Abstract
Psoriatic arthritis (PsA) is a type of chronic inflammatory arthritis which is associated with psoriasis. The early recognition and treatment for PsA are of critical importance. Janus kinase (JAK) inhibitors, as a kind of orally small molecules, have emerged as an encouraging class of drug in PsA treatment. This review provides a discussion of the role and current status of JAK inhibitors in the control of PsA. There are three JAK inhibitors approved for use in autoimmune diseases, for example, tofacitinib, baricitinib, and upadacitinib, and only tofacitinib has been approved in PsA treatment. The clinical trials of upadacitinib and filgotinib in PsA patients are undergoing. The efficacy and safety of these agents were briefly discussed. Although there are still issues in terms of their efficacy and safety currently, JAK inhibitors are expected to benefit more PsA patients in future.
Keywords: Janus kinase, Janus kinase inhibitors, Psoriatic arthritis, Drug treatment, Tofacitinib
Introduction
Psoriatic arthritis (PsA) is a type of chronic inflammatory arthritis which is associated with psoriasis.[1] As a heterogeneous disease, PsA patients could present a broad spectrum of clinical manifestations including musculoskeletal involvement (peripheral and axial arthritis, enthesitis, and dactylitis) as well as skin and nail lesions.[2] PsA can result in an irreversible joint damage and a reduced quality of life, thus the early recognition and treatment is of critical importance.[3]
Generally, the pharmacological treatment of PsA including non-biological agents and biological drugs.[4,5] The former category refers to non-steroidal anti-inflammatory drugs (NSAIDs), corticosteroids, and conventional synthetic disease-modifying anti-rheumatic drugs (csDMARDs).[6] NSAIDs belong to symptom-relieving agents. Systemic administration of corticosteroids is not recommended in most instances. Although there is poor evidence available for csDMARDs in PsA, the relative lower costs make them a priority in the early treatment.[7,8] In comparison, compelling data show the obvious effectiveness of biological drugs, also called biological disease-modifying anti-rheumatic drugs (bDMARDs), in PsA treatment which are effective for various domains of PsA and are able to change the disease course.[9–12] Although the prominent performance of biologics in disease control, there are still a large portion of PsA patients uncontrolled. According to a systemic review which estimated the prevalence of PsA patients achieving minimal disease activity (MDA) in real-life studies and randomized clinical trials, only one-third of PsA patients evaluated were in MDA.[13] This might partly attribute to the disease heterogeneity.[14] In addition, all the biologics cannot be administrated orally, but some patients prefer to oral administration instead of injection. Janus kinase (JAK) inhibitors as a kind of targeted synthetic DMARDs have emerged as an encouraging class for treatment of inflammatory diseases in the past decade.[15,16] With distinct target and convenient oral administration, they offer another good choice for PsA treatment.[17] This review will provide a discussion of the role and current status of JAK inhibitors in the control of PsA.
Overview of JAKs
JAKs were recognized as a family of non-receptor tyrosine kinases which were composed of four members in mammals: JAK1, JAK2, JAK3 and tyrosine kinase 2 (TYK2). They transmit signals from cell membrane receptors to members of the signal transducer and activator of transcription (STAT) family [Figure 1].[18] The JAK-STAT signaling pathway can be activated by a variety of cytokines, which are major contributors to a lot of immune-mediated diseases.[19,20] Thus, inhibiting JAK-mediated signaling might benefit the treatment of autoimmune diseases.[21–23] In fact, an overwhelming body of evidence has established that inhibition of JAK-STAT pathway is effective in rheumatoid arthritis (RA),[24–27] psoriasis,[28–30] and inflammatory bowel disease (IBD).[31,32] Recently many of the cytokines involved in PsA pathogenesis are also identified to be mediated by JAK-STAT pathway such as those related to the interleukin (IL)-12/23 and IL-17 axes.[33,34]
Figure 1.
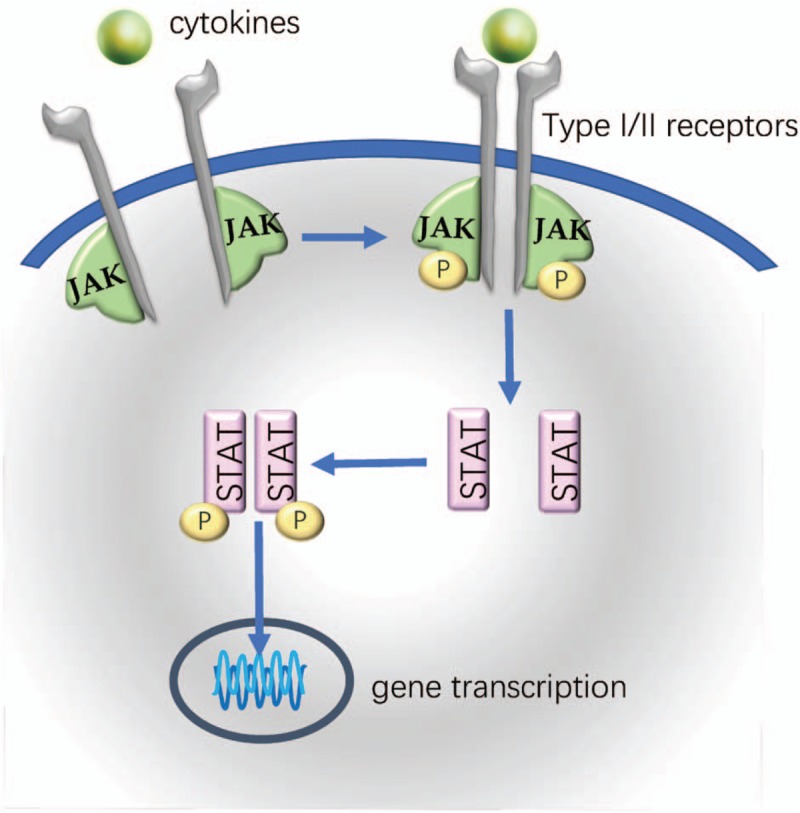
JAK-STAT signaling pathway. The binding of cytokines to the receptors leads to the activation and phosphorylation of JAKs bound to the intracellular domains of these cytokine receptors. STAT molecules are subsequently recruited to form homodimers or heterodimers and phosphorylated by the activated JAKs. The phosphorylated STATs then translocate into the nucleus, where they bind to target DNA and regulate gene transcription. JAK-STAT: Janus kinase-signal transducer and activator of transcription.
Cytokines are not only critical factors for immunoregulation in normal physiological conditions, but also main conductors in the pathogenesis of autoimmune diseases.[35,36] Thus, they are potential treatment targets.[15] Different cytokines bind to their specific receptors, which may share a common chain.[37] Based on either the function or structure of itself or its receptors, a lot of cytokines are divided into two major classes, type I and type II cytokines [Table 1].[38] Type I cytokines include the γ chain cytokines, the β chain cytokines, gp130 related cytokines, dimeric cytokines or hormone-like cytokines. The type II cytokines include the interferon (IFN) family cytokines and IL-10-related cytokines. The γ chain cytokines and the β chain cytokines are two groups of cytokines which individually share a common γ or β chain in their receptors. The gp130 related cytokines are a group of cytokines which signals through the gp130 receptor sub-unit including IL-6, IL-11, and IL-27. The dimeric cytokines are composed of heterodimers with similar structure including IL-12, IL-23, and IL-35, which are also called IL-12 famlily cytokines. The hormone-like cytokines including growth hormone, leptin, and cytokines which play important roles in hematopoietic cells, such as granulocyte colony-stimulating factor, erythropoietin, and thrombopoietin. To transfer their intracellular signals, type I and II cytokine receptors need to cooperate with kinases, such as JAKs.[39,40] The JAKs were paired to form various complexes, mediating distinct cytokine signaling pathways.[16,41] For example, the pair of JAK3 and JAK1 binds to γ-common chain of receptors and controls the signaling for IL-2, IL-4, IL- 7, IL-9, IL-15, and IL-21, which are essential for lymphocyte proliferation and homeostasis.[42] The signaling of IL-6 involved in acute phase response and differentiation of T cells is mediated by JAK1, JAK2, and TYK2. JAK2, the only member that can be paired with itself,[43] regulates the signaling of hormone-like cytokines and then plays an important role in hematopoietic cells.[44] The pair of JAK1 and TYK2 controls the signaling of IL-10 and type I IFN including IFNα and IFNβ, while IFNγ signaling is regulated by the pair of JAK1 and JAK2. The p40-containing cytokines IL-12 and IL-23, which play an important role in the differentiation of Th17 cells, signals through JAK2/TYK2 pair [Table 1].[22,45] However, not all cytokines are dependent upon JAKs for signaling, such as tumor necrosis factor (TNF), IL-1, and IL-17, whose receptors are structurally distinct from type I/II cytokine receptors.[38] Therefore, TNF signaling is not regulated by JAK inhibitors directly.[46]
Table 1.
Summary of the cytokines with signaling through JAKs.
Rationale for JAK Inhibitors for the Treatment of PsA
Although the pathophysiology of PsA is not fully defined, the innate and adaptive cells and proinflammatory cytokines are involved affirmably.[47] In PsA, the infiltration of immune cells into joints, such as activated T cells, dendritic cells, macrophages, and innate lymphoid cells, leads to the production of numerous proinflammatory cytokines.[48] These proinflammatory mediators can further recruit and stimulate the proliferation of immune cells contributing to synovial hypertrophy and bone destruction.[14] While many of these cytokines and immune cell responses involved in this process are regulated by JAK/STAT signaling pathways, thus making JAK to be a therapeutic target of PsA reasonably.[14,34]
The γ-common chain cytokines (IL-2, IL-4, IL-7, IL-9, IL-15, and IL-21) which modulate the adaptive immune functions, including Th cell differentiation and function, were regulated by JAK1/JAK3.[49] The signaling of IFNγ and IL-12 involved in Th1 cell response and TNFα production by macrophages is controlled by JAK1/JAK2 and JAK2/TYK2. IL-6 is also an important pro-inflammatory cytokine in the pathogenesis of PsA, which was demonstrated to be elevated in the patients with PsA.[50] IL-6 can stimulate the activation of Th17 cells and the production of IL-17.[51] The signaling of IL-6 is mediated by JAK1/JAK2. The role of IL-23/IL-17 axis has been recognized and highlighted in the pathogenesis of PsA.[52,53] Signaling of IL-17 is not mediated by JAKs, while signaling of IL-23 is modulated by JAK2/TYK2. The high level of IL-23 were observed in the patients with PsA.[54] IL-23 contributes to the differentiation of Th17 cells which secrete pro-inflammatory cytokines, like IL-17A, IL-22, and TNFα, contributing to joint inflammation, bone erosion and possibly new bone formation.[55] Taken together, JAKs mediate the intracellular signals of many cytokines involved in the pathophysiology of PsA, offering the molecular basis of therapeutic usage of JAK inhibitors.[14,34]
Several studies revealed the mechanisms for the role of JAK-STAT kinase system in the pathogenesis of PsA. One of them has shown that the enhanced activation of JAK1/STAT1/STAT3/STAT5 network may drive the expansion of CD4+IL-17+ T cells and CD4+IL-23R+ T cells, which are considered as pathogenic effector Th17 cells, in synovial fluids of clinically active joints of PsA patients.[56] Gao et al[57] found that tofacitinib, a non-selective inhibitor of JAK1/3, significantly decreased both phosphorylated STAT1 (pSTAT1) and pSTAT3 in the fibroblast-like synoviocytes (FLS) and synovial explant cultures isolated from PsA patients. In cultured PsA-FLS, invasion, network formation, and migration were also significantly inhibited by tofacitinib functionally. Furthermore, tofacitinib showed significant inhibition on the secretion of proinflammatory cytokines (IL-6, IL-8, and monocyte chemoattractant protein-1) and the expression of nuclear factor kappa-B p65. In another study, tofacitinib markedly inhibited the phosphorylation of JAK2 induced by IL-23 in mononuclear cells of peripheral blood isolated from PsA patients. The upregulation of IL-17 in the CD4+ memory T cells induced by IL-23 could also be significantly inhibited by tofacitinib.[58] These data reveal a plausible mechanism of action of JAK inhibitors, to inhibit the IL-23/IL-17 axis by modulating the IL-23-induced JAK signaling. All of these studies support JAK inhibition to be a therapeutic target for the treatment of PsA.
Clinical Aspects of JAK Inhibitors
Up to date, there are three small molecule JAK inhibitors approved for use in autoimmune diseases, for example, tofacitinib, baricitinib, and upadacitinib, which can be orally administrated. Their inhibition of JAK pairs are dose-dependent, and at higher doses they may lead to the non-selective pan JAK inhibition. Tofacitinib inhibits predominantly JAK1/3 and shows a lower activity on JAK2 at higher doses.[46,59] Baricitinib shows major inhibition of JAK1 and JAK2, as well as a much lesser inhibition of TYK2.[60,61] The non-selective pan-JAK blockade may lead to unwanted adverse effects such as higher incidence of infection and cytopenia. Thus, the second generation of JAK inhibitors with more selectivity for a specific JAK were designed, such as upadacitinib and filgotinib [Figure 2].[62,63]
Figure 2.
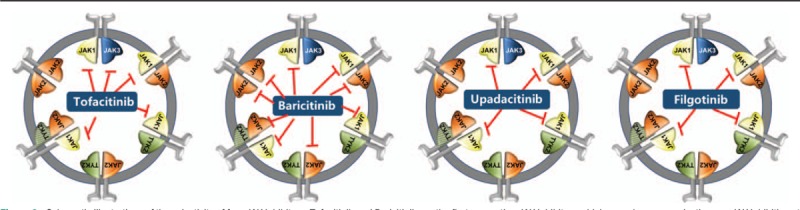
Schematic illustrations of the selectivity of four JAK inhibitors. Tofacitinib and Baricitinib are the first generation JAK inhibitors which may show non-selective pan JAK inhibition at higher dosages. At the recommended therapeutic dosages, tofacitinib inhibits predominantly JAK1 and JAK3, while baricitinib shows major inhibition of JAK1 and JAK2. The second generation of JAK inhibitors has more selectivity for a specific JAK, such as upadacitinib and filgotinib, which selectively inhibit JAK1. JAK: Janus kinase.
Among the various JAK inhibitors, only tofacitinib has been approved in PsA treatment by the USA Food and Drug Administration (FDA) in 2017 and the European Medicines Agency (EMA) in 2018. Baricitinib and upadacitinib have been approved only in RA recently. The efficacy and safety of these agents in PsA patients were discussed in the following.
Tofacitinib
Efficacy
There were two phase III studies (Oral Psoriatic Arthritis Trial [OPAL] Broaden and OPAL Beyond) evaluating the use of tofacitinib in patients with PsA [Table 2].[64,65] Both of them were randomized, double-blind, and multi-center studies to evaluate the efficacy and safety of tofacitinib in PsA patients. OPAL Broaden was a placebo and active-controlled 12-month trial conducted in TNF inhibitor (TNFi)-naïve patients with active PsA who had an inadequate response to at least one csDMARD.[64] OPAL Beyond was a placebo-controlled 6-month study conducted in active PsA patients who had an inadequate response to TNFi therapy.[65] All the patients fulfilled the Classification Criteria for Psoriatic Arthritis, and presented with ≥3 joints affected.
Table 2.
Therapeutic efficacy of tofacitinib in randomized clinical trials in PsA patients.
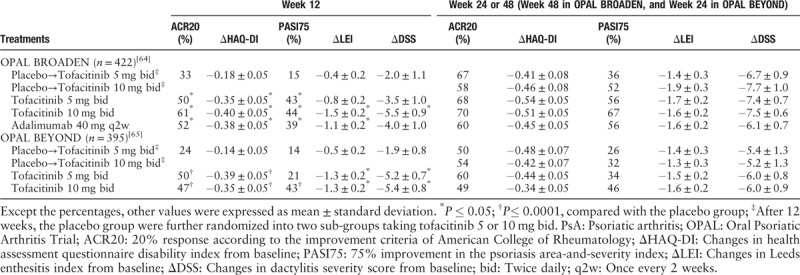
In both studies, significantly greater improvement in the primary efficacy endpoint of American College of Rheumatology 20% (ACR20) improvement criteria responder rates and significantly greater change from baseline in health assessment questionnaire disability index (ΔHAQ-DI) were observed for both dose groups (5 and 10 mg) of tofacitinib in comparison with placebo in month 3. This superiority in ACR20 was shown as early as the first assessment on week 2. Both doses of tofacitinib elicited improvements in the various domains (skin, enthesitis, and dactylitis) of PsA, which were respectively assessed by 75% improvement in psoriasis area-and-severity index (PASI) from baseline (PASI75), changes in Leeds enthesitis index from baseline (ΔLEI) and changes in dactylitis severity score from baseline (ΔDSS). Significant differences in efficacy between two doses of tofacitinib were only observed in ΔLEI and ΔDSS in OPAL Broaden and PASI75 in OPAL Beyond at month 3. In OPAL Broaden, more than 90% of patients receiving tofacitinib met radiographic non-progression criteria at month 12 which was defined as the change of van der Heijde-modified Total Sharp Score from baseline (ΔmTSS) ≤0.5. The efficacy of tofacitinib sustained up to month 30 in the currently ongoing long-term extension (LTE) study OPAL Balance, which included patients from OPAL Broaden and OPAL Beyond who had been treated with tofacitinib.[66] In a post-hoc analysis of pooled data from OPAL Broaden and OPAL Beyond, tofacitinib was superior to placebo at month 3 across four PsA domains: peripheral arthritis, psoriasis, enthesitis, and dactylitis; and the efficacy was maintained to month 6.[67]
Safety
The most commonly reported adverse events (AE) in OPAL Broaden and OPAL Beyond were headache, nasopharyngitis, and upper respiratory tract infection, which were consistent with the safety file of tofacitinib in RA and psoriasis patients. The incidences of overall AE and serious AE were similar among the different treatment groups in two studies. The most infections associated with tofacitinib were viral infections, especially herpes zoster (HZ) infection [Table 3].[64,65] Furthermore, the incidence rate of HZ infection in tofacitinib groups was dose-dependent and varied by region/countries which were observed in RA patients. The highest incidences of HZ infection were reported in Asia population. No cases of tuberculosis were reported in both studies. The elevations in serum lipid and liver enzyme levels were more common in tofacitinib treated groups than placebo. The increases in lipid profile parameters including total cholesterol, triglycerides, low-density lipoprotein (LDL), and high-density lipoprotein cholesterol (HDL) were observed in both the phase III studies and the LTE study in tofacitinib treated groups than placebo.
Table 3.
Summary of adverse events of JAK inhibitors in PsA or RA patients.∗
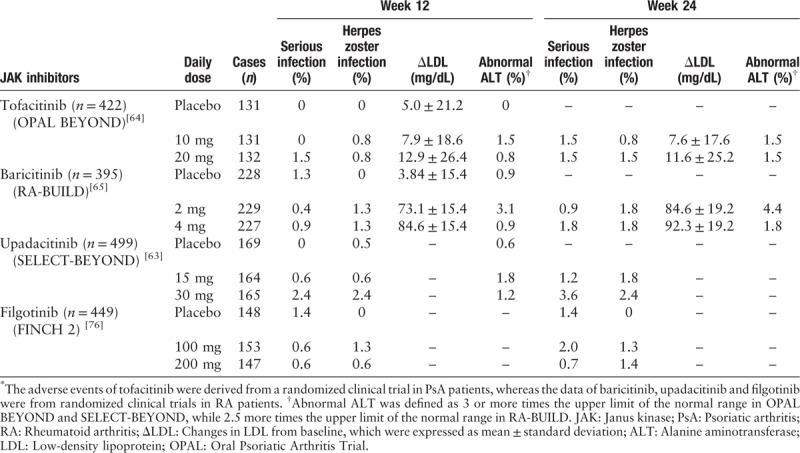
A real world study was conducted to compare the safety data of tofacitinib in clinical trials to other systemic agents for PsA.[68] The cohort of patients treated with tofacitinib were enrolled from OPAL Broaden, OPAL Beyond, or OPAL Balance. The real-world cohort treated with other systemic agents consisted of patients from the US Truven Market Scan Insurance claims database with moderate to severe PsA. Between the two cohorts, incidence rates were similar for each of the sub-categories of serious infection events, including required treatment with parenteral antibiotics, hospitalization, or parenteral antibiotics with hospitalization. However, tofacitinib treated patients had a higher incidence rate of HZ vs. the comparison cohort.
Baricitinib
Baricitinib was approved for RA treatment by EMA in 2017. FDA approved the daily 2 mg dose of baricitinib in 2018 but declined the approval of the daily 4 mg dose in concern of safety issues. However, baricitinib has not been approved for the treatment of PsA up to now. Baricitinib can inhibit the signaling of IFN-γ and IL-6 by acting on JAK1/JAK2, as well as IL-12/23 via JAK2/TYK2.[23]
Efficacy
In four phase III trials (RA Begin, Build, Beam, and Beacon), baricitinib showed unequivocal efficacy in the patients with active RA.[61,69–71] Baricitinib significantly improved the ACR 20/50/70, DAS28, and HAQ-DI in RA patients compared to placebo. In addition, the efficacy and safety of baricitinib in the patients with moderate-to-severe psoriasis were evaluated in a randomized, double-blind, placebo-controlled, dose-ranging phase IIb study.[72] The PASI75 response rates in the patients receiving baricitinib 8 and 10 mg once daily were significantly higher than that in placebo (43% and 54% vs. 17% respectively) at week 12, and this superiority was maintained through 24 weeks. However, the efficacy of baricitinib 2 mg once daily in psoriasis has not been proved yet and there is no reported clinical data of baricitinib in PsA patients.
Safety
The available safety profile data of baricitinib were mainly obtained from RA treatment trials.[61,69–71] Similar to tofacitinib, the incidence rates of infection especially HZ infection were higher in baricitinib compared with placebo [Table 3]. Most of HZ infection were reported in Asia population. Otherwise, the reductions in neutrophil counts and elevations in serum levels of alanine aminotransferase, creatinine, creatine phosphokinase (CPK) and lipids (LDL and HDL) were more common in baricitinib vs. placebo. Modest increases in platelet counts were seen in baricitinib, but there was no significant difference among different groups in incidences of thrombocytosis defined as more than 600,000 per cubic millimeter.
The differences in baricitinib's safety profile from tofacitinib lie in the influence on cardiovascular events. The incidences of deep vein thrombosis (DVT) and pulmonary embolism (PE) were higher in baricitinib compared to placebo. In a study to evaluate the safety profile of baricitinib in patients with active RA in an integrated database which included eight phase III/II/Ib trials and one LTE study, infections including HZ were significantly more frequent for baricitinib 4 mg once daily vs. placebo. DVT and PE were reported in baricitinib 4 mg daily dose group but not in placebo.[73] The similar results were observed in another study to assess the cardiovascular safety of baricitinib in RA patients.[74]
Filgotinib
Filgotinib is an oral, selective inhibitor of JAK1. It is developed for the treatment of many inflammatory diseases, including PsA, RA, ankylosing spondylitis and ulcerative colitis, and has not been approved for marketing yet. Recently filgotinib has been proposed for the New Drug Application in United States and Japan for the RA treatment in virtue of its efficacy in Phase III and II trials.
Efficacy
The trial data of filgotinib for PsA treatment is limited now. There was a randomized, double-blind, placebo-controlled phase II trial (EQUATOR) evaluating the efficacy and safety of filgotinib in the active moderate-to-severe PsA patients with an inadequate response to csDMARD.[75] Filgotinib showed significantly better efficacy in terms of ACR20 response rate at week 16 compared with placebo. This difference was observed as early as week 1, supporting that the onset of filgotinib action was rapid. In addition, the significant improvement in enthesitis, psoriasis, and overall PsA disease control were also demonstrated. The improvement in nail psoriasis at week 16 did not reach statistical significance, probably owing to the limited follow-up duration and relatively small number of patients with nail psoriasis at baseline.
Safety
The safety profile of filgotinib in PsA was similar with that in RA.[75,76] Filgotinib showed well tolerance generally in EQUATOR study.[75] The incidence of treatment-emergent AE was similar in filgotinib compared with placebo, most of which were mild or moderate. The incidence of infections was similar between the groups through to 16 weeks. There was only one single case of HZ reported, and no malignancies, thromboembolic events, or opportunistic infections, including tuberculosis. In terms of laboratory parameters, increases of hemoglobin, HDL and lymphocyte counts, and decrease of platelets were observed in filgotinib. The efficacy and safety of filgotinib in PsA patients need to be confirmed in the future Phase III trials, in which PsA patients will be treated with filgotinib for up to an additional 148 weeks.
Upadacitinib
Upadacitinib is another oral JAK1-selective inhibitor. It has been approved by FDA to treat RA in August 2019 and is under development in use of other auto-immune diseases including PsA, AS, systemic lupus erythematosus, atopic dermatitis, and IBD. There was no data published on the efficacy and safety of upadacitinib in PsA patients. The phase III study of upadacitinib in PsA patients with an inadequate response to csDMARDs is ongoing and will be completed in 2022.
In the four-phase III clinical trials in RA patients (SELECT-COMPARE, SELECT-BEYOND, SELECT-MONOTHERAPY, and SELECT-NEXT),[63,77–80] both upadacitinib 15 and 30 mg daily demonstrated significant improvements in clinical signs and symptoms compared with placebo and adalimumab. Furthermore, upadacitinib inhibited radiographic progression in SELECT-COMPARE study vs. placebo or adalimumab.[77] In SELECT-BEYOND study, upadacitinib led to rapid and significant improvements compared with placebo over 12 weeks in refractory RA patients which had inadequate response to bDMARDs.[63] In terms of safety, as with other JAK inhibitors, dose-independent higher incidences of HZ infection and elevations in lipid profile (LDL-C and HDL-C) and CPK were observed in the upadacitinib treated groups in the above mentioned trials. However, the relationship between the risk for venous thromboembolism (VTE) and upadacitinib treatment was still unclear, considering the not exactly same incidence of VTE in trials between groups and higher background risk for VTE in RA irrespective of the treatment.
Concluding Remarks and Future Perspectives
In the past decade, the development and application of JAK inhibitors are a breakthrough in the treatment of autoimmune diseases. Their wide range of effects on multiple cytokines makes it possible for them in the control of a broad spectrum of diseases, including PsA. Clinical trials of various JAK inhibitors exploded in recent years and then demonstrated their outstanding performance in improvement of diseases. As their oral administration and distinct action mechanism from bDMARDs, JAK inhibitors offer a new therapeutic strategy for rheumatologists. In the treatment of PsA, various JAK inhibitors may improve all aspects of the disease, especially in the control of polyarthritis. In terms of safety, these agents are well-tolerated overall. The AE of special interest are cytopenia and infections, especially the HZ infection which are distinct from those of bDMARDs.
And yet for all that, there are still some issues worth attention and discussion in future. First, among all the JAK inhibitors, only tofacitinib has been approved for PsA. The experience with this kind drugs in practice is limited, which obstruct their fully recommendation in the existing guidelines. In addition, the safety profile needs to be verified further. Their effects on the malignancies and cardiovascular events are still not clarified. Furthermore, some unexpected AE considering their action mechanism have been observed in the available trials like the increase of lipid profile and platelet counts. This might be attributed to the unexpected complexity of JAK-STAT pathway. Thus, more large-scale as well as long-term clinical trials and real-world studies are needed to reveal the safety profile of these agents especially the second generation JAK inhibitors. Second, the second generation of JAK inhibitors was initially developed to increase selectivity while ensuring efficacy. However, considering the demonstrated benefits and risks, the necessity and feasibility to pursue this selectivity is worth further discussion. Their expected superiority in safety to the non-selective JAK inhibitors needs to be confirmed in head-to-head studies with comparison of them to the first generation of JAK inhibitors. Last, the other issues like high cost and the safety of combined therapy with other DMARDs in refractory patients also need to be conquered.
Overall, with the development of a new kind of drugs and an increased understanding of drug action, we believe that further evidence upon their application will be revealed. As a kind of expecting and promising agents, JAK inhibitors might benefit more PsA patients in future.
Funding
This project was supported by grants from the National Natural Science Foundation of China (No. 81771746 and No. 81900795).
Conflicts of interest
None.
Footnotes
How to cite this article: Chen M, Dai SM. A novel treatment for psoriatic arthritis: Janus kinase inhibitors. Chin Med J 2020;133:959–967. doi: 10.1097/CM9.0000000000000711
References
- 1.Olivieri I, D’Angelo S, Palazzi C, Padula A. Advances in the management of psoriatic arthritis. Nat Rev Rheumatol 2014; 10:531–542. doi: 10.1038/nrrheum.2014.106. [DOI] [PubMed] [Google Scholar]
- 2.Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med 2017; 376:957–970. doi: 10.1056/NEJMra1505557. [DOI] [PubMed] [Google Scholar]
- 3.Gladman DD, Stafford-Brady F, Chang CH, Lewandowski K, Russell ML. Longitudinal study of clinical and radiological progression in psoriatic arthritis. J Rheumatol 1990; 17:809–812. [PubMed] [Google Scholar]
- 4.Singh JA, Guyatt G, Ogdie A, Gladman DD, Deal C, Deodhar A, et al. Special Article: 2018 American College of Rheumatology/National Psoriasis Foundation guideline for the treatment of psoriatic arthritis. Arthritis Rheumatol 2019; 71:5–32. doi: 10.1002/art.40726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Coates LC, Kavanaugh A, Mease PJ, Soriano ER, Acosta-Felquer ML, Armstrong AW, et al. Group for research and assessment of psoriasis and psoriatic arthritis 2015 treatment recommendations for psoriatic arthritis. Arthritis Rheumatol 2016; 68:1060–1071. doi: 10.1002/art.39573. [DOI] [PubMed] [Google Scholar]
- 6.Acosta Felquer ML, Coates LC, Soriano ER, Ranza R, Espinoza LR, Helliwell PS, et al. Drug therapies for peripheral joint disease in psoriatic arthritis: a systematic review. J Rheumatol 2014; 41:2277–2285. doi: 10.3899/jrheum.140876. [DOI] [PubMed] [Google Scholar]
- 7.Kingsley GH, Kowalczyk A, Taylor H, Ibrahim F, Packham JC, McHugh NJ, et al. A randomized placebo-controlled trial of methotrexate in psoriatic arthritis. Rheumatology (Oxford) 2012; 51:1368–1377. doi: 10.1093/rheumatology/kes001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaltwasser JP, Nash P, Gladman D, Rosen CF, Behrens F, Jones P, et al. Efficacy and safety of leflunomide in the treatment of psoriatic arthritis and psoriasis: a multinational, double-blind, randomized, placebo-controlled clinical trial. Arthritis Rheum 2004; 50:1939–1950. doi: 10.1002/art.20253. [DOI] [PubMed] [Google Scholar]
- 9.Antoni C, Krueger GG, de Vlam K, Birbara C, Beutler A, Guzzo C, et al. Infliximab improves signs and symptoms of psoriatic arthritis: results of the IMPACT 2 trial. Ann Rheum Dis 2005; 64:1150–1157. doi: 10.1136/ard.2004.032268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saraceno R, Bavetta M, Zangrilli A, Chiricozzi A, Potenza C, Chimenti S, et al. Adalimumab in the treatment of plaque-type psoriasis and psoriatic arthritis. Expert Opin Biol Ther 2013; 13:1325–1334. doi: 10.1517/14712598.2013.820701. [DOI] [PubMed] [Google Scholar]
- 11.Kavanaugh A, McInnes I, Mease P, Krueger GG, Gladman D, Gomez-Reino J, et al. Golimumab, a new human tumor necrosis factor alpha antibody, administered every four weeks as a subcutaneous injection in psoriatic arthritis: twenty-four-week efficacy and safety results of a randomized, placebo-controlled study. Arthritis Rheum 2009; 60:976–986. doi: 10.1002/art.24403. [DOI] [PubMed] [Google Scholar]
- 12.Mease PJ, Kivitz AJ, Burch FX, Siegel EL, Cohen SB, Ory P, et al. Etanercept treatment of psoriatic arthritis: safety, efficacy, and effect on disease progression. Arthritis Rheum 2004; 50:2264–2272. doi: 10.1002/art.20335. [DOI] [PubMed] [Google Scholar]
- 13.Zardin-Moraes M, Azeredo-da-Silva ALF, Saldanha C, Kohem CL, Coates L, Henriques LR, et al. Prevalence of psoriatic arthritis patients achieving minimal disease activity in real-life studies and randomized clinical trials: systematic review with metanalysis. J Rheumatol 2019; [Epub ahead of print]. doi: 10.3899/jrheum.190677. [DOI] [PubMed] [Google Scholar]
- 14.Chimenti MS, Triggianese P, De Martino E, Conigliaro P, Fonti GL, Sunzini F, et al. An update on pathogenesis of psoriatic arthritis and potential therapeutic targets. Expert Rev Clin Immunol 2019; 15:823–836. doi: 10.1080/1744666X.2019.1627876. [DOI] [PubMed] [Google Scholar]
- 15.Clark JD, Flanagan ME, Telliez JB. Discovery and development of Janus kinase (JAK) inhibitors for inflammatory diseases. J Med Chem 2014; 57:5023–5038. doi: 10.1021/jm401490p. [DOI] [PubMed] [Google Scholar]
- 16.Alunno A, Padjen I, Fanouriakis A, Boumpas DT. Pathogenic and therapeutic relevance of JAK/STAT signaling in systemic lupus erythematosus: integration of distinct inflammatory pathways and the prospect of their inhibition with an oral agent. Cells 2019; 8:898.doi: 10.3390/cells8080898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schwartz DM, Kanno Y, Villarino A, Ward M, Gadina M, O'Shea JJ. JAK inhibition as a therapeutic strategy for immune and inflammatory diseases. Nat Rev Drug Discov 2017; 16:843–862. doi: 10.1038/nrd.2017.201. [DOI] [PubMed] [Google Scholar]
- 18.Yamaoka K, Saharinen P, Pesu M, Holt VE, 3rd, Silvennoinen O, O'Shea JJ. The Janus kinases (Jaks). Genome Biol 2004; 5:253.doi: 10.1186/gb-2004-5-12-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang Y, Levy DE. Comparative evolutionary genomics of the STAT family of transcription factors. JAKSTAT 2012; 1:23–33. doi: 10.4161/jkst.19418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Murray PJ. The JAK-STAT signaling pathway: input and output integration. J Immunol 2007; 178:2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- 21.Hammaren HM, Virtanen AT, Raivola J, Silvennoinen O. The regulation of JAKs in cytokine signaling and its breakdown in disease. Cytokine 2019; 118:48–63. doi: 10.1016/j.cyto.2018.03.041. [DOI] [PubMed] [Google Scholar]
- 22.O'Shea JJ, Schwartz DM, Villarino AV, Gadina M, McInnes IB, Laurence A. The JAK-STAT pathway: impact on human disease and therapeutic intervention. Annu Rev Med 2015; 66:311–328. doi: 10.1146/annurev-med-051113-024537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.O'Shea JJ, Kontzias A, Yamaoka K, Tanaka Y, Laurence A. Janus kinase inhibitors in autoimmune diseases. Ann Rheum Dis 2013; 72: Suppl 2: ii111–ii115. doi: 10.1136/annrheumdis-2012-202576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maeshima K, Yamaoka K, Kubo S, Nakano K, Iwata S, Saito K, et al. The JAK inhibitor tofacitinib regulates synovitis through inhibition of interferon-gamma and interleukin-17 production by human CD4+ T cells. Arthritis Rheum 2012; 64:1790–1798. doi: 10.1002/art.34329. [DOI] [PubMed] [Google Scholar]
- 25.Fridman JS, Scherle PA, Collins R, Burn TC, Li YL, Li J, et al. Selective inhibition of JAK1 and JAK2 is efficacious in rodent models of arthritis: preclinical characterization of INCB028050. J Immunol 2010; 184:5298–5307. doi: 10.4049/jimmunol.0902819. [DOI] [PubMed] [Google Scholar]
- 26.Burmester GR, Blanco R, Charles-Schoeman C, Wollenhaupt J, Zerbini C, Benda B, et al. Tofacitinib (CP-690,550) in combination with methotrexate in patients with active rheumatoid arthritis with an inadequate response to tumour necrosis factor inhibitors: a randomised phase 3 trial. Lancet 2013; 381:451–460. doi: 10.1016/S0140-6736(12)61424-X. [DOI] [PubMed] [Google Scholar]
- 27.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med 2012; 367:495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 28.Galluzzo M, D’Adamio S, Servoli S, Bianchi L, Chimenti S, Talamonti M. Tofacitinib for the treatment of psoriasis. Expert Opin Pharmacother 2016; 17:1421–1433. doi: 10.1080/14656566.2016.1195812. [DOI] [PubMed] [Google Scholar]
- 29.Papp KA, Menter A, Strober B, Langley RG, Buonanno M, Wolk R, et al. Efficacy and safety of tofacitinib, an oral Janus kinase inhibitor, in the treatment of psoriasis: a Phase 2b randomized placebo-controlled dose-ranging study. Br J Dermatol 2012; 167:668–677. doi: 10.1111/j.1365-2133.2012.11168.x. [DOI] [PubMed] [Google Scholar]
- 30.Bachelez H, van de Kerkhof PC, Strohal R, Kubanov A, Valenzuela F, Lee JH, et al. Tofacitinib versus etanercept or placebo in moderate-to-severe chronic plaque psoriasis: a phase 3 randomised non-inferiority trial. Lancet 2015; 386:552–561. doi: 10.1016/S0140-6736(14)62113-9. [DOI] [PubMed] [Google Scholar]
- 31.Bradley CA. IBD: tofacitinib effective in ulcerative colitis. Nat Rev Gastroenterol Hepatol 2017; 14:388.doi: 10.1038/nrgastro.2017.66. [DOI] [PubMed] [Google Scholar]
- 32.Sandborn WJ, Su C, Sands BE, D’Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med 2017; 376:1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 33.Fragoulis GE, Siebert S, McInnes IB. Therapeutic targeting of IL-17 and IL-23 cytokines in immune-mediated diseases. Annu Rev Med 2016; 67:337–353. doi: 10.1146/annurev-med-051914-021944. [DOI] [PubMed] [Google Scholar]
- 34.Veale DJ, McGonagle D, McInnes IB, Krueger JG, Ritchlin CT, Elewaut D, et al. The rationale for Janus kinase inhibitors for the treatment of spondyloarthritis. Rheumatology (Oxford) 2019; 58:197–205. doi: 10.1093/rheumatology/key070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Banerjee S, Biehl A, Gadina M, Hasni S, Schwartz DM. JAK-STAT signaling as a target for inflammatory and autoimmune diseases: current and future prospects. Drugs 2017; 77:521–546. doi: 10.1007/s40265-017-0701-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O'Shea JJ, Ma A, Lipsky P. Cytokines and autoimmunity. Nat Rev Immunol 2002; 2:37–45. doi: 10.1038/nri702. [DOI] [PubMed] [Google Scholar]
- 37.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol 2017; 13:234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 38.Schwartz DM, Bonelli M, Gadina M, O'Shea JJ. Type I/II cytokines, JAKs, and new strategies for treating autoimmune diseases. Nat Rev Rheumatol 2016; 12:25–36. doi: 10.1038/nrrheum.2015.167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Villarino AV, Kanno Y, O'Shea JJ. Mechanisms and consequences of Jak-STAT signaling in the immune system. Nat Immunol 2017; 18:374–384. doi: 10.1038/ni.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Leonard WJ, O'Shea JJ. Jaks and STATs: biological implications. Annu Rev Immunol 1998; 16:293–322. doi: 10.1146/annurev.immunol.16.1.293. [DOI] [PubMed] [Google Scholar]
- 41.Baker KF, Isaacs JD. Novel therapies for immune-mediated inflammatory diseases: what can we learn from their use in rheumatoid arthritis, spondyloarthritis, systemic lupus erythematosus, psoriasis, Crohn's disease and ulcerative colitis? Ann Rheum Dis 2018; 77:175–187. doi: 10.1136/annrheumdis-2017-211555. [DOI] [PubMed] [Google Scholar]
- 42.Hofmann SR, Ettinger R, Zhou YJ, Gadina M, Lipsky P, Siegel R, et al. Cytokines and their role in lymphoid development, differentiation and homeostasis. Curr Opin Allergy Clin Immunol 2002; 2:495–506. doi: 10.1097/00130832-200212000-00004. [DOI] [PubMed] [Google Scholar]
- 43.Deon D, Ahmed S, Tai K, Scaletta N, Herrero C, Lee IH, et al. Cross-talk between IL-1 and IL-6 signaling pathways in rheumatoid arthritis synovial fibroblasts. J Immunol 2001; 167:5395–5403. doi: 10.4049/jimmunol.167.9.5395. [DOI] [PubMed] [Google Scholar]
- 44.O'Shea JJ, Holland SM, Staudt LM. JAKs and STATs in immunity, immunodeficiency, and cancer. N Engl J Med 2013; 368:161–170. doi: 10.1056/NEJMra1202117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.El Jammal T, Gerfaud-Valentin M, Seve P, Jamilloux Y. Inhibition of JAK/STAT signaling in rheumatologic disorders: the expanding spectrum. Joint Bone Spine 2020; 87:119–129. doi: 10.1016/j.jbspin.2019.09.005. [DOI] [PubMed] [Google Scholar]
- 46.Ghoreschi K, Jesson MI, Li X, Lee JL, Ghosh S, Alsup JW, et al. Modulation of innate and adaptive immune responses by tofacitinib (CP-690,550). J Immunol 2011; 186:4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bravo A, Kavanaugh A. Bedside to bench: defining the immunopathogenesis of psoriatic arthritis. Nat Rev Rheumatol 2019; 15:645–656. doi: 10.1038/s41584-019-0285-8. [DOI] [PubMed] [Google Scholar]
- 48.Veale DJ, Fearon U. The pathogenesis of psoriatic arthritis. Lancet 2018; 391:2273–2284. doi: 10.1016/S0140-6736(18)30830-4. [DOI] [PubMed] [Google Scholar]
- 49.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev 2009; 228:273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Przepiera-Bedzak H, Fischer K, Brzosko M. Serum IL-6 and IL-23 levels and their correlation with angiogenic cytokines and disease activity in ankylosing spondylitis, psoriatic arthritis, and SAPHO syndrome. Mediators Inflamm 2015; 2015:785705.doi: 10.1155/2015/785705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tesmer LA, Lundy SK, Sarkar S, Fox DA. Th17 cells in human disease. Immunol Rev 2008; 223:87–113. doi: 10.1111/j.1600-065X.2008.00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kirkham BW, Kavanaugh A, Reich K. Interleukin-17A: a unique pathway in immune-mediated diseases: psoriasis, psoriatic arthritis and rheumatoid arthritis. Immunology 2014; 141:133–142. doi: 10.1111/imm.12142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sakkas LI, Zafiriou E, Bogdanos DP. Mini review: new treatments in psoriatic arthritis. Focus on the IL-23/17 axis. Front Pharmacol 2019; 10:872.doi: 10.3389/fphar.2019.00872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vignali DA, Kuchroo VK. IL-12 family cytokines: immunological playmakers. Nat Immunol 2012; 13:722–728. doi: 10.1038/ni.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Sherlock JP, Joyce-Shaikh B, Turner SP, Chao CC, Sathe M, Grein J, et al. IL-23 induces spondyloarthropathy by acting on ROR-gamma t+ CD3+CD4-CD8- entheseal resident T cells. Nat Med 2012; 18:1069–1076. doi: 10.1038/nm.2817. [DOI] [PubMed] [Google Scholar]
- 56.Fiocco U, Accordi B, Martini V, Oliviero F, Facco M, Cabrelle A, et al. JAK/STAT/PKCdelta molecular pathways in synovial fluid T lymphocytes reflect the in vivo T helper-17 expansion in psoriatic arthritis. Immunol Res 2014; 58:61–69. doi: 10.1007/s12026-013-8481-0. [DOI] [PubMed] [Google Scholar]
- 57.Gao W, McGarry T, Orr C, McCormick J, Veale DJ, Fearon U. Tofacitinib regulates synovial inflammation in psoriatic arthritis, inhibiting STAT activation and induction of negative feedback inhibitors. Ann Rheum Dis 2016; 75:311–315. doi: 10.1136/annrheumdis-2014-207201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Raychaudhuri SK, Abria C, Raychaudhuri SP. Regulatory role of the JAK STAT kinase signalling system on the IL-23/IL-17 cytokine axis in psoriatic arthritis. Ann Rheum Dis 2017; 76:e36.doi: 10.1136/annrheumdis-2016-211046. [DOI] [PubMed] [Google Scholar]
- 59.Dowty ME, Lin J, Ryder TF, Wang W, Walker GS, Vaz A, et al. The pharmacokinetics, metabolism, and clearance mechanisms of tofacitinib, a janus kinase inhibitor, in humans. Drug Metab Dispos 2014; 42:759–773. doi: 10.1124/dmd.113.054940. [DOI] [PubMed] [Google Scholar]
- 60.Norman P. Selective JAK inhibitors in development for rheumatoid arthritis. Expert Opin Investig Drugs 2014; 23:1067–1077. doi: 10.1517/13543784.2014.918604. [DOI] [PubMed] [Google Scholar]
- 61.Taylor PC, Keystone EC, van der Heijde D, Weinblatt ME, Del Carmen Morales L, Gonzaga JR, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med 2017; 376:652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 62.Serhal L, Edwards CJ. Upadacitinib for the treatment of rheumatoid arthritis. Expert Rev Clin Immunol 2019; 15:13–25. doi: 10.1080/1744666X.2019.1544892. [DOI] [PubMed] [Google Scholar]
- 63.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet 2018; 391:2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 64.Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med 2017; 377:1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 65.Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF inhibitors. N Engl J Med 2017; 377:1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 66. Nash P, Coates LC, Kivitz AJ, Mease PJ, Gladman DD, Covarrubias-Cobos JA, et al. Safety and efficacy of tofacitinib, an oral Janus kinase inhibitor, up to 36 months in patients with active psoriatic arthritis: data from the third interim analysis of OPAL Balance, an open-label, long-term extension study. Poster presented at the Annual European Congress of Rheumatology, Amsterdam, Netherlands 2018; 13–16. doi: 10.1136/annrheumdis-2018-eular. 3115. [Google Scholar]
- 67.Nash P, Coates LC, Fleischmann R, Papp KA, Gomez-Reino JJ, Kanik KS, et al. Efficacy of tofacitinib for the treatment of psoriatic arthritis: pooled analysis of two phase 3 studies. Rheumatol Ther 2018; 5:567–582. doi: 10.1007/s40744-018-0131-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Curtis JR, Yun H, FitzGerald O, Winthrop K, Azevedo VF, Burmester G, et al. Comparing tofacitinib safety profile in patients with psoriatic arthritis in clinical studies with real-world data. Arthritis Rheum 2017; 69 (S10):833–835. doi: 10.1136/annrheumdis-2017-eular.2448. [Google Scholar]
- 69.van der Heijde D, Durez P, Schett G, Naredo E, Østergaard M, Meszaros G, et al. Structural damage progression in patients with early rheumatoid arthritis treated with methotrexate, baricitinib, or baricitinib plus methotrexate based on clinical response in the phase 3 RA-BEGIN study. Clin Rheumatol 2018; 37:2381–2390. doi: 10.1007/s10067-018-4221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Dougados M, van der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis 2017; 76:88–95. doi: 10.1136/annrheumdis-2016-210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med 2016; 374:1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 72.Papp KA, Menter MA, Raman M, Disch D, Schlichting DE, Gaich C, et al. A randomized phase 2b trial of baricitinib, an oral Janus kinase (JAK) 1/JAK2 inhibitor, in patients with moderate-to-severe psoriasis. Br J Dermatol 2016; 174:1266–1276. doi: 10.1111/bjd.14403. [DOI] [PubMed] [Google Scholar]
- 73.Smolen JS, Genovese MC, Takeuchi T, Hyslop DL, Macias WL, Rooney T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol 2019; 46:7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 74.Taylor PC, Weinblatt ME, Burmester GR, Rooney TP, Witt S, Walls CD, et al. Cardiovascular safety during treatment with baricitinib in rheumatoid arthritis. Arthritis Rheumatol 2019; 71:1042–1055. doi: 10.1002/art.40841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mease P, Coates LC, Helliwell PS, Stanislavchuk M, Rychlewska-Hanczewska A, Dudek A, et al. Efficacy and safety of filgotinib, a selective Janus kinase 1 inhibitor, in patients with active psoriatic arthritis (EQUATOR): results from a randomised, placebo-controlled, phase 2 trial. Lancet 2018; 392:2367–2377. doi: 10.1016/S0140-6736(18)32483-8. [DOI] [PubMed] [Google Scholar]
- 76.Genovese MC, Kalunian K, Gottenberg JE, Mozaffarian N, Bartok B, Matzkies F, et al. Effect of filgotinib vs placebo on clinical response in patients with moderate to severe rheumatoid arthritis refractory to disease-modifying antirheumatic drug therapy: the FINCH 2 randomized clinical trial. JAMA 2019; 322:315–325. doi: 10.1001/jama.2019.9055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol 2019; 71:1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 78.Fleischmann RM, Genovese MC, Enejosa JV, Mysler E, Bessette L, Peterfy C, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis 2019; 78:1454–1462. doi: 10.1136/annrheumdis-2019-215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet 2019; 393:2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 80.Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2018; 391:2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]


