Abstract
CCRL2 is a non-signaling seven-transmembrane domain receptor. CCRL2 binds chemerin, a protein that promotes chemotaxis of selected leukocyte subsets including NK cells. In addition, CCRL2 controls the inflammatory response in different pathological settings. Here we investigated the role of CCRL2 in the regulation of cancer-related inflammation focusing on models of lung cancer. The genetic deletion of CCRL2 promoted tumor progression in urethane-induced and in KrasG12D/+/p53LoxP lung tumor models. Similarly, CCRL2-deficient mice have enhanced growth of a kras mutant lung tumor cell line. This phenotype was associated with impaired recruitment of NK cells to the lung. Bone marrow chimeras highlighted the role of CCRL2 expression by non-hematopoietic cells. In human and mouse lung, CCRL2 is expressed by a fraction of CD31+ endothelial but not by NK cells. Elevated CCRL2 expression in biopsies from human lung adenocarcinoma positively correlated with clinical outcome. These results provide evidence for a crucial role of CCRL2 in NK cell-dependent resistance against lung tumor growth.
Introduction
Lung cancer is the leading cause of cancer-related deaths worldwide, with non-small cell lung carcinoma (NSCLC) being approximately 85% of all lung cancers (Reck and Rabe, 2017; Siegel et al., 2017). Lung adenocarcinoma (LUAD) and squamous cell carcinoma (LUSC) are the most common NSCLC histological subsets (Molina et al., 2008; Travis et al., 2013). NSCLC subtypes are associated with several genetic alterations, such as activating mutations of EGFR or KRAS and loss-of-function mutations, like TP53 (Gridelli et al., 2015). Growing evidence clearly shows that in addition to the intrinsic properties of cancer cells, the tumor microenvironment (TME) plays a relevant role in the definition of tumor phenotype. Indeed, cancer-related inflammation is considered a key aspect of tumor growth and dissemination (Mantovani et al., 2008; Ribatti, 2017). Chemokines and related chemotactic factors are responsible for leukocyte tumor infiltration and control several aspects of tumor biology, including angiogenesis, cancer cell proliferation and migration (Balkwill, 2004; Del Prete et al., 2017b). In tumors, chemokine expression is often dysregulated by cancer-associated genetic alterations (Mantovani et al., 2008).
Chemotactic factors bind seven-transmembrane G protein-coupled receptors and promote directional cell migration through the induction of a cascade of intracellular signaling events. Chemotactic proteins also bind a subset of receptors referred to as Atypical Chemokine Receptors (ACKRs) which lack chemotactic activity and are believed to control inflammation through their ligand scavenging functions (Bachelerie et al., 2014a; Bachelerie et al., 2014b). ACKRs play a role in inflammation and in tumor biology, being able to either promote or limit tumor growth and dissemination (Bachelerie et al., 2014b; Massara et al., 2016).
CCRL2 is a 7-transmembrane protein, closely related to chemokine receptors (e.g. CCR5, CCR2, CX3CR1, CCR3 and CCR8) that share many characteristics with the ACKRs, including the lack of certain consensus sequences and the inability to induce functional responses (Bachelerie et al., 2014a; Del Prete et al., 2013). CCRL2 is expressed by a large variety of leukocyte subsets, including activated monocyte/macrophages, neutrophils, dendritic cells, lymphocytes, mast cells, CD34+ precursor cells and by barrier cells, such as vascular and lymphatic endothelium and some epithelium (Catusse et al., 2010; Del Prete et al., 2017a; Gonzalvo-Feo et al., 2014; Mazzon et al., 2016; Migeotte et al., 2002; Monnier et al., 2012; Oostendorp et al., 2004; Otero et al., 2010; Yoshimura and Oppenheim, 2011; Zabel et al., 2008). CCRL2 binds chemerin, a non-chemokine chemotactic protein (Zabel et al., 2008), and unlike other ACKRs, it does not bind chemokines and is devoid of ligand scavenging functions (De Henau et al., 2016; Mazzotti et al., 2017). Rather, CCRL2 functions as a chemerin presenting molecule on the surface of endothelial cells (Gonzalvo-Feo et al., 2014; Monnier et al., 2012) and in leukocytes, it can regulate the function of chemokine receptors, such as CXCR2 (Del Prete et al., 2017a). Through these functions, CCRL2 was shown to tune the inflammatory response in different pathological settings, such as hypersensitivity, inflammatory arthritis and experimental autoimmune encephalitis (Del Prete et al., 2017a; Mazzon et al., 2016; Otero et al., 2010; Zabel et al., 2008).
The present study was performed to investigate the possible role of CCRL2 in the regulation of host defence cells in the TME. To test this hypothesis, the genetic mouse model of KrasG12D/+; p53LoxP (TK) mice, the urethane chemically-induced model and the transplantable LG1233 cell line were used as experimental models of lung cancer with molecular and histopathological similarities with human Kras-driven lung carcinomas, such as LUAD. The results here reported reveal a crucial role of CCRL2 in the orchestration of an NK cell-dependent control of tumor development and progression.
Results and Discussion
CCRL2 deficiency increased tumor burden in urethane-induced carcinogenesis
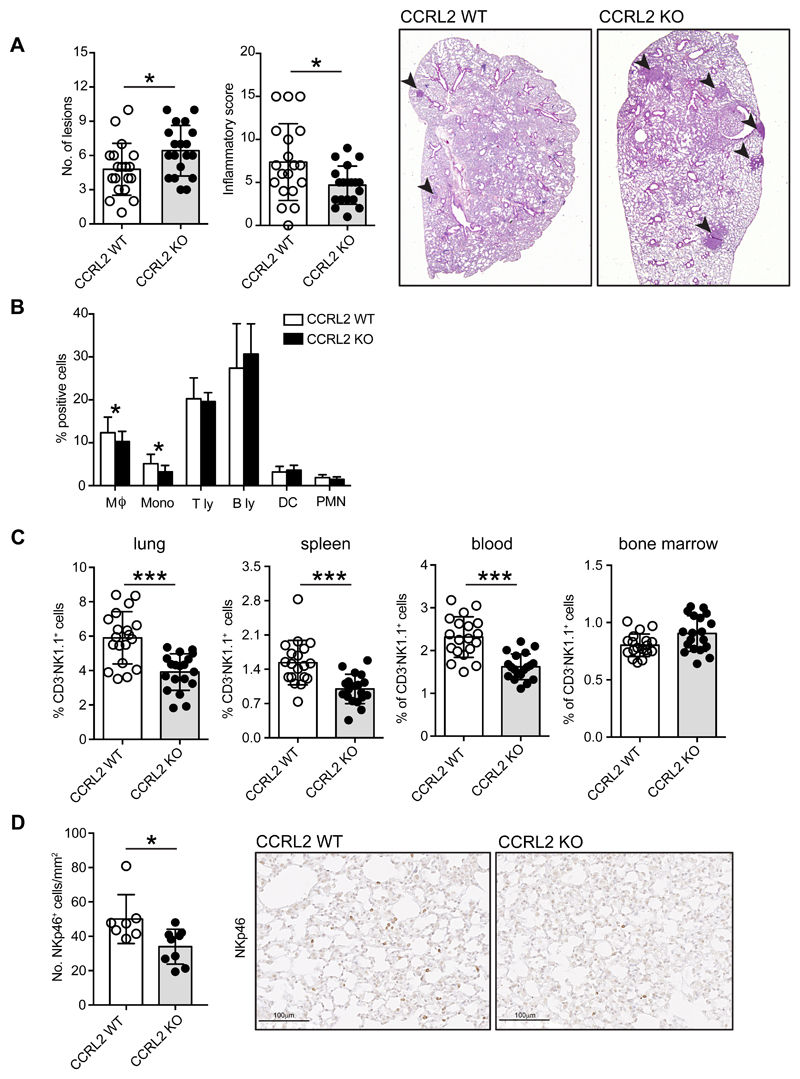
Urethane-induced tumors are known to recapitulate many aspects of human lung cancer including some gene mutations and histological features (Miller et al., 2003). CCRL2 WT and CCRL2 deficient mice were weekly administered i.p. with urethane (1 mg/g) for a total of ten doses, and sacrificed 30 weeks later, as previously described (Miller et al., 2003). The histological analysis of murine lungs showed a significant increase of tumor multiplicity in CCRL2 deficient mice compared to control animals (6.4 ± 0.5 vs. 4.8 ± 0.5; p=0.03) (Figure 1A). The increased tumor burden was associated with the reduction of total inflammatory infiltrate, including interstitial perivascular and peribronchiolar lymphoid infiltrates and aggregates, as evaluated by histological analysis (Figure 1A). Characterization of the lung tumor microenvironment, performed by FACS analysis, revealed that CCRL2 deficiency was not associated with a gross change in the frequency of infiltrating leukocytes, although the frequency of some myeloid cells (i.e. monocytes and macrophages) was decreased, whereas the distribution of dendritic cells, T and B lymphocytes was not affected (Figure 1B). Neutrophils, a leukocyte subset known to be associated with lung tumor growth (Eruslanov et al., 2014) and to be regulated by CCRL2, (Del Prete et al., 2017a) were also not affected, with only a tendency to be reduced in CCRL2 deficient mice (Figure 1B and Figure S1). The leukocyte subset that resulted most consistently reduced in the lung of CCRL2 deficient mice was NK cells. This reduction could be documented both by FACS analysis (Figure 1C; p<0.0001) and by immunohistochemistry in histological sections using an anti-NKp46 (Figure1D; p<0.05). NK cell frequency was severely impaired not only in the lung, but also in the spleen and in the blood compartments of tumor-bearing CCRL2 deficient mice. Conversely, no difference was found in the number of NK cells in the bone marrow of CCRL2 deficient mice (Figure 1C). Of note, no difference in NK cell distribution was observed between healthy WT and CCRL2-deficient mice (data not shown). NK cells are the main innate lymphoid subsets that mediate anti-tumor immune response (Cerwenka and Lanier, 2016; Chiossone et al., 2018; Hayakawa and Smyth, 2006; Waldhauer and Steinle, 2008). A defect of cytotoxic activity of human circulating NK was correlated with increased cancer risk (Imai et al., 2000) and growing evidence proposes a role for NK cells in the control of hematological malignancies (Gismondi et al., 2015). Conversely, the ability of NK cells to control solid tumor progression has only recently becoming recognized (Malmberg et al., 2017; Stojanovic and Cerwenka, 2011) Molgora et al, 2017;Barry et al, 2018). Taking together our results support a protective role of CCRL2 in urethane-induced lung carcinogenesis through its ability to regulate the inflammatory tumor microenvironment.
Figure 1. CCRL2 deficiency increases tumor burden in urethane-induced carcinogenesis.
(A) Number of neoplastic lesions and inflammatory score at the time of sacrifice (30 weeks after the first urethane injection) of CCRL2 WT and CCRL2 deficient (KO) mice. Evaluation was performed microscopically on histological preparations of the lungs. Grading of inflammatory infiltrate: 0=absence of lymphoid infiltrates/aggregates; 1=1-2 foci of interstitial perivascular/peribronchiolar lymphoid infiltrates/aggregates; 2=3-5 foci, 3=>5 foci. *p<0.05, CCRL2 WT (n=19) vs. CCRL2 KO (n=19) by Mann-Whitney test. Representative H&E images of CCRL2 WT and CCRL2 KO lung sections show the presence of neoplastic lesions (indicated with arrowheads). Magnification 12.5x. (B) Analysis of the inflammatory infiltrate by FACS. Single cell suspension was obtained by lung mechanical and enzymatic treatment, the percentage of each leukocyte subpopulation was evaluated within the CD45+ positive population. *p<0.05, CCRL2 WT (n=19) vs. CCRL2 KO (n=19) by Student t-test. (C) NK cell presence in the different organs of CCRL2 WT and CCRL2 KO tumor-bearing mice by FACS analysis. The percentage of CD3-NK1.1+ cells in the CD45+ population was evaluated in lung, spleen, blood and bone marrow. *** p<0.001, CCRL2 WT (n=19) vs. CCRL2 KO (n=19) by Student t-test. (D) Histological evaluation of NKp46+ NK cells, quantified as number of positive cells (left panel) evaluated in 4 random 10x microscopic fields selected throughout the lung parenchyma; right panels are representative sections stained with the anti-NKp46 antibody. *p<0.05, CCRL2 WT (n=7) vs. CCRL2 KO (n=9) by Mann-Whitney test.
CCRL2 deficiency promotes tumor progression in a genetic model of Kras mutated lung tumor
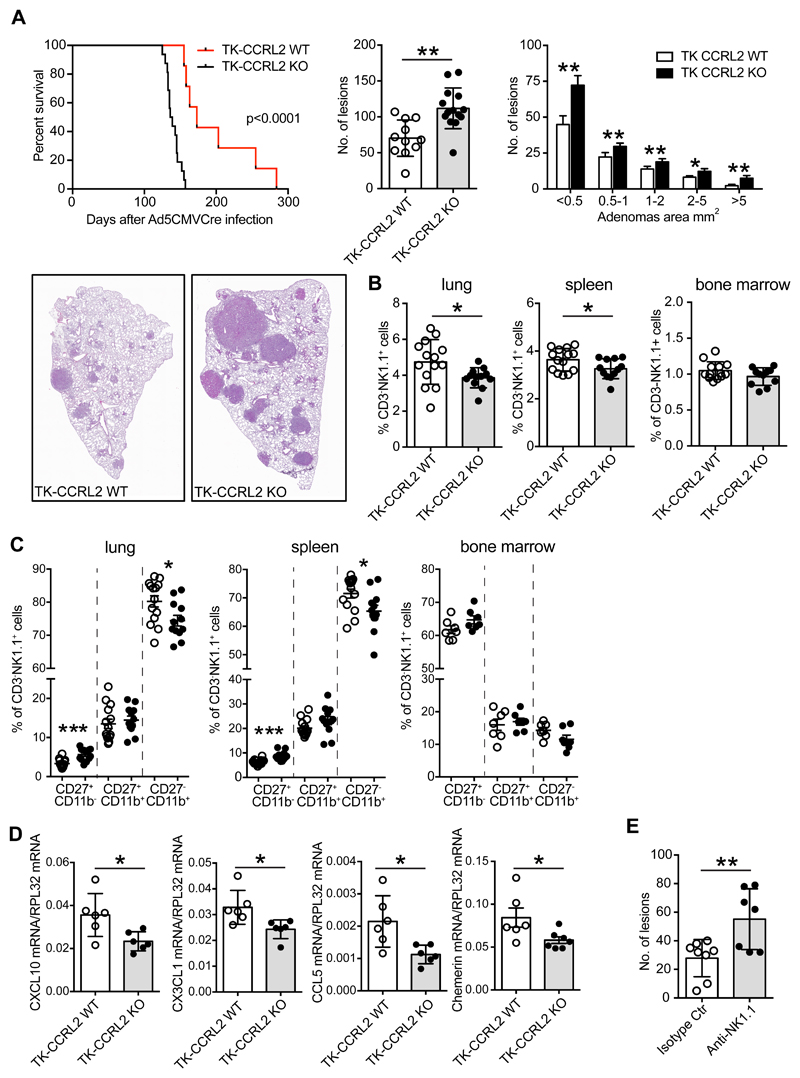
Since urethane-induced lung carcinogenesis is associated with Kras mutations (Dwyer-Nield et al., 2010; Miller et al., 2003), the role of CCRL2 in Kras mutation-dependent lung cancer was further investigated crossing KrasG12D/+; p53LoxP (TK) with CCRL2 deficient mice; carcinogenesis was induced by intranasal delivery of replication-deficient adenoviral vector with Cytomegalovirus promoter driving the expression of the Cre recombinase protein (Ad5CMVCre) as previously described (DuPage et al., 2009). The survival of TK-CCRL2 deficient mice was dramatically reduced compared to TK-CCRL2 WT mice (median survival, 173.0 days vs. 137.5 days, respectively; p<0.0001) with the lungs of TK-CCRL2 deficient mice showing an increased number and larger size of tumor lesions compared to WT animals (Figure 2A). Multiparametric flow cytometry of tumor microenvironment confirmed NK cells as the main cell population affected by CCRL2 deficiency. Figure 2B shows that a statistically significant reduction of CD3-NK1.1+ cells was detected in the lung and in the spleen of TK-CCRL2 deficient mice, while no difference was observed in bone marrow. Immunohistochemistry performed on TK-CCRL2 deficient mice confirmed the reduction of lung NK cells evaluated as NKp46+ cells (Figure S2 A). Independently of their relative frequency, the distribution of NK cells was found similar in TK-CCRL2 WT and KO mice, being mostly confined to the peritumoral areas (invasive margins), with very few NKp46+ cells present within the tumor lesions (Figure S2 A), a distribution pattern similar to that previously reported in human lung cancer (Platonova et al., 2011). NK cell subsets were evaluated by the reciprocal expression of surface markers CD27 and CD11b, with CD27+CD11b- cells being the most immature subset, double positive cells (CD27+CD11b+) at an intermediated stage of maturation, and CD27-CD11b+ cells the most mature subset (Abel et al., 2018). Figure 2C shows that TK-CCRL2 deficient mice have an altered distribution of NK cell subsets both in the lung and spleen with a decrease in the frequency of the most mature CD27-CD11b+ population and the increase of CD27+CD11b- NK cells. No difference was observed in NK subset distribution in the bone marrow, suggesting that CCRL2 has a role in the recruitment of NK cell subsets to peripheral organs rather than in their functional maturation. This hypothesis is supported by the reduced expression of the NK cell chemotactic proteins CXCL10, CX3CL1, CCL5 and chemerin, in the lungs of tumor bearing mice (Figure 2D). These chemotactic factors were expressed in a similar manner in the lungs of normal TK-CCRL2 deficient and TK-CCRL2 WT mice (data not shown). Tumor growth caused a significant reduction of CCL5, CX3CL1 and chemerin mRNA expression in both TK-WT and TK-CCRL2 deficient mice, although the reduction was more marked in TK-CCRL2 deficient compared WT mice.
Figure 2. CCRL2 deficiency promotes tumor progression in KrasG12D/+;p53LoxP (TK) mice.
(A) Left panel indicates percent survival (median survival, TK-CCRL2 WT 173.0 days vs. TK-CCRL2 KO 137.5 days, respectively; p<0.0001) after intranasal inoculation of 2.5x107 infectious particles of Ad5CMVCre recombinase. Central and right panels indicate the number and dimension of lesions 10 weeks after Ad5CMVCre infection. *p<0.05, **p<0.01 TK-CCRL2 WT (n=11) vs. TK-CCRL2 KO (n=14), Mann-Whitney test. Representative histological sections from TK-CCRL2 WT and TK-CCRL2 KO mice are presented. Magnification 10x. (B) The percentage of CD3-NK1.1+ cells in the CD45+ cells evaluated in lung, spleen, and bone marrow, 10 weeks after Ad5CMVCre recombinase infection. *p<0.05, TK-CCRL2 WT (n=14 lung and spleen, n=13 bone marrow) vs. TK-CCRL2 KO (n=12 lung and spleen, n=11 bone marrow) by Student t-test. (C) FACS analysis of the NK main subsets, CD27+CD11b-, CD27+CD11b+, CD27-CD11b+ evaluated CD3-NK1.1+ gated cells from lung, spleen and bone marrow. *p<0.05, ***p<0.001TK-CCRL2 WT (n=14 lung and spleen, n=7 bone marrow) vs. TK CCRL2 KO (n=12 lung and spleen, n=7 bone marrow) by Student t-test. (D) Evaluation of the NK chemotactic factors CXCL10, CX3CL1, CCL5, Chemerin mRNA levels by qPCR from lung tissue. *p<0.05, TK-CCRL2 WT (n=6) vs. TK-CCRL2 KO (n=6) by Student t-test. (E) Number of lung lesions in TK-CCRL2 WT mice treated with anti-NK1.1 moAb or isotype control moAb (100μg per mouse i.p. 1 day before Ad5CMVCre infection and then once a week for the duration of the experiment). **p<0.01, anti-NK1.1 moAb (n=8) vs. Isotype moAb (n=7) by Mann-Whitney test.
To investigate the possible role of NK cells in Kras/Tp53-induced lung cancer, NK cells were depleted in TK-CCRL2 WT mice. The administration of an anti-NK1.1 moAb during tumor progression, efficiently depleted NK cells in the lung, spleen and blood compartments (Figure S2 B) and significantly increased the number of tumor lesions, recapitulating the phenotype of TK-CCRL2-deficient mice (Figure 2E; p<0.01). Taking together, these results demonstrate that NK cells represent a non-redundant effector mechanism in the control of Kras/Tp53-dependend lung tumors and that CCRL2 plays a crucial role in NK cell-mediated immune-surveillance. Of note, NK cells from TK-CCRL2 WT naïve or tumor-bearing mice did not express membrane CCRL2 at the time of purification or after overnight stimulation with the activating cytokines IL-12 and IL-15 (Figure S2 C). In addition, CCRL2 deficiency did not affect NK cell membrane phenotype and function, such as the expression of chemotactic receptors (e.g. CKMLR1, CXCR3, CX3CR1 and CCR5), effector proteins (i.e. IFNγ, Granzyme B and Perforin), immune checkpoint molecules (i.e. PD-1, PDL-1, PDL-2 and LAG3), cytotoxic and chemotactic activity, as evaluated in sorted NK cells from lung and spleen (Figure S3 A and B and data not shown).
CCRL2 deficiency promotes the orthotopic growth of LG1233 cells
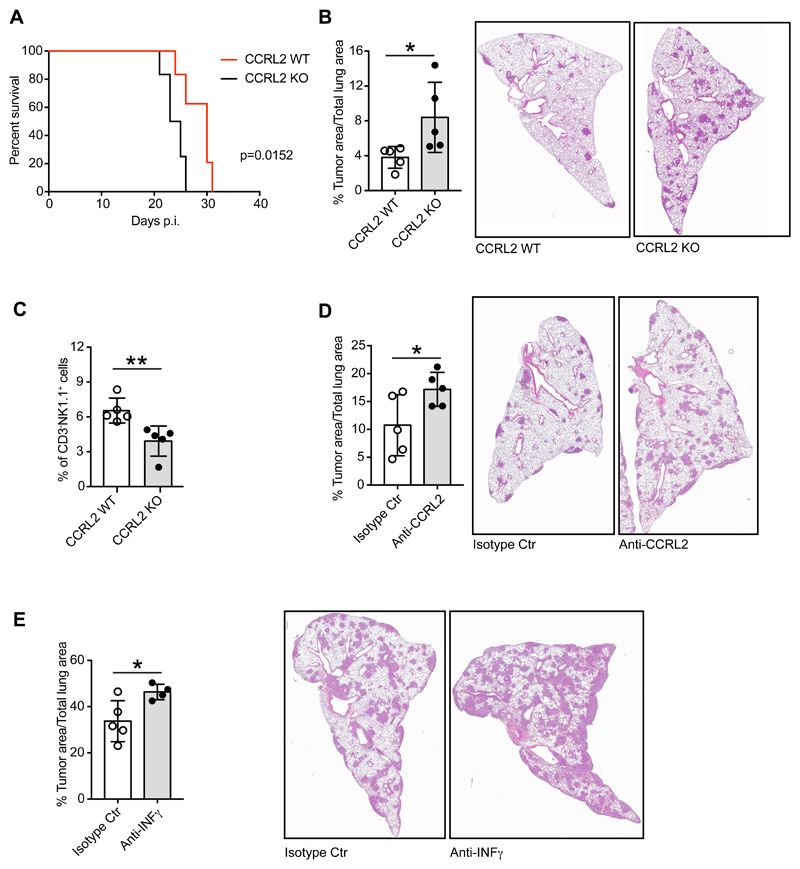
The role of CCRL2 in tumor progression was further investigated by the administration of LG1233 cells, a cell line derived from TK-mice (Caronni et al., 2018; Dimitrova et al., 2016). CCRL2-deficient mice, intravenously injected with LG1233 cells, showed significant reduction of the survival, compared with CCRL2 WT animals, that was associated with increased tumor burden, as evaluated by histological analysis of lung sections at 13 days post-inoculum (Figure 3A and B). Since no difference in tumor load was detectable at earlier time points (e.g. 7 days post-inoculum; data not shown) it is likely that the difference in tumor development is related to the effect of CCRL2 on tumor growth rather than on tumor seeding of injected LG1233 cells. Also in this experimental model, CCRL2-deficient mice showed a reduced frequency of lung CD3-NK1.1+ NK cells confirming the crucial role of CCRL2 in NK cell-dependent immune surveillance in lung tumor growth (Figure 3C). As previously observed (Del Prete et al., 2017a), also in this experimental model the administration of an anti-CCRL2 moAb recapitulated the CCRL2-deficient phenotype further confirming the pivotal role of CCRL2 in lung tumor growth (Figure 3D). Similarly, the administration of a neutralizing anti-IFNγ moAb to WT animals, exacerbated LG1233 lung tumor growth (Figure 3E). Although in the tumor microenvironment IFNγ can be produced by both innate and adaptive immune cells, such as NK, NKT, CD4+ cells and CTLs (Ikeda et al., 2002), this result supports a role for NK cells in the protective phenotype observed in TK-CCRL2 deficient mice.
Figure 3. CCRL2 deficiency enhances orthotopic growth of Kras mutant LG1233 cell line.
(A) Percent survival of CCRL2 WT and CCRL2 KO mice injected intravenously with 105 LG1233 cells. p=0.0152, CCRL2 WT vs. CCRL2 KO. (B) Percentage of area occupied by tumor lesions on total lung area of CCRL2 WT vs. CCRL2 KO mice sacrificed 13 days after tumor injection. *p<0.05, CCRL2 WT (n=5) vs. CCRL2 KO (n=5) by Student t-test. Representative images of H&E staining of CCRL2 WT and CCRL2 KO lungs. Magnification 10x. (C) FACS analysis of CD3-NK1.1+ in the CD45+ population from mechanically and enzymatically treated CCRL2 WT and CCRL2 KO lungs. **p<0.01, CCRL2 WT (n=5) vs. CCRL2 KO (n=5) by Student t-test. (D) Effect of the treatment of CCRL2 WT mice with anti-CCRL2 moAb and isotype control moAb, 100μg/ml given i.p. one day before LG1233 injection and then three times a week for the entire experiment. Percentage of area occupied by tumor lesions on total lung area 13 days after tumor injection (left panel) and representative H&E images (right panel). Magnification 10x. *p<0.05, anti-CCRL2 moAb (n=5) vs. isotype control moAb (n=5) by Student t-test. (E) Effect of the treatment of CCRL2 WT mice with anti-IFNγ moAb and isotype control moAb, 200μg/ml given i.p. one day before LG1233 and then 100μg/ml three times a week for the entire experiment. Percentage of area occupied by tumor lesions on total lung area 13 days after tumor injection (left panel) and representative H&E images (right panel). Magnification 10x. *p<0.05, anti-IFNγ moAb (n=4) vs. isotype control moAb (n=5) by Student t-test.
Role of CCRL2 expression by non-hematopoietic cells in lung tumor growth in TK-mice
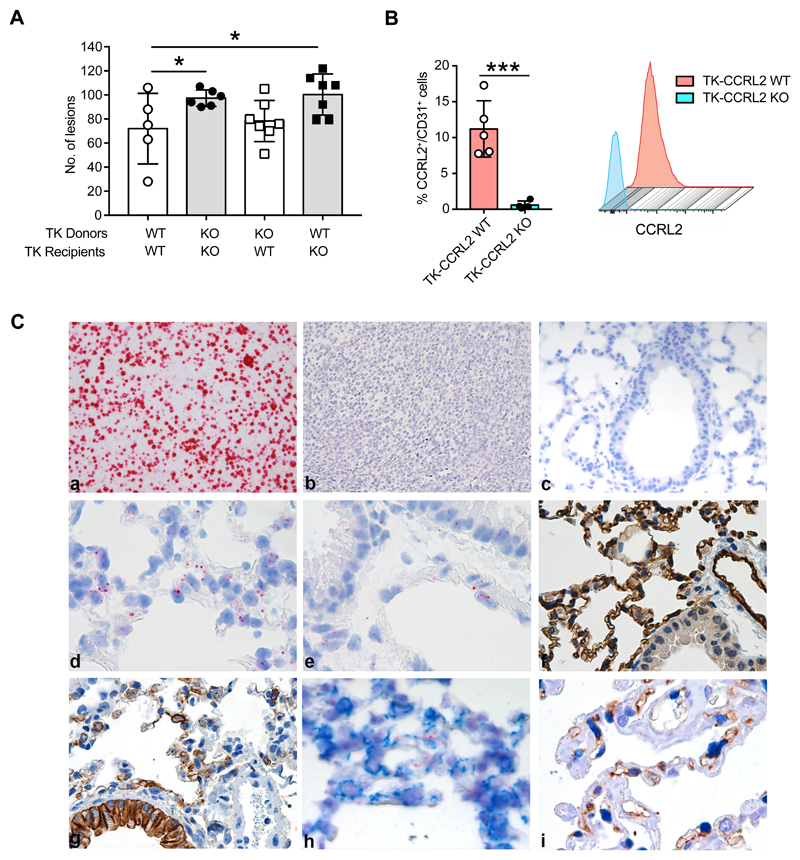
Since CCRL2 is expressed both by hematopoietic and non-hematopoietic cells, bone marrow chimaeras were used to evaluate the possible contribution of CCRL2 expression by endothelial/epithelial cells in lung tumor growth. Figure 4A shows that the injection of bone marrow obtained from TK-CCRL2 WT animals to lethally irradiated TK-CCRL2 deficient mice did not revert the tumor-promoting phenotype, suggesting that CCRL2 expression by the non-hematopoietic cell compartment has a role in the increased tumor formation observed in TK-CCRL2-deficient mice. Figure 4B shows that within the CD45- cell compartment, a fraction of CD31+ cells (11.2%+1.7; n=5) expresses CCRL2, while only a negligible percentage of Epcam+/CCRL2+ cells was detectable (data not shown). Previous work has documented that CCRL2 expression by endothelial cells supports endothelial transmigration of CMKLR1 positive leukocytes to inflammatory sites, with CCRL2 acting as a presenting molecule for chemerin, the chemotactic ligand shared by CCRL2 and CMKLR1 (Gonzalvo-Feo et al., 2014; Monnier et al., 2012). Therefore, this result strongly suggests that CCRL2 promotes NK cell migration acting as a chemerin presenting molecule for CMKLR1+ NK cells. CCRL2 was also described to be expressed by mouse bronchial epithelium in a model of ovalbumin-induced airway inflammation (Oostendorp et al., 2004). Since in the mouse both lung endothelial and epithelial cells can express CD31+ (Bantikassegn et al., 2015), the identity of CCRL2-expressing cells was further investigated by RNAscope. The specificity of the CCRL2 mRNA probe was first confirmed using sections of formalin-fixed cell blocks obtained from CCRL2-transfected L1.2 cells as a positive control (Figure 4C-a). On the contrary, no CCRL2 mRNA signal could be detected in sections of formalin-fixed cell blocks prepared with L1.2 mock cells (Figure 4C-b). A completely negative signal was also obtained using lung sections from CCRL2 deficient mice (Figure 4C-c). In the lung sections of WT mice, CCRL2 mRNA was detected as a diffuse signal in the alveolar wall and in endothelial cells of large vessels (Figure 4C-d, -e). The larger fraction of alveolar cells is composed by CD31+ endothelial cells and a minor fraction by cytokeratin+ pneumocytes (Figure 4C-f, -g). The combination of distribution, morphology and the lack of co-staining of RNAscope slides with an anti-cytokeratin moAb (Figure 4C-h) allow to identify CCRL2-expressing cells as endothelial cells. Of note, CCRL2 expression by peritumoral endothelial cells was also observed by immunohistochemistry in human LUAD biopsies (Fig. 4C-i).
Figure 4. CCRL2 expressed by non-hematopoietic cells plays a role in the control of tumor growth.
(A) TK-CCRL2 WT and TK-CCRL2 KO mice were lethally irradiated, then reconstituted with total bone marrow cells from TK-CCRL2 WT and TK-CCRL2 KO donor mice. Eight weeks after reconstitution, tumor development was induced by intranasal inoculation with 2.5x107 infectious particles of Ad5CMVCre recombinase, then mice were sacrificed after 10 weeks. The number of lesions is indicated. *p<0.05, by Generalized Linear Mixed Model in family Poisson (TK-CCRL2 WT recipients of TK-CCRL2 WT donors, n=5; TK-CCRL2 KO recipients of TK-CCRL2 KO donors, n=6; TK-CCRL2 WT recipients of TK-CCRL2 KO, n=7; TK-CCRL2 KO recipients of TK-CCRL2 WT donors, n=7) (B) CCRL2 expression on CD31+ in CD45- gated cells of lungs from tumor-bearing mice and representative histograms. ***p<0.001, TK-CCRL2 WT (n=5) vs. TK-CCRL2 KO (n=4) by Student t-test. (C) CCRL2 mRNA expression was investigated by RNAscope. The CCRL2 mRNA probe was tested on formalin-fixed blocks from CCRL2 transfected L1.2 cells as a positive control (a) and L1.2 mock cells (b) and CCRL2 KO lung as negative controls (c). CCRL2 mRNA in alveolar wall (d), endothelial cells of large vessel (e) and immunohistochemistry for CD31 (f) and cytokeratin (g) staining of CCRL2 WT lung. (h) CCRL2 mRNA co-stained with anti-cytokeratin antibody (i) double immunohistochemistry of human lung adenocarcinoma (LUAD) biopsy with anti-CCRL2 and anti-ERG moAbs shows double positive endothelial cells in the peritumoral area.
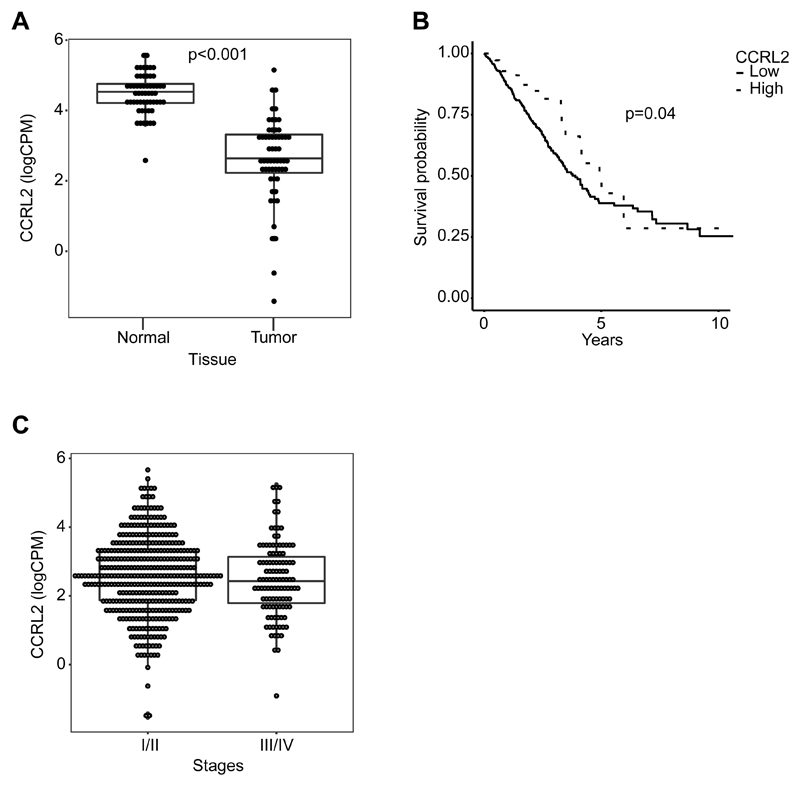
CCRL2 expression in primary human lung adenocarcinoma correlates with improved survival
To gain insights in the clinical significance of our observations, CCRL2 gene expression was assessed in primary human lung adenocarcinoma TGCA/GDC data sets. CCRL2 expression was significantly decreased in LUAD tumors compared to paired-normal samples (n=57) (fold change normal vs. tumor 3.22, p<0.001, Figure 5A). However, no statistically relevant difference in CCRL2 expression could be associated to tumor clinical stages (Figure 5B). CCRL2 expression was then evaluated as a possible prognostic biomarker. High and low CCRL2-expressing groups were defined using the optimal threshold separating CCRL2 expression between normal vs. tumor samples, with high-CCRL2 expressing tumor samples corresponding to the expression levels present in normal tissues. Within a cohort of 500 patients, a higher CCRL2 expression correlated with a better clinical outcome, especially at early observational times (Figure 5C). This positive correlation was not anymore observed at later time points. Taking together, these results support a role for CCRL2 in effective immune surveillance during the early phase of tumor development, possibly by promoting the recruitment of NK cells.
Figure 5. CCRL2 expression in primary human lung adenocarcinoma correlates with improved patient survival.
(A) Distribution of CCRL2 expression values, reported as counts per million (CPM) on log-2 scale, both for Tumor and matched Normal tissues. A significant reduction in CCRL2 expression is evident (p< 0.001, n=57). (B) CCRL2 expression values for tumor samples only grouped based on tumor Stage (I/II and III/IV Stages). (C) Kaplan-Meir curves for overall survival for high and low CCRL2 expression. High expression group was defined as those samples with CCRL2 expression exceeding the optimal threshold separating normal from tumor samples. Therefore, high expression can be considered as having normal-like expression level (p=0.004, n=500).
Concluding Remarks
NK cells are part of the complex network of the innate lymphoid cells (ILCs) and are the only components of this family endowed with cytotoxic activity (Eberl et al., 2015; Waldhauer and Steinle, 2008). Based on this effector function, NK cells have been for long time implicated in the immune defense against tumors (Cerwenka and Lanier, 2016; Marcus et al., 2014). Recent evidence have highlighted the role of NK cells not only in the control of hematologic malignancies and metastatic spread but also for their role against solid tumors, including lung tumors, and as a potential target of checkpoint blockade therapies (Delconte et al., 2016; Guillerey et al., 2016; Hsu et al., 2018; Iannello et al., 2016; Lopez-Soto et al., 2017; Malmberg et al., 2017; Putz et al., 2017; Ruggeri et al., 2002)Molgora et al, 201). Here we report that NK cells play a non-redundant role in limiting lung tumor growth in genetic and chemically-induced experimental models of kras-driven lung carcinogenesis. Furthermore, the results presented here show that CCRL2 expression by endothelial cells represents a key factor in the recruitment of effector NK cells to the lung.
CCRL2 is a non-signaling receptor for chemerin, the ligand of the chemotactic receptor CMKLR1 (Wittamer et al., 2003; Zabel et al., 2008). CMKLR1 is expressed by several leukocyte subsets, including NK cells (Bondue et al., 2011; Parolini et al., 2007; Sozzani et al., 2010). The binding of chemerin to CCRL2 expressed on the surface of endothelial cells was shown to promote leukocyte transmigration (Gonzalvo-Feo et al., 2014; Monnier et al., 2012). The results here presented highlight the importance of CCRL2 for the localization of NK cells to the lung. Multiple evidence support a role for endothelial cells in lung cancer (Kim et al., 2009). Since CCRL2 is also expressed by human lung microendothelial cells, it is likely that CCRL2 also functions in the recruitment of NK cells in humans.
Although in our experimental conditions, CCRL2 is not expressed by lung tumor cells, other studies have reported CCRL2 expression by cancer cells, such as in multiple myeloma, prostate and breast carcinomas, liver metastasis, glioblastoma and salivary adenoid cystic carcinoma (Akram et al., 2016; Mays et al., 2016; Reyes et al., 2017; Sarmadi et al., 2015; Wang et al., 2015; Westhrin et al., 2018; Yin et al., 2012). In these tumors, CCRL2 was described either as a tumor suppressor, such as in breast cancer (Wang et al., 2015), or as a tumor promoter, such as in glioblastoma (Yin et al., 2012), suggesting that CCRL2 might have a different role in different cellular contexts. The exact function of CCRL2 expression in these tumors still needs to be elucidated.
TGCA/GDC data expression analysis in human primary LUAD show correlation of CCRL2 expression with clinical outcome, with higher levels having a protective role, at least at the early observational times. In light of this result it is tempting to speculate that CCRL2-dependent recruitment of NK cells might have a role in limiting tumor growth also in human lung cancer. A protective role of NK cells was previously described in colorectal, gastric, renal, prostate, and hepatic human tumors (Coca et al., 1997; Ishigami et al., 2000; Malmberg et al., 2017; Molgora et al., 2017; Pasero et al., 2015; Schleypen et al., 2006; Villegas et al., 2002) and NK cells are emerging as important targets in checkpoint blockade therapies (Barry et al., 2018; Beldi-Ferchiou and Caillat-Zucman, 2017; Hsu et al., 2018; Iraolagoitia et al., 2016);Pesce et al, 2017). Therefore, our results candidate CCRL2 expression not only as a prognostic marker in LUAD patients but also as a key molecule for directing NK cell localization to the tumor site. Whether this crucial role of CCRL2 is restricted to the lung compartment or represents a general function present in other tissues is at the moment a matter under investigation.
Materials and Methods
Animals
Procedures involving animal handling and care were conformed to protocols approved by the Humanitas Clinical and Research Center (Rozzano, Milan, Italy) in compliance with national (D.L. N.116, G.U., suppl. 40, 18-2-1992 and N. 26, G.U. March 4, 2014) and international law and policies (EEC Council Directive 2010/63/ EU, OJ L 276/33, 22-09-2010; National Institutes of Health Guide for the Care and Use of Laboratory Animals, US National Research Council, 2011). The study was approved by the Italian Ministry of Health (approval number 35/2013-B, issued on 07/02/2013 and number 165/2017-PR, issued on 20/02/2017). All efforts were made to minimize the number of animals used and their suffering. All mice used (WT and CCRL2 KO) (Otero et al., 2010) were on a C57BL/6J genetic background; all colonies were housed and bred in Charles River Laboratories, Calco, Italy, or in the SPF animal facility of Humanitas Clinical and Research Center. B6.129P2-Trp53tm1Brn/J (p53LoxP) and Krastm4Tyj/J (KrasLSL-G12D) were obtained from Jackson Laboratory (cod 008462 and 008179 respectively) and initially crossed to obtain p53LoxP/KrasG12D/+. p53LoxP/KrasG12D/+ mice were then crossed with CCRL2 KO to obtain the two lines p53LoxP/KrasG12D/+/ccrl2+/+ (TK-CCRL2 WT) and p53LoxP//KrasG12D/+/ccrl2-/- (TK-CCRL2 KO). Mice genotyping was performed by PCR.
Urethane-Induced Lung Carcinogenesis Model
Six to seven-week-old WT and CCRL2 KO male mice were intraperitoneally injected with urethane (1mg per g body weight; Sigma) dissolved in saline weekly for 10 weeks as previously described (Miller et al., 2003) and fed with antioxidant free lab diet chow (Teklad Global 19% Protein Extruded Rodent Diet, ENVIGO). Mice were sacrificed 30 weeks after the first urethane injection and lungs, spleen, blood and bone marrow were harvested. Briefly, lungs were collected upon intracardiac perfusion with cold PBS. Right lung lobes were mechanically cut into small pieces and then enzymatically treated with Collagenase D (IV type, Clostridium Histolyticum, Sigma) 1mg/ml and DNase I (from bovine pancreas grade II, Roche) 0.02mg/ml at 37°C for 30 minutes. Enzymatic reaction was stopped by EDTA (Sigma) and single cell suspension was filtered through 70μm cell strainer and stained for cytofluorimetric analysis. Left lung lobe was formalin fixed for 24h, dehydrated and paraffin embedded for histological analysis. Spleens were smashed through 70μm cell strainer to obtain a single cell suspension, bone marrow cells were obtained by flushing of the cavity of freshly dissected femurs filtered through 70μm cell strainer and blood was collected from retro orbital sinus. All single cell suspensions were then stained for cytofluorimetric analysis after red blood cells lysis with AKC Lysing buffer (Lonza).
Kras/p53 driven Lung Cancer Model
TK CCRL2 WT and TK CCRL2 KO, at 8wks of age, were intranasally inoculated with 2.5x107 infectious particles of replication-deficient adenoviral vector with Cytomegalovirus promoter driving the expression of the Cre recombinase protein (Ad5CMVCre) to induce sporadic mutations and lung tumor development as previously described (DuPage et al., 2009). Mice were sacrificed 10 weeks after the adenovirus inoculation and lungs, spleen, and bone marrow were collected and treated as described for urethane model. Furthermore, the accessory lung lobe was immediately frozen at -80°C and used for Real-Time PCR.
Flow Cytometry/Intracellular staining
Single-cell suspensions from bone marrow, blood, spleen, lung were stained with the following antibodies: CD45-BV605 (clone: 30-F11) or –VioGreen (clone: REA737); NK1.1-PECF594 or - APC (clone: PK136); CD11b-APCCy7 or PE-Vio770 (clone: M1/70); CD27-PECy7 (clone: LG.7F9); CD4-FITC or –PE-Vio770 or -AF700 (clone RM 4-5); CD8-BV570 or -VioBlue (clone 53-6.7); Granzyme B-PEeF610 (clone: NGZB); Perforin-PE (clone: eBioOMAK-D); IFNγ-Alexa700 (clone: XMG1.2); CD107a-BV786 (clone: H4A3); Eomes AF488 (clone:Dan 11 mag); CD49b-APC (clone: DX5); CD49a-BV711 (clone: Ha31/8); TCRβ-PerCp (clone: H57-597); TCRγδ-BV421 (clone: GL3); Ly6G-FITC (clone1A8); Ly6C-PE (clone REA796); CD19-VioBlue (clone REA749); F4/80-PercP-Vio770(clone REA126); CD45RA-APC-Vio770 or PE-Vio770 (clone T6D11); MHCII-FITC or –VioBlue (clone REA564); CCRL2-PE (clone BZ2E3); SiglecH-APC (clone 551.3D3); CD11c-PercP-Cy5.5 (clone: REA 754); CD3-PE (clone 145-2C11) or PercP-Cy5.5 (clone17A2); SiglecF-FITC (clone: REA 798); CD31-APC (clone: MEC 13.3); EpCam-VioBlue (clone REA977); CD127-BV786 (clone: 5B/199) from BD Bioscience, eBioscience, BioLegend, Miltenyi Biotec, and Invitrogen. Cell viability was determined by Aqua LIVE/Dead-405 nm or LIVE/Dead -633 nm or LIVE/Dead-488 nm staining (Invitrogen); negative cells were considered viable. A Foxp3/Transcription Factor Staining Buffer Set (eBioscience) was used for intracellular staining of granzyme B, perforin, IFNg and CD107. Cells were analyzed on an LSR Fortessa (BD Bioscience) or MACSQuant (Miltenyi) and analyzed with FlowJo software (Treestar).
Lung histology and immunohistochemistry
Histology was performed on 4 μm five (urethane-induced model) or seven (Kras/p53 driven model) longitudinal serial sections (150 μm apart) from each left lung stained with H&E and scanned by VS120 Dot-Slide BX61 virtual slide microscope (Olympus Optical). Total number and area of lesions were obtained by manually tracing the perimeter of lesions using the Image Pro-Premiere software (Media Cybernetics).
Immunohistochemistry
NKp46 immunohistochemistry staining was performed on tissue slides (4 μm) that were rehydrated and placed in citrate buffer 1M (15min microwave) for antigen retrieval. Endogenous peroxidase activity was quenched with 3% H2O2 for 20 min and unspecific binding sites were blocked for 30 min with Rodent Block M (Biocare Medical). Samples were then incubated 1h with Goat Anti-Mouse NKp46 (AF2225 R&D) and detected by Goat on rodent Polymer kit (Biocare), followed by DAB Chromogen Kit (Biocare). Matched IgG was used for negative control. For Ly6G immunostaining, Rat Anti-Mouse Ly6G (BD Biosciences) was used. To localize CCRL2 positive cells in the lungs, tissues were analyzed with RNAscope assay (Advanced Cell Diagnostics, Newark, CA, USA) using RNAscope 2.5 HD Assay-RED kit and Mm-Ccrl2-No-Xhs probes. Sections from fixed mouse tissue blocks were treated following the manufacturer’s instructions. Briefly, freshly cut 3μm sections were deparaffinized and treated with the peroxidase block solution for 10 min at room temperature followed by the retrieval solution for 15 min at 98°C and by protease plus at 40 °C. Control probes included positive control Mm-Polr2a and negative control dapB. The hybridization was performed for 2 h at 40°C. The signal was revealed using RNAscope 2.5 HD Detection Reagent and FAST RED.
LG1233 cells
To perform the lung orthotopic tumor model, the Kras mutant (KrasG12D/+; p53-/-)-derived cell line (LG1233) was used (Caronni et al., 2018; Dimitrova et al., 2016). Cells were harvested and wash three times with PBS and then 1x105 cells in 100μL of PBS were injected intravenously in the caudal vein. Lungs were collected 13 days after engraftment and used for histology and FACS analysis.
Specific depletion or blocking experiments
The anti-NK1.1 depletion experiment was performed in Kras/p53 driven lung cancer model, while IFNγ and CCRL2 blocking in the LG cell line lung metastasis model. Mice were treated at day -1 intraperitoneally with 100 μg of anti-NK1.1 (clone PK136) or isotype control (clone C1.18.4) and anti-CCRL2, generated in the lab (Otero et al., 2010), or isotype control (clone MPC-11); and 200 μg of anti-IFNγ (clone XMG1.2) or isotype control (IgG1k HRPN) (all from BioXCell). Mice were then treated with 100 μg once (anti-NK1.1) or three times (anti-CCRL2; anti-IFNγ) a week for the entire duration of the experiment.
Bone Marrow Transplantation
TK CCRL2 WT and KO mice were lethally irradiated with a total dose of 9 Gy. Then, 2 hrs later, mice were injected in the retro-orbital plexus with 5×106 nucleated bone marrow cells obtained by flushing of the cavity of freshly dissected femurs from TK CCRL2 WT and KO mice. Eight weeks after bone marrow transplantation, mice were intranasally inoculated with 2.5x107 infectious particles of Ad5CMVCre. After 10 weeks, mice were sacrificed and lung perfused, collected and processed as previously described.
Cytotoxicity assay
Splenic NK cell cytotoxicity was determined after 24 h of incubation in the presence of IL-12 (50 ng/ml) and IL-15 (50 ng/ml), against the YAC-1 target cells labelled with 1 μM of CFSE. Effector cells (E) were mixed with target cells (T) at E/T ratios ranging from 1/1 to 50/1. After 4 h of incubation at 37° C, the percentage of killed target cells was evaluated by FACS cytometry as CFSE positive inside the Live DEAD-405 nm positive cells.
Gene Expression (qPCR)
FACS-sorted lung and splenic cell populations from TK CCRL2 WT and KO mice were analyzed for mRNA expression of the indicated genes by qPCR. RNA was extracted with the RNeasy Kit (Qiagen) according to the manufacturer’s instructions. After RNA purification, reverse transcription was performed using random hexamers and MMLV RT (Thermo Fisher Scientific). Gene-specific primers used were as follows: mCXCL10 (forward: 5’- -3’, reverse: 5’- -3’), mCX3CL1 (forward: 5’-catccgctatcagctaaacca-3’, reverse: 5’-cagaagcgtctgtgctgtgt-3’), mCCL5 (forward: 5’- -3’, reverse: 5’- -3’), mChemerin (forward: 5’-ggagtgcacaatcaaaccaa-3’, reverse: 5’-ttttacccttggggtccatt-3’), mChemR23 (forward: 5’-ccatgt gcaagatcagcaac-3’, reverse: 5’-gcaggaagacgctggtgta -3’), mCCR5 (forward: 5’- -3’, reverse: 5’- -3’), mCXCR3 (forward: 5’- -3’, reverse: 5’- -3’), mCX3CR1 (forward: 5’- -3’, reverse: 5’- -3’), mIFNγ (forward: 5’- -3’, reverse:5’- -3’), mGrB (forward: 5’- -3’, reverse: 5’- -3’), mPerforin (forward: 5’- -3’, reverse: 5’- -3’), mPD-1 (forward: 5’-tgcagttgagctggcaat-3’, reverse: 5’-ggctgggtagaaggtgagg-3’), mPDL1 (forward: 5’-aaatcgtggtccccaagc-3’, reverse: 5’-aatatcctcatgttttgggaactatc-3’), mPDL2 (forward: 5’-gcatgttctggaatgctcac-3’, reverse: 5’-ctttgggttccatccgact-3’), mLag3 (forward: 5’-cacctgtagcatccatctgc-3’, reverse: 5’-ccaggtaacccgaaggattt-3’) and mRPL32 (forward: 5’-gctgccatctgttttacgg-3’, reverse: 5’-tgactggtgcctgatgaact-3’). The SsoAdvanced Universal SYBR Green Supermix (Bio-Rad Laboratories) for quantitative real-time PCR was used according to the manufacturer’s instructions. Reactions were run on a StepOne Plus Real-Time PCR System (Applied Biosystems) and the generated products analyzed by the StepOne Plus Software (Version 2.3, Applied Biosystems). Gene expression was normalized based on RPL32 mRNA content.
TGCA datasets
Data were downloaded from TCGA/GDC using the “harmonized” set as count data using R/Bioconductor package-TCGAbiolinks (version 2.8.4). Paired data (Solid Tumor and Solid Normal) where modelled using weighted linear models accounting for variance-mean relationship after TMM normalization. In total we considered 57 LUAD samples with matched Solid Normal Tissue and Solid Tumor Tissue. When considering only tumors we analyzed a total of 500 LUAD samples. When used as a biomarker, CCRL2 expression was computed as log2 counts-per-million (CPM).
Statistics
Statistical analyses were performed by Student t-test, Mann-Whitney test, as appropriate. Count data (number of lesions) were modelled using Poisson regressions. When multiple counts (lung slice) were available for each sample, data were modelled using Generalized Linear Mixed Models to account for within sample correlation. If zero inflation was detected, due to excess in zero counts, models were adjusted for zero-inflation. Results were analyzed by using GraphPad PRISM 5.0 and R (version 3.51) softwares.
Supplementary Material
Acknowledgements
This work was supported by the Italian Association for Cancer Research (AIRC IG-2016 grant n. 721-19014, AIRC 5x1000 grant n. 9962 and AIRC 5x1000 grant n. 21147 to AM; IG-20776 to SS); Fondazione Berlucchi and the Interuniversity Attraction Poles (IAP) 7-40 program. European Commission (ERC project PHII-669415; FP7 project 281608 TIMER; ESA/ITN, H2020-MSCAITN-2015-676129), Ministero dell’Istruzione, dell’Università e della Ricerca (MIUR) (project FIRB RBAP11H2R9), and the Italian Ministry of Health are gratefully acknowledged. VS was the recipient of a fellowship from Fondazione Italiana Ricerca sul Cancro (FIRC).
Footnotes
Author contribution
A.D-P, F.S., T.S., A.P., M.B., V.S., B.B. performed the experiments and analyzed the results, W.V., G.B., F.B., A.V. conceived the experiments and analyzed the results; S.C. performed the statistical data and bioinformatics analysis; A.M. evaluated the results and contributed to manuscript drafting; A.D-P and S.S. conceived the experiments, analyzed the results and wrote the manuscript.
References
- Abel AM, Yang C, Thakar MS, Malarkannan S. Natural Killer Cells: Development, Maturation, and Clinical Utilization. Front Immunol. 2018;9:1869. doi: 10.3389/fimmu.2018.01869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akram IG, Georges R, Hielscher T, Adwan H, Berger MR. The chemokines CCR1 and CCRL2 have a role in colorectal cancer liver metastasis. Tumour Biol. 2016;37:2461–2471. doi: 10.1007/s13277-015-4089-4. [DOI] [PubMed] [Google Scholar]
- Bachelerie F, Ben-Baruch A, Burkhardt AM, Combadiere C, Farber JM, Graham GJ, Horuk R, Sparre-Ulrich AH, Locati M, Luster AD, Mantovani A, et al. International Union of Basic and Clinical Pharmacology. [corrected]. LXXXIX. Update on the extended family of chemokine receptors and introducing a new nomenclature for atypical chemokine receptors. Pharmacol Rev. 2014a;66:1–79. doi: 10.1124/pr.113.007724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachelerie F, Graham GJ, Locati M, Mantovani A, Murphy PM, Nibbs R, Rot A, Sozzani S, Thelen M. New nomenclature for atypical chemokine receptors. Nat Immunol. 2014b;15:207–208. doi: 10.1038/ni.2812. [DOI] [PubMed] [Google Scholar]
- Balkwill F. Cancer and the chemokine network. Nat Rev Cancer. 2004;4:540–550. doi: 10.1038/nrc1388. [DOI] [PubMed] [Google Scholar]
- Bantikassegn A, Song X, Politi K. Isolation of epithelial, endothelial, and immune cells from lungs of transgenic mice with oncogene-induced lung adenocarcinomas. Am J Respir Cell Mol Biol. 2015;52:409–417. doi: 10.1165/rcmb.2014-0312MA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry KC, Hsu J, Broz ML, Cueto FJ, Binnewies M, Combes AJ, Nelson AE, Loo K, Kumar R, Rosenblum MD, Alvarado MD, et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat Med. 2018;24:1178–1191. doi: 10.1038/s41591-018-0085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beldi-Ferchiou A, Caillat-Zucman S. Control of NK Cell Activation by Immune Checkpoint Molecules. Int J Mol Sci. 2017;18 doi: 10.3390/ijms18102129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bondue B, Wittamer V, Parmentier M. Chemerin and its receptors in leukocyte trafficking, inflammation and metabolism. Cytokine Growth Factor Rev. 2011;22:331–338. doi: 10.1016/j.cytogfr.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Caronni N, Simoncello F, Stafetta F, Guarnaccia C, Ruiz-Moreno JS, Opitz B, Galli T, Proux-Gillardeaux V, Benvenuti F. Downregulation of Membrane Trafficking Proteins and Lactate Conditioning Determine Loss of Dendritic Cell Function in Lung Cancer. Cancer Res. 2018;78:1685–1699. doi: 10.1158/0008-5472.CAN-17-1307. [DOI] [PubMed] [Google Scholar]
- Catusse J, Leick M, Groch M, Clark DJ, Buchner MV, Zirlik K, Burger M. Role of the atypical chemoattractant receptor CRAM in regulating CCL19 induced CCR7 responses in B-cell chronic lymphocytic leukemia. Mol Cancer. 2010;9:297. doi: 10.1186/1476-4598-9-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerwenka A, Lanier LL. Natural killer cell memory in infection, inflammation and cancer. Nat Rev Immunol. 2016;16:112–123. doi: 10.1038/nri.2015.9. [DOI] [PubMed] [Google Scholar]
- Chiossone L, Dumas PY, Vienne M, Vivier E. Natural killer cells and other innate lymphoid cells in cancer. Nat Rev Immunol. 2018;18:671–688. doi: 10.1038/s41577-018-0061-z. [DOI] [PubMed] [Google Scholar]
- Coca S, Perez-Piqueras J, Martinez D, Colmenarejo A, Saez MA, Vallejo C, Martos JA, Moreno M. The prognostic significance of intratumoral natural killer cells in patients with colorectal carcinoma. Cancer. 1997;79:2320–2328. doi: 10.1002/(sici)1097-0142(19970615)79:12<2320::aid-cncr5>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- De Henau O, Degroot GN, Imbault V, Robert V, De Poorter C, McHeik S, Gales C, Parmentier M, Springael JY. Signaling Properties of Chemerin Receptors CMKLR1, GPR1 and CCRL2. PLoS One. 2016;11:e0164179. doi: 10.1371/journal.pone.0164179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete A, Bonecchi R, Vecchi A, Mantovani A, Sozzani S. CCRL2, a fringe member of the atypical chemoattractant receptor family. Eur J Immunol. 2013;43:1418–1422. doi: 10.1002/eji.201243179. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Martinez-Munoz L, Mazzon C, Toffali L, Sozio F, Za L, Bosisio D, Gazzurelli L, Salvi V, Tiberio L, Liberati C, et al. The atypical receptor CCRL2 is required for CXCR2-dependent neutrophil recruitment and tissue damage. Blood. 2017a;130:1223–1234. doi: 10.1182/blood-2017-04-777680. [DOI] [PubMed] [Google Scholar]
- Del Prete A, Schioppa T, Tiberio L, Stabile H, Sozzani S. Leukocyte trafficking in tumor microenvironment. Curr Opin Pharmacol. 2017b;35:40–47. doi: 10.1016/j.coph.2017.05.004. [DOI] [PubMed] [Google Scholar]
- Delconte RB, Kolesnik TB, Dagley LF, Rautela J, Shi W, Putz EM, Stannard K, Zhang JG, Teh C, Firth M, Ushiki T, et al. CIS is a potent checkpoint in NK cell-mediated tumor immunity. Nat Immunol. 2016;17:816–824. doi: 10.1038/ni.3470. [DOI] [PubMed] [Google Scholar]
- Dimitrova N, Gocheva V, Bhutkar A, Resnick R, Jong RM, Miller KM, Bendor J, Jacks T. Stromal Expression of miR-143/145 Promotes Neoangiogenesis in Lung Cancer Development. Cancer Discov. 2016;6:188–201. doi: 10.1158/2159-8290.CD-15-0854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DuPage M, Dooley AL, Jacks T. Conditional mouse lung cancer models using adenoviral or lentiviral delivery of Cre recombinase. Nat Protoc. 2009;4:1064–1072. doi: 10.1038/nprot.2009.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dwyer-Nield LD, McQuillan J, Hill-Baskin A, Radcliffe RA, You M, Nadeau JH, Malkinson AM. Epistatic interactions govern chemically-induced lung tumor susceptibility and Kras mutation site in murine C57BL/6J-ChrA/J chromosome substitution strains. Int J Cancer. 2010;126:125–132. doi: 10.1002/ijc.24743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eberl G, Colonna M, Di Santo JP, McKenzie AN. Innate lymphoid cells. Innate lymphoid cells: a new paradigm in immunology. Science. 2015;348 doi: 10.1126/science.aaa6566. aaa6566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eruslanov EB, Bhojnagarwala PS, Quatromoni JG, Stephen TL, Ranganathan A, Deshpande C, Akimova T, Vachani A, Litzky L, Hancock WW, Conejo-Garcia JR, et al. Tumor-associated neutrophils stimulate T cell responses in early-stage human lung cancer. J Clin Invest. 2014;124:5466–5480. doi: 10.1172/JCI77053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gismondi A, Stabile H, Nisti P, Santoni A. Effector Functions of Natural Killer Cell Subsets in the Control of Hematological Malignancies. Front Immunol. 2015;6:567. doi: 10.3389/fimmu.2015.00567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalvo-Feo S, Del Prete A, Pruenster M, Salvi V, Wang L, Sironi M, Bierschenk S, Sperandio M, Vecchi A, Sozzani S. Endothelial cell-derived chemerin promotes dendritic cell transmigration. J Immunol. 2014;192:2366–2373. doi: 10.4049/jimmunol.1302028. [DOI] [PubMed] [Google Scholar]
- Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, Petrella F, Spaggiari L, Rosell R. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. doi: 10.1038/nrdp.2015.9. [DOI] [PubMed] [Google Scholar]
- Guillerey C, Huntington ND, Smyth MJ. Targeting natural killer cells in cancer immunotherapy. Nat Immunol. 2016;17:1025–1036. doi: 10.1038/ni.3518. [DOI] [PubMed] [Google Scholar]
- Hayakawa Y, Smyth MJ. Innate immune recognition and suppression of tumors. Adv Cancer Res. 2006;95:293–322. doi: 10.1016/S0065-230X(06)95008-8. [DOI] [PubMed] [Google Scholar]
- Hsu J, Hodgins JJ, Marathe M, Nicolai CJ, Bourgeois-Daigneault MC, Trevino TN, Azimi CS, Scheer AK, Randolph HE, Thompson TW, Zhang L, et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J Clin Invest. 2018;128:4654–4668. doi: 10.1172/JCI99317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iannello A, Thompson TW, Ardolino M, Marcus A, Raulet DH. Immunosurveillance and immunotherapy of tumors by innate immune cells. Curr Opin Immunol. 2016;38:52–58. doi: 10.1016/j.coi.2015.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda H, Old LJ, Schreiber RD. The roles of IFN gamma in protection against tumor development and cancer immunoediting. Cytokine Growth Factor Rev. 2002;13:95–109. doi: 10.1016/s1359-6101(01)00038-7. [DOI] [PubMed] [Google Scholar]
- Imai K, Matsuyama S, Miyake S, Suga K, Nakachi K. Natural cytotoxic activity of peripheral-blood lymphocytes and cancer incidence: an 11-year follow-up study of a general population. Lancet. 2000;356:1795–1799. doi: 10.1016/S0140-6736(00)03231-1. [DOI] [PubMed] [Google Scholar]
- Iraolagoitia XL, Spallanzani RG, Torres NI, Araya RE, Ziblat A, Domaica CI, Sierra JM, Nunez SY, Secchiari F, Gajewski TF, Zwirner NW, et al. NK Cells Restrain Spontaneous Antitumor CD8+ T Cell Priming through PD-1/PD-L1 Interactions with Dendritic Cells. J Immunol. 2016;197:953–961. doi: 10.4049/jimmunol.1502291. [DOI] [PubMed] [Google Scholar]
- Ishigami S, Natsugoe S, Tokuda K, Nakajo A, Che X, Iwashige H, Aridome K, Hokita S, Aikou T. Prognostic value of intratumoral natural killer cells in gastric carcinoma. Cancer. 2000;88:577–583. [PubMed] [Google Scholar]
- Kim WY, Perera S, Zhou B, Carretero J, Yeh JJ, Heathcote SA, Jackson AL, Nikolinakos P, Ospina B, Naumov G, Brandstetter KA, et al. HIF2alpha cooperates with RAS to promote lung tumorigenesis in mice. J Clin Invest. 2009;119:2160–2170. doi: 10.1172/JCI38443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Soto A, Gonzalez S, Smyth MJ, Galluzzi L. Control of Metastasis by NK Cells. Cancer Cell. 2017;32:135–154. doi: 10.1016/j.ccell.2017.06.009. [DOI] [PubMed] [Google Scholar]
- Malmberg KJ, Carlsten M, Bjorklund A, Sohlberg E, Bryceson YT, Ljunggren HG. Natural killer cell-mediated immunosurveillance of human cancer. Semin Immunol. 2017;31:20–29. doi: 10.1016/j.smim.2017.08.002. [DOI] [PubMed] [Google Scholar]
- Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- Marcus A, Gowen BG, Thompson TW, Iannello A, Ardolino M, Deng W, Wang L, Shifrin N, Raulet DH. Recognition of tumors by the innate immune system and natural killer cells. Adv Immunol. 2014;122:91–128. doi: 10.1016/B978-0-12-800267-4.00003-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massara M, Bonavita O, Mantovani A, Locati M, Bonecchi R. Atypical chemokine receptors in cancer: friends or foes? J Leukoc Biol. 2016;99:927–933. doi: 10.1189/jlb.3MR0915-431RR. [DOI] [PubMed] [Google Scholar]
- Mays AC, Feng X, Browne JD, Sullivan CA. Chemokine and Chemokine Receptor Profiles in Metastatic Salivary Adenoid Cystic Carcinoma. Anticancer Res. 2016;36:4013–4018. [PubMed] [Google Scholar]
- Mazzon C, Zanotti L, Wang L, Del Prete A, Fontana E, Salvi V, Poliani PL, Sozzani S. CCRL2 regulates M1/M2 polarization during EAE recovery phase. J Leukoc Biol. 2016;99:1027–1033. doi: 10.1189/jlb.3MA0915-444RR. [DOI] [PubMed] [Google Scholar]
- Mazzotti C, Gagliostro V, Bosisio D, Del Prete A, Tiberio L, Thelen M, Sozzani S. The Atypical Receptor CCRL2 (C-C Chemokine Receptor-Like 2) Does Not Act As a Decoy Receptor in Endothelial Cells. Front Immunol. 2017;8:1233. doi: 10.3389/fimmu.2017.01233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Migeotte I, Franssen JD, Goriely S, Willems F, Parmentier M. Distribution and regulation of expression of the putative human chemokine receptor HCR in leukocyte populations. Eur J Immunol. 2002;32:494–501. doi: 10.1002/1521-4141(200202)32:2<494::AID-IMMU494>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Miller YE, Dwyer-Nield LD, Keith RL, Le M, Franklin WA, Malkinson AM. Induction of a high incidence of lung tumors in C57BL/6 mice with multiple ethyl carbamate injections. Cancer Lett. 2003;198:139–144. doi: 10.1016/s0304-3835(03)00309-4. [DOI] [PubMed] [Google Scholar]
- Molgora M, Bonavita E, Ponzetta A, Riva F, Barbagallo M, Jaillon S, Popovic B, Bernardini G, Magrini E, Gianni F, Zelenay S, et al. IL-1R8 is a checkpoint in NK cells regulating anti-tumour and anti-viral activity. Nature. 2017;551:110–114. doi: 10.1038/nature24293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina JR, Yang P, Cassivi SD, Schild SE, Adjei AA. Non-small cell lung cancer: epidemiology, risk factors, treatment, and survivorship. Mayo Clin Proc. 2008;83:584–594. doi: 10.4065/83.5.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monnier J, Lewen S, O'Hara E, Huang K, Tu H, Butcher EC, Zabel BA. Expression, regulation, and function of atypical chemerin receptor CCRL2 on endothelial cells. J Immunol. 2012;189:956–967. doi: 10.4049/jimmunol.1102871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oostendorp J, Hylkema MN, Luinge M, Geerlings M, Meurs H, Timens W, Zaagsma J, Postma DS, Boddeke HW, Biber K. Localization and enhanced mRNA expression of the orphan chemokine receptor L-CCR in the lung in a murine model of ovalbumin-induced airway inflammation. J Histochem Cytochem. 2004;52:401–410. doi: 10.1177/002215540405200311. [DOI] [PubMed] [Google Scholar]
- Otero K, Vecchi A, Hirsch E, Kearley J, Vermi W, Del Prete A, Gonzalvo-Feo S, Garlanda C, Azzolino O, Salogni L, Lloyd CM, et al. Nonredundant role of CCRL2 in lung dendritic cell trafficking. Blood. 2010;116:2942–2949. doi: 10.1182/blood-2009-12-259903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parolini S, Santoro A, Marcenaro E, Luini W, Massardi L, Facchetti F, Communi D, Parmentier M, Majorana A, Sironi M, Tabellini G, et al. The role of chemerin in the colocalization of NK and dendritic cell subsets into inflamed tissues. Blood. 2007;109:3625–3632. doi: 10.1182/blood-2006-08-038844. [DOI] [PubMed] [Google Scholar]
- Pasero C, Gravis G, Granjeaud S, Guerin M, Thomassin-Piana J, Rocchi P, Salem N, Walz J, Moretta A, Olive D. Highly effective NK cells are associated with good prognosis in patients with metastatic prostate cancer. Oncotarget. 2015;6:14360–14373. doi: 10.18632/oncotarget.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pesce S, Greppi M, Tabellini G, Rampinelli F, Parolini O, Olive D, Moretta L, Moretta A, Marcenaro E. Identification of a subset of human natural killer cells expressing high levels of programmed death 1: A phenotypic and functional characterization. J Allergy Clin Immunol. 2017;139:335–346.e3. doi: 10.1016/j.jaci.2016.04.025. [DOI] [PubMed] [Google Scholar]
- Platonova S, Cherfils-Vicini J, Damotte D, Crozet L, Vieillard V, Validire P, Andre P, Dieu-Nosjean MC, Alifano M, Regnard JF, Fridman WH, et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res. 2011;71:5412–5422. doi: 10.1158/0008-5472.CAN-10-4179. [DOI] [PubMed] [Google Scholar]
- Putz EM, Mayfosh AJ, Kos K, Barkauskas DS, Nakamura K, Town L, Goodall KJ, Yee DY, Poon IK, Baschuk N, Souza-Fonseca-Guimaraes F, et al. NK cell heparanase controls tumor invasion and immune surveillance. J Clin Invest. 2017;127:2777–2788. doi: 10.1172/JCI92958. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Reck M, Rabe KF. Precision Diagnosis and Treatment for Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2017;377:849–861. doi: 10.1056/NEJMra1703413. [DOI] [PubMed] [Google Scholar]
- Reyes N, Benedetti I, Rebollo J, Correa O, Geliebter J. Atypical chemokine receptor CCRL2 is overexpressed in prostate cancer cells. J Biomed Res. 2017 doi: 10.7555/JBR.32.20170057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribatti D. The concept of immune surveillance against tumors. The first theories. Oncotarget. 2017;8:7175–7180. doi: 10.18632/oncotarget.12739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggeri L, Capanni M, Urbani E, Perruccio K, Shlomchik WD, Tosti A, Posati S, Rogaia D, Frassoni F, Aversa F, Martelli MF, et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science. 2002;295:2097–2100. doi: 10.1126/science.1068440. [DOI] [PubMed] [Google Scholar]
- Sarmadi P, Tunali G, Esendagli-Yilmaz G, Yilmaz KB, Esendagli G. CRAM-A indicates IFN-gamma-associated inflammatory response in breast cancer. Mol Immunol. 2015;68:692–698. doi: 10.1016/j.molimm.2015.10.019. [DOI] [PubMed] [Google Scholar]
- Schleypen JS, Baur N, Kammerer R, Nelson PJ, Rohrmann K, Grone EF, Hohenfellner M, Haferkamp A, Pohla H, Schendel DJ, Falk CS, et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin Cancer Res. 2006;12:718–725. doi: 10.1158/1078-0432.CCR-05-0857. [DOI] [PubMed] [Google Scholar]
- Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- Sozzani S, Vermi W, Del Prete A, Facchetti F. Trafficking properties of plasmacytoid dendritic cells in health and disease. Trends Immunol. 2010;31:270–277. doi: 10.1016/j.it.2010.05.004. [DOI] [PubMed] [Google Scholar]
- Stojanovic A, Cerwenka A. Natural killer cells and solid tumors. J Innate Immun. 2011;3:355–364. doi: 10.1159/000325465. [DOI] [PubMed] [Google Scholar]
- Travis WD, Brambilla E, Riely GJ. New pathologic classification of lung cancer: relevance for clinical practice and clinical trials. J Clin Oncol. 2013;31:992–1001. doi: 10.1200/JCO.2012.46.9270. [DOI] [PubMed] [Google Scholar]
- Villegas FR, Coca S, Villarrubia VG, Jimenez R, Chillon MJ, Jareno J, Zuil M, Callol L. Prognostic significance of tumor infiltrating natural killer cells subset CD57 in patients with squamous cell lung cancer. Lung Cancer. 2002;35:23–28. doi: 10.1016/s0169-5002(01)00292-6. [DOI] [PubMed] [Google Scholar]
- Waldhauer I, Steinle A. NK cells and cancer immunosurveillance. Oncogene. 2008;27:5932–5943. doi: 10.1038/onc.2008.267. [DOI] [PubMed] [Google Scholar]
- Wang LP, Cao J, Zhang J, Wang BY, Hu XC, Shao ZM, Wang ZH, Ou ZL. The human chemokine receptor CCRL2 suppresses chemotaxis and invasion by blocking CCL2-induced phosphorylation of p38 MAPK in human breast cancer cells. Med Oncol. 2015;32:254. doi: 10.1007/s12032-015-0696-6. [DOI] [PubMed] [Google Scholar]
- Westhrin M, Moen SH, Kristensen IB, Buene G, Mylin AK, Turesson I, Abildgaard N, Waage A, Standal T. Chemerin is elevated in multiple myeloma patients and is expressed by stromal cells and pre-adipocytes. Biomark Res. 2018;6:21. doi: 10.1186/s40364-018-0134-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittamer V, Franssen JD, Vulcano M, Mirjolet JF, Le Poul E, Migeotte I, Brezillon S, Tyldesley R, Blanpain C, Detheux M, Mantovani A, et al. Specific recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J Exp Med. 2003;198:977–985. doi: 10.1084/jem.20030382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin F, Xu Z, Wang Z, Yao H, Shen Z, Yu F, Tang Y, Fu D, Lin S, Lu G, Kung HF, et al. Elevated chemokine CC-motif receptor-like 2 (CCRL2) promotes cell migration and invasion in glioblastoma. Biochem Biophys Res Commun. 2012;429:168–172. doi: 10.1016/j.bbrc.2012.10.120. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Oppenheim JJ. Chemokine-like receptor 1 (CMKLR1) and chemokine (C-C motif) receptor-like 2 (CCRL2); two multifunctional receptors with unusual properties. Exp Cell Res. 2011;317:674–684. doi: 10.1016/j.yexcr.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zabel BA, Nakae S, Zuniga L, Kim JY, Ohyama T, Alt C, Pan J, Suto H, Soler D, Allen SJ, Handel TM, et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-mediated passive cutaneous anaphylaxis. J Exp Med. 2008;205:2207–2220. doi: 10.1084/jem.20080300. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.