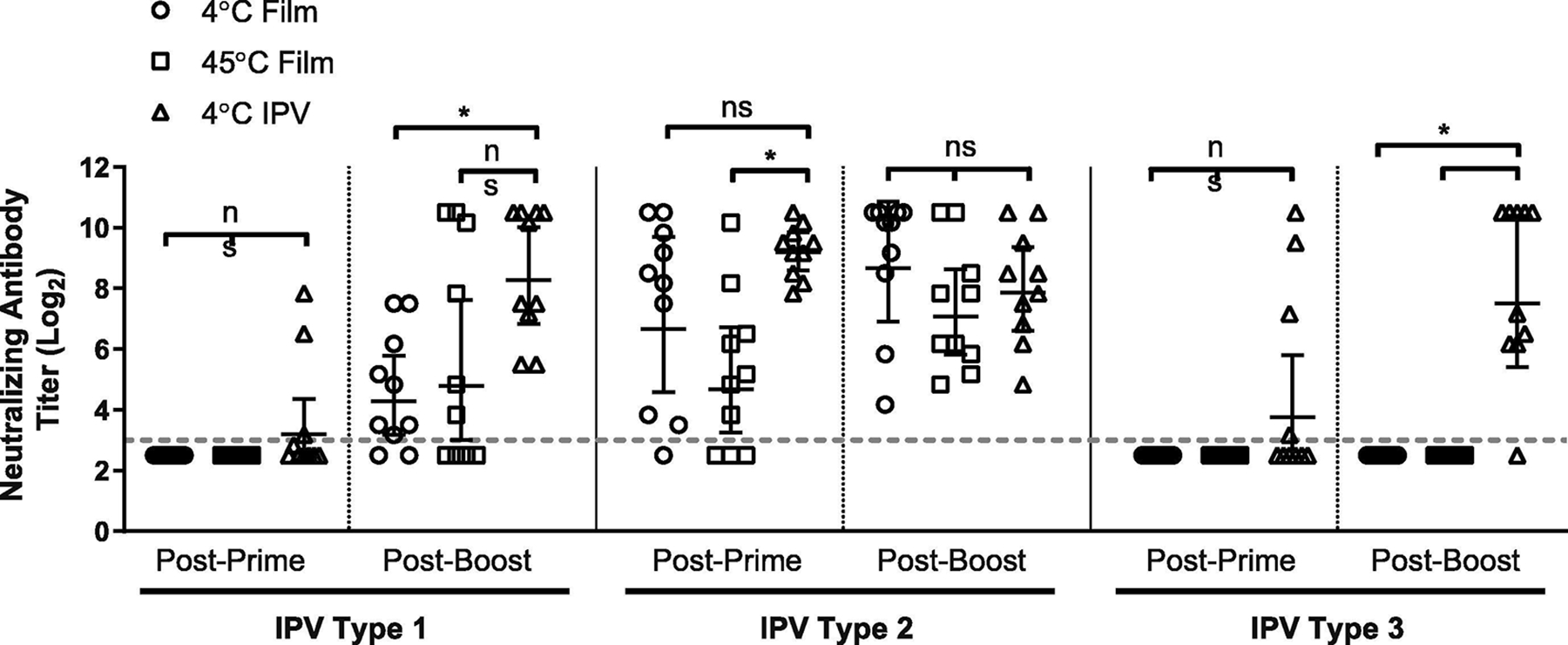
Immunogenicity of IPV antigens stored in thin silk fibroin film format. Formulated IPV films were stored for two weeks at either an elevated temperature (45°C) or refrigerated (4°C) prior to reconstitution and injection to Wistar rats (n=10) to assess retained immunogenicity. IPV (IPOL®, Sanofi-Pasteur, Swiftwater PA) was stored refrigerated and used as an unmodified liquid control for immunization. Neutralizing antibody titers were assessed via micro-neutralization assay on serum collected 4 weeks after primary immunization (day 28, “Post-Prime”) and 4 weeks after booster immunization (day 56, “Post-Boost”). Dashed line represents neutralization titer threshold for seroconversion; geometric means and 95% confidence interval displayed. Significance (ns: not significant, *: p<0.05) was determined using unpaired, two-tailed Student’s T-test.
Silk fibroin IPV film tolerance and safety
As a further aim of the immunogenicity study, we sought to examine the general safety of silk fibroin as a novel vaccine excipient. In solid formats, silk fibroin has been described as biocompatible with minimal inflammatory responses [16]. Following immunization with reconstituted silk films, rats were monitored for ill effects of administrations. We observed no differences in local reactogenicity at the site of injection nor any differences in systemic effects across all vaccine administrations, suggesting the silk fibroin protein was well-tolerated. Furthermore, we assessed the extent of antibodies raised against silk fibroin protein using an ELISA. Anti-silk responses from rats immunized with silk fibroin IPV films were compared to silk-naïve rats (pre-immune serum, vaccine-only serum). No significant differences in anti-silk IgG endpoint titer were observed between the silk-exposed and non-exposed groups after both the prime and boost immunizations (Figure 6; one-way ANOVA with Tukey’s test; p=0.1107 post-prime, p=0.1011 post-boost, analysis in Prism by GraphPad). These results indicate that air-dried silk fibroin films are a safe format for IPV stabilization and delivery.
Figure 6.
Assessment of generated antibody responses towards silk fibroin using ELISA. Serum from Wistar rats immunized using the IPV-loaded silk fibroin films was analyzed for the presence of antibodies directed against the silk protein after primary and booster immunization time points. These responses were compared against vaccine-only control (“4°C IPV”) and naïve animal serum collected prior to the study as negative control. Displayed are individual titers from individual Wistar rats (n=10) with geometric mean and 95% confidence interval. ns indicates no significant difference between treatment groups (one-way ANOVA with Tukey’s multiple comparisons test, p<0.05 for significance).
DISCUSSION
With the eradication of polio within reach, technologies to support global access to polio vaccines are critical to the success of this effort as well as post-eradication immunization to maintain eradication. One of the key challenges of conducting vaccine campaigns in the developing world is ‘last-mile’ distribution, encompassing the final step of the cold chain from the vaccine storage depot to remote vaccination sites. Improving the temperature stability of the vaccine product in order to remove refrigeration requirements during last-mile distribution would significantly improve the reach of immunization campaigns in the remaining polio-endemic regions. A similar case study with Meningitis A vaccine demonstrated that re-labeling the product for excursions of up to 40°C for up to four days helped simplify the logistics of vaccine distribution and led to less than 0.1% of vaccine vials being wasted [29].
In order to address this need, we developed a heat-stable inactivated polio vaccine formulation in an air-dried vaccine format using silk fibroin protein. Our approach began with the selection of an optimal silk condition for film formation, identified through screening of droplet casting parameters. Silk fibroin protein was then combined with a range of excipients and IPV to assess losses through processing and storage, allowing us to down select a lead formulation for long-term testing and assessment of immunogenicity in vivo. We have demonstrated that this lead formulation maintains IPV D-antigen content over 25-times longer than current liquid product at 45°C and has no significant effect on product efficacy, as measured by seroconversion, for two of the three poliovirus serotypes. Moreover, this silk fibroin formulation outperforms recent stable IPV formulations, such as an air-dried IPV formulation for microneedle-based delivery that retained more than 60% D-antigen content after one month of storage at room temperature [9] and another microneedle formulation with retained D-antigen content greater than 40% after two months at 40°C [11]. Here, the silk fibroin IPV film format maintains greater than 70% potency after nearly three years of storage at room temperature. Similar improvements in the stability profile of the silk fibroin IPV film format are observed at 37°C storage when compared to formulations previously described [7, 8, 10]. Such an outcome is an encouraging step towards the development of a marketable heat stable IPV product.
This was the first study we are aware of to consider three specific performance characteristics of silk fibroin films: solubility, mechanics for substrate removal, and ultimately long-term stability in the context of other formulation components. Constraining the parameter space to include formulations capable of mechanical lift-off resulted in ideal constructs with a silk-to-surface area ratio of 10 μg/mm2 and a fibroin casting concentration of roughly 2% (w/v). Furthermore, precise control of silk fibroin molecular weight was demonstrated to have a significant effect on the key parameters of our system and fundamentally enable the successful creation of an air-dried stable polio vaccine format. Due to its impressive ability to maintain the stability of the IPV vaccine, we worked to refine the method of passive drying. As an alternative and potentially more efficient method to deploy these silk-laden formulations, we investigated a process whereby the solutions were cast and subsequently vacuum dried directly in serum vials, resulting in thin conformal coating on the bottom surface of the vials (Supplemental Figure 5). We observed similar solubility profiles of these formats to the free-standing films when reconstituted with the identical fill volume of the initial formulation. This methodology could potentially be more readily scalable by utilizing existing lyophilization equipment and infrastructure, while allowing us to circumvent mechanical film constraints in future product development efforts.
Having established a repeatable and effective silk fibroin based dry film format for vaccine formulation, we then assessed the long-term stability of our samples. Building upon extensive work from other groups, we utilized the previously established stability enhancement of divalent cations and sugar excipients to further the stabilizing capabilities of silk fibroin protein [7, 8]. We had hypothesized that glassy silk protein films could reduce mobility of IPV antigens, and that addition of excipients could provide synergistic enhancements to IPV antigen stability. This hypothesis was evaluated under accelerated and real-time stability studies to display the long-term storage potential of the silk fibroin and IPV films. ELISA recovery results offer evidence for the in vitro stability of the dried film format, which maintained significant potency at room temperature for almost three years. Importantly, we did not observe any major changes in the physical properties of films over this storage period, including the reconstitution properties of the films, discoloration, or gross changes in size. While silk-based protection of IPV against freeze-thaw stress was not explored as part of this work, stabilization in the context of freeze sensitivity remains an area of focus to address additional challenges in global IPV distribution.
Despite the high recovery of antigen after storage at elevated temperature, we also sought to establish that the developed silk fibroin formulation does not impact vaccine immunogenicity. As such, we challenged samples for two weeks at 45°C prior to in vivo administration to female Wistar rats and observed seroconversion against both type 1 and 2 polio antigens. However, we also observed nearly a complete loss of type 3 poliovirus neutralizing antibody responses in animals despite ELISA results suggesting maintenance of >90% type 3 antigen stability at all temperatures measured for the film format. This finding is not unexpected based on numerous similar reports in the literature. Although promising dried poliovirus type 3 formulations have been demonstrated in vitro, none have translated to type 3 neutralizing antibody responses sufficient for seroconversion in vivo [7, 8, 9]. Furthermore, a number of these formulations have been tested as coatings on microneedles, which deliver the vaccine intradermally and may confound formulation benefits with route of administration benefits [5, 26]. Otherwise, strategies to include additional doses of dried vaccine or to include an adjuvant have been required to generate comparable type 3 seroconversion and neutralizing antibody titers versus an intramuscular injection of vaccine [5, 30]. Some references describe cross-reactivity of poliovirus type 3 D-antigen (neutralizing) and C-antigen (non-neutralizing) with monoclonal antibodies [31]. We have treated vaccine at 56°C to force D-to-C antigen conversion [32] and have observed significant signal on the type 3 ELISA, suggesting the monoclonal antibody used may cross-react with both type 3 poliovirus conformations (data not shown). As such, investigation of additional type 3 D-antigen monoclonal antibodies [33] or development of a complementary type 3 C-antigen ELISA is warranted.
Finally, host responses to silk fibroin protein were found to be low and not significantly different than silk-naïve animals in the immunogenicity study with IPV films. This result is an important step towards demonstrating the safety of silk fibroin protein in a soluble format, which differs from FDA-approved fibroin-based surgical sutures (Ethicon Perma-Hand®; Johnson & Johnson, New Brunswick, NJ) and mesh scaffolds (SERI® Surgical Scaffold; Sofregen, Medford, MA). Silk fibroin protein exposure in rats was nearly two orders of magnitude higher in concentration than IPV exposure and was well-tolerated. This result is consistent with the non-immunodominant nature of silk fibroin protein previously reported with other vaccine antigens [28]. As a stabilizer, silk offers similar properties to gelatin but without the cultural concerns associated with mammalian sources of gelatin. However, prior to adoption of silk fibroin as a common excipient like gelatin, additional studies will be required to better characterize the safety profile of the protein and ensure no allergic responses are generated after repeat exposure. In addition, scalable production techniques for both silk fibroin solution and thin films will be needed to apply the stabilization technology to other vaccine targets. Thin films offer advantages over other dried product formats, such as lyophilized cakes, due to more rapid drying. As silk fibroin thin films can be prepared using existing lyophilization equipment and will not require development of new manufacturing infrastructure, heat-stable IPV films could be rapidly scaled-up upon full safety characterization of silk fibroin protein.
CONCLUSIONS
We have presented here the development of novel air-dried silk films for temperature stabilization of IPV. After engineering films for silk fibroin solubility and mechanical features as the backbone of the film formulation, an additional multi-component excipient screening effort was pursued to optimize long-term stability. This effort ultimately resulted in a dried IPV product with minimal antigen loss through drying that vastly outperformed the stability of the existing liquid vaccine. Maintenance of immunogenicity in the silk film format after storage at 45°C demonstrates the potential for last-mile distribution without refrigeration as a key means to increase access to IPV. Additional development is required to optimize the type 3 formulation for in vivo responses. In the future, the underlying formulation and air-drying approach used for silk fibroin films could be complementary with more disruptive delivery formats, including microneedles, to enable simplified distribution and administration of vaccines.
Supplementary Material
HIGHLIGHTS.
Novel polio vaccine format developed for improved product stability
Drying of antigen with short process time and high recovery enabled by silk fibroin
Minimal antigen loss after nearly three years of storage at room temperature
IPV-1 and IPV-2 seroconversion in rats achieved using vaccine stressed at 45°C
Format is compatible with novel delivery systems such as microneedle patches
ACKNOWLEDGEMENTS
The research reported in this publication was supported in part by the National Institute of Allergy and Infectious Diseases of the National Institutes of Health under Award Number R43AI118107. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CONFLICT OF INTEREST STATEMENT
All authors (except those with CDC affiliation) were employees of Vaxess at the time of the study. Employees and contract staff to CDC have no conflicts of interest.
Declaration of interests
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
REFERENCES
- [1].Morales M, Tangermann RH, Wassilak SG. Progress toward polio eradication – worldwide, 2015–2016. MMWR Morb Mortal Wkly Rep 2016; 65:470–3. (DOI: 10.15585/mmwr.mm6518a4) [DOI] [PubMed] [Google Scholar]
- [2].World Health Organization. Global Wild Poliovirus 2013–2018, 2018. Available at: http://polioeradication.org/wp-content/uploads/2018/10/global-wild-poliovirus-2013-2018-20181009.pdf, Accessed October 17, 2018.
- [3].Garon JR, Orenstein WA. A worldwide shift in polio vaccines for routine immunization. Lancet 2015; 386:2375–7. (DOI: 10.1016/S0140-6736(15)00243-3) [DOI] [PubMed] [Google Scholar]
- [4].Le Bouvier GL. The modification of poliovirus antigens by heat and ultraviolet light. Lancet 1955; 269:1013–6. (DOI: 10.1016/S0140-6736(55)93435-8) [DOI] [PubMed] [Google Scholar]
- [5].Kraan HS, Ploemen I, van de Wijdeven G, Que I, Loewik C, Kersten G, Amorij J-P. Alternative delivery of a thermostable inactivated polio vaccine. Vaccine 2015; 33:2030–7. (DOI: 10.1016/j.vaccine.2015.03.011) [DOI] [PubMed] [Google Scholar]
- [6].Kanra G, Silier T, Yurdakök K, Yavuz T, Baskan S, Ulukol B, Ceyhan M, Özmert E, Türkay F, Pehlivan T. Immunogenicity study of a combined diphtheria, tetanus, acellular pertussis, inactivated poliomyelitis vaccine used to reconstitute a freeze-dried Haemophilus influenzae type b vaccine (DTaP-IPV//PRP-T) administered simultaneously with a hepatitis B vaccine at two, three and four months of life. Vaccine 1999; 18(9–10):947–954. (DOI: 10.1016/S0264-410X(99)00331-X) [DOI] [PubMed] [Google Scholar]
- [7].Kraan H, van Herpen P, Kersten G, Amorij J-P. Development of thermostable lyophilized inactivated polio vaccine. Pharm Res 2014; 31:2618–29. (DOI: 10.1007/s11095-014-1359-6) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Tzeng SY, Guarecuco R, McHugh KJ, Rose S, Rosenberg EM, Zeng Y, Langer R, Jaklenec A. Thermostabilization of inactivated polio vaccine in PLGA-based microspheres for pulsatile release. J Control Release 2016; 233:101–13. (DOI: 10.1016/j.jconrel.2016.05.012) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Wan Y, Hickey JM, Bird C, Withan K, Fahey P, Forster A, Joshi SB, Volkin DB. Development of stabilizing formulations of a trivalent inactivated poliovirus vaccine in a dried state for delivery in the Nanopatch™ microprojection array. J Pharm Sci 2018; S0022–3549(18)30071–6. (DOI: 10.1016/j.xphs.2018.01.027) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Qi W, Orgel S, Francon A, Randolph TW, Carpenter JF. Urea Improves Stability of Inactivated Polio Vaccine Serotype 3 During Lyophilization and Storage in Dried Formulations. J Pharm Sci 2018; 107: 2070–2078. (DOI: 10.1016/j.xphs.2018.04.019) [DOI] [PubMed] [Google Scholar]
- [11].Kolluru C, Gomaa Y, Prausnitz MR. Development of a thermostable microneedle patch for polio vaccination. Drug Deliv Trans Res 2019; 9:192–203. (DOI: 10.1007/s13346-018-00608-9) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Lawrence BD, Cronin-Golomb M, Georgakoudi I, Kaplan DL, Omenetto FG. Bioactive silk protein biomaterial systems for optical devices. Biomacromolecules 2008; 9:1214–20. (DOI: 10.1021/bm701235f) [DOI] [PubMed] [Google Scholar]
- [13].Zhou C-Z, Confalonieri F, Jacquet M, Perasso R, Li Z-G, Janin J. Silk fibroin: structural implications of a remarkable amino acid sequence. Proteins 2001; 44:119–22. (DOI: 10.1002/prot.1078) [DOI] [PubMed] [Google Scholar]
- [14].Perrone GS, Leisk GG, Lo TJ, Moreau JE, Haas DS, Papenburg BJ, Golden EB, Partlow BP, Fox SE, Ibrahim AMS, Lin SJ, Kaplan DL. The use of silk-based devices for fracture fixation. Nat Commun 2014; 5:3385 (DOI: 10.1038/ncomms4385) [DOI] [PubMed] [Google Scholar]
- [15].Lu Q, Hu X, Wang X, Kluge JA, Lu S, Cebe P, Kaplan DL. Water-insoluble silk films with silk I structure. Acta Biomater 2010; 6:1380–7. (DOI: 10.1016/j.actbio.2009.10.041) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Thurber AE, Omenetto FG, Kaplan DL. In vivo bioresponses to silk proteins. Biomaterials 2015; 71:145–57. (DOI: 10.1016/j.biomaterials.2015.08.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Kluge JA, Li AB, Kahn BT, Michaud DS, Omenetto FG, Kaplan DL. Silk-based blood stabilization for diagnostics. Proc Natl Acad Sci USA 2016; 113:5892–7. (DOI: 10.1073/pnas.1602493113) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Li AB, Kluge JA, Zhi M, Cicerone MT, Omenetto FG, Kaplan DL. Enhanced stabilization in dried silk fibroin matrices. Biomacromolecules 2017; 18:2900–5. (DOI: 10.1021/acs.biomac.7b00857) [DOI] [PubMed] [Google Scholar]
- [19].Lu S, Wang X, Lu Q, Hu X, Uppal N, Omenetto FG, Kaplan DL. Stabilization of enzymes in silk films. Biomacromolecules 2009; 10:1032–42. (DOI: 10.1021/bm800956n) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Lu Q, Wang X, Hu X, Cebe P, Omenetto FG, Kaplan DL. Stabilization and release of enzymes from silk films. Macromol Biosci 2010; 10:359–68. (DOI: 10.1002/mabi.200900388) [DOI] [PubMed] [Google Scholar]
- [21].Guziewicz NA, Massetti AJ, Perez-Ramirez BJ, Kaplan DL. Mechanisms of monoclonal antibody stabilization and release from silk biomaterials. Biomaterials 2013; 34:7766–75. (DOI: 10.1016/j.biomaterials.2013.06.039) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Li AB, Kluge JA, Guziewicz NA, Omenetto FG, Kaplan DL. Silk-based stabilization of biomacromolecules. J Control Release 2015; 219:416–30. (DOI: 10.1016/j.conrel.2015.09.037) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Rockwood DN, Preda RC, Yucel T, Wang X, Lovett ML, Kaplan DL. Materials fabrication from Bombyx mori silk fibroin. Nat Protoc 2013; 6:1612–31. (DOI: 10.1038/nprot.2011.379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Kluge JA, Kahn BT, Brown JE, Omenetto FG, Kaplan DL. Optimizing molecular weight of lyophilized silk as a shelf-stable source material. ACS Biomater Sci Eng 2016; 2:595–605. (DOI: 10.1021/acsbiomaterials.5b00556) [DOI] [PubMed] [Google Scholar]
- [25].Yucel T, Lovett ML, Giangregorio R, Coonahan E and Kaplan DL, 2014. Silk fibroin rods for sustained delivery of breast cancer therapeutics. Biomaterials, 35(30), pp.8613–8620. [DOI] [PubMed] [Google Scholar]
- [26].Edens C, Dybdahl-Sissoko NC, Weldon WC, Oberste MS, Prausnitz MR. Inactivated polio vaccination using a microneedle patch is immunogenic in the rhesus macaque. Vaccine 2015; 33:4683–90. (DOI: 10.1016/j.vaccine.2015.01.089) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Weldon WC, Oberste MS, Pallansch MA. Standardized methods for detection of poliovirus antibodies. Methods Mol Biol 2016; 1387:145–76. (DOI: 10.1007/978-1-4939-3292-4_8) [DOI] [PubMed] [Google Scholar]
- [28].Stinson JA, Raja WK, Lee S, Kim HB, Diwan I, Tutunjian S, Panilaitis B, Omenetto FG, Tzipori S, Kaplan DL. Silk fibroin microneedles for transdermal vaccine delivery. ACS Biomater Sci Eng 2017; 3:360–9. (DOI: 10.1021/acsbiomaterials.6b00515) [DOI] [PubMed] [Google Scholar]
- [29].Zipursky S, Djingarey MH, Lodjo J-C, Olodo L, Tiendrebeogo S, Ronveaux O. Benefits of using vaccines out of the cold chain: Delivering Meningitis A vaccine in a controlled temperature chain during the mass immunization campaign in Benin. Vaccine 2014; 32:1431–5. (DOI: 10.1016/j.vaccine.2014.01.038) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Muller DA, Fernando GJP, Owens NS, Agyei-Yeboah C, Wei JCJ, Depelsenaire ACI, Forster A, Fahey P, Weldon WC, Oberste MS, Young PR, Kendall MAF. High-density microprojection array delivery to rat skin of low doses of trivalent inactivated poliovirus vaccine elicits potent neutralizing antibody responses. Sci Rep 2017; 7:12644 (DOI: 10.1038/s41598-017-13011-0) [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Singer C, Knauert F, Bushar G, Klutch M, Lundquist R, Quinnan GV Jr. Quantitation of poliovirus antigens in inactivated viral vaccines by enzyme-linked immunosorbent assay using animal sera and monoclonal antibodies. J Biological Standardization 1989; 17:137–50. (DOI: 10.1016/0092-1157(89)90004-8) [DOI] [PubMed] [Google Scholar]
- [32].Beale AJ. The D-antigen content in poliovaccine as a measure of potency. Lancet 1961; 278:1166–68. (DOI: 10.1016/S0140-6736(61)90843-1) [DOI] [PubMed] [Google Scholar]
- [33].Sawyer LA, Wood D, Ferguson M, Crainic R, Coen Beaver E, McInnis J, Albrecht P. Potency of wild-type or Sabin trivalent inactivated poliovirus vaccine, by enzyme-linked immunosorbent assay using monoclonal antibodies specific for each antigenic site. Biologicals 1997; 25:299–306. (DOI: 10.1006/biol.1997.0100) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


