Abstract
A frequent side effect of many drugs includes the occurrence of cholestatic liver toxicity. Over the past couple of decades, drug-induced cholestasis has gained considerable attention, resulting in a plethora of data regarding its prevalence and mechanistic basis. Likewise, several food additives and dietary supplements have been reported to cause cholestatic liver insults in the past few years. The induction of cholestatic hepatotoxicity by other types of chemicals, in particular synthetic compounds, such as industrial chemicals, biocides and cosmetic ingredients, has been much less documented. Such information can be found in occasional clinical case reports of accidental intake or suicide attempts as well as in basic and translational study reports on mechanisms or testing of new therapeutics in cholestatic animal models. This paper focuses on such non-pharmaceutical and non-dietary synthetic chemical inducers of cholestatic liver injury, in particular alpha-naphthylisocyanate, 3,5-diethoxycarbonyl-1,4-dihydrocollidine, methylenedianiline, paraquat, tartrazine, triclosan, 2-octynoic acid and 2-nonynoic acid. Most of these cholestatic compounds act by similar mechanisms. This could open perspectives for the prediction of cholestatic potential of chemicals.
Keywords: hepatotoxicity, cholestasis, industrial chemical, biocide, cosmetic ingredient
1. Introduction
Cholestasis is derived from the Greek words chole meaning bile and stasis indicating halting, and denotes any situation of impaired bile secretion with concomitant accumulation of bile acids in the liver or in the systemic circulation.1,2 Depending on the location and cause of the obstruction, a distinction can be made between intrahepatic and extrahepatic cholestasis. Clinically, cholestasis is routinely diagnosed based on biochemical parameters, including increased serum levels of alkaline phosphatase, gamma-glutamyltransferase and bilirubin.3
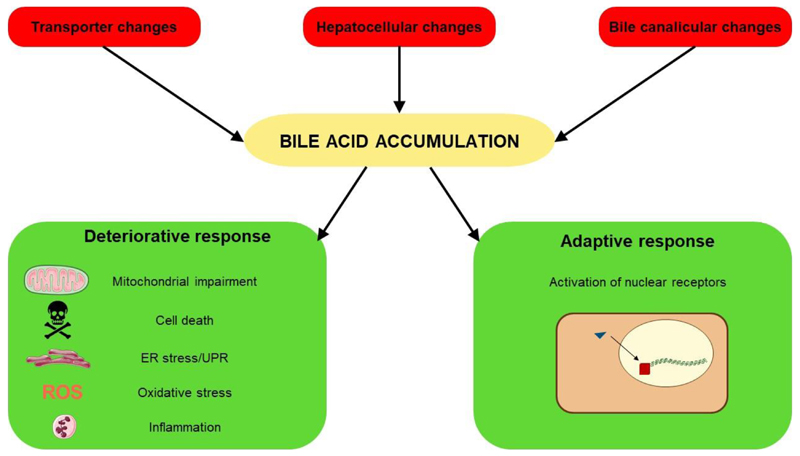
From the mechanistic perspective, cholestatic liver injury can be induced by 3 types of stimuli. First, reduced functionality, expression and/or aberrant subcellular localization of transporters responsible for conveying bile acids and/or drugs may occur, such as the bile salt export pump, multidrug resistance-associated protein 2/3/4 and multidrug resistance protein 3. Second, various hepatocellular changes, including compromised cytoskeletal architecture, disruption of tight junctions and decreased membrane fluidity, may take place. Third, bile canaliculi dynamics may alter. These 3 types of cholestatic triggers induce 2 cellular responses. A first adverse response is elicited by bile acid accumulation and characterized by the occurrence of inflammation, oxidative stress, endoplasmic reticulum stress, mitochondrial impairment and different cell death modes, including necrosis, apoptosis, necroptosis and autophagy. A second adaptive response is aimed at decreasing the uptake and increasing the export of bile acids into and from hepatocytes, respectively, which relies on the activation of nuclear receptors, including the farnesoid X receptor, the pregnane X receptor and the constitutive androstane receptor. These nuclear receptors activate the expression of a number of transporters and enzymes that remove bile acids (Figure 1).2,4 It should be stressed that the adaptive response is not restricted to the liver, but also takes place in the intestine, kidney and epithelia of the bile duct.5 In this respect, proliferation of cholangiocytes leads to corrugations of the luminal duct surface. Accordingly, the surface area increases, duct elongates, branches sprout and loops are formed. Alterations in the bile duct morphology strive to maintain the proximal position of the bile duct relative to the portal vein, which is essential for bile acid transport. Furthermore, this remodeling process enhances resorption of bile acids from the bile duct lumen and transportation to the portal vein.6,7
Figure 1.
Cholestatic liver injury can be initiated by 3 types of triggering factors, namely (i) transporter changes, such as transport inhibition, reduced expression and/or aberrant subcellular localization of bile transporters, (ii) hepatocellular changes, including compromised cytoskeletal architecture, disruption of tight junctions and decreased membrane fluidity, and (iii) altered bile canaliculi dynamics, namely dilatation or constriction of bile canaliculi. These stimuli induce bile accumulation, which subsequently activates 2 cellular responses, a deteriorative response and an adaptive response. The deteriorative response is typified by the occurrence of mitochondrial impairment, different cell death modes, endoplasmic reticulum (ER) stress with unfolded protein responses (UPR), oxidative stress and inflammation. The adaptive response strives to counteract bile acid accumulation via activation of a number of nuclear receptors.
Drug treatment, mainly involving anti-infectious drugs, anti-diabetics, anti-inflammatory drugs, psychotropic drugs, cardiovascular drugs and steroids, frequently underlies the onset of intrahepatic cholestasis.8,9 As such, drug-induced cholestasis constitutes a subgroup of drug-induced liver injury. The latter is a major reason of drug failure during premarketing and postmarketing phases, accounting for up to 29% of all drug withdrawals.10,11 In addition to its pharmaceutical relevance, drug-induced liver injury is also of high clinical concern. Indeed, drug-induced liver injury is frequently misdiagnosed, yet it has been estimated to develop in 1 in 100 patients during hospitalization.12 Furthermore, drug-induced liver injury is responsible for more than 50% of all cases of acute liver failure.13
Given its considerable prevalence, drug-induced cholestasis has become well documented throughout the years. This is unlike other types of synthetic chemicals, for which clinical case studies of cholestatic hepatotoxicity are published only sporadically or that are restricted for use in laboratory animals for basic and translational cholestasis research purposes. The present paper gives a concise overview of such atypical synthetic chemical triggers of cholestatic liver injury from the industrial, biocide and/or cosmetic areas.
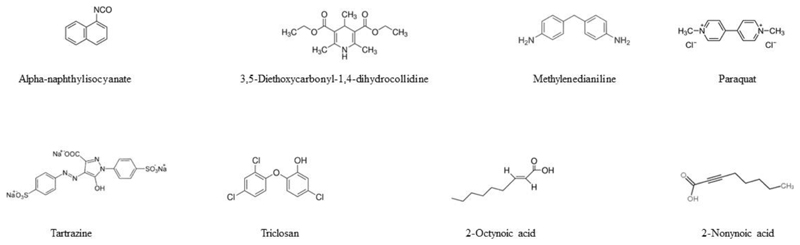
2. Alpha-naphthylisocyanate
Alpha-naphthylisocyanate (Figure 2) is used in the preparation of cationic aromatic urethane. It is a model compound to induce intrahepatic cholestasis in laboratory animals, in particular rodents, associated with increased serum levels of alkaline phosphatase and gamma-glutamyltransferase.14,15
Figure 2.
Structure of chemical inducers of cholestatic liver injury.
Metabolomic screening in rat liver following alpha-naphthylisocyanate administration substantiates an overall cholestatic profile, in particular manifested as effects on primary bile acid biosynthesis.16 Alpha-naphthylisocyanate primarily targets bile duct epithelial cells, but also causes hepatocyte necrosis.17 In rats, alpha-naphthylisocyanate evokes hepatic inflammation18, oxidative stress14, endoplasmic reticulum stress19 and mitochondrial toxicity20, all being key events in cholestatic liver injury.2,4 Alpha-naphthylisocyanate alters the expression and/or activity of a number of hepatic transporters, including the bile salt export pump, multidrug resistance-associated protein 2/3 and multidrug resistance protein 3.21–23 Furthermore, alpha-naphthylisocyanate disrupts hepatic tight junctions24,25, decreases membrane fluidity26 and causes bile canaliculi dilatation27. Alpha-naphthylisocyanate decreases levels of glutathione through involvement of phosphoinositide-3-kinase/protein kinase B and nuclear factor erythroid 2-related factor 2 signaling in rat liver, thereby boosting oxidative stress in cholestasis.28 Alpha-naphthylisocyanate actives the farnesoid X receptor and the pregnane X receptor in mouse liver,29 which mediate the adaptive response in cholestatic injury.2–4
3. 3,5-Diethoxycarbonyl-1,4-dihydrocollidine
3,5-diethoxycarbonyl-1,4-dihydrocollidine (Figure 2) is a porphyrinogenic agent and a powerful inducer of delta-aminolevulinate synthetase.30 It has been used since many decades to experimentally induce cholestasis in rodents. Thus, administration of 3,5-diethoxycarbonyl-1,4-dihydrocollidine to mice increases serum levels of alkaline phosphatase and bilirubin, and triggers histopathological features of inflammation and cell death.31,32
Metabolic profiling has confirmed elevated bile acid levels in mice following 3,5-diethoxycarbonyl-1,4-dihydrocollidine treatment.33 Furthermore, 3,5-diethoxycarbonyl-1,4-dihydrocollidine causes inflammation34, oxidative stress35,36, endoplasmic reticulum stress37, mitochondrial toxicity35 and apoptosis38 in mouse liver.
3,5-diethoxycarbonyl-1,4-dihydrocollidine has differential effects on hepatic transporters, including the bile salt export pump and multidrug resistance-associated protein 239,40, and suppresses expression of tight junction proteins in liver40 following administration to mice. In addition, 3,5-diethoxycarbonyl-1,4-dihydrocollidine triggers bile canaliculi dilatation40 as well as marked derangement of the hepatic cytoskeletal network.41
4. Methylenedianiline
Methylenedianiline (Figure 2) is used in the production of polyamides and epoxy resins as well as in the synthesis of 4,4’-methylenediphenyl diisocyanate, being a major component of polyurethanes. These polymers are applied in the manufacturing of insulation materials, automotive and aircraft parts, and medical devices.42,43
Occupational or accidental exposure to methylenedianiline causes injury to bile ducts with subsequent cholestasis, clinically manifested as jaundice, skin rash and elevated serum quantities of alkaline phosphatase and gamma-glutamyltransferase.44–46 This has been historically termed “Epping jaundice” referring to an accidental mass poisoning in the vicinity of Epping in the United Kingdom in 1965, during which 84 individuals were poisoned through methylenedianiline-contaminated flour used to make bread.45,47
Methylenedianiline diminishes bile flow48, and causes inflammation, oxidative stress, apoptosis and necrosis in liver upon administration to rats49 and mice50. Methylenedianiline compromises hepatic tight junction integrity and functionality,51 an effect that only takes place following mitochondrial dysfunction52. Recently, a transcriptomic signature of methylenedianiline-induced liver toxicity has been established in rat, thereby confirming cholestasis as a main mechanism of adversity, including effects on the peroxisome proliferator-activated receptor alpha, the liver X receptor, the retinoid X receptor and induction of oxidative stress.53
5. Paraquat
Paraquat (Figure 2) is a potent herbicide that has been widely used in agriculture as a weed control agent for farmlands and pastures. Accidental or intended ingestion of paraquat results in multiple organ injuries, yet it especially targets the lungs.54 Nevertheless, paraquat poisoning in humans equally causes cholestatic liver toxicity with extensive jaundice55 and high serum levels of alkaline phosphatase, gamma-glutamyltranferase and bilirubin56–61.
Upon administration to mice or rats, paraquat induces hepatic inflammation62, oxidative stress63–65 and mitochondrial toxicity66,67. This is associated with bile canaliculi dilatation, apoptosis, necrosis and autophagy in liver.61 Paraquat damages both hepatocytes and bile duct epithelial cells.68 Paraquat was found to decrease membrane fluidity in mouse liver homogenates.69 In other cell types, paraquat has shown to disrupt microfilaments70, to affect liver X receptor activity71 and to induce Rho-associated protein kinase72 and c-Jun N-terminal/p38 signaling71. Although solid scientific evidence is currently lacking, these alterations in kinase activity could underlie induction of inflammation and cell death in cholestasis.
6. Triclosan
Triclosan (Figure 2) is a broad-spectrum antimicrobial agent present as an ingredient in several types of personal care products, such as soaps and toothpastes, as well as in detergents, toys, surgical cleaning products and pharmaceuticals.73
Triclosan displays antibiotic and antimycotic properties, and acts by interfering with fatty acid synthesis.74 The latter is not limited to micro-organisms, as triclosan has been shown to induce hallmarks of fatty liver disease in toads75, frogs76 and fish77. This has been associated with activation of hepatic nuclear receptors, including the pregnane X receptor78 and the constitutive androstane receptor79. These nuclear receptors equally play a key role in cholestasis, in particular by mediating the adverse response and inducing the expression of genes involved in counteracting bile acid accumulation, including those coding for hepatic transporters and biotransformation enzymes.2,4
Triclosan causes hepatic inflammation80, oxidative stress81, mitochondrial toxicity82,83, cell cycle arrest and apoptosis81,84. Triclosan also suppresses microfilament remodeling and cell membrane ruffling85, and affects a number of signaling cascades, including protein kinase B86,87 and extracellular signal-regulated kinases 1/287, all that collectively promote cholestatic liver injury. In fact, in oral repeated dose toxicity studies in rodents, triclosan triggers typical diagnostic features of cholestasis, including increased serum levels of alkaline phosphatase, gamma-glutamyltransferase and bilirubin, and induction of liver cell necrosis.80
7. Tartrazine
Tartrazine (Figure 2) is an orange-colored dye used in cosmetics, textiles, pharmaceuticals and foods.88 Tartrazine has been linked to primary biliary cholangitis, occurring most frequently in postmenopausal women.89
Upon administration to rats or mice, tartrazine causes increases in alkaline phosphatase serum levels, and evokes inflammation, oxidative stress and necrosis in liver.90–94 Furthermore, tartrazine activates c-Jun N-terminal signaling95 and mitochondrial toxicity96 in liver. Tartrazine as well as its sulfonated metabolites act as inhibitors of sulphotransferases.93,97 Since sulphotransferases play critical roles in sulphatation and hence in secretion of bile acids, inhibition of sulphotransferases is thought to be the main trigger that eventually leads to tartrazine-associated liver toxicity.93
8. 2-Octynoic Acid and 2-Nonynoic Acid
Primary biliary cholangitis is a chronic progressive cholestatic liver disease accompanied by an anti-mitochondrial antibody response in the vast majority of patients. The auto-antigens recognized by these antibodies are members of 2-oxo-dehydrogenase complexes, particularly the E2 component of pyruvate dehydrogenase. The epitope in the latter includes a lipoyl domain. As a matter of fact, these antibodies also crossreact with a number of chemically modified mimics conjugated to this lipoyl domain,98,99 among which are 2-octynoic acid98 and 2-nonynoic acid99 (Figure 2).
2-Octynoic acid and 2-nonynoic acid are widely used in perfumes, soaps, detergents, lipsticks, toilet waters, facial creams and other perfumed cosmetics because of their violet scent. 2-octynoic acid and 2-nonynoic acid are also applied as food additives, more specifically in flavor compositions for cucumber, berry complexes, fruit blends, peach imitation as well as liqueur flavorings.98 Immunization of mice with 2-octynoic acid coupled to albumin evokes auto-immune cholangitis.100,101 Importantly, sera of patients suffering from primary biliary cholangitis present high antibody reactivity against the E2 component of pyruvate dehydrogenase coupled to 2-octynoic acid, thus underscoring human relevance.98
9. Various Synthetic Chemicals
A number of additional chemical compounds, mainly biocides, have been reported, yet less documented, to induce cholestasis. In this regard, several pesticides, including allethrin and tetramethrin, inhibit various human hepatic transporters, some of which play critical roles in bile acid homeostasis.102,103 The herbicide quizalofop-p-ethyl was found to induce cholestasis in a patient, associated with increased serum levels of alkaline phosphatase, gamma-glutamyltransferase and bilirubin as well as with histopathologically manifested inflammation.104 A recent study showed that pesticides, such as permethrin and N,N-diethyl-meta-toluamide, aggravate cholestasis in rodents.105 Yellow phosphorus, an ingredient of certain pesticide pastes and fireworks, is well known to cause hepatotoxicity. In a clinical case study, ingestion of yellow phosphorus was described to increase alkaline phosphatase, gamma-glutamyltransferase and bilirubin serum amounts. Concomitant histopathological examination of the liver revealed intrahepatic cholestasis with inflammation and hepatocyte necrosis.106
Nonylphenols are used in manufacturing anti-oxidants, lubricating oil additives, laundry and dish detergents, emulsifiers and solubilizers. They also serve as precursors for the commercially important non-ionic surfactants alkylphenol ethoxylates and nonylphenol ethoxylates, which are used in plastics, pesticides, paints, detergents and personal care products. Polyoxyethylene nonylphenol, as an ingredient of a fungicide product, has been reported to induce irreversible hepatic injury associated with intracytoplasmic and intracanalicular cholestasis in a patient. Subsequent in vitro testing showed the occurrence of necrosis in cultured human hepatocytes exposed to polyoxyethylene nonylphenol.107
Diethylhexyl phthalate, also called dioctyl phthalate or bis(2-ethylhexyl)phthalate, is used as a plasticizer in the manufacturing of items made of polyvinylchloride. It is also applied as a hydraulic fluid, as a dielectric fluid in capacitors and as a solvent in glowsticks. Recent evidence suggests cholestatic properties of diethylhexyl phthalate.108
Sunset Yellow FCF is a cosmetic, drug and food dye that has been linked to primary biliary cholangitis, occurring most frequently in postmenopausal women.89 Basic Red 51 is an oxidative and semi-permanent hair dye. In oral repeated dose toxicity studies in rodents, Basic Red 51 increased serum levels of alkaline phosphatase, gamma-glutamyltransferase and bilirubin, and induced liver cell necrosis.80
10. Conclusions and Perspectives
Over the past decades, several compounds from a broad chemical space and diverse application areas have been found to induce unexpected and/or unpredicted cholestatic effects. This calls for awareness, not only with risk assessors in governmental agencies and industry, but also in society as a whole. The present paper has reviewed relevant non-pharmaceutical and non-dietary synthetic chemicals that have been described to elicit cholestatic liver insults. For several of these compounds, observations on induction of cholestatic effects directly comes from clinical settings. For other compounds, however, cholestasis-inducing potential has been studied only in laboratory animals and/or in human-based cell cultures, and therefore may need to be verified for actual clinical relevance. Interestingly, most cholestatic compounds act by similar mechanisms, especially regarding the induction of the deteriorative cholestatic response (Table 1). This could open perspectives for the prediction of cholestatic potential of chemicals of any type and origin. Indeed, current in vitro detection of cholestatic compounds is typically based on testing single parameters, such as hepatic transporter inhibition, or on the use of single techniques, like microarrays, yet this has shown to be problematic due to poor predictivity109 or low sensitivity11. The future lies in combining biomarkers and methods that are fully anchored in the mechanistic basis of cholestatic hepatotoxicity. Furthermore, in vitro test approaches should be complemented with emerging in silico methods that computationally predict cholestatic properties by relying on chemical structure and/or physico-chemical profiles.2 It can be anticipated that full integration of these methodologies in the upcoming years will enable early and accurate detection of cholestatic chemicals. Such pragmatic strategy can also be of great value for next generation hazard identification of nanomaterials, including nanotitanium oxide, used in household materials, foods and cosmetic products, which might equally evoke cholestatic liver toxicity.110
Table 1. Induction of cholestatic stimuli and responses by industrial, biocide and cosmetic chemicals.
| Effects on transporters | Hepatocellular changes | Altered bile canaliculi dynamics | Inflammation | Oxidative stress | Endoplasmic reticulum stress | Mitochondrial toxicity | Cell death | Nuclear receptor activation | |
|---|---|---|---|---|---|---|---|---|---|
| Alpha-naphthylisocyanate | X | X | X | X | X | X | X | X | X |
| 3,5-diethoxycarbonyl-1,4-dihydrocollidine | X | X | X | X | X | X | X | X | |
| Methylenedianiline | X | X | X | X | X | ||||
| Paraquat | X | X | X | X | X | X | |||
| Triclosan | X | X | X | X | X | X | |||
| Tartrazine | X | X | X | X |
Funding sources
This work was financially supported by the grants of the Center for Alternatives to Animal Testing (CAAT) at Johns Hopkins University Baltimore-USA, the European Marie-Curie Sklodowska Action program (MSCA; Individual Fellowship 833095), the European Research Council (ERC; Starting Grant 335476), the Fund for Scientific Research-Flanders (FWO-Vlaanderen) and the University Hospital of the Vrije Universiteit Brussel-Belgium (Willy Gepts Fonds UZ-Brussel).
Biographies
Biographies
Vânia Vilas-Boas
Vânia Vilas-Boas graduated as a pharmacist (Pharm.D.) and obtained a doctoral degree in toxicology (Ph.D.) at the University of Porto-Portugal. She is current a postdoctoral researcher at Vrije Universiteit Brussel-Belgium. Her current research interest lies in the application of tridimensional in vitro models for therapeutic and (nano)toxicological studies, with specific focus on drug-induced cholestatic injury. She is the author of 18 peer-reviewed publications in international journals.
Eva Gijbels
Eva Gijbels is a doctoral student at Vrije Universiteit Brussel-Belgium. She has a background in pharmaceutical sciences and holds a master degree in drug development (Pharm.D.). Her master thesis was conducted at Columbia University in New York-USA. After her graduation, she started her doctoral thesis project to elucidate the mechanisms of drug-induced cholestasis as the basis for improved animal-free prediction of drug-induced liver injury. She published 2 review papers and 2 book chapters as first author, and co-authored 2 research publications in international peer-reviewed journals.
Axelle Cooreman
Axelle Cooreman is a doctoral student at Vrije Universiteit Brussel-Belgium. She has a background in pharmaceutical sciences and holds a master degree in pharmaceutical care (Pharm.D.). After her graduation, she started her doctoral thesis project to elucidate the role of connexin and pannexin signaling in cholestasis. She is (co-)author of 4 publications in international peer-reviewed journals.
Raf Van Campenhout
Raf Van Campenhout is a doctoral student at Vrije Universiteit Brussel-Belgium. He has a background in pharmaceutical sciences and holds a master degree in drug development (Pharm.D.). After his graduation, he started his doctoral thesis project, which is focused on the production of novel inhibitors of pannexin signaling. He is co-author of 4 publications in international peer-reviewed journals.
Emma Gustafson
Emma Gustafson graduated as a master in medical science with major in toxicology at the Karolinska Institutet-Sweden. She conduced her master thesis in the area of translational nanomedicine at Trinity College-Ireland. She currently is a doctoral student at Vrije Universiteit Brussel-Belgium. Her project aims to test the applicability of a generic strategy using in vitro and in silico tools for animal-free risk evaluation of chemicals with a focus on hepatotoxicity in a repeated exposure scenario. She co-authored 2 publications in international peer-reviewed journals.
Kaat Leroy
Kaat Leroy graduated as a master of science in biomedical sciences at Universiteit Gent-Belgium and currently is a doctoral student at Vrije Universiteit Brussel-Belgium. Her doctoral thesis project specifically addresses the role of connexin and pannexin signaling in liver cancer. This project aims to identify new biomarkers, drug targets and therapeutics for a better prognosis and treatment of liver cancer.
Mathieu Vinken
Mathieu Vinken is an associate professor affiliated to the Vrije Universiteit Brussel-Belgium. He has a background in pharmaceutical sciences (Pharm.D.), holds a doctoral degree in experimental in vitro toxicology (Ph.D.) and is a European Registered Toxicologist (E.R.T.). He is President of the European Society of Toxicology In Vitro. He is author of more than 150 publications in international peer-reviewed journals and books. He is editor of 3 books. He is associate editor of the journals Toxicology In Vitro and Archives of Toxicology as well as European editor of the journal Applied In Vitro Toxicology.
Footnotes
Author contributions
All authors have given approval to the final version of the manuscript.
References
- 1.Noor F. A shift in paradigm towards human biology-based systems for cholestatic-liver diseases. J Physiol. 2015;593:5043–5055. doi: 10.1113/JP271124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vinken M. In vitro prediction of drug-induced cholestatic liver injury: a challenge for the toxicologist. Arch Toxicol. 2018;92:1909–1912. doi: 10.1007/s00204-018-2201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Vinken M, Landesmann B, Goumenou M, et al. Development of an adverse outcome pathway from drug-mediated bile salt export pump inhibition to cholestatic liver injury. Toxicol Sci. 2013;136:97–106. doi: 10.1093/toxsci/kft177. [DOI] [PubMed] [Google Scholar]
- 4.Gijbels E, Vilas-Boas V, Deferm N, et al. Mechanisms and in vitro models of drug-induced cholestasis. Arch Toxicol. 2019 doi: 10.1007/s00204-019-02437-2. in press. [DOI] [PubMed] [Google Scholar]
- 5.Wagner M, Zollner G, Trauner M. New molecular insights into the mechanisms of cholestasis. J Hepatol. 2009;51:565–580. doi: 10.1016/j.jhep.2009.05.012. [DOI] [PubMed] [Google Scholar]
- 6.Jansen PL, Ghallab A, Vartak N, et al. The ascending pathophysiology of cholestatic liver disease. Hepatology. 2017;65:722–738. doi: 10.1002/hep.28965. [DOI] [PubMed] [Google Scholar]
- 7.Vartak N, Damle-Vartak A, Richter B, et al. Cholestasis-induced adaptive remodeling of interlobular bile ducts. Hepatology. 2016;63:951–964. doi: 10.1002/hep.28373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhamidimarri KR, Schiff E. Drug-induced cholestasis. Clin Liver Dis. 2013;17:519–531. doi: 10.1016/j.cld.2013.07.015. [DOI] [PubMed] [Google Scholar]
- 9.Parmentier C, Couttet P, Wolf A, et al. Evaluation of transcriptomic signature as a valuable tool to study drug-induced cholestasis in primary human hepatocytes. Arch Toxicol. 2017;91:2879–2893. doi: 10.1007/s00204-017-1930-0. [DOI] [PubMed] [Google Scholar]
- 10.Lee WM. Drug-induced acute liver failure. Clin Liver Dis. 2013;17:575–586. doi: 10.1016/j.cld.2013.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Van den Hof WF, Coonen ML, van Herwijnen M, et al. Classification of hepatotoxicants using HepG2 cells: A proof of principle study. Chem Res Toxicol. 2014;27:433–442. doi: 10.1021/tx4004165. [DOI] [PubMed] [Google Scholar]
- 12.Meier Y, Cavallaro M, Roos M, et al. Incidence of drug-induced liver injury in medical inpatients. Eur J Clin Pharmacol. 2005;61:135–143. doi: 10.1007/s00228-004-0888-z. [DOI] [PubMed] [Google Scholar]
- 13.Goldberg DS, Forde KA, Carbonari DM, et al. Population-representative incidence of drug-induced acute liver failure based on analysis of an integrated health care system. Gastroenterology. 2015;148:1353–1561. doi: 10.1053/j.gastro.2015.02.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yao H, Xu Y, Yin L, et al. Dioscin protects ANIT-induced intrahepatic cholestasis through regulating transporters, apoptosis and oxidative stress. Front Pharmacol. 2017;8:116. doi: 10.3389/fphar.2017.00116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhao Y, He X, Ma X, et al. Paeoniflorin ameliorates cholestasis via regulating hepatic transporters and suppressing inflammation in ANIT-fed rats. Biomed Pharmacother. 2017;89:61–68. doi: 10.1016/j.biopha.2017.02.025. [DOI] [PubMed] [Google Scholar]
- 16.Chen Z, Zhu Y, Zhao Y, et al. Serum metabolomic profiling in a rat model reveals protective function of paeoniflorin against ANIT induced cholestasis. Phytother Res. 2016;30:654–662. doi: 10.1002/ptr.5575. [DOI] [PubMed] [Google Scholar]
- 17.Joshi N, Kopec AK, Ray JL, et al. Fibrin deposition following bile duct injury limits fibrosis through an alphaMbeta2-dependent mechanism. Blood. 2016;127:2751–2762. doi: 10.1182/blood-2015-09-670703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wang T, Zhou ZX, Sun LX, et al. Resveratrol effectively attenuates α-naphthylisothiocyanate-induced acute cholestasis and liver injury through choleretic and anti-inflammatory mechanisms. Acta Pharmacol Sin. 2014;35:1527–1536. doi: 10.1038/aps.2014.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yao X, Li Y, Cheng X, et al. ER stress contributes to alpha-naphthyl isothiocyanate-induced liver injury with cholestasis in mice. Pathol Res Pract. 2016;212:560–567. doi: 10.1016/j.prp.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 20.Palmeira CM, Ferreira FM, Rolo AP, et al. Histological changes and impairment of liver mitochondrial bioenergetics after long-term treatment with alpha-naphtyl-isothiocyanate (ANIT) Toxicology. 2003;190:185–196. doi: 10.1016/s0300-483x(03)00163-x. [DOI] [PubMed] [Google Scholar]
- 21.Guo C, He L, Yao D, et al. Alpha-naphthylisothiocyanate modulates hepatobiliary transporters in sandwich cultured rat hepatocytes. Toxicol Lett. 2014;224:93–100. doi: 10.1016/j.toxlet.2013.09.019. [DOI] [PubMed] [Google Scholar]
- 22.Li X, Liu R, Yu L, et al. Alpha-naphthylisothiocyanate impairs bile acid homeostasis through AMPK-FXR pathways in rat primary hepatocytes. Toxicology. 2016;370:106–115. doi: 10.1016/j.tox.2016.09.020. [DOI] [PubMed] [Google Scholar]
- 23.Zhang A, Jia Y, Xu Q, et al. Dioscin protects against ANIT-induced cholestasis via regulating Oatps, Mrp2 and Bsep expression in rats. Toxicol Appl Pharmacol. 2016;305:127–135. doi: 10.1016/j.taap.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 24.Kan KS, Coleman R. 1-Naphthylisothiocyanate-induced permeability of hepatic tight junctions to proteins. Biochem J. 1986;238:323–328. doi: 10.1042/bj2380323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yang T, Mei H, Xu D, et al. Early indications of ANIT-induced cholestatic liver injury: alteration of hepatocyte polarization and bile acid homeostasis. Food Chem Toxicol. 2017;110:1–12. doi: 10.1016/j.fct.2017.09.051. [DOI] [PubMed] [Google Scholar]
- 26.Calvo JR, Reiter RJ, Garcia JJ, et al. Characterization of the protective effects of melatonin and related indoles against alpha-naphtylisothiocyanate-induced liver injury in rats. J Cell Biochem. 2001;80:461–470. doi: 10.1002/1097-4644(20010315)80:4<461::aid-jcb1000>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 27.Yoshino K. Scanning electron microscopy on the rat liver with alpha-naphtylisothiocyanate-induced cholestasis. Gastroenterol Jpn. 1980;15:550–563. doi: 10.1007/BF02773758. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, Ma X, Zhu Y, et al. Paeoniflorin ameliorates ANIT-induced cholestasis by activating Nrf2 through an PI3K/Akt-dependent pathway in rats. Phytother Res. 2015;29:1768–1775. doi: 10.1002/ptr.5431. [DOI] [PubMed] [Google Scholar]
- 29.Cui YJ, Aleksunes LM, Tanaka Y, et al. Compensatory induction of liver efflux transporters in response to ANIT-induced liver injury is impaired in FXR-null mice. Toxicol Sci. 2009;110:47–60. doi: 10.1093/toxsci/kfp094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gayathri AK, Padmanaban G. Biochemical effects of 3,5-diethoxycarbonyl-1,4-dihydrocollidine in mouse liver. Biochem Pharmacol. 1974;23:2713–2725. doi: 10.1016/0006-2952(74)90042-2. [DOI] [PubMed] [Google Scholar]
- 31.Jiang A, Okabe H, Popovic B, et al. Loss of Wnt secretion by macrophages promotes hepatobiliary injury after administration of 3,5-diethoxycarbonyl-1,4-dihydrocollidine diet. Am J Pathol. 2019;189:590–603. doi: 10.1016/j.ajpath.2018.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kim KH, Sung HJ, Lee WR, et al. Effects of melittin treatment in cholangitis and biliary fibrosis in a model of xenobiotic-induced cholestasis in mice. Toxins. 2015;7:3372–3387. doi: 10.3390/toxins7093372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yang R, Zhao Q, Hu DD, et al. Metabolomic analysis of cholestatic liver damage in mice. Food Chem Toxicol. 2018;120:253–260. doi: 10.1016/j.fct.2018.07.022. [DOI] [PubMed] [Google Scholar]
- 34.Fickert P, Thueringer A, Moustafa T, et al. The role of osteopontin and tumor necrosis factor alpha receptor-1 in xenobiotic-induced cholangitis and biliary fibrosis in mice. Lab Invest. 2010;90:844–852. doi: 10.1038/labinvest.2010.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nikam A, Patankar JV, Lackner C, et al. Transition between acute and chronic hepatotoxicity in mice is associated with impaired energy metabolism and induction of mitochondrial heme oxygenase-1. PLoS One. 2013;8:e66094. doi: 10.1371/journal.pone.0066094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sukhotnik I, Kuscuoglu U, Altindag B, et al. Intestinal involvement during 3,5-diethoxycarbonyl-1,4-dihydrocollidine-induced chronic liver injury in a mouse model. J Pediatr Surg. 2011;46:1495–1502. doi: 10.1016/j.jpedsurg.2011.04.007. [DOI] [PubMed] [Google Scholar]
- 37.Robin MJD, Appelman MD, Vos HR, et al. Calnexin depletion by endoplasmic reticulum stress during cholestasis inhibits the Na-taurocholate cotransporting polypeptide. Hepatol Commun. 2018;2:1550–1566. doi: 10.1002/hep4.1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaudhary K, Liedtke C, Wertenbrunch S, et al. Caspase 8 differentially controls hepatocytes and non-parenchymal liver cells during chronic cholestatic liver injury in mice. J Hepatol. 2013;59:1292–1298. doi: 10.1016/j.jhep.2013.07.026. [DOI] [PubMed] [Google Scholar]
- 39.Fickert P, Stöger U, Fuchsbichler A, et al. A new xenobiotic-induced mouse model of sclerosing cholangitis and biliary fibrosis. Am J Pathol. 2007;171:525–536. doi: 10.2353/ajpath.2007.061133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Pradhan-Sundd T, Vats R, Russell JO, et al. Dysregulated bile transporters and impaired tight junctions during chronic liver injury in mice. Gastroenterology. 2018;155:1218–1232. doi: 10.1053/j.gastro.2018.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsunoo C, Harwook TR, Arak S, et al. Cytoskeletal alterations leading to Mallory body formation in livers of mice fed 3,5-diethoxycarbonyl-1,4-dihydrocollidine. J Hepatol. 1987;5:85–97. doi: 10.1016/s0168-8278(87)80065-x. [DOI] [PubMed] [Google Scholar]
- 42.Do Luu HM, Hutter JC. Pharmacokinetic modeling of 4,4'-methylenedianiline released from reused polyurethane dialyzer potting materials. J Biomed Mater Res. 2000;53:276–286. doi: 10.1002/(sici)1097-4636(2000)53:3<276::aid-jbm13>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 43.Moore WM. Methylenedianiline. In: Mark HF, et al., editors. Kirkothmer Encyclopedia of Chemical Technology. Vol. 2. 1978. pp. 338–348. [Google Scholar]
- 44.Bastian PG. Occupational hepatitis caused by methylenedianiline. Med J Aust. 1984;141:533–535. doi: 10.5694/j.1326-5377.1984.tb132915.x. [DOI] [PubMed] [Google Scholar]
- 45.Kopelman H, Robertson MH, Sanders PG, et al. The Epping jaundice. Br Med J. 1966;1:514–516. doi: 10.1136/bmj.1.5486.514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Tillmann HL, van Pelt FN, Martz W, et al. Accidental intoxication with methylene dianiline p,p'-diaminodiphennylmethane: acute liver damage after presumed ecstasy consumption. J Toxicol Clin Toxicol. 1997;35:35–40. doi: 10.3109/15563659709001163. [DOI] [PubMed] [Google Scholar]
- 47.McGill DB, Motto JD. An industrial outbreak of toxic hepatitis due to methylenedianiline. N Engl J Med. 1974;291:278–282. doi: 10.1056/NEJM197408082910604. [DOI] [PubMed] [Google Scholar]
- 48.Kanz MF, Kaphalia L, Kaphalia BS, et al. Methylene dianiline: acute toxicity and effects on biliary function. Toxicol Appl Pharmacol. 1992;117:88–97. doi: 10.1016/0041-008x(92)90221-d. [DOI] [PubMed] [Google Scholar]
- 49.Bailie MB, Mullaney TP, Roth RA. Characterization of acute 4,4’-methylene dianiline hepatotoxicity in the rat. Environ Health Perspect. 1993;101:130–133. doi: 10.1289/ehp.93101130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kwon SB, Park JS, Yi JY, et al. Time- and dose-based gene expression profiles produced by a bile-duct-damaging chemical, 4,4’-methylene dianiline, in mouse liver in an acute phase. Toxicol Pathol. 2008;36:660–673. doi: 10.1177/0192623308320272. [DOI] [PubMed] [Google Scholar]
- 51.Santa Cruz V, Liu H, Kaphalia L, et al. Effects of methylenedianiline on tight junction permeability of biliary epithelial cells in vivo and in vitro. Toxicol Lett. 2007;169:13–25. doi: 10.1016/j.toxlet.2006.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Santa Cruz V, Dugas TR, Kanz MF. Mitochondrial dysfunction occurs before transport or tight junction deficits in biliary epithelial cells exposed to bile from methylenedianiline-treated rats. Toxicol Sci. 2005;84:129–138. doi: 10.1093/toxsci/kfi061. [DOI] [PubMed] [Google Scholar]
- 53.Rao MS, Van Vleet TR, Ciurlionis R, et al. Comparison of RNA-Seq and microarray gene expression platforms for the toxicogenomic evaluation of liver from short-term rat toxicity studies. Front Genet. 2018;9:636. doi: 10.3389/fgene.2018.00636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun B, Chen YG. Advances in the mechanism of paraquat-induced pulmonary injury. Eur Rev Med Pharmacol Sci. 2016;20:1597–1602. [PubMed] [Google Scholar]
- 55.Dinis-Oliveira RJ, de Pinho PG, Santos L, et al. Postmortem analyses unveil the poor efficacy of decontamination, anti-inflammatory and immunosuppressive therapies in paraquat human intoxications. PLoS One. 2009;4:e7149. doi: 10.1371/journal.pone.0007149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bataller R, Bragulat E, Nogué S, et al. Prolonged cholestasis after acute paraquat poisoning through skin absorption. Am J Gastroenterol. 2000;95:1340–1343. doi: 10.1111/j.1572-0241.2000.02021.x. [DOI] [PubMed] [Google Scholar]
- 57.Matsumoto T, Matsumori H, Kuwabara N, et al. A histopathological study of the liver in paraquat poisoning: an analysis of fourteen autopsy cases with emphasis on bile duct injury. Acta Pathol Jpn. 1980;30:859–870. doi: 10.1111/j.1440-1827.1980.tb03276.x. [DOI] [PubMed] [Google Scholar]
- 58.Mullick FG, Ishak KG, Mahabir R. Hepatic injury associated with paraquat toxicity in humans. Liver. 1981;1:209–221. doi: 10.1111/j.1600-0676.1981.tb00035.x. [DOI] [PubMed] [Google Scholar]
- 59.Muthu V, Das A, Bal A, et al. Severe cholestasis and hepatic dysfunction in a case of fatal paraquat poisoning. Clin Res Hepatol Gastroenterol. 2015;39:e7–9. doi: 10.1016/j.clinre.2014.07.013. [DOI] [PubMed] [Google Scholar]
- 60.Soontornniyomkij V, Bunyaratvej S. Fatal paraquat poisoning: a light microscopic study in eight autopsy cases. J Med Assoc Thai. 1992;1:98–105. [PubMed] [Google Scholar]
- 61.Takegoshi K, Nakanuma Y, Ohta M, et al. Light and electron microscopic study of the liver in paraquat poisoning. Liver. 1988;8:330–336. doi: 10.1111/j.1600-0676.1988.tb01012.x. [DOI] [PubMed] [Google Scholar]
- 62.Ahmad L, Shukla S, Kumar A, et al. Biochemical and molecular mechanisms of N-acetyl cysteine and silymarin-mediated protection against maneb- and paraquat-induced hepatotoxicity in rats. Chem Biol Interact. 2013;201:9–18. doi: 10.1016/j.cbi.2012.10.027. [DOI] [PubMed] [Google Scholar]
- 63.Atashpour S, Jahromi HK, Jahromi ZK, et al. Antioxidant effects of aqueous extraction of alep on paraquat-induced rat liver injury. World J Hepatol. 2017;9:209–2016. doi: 10.4254/wjh.v9.i4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Cao L, Waldon D, Teffera Y, et al. Ratios of biliary glutathione disulfide (GSSG) to glutathione (GSH): a potential index to screen drug-induced hepatic oxidative stress in rat and mice. Anal Bioanalytical Chem. 2013;405:2635–2642. doi: 10.1007/s00216-012-6661-8. [DOI] [PubMed] [Google Scholar]
- 65.Madhu C, Gregus Z, Cheng CC, et al. Identification of the mixed disulfide of glutathione and cysteinylglycine in bile: dependence on gamma-glutamyl transferase and responsiveness to oxidative stress. J Pharmacol Exp Ther. 1992;262:896–900. [PubMed] [Google Scholar]
- 66.Han J, Zhang Z, Yang S, et al. Betanin attenuates paraquat-induced liver toxicity through a mitochondrial pathway. Food Chem Toxicol. 2014;70:100–106. doi: 10.1016/j.fct.2014.04.038. [DOI] [PubMed] [Google Scholar]
- 67.Mohammadi-Bardbori A, Ghazi-Khansari M. Comparative measurement of cyanide and paraquat mitochondrial toxicity using two different mitochondrial toxicity assays. Toxicol Mech Methods. 2007;17:87–91. doi: 10.1080/15376510600822664. [DOI] [PubMed] [Google Scholar]
- 68.Vadnay I. Hepatic alterations in experiment paraquat intoxication. Exp Toxicol Pathol. 1993;45:355–364. doi: 10.1016/S0940-2993(11)80428-8. [DOI] [PubMed] [Google Scholar]
- 69.Barabás K, Szabó L, Matkovics B, et al. The effect of light on the toxicity of paraquat in the mouse. Gen Pharmacol. 1986;17:359–362. doi: 10.1016/0306-3623(86)90055-8. [DOI] [PubMed] [Google Scholar]
- 70.Cappelletti G, Incani C, Maci R. Paraquat induces irreversible actin cytoskeleton disruption in cultured human lung cells. Cell Biol Toxicol. 1994;4:255–263. doi: 10.1007/BF00756765. [DOI] [PubMed] [Google Scholar]
- 71.Hu X, Shen H, Wang Y, et al. Liver X receptor agonist TO901317 attenuates paraquat-induced acute lung injury through inhibition of NF-κB and JNK/p38 MAPK signal pathways. Biomed Res Int. 2017;2017 doi: 10.1155/2017/4652695. 4652695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Chen T, Wang R, Jiang W, et al. Protective effect of astragaloside IV against paraquat-induced lung injury in mice by suppressing rho signaling. Inflammation. 2016;39:483–492. doi: 10.1007/s10753-015-0272-4. [DOI] [PubMed] [Google Scholar]
- 73.Olaniyan LW, Mkwetshana N, Okoh AI. Triclosan in water, implications for human and environmental health. Springerplus. 2016;5:1639. doi: 10.1186/s40064-016-3287-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Dhillon GS, Kaur S, Pulicharla R, et al. Triclosan: current status, occurrence, environmental risks and bioaccumulation potential. Int J Environ Res Public Health. 2015;12:5657–5684. doi: 10.3390/ijerph120505657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Chai L, Chen A, Luo P, et al. Histopathological changes and lipid metabolism in the liver of Bufo gargarizans tadpoles exposed to triclosan. Chemosphere. 2017;182:255–266. doi: 10.1016/j.chemosphere.2017.05.040. [DOI] [PubMed] [Google Scholar]
- 76.Regnault C, Willison J, Veyrenc S, et al. Metabolic and immune impairments induced by the endocrine disruptors benzo[a]pyrene and triclosan in Xenopus tropicalis. Chemosphere. 2016;155:519–527. doi: 10.1016/j.chemosphere.2016.04.047. [DOI] [PubMed] [Google Scholar]
- 77.Haggard DE, Noyes PD, Waters KM, et al. Phenotypically anchored transcriptome profiling of developmental exposure to the antimicrobial agent, triclosan, reveals hepatotoxicity in embryonic zebrafish. Toxicol Appl Pharmacol. 2016;308:32–45. doi: 10.1016/j.taap.2016.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jacobs MN, Nolan GT, Hood SR. Lignans, bacteriocides and organochlorine compounds activate the human pregnane X receptor (PXR) Toxicol Appl Pharmacol. 2005;209:123–133. doi: 10.1016/j.taap.2005.03.015. [DOI] [PubMed] [Google Scholar]
- 79.Yueh MF, Taniguchi K, Chen S, et al. The commonly used antimicrobial additive triclosan is a liver tumor promoter. Proc Natl Acad Sci USA. 2014;111:17200–17205. doi: 10.1073/pnas.1419119111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Vinken M, Pauwels M, Ates G, et al. Screening of repeated dose toxicity data present in SCC(NF)P/SCCS safety evaluations of cosmetic ingredients. Arch Toxicol. 2012;86:405–412. doi: 10.1007/s00204-011-0769-z. [DOI] [PubMed] [Google Scholar]
- 81.Wang F, Xu R, Zheng F, et al. Effects of triclosan on acute toxicity, genetic toxicity and oxidative stress in goldfish (Carassius auratus) Exp Anim. 2018;67:219–227. doi: 10.1538/expanim.17-0101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Newton AP, Cadena SM, Rocha ME, et al. Effect of triclosan (TRN) on energy-linked functions of rat liver mitochondria. Toxicol Lett. 2005;160:49–59. doi: 10.1016/j.toxlet.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 83.Teplova VV, Belosludtsev KN, Kruglov AG. Mechanism of triclosan toxicity: mitochondrial dysfunction including complex II inhibition, superoxide release and uncoupling of oxidative phosphorylation. Toxicol Lett. 2017;275:108–117. doi: 10.1016/j.toxlet.2017.05.004. [DOI] [PubMed] [Google Scholar]
- 84.Wang L, Mao B, He H, et al. Comparison of hepatotoxicity and mechanisms induced by triclosan (TCS) and methyl-triclosan (MTCS) in human liver hepatocellular HepG2 cells. Toxicol Res. 2018;8:38–45. doi: 10.1039/c8tx00199e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Palmer RK, Hutchinson LM, Burpee BT, et al. Antibacterial agent triclosan suppresses RBL-2H3 mast cell function. Toxicol Appl Pharmacol. 2012;258:99–108. doi: 10.1016/j.taap.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 86.Winitthana T, Lawanprasert S, Chanvorachote P. Triclosan potentiates epithelial-to-mesenchymal transition in anoikis-resistant human lung cancer cells. PLoS One. 2014;9:e110851. doi: 10.1371/journal.pone.0110851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wu Y, Beland FA, Chen S, et al. Extracellular signal-regulated kinases 1/2 and Akt contribute to triclosan-stimulated proliferation of JB6 Cl 41-5a cells. Arch Toxicol. 2015;89:1297–1311. doi: 10.1007/s00204-014-1308-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rovina K, Siddiquee S, Shaarani SM. A review of extraction and analytical methods for the determination of tartrazine (E 102) in foodstuffs. Crit Rev Anal Chem. 2017;47:309–324. doi: 10.1080/10408347.2017.1287558. [DOI] [PubMed] [Google Scholar]
- 89.Axon A, May FE, Gaughan LE, et al. Tartrazine and sunset yellow are xenoestrogens in a new screening assay to identify modulators of human oestrogen receptor transcriptional activity. Toxicology. 2012;298:40–51. doi: 10.1016/j.tox.2012.04.014. [DOI] [PubMed] [Google Scholar]
- 90.Amin KA, Abdel Hameid H, Abd Elsttar AH. Effect of food azo dyes tartrazine and carmoisine on biochemical parameters related to renal, hepatic function and oxidative stress biomarkers in young male rats. Food Chem Toxicol. 2010;48:2994–2999. doi: 10.1016/j.fct.2010.07.039. [DOI] [PubMed] [Google Scholar]
- 91.El-Desoky GE, Abdel-Ghaffar A, Al-Othman ZA, et al. Curcumin protects against tartrazine-mediated oxidative stress and hepatotoxicity in male rats. Eur Rev Med Pharmacol Sci. 2017;21:635–645. [PubMed] [Google Scholar]
- 92.Khayyat L, Essawy A, Sorour J, et al. Tartrazine induces structural and functional aberrations and genotoxic effects in vivo. PeerJ. 2017;5:e3041. doi: 10.7717/peerj.3041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Meyer SK, Probert PME, Lakey AF, et al. Hepatic effects of tartrazine (E 102) after systemic exposure are independent of oestrogen receptor interactions in the mouse. Toxicol Lett. 2017;273:55–68. doi: 10.1016/j.toxlet.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Saxena B, Sharma S. Serological changes induced by blend of sunset yellow, metanil yellow and tartrazine in swiss albino rat, rattus norvegicus. Toxicol Int. 2014;21:65–68. doi: 10.4103/0971-6580.128798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Raposa B, Pónusz R, Gerencsér G, et al. Food additives: sodium benzoate, potassium sorbate, azorubine, and tartrazine modify the expression of NFκB, GADD45α, and MAPK8 genes. Physiol Int. 2016;103:334–343. doi: 10.1556/2060.103.2016.3.6. [DOI] [PubMed] [Google Scholar]
- 96.Reyes FG, Valim MF, Vercesi AE. Effect of organic synthetic food colours on mitochondrial respiration. Food Addit Contam. 1996;13:5–11. doi: 10.1080/02652039609374376. [DOI] [PubMed] [Google Scholar]
- 97.Bamforth KJ, Jones AL, Roberts RC, et al. Common food additives are potent inhibitors of human liver 17 alpha-ethinyloestradiol and dopamine sulphotransferases. Biochem Pharmacol. 1993;46:1713–1720. doi: 10.1016/0006-2952(93)90575-h. [DOI] [PubMed] [Google Scholar]
- 98.Amano K, Leung PS, Rieger R, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174:5874–5883. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 99.Rieger R, Leung PS, Jeddeloh MR, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27:7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 100.Wakabayashi K, Lian ZX, Leung PS, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48:531–540. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wakabayashi K, Yoshida K, Leung PS, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD).1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155:577–586. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Chedik L, Bruyere A, Le Vee M, et al. Inhibition of human drug transporter activities by the pyrethroid pesticides allethrin and tetramethrin. PLoS One. 2017;12:e0169480. doi: 10.1371/journal.pone.0169480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Fardel O, Kolasa E, Le Vee M. Environmental chemicals as substrates, inhibitors or inducers of drug transporters: implication for toxicokinetics, toxicity and pharmacokinetics. Expert Opin Drug Metab Toxicol. 2012;8:29–46. doi: 10.1517/17425255.2012.637918. [DOI] [PubMed] [Google Scholar]
- 104.Elefsiniotis IS, Liatsos GD, Stamelakis D, et al. Case report: mixed cholestatic/hepatocellular liver injury induced by the herbicide quizalofop-p-ethyl. Environ Health Perspect. 2007;115:1479–1481. doi: 10.1289/ehp.9968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Petrescu AD, Grant S, Frampton G, et al. Gulf war illness-related chemicals increase CD11b/c+ monocyte infiltration into the liver and aggravate hepatic cholestasis in a rodent model. Sci Rep. 2018;8 doi: 10.1038/s41598-018-31599-9. 13147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Lakshmi CP, Goel A, Basu D. Cholestatic presentation of yellow phosphorus poisoning. J Pharmacol Pharmacother. 2014;5:67–69. doi: 10.4103/0976-500X.124430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Min J, Han J, Kim K, et al. Human cholestatic hepatitis owing to polyoxyethylene nonylphenol ingestion. Medicine. 2017;96:e7737. doi: 10.1097/MD.0000000000007737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Gaitantzi H, Hakenberg P, Theobald J, et al. Di (2-ethylhexyl) phthalate and its role in developing cholestasis: an in vitro study on different liver cell types. J Pediatr Gastroenterol Nutr. 2018;66:e28–e35. doi: 10.1097/MPG.0000000000001813. [DOI] [PubMed] [Google Scholar]
- 109.Ali I, Welch MA, Lu Y, et al. Identification of novel MRP3 inhibitors based on computational models and validation using an in vitro membrane vesicle assay. Eur J Pharm Sci. 2017;103:52–59. doi: 10.1016/j.ejps.2017.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Yang Y, Niu S, Han D, et al. Progress in developing metal oxide nanomaterials for photoelectrochemical water splitting. Adv Energy Mater. 2017;7 1700555. [Google Scholar]