Endangered staghorn and elkhorn corals began disappearing from Caribbean reefs decades before climate change impacts.
Abstract
The mass mortality of acroporid corals has transformed Caribbean reefs from coral- to macroalgal-dominated habitats since systematic monitoring began in the 1970s. Declines have been attributed to overfishing, pollution, sea urchin and coral disease, and climate change, but the mechanisms are unresolved due to the dearth of pre-1970s data. We used paleoecological, historical, and survey data to track Acropora presence and dominance throughout the Caribbean from the prehuman period to present. Declines in dominance from prehuman values first occurred in the 1950s for Acropora palmata and the 1960s for Acropora cervicornis, decades before outbreaks of acroporid disease or bleaching. We compared trends in Acropora dominance since 1950 to potential regional and local drivers. Human population negatively affected and consumption of fertilizer for agriculture positively affected A. palmata dominance, the latter likely due to lower human presence in agricultural areas. The earlier, local roots of Caribbean Acropora declines highlight the urgency of mitigating local human impacts.
INTRODUCTION
The living cover of Caribbean reef-building corals has declined by 50% since systematic reef monitoring began in the late 1970s (1). During this time, the majority of Caribbean reefs have been transformed from habitats dominated by reef-building corals into habitats dominated by macroalgae, sponges, and/or non–reef-building invertebrates (2–4). The decline in corals has been attributed to fishing, land-based pollution, anthropogenic ocean warming, and outbreaks of coral and sea urchin diseases (1, 5, 6).
Modern ecological studies of Caribbean reefs began in the late 1960s, less than a decade before a series of acute events acted in synergy to rapidly transform coral communities. Outbreaks of “white-band disease” (WBD) appeared on many reefs in the late 1970s and early 1980s, eventually killing over 80% of the populations of the elkhorn coral Acropora palmata, which previously dominated reef crest zones, and the staghorn coral Acropora cervicornis, which previously dominated midslope zones (1, 7–9). Mass mortality of the sea urchin Diadema antillarum in 1983–1984 due to an unidentified pathogen removed this keystone herbivore from reefs that were already largely devoid of large herbivorous fish because of overfishing (1, 10, 11). Diadema mortality exceeded 90% (12), precipitating an explosion of macroalgae on reefs across the Caribbean. These events were followed by local outbreaks of coral bleaching beginning in the late 1980s followed by regional outbreaks in the 1990s, leading to further increases in coral disease and, in some instances, a further replacement of corals by macroalgae (1, 2, 13).
Despite decades of research, the origin and transmission of WBD in Caribbean Acropora are still poorly understood. However, multiple anthropogenic stressors appear to have played a role. Recent observations (1997–2004) of the presence of WBD on Acropora corals across the Caribbean show a link between contemporary WBD and elevated sea surface temperature from anthropogenic climate change (14). Although the coverage of early survey data is insufficient to investigate causes of the initial WBD epidemics of the late 1970s and early 1980s, they may also have been related to temperature stress: Anthropogenic warming of sea surface waters in the Caribbean first became pronounced in the 1970s (15, 16). Initial and subsequent WBD outbreaks may also have been caused by increased macroalgal abundance related to overfishing of reef herbivores and/or reef eutrophication, as numerous experiments have found increased disease prevalence in other Caribbean scleractinian coral species associated with macroalgal contact (17–19). Nutrient enrichment from land-based runoff has also likely exacerbated WBD outbreaks by suppressing coral immunity and encouraging growth of pathogenic microbes and allelopathic algae (19–21). Another hypothesis is that declines in acroporid and other corals in the Caribbean are related to an increase in hurricane frequency and intensity due to climate change, which could limit Acropora recovery on reefs already degraded by overfishing and nutrification. Although hurricanes have been a natural occurrence on low-latitude Caribbean coral reefs for millions of years, corals in this region have increasingly failed to recover following major storms (2, 22).
The loss of acroporids has profoundly altered the structure and functioning of Caribbean reef ecosystems, as this genus grows up to 5 to 10 times faster and taller than other Caribbean coral species and disproportionately contributes to reef architectural complexity and carbonate production (23, 24). The virtual absence of these species at the shallow zones of most reefs today represents an unnatural state for modern Caribbean reef ecosystems. Studies of uplifted Pleistocene and Holocene reefs revealed that Acropora dominance has persisted for at least the past ~250,000 years despite marked fluctuations in temperature and sea level (25–27). Isolated surveys of altered reefs have tracked an increase in the relative abundance of disturbance-tolerant and low-relief “weedy” species such as Porites and Agaricia over the past few decades following the loss of Acropora, indicating an unprecedented shift from dominance of superior competitors to that of stress-tolerant and weedy species (6, 28–30).
The scarcity of quantitative ecological surveys before the 1970s leaves open the question of when the declines in acroporids may have initially begun. Two isolated studies based on historical and paleontological data suggest that acroporids began to decline well before the 1970s, most likely due to increases in coastal runoff from land clearing for agriculture (30, 31), but the geographic extent of these declines is unknown. In contrast, another isolated paleocological study found that A. cervicornis dominance was continuous over the past thousands of years until its abrupt decline beginning in the 1980s (5). To resolve the initial timing of the widespread decline in Caribbean acroporids, we compiled an extensive dataset of qualitative and quantitative observations of the presence and dominance of both staghorn (A. cervicornis) and elkhorn (A. palmata) corals within reef crest and forereef zones at a number of reef sites throughout the Caribbean Sea, spanning the prehuman Late Pleistocene epoch (~125,000 years before present) to present (2011 AD). To explore the possible causes of declines in these corals, we related Acropora dominance since the 1950s to available proxies of potential regional and local disturbances.
MATERIALS AND METHODS
Coral community composition database
Occurrence data of Acropora corals from the Pleistocene to 2011 were obtained in a variety of ways. Semiquantitative (number of colonies, species abundance rankings, and percent weight of coral skeletons in reef matrix cores) and qualitative (observations of dominance/commonness/rarity or species’ presence/absence) data were compiled from the primary peer-reviewed scientific literature, government reports, and (less commonly) historical literature, including field notes from early explorers. Quantitative data (percent fossil abundance, percent living cover) were compiled from surveys of uplifted fossil reefs or underwater survey data of modern reefs that were received directly from contributors or gleaned from peer-reviewed literature to construct the Global Coral Reef Monitoring Network (GCRMN) database that assessed trends in Caribbean reef benthic communities from 1970 to 2011 (1). For data from the historical and modern periods (1500–2011 AD), presence and dominance values were reported from a single survey encompassing a single day or from a series of multiyear surveys spanning up to 6 years. For paleoecological data from the Pleistocene and Holocene epochs, presence and dominance values were reported from samples of fossil coral assemblages that represent one or more centuries of reef growth to entire geologic units that represent up to 60,000 years of reef growth. Fossil data were gathered from reef matrix cores collected below current sea level for the Holocene and from transect surveys of uplifted reefs for the Pleistocene, while modern data were primarily derived from underwater field surveys, although a small number were from boat-based observations and high-resolution aerial photographs (see table S1 for a complete list of data sources).
To strike a balance between providing sufficient temporal resolution and ensuring adequate sample sizes and geographic coverage when assessing the original timing of declines in both Acropora species, data were grouped into 12 time bins: Pleistocene (~125,000 to 12,000 years ago), Holocene (~9,100 years ago to 1500 AD), 1500 to 1949, 1950 to 1959, 1960 to 1969, 1970 to 1979, 1980 to 1984, 1985 to 1989, 1990 to 1994, 1995 to 1999, 2000 to 2004, and 2005 to 2011. Bins were reduced to 5-year increments after 1980 (except for a 6-year increment for the most recent bin) due to the large increase in reef survey effort following the mass die-off of the sea urchin D. antillarum.
Data from the literature were extracted from text, tables, figures, and maps. Qualitative data were included in the database if, in addition to presence/absence and/or dominance information for at least one Acropora species, the following set of associated information was also available: (i) age of fossil assemblage or year of observation of modern data, (ii) original source of data, (iii) country and island, coastline, or reef site, and (iv) water depth or reef zone. Data were recorded at survey level, with a survey constituting a unique combination of reef site, depth zone, and year/time period. Surveys constituted individual reef “sites” in some cases and encompassed more extensive areas such as entire reef tracts, bays, or banks in other cases.
Data were compiled from “reef crest” and “midslope” reef zones, the zones where Acropora were noted to occur in early Caribbean reef surveys (32, 33). Generally, reef crest data spanned 0- to 6-m water depth, and midslope data spanned between 6 and 20 m, as 6 m was the depth at which dominance typically transitioned from A. palmata to A. cervicornis in the semiquantitative and quantitative data. However, the reef crest/midslope zone delineation was made on a location-by-location basis by first considering water depth, and when available, considering additional environmental characteristics such as wave exposure and reef morphology. For some offshore reef locations with presumably higher water clarity, the boundary between reef zones was closer to 10 m, the same value used in a coarser analysis in Jackson et al. (28). When a precise water depth was not available, we used Acropora species presence and/or dominance in addition to environmental characteristics to delineate between zones. When not reported in the papers from which data were extracted, paleo water depths were determined by (i) defining sea surface as the top of the Pleistocene fossil reef terrace sampled and (ii) constructing a composite Caribbean-wide sea level curve for the Holocene using data presented in (34–38). Surveys from backreef habitats, reef flats, and reef pavements were excluded, as these reef zones are not the preferred environments for Acropora in the Caribbean (33).
Changes in Acropora presence and dominance were assessed from analysis of percent living coral cover, abundance rankings, and presence/absence data. Species were ranked by percent coral cover values, species rankings, and qualitative descriptions of relative abundance (e.g., “principal reef-building coral” = 1, “second most commonly found coral” = 2). Presence and ranking values were assigned for subordinate (nondominant) species only if a source contained abundance or relative abundance data for at least two species. For data sources that only listed the presence or dominance of one species, the remaining species were assumed to be nondominant (i.e., ranking = 0), but their presence or absence could not be determined [i.e., presence = not applicable (NA)].
Initial timing of Acropora decline
Temporal changes in the presence and dominance of Acropora across the Caribbean were tracked by computing the proportion of sites with each species present and dominant in each time bin. To account for the uneven geographic distribution of samples across time bins, we assigned each reef site to an island group, large reef tract, or country (which we refer to as “country”; tables S2 and S3), and we used binomial generalized linear mixed-effects models (GLMMs) that included time bin as fixed effect and country as random effect (see table S4 for model specification). We chose the country level to represent spatial structure in the data because (i) the imprecision of the geographic locations noted in some of the historical records and sparser sample sizes in the historical and early survey periods (Fig. 1) did not permit a finer geographic partitioning, and (ii) the only reliable, multidecadal proxy for coastal pollution available, fertilizer consumption compiled by the United Nations Food and Agriculture Organization (FAO), is reported at this same spatial resolution (see next section). It was not possible to analyze temporal trends within individual countries, as data were too sparse for most countries (figs. S1 to S4). Separate models were run for both species in the reef crest and midslope zones. We determined the earliest timing of significant change in the presence and dominance of each Acropora coral species compared with the prehuman baseline state (the Pleistocene epoch) from Wald’s Z tests of differences between Pleistocene values with those from other time bins (tables S5 to S8) (39). To account for the possibility that high Acropora presence and dominance values in Pleistocene and Holocene periods may be related to the relatively longer time spans these periods encompass, we excluded these periods and ran additional GLMMs of the same form described above for A. palmata presence and dominance at the reef crest and A. cervicornis presence and dominance at the midslope zone. Estimates of uncertainty for percent presence and dominance were computed from binomial confidence intervals.
Fig. 1. Distribution of dominance and presence/absence data for acroporid corals.
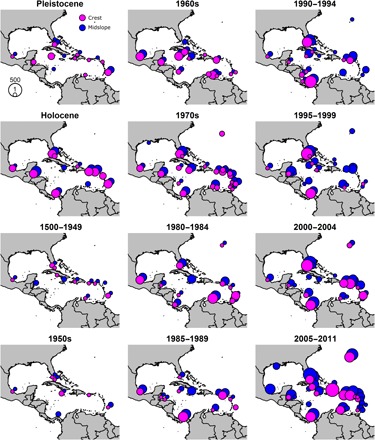
Data from reef crest zones in magenta; data from midslope zones in blue. Size of dot proportional to total number of surveys across both reef zones and all bins combined (range, 1 to 541).
Potential drivers of Acropora decline
To explore the possible causes of initial declines in Acropora, we assessed the influence of a suite of local and regional stressors on Acropora dominance from 1950 to 2011 using binomial GLMMs. We compiled data for four potential drivers for which reliable data were available from at least the 1960s to the present: temperature stress represented by degree heating months (DHMs), hurricane activity represented by average number of hurricanes per year, coastal agricultural pollution represented by total fertilizer consumption, and a general proxy of anthropogenic disturbance (including fishing) represented by human population density. We were not able to include an independent measure of fishing effort in our analysis due to the lack of reliable data over the broad geographic and temporal scale investigated in this study.
Temperature data were compiled from the Hadley Centre Global Sea Ice and Sea Surface Temperature (HadISST) dataset, a product that blends historical shipboard temperature records with satellite records (http://hadobs.metoffice.com/hadisst/). Monthly sea surface temperature averaged over 1 × 1–degree cells was extracted for each year from 1950 to 2011, and DHMs were computed from latitude and longitude coordinates for each year and reef site by summing the positive monthly temperature anomalies relative to the maximum monthly temperature from the climatological base period from 1900 to 2011. Average and maximum DHMs were then computed for each reef site and time bin. Geographic coordinates were obtained from Google Earth for reef sites with a specific identifiable location indicated, while coordinates for the center of a more general region or island were used for entries without a specific reef site name recorded. Although we calculated average and maximum DHM for each reef site, we chose to include the latter in our analyses because it often included values >2°C and, thus, was a more appropriate proxy of acute temperature stress (40).
Hurricane data were compiled by tallying the number of unique hurricanes (from categories 1 to 5) that were reported to cross within approximately 20 km of each reef site or island [an area that typically encompasses maximum wind speeds; (41)] during a time bin and then dividing by the number of years included in that bin. We chose to include hurricanes from all categories because Caribbean acroporids are susceptible to fragmentation from lower-intensity storms (42) and chose to focus on hurricane frequency because this variable greatly affects Acropora recovery potential (43). Data were obtained from http://stormcarib.com/climatology/, whose analyses were based on “best track” data taken from the National Oceanic and Atmospheric Administration’s (NOAA’s) National Hurricane Center’s North Atlantic hurricane database reanalysis project (HURDAT; www.aoml.noaa.gov/hrd/data_sub/re_anal.html).
Fertilizer consumption data were computed from the quantity of fertilizer (in metric tons) of plant nutrient consumed in agriculture by a country annually from 1961 to 2002 [from the Food and Agriculture Organization of the United Nations’ Statistics Division (FAOSTAT), www.fao.org/faostat/en/#data/RA]. Annual estimates were averaged across each time bin. For island nations, the value reported for the entire country was used, while for large continental countries and islands (Colombia, Costa Rica, Cuba, Mexico, Panama, and Venezuela), the total country value was multiplied by the fraction of total country area comprised by the provinces, states, or departments in which the reef sites included in our database were located. Because the composition of reef sites varied across time bins, these calculations were performed separately for each bin. Reef sites claimed by a continental country but located on small islands well (>70 km) offshore (San Andrés, Providencia, and Santa Catalina archipelago of Colombia, Corn Islands of Honduras, and Los Roques archipelago of Nicaragua) were assigned a fertilizer consumption value of zero. Because of the exceptionally high rate of agricultural fertilizer usage and geographic extent of the United States and our inability to locate state-level fertilizer consumption data for the United States that were reported in a comparable manner to the country-level FAO data, we excluded Florida reefs from the drivers analyses.
Human population density data (people/km2) were obtained by country for 5-year increments from 1950 to 2010 (e.g., 1950, 1955, 1960, 1965, 1970, 1975, 1980, 1985, 1990, 1995, 2000, 2005, and 2010) from the United Nations Population Division [https://population.un.org/wpp/; (44)]. Quinquennial or annual values were averaged to compute density values for each of the time bins considered in our analyses (see next paragraph).
To ensure adequate sample size and comparable spatial extent of data on Acropora dominance through time and to separate the pre- and post-WBD periods (late 1970s to early 1980s) and pre- and post-Diadema die-off periods (~1984), we computed coral dominance and driver values within four time bins: 1950–1969, 1970–1984, 1985–1994, and 1995–2011. (Acropora presence and dominance data were too sparse to conduct separate analyses for each of these countries or time bins or to partition data into finer-resolution time bins; figs. S1 to S4.) Separate binomial GLMMs were formulated to predict A. palmata dominance at the reef crest zone and A. cervicornis at the midslope zone as a function of (i) all four of the potential drivers, (ii) time bin, and (iii) country. While individual potential drivers were included as fixed effects, time bin and country were included as random effects to account for temporal autocorrelation and the uneven geographic distribution of samples across time bins, respectively (see table S5 for model specification).
To determine the models that best described patterns of change in A. palmata dominance at the reef crest and A. cervicornis dominance at the midslope zones since 1950, we (i) ran an initial “full” model that included all four potential fixed effects and both random effects, (ii) inspected the significance of each fixed effect (generally, at P < 0.05), and (iii) ran a “final” model that included significant fixed effects and both random effects. In the case that a fixed effect was found to be “nearly significant” (i.e., its P value was <0.1), R2 values were compared across models that included and excluded this effect to determine the best-fit model. We inspected both the marginal R2 (variance explained by fixed effects) and conditional R2 (variance explained by the entire model, including fixed and random effects) using the r.squaredGLMM function in the MuMIn package in R [which uses the methods described by Nakagawa and Schielzeth (45)]. Before running the GLMM analyses, the distributions of all four continuous predictor variables were log transformed to reduce the influence of extreme large values and improve model convergence. Linear correlations between potential drivers were assessed by conducting Spearman rank correlation tests. Because of the lack of discernable mechanistic causes for any correlative relationships between potential drivers and our interest in assessing the effects of each of these stressors, all four drivers were included as predictor variables in the initial models.
For the time series and drivers analyses, model performance was assessed via diagnostic plots of model residuals (quantile-quantile plots, pooled residuals versus predicted values, and residuals of random and all significant fixed effects versus predicted values) and via goodness-of-fit tests on pooled residuals (uniformity, outliers, and dispersion). Diagnostic plots and goodness-of-fit tests were produced for each model using the DHARMa package in R. Tests were carried out via a simulation-based approach that transformed model residuals to a standardized scale (46). For each test, 1000 simulations were conducted. All statistical analyses were performed using the program Rv3.4 (47).
RESULTS
Presence and dominance of Acropora from the prehuman period to present
Presence and dominance data for A. cervicornis and A. palmata were compiled from 2459 reef sites from 27 countries for the reef crest zone and 5185 reef sites from 30 countries for the midslope zone (tables S2 and S3). Each of the 12 time bins contained data from a broad geographic area that spanned the greater Caribbean, including sites from the Lesser Antilles, Greater Antilles, Gulf of Mexico, Florida, and mainland coast of Central or South America (Fig. 1). The number of reef sites exceeded 100 in both reef zones within each time bin except for the Pleistocene, 1500–1949, and 1950–1959 periods. Two time bins, 1500–1949 and 1950–1959, contained the fewest number of reef sites at both reef zones (Fig. 1) and accordingly contained the largest uncertainty values (Fig. 2).
Fig. 2. Long-term trends in presence and dominance of Caribbean acroporid corals.
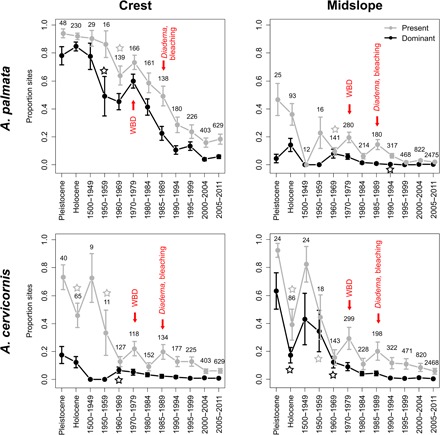
Proportion of reef sites with species present (gray) and dominant (black), determined from a binomial GLMM that included country as a random effect. Vertical bars are standard errors of mean-fitted values; stars indicate earliest significant declines since the historical period compared with Pleistocene values; numbers are reef sites with species presence/absence data for each time bin. Arrows indicate first recorded occurrence of major novel disturbances, with timing of coral bleaching signifying bleaching at several locations across the Caribbean. Diadema, mass die-off of sea urchin D. antillarum.
At the reef crest zone, the model-estimated percent of reef sites dominated by the elkhorn coral A. palmata declined from the Pleistocene to the present, from 78 to 6% of sites (Fig. 2). Wald’s Z tests indicated that the first significant decline from baseline dominance values in the Pleistocene occurred in the 1950s, by which point, A. palmata was dominant at only 49% of reef sites (Table 1 and fig. S6). Dominance levels for A. palmata remained significantly lower than Pleistocene values from the 1950s to the present, although dominance increased to 60% in the 1970s (table S6). During the 1980–1984 time period, the percent of sites with A. palmata dominance shrunk to only 52% of the number of sites it dominated in the Pleistocene. The impact of initial WBD outbreaks in the late 1970s/early 1980s and the Diadema die-off in the early 1980s was reflected in the continued decline in dominance of A. palmata from 41 to 23% of sites between the 1980–1984 and 1985–1989 time periods (Fig. 2 and table S6). The staghorn coral A. cervicornis experienced a coincident but less marked decline in dominance at the reef crest zone. The percent of sites with A. cervicornis dominance declined significantly from Pleistocene values beginning in the 1960s (from 18 to 7% of sites; Fig. 2 and table S7) and remained significantly lower than Pleistocene levels from that point on. By the time of the Diadema die-off, the percent of reef sites with A. cervicornis dominating the reef crest zone had already declined to only 3% (Fig. 2 and table S7).
Table 1. Initial timing of decline in Acropora presence and dominance relative to prehuman baseline.
Earliest occurrences of significant increase or decrease in proportion of sites with Acropora present and dominant relative to Pleistocene baseline time bin (from GLMM models). Presence and dominance for A. cervicornis declined significantly between the Pleistocene and Holocene periods as well as between Pleistocene and mid-20th century.
| Reef zone | Species |
Abundance measure |
Earliest change relative to Pleistocene |
Change | Z | P level | Sites | Countries |
| Crest | A. palmata | Presence | 1960–1969 | 94–65% | −3.8 | 0.001 | 2365 | 27 |
| Dominance | 1950–1959 | 78–49% | −2.0 | 0.05 | 2343 | |||
| A. cervicornis | Presence | Holocene | 73–46% | −2.3 | 0.05 | 2090 | ||
| 1950–1959 | 73–33% | −2.1 | 0.05 | – | ||||
| Dominance | 1960–1969 | 18–7% | −2.0 | 0.05 | 2081 | |||
| Midslope | A. cervicornis | Presence | Holocene | 92–39% | −4.2 | 0.001 | 5101 | 30 |
| 1950–1959 | 92–44% | −3.7 | 0.001 | – | ||||
| Dominance | Holocene | 63–17% | −3.8 | 0.001 | 5185 | |||
| 1960–1969 | 63–12% | −4.4 | 0.001 | – | ||||
| A. palmata | Presence | 1960–1969 | 47–10% | −4.1 | 0.001 | 5043 | ||
| Dominance | 1990–1994 | 4–<1% | −2.5 | 0.01 | 5150 |
A. cervicornis was the most abundant species at the midslope zone across the Caribbean during the prehuman and historical periods, dominating 63% of sites in the Pleistocene (Fig. 2). Wald’s Z tests indicated that presence and dominance of A. cervicornis at the midslope zone declined significantly between the Pleistocene and Holocene periods. The second significant decline from the baseline dominance values in the Pleistocene occurred in the 1960s, by which time the species was dominant at only 12% of sites (Fig. 2, Table 1, and fig. S8). By the time of the Diadema die-off in the early 1980s, A. cervicornis dominated only 4% of reefs at the midslope zone and did not significantly decline post-Diadema die-off (Fig. 2). This species dominated the midslope zone at <1% of the 3293 reef sites included in the most recent time bin. A. palmata dominance at the midslope zone significantly declined from 4 to <1% of sites from the Pleistocene to the early 1990s and has since remained significantly lower (at <1% of sites) than Pleistocene values (Fig. 2 and table S9).
When Acropora presence and dominance values from the historical period (1500–1949 AD) were considered the baseline, results were similar to analyses that considered the Pleistocene period as the baseline. The first significant declines in A. palmata presence and dominance in the reef crest zone from the baseline period 1500–1949 occurred in the 1960s and 1950s, respectively (compared with the 1950s when the Pleistocene period was considered the baseline), and the first significant declines in A. cervicornis presence and dominance in the midslope zone from the baseline period 1500–1949 occurred in the 1950s (compared with the 1950s for A. cervicornis presence and the 1960s for A. cervicornis dominance when the Pleistocene period was considered the baseline; Fig. 2 and table S10).
Potential drivers of Acropora decline
Because of insufficient or incompatible data for one or more potential drivers, the analysis of potential drivers of change in Acropora dominance since 1950 included a subset of the countries and reef sites that were included in the time series (Tables 1 and 2). Relating declines in Acropora dominance since 1950 to a suite of potential disturbances suggested that local human stressors played a significant role in pre-WBD declines of A. palmata. At the reef crest, human population density had a significant negative effect on A. palmata dominance, and fertilizer consumption had a nearly significant positive effect on A. palmata dominance (Fig. 3 and Table 2). While the GLMM (including country and time bin as random effects and population density and fertilizer consumption as fixed effects) explained 30% of the variance in A. palmata dominance, the fixed effects alone only explained 7% of the variance in A. palmata dominance (Table 2). At the midslope zone, none of the fixed effects were found to have a significant effect on A. cervicornis dominance (Table 2).
Table 2. Results from analyses of possible drivers of change in Acropora dominance from 1950 to 2011.
Marginal R2 is variance explained by significant fixed effect(s); conditional R2 is variance explained by entire GLMM, including random effects and significant fixed effects. PopDens, population density; FertCons, fertilizer consumption. None of the possible drivers were significant predictors of A. cervicornis dominance at the midslope zone.
| Reef zone | Species |
Significant fixed effect |
Effect estimate | SE |
Prob (>|z|) |
Mar R2 | Cond R2 | Sites | Countries |
| Crest | A. palmata | Log(PopDens) | −0.50 | 0.17 | 0.004 | ||||
| Log(FertCons) | 0.01 | 0.05 | 0.073 | 0.08 | 0.30 | 1220 | 20 | ||
| Mid-slope | A. cervicornis | – | – | – | – | 0.43 | 3183 | 21 |
Fig. 3. Significant drivers of change in dominance of A. palmata at the reef crest zone from 1950 to 2011.
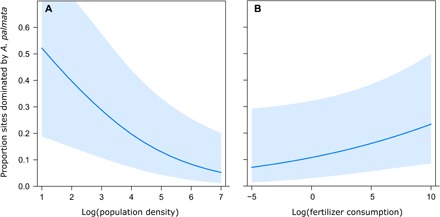
(A) Effect of human population density. (B) Effect of fertilizer consumption. Partial effects determined from a binomial generalized linear mixed effects model that included time bin and country as random effects. Fixed effects were log transformed to reduce the influence of extreme large values and improve model convergence. Blue bands represent 95% confidence intervals.
Comparisons of country-level temporal trends in potential drivers since 1950 revealed differing patterns across variables. In the datasets for A. palmata dominance at the reef crest and A. cervicornis dominance at the midslope zone, population density increased across all four time periods in each country, while values of the other three potential drivers fluctuated over time (figs. S7 and S8). DHM was highest within the 1970–1984 and 1995–2011 time periods for most countries, reflecting major El Niño–Southern Oscillation (ENSO) events in 1982–1983 and 1997–1998, respectively. Fertilizer consumption increased in most countries until the 1985–1994 or 1995–2011 time periods. Hurricanes per year fluctuated across the earliest three time periods, from 1950 to 1994, and reached peak values in most countries during the 1995–2011 period. The analysis of relationships among potential drivers revealed that reef locations are exposed to varying combinations of stressors and that reefs with high exposure to one or more stressors have a low degree of exposure to others. In both the reef crest and midslope zone datasets, all drivers were significantly correlated with one another (figs. S5 and S6), although many of these correlations have no obvious ecological explanation.
DISCUSSION
Early decline in acroporid corals
The timeline of change in Acropora presence and dominance across the Caribbean from the prehuman period to the present revealed that the dominance of Acropora (A. palmata at reef crest and A. cervicornis at midslope) began to decline significantly by the 1950s and 1960s, predating the first recorded instance of WBD by 10 to 30 years (8), the Diadema die-off by about 25 to 35 years (10), and large-scale coral bleaching epidemics by 20 to 40 years (Fig. 2) (48). This pattern holds whether the Pleistocene period, Pleistocene/Holocene periods combined, or historical period (1500–1949 AD) are treated as the baseline time bin (Fig. 2, fig. S9, and table S10). Our long-term dataset shows that by the time the earliest widespread coral bleaching occurred in the Caribbean in the late 1980s, Acropora corals were relatively rare in the Caribbean: The proportion of sites dominated by A. palmata at the reef crest had already declined from 78 to 22%, and the proportion of sites dominated by A. cervicornis at the midslope had already declined from 63 to 4% since the Pleistocene. Acropora presence and dominance values briefly increased in the 1970s and were particularly notable for A. palmata (Fig. 2). This period also encompasses the first reported instances of WBD (7–9) and initial pronounced anthropogenic ocean warming in the Caribbean (17, 18), precluding any obvious ecological explanation for Acropora recovery.
Exploring possible drivers of Acropora decline
The analysis of potential drivers of Acropora loss for which reliable long-term data exist—temperature stress, fertilizer consumption for agriculture (as a proxy for eutrophication), hurricanes, and population density—suggests that local human impacts may have played a role in the high levels of Acropora mortality that occurred decades before WBD and widespread coral bleaching. The strong negative effect of human population density on A. palmata dominance at the reef crest zone since 1950 (Fig. 3) could implicate either land-based pollution or fishing effects—the importance of now-depleted herbivorous reef fish to coral health is well established (49–51)—but there are no reliable proxies of fishing effort or reef fish abundance at the broad temporal and spatial scales explored here. The weak positive effect of fertilizer consumption on A. palmata dominance at the reef crest was likely related to lower human presence in agricultural areas, as values of fertilizer consumption were greatest at low levels of human population density in this dataset (fig. S5). In contrast, neither population density nor fertilizer consumption was a significant predictor of A. cervicornis dominance in the midslope zone, suggesting that other factors played a role in the historical decline of this coral.
We did not find evidence that hurricanes and ocean warming were responsible for the initial decline in Acropora dominance that occurred across the Caribbean in the mid-20th century. Earlier work has linked anthropogenic ocean warming to outbreaks of WBD that caused massive die-offs of Acropora beginning in the late 1980s/early 1990s (14); however, the analyses upon which these conclusions were based could not identify earlier causes of initial decline, because they did not include pre-WBD abundance or dominance data. Our long record of Acropora dominance since the prehuman period reveals that early Acropora declines predate region-wide coral disease outbreaks, indicating that coral populations across the Caribbean were substantially altered before catastrophic climate change impacts. The lack of association between declines in Acropora over the past half-century and hurricane exposure may be because storm events assist in the primary mode of Acropora reproduction, asexual propagation via colony fragmentation (42). It is possible that the recent lack of Acropora recovery following hurricanes is related to loss of herbivory on reefs rather than the hurricanes themselves—an analysis of total living coral cover on reefs across the Caribbean since 1970 found that coral cover was not related to hurricane exposure until after the die-off of the keystone herbivore Diadema urchin (1).
The role of land-based runoff in early coral declines
Coral declines in the Caribbean have commonly been attributed, in part, to declining water quality due to inputs of land-based sediments and pollutants. Water turbidity has almost certainly increased across the Caribbean as agricultural and industrial activities have increased over the past century, but this trend has gone undetected due to the lack of established water quality monitoring programs for reef environments. The only long-term water clarity surveys reported for the Caribbean, from Belize and Puerto Rico, revealed a significant increase in turbidity from 1993 to 2012 attributed to land-based runoff (1). The earlier decline to close to 0% dominance of A. cervicornis at the midslope (early 1980s) compared with A. palmata at the reef crest (early 2000s; Fig. 2) and the failure of A. cervicornis populations to experience the isolated recoveries that have been recorded for A. palmata over the past one to two decades [e.g., (52, 53)] may be related to declining water quality, as increased turbidity would most likely have a greater effect on the midslope compared with the reef crest zone due to increasing light attenuation with depth. Prolonged shading resulting from increased water turbidity negatively affects zooxanthellate coral survival and growth, particularly in A. cervicornis (54). Beyond shading effects, contaminants in land-based pollution have also been linked to coral declines—microbial pathogens from human sewage have been identified as a cause of a white pox disease epidemic that has infected many of the remaining A. palmata colonies over the past decade (55), and nitrate and phosphate enrichment has been shown to increase the severity of disease epidemics that affect other scleractinian corals (20, 56).
Unfortunately, the only proxy for land-based pollution available at the broad temporal and spatial scales explored in this study—fertilizer consumption used in agriculture—was reported at the country level rather than the more hydrographically appropriate watershed level. Therefore, this proxy may not have adequately captured large variations in agrochemical runoff on reefs within a country. In addition, the fertilizer consumption dataset begins in 1961, a decade after initial declines in dominance of A. palmata at the reef crest zone and declines in the presence of A. cervicornis at the midslope zone. These discrepancies may explain why fertilizer consumption had a weak positive effect on A. palmata dominance at the reef crest zone and no effect on A. cervicornis dominance at the midslope zone, rather than having a substantial negative effect (Fig. 2 and Table 2). Although it would be desirable to use fertilizer data reported at a finer spatial scale, we are not aware of any such long-term datasets available for the wider Caribbean region.
Although not directly analyzed in this study, the early Acropora declines documented here may be related to other types of agrochemical pollution. The timing of initial significant Acropora declines in the 1950s coincides with the widespread application of synthetic pesticides on agricultural crops in the Caribbean. Chronic exposure to common herbicides used in industrial agricultural operations has been shown to interfere with a variety of scleractinian coral biological processes. These include (i) reduction in reproductive output via photoinhibition of Symbiodinium (57), (ii) mortality of coral polyps and planulae (58, 59), and (iii) inhibition of settlement and metamorphosis of Acropora planulae (60). Both fertilizer use and pesticide imports increased noticeably between the 1960s and 1970s, the earliest times for which data are available [FAOStat data; (61)]. It is estimated that 90% of the pesticides used today in the wider Caribbean do not meet their intended target, and a high percentage enters the marine environment through surface and drainage runoff, erosion, misapplication, and atmospheric transport (62). High levels of heavy metals associated with synthetic agrochemicals, mining, sewage, and oil spills have been found within scleractinian skeletons in both degraded nearshore and more intact offshore reefs along the Central American coast (63). Resolving the role of pesticide/agrochemical usage in Acropora and other coral declines in the Caribbean will require the analysis of chemical signatures of agrochemicals and other contaminants from coral skeletal material [e.g., (64)].
Assessment of long-term trends
To track long-term change in Acropora corals, we used data from multiple sources, including uplifted fossil reefs, reef matrix cores, qualitative historical data, and underwater survey data. However, the timing and nature of trends we observed in Acropora presence and dominance suggest that results were not artifacts of comparing differing data types. One possible exception is the significant decline in the presence and dominance of A. cervicornis at the midslope zone between the Pleistocene (data obtained mainly from transect surveys of uplifted reefs) and Holocene (data obtained mainly from reef matrix cores). Although this trend could conceivably be a response to declining rates of sea level rise during the late Holocene, which would favor increased dominance of more slowly growing coral species such as massive colony forms (65), we did not observe a significant increase in massive coral dominance during this time in our dataset (fig. S10). Low A. cervicornis dominance in the Holocene could also be because of inaccurate paleodepth estimates and/or underestimation of A. cervicornis abundance from narrow-diameter core tubes [e.g., comparison of A. cervicornis abundance estimates from narrow cores versus large pits (29, 30)]. In contrast, A. palmata dominance increased slightly at the reef crest between the Pleistocene and Holocene, demonstrating that there was no bias against sampling this coral in the Holocene reef cores. A second bias could have arisen from comparison of fossil and survey data because of the longer time span represented by the former. While we acknowledge the greater likelihood of recording the presence of Acropora within highly time-averaged samples, this bias would not be expected to affect dominance values. Our analysis of change using the historical period (1500–1949 AD) as the baseline also showed initial declines in Acropora presence and dominance in the 1950s and 1960s (table S10). Other studies from the Caribbean Sea that compared coral community composition between Pleistocene and modern reefs showed remarkable comparability despite variation in growth rates among coral species, the higher degree of time-averaging in fossil assemblages, and possible transport and mixing of fossil material (25, 27). A third bias could have arisen from the comparison of qualitative historical data (from the 1500–1949 and 1950–1959 time periods) and quantitative survey data (from 1960 to 2011). However, dominance values for the historical periods for A. palmata at reef crest and A. cervicornis at midslope generally fell within the range of values for the fossil and underwater survey periods (Fig. 2).
Implications for reef conservation
The ecological consequences of the loss of Acropora corals on Caribbean coral reefs are difficult to overstate. Since the disappearance of these once ubiquitous species, reefs are but a shadow of their former selves. Reductions in architectural complexity (23), carbonate production (24), and biodiversity (7, 30) have been profound and essentially unidirectional since the loss of these corals. The early timing of Acropora declines revealed from this study indicates that ongoing efforts to repopulate reefs with these corals must include mitigation of local anthropogenic stressors in addition to immediate reductions in carbon emissions.
Supplementary Material
Acknowledgments
We thank P. Alcolado, J. Alemu, E. Arias, A. Atkinson, R. Bak, C. Bastidas, P. Blanchon, C. Bouchon, A. Brathwaite, J. Bruno, K. Buchan, P. Bush, C. Caldow, B. Charpentier, M. Chiappone, M. Colella, M. Creary, C. McCoy, A. Croquer, K. De Meyer, P. Dustan, P. Edmunds, D. Fenner, G. Forrester, A. Friedlander, P. Gayle, H. Guzmán, A. Harborne, M. Hardt, Z. Hilis-Starr, E. Hochberg, T. Hughes, W. Jaap, M. Jordan, K. Koltes, J. Lang, Y. Loya, I. Lundgren, C. Manfrino, M. McField, J. Miller, J. Mitchell, P. Mumby, T. Murdoch, I. Nagelkerkan, R. Nemeth, M. Nugues, H. Oxenford, J. Pandolfi, G. Paredes, H. Reyes Bonilla, R. Rodriguez-Martinez, A. Rodriguez-Ramirez, C. Rogers, R. Ruzicka, T. Smith, B. Sommer, B. Steneck, M. Vermeij, and E. Weil for contributing their survey data to the GCRMN, J. Carilli for extracting the Hadley SST dataset, S. Sandin and R. Norris for providing helpful insights on statistical methods and interpretation of results, and B. Ruttenberg for assistance with producing maps. Funding: This project was funded by GCRMN. Author contributions: All authors assembled the database of Acropora presence/dominance. K.L.C., J.B.C.J., B.J.G., C.A.K., G.M.C., and J.M.P. designed the study. K.L.C. and M.K.D. performed modeling and statistical analyses. K.L.C. compiled data on potential drivers of Acropora decline, made figures and tables, and wrote the initial draft of the manuscript. All authors contributed substantially to subsequent manuscript revisions. Competing interests: The authors declare that they have no competing interests. Data and materials availability: Data will be deposited on Dryad. Additional data may be requested from the corresponding author.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/17/eaax9395/DC1
REFERENCES AND NOTES
- 1.J. B. C. Jackson, M. K. Donovan, K. L. Cramer, V. Y. Y. Lam, Status and Trends of Caribbean Coral Reefs: 1970–2012 (Global Coral Reef Monitoring Network, IUCN, 2014). [Google Scholar]
- 2.Hughes T. P., Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 265, 1547–1551 (1994). [DOI] [PubMed] [Google Scholar]
- 3.Norström A. V., Nyström M., Lokrantz J., Folke C., Alternative states on coral reefs: Beyond coral–macroalgal phase shifts. Mar. Ecol. Prog. Ser. 376, 295–306 (2009). [Google Scholar]
- 4.Loh T.-L., McMurray S. E., Henkel T. P., Vicente J., Pawlik J. R., Indirect effects of overfishing on Caribbean reefs: Sponges overgrow reef-building corals. PeerJ 3, e901 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aronson R. B., Precht W. F., White-band disease and the changing face of Caribbean coral reefs. Hydrobiologia 460, 25–38 (2001). [Google Scholar]
- 6.Hughes T. P., Baird A. H., Bellwood D. R., Card M., Connolly S. R., Folke C., Grosberg R., Hoegh-Guldberg O., Jackson J. B. C., Kleypas J., Lough J. M., Marshall P., Nyström M., Palumbi S. R., Pandolfi J. M., Rosen B., Roughgarden J., Climate change, human impacts, and the resilience of coral reefs. Science 301, 929–933 (2003). [DOI] [PubMed] [Google Scholar]
- 7.Gladfelter W. B., Gladfelter E. H., Fish community structure as a function of habitat structure on West Indian patch reefs. Rev. BioI. Trop. 26 (suppl. 1), 65–84 (1978). [Google Scholar]
- 8.Gladfelter W. B., White-band disease in Acropora palmata: Implications for the structure and growth of shallow reefs. Bull. Mar. Sci. 32, 639–643 (1982). [Google Scholar]
- 9.E. Weil, Coral reef diseases in the wider Caribbean, in Coral Health and Disease, E. Rosenberg, Y. Loya, Eds. (Springer-Verlag, 2004), pp. 35–68. [Google Scholar]
- 10.Lessios H. A., Robertson D. R., Cubit J. D., Spread of Diadema mass mortality through the Caribbean. Science 226, 335–337 (1984). [DOI] [PubMed] [Google Scholar]
- 11.Pandolfi J. M., Bradbury R. H., Sala E., Hughes T. P., Bjorndal K. A., Cooke R. G., McArdle D., McClenachan L., Newman M. J. H., Paredes G., Warner R. R., Jackson J. B. C., Global trajectories of the long-term decline of coral reef ecosystems. Science 301, 955–958 (2003). [DOI] [PubMed] [Google Scholar]
- 12.Lessios H. A., Mass mortality of Diadema antillarum in the Caribbean: What have we learned? Annu. Rev. Ecol. Syst. 19, 371–393 (1988). [Google Scholar]
- 13.Glynn P. W., Coral reef bleaching: Ecological perspectives. Coral Reefs 12, 1–17 (1993). [Google Scholar]
- 14.Randall C. J., van Woesik R., Contemporary white-band disease in Caribbean corals driven by climate change. Nat. Clim. Chang. 5, 375–379 (2015). [Google Scholar]
- 15.Sheppard C., Rioja-Nieto R., Sea surface temperature 1871–2099 in 38 cells in the Caribbean region. Mar. Environ. Res. 60, 389–396 (2005). [DOI] [PubMed] [Google Scholar]
- 16.Knutson T. R., Delworth T. L., Dixon K. W., Held I. M., Lu J., Ramaswamy V., Schwarzkopf M. D., Stenchikov G., Stouffer R. J., Assessment of twentieth-century regional surface temperature trends using the GFDL CM2 coupled models. J. Climate 19, 1624–1651 (2006). [Google Scholar]
- 17.Nugues M. M., Smith G. W., van Hooidonk R. J., Seabra M. I., Bak R. P. M., Algal contact as a trigger for coral disease. Ecol. Lett. 7, 919–923 (2004). [Google Scholar]
- 18.Smith J. E., Shaw M., Edwards R. A., Obura D., Pantos O., Sala E., Sandin S. A., Smriga S., Hatay M., Rohwer F. L., Indirect effects of algae on coral: Algae-mediated, microbe-induced coral mortality. Ecol. Lett. 9, 835–845 (2006). [DOI] [PubMed] [Google Scholar]
- 19.Rasher D. B., Hay M. E., Chemically rich seaweeds poison corals when not controlled by herbivores. Proc. Natl. Acad. Sci. U.S.A. 107, 9683–9688 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bruno J. F., Petes L. E., Harvell C. D., Hettinger A., Nutrient enrichment can increase the severity of coral diseases. Ecol. Lett. 6, 1056–1061 (2003). [Google Scholar]
- 21.Kuntz N. M., Kline D. I., Sandin S. A., Rohwer F., Pathologies and mortality rates caused by organic carbon and nutrient stressors in three Caribbean coral species. Mar. Ecol. Prog. Ser. 294, 173–180 (2005). [Google Scholar]
- 22.Gardner T. A., Côté I. M., Gill J. A., Grant A., Watkinson A. R., Long-term region-wide declines in Caribbean corals. Science 301, 958–960 (2003). [DOI] [PubMed] [Google Scholar]
- 23.Alvarez-Filip L., Dulvy N. K., Gill J. A., Côté I. M., Watkinson A. R., Flattening of Caribbean coral reefs: Region-wide declines in architectural complexity. Proc. Biol. Sci. 276, 3019–3025 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Perry C. T., Steneck R. S., Murphy G. N., Kench P. S., Edinger E. N., Smithers S. G., Mumby P. J., Regional-scale dominance of non-framework building corals on Caribbean reefs affects carbonate production and future reef growth. Glob. Chang. Biol. 21, 1153–1164 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Jackson J. B. C., Pleistocene perspectives on coral reef community structure. Am. Zool. 32, 719–731 (1992). [Google Scholar]
- 26.Greenstein B. J., Curran H. A., Pandolfi J. M., Shifting ecological baselines and the demise of Acropora cervicornis in the western North Atlantic and Caribbean Province: A Pleistocene perspective. Coral Reefs 17, 249–261 (1998). [Google Scholar]
- 27.Pandolfi J. M., Jackson J. B. C., Ecological persistence interrupted in Caribbean coral reefs. Ecol. Lett. 9, 818–826 (2006). [DOI] [PubMed] [Google Scholar]
- 28.Jackson J. B. C., Kirby M. X., Berger W. H., Bjorndal K. A., Botsford L. W., Bourque B. J., Bradbury R. H., Cooke R., Erlandson J., Estes J. A., Hughes T. P., Kidwell S., Lange C. B., Lenihan H. S., Pandolfi J. M., Peterson C. H., Steneck R. S., Tegner M. J., Warner R. R., Historical overfishing and the recent collapse of coastal ecosystems. Science 292, 629–637 (2001). [DOI] [PubMed] [Google Scholar]
- 29.Aronson R. B., Macintyre I. G., Wapnick C. M., O’Neill M. W., Phase shifts, alternative states, and the unprecedented convergence of two reef systems. Ecology 85, 1876–1891 (2004). [Google Scholar]
- 30.Cramer K. L., Jackson J. B. C., Angioletti C. V., Leonard-Pingel J., Guilderson T. P., Anthropogenic mortality on coral reefs in Caribbean Panama predates coral disease and bleaching. Ecol. Lett. 15, 561–567 (2012). [DOI] [PubMed] [Google Scholar]
- 31.Lewis J. B., The Acropora inheritance: A reinterpretation of the development of fringing reefs in Barbados, West Indies. Coral Reefs 3, 117–122 (1984). [Google Scholar]
- 32.Goreau T. F., The ecology of Jamaican coral reefs I. Species composition and zonation. Ecology 40, 67–90 (1959). [Google Scholar]
- 33.Milliman J. D., Four southwestern Caribbean atolls: Courtown Cays, Albuquerque Cays, Roncador Bank and Serrana Bank. Atoll Res. Bull. 129, 1–26 (1969). [Google Scholar]
- 34.R. B. Halley, H. L. Vacher, E. A. Shinn, Geology and hydrogeology of the Florida Keys, in Geology and Hydrogeology of Carbonate Islands, H. L. Vacher, T. Quinn, Eds. (Elsevier, 1997), pp. 217–248. [Google Scholar]
- 35.Toscano M. A., Macintyre I. G., Corrected western Atlantic sea-level curve for the last 11,000 years based on calibrated 14C dates from Acropora palmata framework and intertidal mangrove peat. Coral Reefs 22, 257–270 (2003). [Google Scholar]
- 36.Gischler E., Hudson J.-H., Holocene development of the Belize barrier reef. Sediment. Geol. 164, 223–236 (2004). [Google Scholar]
- 37.Peltier W. R., Fairbanks R. G., Global glacial ice volume and Last Glacial Maximum duration from an extended Barbados sea level record. Quatern. Sci. Rev. 25, 3322–3337 (2006). [Google Scholar]
- 38.Jessen C. A., Pedersen J. B. T., Bartholdy J., Seidenkrantz M.-S., Kuijpers A., A late Holocene palaeoenvironmental record from Altona Bay, St. Croix, US Virgin Islands. Geografisk Tidsskrift-Danish J. Geogr. 108, 59–70 (2008). [Google Scholar]
- 39.Bolker B. M., Brooks M. E., Clark C. J., Geange S. W., Poulsen J. R., Stevens M. H. H., White J.-S. S., Generalized linear mixed models: A practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009). [DOI] [PubMed] [Google Scholar]
- 40.Donner S. D., Skirving W. J., Little C. M., Oppenheimer M., Hoegh-Guldberg O., Global assessment of coral bleaching and required rates of adaptation under climate change. Glob. Change Biol. 11, 2251–2265 (2005). [DOI] [PubMed] [Google Scholar]
- 41.Treml E., Cogan M., Keevican M., Hurricane disturbance and coral reef development: A geographic information system (GIS) analysis of 501 years of hurricane data from the Lesser Antilles. Proc. Eighth Int. Coral Reef Symp. 1, 541–546 (1997). [Google Scholar]
- 42.Highsmith R. C., Reproduction by fragmentation in corals. Mar. Ecol. Prog. Ser. 7, 207–226 (1982). [Google Scholar]
- 43.Highsmith R. C., Riggs A. C., D'Antonio C. M., Survival of hurricane-generated coral fragments and a disturbance model of reef calcification/growth rates. Oecologia 46, 322–329 (1980). [DOI] [PubMed] [Google Scholar]
- 44.World Population Prospects: The 2012 Revision, United Nations, Department of Economic and Social Affairs, Population Division, New York, 2013.
- 45.Nakagawa S., Schielzeth H., A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013). [Google Scholar]
- 46.Dunn P. K., Smyth G. K., Randomized quantile residuals. J. Comput. Graph. Stat. 5, 236–244 (1996). [Google Scholar]
- 47.R Core Team, R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria (2018); http://www.R-project.org/.
- 48.Brown B. E., Coral bleaching: Causes and consequences. Coral Reefs 16, S129–S138 (1997). [Google Scholar]
- 49.Randall J. E., Overgrazing of algae by herbivorous marine fishes. Ecology 42, 812 (1961). [Google Scholar]
- 50.Hughes T. P., Rodrigues M. J., Bellwood D. R., Ceccarelli D., Hoegh-Guldberg O., McCook L., Moltschaniwskyj N., Pratchett M. S., Steneck R. S., Willis B., Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr. Biol. 17, 360–365 (2007). [DOI] [PubMed] [Google Scholar]
- 51.Cramer K. L., O’Dea A., Clark T. R., Zhao J.-x., Norris R. D., Prehistorical and historical declines in Caribbean coral reef accretion rates driven by loss of parrotfish. Nat. Commun. 8, 14160 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Macintyre I. G., Toscano M. A., The elkhorn coral Acropora palmata is coming back to the Belize Barrier Reef. Coral Reefs 26, 757 (2007). [Google Scholar]
- 53.Zubillaga A. L., Márquez L. M., Cróquer A., Bastidas C., Ecological and genetic data indicate recovery of the endangered coral Acropora palmata in Los Roques, Southern Caribbean. Coral Reefs 27, 63–72 (2008). [Google Scholar]
- 54.Rogers C. S., The effect of shading on coral reef structure and function. J. Exp. Mar. Biol. Ecol. 41, 269–288 (1979). [Google Scholar]
- 55.Sutherland K. P., Porter J. W., Turner J. W., Thomas B. J., Looney E. E., Luna T. P., Meyers M. K., Futch J. C., Lipp E. K., Human sewage identified as likely source of white pox disease of the threatened Caribbean elkhorn coral, Acropora palmata. Environ. Microbiol. 12, 1122–1131 (2010). [DOI] [PubMed] [Google Scholar]
- 56.Kaczmarsky L. T., Draud M., Williams E. H., Is there a relationship between proximity to sewage effluent and the prevalence of coral disease? Caribb. J. Sci. 41, 124–137 (2005). [Google Scholar]
- 57.Cantin N. E., Negri A. P., Willis B. L., Photoinhibition from chronic herbicide exposure reduces reproductive output of reef-building corals. Mar. Ecol. Prog. Ser. 344, 81–93 (2007). [Google Scholar]
- 58.Acevedo R., Preliminary observations on effects of pesticides Carbaryl, Naphthol, and Chlorpyrifos on planulae of the hermatypic Coral Pocillopora damicornis. Pac. Sci. 45, 287–289 (1991). [Google Scholar]
- 59.Te F. T., Preliminary investigations into the effects of Dursban insecticide on Pocillopora damicornis (Scleractinia: Cnidaria). J. Mar. Environ. Eng. 4, 189–199 (1998). [Google Scholar]
- 60.Markey K. L., Baird A. H., Humphrey C., Negri A. P., Insecticides and a fungicide affect multiple coral life stages. Mar. Ecol. Prog. Ser. 330, 127–137 (2007). [Google Scholar]
- 61.Rawlins B. G., Ferguson A. J., Chilton P. J., Arthurton R. S., Rees J. G., Baldock J. W., Review of agricultural pollution in the Caribbean with particular emphasis on small island developing states. Mar. Pollut. Bull. 36, 658–668 (1998). [Google Scholar]
- 62.Fernandez A., Singh A., Jaffé R., A literature review of trace metals and organic compounds of anthropogenic origin in the wider Caribbean region. Mar. Pollut. Bull. 54, 1681–1691 (2007). [DOI] [PubMed] [Google Scholar]
- 63.Guzmán H. M., Jiménez C. E., Contamination of coral reefs by heavy metals along the Caribbean coast of Central America (Costa Rica and Panama). Mar. Pollut. Bull. 24, 554–561 (1992). [Google Scholar]
- 64.Baker D. M., Webster K. L., Kim K., Caribbean octocorals record changing carbon and nitrogen sources from 1862 to 2005. Glob. Chang. Biol. 16, 2701–2710 (2010). [Google Scholar]
- 65.Hongo C., Holocene key coral species in the Northwest Pacific: Indicators of reef formation and reef ecosystem responses to global climate change and anthropogenic stresses in the near future. Quat. Sci. Rev. 35, 82–99 (2012). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/6/17/eaax9395/DC1


