Abstract
Antiphospholipid syndrome (APS) is a thromboinflammatory disease with a variety of clinical phenotypes. Primary thrombosis prophylaxis should take an individualized risk stratification approach. Moderate-intensity vitamin K antagonist such as warfarin remains the primary strategy for secondary thrombosis prophylaxis among APS patients, especially for patients with predominantly venous disease. For now, direct oral anti-coagulants should be avoided in most APS patients, especially those with history of arterial manifestations. Obstetric APS management should be tailored based on an individual patient's antiphospholipid antibody profile, and obstetric and thrombotic history. Pharmacological agents beyond anticoagulants may be considered for the management of microthrombotic and nonthrombotic manifestations of APS, although more data are needed. A relatively recent discovery in the area of APS pathogenesis is the implication of neutrophil extracellular traps in thrombin generation and initiation of inflammatory cascades. APS is a complex thromboinflammatory disease with a broad clinical spectrum. Personalized therapy according to an individual's unique thrombosis and obstetric risk should be advocated.
Keywords: Antiphospholipid syndrome, Antiphospholipid antibodies, Treatment
Introduction
Antiphospholipid syndrome (APS) is an autoimmune thromboinflammatory disorder that can have detrimental and sometimes devastating effects on patients and their families. APS may involve essentially any circulatory bed in the body. While the deep veins of the lower limbs and the arterial circulation of the brain are the most common sites of thrombosis, any tissue or organ can be affected.[1,2] Obstetrical complications are also well recognized in APS, including eclampsia or severe preeclampsia that results in premature birth, as well as fetal demise after the 10th week of gestation.[1,3] Beyond thrombosis and pregnancy complications, other clinical features such as persistent thrombocytopenia, hemolytic anemia, livedo reticularis, APS nephropathy, and cognitive dysfunction have been associated with APS, are clearly associated with APS and often referred to as “non-criteria” or “extra-criteria” manifestations[4] [Table 1]. APS is divided into primary APS that occurs in isolation, and secondary APS that is associated with another autoimmune syndrome, most commonly systemic lupus erythematosus (SLE). Catastrophic antiphospholipid syndrome (CAPS), which is characterized by thrombi in multiple small vascular beds leading to multi-organ failure with high mortality, develops in a small subgroup of APS patients.[5] The estimated population prevalence of APS is 50 cases per 100,000, with an annual incidence of 2.1 per 100,000.[6] Observational studies, which typically lack rigorous follow-up, have shown that antiphospholipid antibodies (aPL) may be positive in as many as 13% of patients with stroke, 11% with myocardial infarction, and 9.5% of patients with deep vein thrombosis.[7] The prevalence of persistently positive aPL among the healthy population is still not known.
Table 1.
Clinical manifestations of antiphospholipid syndrome.

Classification of APS requires a positive test of one or more typical aPL (anticardiolipin [aCL] IgG or IgM, anti-β2glycoprotein-I [aβ2GPI] IgG or IgM, and lupus anticoagulant [LA]) in the context of either a thrombotic event or certain types of pregnancy morbidity [Table 2].[8] However, in daily practice APS may be much more complex and clearly represents a disease spectrum [Figure 1]. While there are some APS patients with seemingly isolated thrombotic or obstetric complications, there are also patients who have persistently positive aPL and only “non-criteria” manifestations. There are also a small group of patients who develop CAPS. We will now review current literature relevant to APS clinical care and briefly describe some updates related to pathophysiology.
Table 2.
Classification criteria for antiphospholipid syndrome.
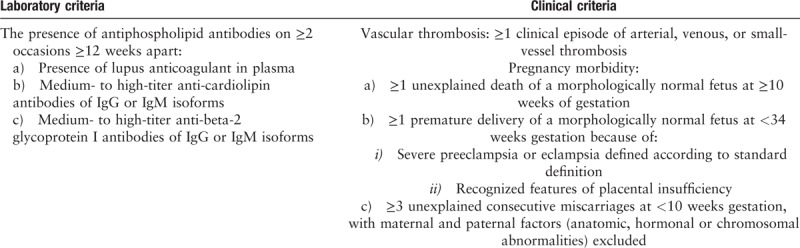
Figure 1.
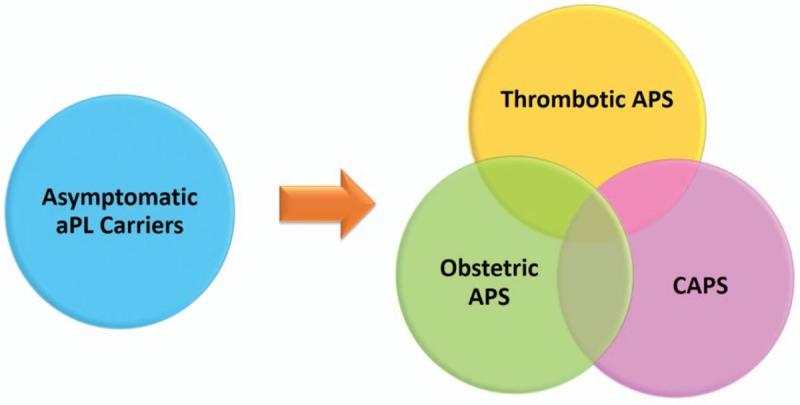
Overlapping clinical spectrum of antiphospholipid syndrome (APS). aPL: Antiphospholipid antibodies; CAPS: Catastrophic antiphospholipid syndrome.
Update on pathophysiology
Antiphospholipid antibodies (aPL) are a heterogeneous group of autoantibodies that play an important role in the pathogenesis of APS via their interactions with plasma protein such as β2-glycoprotein I (β2GPI), prothrombin, thrombomodulin, plasminogen, antithrombin III, protein C, protein S, annexin II, and annexin V.[9–14] Key aPL-mediated prothrombotic mechanisms involve the activation of endothelial cells,[15] monocytes,[16] platelets,[17] coagulation factors, and complement proteins.[18,19] Furthermore, aPL interfere with fibrinolytic and coagulation pathways and trigger placental inflammation and injury.[20,21] As the pathogenesis of APS has been reviewed in detail elsewhere,[22,23] we will here just highlight a few recent studies that may advance our understanding of APS pathogenesis.
MicroRNAs (miRNAs) are single-stranded small non-coding RNAs that play an important role in cellular communication. They act to regulate the expression of messenger RNAs which contain complementary sequence to miRNAs. In recent years, several groups have characterized miRNAs in the pathogenesis of APS.[24–27] One interesting study found that forced overexpression of certain miRNAs (miR-19b and miR-20a) in tissue factor-expressing cell lines reduced levels of tissue factor messenger RNA, along with cellular procoagulant activity.[25] It appears that monocytes from APS patients have significantly lower levels of miR-19b and miR-20a as compared with healthy controls, with low levels of these miRNAs predicting an increased level of tissue factor.[25] In a different study, in vitro treatment of healthy-donor neutrophils, monocytes, and endothelial cells with purified aPL IgG decreased the expression of various miRNAs.[27] At the same time, differential expression of circulating miRNAs can distinguish APS patients from healthy controls[26]; for example, transcriptomic analysis of plasmacytoid dendritic cells from APS and SLE patients suggested that lower miRNA expression (miR-361-5p, miR-128-3p, miR-181a-2-3p, and others) associates with a heightened type I interferon signature.[24] More studies are needed to further elucidate the role that miRNAs play in APS disease modulation, and the extent to which miRNAs may be viable therapeutic targets.
Many studies from the general thrombosis literature have revealed that activated neutrophils, and in particular neutrophil extracellular trap (NET) formation, contribute to the propagation of thrombi affecting arterial, venous, and microscopic vascular beds.[28,29] NETs have also been recently implicated in the pathogenesis of APS. In 2015, our group reported that sera from APS patients, as well as purified aPL, trigger neutrophils to release NETs.[30] The potential in vivo relevance of this observation has been confirmed in mouse models of aPL-mediated large-vein thrombosis in which either depletion of neutrophils or digestion of NETs is protective.[31] Neutrophils from APS patients also appear to have increased adhesive potential, which is dependent upon the activated form of integrin Mac-1. This proadhesive phenotype amplifies neutrophil-endothelium interactions, potentiates NET formation, and potentially lowers the threshold for thrombosis.[32]
Sera from primary APS patients have elevated type I interferon activity,[33] which has been confirmed by many groups.[34–36] Interestingly, transcriptome analysis of neutrophils from APS patients revealed a heightened expression of genes relevant to not only interferon signaling, but also cellular defense and cell-cell adhesion. One particular gene encoding P-selectin glycoprotein ligand-1 (PSGL-1) was strongly upregulated and potentially involved in thrombus formation. Indeed, an in vivo model demonstrated that PSGL-1 deficiency protected mice from aPL-accelerated thrombus formation.[37] The relevance of this pathway in patients has yet to be intensively studied.
Therapies that target NET formation have the potential to treat thrombotic diseases.[29] For example, selective agonism of the adenosine A2A receptor suppresses aPL-mediated NETosis in protein kinase A-dependent fashion.[38] A2A agonism also reduces thrombosis in the inferior vena cava of both control mice and mice treated with aPL. Dipyridamole, which is known to potentiate adenosine signaling by increasing extracellular concentrations of adenosine and interfering with the breakdown of cAMP, also suppresses aPL-mediated NETosis and mitigates venous thrombosis in mice. Interestingly, CD39 and CD73, which convert extracellular ATP first to AMP and then to adenosine protect experimental animals from aPL-induced fetal loss.[39]
In summary, it is likely that heterogeneous mechanisms are at play in the prothrombotic and proinflammatory mechanisms mediated by aPL. Emerging role of miRNAs in APS pathogenesis has attracted growing attention. Neutrophils and NET formation have only recently been investigated, and future research should help us understand the extent to which neutrophils are viable drug targets in patients with APS, as well as how neutrophils interact with other well-accepted players in APS pathophysiology such as endothelial cells and platelets. We speculate that therapies targeting NETs may hold particular promise, at least for a subset of patients with APS.
Primary thrombosis prophylaxis
One of the most significant challenges in APS management is the treatment strategy for asymptomatic aPL-positive individuals. It is well known that persistently positive aPL are associated with an increased risk of arterial and venous thrombosis.[40] However, precise quantification of such risk has been difficult due to inconsistent application of aPL laboratory criteria, the multifactorial nature of thrombosis risk, and various confounding factors such as underlying autoimmune diseases and medication effects.[40,41] Routine primary thrombosis prophylaxis among asymptomatic aPL carriers remains controversial due to limited and low quality data.[41,42] Here we will summarize current evidence and recommendations regarding primary thrombosis prophylaxis as it relates to APS.
Clinically-significant aPL
The first step in risk stratifying an aPL-positive individual is to determine whether a positive aPL test is clinically significant.[40] Transiently positive aPL are common, especially during concomitant infections, and are often not associated with thrombosis. A recent systemic review of 297 infection-associated aPL-positive cases (24.6% fulfilled full Sydney classification criteria) showed that 75.4% of positive aPL detected during an infection are transient.[43] A prospective cohort study of blood samples from healthy donors showed 10% baseline positivity for aCL or LA; however, 12 months later only 1% of those blood samples remained positive for aCL or LA.[44] Determination of whether positive aPL are clinically significant should follow the laboratory criteria in the 2006 revised APS classification criteria.[8] First, a positive aPL needs to be at moderate/high titer, which can be defined as greater than the 99th percentile cut-off derived from samples obtained from healthy controls. Second, a positive aPL should be persistently present for at least 12 weeks. Lupus anticoagulant testing should be based on the International Society of Thrombosis and Hemostasis (ISTH) recommendations.[45] While “criteria aPL” (IgG/IgM of aCL, IgG/IgM of aβ2GPI, and LA) are the most tested and easily assessable in all clinical settings,[46] there are also a number of non-criteria aPL (eg, anti-phosphatidylserine/ prothrombin (anti-PS/PT), anti-domain I aβ2GPI, IgA isotypes of aCL and aβ2GPI, and APhL) which were discovered in the past 20 years and are not part of the revised APS classification criteria.[46] Presently, these are mainly used in research settings and are not readily available for most practitioners. A report from the 15th International Congress on Antiphospholipid Antibodies Task Force summarized the recent clinical evaluations of various non-criteria aPL.[47] While some of these antibodies did show promising clinical utility in identifying APS patients,[47] more data are needed before recommending them for routine testing. Our opinion is that a number of these tests (such as high-titer presence of anti-PS/PT and anti-domain I aβ2GPI) are potential drivers of APS pathogenesis in at least a subset of APS patients and look forward to future multicenter studies that will evaluate their significance.
Thrombosis incidence of aPL positive carriers
The triggers of thrombosis are likely multifactorial. The absolute thrombosis incidence among asymptomatic aPL-positive carriers is therefore difficult to assess as it is affected by various confounders, both known (eg, age, underlying systemic autoimmune disease, cardiovascular risk factors, traditional venous thrombosis risks, medications) and unknown.[48] Available studies are limited by small sample sizes and study designs that do not always control for these various confounders. For example, a prospective study of 178 asymptomatic aPL carriers without any primary prophylaxis did not observe any thrombotic events during 36 months of follow up.[49] Another prospective 4-year observation of 258 asymptomatic patients with confirmed persistent aPL (54.3% on primary prophylaxis) showed a thrombotic incidence rate of 1.86%.[50] Pengo et al[51] conducted a prospective observation of 179 asymptomatic isolated persistent LA carriers (23% on primary prophylaxis) with a total follow up of 552 patient-years. 66% of patients did not have any underlying systemic autoimmune diseases. The observed annual incidence rate of thrombosis was 1.3%. Another prospective observation of 104 triple-positive aPL carriers (63.5% on primary prophylaxis and 47% with an underlying systemic autoimmune disease) with a mean follow up of 4.5 years showed a thrombosis incidence of 5.3%. None of the studies were designed to control for primary prophylaxis use. In summary, thrombosis is multifactorial and the absolute thrombosis incidence among asymptomatic aPL carriers is challenging to assess. Having said that, triple-positive aPL carriers may have a higher annual thrombosis risk. Some experts in the field have suggested that the absolute annual thrombosis incidence in aPL carriers without any other thrombosis risks is less than 1%.[48]
Aspirin
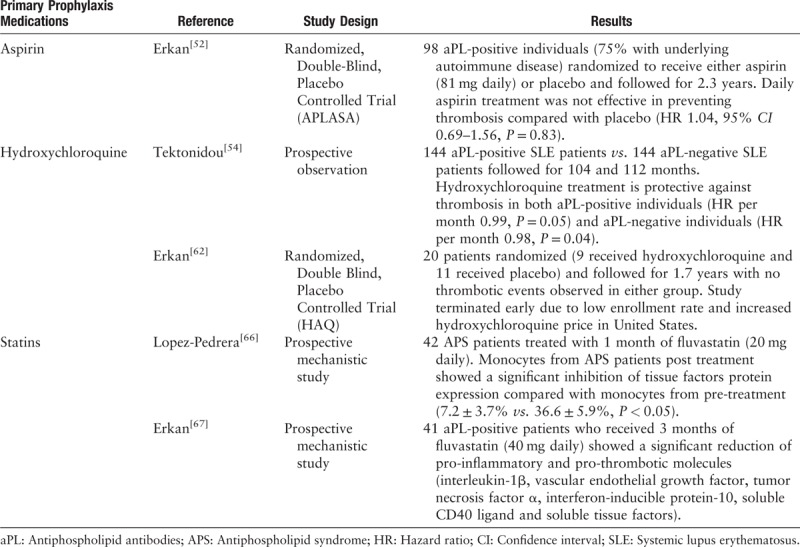
Aspirin's role as a primary thrombosis prophylactic agent among patients with persistently positive aPL remains debatable.[41,42] APLASA is the only randomized controlled trial (RCT) evaluating the effectiveness of aspirin (n = 48) vs. placebo (n = 50) at preventing first thrombotic event among asymptomatic persistently aPL positive carriers. It concluded that daily low dose aspirin (LDA) (81 mg) is no better than placebo at preventing thrombosis (hazard ratio [HR] = 1.04. 95% confidence interval [CI]: 0.69–1.56),[52] albeit with a low event rate as a major limitation of this trial. Thus, many experts argue that it is underpowered to detect any effect of LDA. Data from observational studies do suggest a protective effect of aspirin.[53–55] One such observation of 103 aPL carriers with a mean follow up of 64 months supported the use of LDA as primary thrombosis prophylaxis, particularly in those with either SLE or thrombocytopenia.[55] A recent Cochrane systematic review assessed the effects of antiplatelet or anticoagulant agents vs. placebo at preventing thrombosis among aPL-positive individuals. It included nine studies and 1044 participants, and concluded that there is not sufficient evidence to support the use of aspirin for primary thrombosis prevention among asymptomatic aPL carriers.[56] The 15th International Congress on Antiphospholipid Antibodies recognized that we do not yet have convincing evidence to support the use of aspirin in all patients with persistent aPL; however, a subgroup of patients with concomitant cardiovascular disease risks may benefit from LDA to prevent first thrombosis.[42] Recently published EULAR APS treatment recommendations do endorse the use of LDA for primary prophylaxis among patients with high-risk aPL profiles (persistently positive LA, double- and triple-positive aPL).
It must be remembered that even LDA use is associated with increased risk of bleeding. Data from cardiovascular disease prevention studies (albeit with much older participants than many aPL/APS patients) has suggested that chronic LDA use is associated with increased risk of major gastrointestinal bleeding (OR = 1.58, 95% CI: 1.29–1.95.) and hemorrhagic stroke (OR = 1.27, 95% CI: 0.96–1.68).[57] Another recent population-based 10-year observation of 3166 patients who were on LDA (75 mg daily)[58] suggests that the average annual risk of bleeding among patients on aspirin is 3.36%. This annual bleeding risk increases with age, reaching 4.1% at age 85 or older.[58] The risk of bleeding needs to be weighed against the risk of thrombosis when considering LDA as a primary prophylactic agent.
In summary, convincing evidence to support the use of aspirin for primary thrombosis prophylaxis remains lacking, especially for patients without other systemic autoimmune diseases. Persistent aPL carriers with concomitant cardiovascular disease risks, high-risk aPL profiles, or SLE may benefit from aspirin to lower the risk of first thrombosis. The risk of bleeding from aspirin should always be considered when making a decision about primary thrombosis prophylaxis.
Hydroxychloroquine
Hydroxychloroquine (HCQ) is an important disease-modifying agent for the treatment of systemic autoimmune diseases, particularly SLE. In animal models of APS, treatment with HCQ leads to smaller thrombi and less durable persistence.[59] HCQ may also mediate a reduction of aPL-β2GPI complex binding to phospholipid bilayers and human monocytes.[60] Annexin A5 is an anticoagulant protein that coats phospholipid bilayers and shields them from critical coagulation enzymatic reactions. An in vitro study showed that HCQ treatment can attenuate aPL-mediated disruption of the Annexin A5 shield and thereby conserve its anticoagulant properties.[61] An interesting human study showed that higher type I interferon signature was observed in monocytes from APS patients who were not on HCQ as compared with those who were.[38] A prospective follow up of 144 SLE patients with aPL and 144 sex- and age-matched SLE patients without aPL showed that HCQ use is protective against thrombosis in SLE patients with and without aPL.[54] Unfortunately, an international, prospective, RCT of HCQ for primary thrombosis prevention in persistently aPL-positive carriers (without SLE) was terminated recently due to low recruitment rate and high cost.[62] However, before termination, a total of 20 patients with persistently positive aPL without history of thrombosis were enrolled. Nine patients were randomized to receive HCQ and 11 patients did not receive HCQ. None of the patients in either group developed thrombosis during the 1.7 year follow up.[62] Chronic HCQ usage (>5 years) at higher doses (>6.5 mg/kg/day or >1000-g cumulative dose) is associated with an increased risk (1%) of retinal toxicity.[63] Thus, routine ophthalmological surveillance is warranted among patients who are on long term HCQ.
In summary, mechanistic studies do suggest a potentially protective role of HCQ against thrombosis. HCQ reduces thrombosis risk among aPL-positive SLE patients. No completed studies have yet evaluated its role in primary aPL carriers. HCQ must be considered in aPL carriers with underlying systemic autoimmune diseases.
Statins
Statins, which function as 3-hydroxy-3-methyl glutaryl coenzyme A (HMG-CoA) reductase inhibitors, have been widely used for primary and secondary cardiovascular disease prevention due to their cholesterol lowering, anti-inflammatory, and anti-thrombotic effects.[64] Fluvastatin-treated APS mice have significantly smaller thrombi, decreased inflammatory molecules (intercellular cell adhesion molecule [ICAM]-1), and reduced leukocyte adhesion to endothelial cells compared with controls.[65] Monocytes from 42 thrombotic APS patients treated with 1 month of fluvastatin showed a significant inhibition of tissue factor expression in monocytes.[66] A prospective study of 41 aPL-positive individuals who were treated with 40 mg daily of fluvastatin for 3 months showed significantly reduced circulating proinflammatory and prothrombotic biomarkers post treatment.[67] At this time, there is no randomized clinical trial of statins for primary thrombosis prevention among aPL-positive carriers.
In summary, animal and human mechanistic studies suggest that statin-induced alternation of aPL effects on target cells may be a useful strategy for primary thrombosis prophylaxis and warrants further clinical evaluation. aPL carriers who also have other concomitant cardiovascular disease risk factors may be good candidates for statin medications [Table 3].
Table 3.
Summary of important clinical studies for primary thrombosis prophylaxis among aPL positive carriers.
Secondary thrombosis prophylaxis
Secondary thrombosis prophylaxis refers to the treatment of APS patients after an unprovoked arterial and/or venous thrombotic event. Unprovoked thrombotic events are defined as clotting events that are independent from any major transient thrombotic risks such as the usage of estrogen-containing oral contraception, prolonged immobilization, or cancer.[2,68] The current mainstay of treatment for secondary thrombotic prophylaxis is life-long anticoagulation with a vitamin K antagonist, or occasionally low-molecular-weight heparin (LMWH) for patients who have a contraindication to or do not tolerate vitamin K antagonist. Recently, direct oral anticoagulants (DOACs) have been evaluated as an alternative agent for secondary thrombosis prophylaxis among APS patients.
Vitamin K antagonists
Vitamin K antagonists such as warfarin have historically been the primary treatment for thrombotic APS. Their efficacy in preventing recurrent thrombosis has been supported by multiple studies. One systemic review suggested that anticoagulation with moderate intensity warfarin (INR between 2.0 and 3.0) reduced the risk of recurrent venous thrombosis by 80% to 90%.[69] Substantial debate has surrounded the intensity of vitamin K antagonist therapy in patients with APS. Several early observation studies suggested that optimal anticoagulation regimens were those that maintained the INR between 3.0 and 4.0.[70–72] However, two randomized controlled trials in 2000s suggested otherwise. A randomized, double-blind trial of 114 APS patients in which patients were assigned to receive vitamin K antagonist to achieve an INR of 2.0 to 3.0 (moderate intensity) or 3.1 to 4.0 (high intensity), showed that high-intensity vitamin K antagonist was not superior to moderate-intensity for secondary thromboprophylaxis.[73] A second trial of 109 patients confirmed that high-intensity vitamin K antagonist therapy was not superior to standard treatment in preventing recurrent thrombosis in patients with APS and was associated with an increased rate of hemorrhagic complications.[74] Based on the evidence of the above two trials, the current standard of care for initial management of thrombotic APS is moderate-intensity vitamin K antagonist. Critics of those two trials argue that the proportion of participants persistently achieved the higher INR target was low and very few APS patients with arterial thrombosis were enrolled. Many experts in the field continue to endorse the use of high-intensity vitamin K antagonist among APS patients with recurrent thrombosis based on anecdotal data and personal experience. Though evidence is limited, recent EULAR APS management guideline considers high-intensity vitamin K antagonist as an alternative option to LMWH for APS patients with recurrent thrombosis.[75] A final point is that, in many centers, LDA is combined with a vitamin K antagonist for secondary prevention in patients with arterial thromboembolic events and one recent retrospective observation supports combination therapy.[76]
Direct oral anti-coagulants (DOACs)
Oral direct thrombin or direct factor Xa inhibitors such as rivaroxaban or apixaban have recently received widespread use for thrombosis prevention among patients with atrial fibrillation and those receiving hip or knee replacement, as well as for treatment of deep venous thrombosis.[77] Retrospective observations have reported on DOAC use for secondary thrombosis prophylaxis among APS patients and have demonstrated conflicting results regarding its efficacy.[78] Three randomized controlled trials to date have evaluated the effectiveness of DOAC for secondary thrombosis prophylaxis among APS patients. The first study was an open-label randomized controlled non-inferiority study of 166 (28% triple positive) APS patients comparing rivaroxaban with warfarin. The primary outcome was not clinical, but rather the percentage change in endogenous thrombin potential (ETP) from randomization to day 42, with non-inferiority set at less than 20% difference from the warfarin treatment arm.[79] The result of this study did not meet its primary endpoint, which was the set non-inferiority threshold. Importantly, no thrombosis or major bleeding were observed in either group. Another randomized open-label study evaluated the comparative effectiveness of rivaroxaban DOAC with warfarin among 120 high-risk (defined by triple-positive aPL) APS patients with a mean follow up of 569 days.[80] The primary outcomes were cumulative thrombotic events, major bleeding, and vascular mortality, which were noted to be significantly higher in the rivaroxaban group compared with warfarin (HR 7.4, 95% CI 1.7–32.9, P = 0.008) during interim analysis. Seven arterial thromboses and one venous thrombosis occurred in the rivaroxaban group whereas none occurred in the warfarin group. Considering the excessive risk and no apparent benefits of rivaroxaban among high-risk APS patients, this study was terminated early.[80] The most recent trial, a randomized non-inferiority study published in October 2019, again did not demonstrate non-inferiority of rivaroxaban compared with warfarin as a secondary thrombosis prophylaxis agent.[81] A slightly increased risk of arterial thrombosis was also observed (RR 19, 95% CI 1.1–321.9).[81] There is one more ongoing randomized controlled trial (ASTRO-APS) evaluating DOAC use for APS secondary thrombosis prophylaxis.[78] In summary, we do not currently have data to support the use of DOACs for thrombotic APS. Furthermore, there is evidence against the use of DOACs for secondary thrombosis prophylaxis among high-risk APS patients and especially any patient with a history of arterial manifestations (which is perhaps not surprising as DOACs are not approved for arterial indications in the general population). The reasons DOACs have failed to prevent recurrent thrombosis in APS remains unclear. Suboptimal dosing and uncontrolled anticoagulation intensity (none of the trials standardized anticoagulation intensity with anti-Xa factor activity) could be contributing factors. It is possible that these agents may eventually find a role in a subgroup of APS patients, but further study is certainly needed before that is the case.
In summary, moderate-intensity warfarin remains the primary strategy for secondary thrombosis prophylaxis among APS patients. LDA can also be added for patients with arterial thrombosis. For the subgroup of patients who develop thrombosis while on warfarin, alternative therapy with either LMWH or high-intensity warfarin can be considered. No randomized control data at this time support the use of DOACs among thrombotic APS patients, and DOACs should likely be avoided among high-risk APS patients unless the clinician is dealing with special circumstances.
Obstetric APS management
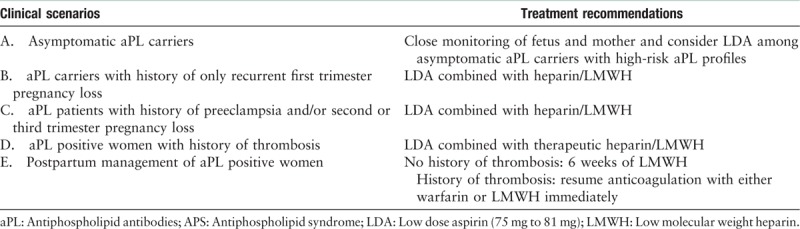
Pregnancy management strategies for patients with aPL or APS are largely based on small trials, observational studies, and expert opinions. Here we will summarize current recommended treatment strategies and available evidence for five clinical APS related obstetric scenarios [Table 4].
Table 4.
Obstetric management of APS.
Asymptomatic aPL carriers
There are conflicting data regarding how to best manage those patients with persistently high-titer aPL who have never had pregnancy complications nor thromboses. Two randomized control trials and one retrospective observation of pregnant women with positive aPL, but without SLE did not show a difference in live birth rate with the use of LDA (defined as between 75 mg daily to 81 mg daily).[82–84] One large randomized control trial of general high-risk pregnancy population (n = 1176), including advanced maternal age, smoking, hypertension, diabetes, low-level pregnancy related plasma protein A, and positive aPL, demonstrated that LDA resulted in significantly lower incidence of preterm preeclampsia.[85] Current expert consensus recommends close monitoring of fetus and mother and considering LDA among asymptomatic aPL carriers with high-risk aPL profiles such as triple-positive aPL or persistently-positive LA.[2,75]
aPL carriers with history of only recurrent first trimester pregnancy loss (no thrombosis history)
There are again conflicting data regarding how to best manage this group of women. Three RCTs suggested a significantly higher live birth rate with the combination of LDA and either LMWH or heparin.[86–88] Two RCTs did not observe a difference in live birth rate between LDA alone and LDA and heparin combination.[89,90] A meta-analysis of all completed trials slightly favors the use of LDA with heparin/LMWH.[3] Current recommendations endorse the addition of prophylaxis heparin/LMWH to LDA during pregnancy for those women with history of recurrent first-trimester pregnancy loss.[75]
aPL carriers with history of preeclampsia and/or second or third trimester pregnancy loss (no thrombosis history)
One randomized control trial that evaluated 110 aPL-positive women with prior history of preeclampsia, placental abruption or late term pregnancy loss suggested that the combination of LDA and LMWH was associated with significantly lower rate of severe preeclampsia, placenta rupture, and low birth weight.[91] Thus, it is recommended to use LDA and heparin/LMWH for this group of women during pregnancy.[2,75]
aPL-positive women with history of thrombosis
One small observational study of 20 pregnant women with thrombotic APS who received 100 mg daily aspirin and therapeutic LMWH showed a live birth rate of 91.3%.[92] However, a high incidence of obstetric complications (preeclampsia 32.8% and premature delivery 42.9%) continued to be observed.[92] It is well known that thromboembolic events among APS patients are significantly associated with a heightened future thrombosis risk and obstetric complications. The obstetric management consensus for aPL-positive women with prior thromboembolic events is to treat with a combination of LDA and therapeutic LMWH during pregnancy.[48,75]
Postpartum management of aPL-positive women
Even in general population, women during the postpartum period have a high risk of thrombosis.[93] It is recommended that aPL-positive women who have never had thrombosis receive 6 weeks of prophylactic LMWH and women with APS who had history of thrombosis resume therapeutic anticoagulation (either warfarin or LMWH) immediately to prevent postpartum thrombosis.[2,48]
In summary, obstetric APS management is complex and current recommendations are often based on lower-quality data and expert consensus. Patient education and counseling of obstetric risk is important. Treatment should be tailored based on an individual patient's aPL profile and obstetric and thrombotic history.
Potential new treatments/pathways for consideration
APS is a complex multisystem disease. Recent pathophysiology studies have implicated many non-thrombotic pathways that contribute to various APS clinical manifestations. Traditional anticoagulation is often not effective for “non-criteria” APS clinical manifestations, which may often have their origins in the microvasculature. Here we summarize current evidence regarding treatment of APS with medications other than anticoagulants, and some emerging considerations in pharmacological management.
Rituximab
B cells play an important role in APS pathogenesis. In vivo studies have shown that B cell inhibition prevents disease onset and prolongs survival in APS murine models.[94] Several case reports have described the successful use of rituximab in APS patients with severe thrombocytopenia, hemolytic anemia, skin ulcers or necrosis, aPL nephropathy, and catastrophic APS.[95] The RITAPS trial was a pilot open-label Phase II study that aimed to evaluate the safety of rituximab in adult primary APS patients.[96] The findings suggested that rituximab is safe to use in APS patients and may be effective in controlling non-criteria manifestations such as thrombocytopenia, skin ulcers, and APS nephropathy.[96]
Eculizumab
Complement activation plays an important role in APS pathogenesis. For example, murine studies have shown that complement activation is required for aPL-mediated fetal loss.[20] In these models, complement inhibition prevents fetal growth restriction and can also reduce aPL-mediated thrombus formation.[20,94] Eculizumab is a humanized monoclonal antibody currently approved for treatment of atypical hemolytic uremic syndrome and paroxysmal nocturnal hematuria.[97] The antibody binds to C5 and prevents C5 cleavage to C5a and C5b.[97] Multiple case series have suggested its efficacy in treating refractory APS, CAPS, and SLE thrombotic microangiopathy.[94,97] There is an ongoing clinical trial aimed at evaluating the safety and tolerability of eculizumab among APS renal transplant patients and evaluating its effect on thrombosis prevention (ClinicalTrials.gov Identifier: NCT01029587).
Defibrotide and adenosine receptor agonists
Defibrotide is a mixture of oligonucleotides derived from the controlled depolymerization of porcine intestinal mucosal DNA with antithrombotic, anti-ischemic, and anti-inflammatory activities. It binds to the vascular endothelium, modulates platelet activity, promotes fibrinolysis, decreases thrombin generation and activity, and reduces circulating levels of plasminogen activator inhibitor type 1 (PAI-1).[98–102] It may also act as an adenosine receptor agonist and is thought to have particular affinity for receptors A1 and A2.[103]
A number of studies have suggested the potential efficacy of defibrotide in vascular disorders, including peripheral vascular disease, microvascular thrombotic states, and chemotherapy-related hemolytic uremic syndrome.[104–106] Defibrotide was initially approved for the treatment of thrombophlebitis and as prophylaxis for deep vein thrombosis in Italy.[104,107] It has also been approved in the United States and Europe for treatment of severe hepatic veno-occlusive disease (sVOD) following high-dose chemotherapy and autologous bone marrow transplantation. Given its known functions as an endothelium-protective reagent and adenosine receptor agonist, defibrotide has been successfully used to treat at least one refractory CAPS patient.[108] Tolerability of defibrotide appears to be acceptable with a relative lack of systemic anticoagulant activity, which could suggest a possible therapeutic advantage over other available treatments.[109] More research seems warranted to probe the efficacy and safety of defibrotide in APS, especially in treatment-refractory microvascular disease and CAPS.
Other approaches
Coenzyme Q10 (CoQ10) plays an important role in the electron transport chain of the mitochondrial membrane, while adequate CoQ10 levels protect cells from protein oxidation and lipid peroxidation. Supplementation of CoQ10 has been trialed in patients with coronary artery disease, where it decreases the production of proinflammatory cytokines.[110] One recent RCT evaluated the effect of ubiquinol (a reduced CoQ10 supplement) on prothrombotic and inflammatory mediators among APS patients.[111] The study found that ubiquinol improved endothelial function and decreased monocyte expression of prothrombotic mediators among APS patients.[111] No clinically significant side effects were observed in the ubiquinol-treated patients.[111] The authors suggested that ubiquinol might complement current standard APS therapies.[111] During the 2019 International Congress on Antiphospholipid Antibodies other potential therapeutic targets for APS, such as agents targeting plasma cells and interferons, were discussed (http://icapaconference.com). Whether neutralization of antibody-producing plasma cells or interferon might mitigate criteria or non-criteria manifestations of APS awaits further study.
In summary, agents that target B-cell and complement activation have been used and evaluated clinically for the management of “non-criteria” APS manifestations such as thrombocytopenia, nephropathy, and thrombotic microangioapthy. More data are needed before any of these agents can be formally recommended. Recent advancements in our understanding of APS pathogenesis, particularly the role of NETosis in APS, may provide new pathways for targeted therapies that could change, and increasingly personalize, the management of APS.
In conclusion, APS is a complex thromboinflammatory syndrome with various clinical manifestations. Primary thrombosis prophylaxis should take an individualized risk stratification approach to make a personalized decision regarding the addition of LDA, HCQ, and/or statin. Moderate-intensity warfarin remains the primary strategy for secondary thrombosis prophylaxis among APS patients. DOACs should be avoided among triple-positive APS patients, especially those with history of arterial manifestations. Obstetric APS management should be tailored based on individual patient's aPL profile, and obstetric and thrombotic history. Pharmacological agents beyond anticoagulants can be considered for the management of “non-criteria” manifestations, though more data are needed.
Funding
Dr. Knight is supported by a pilot grant for preclinical studies from Jazz Pharmaceuticals.
Conflicts of interest
None.
Footnotes
How to cite this article: Zuo Y, Shi H, Li C, Knight JS. Antiphospholipid syndrome: a clinical perspective. Chin Med J 2020;133:929–940. doi: 10.1097/CM9.0000000000000705
References
- 1.Giannakopoulos B, Krilis SA. The pathogenesis of the antiphospholipid syndrome. N Engl J Med 2013; 368:1033–1044. doi: 10.1056/NEJMra1112830. [DOI] [PubMed] [Google Scholar]
- 2.Schreiber K, Sciascia S, de Groot PG, Devreese K, Jacobsen S, Ruiz-Irastorza G, et al. Antiphospholipid syndrome. Nat Rev Dis Primers 2018; 4:17103.doi: 10.1038/nrdp.2017.103. [DOI] [PubMed] [Google Scholar]
- 3.de Jesus GR, Agmon-Levin N, Andrade CA, Andreoli L, Chighizola CB, Porter TF, et al. 14th International Congress on Antiphospholipid Antibodies Task Force report on obstetric antiphospholipid syndrome. Autoimmun Rev 2014; 13:795–813. doi: 10.1016/j.autrev.2014.02.003. [DOI] [PubMed] [Google Scholar]
- 4.Abreu MM, Danowski A, Wahl DG, Amigo MC, Tektonidou M, Pacheco MS, et al. The relevance of “non-criteria” clinical manifestations of antiphospholipid syndrome: 14th International Congress on Antiphospholipid Antibodies Technical Task Force Report on Antiphospholipid Syndrome Clinical Features. Autoimmun Rev 2015; 14:401–414. doi: 10.1016/j.autrev.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Cervera R, Bucciarelli S, Plasin MA, Gomez-Puerta JA, Plaza J, Pons-Estel G, et al. Catastrophic antiphospholipid syndrome (CAPS): descriptive analysis of a series of 280 patients from the “CAPS Registry”. J Autoimmun 2009; 32:240–245. doi: 10.1016/j.jaut.2009.02.008. [DOI] [PubMed] [Google Scholar]
- 6.Duarte-Garcia A, Pham MM, Crowson CS, Amin S, Moder KG, Pruthi RK, et al. The epidemiology of antiphospholipid syndrome. A population-based study. Arthritis Rheumatol 2019; 71:1545–1552. doi: 10.1002/art.40901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elsevier, Durcan L, Petri M. Ricard Cervera GE, Munther K. Chapter 2 - Epidemiology of the Antiphospholipid Syndrome. Handbook of Systemic Autoimmune Diseases: 2017. 17–30. [Google Scholar]
- 8.Miyakis S, Lockshin MD, Atsumi T, Branch DW, Brey RL, Cervera R, et al. International consensus statement on an update of the classification criteria for definite antiphospholipid syndrome (APS). J Thromb Haemost 2006; 4:295–306. doi: 10.1111/j.1538-7836.2006.01753.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen KL, Fonseca FV, Betapudi V, Willard B, Zhang J, McCrae KR. A novel pathway for human endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2012; 119:884–893. doi: 10.1182/blood-2011-03-344671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Galli M, Comfurius P, Maassen C, Hemker HC, de Baets MH, van Breda-Vriesman PJ, et al. Anticardiolipin antibodies (ACA) directed not to cardiolipin but to a plasma protein cofactor. Lancet 1990; 335:1544–1547. doi: 10.1016/0140-6736(90)91374-j. [DOI] [PubMed] [Google Scholar]
- 11.Amengual O, Atsumi T, Koike T. Antiprothombin antibodies and the diagnosis of antiphospholipid syndrome. Clin Immunol 2004; 112:144–149. doi: 10.1016/j.clim.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 12.Permpikul P, Rao LV, Rapaport SI. Functional and binding studies of the roles of prothrombin and beta 2-glycoprotein I in the expression of lupus anticoagulant activity. Blood 1994; 83:2878–2892. doi: 10.1182/blood.V83.10.2878.2878. [PubMed] [Google Scholar]
- 13.Chen PP, Giles I. Antibodies to serine proteases in the antiphospholipid syndrome. Cur Rheumatol Rep 2010; 12:45–52. doi: 10.1007/s11926-009-0072-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cesarman-Maus G, Rios-Luna NP, Deora AB, Huang B, Villa R, Cravioto Mdel C, et al. Autoantibodies against the fibrinolytic receptor, annexin 2, in antiphospholipid syndrome. Blood 2006; 107:4375–4382. doi: 10.1182/blood-2005-07-2636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang J, McCrae KR. Annexin A2 mediates endothelial cell activation by antiphospholipid/anti-beta2 glycoprotein I antibodies. Blood 2005; 105:1964–1969. doi: 10.1182/blood-2004-05-1708. [DOI] [PubMed] [Google Scholar]
- 16.Sorice M, Longo A, Capozzi A, Garofalo T, Misasi R, Alessandri C, et al. Anti-beta2-glycoprotein I antibodies induce monocyte release of tumor necrosis factor alpha and tissue factor by signal transduction pathways involving lipid rafts. Arthritis Rheum 2007; 56:2687–2697. doi: 10.1002/art.22802. [DOI] [PubMed] [Google Scholar]
- 17.Shi T, Giannakopoulos B, Yan X, Yu P, Berndt MC, Andrews RK, et al. Anti-beta2-glycoprotein I antibodies in complex with beta2-glycoprotein I can activate platelets in a dysregulated manner via glycoprotein Ib-IX-V. Arthritis Rheum 2006; 54:2558–2567. doi: 10.1002/art.21968. [DOI] [PubMed] [Google Scholar]
- 18.Pierangeli SS, Girardi G, Vega-Ostertag M, Liu X, Espinola RG, Salmon J. Requirement of activation of complement C3 and C5 for antiphospholipid antibody-mediated thrombophilia. Arthritis Rheum 2005; 52:2120–2124. doi: 10.1002/art.21157. [DOI] [PubMed] [Google Scholar]
- 19.Fischetti F, Durigutto P, Pellis V, Debeus A, Macor P, Bulla R, et al. Thrombus formation induced by antibodies to beta2-glycoprotein I is complement dependent and requires a priming factor. Blood 2005; 106:2340–2346. doi: 10.1182/blood-2005-03-1319. [DOI] [PubMed] [Google Scholar]
- 20.Girardi G, Berman J, Redecha P, Spruce L, Thurman JM, Kraus D, et al. Complement C5a receptors and neutrophils mediate fetal injury in the antiphospholipid syndrome. J Clin Invest 2003; 112:1644–1654. doi: 10.1172/JCI18817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Girardi G, Redecha P, Salmon JE. Heparin prevents antiphospholipid antibody-induced fetal loss by inhibiting complement activation. Nat Med 2004; 10:1222–1226. doi: 10.1038/nm1121. [DOI] [PubMed] [Google Scholar]
- 22.Chaturvedi S, Brodsky RA, McCrae KR. Complement in the pathophysiology of the antiphospholipid syndrome. Front Immunol 2019; 10:449.doi: 10.3389/fimmu.2019.00449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arachchillage DRJ, Laffan M. Pathogenesis and management of antiphospholipid syndrome. Br J Haematol 2017; 178:181–195. doi: 10.1111/bjh.14632. [DOI] [PubMed] [Google Scholar]
- 24.van den Hoogen LL, Rossato M, Lopes AP, Pandit A, Bekker CPJ, Fritsch-Stork RDE, et al. microRNA downregulation in plasmacytoid dendritic cells in interferon-positive systemic lupus erythematosus and antiphospholipid syndrome. Rheumatology 2018; 57:1669–1674. doi: 10.1093/rheumatology/key159. [DOI] [PubMed] [Google Scholar]
- 25.Teruel R, Perez-Sanchez C, Corral J, Herranz MT, Perez-Andreu V, Saiz E, et al. Identification of miRNAs as potential modulators of tissue factor expression in patients with systemic lupus erythematosus and antiphospholipid syndrome. J Thromb Haemost 2011; 9:1985–1992. doi: 10.1111/j.1538-7836.2011.04451.x. [DOI] [PubMed] [Google Scholar]
- 26.Perez-Sanchez C, Arias-de la Rosa I, Aguirre MA, Luque-Tevar M, Ruiz-Limon P, Barbarroja N, et al. Circulating microRNAs as biomarkers of disease and typification of the atherothrombotic status in antiphospholipid syndrome. Haematologica 2018; 103:908–918. doi: 10.3324/haematol.2017.184416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Perez-Sanchez C, Aguirre MA, Ruiz-Limon P, Barbarroja N, Jimenez-Gomez Y, de la Rosa IA, et al. ’Atherothrombosis-associated microRNAs in Antiphospholipid syndrome and Systemic Lupus Erythematosus patients’. Sci Rep 2016; 6:31375.doi: 10.1038/srep31375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Jr, et al. Extracellular DNA traps promote thrombosis. Proc Natl Acad Sci U S A 2010; 107:15880–15885. doi: 10.1073/pnas.1005743107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gupta S, Kaplan MJ. The role of neutrophils and NETosis in autoimmune and renal diseases. Nat Rev Nephrol 2016; 12:402–413. doi: 10.1038/nrneph.2016.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yalavarthi S, Gould TJ, Rao AN, Mazza LF, Morris AE, Nunez-Alvarez C, et al. Release of neutrophil extracellular traps by neutrophils stimulated with antiphospholipid antibodies: a newly identified mechanism of thrombosis in the antiphospholipid syndrome. Arthritis Rheumatol 2015; 67:2990–3003. doi: 10.1002/art.39247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Meng H, Yalavarthi S, Kanthi Y, Mazza LF, Elfline MA, Luke CE, et al. In vivo role of neutrophil extracellular traps in antiphospholipid antibody-mediated venous thrombosis. Arthritis Rheumatol 2017; 69:655–667. doi: 10.1002/art.39938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sule G, Kelley WJ, Gockman K, Yalavarthi S, Vreede AP, Banka AL, et al. Increased adhesive potential of antiphospholipid syndrome neutrophils mediated by beta-2 integrin Mac-1. Arthritis Rheumatol 2019; 72:114–124. doi: 10.1002/art.41057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Grenn RC, Yalavarthi S, Gandhi AA, Kazzaz NM, Nunez-Alvarez C, Hernandez-Ramirez D, et al. Endothelial progenitor dysfunction associates with a type I interferon signature in primary antiphospholipid syndrome. Ann Rheum Dis 2017; 76:450–457. doi: 10.1136/annrheumdis-2016-209442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ugolini-Lopes MR, Torrezan GT, Gandara APR, Olivieri EHR, Nascimento IS, Okazaki E, et al. Enhanced type I interferon gene signature in primary antiphospholipid syndrome: association with earlier disease onset and preeclampsia. Autoimmun Rev 2019; 18:393–398. doi: 10.1016/j.autrev.2018.11.004. [DOI] [PubMed] [Google Scholar]
- 35.Palli E, Kravvariti E, Tektonidou MG. Type I interferon signature in primary antiphospholipid syndrome: clinical and laboratory associations. Front Immunol 2019; 10:487.doi: 10.3389/fimmu.2019.00487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van den Hoogen LL, Fritsch-Stork RD, Versnel MA, Derksen RH, van Roon JA, Radstake TR. Monocyte type I interferon signature in antiphospholipid syndrome is related to proinflammatory monocyte subsets, hydroxychloroquine and statin use. Ann Rheumatic Dis 2016; 75:e81.doi: 10.1136/annrheumdis-2016-210485. [DOI] [PubMed] [Google Scholar]
- 37.Knight JS, Meng H, Coit P, Yalavarthi S, Sule G, Gandhi AA, et al. Activated signature of antiphospholipid syndrome neutrophils reveals potential therapeutic target. JCI Insight 2017; 2:e93897.doi: 10.1172/jci.insight.93897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ali RA, Gandhi AA, Meng H, Yalavarthi S, Vreede AP, Estes SK, et al. Adenosine receptor agonism protects against NETosis and thrombosis in antiphospholipid syndrome. Nat Commun 2019; 10:1916.doi: 10.1038/s41467-019-09801-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Samudra AN, Dwyer KM, Selan C, Freddi S, Murray-Segal L, Nikpour M, et al. CD39 and CD73 activity are protective in a mouse model of antiphospholipid antibody-induced miscarriages. J Autoimmun 2018; 88:131–138. doi: 10.1016/j.jaut.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 40.Barbhaiya M, Erkan D. Primary thrombosis prophylaxis in antiphospholipid antibody-positive patients: where do we stand? Curr Rheumatol Rep 2011; 13:59–69. doi: 10.1007/s11926-010-0149-3. [DOI] [PubMed] [Google Scholar]
- 41.Arnaud L, Conti F, Massaro L, Denas G, Chasset F, Pengo V. Primary thromboprophylaxis with low-dose aspirin and antiphospholipid antibodies: Pro's and Con's. Autoimmun Rev 2017; 16:1103–1108. doi: 10.1016/j.autrev.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 42.Springer International Publishing, Crowther M, Legault KJ, Garcia DA, Tektonidou MG, Ugarte A, Bruce IN. DE, ML, et al. Prevention and Treatment of Thrombotic Antiphospholipid Syndrome. Antiphospholipid Syndrome Current Research Highlights, Clinical Insights 2016. 223–233. [Google Scholar]
- 43.Abdel-Wahab N, Lopez-Olivo MA, Pinto-Patarroyo GP, Suarez-Almazor ME. Systematic review of case reports of antiphospholipid syndrome following infection. Lupus 2016; 25:1520–1531. doi: 10.1177/0961203316640912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vila P, Hernandez MC, Lopez-Fernandez MF, Batlle J. Prevalence, follow-up and clinical significance of the anticardiolipin antibodies in normal subjects. Thromb Haemost 1994; 72:209–213. doi: 10.1055/s-0038-1648840. [PubMed] [Google Scholar]
- 45.Pengo V, Tripodi A, Reber G, Rand JH, Ortel TL, Galli M, et al. Update of the guidelines for lupus anticoagulant detection. Subcommittee on Lupus Anticoagulant/Antiphospholipid Antibody of the Scientific and Standardisation Committee of the International Society on Thrombosis and Haemostasis. J Thromb Haemost 2009; 7:1737–1740. doi: 10.1111/j.1538-7836.2009.03555.x. [DOI] [PubMed] [Google Scholar]
- 46.Bertolaccini ML, Amengual O, Andreoli L, Atsumi T, Chighizola CB, Forastiero R, et al. 14th International Congress on Antiphospholipid Antibodies Task Force. Report on antiphospholipid syndrome laboratory diagnostics and trends. Autoimmun Rev 2014; 13:917–930. doi: 10.1016/j.autrev.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 47.Bertolaccini ML, Amengual O, Artim-Eser B, Atsumi T, de Groot PG, de Laat B. Erkan D, Lockshin MD, et al. Clinical and Prognostic Significance of Non-criteria Antiphospholipid Antibody Tests. Antiphospholipid Syndrome: Current Research Highlights and Clinical Insights.. Cham: Springer International Publishing; 2017. 171–187. [Google Scholar]
- 48.Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018; 378:2010–2021. doi: 10.1056/NEJMra1705454. [DOI] [PubMed] [Google Scholar]
- 49.Giron-Gonzalez JA, Garcia del Rio E, Rodriguez C, Rodriguez-Martorell J, Serrano A. Antiphospholipid syndrome and asymptomatic carriers of antiphospholipid antibody: prospective analysis of 404 individuals. J Rheumatol 2004; 31:1560–1567. [PubMed] [Google Scholar]
- 50.Ruffatti A, Del Ross T, Ciprian M, Bertero MT, Sciascia S, Scarpato S, et al. Risk factors for a first thrombotic event in antiphospholipid antibody carriers: a prospective multicentre follow-up study. Ann Rheum Dis 2011; 70:1083–1086. doi: 10.1136/ard.2010.142042. [DOI] [PubMed] [Google Scholar]
- 51.Pengo V, Ruffatti A, Legnani C, Testa S, Fierro T, Marongiu F, et al. Incidence of a first thromboembolic event in asymptomatic carriers of high-risk antiphospholipid antibody profile: a multicenter prospective study. Blood 2011; 118:4714–4718. doi: 10.1182/blood-2011-03-340232. [DOI] [PubMed] [Google Scholar]
- 52.Erkan D, Harrison MJ, Levy R, Peterson M, Petri M, Sammaritano L, et al. Aspirin for primary thrombosis prevention in the antiphospholipid syndrome: a randomized, double-blind, placebo-controlled trial in asymptomatic antiphospholipid antibody-positive individuals. Arthritis Rheum 2007; 56:2382–2391. doi: 10.1002/art.22663. [DOI] [PubMed] [Google Scholar]
- 53.Erkan D, Yazici Y, Peterson MG, Sammaritano L, Lockshin MD. A cross-sectional study of clinical thrombotic risk factors and preventive treatments in antiphospholipid syndrome. Rheumatology 2002; 41:924–929. doi: 10.1093/rheumatology/41.8.924. [DOI] [PubMed] [Google Scholar]
- 54.Tektonidou MG, Laskari K, Panagiotakos DB, Moutsopoulos HM. Risk factors for thrombosis and primary thrombosis prevention in patients with systemic lupus erythematosus with or without antiphospholipid antibodies. Arthritis Rheum 2009; 61:29–36. doi: 10.1002/art.24232. [DOI] [PubMed] [Google Scholar]
- 55.Hereng T, Lambert M, Hachulla E, Samor M, Dubucquoi S, Caron C, et al. Influence of aspirin on the clinical outcomes of 103 anti-phospholipid antibodies-positive patients. Lupus 2008; 17:11–15. doi: 10.1177/0961203307084724. [DOI] [PubMed] [Google Scholar]
- 56.Bala MM, Paszek E, Lesniak W, Wloch-Kopec D, Jasinska K, Undas A. Antiplatelet and anticoagulant agents for primary prevention of thrombosis in individuals with antiphospholipid antibodies. Cochrane Database Syst Rev 2018; 7:CD012534.doi: 10.1002/14651858.CD012534.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitlock EP, Burda BU, Williams SB, Guirguis-Blake JM, Evans CV. Bleeding risks with aspirin use for primary prevention in adults: a systematic review for the U.S. preventive services task force. Ann Intern Med 2016; 164:826–835. doi: 10.7326/M15-2112. [DOI] [PubMed] [Google Scholar]
- 58.Li L, Geraghty OC, Mehta Z, Rothwell PM, Oxford Vascular S. Age-specific risks, severity, time course, and outcome of bleeding on long-term antiplatelet treatment after vascular events: a population-based cohort study. Lancet 2017; 390:490–499. doi: 10.1016/S0140-6736(17)30770-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Edwards MH, Pierangeli S, Liu X, Barker JH, Anderson G, Harris EN. Hydroxychloroquine reverses thrombogenic properties of antiphospholipid antibodies in mice. Circulation 1997; 96:4380–4384. doi: 10.1161/01.CIR.96.12.4380. [DOI] [PubMed] [Google Scholar]
- 60.Rand JH, Wu XX, Quinn AS, Chen PP, Hathcock JJ, Taatjes DJ. Hydroxychloroquine directly reduces the binding of antiphospholipid antibody-beta2-glycoprotein I complexes to phospholipid bilayers. Blood 2008; 112:1687–1695. doi: 10.1182/blood-2008-03-144204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rand JH, Wu XX, Quinn AS, Ashton AW, Chen PP, Hathcock JJ, et al. Hydroxychloroquine protects the annexin A5 anticoagulant shield from disruption by antiphospholipid antibodies: evidence for a novel effect for an old antimalarial drug. Blood 2010; 115:2292–2299. doi: 10.1182/blood-2009-04-213520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Erkan D, Unlu O, Sciascia S, Belmont HM, Branch DW, Cuadrado MJ, et al. Hydroxychloroquine in the primary thrombosis prophylaxis of antiphospholipid antibody positive patients without systemic autoimmune disease. Lupus 2018; 27:399–406. doi: 10.1177/0961203317724219. [DOI] [PubMed] [Google Scholar]
- 63.Ding HJ, Denniston AK, Rao VK, Gordon C. Hydroxychloroquine-related retinal toxicity. Rheumatology 2016; 55:957–967. doi: 10.1093/rheumatology/kev357. [DOI] [PubMed] [Google Scholar]
- 64.Danesh FR, Anel RL, Zeng L, Lomasney J, Sahai A, Kanwar YS. Immunomodulatory effects of HMG-CoA reductase inhibitors. Arch Immunol Ther Exp 2003; 51:139–148. [PubMed] [Google Scholar]
- 65.Ferrara DE, Liu X, Espinola RG, Meroni PL, Abukhalaf I, Harris EN, et al. Inhibition of the thrombogenic and inflammatory properties of antiphospholipid antibodies by fluvastatin in an in vivo animal model. Arthritis Rheum 2003; 48:3272–3279. doi: 10.1002/art.11449. [DOI] [PubMed] [Google Scholar]
- 66.Lopez-Pedrera C, Ruiz-Limon P, Aguirre MA, Barbarroja N, Perez-Sanchez C, Buendia P, et al. Global effects of fluvastatin on the prothrombotic status of patients with antiphospholipid syndrome. Ann Rheum Dis 2011; 70:675–682. doi: 10.1136/ard.2010.135525. [DOI] [PubMed] [Google Scholar]
- 67.Erkan D, Willis R, Murthy VL, Basra G, Vega J, Ruiz-Limon P, et al. A prospective open-label pilot study of fluvastatin on proinflammatory and prothrombotic biomarkers in antiphospholipid antibody positive patients. Ann Rheum Dis 2014; 73:1176–1180. doi: 10.1136/annrheumdis-2013-203622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kearon C, Ageno W, Cannegieter SC, Cosmi B, Geersing GJ, Kyrle PA, et al. Categorization of patients as having provoked or unprovoked venous thromboembolism: guidance from the SSC of ISTH. J Thromb Haemost 2016; 14:1480–1483. doi: 10.1111/jth.13336. [DOI] [PubMed] [Google Scholar]
- 69.Lim W, Crowther MA, Eikelboom JW. Management of antiphospholipid antibody syndrome: a systematic review. JAMA 2006; 295:1050–1057. doi: 10.1001/jama.295.9.1050. [DOI] [PubMed] [Google Scholar]
- 70.Khamashta MA, Cuadrado MJ, Mujic F, Taub NA, Hunt BJ, Hughes GR. The management of thrombosis in the antiphospholipid-antibody syndrome. N Engl J Med 1995; 332:993–997. doi: 10.1056/NEJM199504133321504. [DOI] [PubMed] [Google Scholar]
- 71.Rosove MH, Brewer PM. Antiphospholipid thrombosis: clinical course after the first thrombotic event in 70 patients. Ann Intern Med 1992; 117:303–308. doi: 10.7326/0003-4819-117-4-303. [DOI] [PubMed] [Google Scholar]
- 72.Ruiz-Irastorza G, Khamashta MA, Hunt BJ, Escudero A, Cuadrado MJ, Hughes GR. Bleeding and recurrent thrombosis in definite antiphospholipid syndrome: analysis of a series of 66 patients treated with oral anticoagulation to a target international normalized ratio of 3.5. Arch Intern Med 2002; 162:1164–1169. doi: 10.1001/archinte.162.10.1164. [DOI] [PubMed] [Google Scholar]
- 73.Crowther MA, Ginsberg JS, Julian J, Denburg J, Hirsh J, Douketis J, et al. A comparison of two intensities of warfarin for the prevention of recurrent thrombosis in patients with the antiphospholipid antibody syndrome. N Engl J Med 2003; 349:1133–1138. doi: 10.1056/NEJMoa035241. [DOI] [PubMed] [Google Scholar]
- 74.Finazzi G, Marchioli R, Brancaccio V, Schinco P, Wisloff F, Musial J, et al. A randomized clinical trial of high-intensity warfarin vs. conventional antithrombotic therapy for the prevention of recurrent thrombosis in patients with the antiphospholipid syndrome (WAPS). J Thromb Haemost 2005; 3:848–853. doi: 10.1111/j.1538-7836.2005.01340.x. [DOI] [PubMed] [Google Scholar]
- 75.Tektonidou MG, Andreoli L, Limper M, Amoura Z, Cervera R, Costedoat-Chalumeau N, et al. EULAR recommendations for the management of antiphospholipid syndrome in adults. Ann Rheum Dis 2019; 78:1296–1304. doi: 10.1136/annrheumdis-2019-215213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Jackson WG, Oromendia C, Unlu O, Erkan D, DeSancho MT. Antiphospholipid Syndrome Alliance for Clinical T, et al. Recurrent thrombosis in patients with antiphospholipid antibodies and arterial thrombosis on antithrombotic therapy. Blood Adv 2017; 1:2320–2324. doi: 10.1182/bloodadvances.2017008185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cohen H, Efthymiou M, Gates C, Isenberg D. Direct oral anticoagulants for thromboprophylaxis in patients with antiphospholipid syndrome. Semin Thromb Hemost 2018; 44:427–438. doi: 10.1055/s-0036-1597902. [DOI] [PubMed] [Google Scholar]
- 78.Cohen H, Efthymiou M, Isenberg D. Use of direct oral anticoagulants in antiphospholipid syndrome. J Thromb Haemost 2018; 16:1028–1039. doi: 10.1111/jth.14017. [DOI] [PubMed] [Google Scholar]
- 79.Cohen H, Hunt BJ, Efthymiou M, Arachchillage DR, Mackie IJ, Clawson S, et al. Rivaroxaban versus warfarin to treat patients with thrombotic antiphospholipid syndrome, with or without systemic lupus erythematosus (RAPS): a randomised, controlled, open-label, phase 2/3, non-inferiority trial. Lancet Haematol 2016; 3:e426–436. doi: 10.1016/S2352-3026(16)30079-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Pengo V, Denas G, Zoppellaro G, Jose SP, Hoxha A, Ruffatti A, et al. Rivaroxaban vs warfarin in high-risk patients with antiphospholipid syndrome. Blood 2018; 132:1365–1371. doi: 10.1182/blood-2018-04-848333. [DOI] [PubMed] [Google Scholar]
- 81.Ordi-Ros J, Saez-Comet L, Perez-Conesa M, Vidal X, Riera-Mestre A, Castro-Salomo A, et al. Rivaroxaban Versus Vitamin K antagonist in antiphospholipid syndrome: a randomized noninferiority trial. Ann Intern Med 2019; published ahead of print. doi: 10.7326/M19-0291. [DOI] [PubMed] [Google Scholar]
- 82.Cowchock S, Reece EA. Do low-risk pregnant women with antiphospholipid antibodies need to be treated? Organizing Group of the Antiphospholipid Antibody Treatment Trial. Am J Obstet Gynecol 1997; 176:1099–1100. doi: 10.1016/s0002-9378(97)70409-5. [DOI] [PubMed] [Google Scholar]
- 83.Kahwa EK, Sargeant LA, McCaw-Binns A, McFarlane-Anderson N, Smikle M, Forrester T, et al. Anticardiolipin antibodies in Jamaican primiparae. J Obstet Gynaecol 2006; 26:122–126. doi: 10.1080/01443610500443352. [DOI] [PubMed] [Google Scholar]
- 84.Del Ross T, Ruffatti A, Visentin MS, Tonello M, Calligaro A, Favaro M, et al. Treatment of 139 pregnancies in antiphospholipid-positive women not fulfilling criteria for antiphospholipid syndrome: a retrospective study. J Rheumatol 2013; 40:425–429. doi: 10.3899/jrheum.120576. [DOI] [PubMed] [Google Scholar]
- 85.Rolnik DL, Wright D, Poon LC, O’Gorman N, Syngelaki A, de Paco Matallana C, et al. Aspirin versus placebo in pregnancies at high risk for preterm preeclampsia. N Engl J Med 2017; 377:613–622. doi: 10.1056/NEJMoa1704559. [DOI] [PubMed] [Google Scholar]
- 86.Kutteh WH. Antiphospholipid antibody-associated recurrent pregnancy loss: treatment with heparin and low-dose aspirin is superior to low-dose aspirin alone. Am J Obstet Gynecol 1996; 174:1584–1589. doi: 10.1016/s0002-9378(96)70610-5. [DOI] [PubMed] [Google Scholar]
- 87.Rai R, Cohen H, Dave M, Regan L. Randomised controlled trial of aspirin and aspirin plus heparin in pregnant women with recurrent miscarriage associated with phospholipid antibodies (or antiphospholipid antibodies). BMJ 1997; 314:253–257. doi: 10.1136/bmj.314.7076.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Goel N, Tuli A, Choudhry R. The role of aspirin versus aspirin and heparin in cases of recurrent abortions with raised anticardiolipin antibodies. Med Sci Monit 2006; 12:CR132–CR136. [PubMed] [Google Scholar]
- 89.Laskin CA, Spitzer KA, Clark CA, Crowther MR, Ginsberg JS, Hawker GA, et al. Low molecular weight heparin and aspirin for recurrent pregnancy loss: results from the randomized, controlled HepASA Trial. J Rheumatol 2009; 36:279–287. doi: 10.3899/jrheum.080763). [DOI] [PubMed] [Google Scholar]
- 90.Farquharson RG, Quenby S, Greaves M. Antiphospholipid syndrome in pregnancy: a randomized, controlled trial of treatment. Obstet Gynecol 2002; 100:408–413. doi: 10.1016/s0029-7844(02)02165-8. [DOI] [PubMed] [Google Scholar]
- 91.Rey E, Garneau P, David M, Gauthier R, Leduc L, Michon N, et al. Dalteparin for the prevention of recurrence of placental-mediated complications of pregnancy in women without thrombophilia: a pilot randomized controlled trial. J Thromb Haemost 2009; 7:58–64. doi: 10.1111/j.1538-7836.2008.03230.x. [DOI] [PubMed] [Google Scholar]
- 92.Fischer-Betz R, Specker C, Brinks R, Schneider M. Pregnancy outcome in patients with antiphospholipid syndrome after cerebral ischaemic events: an observational study. Lupus 2012; 21:1183–1189. doi: 10.1177/0961203312451335. [DOI] [PubMed] [Google Scholar]
- 93.De Stefano V, Martinelli I, Rossi E, Battaglioli T, Za T, Mannuccio Mannucci P, et al. The risk of recurrent venous thromboembolism in pregnancy and puerperium without antithrombotic prophylaxis. Br J Haematol 2006; 135:386–391. doi: 10.1111/j.1365-2141.2006.06317.x. [DOI] [PubMed] [Google Scholar]
- 94.Erkan D, Aguiar CL, Andrade D, Cohen H, Cuadrado MJ, Danowski A, et al. 14th International Congress on Antiphospholipid Antibodies: task force report on antiphospholipid syndrome treatment trends. Autoimmun Rev 2014; 13:685–696. doi: 10.1016/j.autrev.2014.01.053. [DOI] [PubMed] [Google Scholar]
- 95.Kumar D, Roubey RA. Use of rituximab in the antiphospholipid syndrome. Curr Rheumatol Rep 2010; 12:40–44. doi: 10.1007/s11926-009-0074-5. [DOI] [PubMed] [Google Scholar]
- 96.Erkan D, Vega J, Ramon G, Kozora E, Lockshin MD. A pilot open-label phase II trial of rituximab for non-criteria manifestations of antiphospholipid syndrome. Arthritis Rheum 2013; 65:464–471. doi: 10.1002/art.37759. [DOI] [PubMed] [Google Scholar]
- 97.Guillot M, Rafat C, Buob D, Coppo P, Jamme M, Rondeau E, et al. Eculizumab for catastrophic antiphospholipid syndrome-a case report and literature review. Rheumatology 2018; 57:2055–2057. doi: 10.1093/rheumatology/key228. [DOI] [PubMed] [Google Scholar]
- 98.Eissner G, Multhoff G, Gerbitz A, Kirchner S, Bauer S, Haffner S, et al. Fludarabine induces apoptosis, activation, and allogenicity in human endothelial and epithelial cells: protective effect of defibrotide. Blood 2002; 100:334–340. doi: 10.1182/blood.v100.1.334. [DOI] [PubMed] [Google Scholar]
- 99.Bracht F, Schror K. Isolation and identification of aptamers from defibrotide that act as thrombin antagonists in vitro. Biochem Biophys Res Commun 1994; 200:933–937. doi: 10.1006/bbrc.1994.1539. [DOI] [PubMed] [Google Scholar]
- 100.Coccheri S, Biagi G, Legnani C, Bianchini B, Grauso F. Acute effects of defibrotide, an experimental antithrombotic agent, on fibrinolysis and blood prostanoids in man. Eur J Clin Pharmacol 1988; 35:151–156. doi: 10.1007/bf00609244. [DOI] [PubMed] [Google Scholar]
- 101.Berti F, Rossoni G, Biasi G, Buschi A, Mandelli V, Tondo C. Defibrotide, by enhancing prostacyclin generation, prevents endothelin-1 induced contraction in human saphenous veins. Prostaglandins 1990; 40:337–350. doi: 10.1016/0090-6980(90)90099-h. [DOI] [PubMed] [Google Scholar]
- 102.Zhou Q, Chu X, Ruan C. Defibrotide stimulates expression of thrombomodulin in human endothelial cells. Thromb Haemost 1994; 71:507–510. doi: 10.1055/s-0038-1642468. [PubMed] [Google Scholar]
- 103.Bianchi G, Barone D, Lanzarotti E, Tettamanti R, Porta R, Moltrasio D, et al. Defibrotide, a single-stranded polydeoxyribonucleotide acting as an adenosine receptor agonist. Eur J Pharmacol 1993; 238:327–334. doi: 10.1016/0014-2999(93)90864-e. [DOI] [PubMed] [Google Scholar]
- 104.Richardson PG, Corbacioglu S, Ho VT, Kernan NA, Lehmann L, Maguire C, et al. Drug safety evaluation of defibrotide. Expert Opin Drug Saf 2013; 12:123–136. doi: 10.1517/14740338.2012.749855. [DOI] [PubMed] [Google Scholar]
- 105.Bonomini V, Frasca GM, Raimondi C, Liviano D’arcangelo G, Vangelista A. Effect of a new antithrombotic agent (defibrotide) in acute renal failure due to thrombotic microangiopathy. Nephron 1985; 40:195–200. doi: 10.1159/000190340. [DOI] [PubMed] [Google Scholar]
- 106.Ulutin ON. Antithrombotic effect and clinical potential of defibrotide. Semin Thromb Hemost 1993; 19: Suppl 1: 186–191. [PubMed] [Google Scholar]
- 107.Pescador R, Capuzzi L, Mantovani M, Fulgenzi A, Ferrero ME. Defibrotide: properties and clinical use of an old/new drug. Vascul Pharmacol 2013; 59:1–10. doi: 10.1016/j.vph.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 108.Burcoglu-O’Ral A, Erkan D, Asherson R. Treatment of catastrophic antiphospholipid syndrome with defibrotide, a proposed vascular endothelial cell modulator. J Rheumatol 2002; 29:2006–2011. [PubMed] [Google Scholar]
- 109.Richardson PG, Elias AD, Krishnan A, Wheeler C, Nath R, Hoppensteadt D, et al. Treatment of severe veno-occlusive disease with defibrotide: compassionate use results in response without significant toxicity in a high-risk population. Blood 1998; 92:737–744. [PubMed] [Google Scholar]
- 110.Garrido-Maraver J, Cordero MD, Oropesa-Avila M, Vega AF, de la Mata M, Pavon AD, et al. Clinical applications of coenzyme Q10. Front Biosci 2014; 19:619–633. doi: 10.2741/4231. [DOI] [PubMed] [Google Scholar]
- 111.Perez-Sanchez C, Aguirre MA, Ruiz-Limon P, Abalos-Aguilera MC, Jimenez-Gomez Y, Arias-de la Rosa I, et al. Ubiquinol effects on antiphospholipid syndrome prothrombotic profile: a randomized, placebo-controlled trial. Arterioscler Thromb Vasc Biol 2017; 37:1923–1932. doi: 10.1161/ATVBAHA.117.309225. [DOI] [PubMed] [Google Scholar]


