Abstract
Background
Despite the recent advances in treatments for rheumatoid arthritis (RA), there are still unmet needs in disease outcomes. This study aimed to analyze the satisfaction with drug therapies for RA according to the levels of disease severity (patient-assessed) and proportions of treatment cost to household income.
Methods
This was a subgroup study of a cross-sectional study in patients with RA and their physicians. The patients were subdivided into different subgroups based on their self-assessed severity of RA and on the proportions of treatment cost to household income (<10%, 10–30%, 31–50%, and >50%). The Treatment Satisfaction Questionnaire for Medication version II was used to assess patients’ treatment satisfaction.
Results
When considering all medications, effectiveness, convenience, and global satisfaction scores were lower in the severe and moderate RA subgroups than those in the mild and extremely mild RA subgroups (all P < 0.001). Effectiveness, side effects, and convenience scores were higher in the <10% subgroup compared to those in the >50% subgroup (all P < 0.05). Global satisfaction score was higher in the <10% subgroup than that in the 31% to 50% subgroup (F = 13.183, P = 0.004). For biological disease-modifying anti-rheumatic drugs, effectiveness and convenience scores were lower in the severe RA subgroup than those in the extremely mild RA subgroup (both P < 0.05). Convenience score was higher in the <10% subgroup compared to that in the 31% to 50% and >50% subgroups (F = 12.646, P = 0.005). Global satisfaction score was higher in the <10% subgroup than that in the 31% to 50% subgroup (F = 8.794, P = 0.032).
Conclusion
Higher disease severity and higher financial burden were associated with lower patient satisfaction.
Keywords: Disease severity, Rheumatoid arthritis, Treatment cost, Treatment satisfaction
Introduction
Deep clinical remission can be achieved in patients with rheumatoid arthritis (RA), but achieving this treatment goal requires intensive management.[1] The treat-to-target (T2T) strategy is recommended for the treatment of RA, which aims at maximizing the long-term quality of life by preventing the structural damage and normalizing the social and work-related activities.[2,3] Nevertheless, recent studies indicated that the level of diagnosis and treatment for RA in China is still in the developmental stage, and a good implementation of the T2T strategy has not been observed yet.[4,5]
Despite the recent advances in treatments for RA, there are still unmet needs in disease outcomes. In fact, studies highlighted that safety issues, therapeutic regimen, remission duration, and compliance still require attention.[6,7] Dissatisfaction with treatment will lead to poor compliance, which will inevitably affect the patients’ outcomes.[8–10] Therefore, a better understanding of patients’ satisfaction can help for the optimal use of medications for RA treatment.
The Chinese Registry of Rheumatoid Arthritis (CREDIT) is so far the largest nationwide cohort of RA in China, showing the prevalence of remission and comorbidities, and the risk factors of comorbidities in patients with RA.[4,5] Nevertheless, the CREDIT study did not address the problem of patients’ satisfaction with RA treatments, which has to be solved to improve patient management.
Therefore, to improve our understanding of the satisfaction of Chinese patients with RA treatment, the present study aimed to analyze the satisfaction with drug therapies for RA according to the levels of disease severity (patient-assessed) and proportions of treatment cost to household income.
Methods
Ethical approval
This study was approved by the research ethics committee of Peking Union Medical College Hospital (No. S-K432), which was accepted by all participating centers as the central institutional review board. All participants signed an informed consent form for participation in the original study and eventual subgroup studies.
Study design and population
This was a subgroup analysis of a cross-sectional study in patients with RA and their physicians that was conducted between March 2018 and April 2018 in 12 hospitals from 11 provinces, municipalities, and autonomous regions in China. In addition, all hospitals, physicians, and patients were participating in the CREDIT registry.[4,5] In the original study, the eligibility criteria were: (1) ≥18 years of age; and (2) having been diagnosed with RA for >6 months according to the 2010 American College of Rheumatology (ACR)/European League Against Rheumatism (EULAR) classification criteria.[11] The exclusion criteria were: (1) had not yet received RA treatment; or (2) had comprehension barriers to reading Chinese.
Subgrouping
This study included two subgroup analyses. The patients were subdivided into different subgroups and then analyzed according to their self-assessed severity of RA (severe, moderate, mild, and extremely mild), or the proportion of treatment cost relative to household income (<10%, 10–30%, 31–50%, and >50%).
Questionnaires
In the original study, a patient questionnaire was used to collect the sociodemographic characteristics, medical history, and factors affecting long-term treatment. The outpatients were surveyed during a routine treatment follow-up visit. Disease activity score in 28 joints (DAS28) and the patient global assessment of RA disease activity were collected by the physicians.
The Treatment Satisfaction Questionnaire for Medication, version II (TSQM-II), was used to assess patients’ treatment satisfaction,[12] which includes 11 questions on four domains: (1) treatment effectiveness; (2) side effects; (3) convenience of administration; and (4) global satisfaction. Each domain is scored from 0 (extremely dissatisfied) to 100 (extremely satisfied). Patients’ satisfaction was assessed using the TSQM-II for all medications and biological disease-modifying anti-rheumatic drugs (bDMARDs) they ever received. Targeted synthetic DMARDs (tsDMARDs) were included in bDMARDs to simplify the questionnaire.
Statistical analysis
SPSS 20.0 (IBM, Armonk, NY, USA) was used for statistical analysis. Continuous variables in accordance with normal distribution were expressed as mean ± standard deviation (SD) and compared using the one-way analysis of variance (ANOVA). Skewed continuous variables were expressed as medians (interquartile range) and compared using Kruskal-Wallis test. Categorical variables were presented as frequencies (percentage) and analyzed using the Chi-squared test. P values <0.05 were considered statistically significant.
Results
Baseline characteristics of patients subgrouped by self-assessed severity of RA
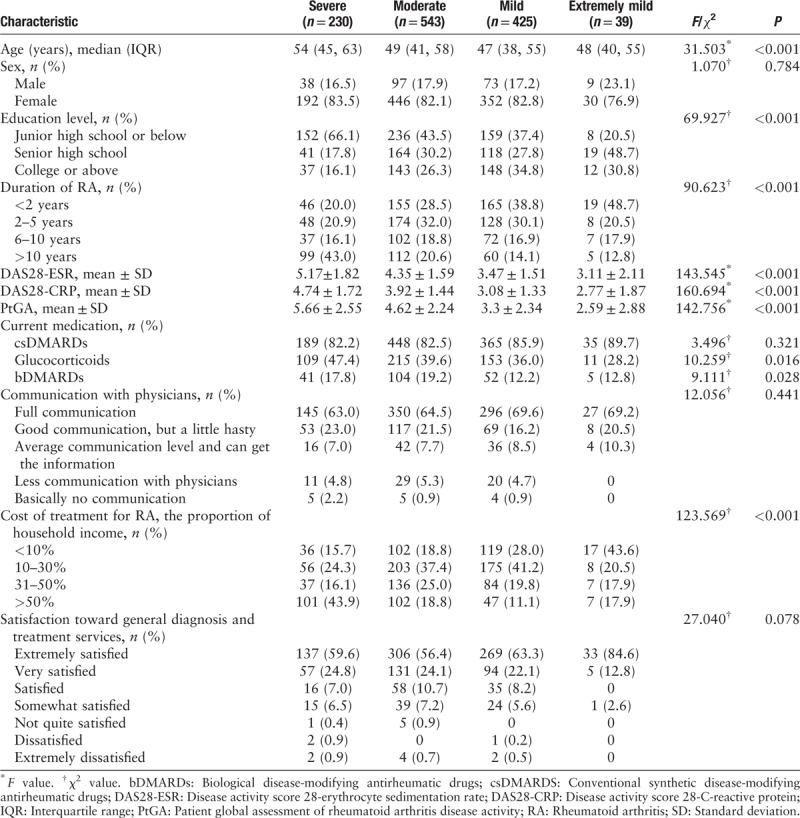
Table 1 presents the characteristics of patients with different self-assessed disease severity. Older age (F = 31.503, P < 0.001), lower education level (χ2 = 69.927, P < 0.001), longer disease duration (χ2 = 90.623, P < 0.001), more use of glucocorticoids (χ2 = 10.259, P = 0.016) and bDMARDs (χ2 = 9.111, P = 0.028), and higher proportion of treatment costs (χ2 = 123.569, P < 0.001) were observed with the increase of patient-assessed disease severity, showing the significant differences among the subgroups. There were no significant differences in sex, use of csDMARDs, communication with physicians, and satisfaction toward general diagnosis and treatment services.
Table 1.
Characteristics of the patients with different self-assessed severity of RA.
Satisfaction summary of patients with various self-assessed severity of RA
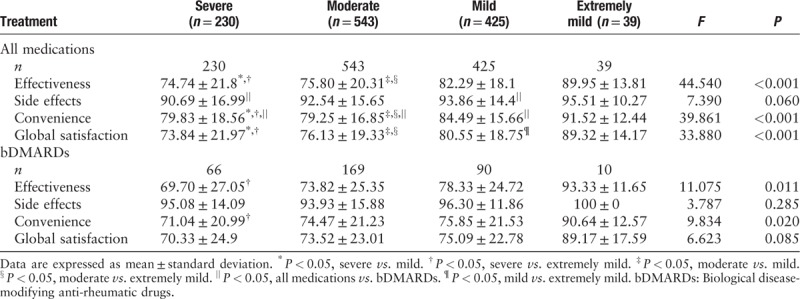
The TSQM-II summary scores of patients with different self-assessed disease severity are shown in Table 2. When considering all medications, effectiveness, convenience, and global satisfaction scores were lower in the severe and moderate RA subgroups compared to those in the mild and extremely mild RA subgroups (all P < 0.001). For bDMARDs, effectiveness and convenience scores were lower in the severe RA subgroup compared to those in the extremely mild RA subgroup (both P < 0.05). No significant differences in side effects score for all medications, or side effects and global satisfaction scores for bDMARDs were observed among the subgroups.
Table 2.
TSQM-II summary scores of patients with different self-assessed severity of rheumatoid arthritis.
Baseline characteristics of patients subgrouped by their proportion of treatment cost to household income
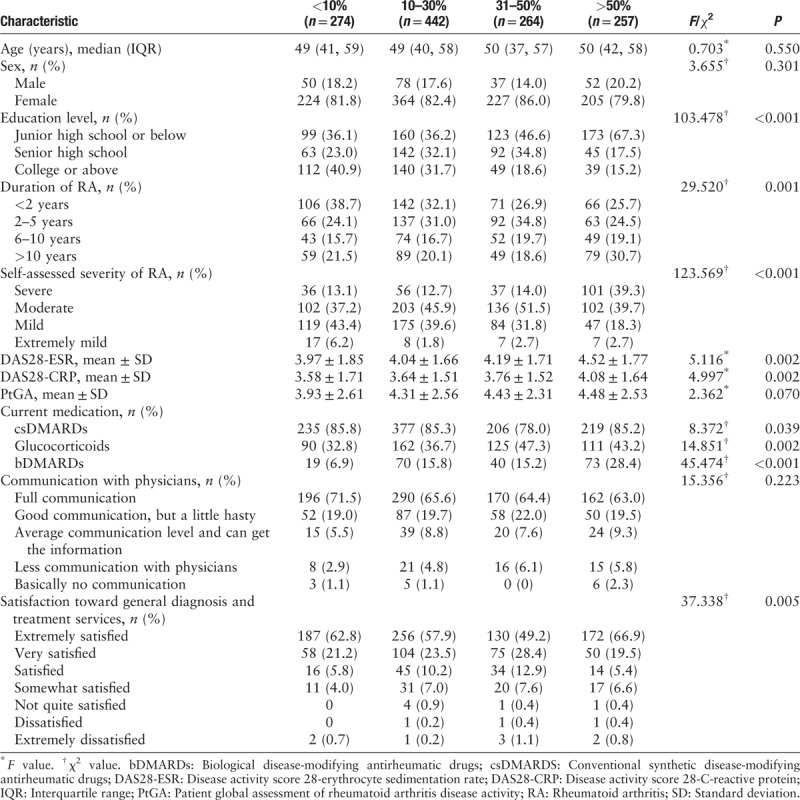
Then, the patients were subgrouped according to the proportion of income devoted to RA treatments. Table 3 shows that lower education level (χ2 = 103.478, P < 0.001), longer disease duration (χ2 = 29.520, P = 0.001), higher self-assessed disease severity (χ2 = 123.569, P < 0.001), more use of csDMARDs (χ2 = 8.372, P = 0.039), glucocorticoids (χ2 = 14.851, P = 0.002) and bDMARDs (χ2 = 45.474, P < 0.001), and better satisfaction toward general diagnosis and treatments (χ2 = 37.338, P = 0.005) were observed with the increase of financial burden, showing the significant differences among the subgroups. There were no significant differences in age, sex, and communication with physicians.
Table 3.
Characteristics of the patients with different proportion of treatment cost to household income.
Satisfaction summary of patients with various proportion of treatment cost to household income
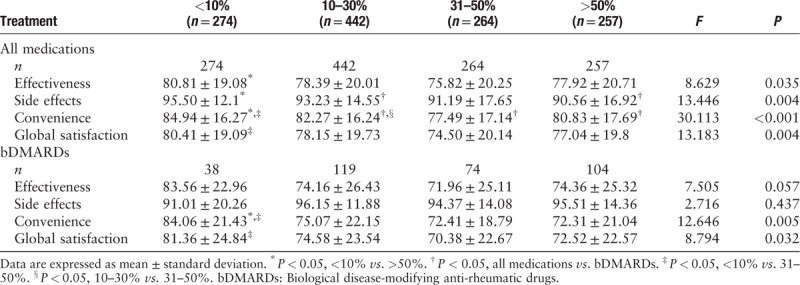
The TSQM-II summary scores of patients with different proportion of income devoted to RA treatments are shown in Table 4. When considering all medications, effectiveness, side effects, and convenience scores were higher in the < 10% subgroup compared to those in the > 50% subgroup (all P < 0.05). Global satisfaction score was higher in the < 10% subgroup than that in the 31–50% subgroup (F = 13.183, P = 0.004). For bDMARDs, convenience score was higher in the < 10% subgroup compared to that in the 31–50% and > 50% subgroups (F = 12.646, P = 0.005). Global satisfaction score was higher in the < 10% subgroup than that in the 31%-50% subgroup (F = 8.794, P = 0.032). There were no significant differences in effectiveness or side effects scores for bDMARDs among the subgroups.
Table 4.
TSQM-II summary scores of the patients with different proportion of treatment cost to household income.
Discussion
The status of patients’ satisfaction with treatments for RA is mostly unknown in China. Therefore, this study aimed to analyze satisfaction with drug therapies for RA according to the levels of disease severity (patient-assessed) and the proportion of treatment cost to household income. The results suggested that higher disease severity and higher costs of treatment relative to the household income were associated with lower patient satisfaction.
The data of the self-assessed disease severity showed that the majority of patients with severe disease had a duration of disease >10 years and paid >50% of their outcome for treatments. In contrast, the patients with extremely mild RA had a duration of disease <2 years and low treatment costs, supporting the concept that early management leads to better outcomes and smaller economic restraints. Similar results were obtained when grouping the patients according to the proportions of treatment cost to household income. Indeed, most patients with high treatment costs (>50% of their income) had a duration of disease >10 years. Some patients with a long duration of disease possibly did not receive early diagnosis and treatment, which might affect the overall prognosis in patients with long disease duration. These results are supported by the current T2T strategy that supports early treatment for the prevention of structural damage progression and for the optimization of quality of life.[2,3] The results are also supported by a number of studies showed that the severity of RA is associated with healthcare costs in different populations around the globe.[13–18]
Therefore, it is reasonable to seek treatments at an early stage of RA to achieve a better prognosis.[2,3] Nevertheless, this concept of early diagnosis and treatment is not widely accepted in Chinese patients.[19,20] In the present study, most patients had at least moderate or severe illness, but only 3.2% of all the patients enrolled were considering themselves as being with an extremely mild disease. High severity reported in most patients was possibly concerned with the fact that patients had low education levels in general and probably low levels of disease alertness, resulting in treatment delay.[21] In addition, we cannot rule out the possibility that some patients from rural areas had difficulty in accessing medical resources, thereby leading to a delay before effective treatment, which potentially resulted in poor prognosis. This hypothesis will have to be confirmed in future studies.
In the present study, there was no significant difference in satisfaction with the side effects of different medications among patients with different disease severity. On the contrary, those with more severe illnesses tended to be more unsatisfied with the effectiveness and convenience of all drugs. Daniel et al[22] showed that convenience was a major determinant of patients’ satisfaction with treatments for RA. De Mits et al[23] showed that patient satisfaction was more dependent upon effectiveness than the route of administration. These results indicated that there was an urgent need to improve the effectiveness and convenience of RA treatment. In consideration of biological agents, a significant difference appeared in the satisfaction with efficacy and convenience only between the severe and extremely mild groups, suggesting that disease severity does not have a remarkable impact on the satisfaction with bDMARDs, as supported by previous studies.[9,24] Nevertheless, more effective and convenient bDMARDs are indeed required for patients with severe RA.
Among all patients, patients with <10% proportion of treatment cost to income were obviously more satisfied with all aspects of various medications, suggesting that the treatment costs were inevitably an important aspect of patient satisfaction, as supported by previous studies.[25–27] A significant decrease in the satisfaction of Chinese patients was apparently correlated with expenditure increase. This could result in poor compliance and prognosis. This relationship between treatment costs, compliance, and prognosis has been reported by many populations all over the world.[28–31] As for biologics, different satisfaction levels in the different treatment cost groups were only observed in the convenience aspect. It can be seen that increasing treatment costs will remarkably affect the compliance of Chinese patients on bDMARDs, thus affecting their therapeutic regimens and prognoses.
This study revealed the relationship between Chinese patients’ satisfaction for RA treatment and their disease severity and treatment cost for the first time, which had great significance in improving patients’ satisfaction and disease outcome. However, there is still room to improve this study. The sample size was not large enough, especially when considering the number of patients with RA in China. Second, disease severity and cost proportion were assessed by the patients, which could lead to a number of subjective biases. The DAS28 scores were determined and were associated with the patients’ evaluation, but other scores such as the Health Assessment Questionnaire (HAQ) or the Sharp score were not determined. Third, as this was a cross-sectional study, the patients’ evaluation was performed only once without follow up. Fourth, the questionnaire focused only on RA. Patients were asked about their satisfaction with their treatment for RA, and no question was asked about any other chronic disease. Based on a previous study from the CREDIT registry,[4] the proportion of Chinese patients with RA with major comorbidities (cardiovascular diseases, fragility fracture, and malignancy) is low (4.2%). Therefore, it could be hypothesized that the influence of other chronic diseases on satisfaction might be small, but this will have to be confirmed. Fifth, the reliability and validity of the Chinese version of TSQM-II were not assessed. Finally, only bDMARDS and whole treatment were evaluated, and csDMARDS and glucocorticoids were not evaluated, as per study design. In addition, the results will have to be consistently revised as new drugs become available in China. Indeed, a recent trial in China demonstrated the efficacy and safety of tofacitinib in patients with RA,[32] and future studies will have to be performed.
In conclusion, this study demonstrated that higher disease severity and higher costs of treatment relative to the household income were associated with lower patient satisfaction. These results improve our understanding of the satisfaction of Chinese patients with RA treatment and could be used to design new strategies to improve compliance and prognosis in patients with RA.
Acknowledgements
The authors acknowledge contributions from the HealthCloud Co., Ltd as the system provider.
Funding
This work was supported by grants from the Chinese National Key Research R&D Program (Nos. 2017YFC0907601, 2017YFC0907604).
Conflicts of interest
None.
Footnotes
How to cite this article: Li HB, Wu LJ, Jiang N, Yang PT, Liu SY, Shi XF, Fang YF, Zhao Y, Xu J, Jiang ZY, Wu ZB, Duan XW, Wang Q, Li MT, Tian XP, Zeng XF. Treatment satisfaction with rheumatoid arthritis in patients with different disease severity and financial burden: a subgroup analysis of a nationwide survey in China. Chin Med J 2020;133:892–898. doi: 10.1097/CM9.0000000000000749
Hong-Bin Li, Li-Jun Wu, and Nan Jiang contributed equally to the work.
References
- 1.Liu JJ, Li R, Gan YZ, Zhang RJ, Li J, Cai YM, et al. Clinical deep remission and related factors in a large cohort of patients with rheumatoid arthritis. Chin Med J 2019; 132:1009–1014. doi: 10.1097/CM9.0000000000000227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Smolen JS, Landewe R, Bijlsma J, Burmester G, Chatzidionysiou K, Dougados M, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2016 update. Ann Rheum Dis 2017; 76:960–977. doi: 10.1136/annrheumdis-2016-210715. [DOI] [PubMed] [Google Scholar]
- 3.Smolen JS, Aletaha D, Barton A, Burmester GR, Emery P, Firestein GS, et al. Rheumatoid arthritis. Nat Rev Dis Primers 2018; 4:18001.doi: 10.1038/nrdp.2018.1. [DOI] [PubMed] [Google Scholar]
- 4.Jin S, Li M, Fang Y, Li Q, Liu J, Duan X, et al. Chinese Registry of rheumatoid arthritis (CREDIT): II. prevalence and risk factors of major comorbidities in Chinese patients with rheumatoid arthritis. Arthritis Res Ther 2017; 19:251.doi: 10.1186/s13075-017-1457-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yu C, Li M, Duan X, Fang Y, Li Q, Wu R, et al. Chinese registry of rheumatoid arthritis (CREDIT): Introduction and prevalence of remission in Chinese patients with rheumatoid arthritis. Clin Exp Rheumatol 2018; 36:836–840. [PubMed] [Google Scholar]
- 6.Baker JF, Sauer B, Teng CC, George M, Cannon GW, Ibrahim S, et al. Initiation of disease-modifying therapies in rheumatoid arthritis is associated with changes in blood pressure. J Clin Rheumatol 2018; 24:203–209. doi: 10.1097/RHU.0000000000000736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wilsdon TD, Hill CL. Managing the drug treatment of rheumatoid arthritis. Aust Prescr 2017; 40:51–58. doi: 10.18773/austprescr.2017.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bowling A, Ebrahim S. Measuring patients’ preferences for treatment and perceptions of risk. Qual Health Care 2001; 10: Suppl 1: i2–i8. doi: 10.1136/qhc.0100002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barton JL. Patient preferences and satisfaction in the treatment of rheumatoid arthritis with biologic therapy. Patient Prefer Adherence 2009; 3:335–344. doi: 10.2147/ppa.s5835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Louder AM, Singh A, Saverno K, Cappelleri JC, Aten AJ, Koenig AS, et al. Patient preferences regarding rheumatoid arthritis therapies: a conjoint analysis. Am Health Drug Benefits 2016; 9:84–93. [PMC free article] [PubMed] [Google Scholar]
- 11.Aletaha D, Neogi T, Silman AJ, Funovits J, Felson DT, Bingham CO, 3rd, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum 2010; 62:2569–2581. doi: 10.1002/art.27584. [DOI] [PubMed] [Google Scholar]
- 12.Atkinson MJ, Kumar R, Cappelleri JC, Hass SL. Hierarchical construct validity of the treatment satisfaction questionnaire for medication (TSQM version II) among outpatient pharmacy consumers. Value Health 2005; 8: Suppl 1: S9–S24. doi: 10.1111/j.1524-4733.2005.00066.x. [DOI] [PubMed] [Google Scholar]
- 13.Baser O, Baser E, Altinbas A, Burkan A. Severity index for rheumatoid arthritis and its association with health care costs and biologic therapy use in Turkey. Health Econ Rev 2013; 3:5.doi: 10.1186/2191-1991-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Standfield L, Norris S, Harvey C, Elliot L, Riordan J, Hall S, et al. Relationship between rheumatoid arthritis disease severity, health-related utility, and resource use in Australian patients: a cross-sectional, multicenter study. Clin Ther 2010; 32:1329–1342. doi: 10.1016/j.clinthera.2010.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Klimes J, Vocelka M, Sedova L, Dolezal T, Mlcoch T, Petrikova A, et al. Medical and productivity costs of rheumatoid arthritis in the Czech Republic: cost-of-illness study based on disease severity. Value Health Reg Issues 2014; 4:75–81. doi: 10.1016/j.vhri.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Wallman JK, Eriksson JK, Nilsson JA, Olofsson T, Kristensen LE, Neovius M, et al. Costs in relation to disability, disease activity, and health-related quality of life in rheumatoid arthritis: observational data from southern Sweden. J Rheumatol 2016; 43:1292–1299. doi: 10.3899/jrheum.150617. [DOI] [PubMed] [Google Scholar]
- 17.Lee TJ, Park BH, Son HK, Song R, Shin KC, Lee EB, et al. Cost of illness and quality of life of patients with rheumatoid arthritis in South Korea. Value Health 2012; 15:S43–S49. doi: 10.1016/j.jval.2011.11.020. [DOI] [PubMed] [Google Scholar]
- 18.Kobelt G, Lindgren P, Lindroth Y, Jacobson L, Eberhardt K. Modelling the effect of function and disease activity on costs and quality of life in rheumatoid arthritis. Rheumatology 2005; 44:1169–1175. doi: 10.1093/rheumatology/keh703. [DOI] [PubMed] [Google Scholar]
- 19.Seca S, Franconi G. Understanding Chinese medicine patterns of rheumatoid arthritis and related biomarkers. Medicines 2018; 5: doi: 10.3390/medicines5010017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang L, Wang J, Zhang Q, Fu T, Yin R, Wang Z, et al. Factors associated with hand joint destruction in Chinese patients with rheumatoid arthritis. BMC Musculoskelet Disord 2017; 18:211.doi: 10.1186/s12891-017-1548-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joplin S, van der Zwan R, Joshua F, Wong PK. Medication adherence in patients with rheumatoid arthritis: the effect of patient education, health literacy, and musculoskeletal ultrasound. BioMed Res Int 2015; 2015:150658.doi: 10.1155/2015/150658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Daniel SR, McDermott JD, Jr, Le C, Pierce CA, Ziskind MA, Ellis LA. A real-world, multi-site, observational study of infusion time and treatment satisfaction with rheumatoid arthritis patients treated with intravenous golimumab or infliximab. J Med Econ 2018; 21:724–731. doi: 10.1080/13696998.2018.1472098. [DOI] [PubMed] [Google Scholar]
- 23.De Mits S, Lenaerts J, Vander Cruyssen B, Mielants H, Westhovens R, Durez P, et al. A nationwide survey on patient's versus physician's evaluation of biological therapy in rheumatoid arthritis in relation to disease activity and route of administration: the be-raise study. PLoS One 2016; 11:e0166607.doi: 10.1371/journal.pone.0166607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Calvo-Alen J, Vela P, Bustabad S, Maceiras F, Carmona L, Cea-Calvo L. Satisfaction, fulfillment of expectations and adherence to subcutaneous biological drugs in patients with rheumatoid arthritis: ARCO study. Reumatol Clin 2018; pii: S1699-258X(18)30076-7. doi: 10.1016/j.reuma.2018.02.015. [DOI] [PubMed] [Google Scholar]
- 25.Lofland JH, Johnson PT, Ingham MP, Rosemas SC, White JC, Ellis L. Shared decision-making for biologic treatment of autoimmune disease: influence on adherence, persistence, satisfaction, and health care costs. Patient Prefer Adherence 2017; 11:947–958. doi: 10.2147/PPA.S133222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Marshall NJ, Wilson G, Lapworth K, Kay LJ. Patients’ perceptions of treatment with anti-TNF therapy for rheumatoid arthritis: a qualitative study. Rheumatology 2004; 43:1034–1038. doi: 10.1093/rheumatology/keh237. [DOI] [PubMed] [Google Scholar]
- 27.Wolfe F, Michaud K. Resistance of rheumatoid arthritis patients to changing therapy: discordance between disease activity and patients’ treatment choices. Arthritis Rheum 2007; 56:2135–2142. doi: 10.1002/art.22719. [DOI] [PubMed] [Google Scholar]
- 28.Bonafede M, Johnson BH, Tang DH, Harrison DJ, Stolshek BS. Compliance and cost of biologic therapies for rheumatoid arthritis. Am J Pharm Benefits 2017; 9:e1–e7. [Google Scholar]
- 29.Marengo MF, Suarez-Almazor ME. Improving treatment adherence in patients with rheumatoid arthritis: what are the options? Int J Clin Rheumatol 2015; 10:345–356. doi: 10.2217/ijr.15.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Curkendall S, Patel V, Gleeson M, Campbell RS, Zagari M, Dubois R. Compliance with biologic therapies for rheumatoid arthritis: do patient out-of-pocket payments matter? Arthritis Rheum 2008; 59:1519–1526. doi: 10.1002/art.24114. [DOI] [PubMed] [Google Scholar]
- 31.Harley CR, Frytak JR, Tandon N. Treatment compliance and dosage administration among rheumatoid arthritis patients receiving infliximab, etanercept, or methotrexate. Am J Manag Care 2003; 9:S136–S143. [PubMed] [Google Scholar]
- 32.Li ZG, Liu Y, Xu HJ, Chen ZW, Bao CD, Gu JR, et al. Efficacy and safety of tofacitinib in Chinese patients with rheumatoid arthritis. Chin Med J 2018; 131:2683–2692. doi: 10.4103/0366-6999.245157. [DOI] [PMC free article] [PubMed] [Google Scholar]


