Supplemental Digital Content is available in the text
Keywords: Hyperuricemia, Gout, Cardiovascular event, Febuxostat, Allopurinol
Abstract
Background
Hyperuricemia and gout have become public health concerns; many important guidelines have recommended xanthine oxidase inhibitors (XOIs) as the first-line urate-lowering therapies (ULTs) to treat chronic gout with hyperuricemia. However, whether treating hyperuricemia and gout with ULTs modifies cardiovascular risks remains controversial. The aim of this study was to assess the incident risk of cardiovascular (CV) events (CVE) in hyperuricemia population, assess the cardiovascular benefit-risk of ULTs in hyperuricemia patients with or without gout in diverse cardiovascular risk sub-groups, and specify the safety of different ULTs.
Methods
We searched PubMed, Embase, the Cochrane Library, Wanfang, Chongqing VIP (CQVIP, en.cqvip.com), and China National Knowledge Infrastructure Database for prospective cohort studies and randomized controlled trials (RCTs) in English and Chinese. Potential medications included XOIs, and uricosurics. RCTs were divided into sub-groups analysis based on blinding status and patients’ history of CV diseases. Risk ratios (RRs) were calculated and were reported with corresponding 95% confidence intervals (CIs) by fixed-effects or random-effects model.
Results
Seven prospective cohort studies and 17 RCT studies were included. The risks of both major adverse cardiovascular events (MACE) (RR = 1.72, 95% CI 1.28–2.33) and CVE (RR = 1.35, 95% CI 1.12–1.62) were higher in the hyperuricemia population than non-hyperuricemia one. In seven RCT studies where XOIs were compared with no-treatment or placebo, the results of five low CV risk studies showed that XOIs lowered the risks of both MACE (RR = 0.35, 95% CI 0.20–0.62) and CVE (RR = 0.61, 95% CI 0.44–0.85); whereas two high CV risk studies showed that XOIs lowered the risk of CVE (RR = 0.69, 95% CI 0.54–0.88) rather than MACE (RR = 0.62, 95% CI 0.29–1.35). In nine RCT studies where the cardiovascular safety between febuxostat and allopurinol were compared, no statistical difference was found in the risk of MACE or CVE.
Conclusions
The hyperuricemia population does have a higher incidence of CVE, and the results suggested that XOIs might reduce the incidence of MACE and total CVE. In addition, from the perspective of cardiovascular safety, febuxostat equaled allopurinol in our meta-analysis.
Introduction
Gout is the most common form of inflammatory arthritis involving the joints characterized by urate crystal deposition. Hyperuricemia is prerequisite in developing gout which is curable by keeping serum uric acid <6 mg/dL in most instances.[1] Many important guidelines, including the 2007 British Society for Rheumatology guidelines,[2] the 2011 Japanese guideline,[3] the 2012 American College of Rheumatology guidelines,[1,4] the 2014 3e guidelines issued by multinational experts[5] and the 2016 European League Against Rheumatism gout treatment guidelines,[6] and so on, have recommended xanthine oxidase inhibitors (XOIs) as the first-line urate-lowering therapy for treating chronic gout with hyperuricemia. Besides gout, hyperuricemia is considered to cause vascular endothelial dysfunction resulting in complications of cerebral, cardiovascular, renal dysfunction, and non-alcoholic fatty liver disease. Therefore, uric acid lowering was widely noted in these related studies.[3,7] However, it remains controversial whether treating hyperuricemia and gout with urate-acid lowering therapies (ULTs) including XOI or uricosuric modifies cardiovascular risks.
Allopurinol and febuxostat are two most widely used XOIs. Compared with allopurinol (approved since 1965),[8] febuxostat is a novel urate-lowering agent (ULT) and was first approved in European Union in April 2008, followed by USA and later in Japan, for the treatment of chronic hyperuricemia where urate deposition occurred (including a history or presence of tophus and/or gouty arthritis).[9,10] In contrast to allopurinol (a purine analog), febuxostat inhibits both the oxidized and reduced forms of xanthine oxidase and decreases the formation of uric acid as a non-purine inhibitor of xanthine oxidase.[11] Febuxostat provides highly selective and potent inhibition of xanthine oxidase and greater hypouricemic activity than commonly used doses of allopurinol.[12] However, there are concerns about more heart-related deaths with febuxostat compared with allopurinol, and drug safety alerts were released by Health Canada on 1 April 2016 and American Food and Drug Administration (FDA) on November 15, 2017.[13,14] Hyperuricemia and gout were reported to be linked with an increased risk of cardiovascular disease, that complicated the interpretation of cardiovascular side effects of ULTs in hyperuricemia/gout patients.[15,16]
With the concerns of cardiovascular safety in hyperuricemia patients, especially in those using ULTs, we conducted this study to compare the incident risk of cardiovascular (CV) events (CVEs) between hyperuricemia and non-hyperuricemia population, assess the cardiovascular benefit-risk of ULTs in hyperuricemia patients with or without gout, and specify the safety of different urate-lowering drugs in patients with diverse cardiovascular risk background in CVE.
Methods
This meta-analysis was performed according to the Cochrane Handbook for Systematic Reviews of Interventions[17] and was reported according to the preferred reporting items for systematic reviews and meta-analyses statement.[18] The protocol for this meta-analysis is available in PROSPERO (CRD42018090238).
Data sources and search strategy
We searched the PubMed, Embase, the Cochrane Library for the Cochrane Database of Systematic Reviews and the Cochrane Central Register of Controlled Trials, Wanfang, Chongqing VIP (CQVIP, en.cqvip.com), and China National Knowledge Infrastructure (CNKI) Database using the keywords “hyperuricemia,” “gout,” “cardiovascular,” “uric acid lowering,” “allopurinol,” “febuxostat,” “benzbromarone,” and the corresponding Chinese words for studies published until October 2019. After the initial electronic retrieval, we manually screened the identified literature.
Inclusion criteria: patients, outcomes, and study design
We used two main outcomes to assess the inclusion criteria. The primary outcome was major adverse cardiovascular events (MACEs), including cardiovascular death, non-fatal myocardial infarction, non-fatal stroke, and unstable angina with urgent coronary revascularization. The secondary outcome was all new-onset CVE, including arrhythmia, heart failure, coronary heart disease (CHD), peripheral vascular disease, and MACE.
To compare the incident risk of CVE between hyperuricemia and non-hyperuricemia population, studies were eligible for inclusion if they (1) were prospective cohort studies of adults, (2) with longer than one year of follow-up, (3) with a sample size of at least 100 subjects, (4) with an inception cohort free of CHD or ongoing ULTs, and (5) reported CVE. Studies reporting interventional and secondary prevention trials were excluded.
To assess the cardiovascular benefit-risk of ULTs in hyperuricemia patients with or without gout, and to specify the safety of different urate-lowering drugs, trials were selected based on the following inclusion criteria: (1) studies of hyperuricemia adults with or without gout, (2) randomized controlled trials (RCTs), (3) eligible trials have to report cardiovascular safety of a ULT, (4) potential medications including allopurinol, febuxostat, and benzbromarone, (5) any dosing regimen and sample size were allowed, (6) with longer than one week of follow-up, and (7) the comparative group can use placebo or another ULT.
Study selection, quality assessment, and data extraction
Three reviewers (Zhao L, Cao L, and Zhao TY) separately reviewed all identified records in duplication. Full text was retrieved if either reviewer thought that a certain study definitely met the criteria or featured possible eligibility. All the reviewers processed subsequent global screening independently, and adequate discussion was held to resolve any disagreement or question about the inclusion during the procedure.
Three reviewers independently assessed the methodological quality of eligible studies using the Cochrane Risk of Bias tool[17] for RCTs or using the Newcastle-Ottawa scale for prospective cohort studies.[19] The Cochrane Risk of Bias tool mainly evaluated the methodological quality of studies from the following aspects: random sequence generation, allocation concealment, blinding of participants or personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other bias factors. The quality of each item was divided into low risk of bias, unclear, high risk of bias. And any disagreement between reviewers was solved through discussion. Final map of judgments of studies was synthesized by Review Manager 5.3 software (Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2014). The Newcastle-Ottawa scale mainly assessed the quality from three domains: the selection of cohorts (including representativeness of the exposed cohort, the selection of the non-exposed cohort, ascertainment of exposure, and demonstration with outcome of interest absent at the start of study); comparability of cohorts on the basis of the design or analysis; outcome status (including assessment of outcomes, whether the follow-up long enough for outcomes to occur, and adequacy of follow-up of cohorts).
One reviewer (Zhao L) was responsible for extracting data from the original literature into the form, and other reviewers (Cao L and Zhao TY) verified the data independently. The data was extracted in five domains: general information including title, authors, publication year, and country; design characteristics of the study such as blinding, group settings; baseline characteristics of the study population as follows: number, sex, cardiovascular risk hierarchy (assessed according to criteria mentioned in the next paragraph), diagnosis; intervention details such as medicine, dose, frequency of administration, as well as duration of treatment; details of outcome measures as mentioned above and results.
Stratification of cardiovascular risk
To better illustrate the cardiovascular benefit-risk of ULT in different population with different cardiovascular risk, we evaluated studies according to patients’ previous CVE, those with any of the following belonged to high CV risk: heart failure, atrial fibrillation, chronic heart disease, myocardial infarction, cerebrovascular accident, or transient ischemic attack.
Planned sub-group, data analysis and synthesis
To illustrate the correlation between outcomes and intervention more clearly and observe the influence of blinding, we conducted a sub-group analysis according to whether double-blinding was exerted.
We performed meta-analysis to calculate risk ratios (RRs) and 95% confidence intervals (CIs) using the Mantel-Haenszel statistical method with Review Manager 5.3 software. Based on the practice recommendation of the Cochrane Handbook,[17] studies with zero event in both the intervention and the control groups were not included in the meta-analysis when RRs were calculated.
Statistical heterogeneity between summarized data was evaluated using the I2 statistic. A fixed-effects model was used to process the data if the I2 value was less than 50%, otherwise, a random-effects model was utilized to reduce errors due to heterogeneity.
Sensitivity analysis and publication bias test
Sensitivity analysis was processed by using Stata MP 14 software (StataCorp LP, College Station, Texas, USA) to exclude each study in turn. To test bias of publication, we performed Begg rank correlation test and Egger linear regression test[20] in Stata. All the data entered into the software and the results of the calculation were verified by all the reviewers independently.
Results
Selection and description of studies
We obtained 3733 records from Pubmed, Embase, Cochrane Library database, and 3013 records from Wanfang, CQVIP, CNKI database for a total of 6746 citations [Figure 1]. Of these, 2768 citations were excluded for duplication. 3890 publications were excluded because they did not fulfill the inclusion criteria based on their titles and abstracts after the separate screening by all three reviewers. For further screening, we obtained full-text articles of the remaining citations. In scrutinizing the articles, we finally identified seven prospective cohort studies[21–27] and 17 RCTs[11,28–43] eligible for meta-analysis. The other 64 publications were excluded for the following reasons: eight were reviews, 15 were not CV or ULT related, 35 failed to present both primary and secondary outcomes, one shared the same queue of articles published by same authors 5 years ago,[44] five were retrospective studies.
Figure 1.
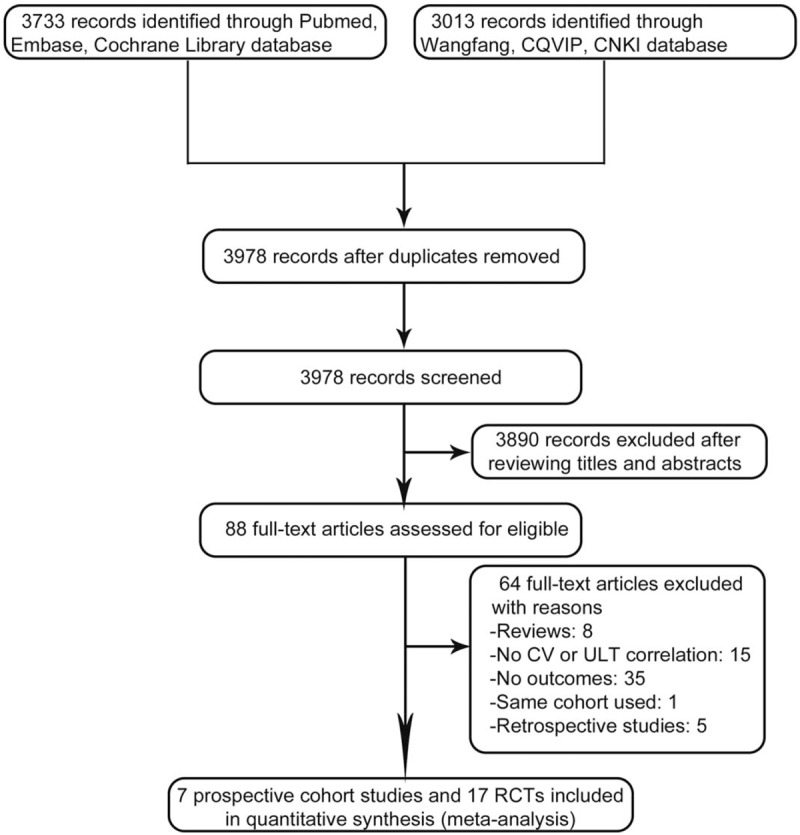
Flow diagram of the selection of randomized controlled trials (RCTs) and prospective cohort studies treating hyperuricemia and gout with urate-lowering therapies. CQVIP: Chongqing VIP; CNKI: China National Knowledge Infrastructure; CV: Cardiovascular; ULT: Urate-lowering agent.
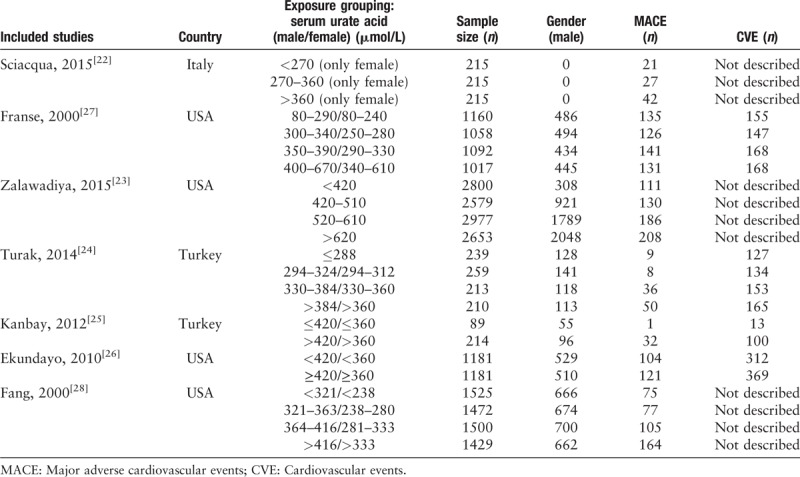
Finally, seven prospective cohort studies and 17 RCTs were included in our study. The seven prospective cohort studies were described according to exposure degree as shown in Table 1. All of these studies reported MACE while three of them[21,22,27] failed to clarify CVE. All studies were grouped according to the method of stratification of exposure factors (serum uric acid). To facilitate statistics, we combined the groups whose serum uric acid exceeded the diagnostic criteria into the hyperuricemia group.
Table 1.
Characteristics of included prospective cohort studies treating hyperuricemia and gout with urate-lowering therapies.
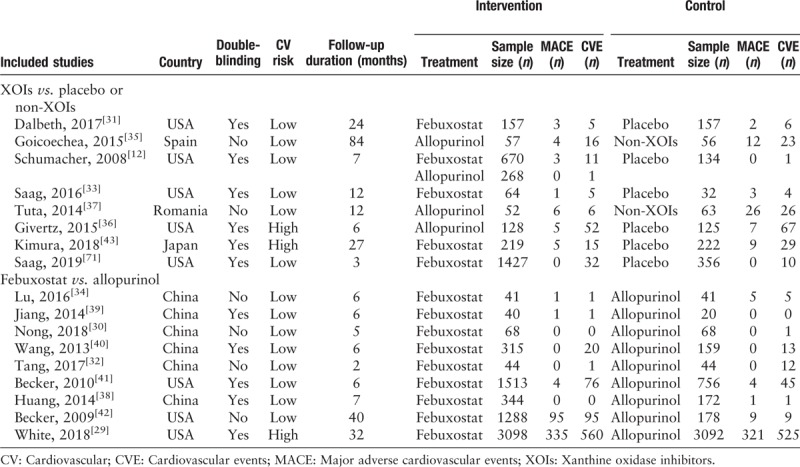
The 17 RCTs from four countries were included in our meta-analysis [Table 2]. The publication years varied from 2014 to 2019, 11 among them were double-blinded. According to the previously mentioned CV risk, only three RCTs recruited subjects fulfilled high CV risk standard, and the rest were described as low CV risk. Then these RCTs were divided into two groups for different analysis purpose, XOIs vs. placebo/non-XOIs and febuxostat vs. allopurinol. There was no eligible study about uricosurics included.
Table 2.
Characteristics of included randomized controlled trials treating hyperuricemia and gout with urate-lowering therapies.
Methodological quality assessment
Different standards of judgement were utilized to assess the methodological quality of prospective cohort studies and RCTs as mentioned previously. The result was presented in Supplementary Table 1 and Supplementary Figure 1.
Hyperuricemia and cardiovascular risk
Seven prospective cohort studies were included in which, inception cohorts were free of cardiovascular diseases or ongoing ULTs. In comparison between hyperuricemia and non-hyperuricemia based on seven prospective cohort studies [Figure 2], the risk of MACE was higher in the hyperuricemia population (RR = 1.72, 95% CI 1.28–2.33) with a significant heterogeneity (I2 = 88%, P < 0.001) [Figure 2A]. Moreover, the pooled RR for CVE cased on four studies[23–26] was 1.35 (95% CI 1.12–1.62) also with a significant heterogeneity (I2 = 81%, P = 0.001) [Figure 2B].
Figure 2.
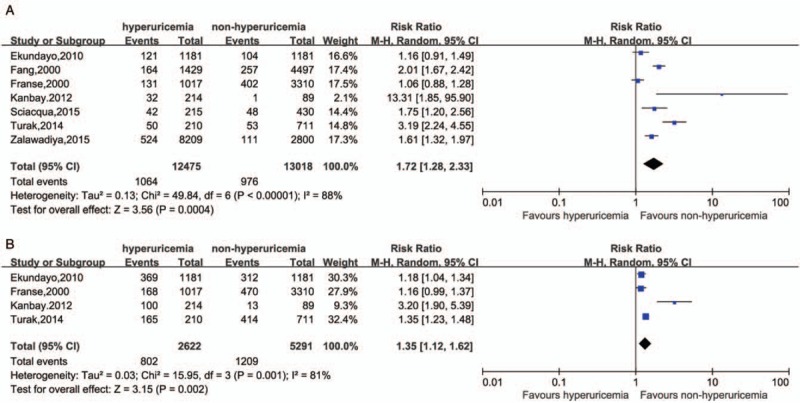
Comparison between hyperuricemia and non-hyperuricemia in MACE (A, P value in Begg test = 0.764, in Egger test = 0.765) and in CVE (B, P value in Begg test = 0.734, in Egger test = 0.402). CVE: Cardiovascular events; MACE: Major adverse cardiovascular events.
Comparison between XOIs and non-XOIs/placebo on cardiovascular risk
The pooled RR of MACE in comparison between XOIs and non-XOIs/placebo with all five low CV risk studies was 0.35 [Figure 3A] (RR = 0.35, 95% CI 0.20–0.62), and a mild heterogeneity was calculated (I2 = 0%, P = 0.43). Interestingly, when we performed a sub-group analysis, the pooled estimated RR indicated no significant difference of MACE incidence between the two groups in double-blinded trials (RR = 0.66, 95% CI 0.22–2.03, I2 = 16%) based on three RCTs,[11,30,32] while the MACE risk of ULTs was reduced by 70% in non-double-blinded sub-group (RR = 0.30, 95% CI 0.16–0.56, I2 = 0%) based on two RCTs.[34,36]
Figure 3.
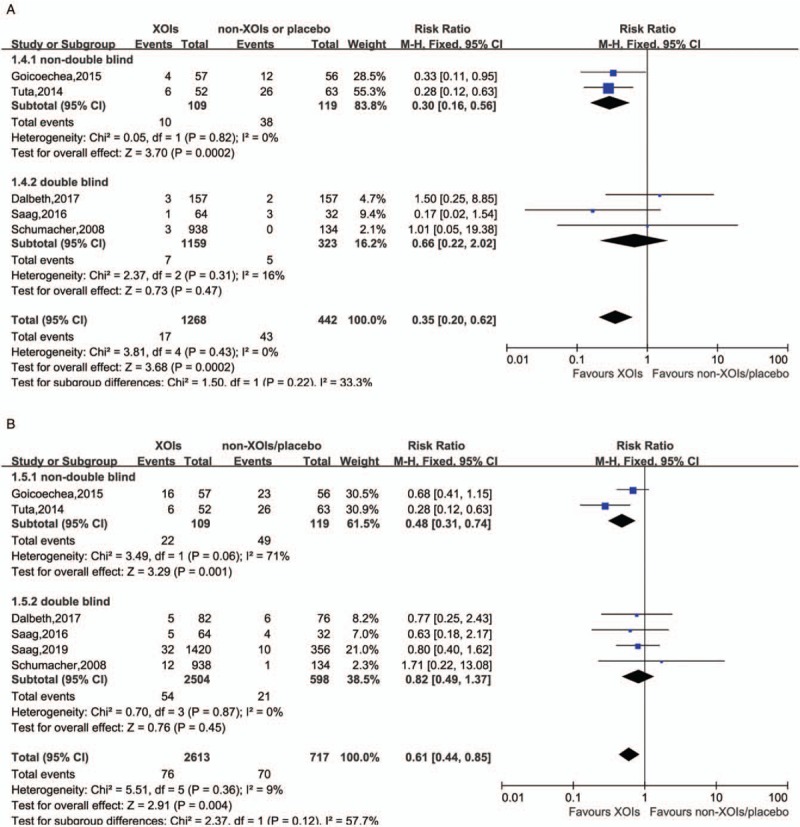
Comparison between XOIs and non-XOIs/placebo in MACE with low CV risk (A, P value in Begg test = 0.462, in Egger test = 0.275), in CVE with low CV risk (B, P value in Begg test = 0.851, in Egger test = 0.687). CV: Cardiovascular; CVE: Cardiovascular events; MACE: Major adverse cardiovascular events; XOIs: Xanthine oxidase inhibitors.
When came to risk of CVE with low CV risk [Figure 3B], the trend seemed to be the same, the reduction of CVE incidence only presented in XOIs groups of non-double-blinded studies (RR = 0.48, 95% CI 0.32–0.74, I2 = 71%) and the whole RCTs (RR = 0.61, 95% CI 0.44–0.85, I2 = 9%) rather than double-blinded trials (RR = 0.82, 95% CI 0.49–1.37, I2 = 0%).
In addition, two high CV risk studies[35,42] which carried out on hyperuricemia subjects with chronic heart failure or stage 3 chronic kidney disease was included. In the analysis of these studies, the RR exhibited divergence between MACE and CVE. Specifically, XOIs lowered the risk of CVE by 31% but failed on MACE (RR = 0.69, 95% CI 0.54–0.88; RR = 0.62, 95% CI 0.29–1.35, respectively) [Figure 4A and 4B].
Figure 4.

Comparison between XOIs and non-XOIs/placebo in MACE with high CV risk (A), in CVE with high CV risk (B). CV: Cardiovascular; CVE: Cardiovascular events; MACE: Major adverse cardiovascular events; XOIs: Xanthine oxidase inhibitors.
Comparison between allopurinol and febuxostat on cardiovascular risk
The pooled estimated RR based on five trials indicated no significant difference in comparison between allopurinol and febuxostat on MACE of low CV risk population (RR = 0.51, 95% CI 0.21–1.24) regardless of blinding with a mild heterogeneity (I2 = 0%, P = 0.56) [Figure 5A]. Similarly, there was no significant difference between the two medications on CVE of low CV risk subjects (RR = 0.84, 95% CI 0.65–1.09) regardless of blinding [Figure 5B].
Figure 5.
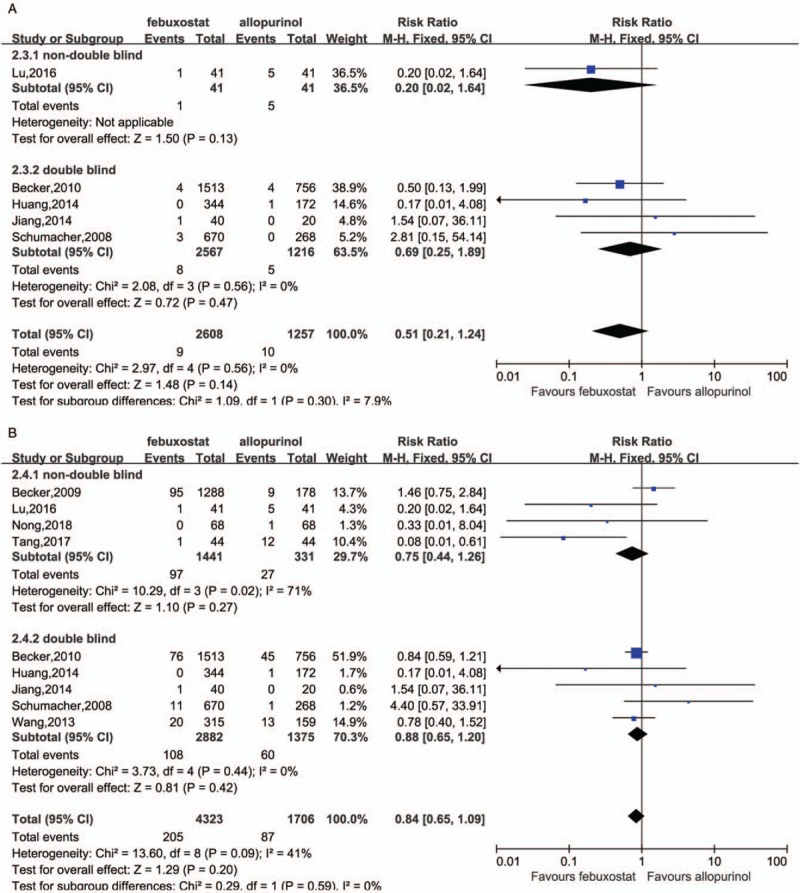
Comparison between febuxostat and allopurinol in MACE with low CV risk (A, P value in Begg test = 0.994, in Egger test = 0.888), in CVE with low CV risk (B, P value in Begg test = 0.348, in Egger test = 0.605). CV: Cardiovascular; CVE: Cardiovascular events; MACE: Major adverse cardiovascular events.
Only one high CV risk study[28] which involved subjects with established cardiovascular comorbidities at baseline, was included. The results were statistically insignificant in both MACE and CVE (RR = 1.05, 95% CI 0.89–1.23; RR = 1.08, 95% CI 0.95–1.23, respectively) [Figure 6A and 6B].
Figure 6.
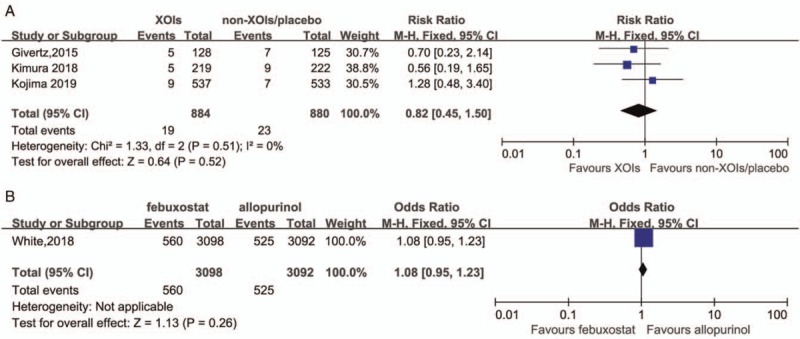
Comparison between febuxostat and allopurinol in MACE with high CV risk (A), in CVE with high CV risk (B). CV: Cardiovascular; CVE: Cardiovascular events; MACE: Major adverse cardiovascular events.
Sensitivity analysis and publication bias
We conducted a sensitivity analysis by observing whether estimated RRs pooled after excluding each study in turn at previous 95% CIs. There were no significant changes [Supplementary Figures 2–4]. The results of Begg rank correlation test and Egger linear regression test calculated to assess the publication bias were attached to descriptions of each figure. Egger publication bias plots, indicated that there was no significant publication bias.
Discussion
Many studies have shown that hyperuricemia was associated with CVE such as hypertension, CHD, peripheral vascular disease, heart failure, metabolic syndrome, and stroke. However uric acid as an anti-oxidant has evolutionary advantages to protect human from oxidative damage and prolong life span, which is in conflict with many epidemiologic studies.[45–52] In our meta-analysis, we included seven prospective cohort studies, which were inception cohorts without cardiovascular diseases or ongoing ULTs. Our result showed that hyperuricemia increased the risk of MACE by 72% compared with that of non-hyperuricemia patients (RR = 1.72, 95% CI 1.28–2.33). This result was in consistency with the meta-analysis conducted by Kim et al, which indicated that hyperuricemia was associated with an increased risk of CHD incidence (RR = 1.34, 95% CI 1.19–1.49) and mortality (RR = 1.46, 95% CI 1.20–1.73).[53] Whether hyperuricemia directly or indirectly increases the risk of cardiovascular disease remains uncertain. However current data suggested more aggressive uric acid management in hyperuricemia patients with potential cardiovascular risk. There was a certain limitation in this part of analysis. Based on the current understanding of biology and pathophysiology of hyperuricemia and gout, it was inappropriate to analyze a mixed population of individuals with gout and asymptomatic hyperuricemia. Although the two have many conditions in common, the impact of gout on cardiovascular disease is different from that of hyperuricemia. All of these studies did not give the information of exclusion of gout in the enrolled population except one study.[42] We believed they were more likely to be a mixed population, and unfortunately we were unable to separate these data into gout vs. asymptomatic hyperuricemia.
Substantial literature reported that the mechanism of hyperuricemia contributing to cardiovascular risk may be linked to vascular endothelial dysfunction.[3,7] The production of uric acid by xanthine oxidase also generates free radicals that might adversely affect mitochondrial function and production of adenosine triphosphate (ATP) which leading to endothelial dysfunction. XOIs are thus supposed to be capable of reducing free radicals’ production and the risk of CVE.[8,51,54–58] In several studies, allopurinol treatment significantly reduced the risk of CV diseases and improved endothelial functions.[59–61] These encouraging clinical data have led to the increased use of allopurinol for these diseases.[62] However, it remains controversial, whether XOIs might improve CV outcomes in hyperuricemia patients. A meta-analysis done by Zhang et al[54] in 2014 suggested that XOIs might improve outcomes of patients with cardiovascular disease but more evidence was required. Then in 2016, the same team updated their meta-analysis in which new studies with large sample size were added.[63] However, the results remained similar to the previous one that XOIs (allopurinol or oxypurinol) did not exert a large reduction in mortality but also could not exclude the possibility of substantial harm or benefit. Our meta-analysis included eight RCTs (six studies in low CV risk patients and two studies in high CV risk patients), potential medications were XOIs (allopurinol or febuxostat), and comparator group used placebo or received no treatment. To avoid investigator bias, we stratified these studies into double-blinded and non-double-blinded sub-groups. Interestingly, analysis of the blinding sub-group came to opposite results. Non-double-blinded RCT studies showed that XOIs lowered the risk of both MACE and CVE in hyperuricemia patients (RR = 0.30, 95% CI 0.16–0.56; RR = 0.48, 95% CI 0.31–0.74, respectively), whereas double-blinded studies showed that XOIs neither lowered the risk of MACE, nor the CVE (RR = 0.66, 95% CI 0.22–2.02; RR = 0.82, 95% CI 0.49–1.37, respectively). Total 13,468 and 1995 patients were included in double-blinded and non-double-blinded studies, respectively. The follow-up time of double-blinded studies varied from 6 to 24 months, while follow-up time of non-blinded studies varied from 2 to 84 months. No significant difference was seen between the baselines of two sub-groups. Our results resemble the results of Zhang et al's study to some extent. XOIs might have their protection properties in lowering cardiovascular risk. However, in the double blinded studies the relative results were not statistically significant due to being underpowered based on only 28 MACE. More double-blinded RCTs are still needed to address this issue.
Would the CV protection properties differ between XOIs? Would this difference exactly lead to the contrary results from the hypothesis? Since early in 2009, the febuxostat's drug labels have already carried a warning and precaution about CVE because the pre-approval clinical trials showed a higher rate of CVE in patients who were treated with febuxostat compared to allopurinol.[12,13,64] The Cardiovascular Safety of Febuxostat and Allopurinol in Patients with Gout and Cardiovascular Morbidities (CARES) trial, was; therefore, conducted as an FDA requirement to better understand these differences. The 2017 FDA drug alert mentioned in the beginning of this article was just due to the preliminary results of this safety trial which was conducted in over 6000 gout patients treated with either febuxostat or allopurinol. The results showed that in patients with gout and major cardiovascular coexisting conditions, febuxostat was non-inferior to allopurinol with respect to rates of adverse CVE. However all-cause mortality and cardiovascular mortality were higher with febuxostat than with allopurinol.[28] This article was published on March, 2018 and was included in our study. Since it was the only one eligible study focused on high CV risk patients, it was analyzed separately, and the result of febuxostat vs. allopurinol was statistically insignificant in both MACE and CVE (RR = 1.05, 95% CI 0.89–1.23; RR = 1.08, 95% CI 0.95–1.23). Choi et al[71] published a review article which talked about the implications of the CARES trial and associated it with the FDA public safety alert, that may help us to understand deeply. CARES did not prove that febuxostat raises CV mortality risk; however, it suggested greater risk with febuxostat than allopurinol. CARES results did not support first line use of febuxostat, and raised questions on febuxostat placement at various pharmacologic ULT decision tree branches. The FDA safety alert highlighted the need for sharing ULT medical decision with gout patients, including discussion of CV safety of febuxostat.[67]
The effect of urate-lowering drugs was different between XOIs and uricosuric. For benzbromarone, there was no eligible study included in our study. Recently, Kim et al[65] sought to examine the effect of ULTs with either probenecid or allopurinol on cardiovascular risk in older patients with gout. Using Medicare to claim data of over a 6-year period, the authors identified a total of 9722 probenecid initiators propensity score-matched to 29,166 allopurinol initiators with mean age of 76 ± 7 years. Treatment with probenecid appeared to be associated with a modestly decreased risk of CV events including MI, stroke, and HF exacerbation compared with allopurinol. From a mechanic view, probenecid was not only a uricosuric, but also an inhibitor of pannexin 1 channels (an ATP release channel) which involved in inflammasome activation and interleukin (IL)-1β release.[66] IL-1β was also known to play a pivotal role in the pathogenesis of gout inflammation and atherosclerosis.[67] Therefore, it was plausible to meet the conclusion that probenecid may have cardioprotective effects in gout patients and this conclusion might challenge the first line choice of XOIs as ULTs. However, it should be considered that only prospective RCTs can prove causality; retrospective analysis can only suggest associations.
There were still several limitations in this analysis. The CVE rates were low. Most RCTs were single-center, which are more prone to investigator bias. There was a highly variable length of follow-up (2 to 84 months). The meta-analysis could be underpowered, as studies were not powered to report CVE. Since the follow-up durations were largely different among the studies, we did a sub-group meta-analysis based on short-term (<12 m) and long-term (≥12 m), separately [Supplementary Figure 5]. Compared with no-treatment or placebo, XOIs lowered the risk of CVE (RR = 0.52, 95% CI 0.38–0.73) and MACE (RR = 0.38, 95% CI 0.23–0.62) in long-term sub-group, but not in short-term sub-group (CVE: RR = 0.79, 95% CI 0.61–1.01; MACE: RR = 0.73, 95% CI 0.26–2.08). When comparing febuxostat and allopurinol, febuxostat slightly lowered CVE (RR = 0.74, 95% CI 0.56–0.99) in short-term sub-group; however, no significant difference was seen in MACE and long-term sub-group. In addition, the doses of XOIs might also be important in their cardiovascular effects. Doses of allopurinol ranged from 100 to 600 mg/day and doses of febuxostat ranged from 40 to 120 mg/day. There might be a dose-dependent relationship between XOIs and cardiovascular effects. However, we were unable to do dosage sub-group analysis since there were less studies and no detailed information as well. Moreover, the CV safety evaluation of XOIs in high CV risk patients is the priority. Unfortunately, there were only two trials with high CV risk patients. The outcomes showed that XOIs lowered the risk of CVE with high CV risk rather than MACE with high CV risk (RR = 0.69, 95% CI 0.54–0.88; RR = 0.62, 95% CI 0.29–1.35, respectively). Inflammation was the key role in developing CVE in hyperuricemia and gout. Recurrent acute gout attack, chronic gouty arthropathy, and uncontrolled high serum uric acid are three major elements which contributed to inflammation. Most of the studies have been not only too small in sample size but also too short to show CV benefits by controlling gout attacks and lowering uric acid. There was some substantive heterogeneity in comparison between hyperuricemia and non-hyperuricemia in MACE and CVE due to large range of population.
Because of the uncertainty, larger clinical trials with a longer follow-up period are needed to determine the cardiovascular safety and efficacy of XOIs in hyperuricemia and gout. Two large RCTs are now underway. The PRIZE study is a multi-center randomized study for evaluating vascular function under uric acid control using febuxostat in 500 patients with asymptomatic hyperuricemia (uric acid >7.0 mg/dL). Participants will be centrally randomized to receive either febuxostat (10–60 mg/day) or non-pharmacological treatment. Follow-up will be continued for 24 months. The PRIZE study will be the first study to provide important data on the effects of febuxostat on atherosclerosis in patients with asymptomatic hyperuricemia.[69] The FAST study is a cardiovascular safety study using the prospective, randomized, open, blinded endpoint design. Recruited patients are aged over 60 years, randomized to either allopurinol or febuxostat. It plans to randomize 5000 patients with at least 3 years of follow-up. The primary endpoint is Anti-Platelet Trialists’ Collaboration composite cardiovascular endpoint of non-fatal myocardial infarction, non-fatal stroke or cardiovascular death.[70] The Febuxostat for Cerebral and caRdiorenovascular Events prEvEntion stuDy was a study to compare febuxostat with allopurinol on the effect of preventing cerebral, cardiovascular, and renal events in patients with hyperuricemia. 1070 patients were followed for at least 3 years.[68] In this study the non-febuxostat group included 533 hyperuricemia patients and 27% of which accepted allopurinol; however, no detailed information was available for the allopurinol sub-group, which made our further analysis impossible. Thus, we excluded it from our study regretfully. The results of these two trails are worth waiting. We are keeping focus on their progress and will update our meta-analysis as soon as these results are available.
Conclusions
Our meta-analysis indicates that patients with hyperuricemia do have an increased risk of CVEs, and XOIs may reduce the incidence of MACE and total CVE. In addition, CV safety between allopurinol and febuxostat has no significant difference; however, all-cause mortality and cardiovascular mortality were higher in patients with febuxostat than allopurinol. Because of the limitations of the previous studies, data of large and long-term ongoing trials are worth waiting.
Funding
This work was supported by a grant from the National Natural Science Foundation of China (No. 81601396).
Conflicts of interest
None.
Supplementary Material
Footnotes
How to cite this article: Zhao L, Cao L, Zhao TY, Yang X, Zhu XX, Zou HJ, Wan WG, Xue Y. Cardiovascular events in hyperuricemia population and a cardiovascular benefit-risk assessment of urate-lowering therapies: a systematic review and meta-analysis. Chin Med J 2020;133:982–993. doi: 10.1097/CM9.0000000000000682
References
- 1.Khanna D, Fitzgerald JD, Khanna PP, Bae S, Singh MK, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res 2012; 64:1431–1446. doi: 10.1002/acr.21772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jordan KM, Cameron JS, Snaith M, Zhang W, Doherty M, Seckl J, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of gout. Rheumatology 2007; 46:1372–1374. doi: 10.1093/rheumatology/kem056a. [DOI] [PubMed] [Google Scholar]
- 3.Yamanaka H. Japanese guideline for the management of hyperuricemia and gout: second edition. Nucleosides Nucleotides Nucleic Acids 2011; 30:1018–1029. doi: 10.1080/15257770.2011.596496. [DOI] [PubMed] [Google Scholar]
- 4.Khanna D, Khanna PP, Fitzgerald JD, Singh MK, Bae S, Neogi T, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 2: therapy and anti-inflammatory prophylaxis of acute gouty arthritis. Arthritis Care Res 2012; 64:1447–1461. doi: 10.1002/acr.21773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sivera F, Andrés M, Carmona L, Kydd AS, Moi J, Seth R, et al. Multinational evidence-based recommendations for the diagnosis and management of gout: integrating systematic literature review and expert opinion of a broad panel of rheumatologists in the 3e initiative. Ann Rheum Dis 2014; 73:328–355. doi: 10.1136/annrheumdis-2013-203325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Richette P, Doherty M, Pascual E, Barskova V, Becce F, Castañeda-Sanabria J, et al. 2016 updated EULAR evidence-based recommendations for the management of gout. Ann Rheum Dis 2017; 76:29–42. doi: 10.1136/annrheumdis-2016-209707. [DOI] [PubMed] [Google Scholar]
- 7.Mercuro G, Vitale C, Cerquetani E, Zoncu S, Deidda M, Fini M, et al. Effect of hyperuricemia upon endothelial function in patients at increased cardiovascular risk. Am J Cardiol 2004; 94:932–935. doi: 10.1016/j.amjcard.2004.06.032. [DOI] [PubMed] [Google Scholar]
- 8.Schlesinger DN. Management of acute and chronic gouty arthritis. Drugs 2004; 64:2399.doi: 10.2165/00003495-200464210-00003. [DOI] [PubMed] [Google Scholar]
- 9.Schlesinger N. New agents for the treatment of gout and hyperuricemia: febuxostat, puricase, and beyond. Curr Rheumatol Rep 2010; 12:130–134. doi: 10.1007/s11926-010-0093-2. [DOI] [PubMed] [Google Scholar]
- 10. TMX-67 (febuxostat) Approved in Japan - Teijin-developed Novel Drug for Chronic Management of Hyperuricemia; 2011. Available from http://www.medicalnewstoday.com/releases/214469.php. [Accessed October 8, 2019] [Google Scholar]
- 11.Schumacher HR, Jr, Becker MA, Wortmann RL, Macdonald PA, Hunt B, Streit J, et al. Effects of febuxostat versus allopurinol and placebo in reducing serum urate in subjects with hyperuricemia and gout: a 28-week, phase III, randomized, double-blind, parallel-group trial. Arthritis Rheum 2008; 59:1540–1548. doi: 10.1002/art.24209. [DOI] [PubMed] [Google Scholar]
- 12.Becker MA, Schumacher HR, Jr, Wortmann RL, MacDonald PA, Eustace D, Palo WA, et al. Febuxostat compared with allopurinol in patients with hyperuricemia and gout. N Engl J Med 2005; 353:2450–2461. doi: 10.1056/NEJMoa050373. [DOI] [PubMed] [Google Scholar]
- 13. FDA Drug Safety Communication: FDA to Evaluate Increased Risk of Heart-related Death and Death From all Causes With the Gout Medicine Febuxostat (Uloric). Available from https://www.fda.gov/drugs/drug-safety-and-availability/fda-drug-safety-communication-fda-evaluate-increased-risk-heart-related-death-and-death-all-causes. [Accessed October 8, 2019] [Google Scholar]
- 14. Health Canada. Summary Safety Review - ULORIC (febuxostat) - Assessing the Potential Risk of Heart Failure. Available from https://www.canada.ca/en/health-canada/services/drugs-health-products/medeffect-canada/safety-reviews/summary-safety-review-uloric-febuxostat-assessing-potential-risk-heart-failure.html. [Accessed October 8, 2019] [Google Scholar]
- 15.Choi HK, Curhan G. Independent impact of gout on mortality and risk for coronary heart disease. Circulation 2007; 116:894–900. doi: 10.1161/CIRCULATIONAHA.107.703389. [DOI] [PubMed] [Google Scholar]
- 16.Krishnan E, Svendsen K, Neaton JD, Grandits G, Kuller LH. MRFIT Research Group. Long-term cardiovascular mortality among middle-aged men with gout. Arch Intern Med 2008; 168:1104–1110. doi: 10.1001/archinte.168.10.1104. [DOI] [PubMed] [Google Scholar]
- 17.Higgins JPT, Thomas J, Chandler J, Cumpston M, Li T, Page MJ, et al. Cochrane Handbook for Systematic Reviews of Interventions. 2nd EditionChichester (UK): John Wiley & Sons; 2019. [Google Scholar]
- 18.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gøtzsche PC, Ioannidis JP. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ 2009; 339:b2700.doi: 10.7326/0003-4819-151-4-200908180-00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mcpheeters ML. Newcastle-Ottawa Quality Assessment Scale. Available from: http://www.ncbi.nlm.nih.gov/pubmedhealth/PMH0049210/. [Accessed October 8, 2019] [Google Scholar]
- 20.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sciacqua A, Perticone M, Tassone EJ, Cimellaro A, Miceli S, Maio R. Uric acid is an independent predictor of cardiovascular events in post-menopausal women. Int J Cardiol 2015; 197:271–275. doi: 10.1016/j.ijcard.2015.06.069. [DOI] [PubMed] [Google Scholar]
- 22.Zalawadiya SK, Veeranna V, Mallikethi-Reddy S, Bavishi C, Lunagaria A, Kottam A, et al. Uric acid and cardiovascular disease risk reclassification: findings from NHANES III. Eur J Prevent Cardiol 2015; 22:513–518. doi: 10.1177/2047487313519346. [DOI] [PubMed] [Google Scholar]
- 23.Turak O, Afsar B, Ozcan F, Canpolat U, Grbovic E, Mendi MA, et al. Relationship between elevated morning blood pressure surge, uric acid, and cardiovascular outcomes in hypertensive patients. J Clin Hypertens 2014; 16:530–535. doi: 10.1111/jch.12359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanbay M, Yilmaz MI, Sonmez A, Solak Y, Saglam M, Cakir E, et al. Serum uric acid independently predicts cardiovascular events in advanced nephropathy. Am J Nephrol 2012; 36:324–331. doi: 10.1159/000342390. [DOI] [PubMed] [Google Scholar]
- 25.Ekundayo OJ, Dell’Italia LJ, Sanders PW, Arnett D, Aban I, Love TE, et al. Association between hyperuricemia and incident heart failure among older adults: a propensity-matched study. Int J Cardiol 2010; 142:279–287. doi: 10.1016/j.ijcard.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Franse LV, Pahor M, Di Bari M, Shorr RI, Wan JY, Somes GW, et al. Serum uric acid, diuretic treatment and risk of cardiovascular events in the systolic hypertension in the elderly program (SHEP). J Hypertens 2000; 18:1149–1154. doi: 10.1097/00004872-200018080-00021. [DOI] [PubMed] [Google Scholar]
- 27.Fang J, Alderman MH. Serum uric acid and cardiovascular mortality: the NHANES I epidemiologic follow-up study, 1971-1992. JAMA 2000; 283:2404–2410. doi: 10.1001/jama.283.18.2404. [DOI] [PubMed] [Google Scholar]
- 28.White WB, Saag KG, Becker MA, Borer JS, Gorelick PB, Whelton A, et al. Cardiovascular safety of febuxostat or allopurinol in patients with gout. N Engl J Med 2018; 378:1200–1210. doi: 10.1056/NEJMoa1710895. [DOI] [PubMed] [Google Scholar]
- 29.Guimin N, Peng H. Clinical study and safety analysis of febuxostat in treatment of gout (in Chinese). Chin J Clin Rational Drug Use 2018; 11:38–39. doi: 10.15887/j.cnki.13-1389/r.2018.01.019. [Google Scholar]
- 30.Dalbeth N, Saag KG, Palmer WE, Choi HK, Hunt B, MacDonald PA, et al. Effects of febuxostat in early gout: a randomized, double-blind, placebo-controlled study. Arthritis Rheumatol 2017; 69:2386–2395. doi: 10.1002/art.40233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Tang YHT. Application and safety of febuxostat in patients with hyperuricemia complicated with chronic renal insufficiency (in Chinese). Chin J Modern Drug Application 2017; 11:124–125. doi: 10.14164/j.cnki.cn11-5581/r.2017.20.066. [Google Scholar]
- 32.Saag KG, Whelton A, Becker MA, MacDonald P, Hunt B, Gunawardhana L. Impact of febuxostat on renal function in gout subjects with moderate-to-severe renal impairment. Arthritis Rheumatol 2016; 68:2035–2043. doi: 10.1002/art.39654. [DOI] [PubMed] [Google Scholar]
- 33.Lu F. The efficacy and safety of febuxostat in the treatment of patients with gout and hyperuricemia (in Chinese). Chin J Health Care Nutrition 2016; 26:179.doi: 10.3969/j.issn.1004-7484.2016.26.245. [Google Scholar]
- 34.Goicoechea M, Garcia S, Verdalles U, Verde E, Macias N, Santos A, et al. Allopurinol and progression of CKD and cardiovascular events: long-term follow-up of a randomized clinical trial. Am J Kidney Dis 2015; 65:543–549. doi: 10.1053/j.ajkd.2014.11.016. [DOI] [PubMed] [Google Scholar]
- 35.Givertz MM, Anstrom KJ, Redfield MM, Deswal A, Haddad H, Butler J, et al. Effects of xanthine oxidase inhibition in hyperuricemic heart failure patients: the xanthine oxidase inhibition for hyperuricemic heart failure patients (EXACT-HF) study. Circulation 2015; 131:1763–1771. doi: 10.1161/CIRCULATIONAHA.114.014536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tuta L, Stanigut A. Allopurinol therapy for hyperuricemia reduces inflammation and progression of renal disease in moderate chronic kidney disease. Nephrol Dial Transplant 2014;29 Suppl 3:iii118 (Conference, 51st ERA-EDTA Congress Amsterdam Netherlands). DOI: 10.1093/ndt/gfu145. [Google Scholar]
- 37.Huang X, Du H, Gu J, Zhao D, Jiang L, Li X, et al. An allopurinol-controlled, multicenter, randomized, double-blind, parallel between-group, comparative study of febuxostat in Chinese patients with gout and hyperuricemia. Int J Rheum Dis 2014; 17:679–686. doi: 10.1111/1756-185X.12266. [DOI] [PubMed] [Google Scholar]
- 38.Jiang LL, Jin X, Shen Y, Ma JH, Wu JD. The efficacy and safety of febuxostat in the treatment of patients with gout and hyperuricemia (in Chinese). J Practical Med 2014; 17:2827–2830. doi: 10.3969/j.issn.1006-5725.2014.17.047. [Google Scholar]
- 39.Wang LY, Zhao Y, Zheng Y, Li XX, Zhang X, Xu JH, et al. Effect and safety of febuxotant versus allopurinol in reducing serum urate in subjects with hyperuricemia and gout: a multi-center, randomized, double-blind, parallel controlled trail (in Chinese). Chin J Clinicians (Electronic Edition) 2013; 7:2798–2803. doi: 10.3877/cma.j.issn.1674-0785.2013.07.012. [Google Scholar]
- 40.Becker MA, Schumacher HR, Espinoza LR, Wells AF, MacDonald P, Lloyd E, et al. The urate-lowering efficacy and safety of febuxostat in the treatment of the hyperuricemia of gout: the CONFIRMS trial. Arthritis Res Ther 2010; 12:R63.doi: 10.1186/ar2978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Becker MA, Schumacher HR, MacDonald PA, Lloyd E, Lademacher C. Clinical efficacy and safety of successful longterm urate lowering with febuxostat or allopurinol in subjects with gout. J Rheumatol 2009; 36:1273–1282. doi: 10.3899/jrheum.080814. [DOI] [PubMed] [Google Scholar]
- 42.Kimura K, Hosoya T, Uchida S, Inaba M, Makino H, Maruyama S, et al. Febuxostat therapy for patients with stage 3 CKD and asymptomatic hyperuricemia: a randomized trial. Am J Kidney Dis 2018; 72:798–810. doi: 10.1053/j.ajkd.2018.06.028. [DOI] [PubMed] [Google Scholar]
- 43.Saag KG, Becker MA, Whelton A, Hunt B, Castillo M, Kisfalvi K, et al. Efficacy and safety of febuxostat extended and immediate release in patients with gout and renal impairment: a phase III placebo-controlled study. Arthritis Rheumatol 2019; 1:143–153. doi: 10.1002/art.40685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Goicoechea M, de Vinuesa SG, Verdalles U, Ruiz-Caro C, Ampuero J, Rincón A, et al. Effect of allopurinol in chronic kidney disease progression and cardiovascular risk. Clin J Am Soc Nephrol 2010; 5:1388–1393. doi: 10.2215/CJN.01580210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Baker JF, Krishnan E, Chen L, Schumacher HR. Serum uric acid and cardiovascular disease: recent developments, and where do they leave us? Am J Med 2005; 118:816–826. doi: 10.1016/j.amjmed.2005.03.043. [DOI] [PubMed] [Google Scholar]
- 46.Becker MA, Jolly M. Hyperuricemia and associated diseases. Rheum Dis Clin North Am 2006; 32:275–293. doi: 10.1016/j.rdc.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 47.Abeles AM. Hyperuricemia, gout, and cardiovascular disease: an update. Curr Rheumatol Rep 2015; 17:13.doi: 10.1007/s11926-015-0495-2. [DOI] [PubMed] [Google Scholar]
- 48.Tseng CH. Independent association of uric acid levels with peripheral arterial disease in Taiwanese patients with type 2 diabetes. Diabet Med 2004; 21:724–729. doi: 10.1111/j.1464-5491.2004.01239.x. [DOI] [PubMed] [Google Scholar]
- 49.Baker JF, Schumacher HR, Krishnan E. Serum uric acid level and risk for peripheral arterial disease: analysis of data from the multiple risk factor intervention trial. Angiology 2007; 58:450–457. doi: 10.1177/0003319707303444. [DOI] [PubMed] [Google Scholar]
- 50.Kim SY, De Vera MA, Choi HK. Gout and mortality. Clin Exp Rheumatol 2008; 26:S115.doi: 10.1002/art.24044. [PubMed] [Google Scholar]
- 51.Feig DI, Kang DH, Johnson RJ. Uric acid and cardiovascular risk. New Engl J Med 2008; 359:1811–1821. doi: 10.1056/NEJMra0800885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Braga F, Pasqualetti S, Ferraro S, Panteghini M. Hyperuricemia as risk factor for coronary heart disease incidence and mortality in the general population: a systematic review and meta-analysis. Clin Chem Lab Med 2015; 54:7–15. doi: 10.1515/cclm-2015-0523. [DOI] [PubMed] [Google Scholar]
- 53.Kim SY, Guevara JP, Kim KM, Choi HK, Heitjan DF, Albert DA. Hyperuricemia and coronary heart disease: a systematic review and meta-analysis. Arthritis Care Res 2010; 62:170–180. doi: 10.1002/acr.20065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhang J, Dierckx R, Cleland JG. Xanthine oxidase inhibition for the treatment of cardiovascular disease: a systematic review and meta-analysis. Cardiovasc Therapeutics 2014; 32:57–58. doi: 10.1111/1755-5922.12059. [DOI] [PubMed] [Google Scholar]
- 55.Bredemeier M, Lopes LM, Eisenreich MA, Sheila H, Guilherme KB, d’Avila R, et al. Xanthine oxidase inhibitors for prevention of cardiovascular events: a systematic review and meta-analysis of randomized controlled trials. BMC Cardiovascular Disord 2018; 18:24.doi: 10.1186/s12872-018-0757-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saavedra WF, Paolocci N, St John ME, Skaf MW, Stewart GC, Xie JS, et al. Imbalance between xanthine oxidase and nitric oxide synthase signaling pathways underlies mechanoenergetic uncoupling in the failing heart. Circ Res 2002; 90:297–304. doi: 10.1161/hh0302.104531. [DOI] [PubMed] [Google Scholar]
- 57.Anker SD, Doehner W, Rauchhaus M, Sharma R, Francis D, Knosalla C, et al. Uric acid and survival in chronic heart failure validation and application in metabolic, functional, and hemodynamic staging. Circulation 2003; 41:1991–1997. doi: 10.1161/01.CIR.0000065637.10517.A0. [DOI] [PubMed] [Google Scholar]
- 58.Krishnan E. Gout and the risk for incident heart failure and systolic dysfunction. BMJ Open 2012; 2:e000282.doi: 10.1136/bmjopen-2011-000282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Dubreuil M, Zhu Y, Zhang Y, Seeger JD, Lu N, Rho YH, et al. Allopurinol initiation and all-cause mortality in the general population. Ann Rheum Dis 2014; 74:1368–1372. doi: 10.1136/annrheumdis-2014-205269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Khosla UM, Zharikov S, Finch JL, Nakagawa T, Roncal C, Mu W, et al. Hyperuricemia induces endothelial dysfunction. Kidney Int 2005; 67:1739–1742. doi: 10.1111/j.1523-1755.2005.00273.x. [DOI] [PubMed] [Google Scholar]
- 61.Grimaldi-Bensouda L, Alpérovitch A, Aubrun E, Danchin N, Rossignol M, Abenhaim L, et al. Impact of allopurinol on risk of myocardial infarction. Ann Rheum Dis 2015; 74:836–842. doi: 10.1136/annrheumdis-2012-202972. [DOI] [PubMed] [Google Scholar]
- 62.Struthers A, Shearer F. Allopurinol: novel indications in cardiovascular disease. Heart 2012; 98:1543–1545. doi: 10.1136/heartjnl-2012-302249. [DOI] [PubMed] [Google Scholar]
- 63.Zhang J, Dierckx R, Mohee K, Clark AL, Cleland JG. Xanthine oxidase inhibition for the treatment of cardiovascular disease: an updated systematic review and meta-analysis. ESC Heart Fail 2017; 4:40–45. doi: 10.1002/ehf2.12112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Schumacher HR, Jr, Becker MA, Lloyd E, MacDonald PA, Lademacher C. Febuxostat in the treatment of gout: 5-yr findings of the FOCUS efficacy and safety study. Rheumatology 2009; 48:188–194. doi: 10.1093/rheumatology/ken457. [DOI] [PubMed] [Google Scholar]
- 65.De Vera MA, Rahman MM, Bhole V, Kopec JA, Choi HK. Independent impact of gout on the risk of acute myocardial infarction among elderly women: a population-based study. Ann Rheum Dis 2010; 69:1162–1164. doi: 10.1136/ard.2009.122770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Silverman W, Locovei S, Dahl G. Probenecid, a gout remedy, inhibits pannexin 1 channels. Am J Physiol Cell Physiol 2008; 295:C761–C767. doi: 10.1152/ajpcell.00227.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Fearon WF, Fearon DT. Inflammation and cardiovascular disease: role of the interleukin-1 receptor antagonist. Circulation 2008; 117:2577–2579. doi: 10.1161/CIRCULATIONAHA.108.772491. [DOI] [PubMed] [Google Scholar]
- 68.Kojima S, Matsui K, Ogawa H, Jinnouchi H, Hiramitsu S, Hayashi T, et al. Rationale, design, and baseline characteristics of a study to evaluate the effect of febuxostat in preventing cerebral, cardiovascular, and renal events in patients with hyperuricemia. J Cardiol 2017; 69:169–175. doi: 10.1016/j.jjcc.2016.02.015. [DOI] [PubMed] [Google Scholar]
- 69.Oyama J, Tanaka A, Sato Y, Tomiyama H, Sata M, Ishizu T, et al. Rationale and design of a multicenter randomized study for evaluating vascular function under uric acid control using the xanthine oxidase inhibitor, febuxostat: the PRIZE study. Cardiovasc Diabetol 2016; 15:87.doi: 10.1186/s12933-016-0409-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.MacDonald TM, Ford I, Nuki G, Mackenzie IS, De Caterina R, Findlay E, et al. Protocol of the febuxostat versus allopurinol streamlined trial (FAST): a large prospective, randomised, open, blinded endpoint study comparing the cardiovascular safety of allopurinol and febuxostat in the management of symptomatic hyperuricaemia. BMJ Open 2014; 4:e005354.doi: 10.1136/bmjopen-2014-005354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Choi H, Neogi T, Stamp L, Dalbeth N, Terkeltaub R. New perspectives in rheumatology: implications of the cardiovascular safety of febuxostat and allopurinol in patients with gout and cardiovascular morbidities trial and the associated Food and Drug Administration public safety alert. Arthritis Rheumatol 2018; 70:1702–1709. doi: 10.1002/art.40583. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


