Figure 1.
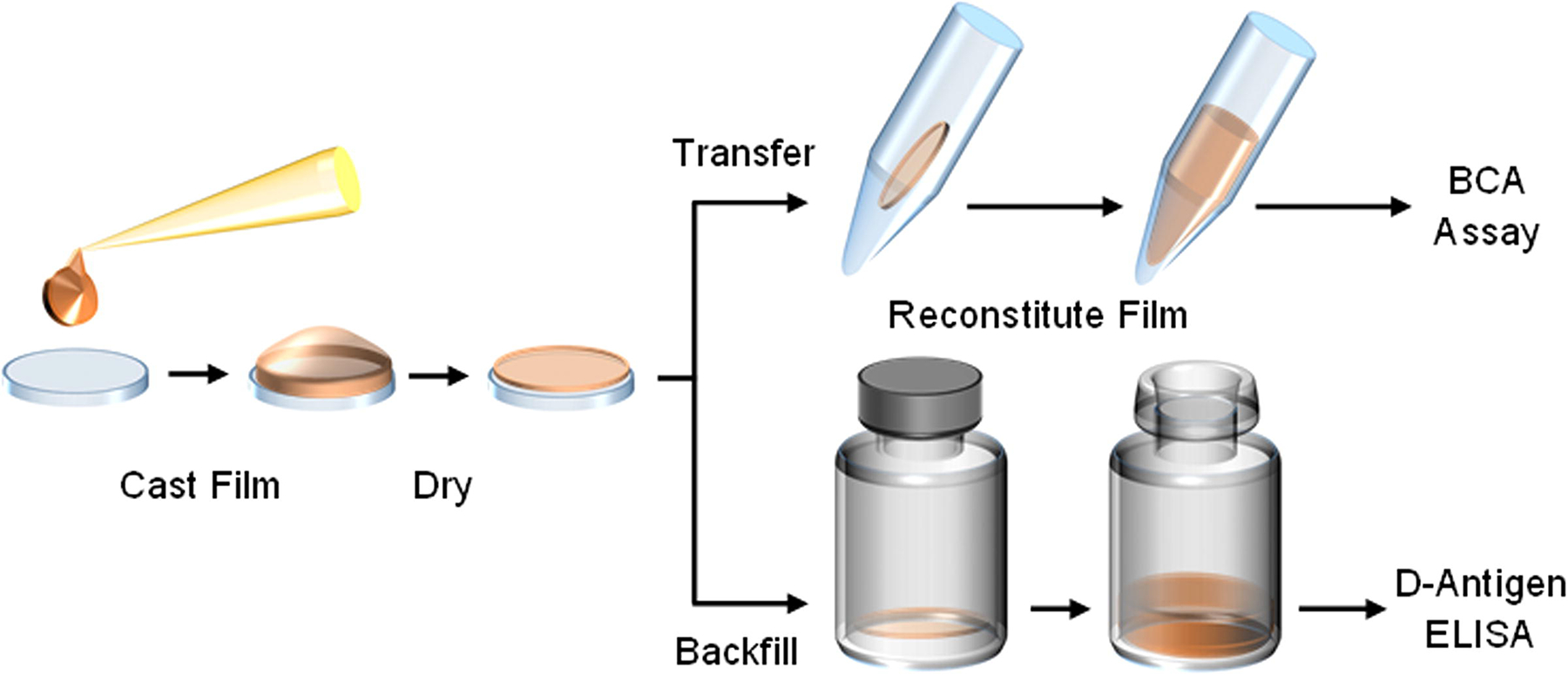
Thin silk fibroin film processing begins by pipetting a volume of neat or IPV-containing silk fibroin solution onto a sterile polydimethylsiloxane (PDMS) chip and spreading evenly across the surface. This volume is air-dried in a biosafety cabinet, and then transferred into an appropriate container for assessment. For baseline solubility assessments of thin silk fibroin films, the material is transferred to a capped tube, reconstituted using sterile buffer, and protein content measured through a bicinchoninic acid (BCA) assay. For stability assessments of IPV, films are stored in sterile glass serum vials and backfilled under inert nitrogen for storage. Upon reaching storage time points, films are reconstituted with buffer and assayed on the D-antigen ELISA.
