Abstract
Introduction:
Neuromotor control of diaphragm muscle and the recovery of diaphragm activity following spinal cord injury has been narrowly focused on ventilation. By contrast, the understanding of neuromotor control for non-ventilatory expulsive/straining maneuvers (including coughing, defecation and parturition) is relatively impoverished. This variety of behaviours is achieved via the recruitment of the diverse array of motor units that comprise the diaphragm muscle.
Areas covered:
The neuromotor control of ventilatory and non-ventilatory behaviors in health and in the context of spinal cord injury is explored. Particular attention is played to the neuroplasticity of phrenic motor neurons in various models of cervical spinal cord injury.
Expert opinion:
There is a remarkable paucity in our understanding of neuromotor control of maneuvers in spinal cord injury patients. Dysfunction of these expulsive/straining maneuvers reduces patient quality of life and contributes to severe morbidity and mortality. As spinal cord injury patient life expectancies continue to climb steadily, a nexus of spinal cord injury and age-associated comorbidities is likely to occur. While current research remains concerned only with the minutiae of ventilation, the major functional deficits of this clinical cohort will persist intractably. We posit some future research directions to avoid this scenario.
Keywords: phrenic motor neurons, motor unit, recruitment, contusion, hemisection, neural circuit, skeletal muscle
1. Introduction
For far too long diaphragm neuromotor control has been slavishly focused on breathing and the response of ventilation to a variety of interventions. While undoubtedly worthwhile with regard to central pattern generation control of eupnea and responses to ventilatory challenge, misguided reductionists have led to the adoption of the idea that diaphragm motor units are merely the homogeneous and monolithic intermediates (the ‘inspiratory motor neurons’) between brainstem respiratory centers and the generation of tidal volumes. In reality, the diaphragm muscle forms the posterior partition for the thoracic cavity and the anterior partition of the abdominal cavity and comprises a mixed motor unit population that allows for generation of transdiaphragmatic pressures (Pdi) [1]. These pressures (negative in the thorax and positive in the abdomen), are essential to a variety of behaviors, with quiet breathing accounting for ~15% of the maximum pressure generating capacity of the diaphragm muscle (Pdimax) in almost all species assessed [1, 2], including humans [1, 2, 3]. In the context of spinal cord injury, the nature of functional impairments may differ along with the therapeutic approach and consequences.
Here we take a nuanced view of diaphragm neuromotor control across four key areas relevant to spinal cord injury: i) distinguishing the three broad categories of diaphragm behavior and the requirements for recruitment of different motor unit types; ii) identifying how different models of spinal cord injury disrupt motor circuitry; iii) exploring the different neuroplastic responses of phrenic motor neurons to various spinal cord injury models; and iv) quantifying the functional outcomes and motor-unit specific underpinnings of deficits observed in different spinal cord injury models, and a brief exploration of therapies designed to improve non-ventilatory activities in patients.
2. Neuromotor control of diaphragm motor units
In addition to its non-trivial role as an anatomic barrier between the thoracic and abdominal cavities [1], the diaphragm muscle serves to effect three main behavioral functions: i) to ventilate the lungs for gas exchange (i.e., breathing); ii) to facilitate pressure generation for effective expulsive maneuvers (e.g. coughing sneezing, parturition and defecation); and iii) to accomplish social, language and emotional tasks (e.g. vocalizations or calls in all mammals and speech and musicianship in humans).
The indefatigable requirement for tidal breathing necessitates generation of nonfatiguing Pdi, at a high duty cycle (time active versus inactive) approaching 40% [4, 5] that is sustained for the entirety of one’s life. This is achieved via the generation of small negative intrathoracic pressures (Pth) resulting from relatively moderate caudal excursions of the diaphragm muscle [6, 7]. Typically, expiration is passive during eupnea, driven by the elastic recoil of the lungs and chest wall that generate a positive Pab [6]. The Pdi necessary for ventilation of the lung is generally around 15% of Pdimax during eupnea and approaches 30-40% Pdimax during maximal ventilatory efforts against an occluded airway [3, 4, 5, 8]. Indeed, during maximum voluntary hyperpnea with controlled CO2, Pdi does not exceed 60% Pdimax [9, 10]. Thus, ventilatory requirements for diaphragm muscle activation are submaximal with considerable reserve capacity for force generation.
The second category of diaphragm muscle activation is during expulsive behaviors that often involve coordinated co-contractions of the diaphragm with abdominal and/or upper airway muscles or lower sphincter muscles [11, 12, 13, 14, 15, 16], depending on the orifice of ejection [1]. These behaviors are driven by central pattern generators that are likely distinct from but interact with the respiratory pattern generator responsible for ventilation [2, 11, 13, 17, 18, 19]. It is important to recognize that these expulsive behaviors are far less frequent, are of shorter duration (i.e., a lower duty cycle), and involve recruitment of more fatigable diaphragm motor units in order to generate higher Pdi, that at times approach Pdimax. Thus, they may involve selective/distinct inputs to larger phrenic motor neurons that comprise more fatigable diaphragm motor units.
The social and emotional functions of the diaphragm muscle also require coordinated activation with other muscles such as the tongue and laryngeal muscles. The functional requirements during these motor behaviors do not require additional diaphragm motor unit types, but rather pattern generators and premotor inputs that are distinct from the ventilatory pattern generator. However, the seamless interruption of breathing during speech and vocalisation indicates interactions with the ventilatory pattern generator and/or premotor circuits, as well as with sympathetic and parasympathetic motor pathways [20, 21]. In humans, speech extends the post-inspiratory phase and reduces duration of the inspiratory phase, allowing for speech to occur relatively unpunctuated [20, 22].
2.1. Diaphragm motor units
To accommodate the diverse pressure generation requirements of these motor behaviors, the diaphragm muscle comprises different motor unit types: slow (type S), fast fatigue resistant (type FR), fast fatigue intermediate (FInt) and fast fatiguable [1, 2, 5, 10, 23, 24, 25, 26] (Figure 1). These mixed motor unit types furnish two broad groups of motor units whose properties denote their behavioral functions: a first set of lower force but highly fatigue resistant motor units (type S and FR) that efficiently generate adequate Pdi to sustain breathing even under more extreme conditions [10, 27], and a second set of higher force but more fatigable motor units (type FInt and FF) that are optimally primed for short duration bursts of force generation approaching Pdimax [1]. The first set of inspiratory-related motor units is best considered as ‘the tidal pool’, responsible for generating the tidal volume during eupnea. The FInt and FF diaphragm motor units are not required for ventilation, despite their motor neurons residing in the phrenic pool and their constituent muscle fibers being distributed throughout the diaphragm muscle [28].
Figure 1:
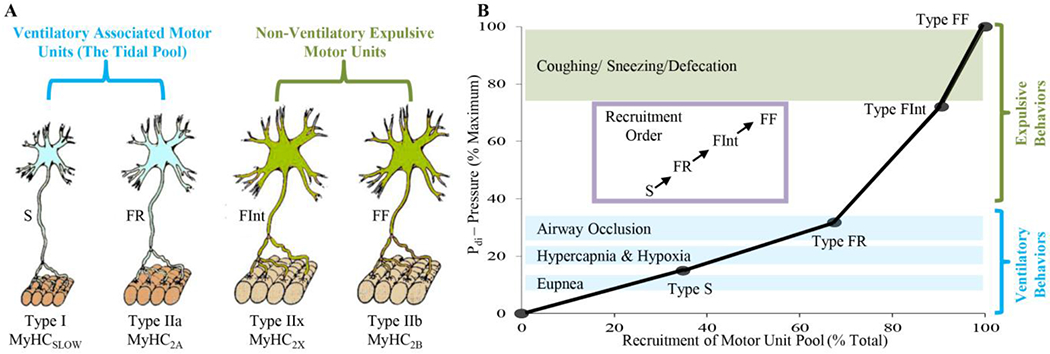
Different diaphragm muscle motor unit types are distinguished by their intrinsic, mechanical, and fatigue properties, and are classified as type S, FR, FInt, and FF. Within an individual motor unit, all constituent muscle fibers exhibit homogeneous myosin heavy chain (MyHC) expression. In the diaphragm muscle of most species, type I and IIa muscle fibers have smaller cross-sectional areas than those of type IIx and/or IIb fibers. Forces produced by type I fibers are less than forces produced by type IIa fibers that are less than forces produced by IIx and/or IIb fibers. Recruitment of diaphragm muscle motor units is in an orderly fashion, necessary to accomplish a range of motor behaviors. Ventilation is accomplished by recruitment type S and FR motor units, whereas higher-force airway clearance behaviors and straining/expulsive manoeuvres require recruitment of more fatigueable type FInt and FF motor units.
Type S diaphragm motor units comprise type I muscle fibers (expressing the slow myosin heavy chain – MyHCslow – isoform) that generate less force but are fatigue resistant and have a higher oxidative capacity (increased mitochondrial volume density) [23, 24, 29, 30, 31, 32]. It is likely that smaller phrenic motor neurons innervate type S motor unit fibers (Figure 1) [33, 34, 35], although this has not been directly established. Type FR diaphragm motor units comprise type IIa fibers (expressing the MyHC2A isoform) that also generate lower specific force compared to other type II fibers but greater than type I fibers and have a higher oxidative capacity accounting for their fatigue resistance (Figure 1) [23, 24, 29, 30, 31, 32]. It is likely that smaller phrenic motor neurons also innervate type FR motor unit fibers [33, 34, 35]. Type FR motor units are more functionally similar to type S motor units than to either type FInt of FF motor units (Figure 1) [1, 5, 10].
More fatiguable type FInt of FF motor units comprise type IIx and/or IIb diaphragm muscle fibers (co-expressing MyHC2X and MyHC2B isoforms in varying proportions) with larger cross-sectional areas (Figure 1) [4, 23, 25, 30, 31, 32, 36]. Single fiber studies have shown that type IIa, IIx and IIb diaphragm fibers generate greater specific forces compared to type I fibers [4, 23, 25, 29, 30, 31, 32, 36]. However, type IIx and/or IIb diaphragm muscle fibers have much lower oxidative capacities that contribute to their increased susceptibility to fatigue [4, 23, 25, 29, 30, 31, 32, 36]. Thus, although the contribution of type IIx and/or IIb fibers to total diaphragm muscle force is proportionately greater, their force contribution cannot be sustained. Phrenic motor neurons innervating type FInt and FF units are also likely to be larger than those of S and FR units [33, 34, 35] and this distinction is important in the appropriate recruitment of motor units during different behaviors (Figure 1).
2.2. Phrenic motor neurons and their recruitment
Phrenic motor neurons are located in the cervical spinal cord (C3-C6 depending on species). In rodents there are ~200-240 on each side [34], providing a total of ~450 diaphragm motor units. In the adult rat, phrenic motor neuron sizes vary, with somal surface areas ranging from ~500 to 8,000 μm 2 [34, 37]. This size variability plays an important role in motor control, with the orderly recruitment of diaphragm motor units being highly dependent on the total neuronal membrane surface area (capacitance). For a given synaptic input, the change in membrane potential (ΔVm/Δt) of smaller (lower capacitance) motor neurons is greater leading to earlier generation of action potentials (i.e., they are more excitable and recruited earlier) compared to larger (higher capacitance) motor neurons with lower intrinsic excitability – the Size Principle [5, 38, 39]. The size of motor neurons is also reflected by axonal diameters and axonal conduction velocities. Indeed, the Size Principle first proposed by Henneman was based on the observation that earlier recruited motor units displayed slower conduction velocities (i.e., smaller axonal diameters) [38, 39]. This distinction in the recruitment order of diaphragm motor units based on axonal conduction velocities was later validated by Dick et al [40]. Importantly, within a given motor unit type, the intrinsic properties of motor neurons are less variable, including properties such as axonal conduction velocity, discharge rates, somal surface areas, dendritic arborization and innervation ratio [34, 35, 40, 41, 42, 43, 44, 45, 46]. Accordingly, we introduced a model of diaphragm motor unit recruitment that assumes an orderly recruitment of type S, then type FR, followed by type FInt and FF units to accomplish increasing Pdi generation across different behavioral requirements (Figure 1) [1, 5, 10, 26, 40, 46, 47].
There are five main circuit components that are included in the neuromotor control of diaphragm muscle during different motor behaviors (Figure 2): i) phrenic motor neurons; ii) central pattern generator responsible for the timing of diaphragm activation (and co-activation of other muscles) during the specific motor behavior; iii) premotor neurons responsible for transmitting the output of the central pattern generator and integrating sensory and other inputs; iv) interneurons (both local and ascending) responsible for modulating premotor neuron and/or phrenic motor neuron excitability; and v) direct cortical premotor input to phrenic motor neurons via the corticospinal pathway.
Figure 2:
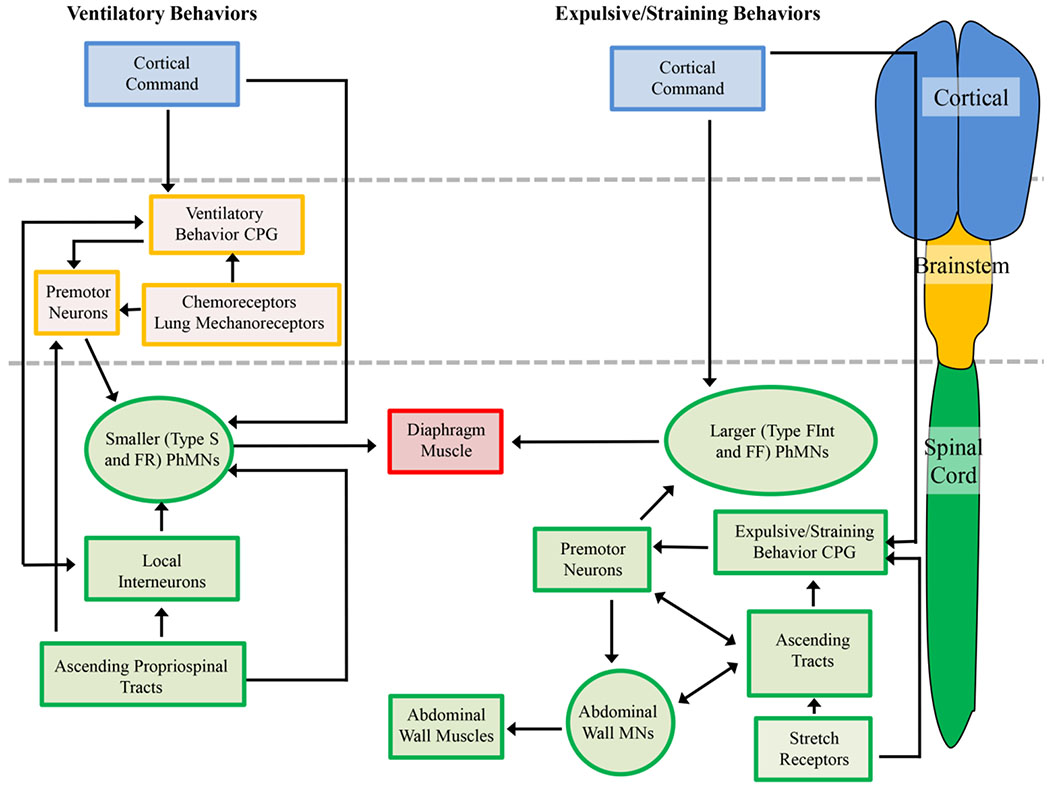
Neuromotor control of diaphragm muscle ventilatory and expulsive/straining behaviors requires cortical (blue boxes), brainstem (orange boxes) and spinal cord (green boxes) centers. Ventilatory behaviors are the well characterized and require the recruitment of predominantly type S and FR motor units. Cortical pathways are able to modulate the eupnic rhythm by interactions with the ventilatory central pattern generator (CPG) or directly via synapses onto phrenic motor neurons (PhMNs). The ventilatory CPG activates brainstem premotor neurons that in turn innervate the PhMNs. Activity of PhMNs during ventilation is also modulated (directly and indirectly) by spinal cord ascending tracts and interneurons. Brainstem chemoreceptors and lung mechanoreceptors regulate the activity of premotor neurons, and act to increase premotor neuron discharge (and thus PhMN activity) during hypoxia/hypercapnia. In the case of expulsive/straining behaviors, the majority of control centers are located within the spinal cord, and recruitment of type FInt and FF motor units (higher-force producing units) is necessitated. Some cortical control of the PhMNs and spinal expulsive/straining CPG may be evident, but rectal and vaginal stretch receptors also elicit strong Pab generation. There may be shared spinal premotor neurons within the spinal cord for co-activations of PhMNs and abdominal muscle MNs, with a variety of ascending projections coordinating these activities.
The neuromotor circuitry involved in activation of the diaphragm muscle during ventilatory behaviors has been very well-described (Figure 2). These previous studies reflect an intense focus on the ventilatory central pattern generator in the PreBötzinger complex, which represent the spontaneously active ‘kernel’ of neurons for the metronomic drive for inspiratory activation of the diaphragm [2, 48]. The location of inspiratory premotor neurons in the ventrolateral medulla (ventral respiratory group) and dorsomedial medulla (dorsal respiratory group) has been well documented. These medullary premotor neurons provide a predominantly ipsilateral monosynaptic drive to phrenic motor neurons during inspiration (Figure 3) [49, 50, 51, 52, 53, 54, 55]. If this descending bulbospinal presynaptic input is uniformly distributed, the recruitment of phrenic motor neurons would solely depend on intrinsic, size-dependent electrophysiological properties of motor neurons (i.e., the Size Principle). However, in a recent study, we found that glutamatergic presynaptic terminal density is higher on smaller phrenic motor neurons [37], that likely innervate type S and FR motor units that are involved in ventilatory behaviors. Similarly, we recently reported that expression of glutamatergic NMDA and AMPA receptors depends on phrenic motor neuron size with smaller motor neurons having a greater density of NMDA and AMPA receptor mRNA transcripts compared to larger motor neurons [56]. Thus, in addition to intrinsic motor neuron properties, the recruitment of fatigue resistant type S and FR motor units is guaranteed by the differential distribution of excitatory bulbospinal glutamatergic drive to smaller phrenic motor neurons (Figure 3).
Figure 3:
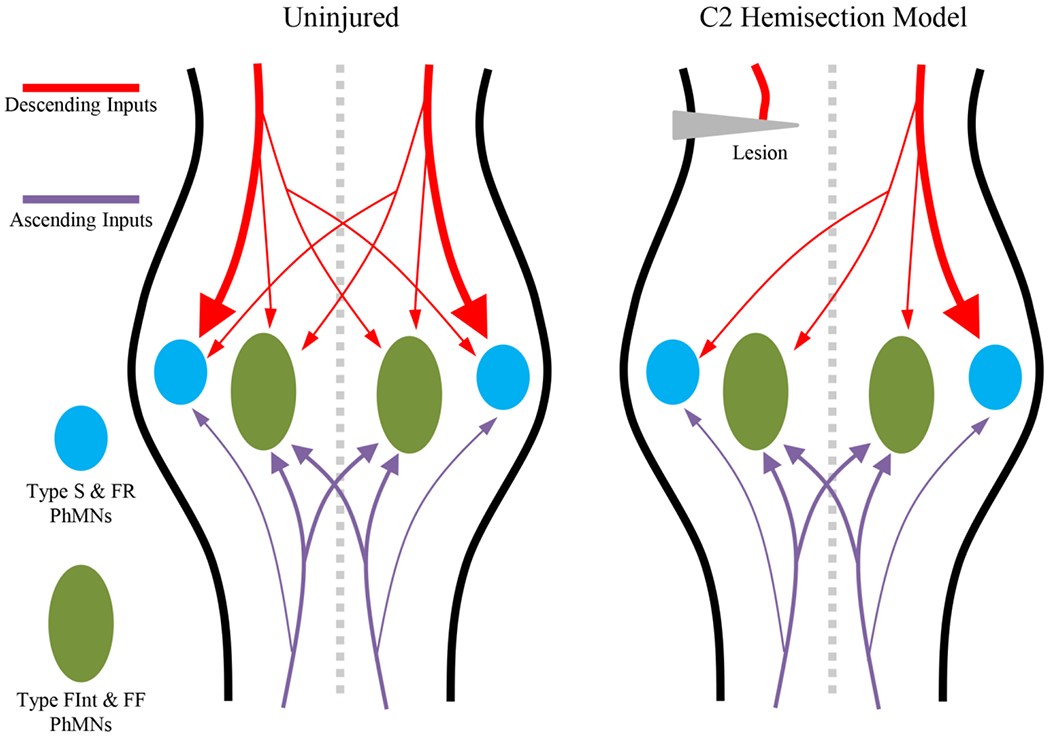
The majority of phrenic motor neuron glutamatergic inputs are derived from descending (red) and ascending (purple) tracts. In the uninjured spinal cord, the majority of the bulbospinal descending inputs are distributed ipsilaterally to type S and FR phrenic motor neurons (PhMNs, blue), with small amounts of contralateral input to type S and FR PhMNs and a modicum of ipsi- and contralateral inputs to type FInt and FF motor units (green). Ascending inputs are primarily activated by co-contractions with abdominal muscles and both ipsi- and potentially contralateral inputs are predominantly on type FInt and FF PhMNs. Following unilateral C2 cervical hemisection, the inputs transected are the ipsilateral bulbospinal descending projections onto type S and FR PhMNs, accounting for impairments in ventilatory behaviors. In this model, the majority of inputs to type FInt and FF PhMNs remain, accounting for the preserved Pdimax.
Peripheral and central chemoreceptors are found in the carotid bodies and brainstem, respectively, and increase ventilatory drive in response to hypoxia and/or hypercapnia, respectively [2]. Lung mechanoreceptors are sensitive to lung inflation and act to prevent airway over-inflation [2]. Local inhibition of phrenic motor neurons from interneurons within the spinal cord has also been characterized [57, 58]. Additionally there are direct corticospinal inputs [59, 60] that allow for the voluntary control of ventilation or expulsive maneuvers, as well as during social and emotional activities [21, 61].
By stark contrast to the neural control of breathing, the circuits involved in expulsive/straining behaviors remain poorly characterised (Figure 2) [1, 2]. In expulsive maneuvers such defecation, vomiting, coughing and childbirth, the diaphragm muscle is often co-activated with upper airway, chest wall and/or abdominal muscles. The pattern generators (reflex centers) for these expulsive/straining behaviors are poorly defined but are likely located in the brainstem or spinal cord, similar to other pattern generators for vomiting [11, 12], sigh [62], swallowing [63], locomotion [64], micturition [65, 66] and ejaculation [67]. In addition, how these neural circuits interact or perhaps share common components with the ventilatory circuits [11, 13, 17, 18, 19, 68] or instead project directly to phrenic motor neurons [69, 70, 71] is obscure. Regardless, these expulsive/straining behaviors require the recruitment of the higher pressure generating but more fatigueable FInt and FF diaphragm motor units, with Pdi often in excess of 200 cm H2O [72, 73, 74, 75]. Perhaps our best knowledge of the neuromotor circuitry associated with these behaviors comes from studies investigating spinal cord injury and neuroplasticity in both animal models and clinical patients. The remainder of this review is concerned with these insights.
3. Models of spinal cord injury
Almost 17,000 new cases of spinal cord injury are diagnosed in the USA each year. The majority of these injuries involve the cervical spinal cord and result in significant impairment of diaphragm muscle activity [76]. A subset of these patients are unable to maintain adequate ventilation and become dependent on mechanical ventilation, a situation associated with substantial morbidity and mortality [77, 78]. Most spinal cord injuries are incomplete (~70%), with incomplete tetraplegia (cervical injury) accounting for 20.4% of spinal cord injury since 2015, with complete tetraplegia accounting for 11.5% of all injury [79]. Incomplete injuries largely affect only ipsilateral injury side portions of the descending bulbospinal excitatory inspiratory drive from the medulla to the phrenic motor neuron pool, although high level lesions (C2) can affect both sides of the diaphragm muscle and chest wall [80]. These defects cause partial or complete paralysis of one side of the diaphragm during eupnea [76]. Post injury, variable recovery of ventilatory-related diaphragm muscle activity is observed [81], although the precise mechanisms underlying recovery of ventilatory function are not fully described. Overall, deficits in ventilatory activity following spinal cord injury range from 40-60% of expected forced vital capacity [76, 82], however, the contribution of diaphragm muscle alone to these deficits is confounded by increased airway secretions, reduced chest wall stiffness, reduced chest wall compliance, atelectasis and altered abdominal muscle tone, impairing Pdi generation [76]. The impact of spinal cord injury on expulsive/straining behaviors is poorly understood, but dysfunction of these behaviors underlies diminished quality of life for spinal cord injury patients. Two models of spinal cord injury are routinely used to explore the mechanisms underlying diaphragm muscle dysfunction and recovery, namely, contusion injury and cervical spinal hemisection.
3.1. Cervical contusion injury model
Contusion injuries are the most common type of spinal cord injury, with substantial effects on long-term morbidity as well as mortality [76]. Animal studies of traumatic spinal cord injury commonly employ cervical contusion models [83, 84], including unilateral lesions [85, 86, 87, 88, 89, 90]. These models typically involve a cervical dorsal laminectomy, followed by collision with an impactor (with varying tip sizes ~1-2 mm) producing an impact force of 100-400 kDy. In addition to a range of forces, contusion models also differ in their placement, with midline and unilateral lesions employed. Despite faithful recapitulation of various histopathological hallmarks of spinal cord injury [86, 91], cervical contusion models are inconsistent in demonstrating alterations in diaphragm motor unit activity and ventilatory or non-ventilatory behaviors [83, 84, 85, 87, 88, 90, 92]. Inconsistencies in the effects of contusion models relate to differences in the extent of phrenic motor neuron and interneuron death, as well as the involvement of white matter tracts (ascending, descending and segmental) [86] that play major roles in neuromotor control of diaphragm motor units (see Figure 3) [1, 2]. The inconsistencies in these models limit their utility to provide mechanistic insight or as a key prognostic indicator for human functional outcomes [93]. For example, we showed that following unilateral mid-cervical contusion injury in rats, there is extensive loss of ~50% of phrenic motor neurons on the affected side [86, 88, 89, 90, 94] and a ~30% decrease in Pdimax evoked by bilateral phrenic stimulation [85]; yet there is very little effect on the performance of ventilatory behaviors [86, 88, 89, 90, 94]. Indeed, unilateral denervation of the diaphragm muscle with total unilateral paralysis does not affect the performance of ventilatory behaviors [95]. This is not surprising since ventilatory behaviors of the diaphragm muscle require less than 50% of Pdimax [1, 4, 10, 85]. Importantly, without a clear loss of ventilatory function in the contusion model, the ability of this model to provide insight for the development of therapeutic approaches to promote functional recovery is severely limited. However, with a decrease in neural output to the diaphragm (reflected by a decrease in evoked Pdimax), it is likely that there are deficits in expulsive/straining behaviors of the diaphragm muscle in contusion models of spinal cord injury. Unfortunately, deficits in these expulsive/straining diaphragm muscle behaviors have not been adequately demonstrated.
3.2. Cervical spinal hemisection model
In 1895, John Porter introduced the cervical spinal hemisection animal model to investigate neuroplasticity of diaphragm muscle neuromotor control following spinal cord injury [96]. The cervical spinal hemisection technique is commonly performed at the C2 level, rostral to the phrenic motor pool, thereby disrupting ipsilateral premotor input to phrenic motor neurons. Similarly to the contusion model, this model involves a dorsal laminectomy followed by unilateral transection transecting anteriolateral projections, specifically the ipsilateral bulbospinal pathways (Figure 3) [49, 50, 51, 52, 53, 54, 55]. Despite the presence of a modicum of contralateral descending inputs in addition to ascending columns projecting to cervical motor pools (Figure 3) [2], unilateral paralysis of eupneic diaphragm muscle activity ensues following transection [96, 97, 98, 99, 100, 101, 102, 103, 104, 105, 106, 107, 108, 109]. The absence of inspiratory-related diaphragm muscle EMG activity is validated at the time of surgery, providing a clear marker for the subsequent spontaneous recovery of ventilatory function over time (neuroplasticity) [101, 102, 103, 104, 105, 106, 107, 109, 110, 111]. A number of laboratories have used the cervical spinal hemisection model, and thus it is well-characterized and lacks many of the vagaries (scarring, inflammation, penumbra of injury) of the contusion model. Importantly, there is no loss of phrenic motor neurons in the cervical spinal hemisection model of injury, despite unsubstantiated reports from some laboratories. Furthermore, axotomy of the descending corticospinal and bulbospinal tracts does not result in marked death of premotor neurons [112, 113, 114, 115]. Recently, we did report that by two weeks following C2 spinal cord hemisection, the size (somal surface areas) of phrenic motor neurons decreases, consistent with an increase in excitability that may underlie functional recovery.
4. Phrenic motor neurons and spinal cord injury
The primary prognostic determinant of neuromotor deficits following cervical spinal cord injuries is the extent of motor pathway disruption [76, 116]. For diaphragm motor units, the types of functional deficits present are largely dependent on the extent of bulbospinal disruption and the extent of phrenic motor neuron loss [76, 86]. There is a substantial capacity for neuroplasticity exhibited by the diaphragm neuromotor control system that may be harnessed for recovery. This plasticity is provided for by multiple substrates, including axonal sprouting of spared bulbospinal or interneuronal tracts, altered intrinsic properties of phrenic motor neurons (e.g., morphological changes) and enhancement of neurotrophic signaling pathways (e.g., brain-derived neurotrophic factor – BDNF/tropomyocin-receptor kinase B – TrkB).
4.1. Presynaptic neuroplasticity at phrenic motor neurons following spinal cord injury
There is considerable regenerative neuroplastic capacity in the neural inputs onto phrenic motor neurons following spinal cord injury. These involve inputs from contralateral bulbospinal projections, local segment interneurons and ascending projections from more caudal regions of the spinal cord [52, 99, 101, 102, 103, 104, 109, 111, 117, 118, 119]. In cases where ventilatory behaviors are impaired, such as in the cervical spinal hemisection model, synaptic stripping occurs for the axotomized inputs, with a marked reduction in the number of excitatory glutamatergic pre-synaptic terminals remaining on phrenic motor neurons [120]. In a preliminary study from our group, we found that following unilateral cervical spinal hemisection, the extent of loss of glutamatergic presynaptic terminals (synaptic stripping) is much greater on smaller phrenic motor neurons. This obsevation confirms that descending inspiratory presynaptic drive for ventilatory behaviors is primarily ipsilateral [49, 50, 51, 52, 53, 54, 55] and further suggests this drive is distributed disproportionately to smaller phrenic motor neurons. It is possible, that a signficant proportion of presynaptic drive for expulsive /straining behaviors does not emanate from a supraspinal origin. In limb locomotor control, the central pattern generator and presynpatic neurons are located segmentally in the spinal cord [1]. This may also be the case for expulsive/straining behaviors of the diaphragm [11, 13, 17, 18, 19, 68], particularly those that involve co-activation of chest wall and abodminal muscles (Figure 3).
4.2. Postsynaptic neuroplasticity of phrenic motor neurons following spinal cord injury
Postsynaptic neuroplasticity of phrenic motor neurons may be divided into alterations of intrinsic neuronal properties, primarily through morphological adaptations, and alterations of neurotransmitter receptors and/or the expression of various subtypes.
Phrenic motor neurons display a wide range of neuronal surface areas, with their intrinsic excitability dictated by their capacitance (see section 1.2), as determined by the overall neuronal membrane surface area. Following spinal cord injury, the somal and dendritic compartments respond to alter intrinsic excitability. In the cervical spinal cord hemisection model, phrenic motor neurons exhibit reduced somal surface areas [121] and maintenance of the dendritic compartment surface area [121]. These changes are consistent with increased excitability of phrenic motor neurons thereby promoting recovery of eupneic activity. Alterations in somal or dendritic morphology following contusion injury have not been systematically examined. There is little reason to expect that contusion injury would have a selective size-dependent loss of phrenic motor neurons. By comparison, other conditions where phrenic motor neurons loss occurs over a longer time period affect primarily larger motor neurons [2, 33, 34, 122].
In addition to the remarkable structural neuroplasticity that is evident subsequent to spinal cord injury, modulation of receptor expression plays a key role in priming phrenic motor neurons for recovery. As mentioned above, smaller phrenic motor neurons have a greater density of NMDA and AMPA receptor mRNA transcripts compared to larger motor neurons [56]. Early evidence for receptor-mediated neuroplasticity came from ultrastructural studies documenting increased synaptic apposition and double synapses [123, 124]. More recently, we reported a robust up-regulation of NMDA glutamatergic receptor mRNA expression in phrenic motor neurons that was associated with spontaneous recovery of eupnea following cervical spinal hemisection [119, 125]. Enhanced serotonergic signalling has also shown to be of importance in the recovery of diaphragm muscle ventilatory behaviors following cervical spinal hemisection [125, 126, 127, 128, 129] and contusion [92]. The precise interactions between phrenic motor neuron size, recovery of ventilatory behaviors and the neuroplastic enhancement by neurotrophins (including BDNF) [101, 102, 103, 109, 110, 111] is not fully determined. To this end, recent preliminary work by our group shows that smaller phrenic motor neurons express a greater amount of TrkB receptor compared to larger phrenic motor neurons. This finding may account for the ~30% spontaneous recovery from cervical spinal hemisection of ventilatory efforts and the robust effectiveness of neurotrophic enhancements in this particular model.
5. Diaphragm muscle functional deficits following spinal cord injury
The functional outcomes from the differing models of spinal cord injury depend on the model being assessed. In the case of cervical contusion models, the indiscriminate loss of phrenic motor neurons innervating type S, FR, FInt and FF motor units leads to an overall decrease in the generation of Pdimax, with no deficit in the performance of diaphragm muscle function. Unknown, but likely, deficits arise in the performance in the performance of expulsive/straining behaviors [85, 86, 94]. Importantly, the performance of expulsive/straining maneuvers by the diaphragm muscle display very little reserve capacity, with these behaviors necessitating recruitment of almost all diaphragm motor units [1, 2, 5, 10, 26, 27]. These observations are consistent with those in human clinical cohorts, with impaired Valsalva maneuvers [130], weak cough [131, 132, 133, 134], defecation difficulties (constipation, increased transit time) [135, 136, 137] and parturition difficulties (particularly stage 2 of labor) [138]. In patients, increased airway secretions, changes in lung and chest wall mechanics and reduced abdominal muscle activity may impair ventilation, particularly during high cervical injuries [76]. These factors all contribute to a ventilatory phenotype without requiring substantial alterations of S and FR diaphragm motor units.
Although ventilatory efforts of the diaphragm muscle are absent or markedly attenuated following cervical spinal hemisection, Pdimax is preserved. Unfortunately, functional deficits in the performance of higher force expulsive/straining behaviors of the diaphragm have not been examined. However, ventilatory behaviors that require greater Pdi generation (e.g., efforts against an occluded airway) are less impacted by cervical spinal cord hemisection. Instead, the functional changes are primarily in the ventilatory pattern and unilateral diaphragm muscle paralysis during ventilation [51, 96, 99, 101, 102, 103, 104, 110, 111, 117, 119, 139, 140, 141, 142, 143], consistent with the selective disruption of ipsilateral bulbospinal respiratory glutamatergic drive [49, 50, 51, 52, 53, 54, 55]. Spontaneous recovery (~40% by 14 days post-injury) following cervical spinal hemisection does occur, although not to pre-injury levels [103, 104, 110, 117, 139, 140, 141, 142, 143]. The substrate for the recovery of diaphragm muscle activity are latent contralateral, segmental and ascending inputs (Figure 3) [99, 101, 102, 103, 104, 109, 111, 117, 119, 144], although the precise motor unit specifics and pre- versus post-synaptic potencies remain undescribed.
Clinically, a variety of rehabilitative strategies have been developed in order to improve diaphragm muscle function following spinal cord injury. Ventilatory assistance is more common in the acute phase post-injury, related to spinal shock, excessive airway secretions and immediate flaccid paralysis of muscles below the level of injury, including intercostal muscles [82, 133, 145, 146, 147]. This latter results in the ribcage collapsing with negative intrathoracic pressures, substantially impinging on the effectiveness of lung ventilation [82]. However, with little changes in PaCO2 between cervical and lower injuries [148] and only 10% of all spinal cord injury patients being hypoxaemic [149], mechanical ventilation weaning is a priority in all but the most severe cases of diaphragm paralysis following spinal cord injury. In the longer term, and with the goal of providing for expulsive/straining behaviors, a variety of treatment options exist, including manually assisted cough, mechanical insufflation/exsufflation devices and functional electrical stimulation of abdominal muscle. Manually assisted cough and the successful operation of insufflation/exsufflation devices requires the assistance of trained caregivers, which is often expensive or inconvenient. Furthermore these techniques are fail to produce peak cough flows > 4.5 l/s [150, 151], necessary to reduce risk of acute respiratory failure [152]. By contrast, functional electrical stimulation of abdominal muscle results in peak cough flows of >7 l/sec [153, 154], albeit in patients with implanted electrodes, although reasonable peak flows are achieved using surface electrodes ~3L/s, that are improved with muscle training [155]. Aside from the cost of the technology, the adoption of stimulation methods is limited to those who have intact motor neurons in the spinal pools innervating the abdominal muscles. While functional electrical stimulation of abdominal muscles does improve cough effectiveness, it does little to improve the function of the diaphragm muscle, whose inspiratory action is of greater importance than expiratory muscles, as low volumes of inspired air decrease cough capacity, regardless of the effectiveness of active expiratory muscles [156, 157]. Although pressure generation of the oesophagus and gastric compartments remain the best measure of cough effectiveness and muscle strength [4, 85, 158, 159, 160], they are often overlooked in favour of flow measures [161].
6. Conclusions
Diaphragm neuromotor control is about far more than just breathing, with diaphragm muscle activation required to accomplish a wide assortment of expulsive/straining maneuvers. As the emergency and ongoing care of patients with spinal cord injury steadily improves, there will be an increased onus on pre-clinical and clinical researchers to preserve these higher-force airway defence behaviors that are highly correlated with mortality and morbidity. Thus studies in neuromotor control of the diaphragm muscle should be of interest beyond the usual coterie of ventilation-centric studies.
7. Expert opinion
There are two major hindrances to increasing the translational potential of recent discoveries in the neuroplasticity of diaphragm motor units. The first problem is the lack of real data on the burden of expulsive/straining deficits in the spinal cord injury clinical population. To date there have been no exhaustive studies or surveys of abdominal pressure generation in clinical cohorts with spinal cord injury, with the prime clinical assessment being the ability to ventilate. As the life expectancy of spinal cord injury sufferers continues to improve [162], and as the inclusivity of this cohort to activities such as pregnancy and childbirth increase [138], there remains an immediate need for some baseline quantifications of non-ventilatory maneuvers in these patients. Many other conditions of the diaphragm muscle selectively afflict the type FInt and FF diaphragm motor units, including age-associated weakness and atrophy (sarcopenia) [4, 31, 34, 163], malnutrition [36] and chronic obstructive pulmonary disease [164]. The FInt and FF motor units are activated to perform the expulsive/straining maneuvers that we know the least about in spinal cord injury patients. It behoves us a research community to address this issue, as these co-morbidities are going to become much more common as life span and quality of life expectations increase in the spinal cord injury cohort. Additionally, these concerns are not limited to the subset of patients with a cervical injury.
The second problem remains the obdurate focus of preclinical research into the minutiae of ventilation and ventilatory control. In many cases of cervical spinal cord injury, similar to the contusion models, there is little long-term compromise of eupnea. However, the substantial risk of airway infections remains, due to the lack of airway defence and effective clearance [76, 77]. It is important that issues such as weaning from mechanical ventilation continue to be addressed, but these constitute the minority of cases and are likely intractable in the absence of any paradigm-shattering advance.
Over the next five years, an adequate description of the specific motor unit types afflicted in various spinal cord injury models is necessary, along with a precise description of the specific functional deficits associated with injury. Similarly, the effect of therapies and interventions must be assessed in light of the motor units and type of behaviour affected. For too long, successful intervention has been defined as being a return to the normal pattern and tidal volume generation during eupnea. It is essential that assessment of non-ventilatory expulsive/straining maneuvers becomes de rigueur in the testing of interventions aimed at improving diaphragm muscle function. For enrolment of patients, eligibility criteria would not be exclusive to those with a cervical injury. Patients could be funnelled into pressure-generation assessments based on anecdotal histories (i.e., previous complaints of constipation or weak cough). At first blush, a cross-sectional study would provide some actionable data regarding abdominal pressure generation efforts, to be followed up by longitudinal studies and stratification by age and gender. For preclinical studies, various efforts outlined in this review to classify the effectiveness of intrinsic and circuit (input) substrates for recovery in a size-dependent manner are a reasonable start. We wait with bated breath for a wider adoption of motor unit diversity in the design and characterisation of future spinal cord injury research.
Article highlights.
Neuromotor control of the diaphragm muscle is important for both ventilatory and non-ventilatory behaviors.
The neuromotor circuitry that is integrated by phrenic motor neurons is likely to be different for different types of behaviour.
Cervical spinal cord injuries and models of cervical spinal cord injury have differential effects on ventilatory and non-ventilatory behaviour
Non-ventilatory expulsive/straining maneuvers cause the majority of morbidity and mortality in the spinal cord injury population, but very little quantitative data exists regarding these deficits.
We outline some of the urgent pre-clinical and clinical directions to address non-ventilatory outcomes of spinal cord injury.
Acknowledgments
Funding
Ongoing work in this area is provided by the funding agency NIH under grants R01-AG044615 and R01-HL146114.
Footnotes
Declaration of interest
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript. This includes employment, consultancies, honoraria, stock ownership or options, expert testimony, grants or patents received or pending, or royalties. The authors have nothing to disclose and declare no actual or perceived conflicts of interest.
Reviewer disclosures
Peer reviewers on this manuscript have no relevant financial or other relationships to disclose.
References
Papers of special note have been marked as of considerable interest (*) to readers.
- 1.Fogarty MJ, Sieck GC. Evolution and Functional Differentiation of the Diaphragm Muscle of Mammals. Compr Physiol. 2019;9(2):715–766. [DOI] [PMC free article] [PubMed] [Google Scholar]; * This review is contains a synthesis of the best information available regarding the ventilatory and non-ventilatory behaviours of the diaphragm muscle.
- 2.Fogarty MJ, Mantilla CB, Sieck GC. Breathing: Motor Control of Diaphragm Muscle. Physiology (Bethesda). 2018. March 1;33(2):113–126. doi: 10.1152/physiol.00002.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lisboa C, Pare PD, Pertuze J, et al. Inspiratory muscle function in unilateral diaphragmatic paralysis. Am Rev Respir Dis. 1986. September;134(3):488–92. doi: 10.1164/arrd.1986.134.3.488. [DOI] [PubMed] [Google Scholar]
- 4.Khurram OU, Fogarty MJ, Sarrafian TL, et al. Impact of aging on diaphragm muscle function in male and female Fischer 344 rats. Physiol Rep. 2018. July;6(13):e13786. doi: 10.14814/phy2.13786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sieck GC, Fournier M. Diaphragm motor unit recruitment during ventilatory and nonventilatory behaviors. J Appl Physiol. 1989;66(6):2539–2545. [DOI] [PubMed] [Google Scholar]
- 6.Herxheimer H Some observations on the coordination of diaphragmatic and rib movement in respiration. Thorax. 1949. March;4(1):65–72. doi: 10.1136/thx.4.1.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haber K, Asher M, Freimanis AK. Echographic evaluation of diaphragmatic motion in intra-abdominal diseases. Radiology. 1975. January;114(1):141–4. doi: 10.1148/114.1.141. [DOI] [PubMed] [Google Scholar]
- 8.Sadoul N, Bazzy AR, Akabas SR, et al. Ventilatory response to fatiguing and nonfatiguing resistive loads in awake sheep. J Appl Physiol. 1985;59:969–978. [DOI] [PubMed] [Google Scholar]
- 9.Belman MJ, Sieck GC. The ventilatory muscles: Fatigue, endurance and training. Chest. 1982;82:761–766. [DOI] [PubMed] [Google Scholar]
- 10.Fogarty MJ, Sieck GC. Diaphragm muscle adaptations in health and disease. Drug Discovery Today: Disease Models. 2019;In Press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koga T, Fukuda H. Bulbospinal augmenting inspiratory neurons may participate in contractions of the diaphragm during vomiting in decerebrate dogs. Neurosci Lett. 1994. October 24;180(2):257–60. [DOI] [PubMed] [Google Scholar]
- 12.Milano S, Grelot L, Bianchi AL, et al. Discharge patterns of phrenic motoneurons during fictive coughing and vomiting in decerebrate cats. J Appl Physiol. 1992. October;73(4):1626–36.; eng. [DOI] [PubMed] [Google Scholar]
- 13.Fukuda H, Fukai K. Location of the reflex centre for straining elicited by activation of pelvic afferent fibres of decerebrate dogs. Brain Res. 1986. August 20;380(2):287–96. [DOI] [PubMed] [Google Scholar]
- 14.Fukuda H, Fukai K. Postural change and straining induced by distension of the rectum, vagina and urinary bladder of decerebrate dogs. Brain Res. 1986. August 20;380(2):276–86. [DOI] [PubMed] [Google Scholar]; * Seminal study showing co-activations of diaphragm muscle with upper airway and abdominal musculature following an assortment of reflex arc stimulations.
- 15.Shafik A Straining urethral reflex: description of a reflex and its clinical significance. Preliminary report. Acta Anatomica. 1991;140(2):104–7. [DOI] [PubMed] [Google Scholar]
- 16.Shafik A Straining puborectalis reflex: description and significance of a “new” reflex. Anat Rec. 1991. February;229(2):281–4. doi: 10.1002/ar.1092290216. [DOI] [PubMed] [Google Scholar]
- 17.Fukuda H, Fukai K. Discharges of bulbar respiratory neurons during rhythmic straining evoked by activation of pelvic afferent fibers in dogs. Brain Res. 1988. May 24;449(1–2):157–66. [DOI] [PubMed] [Google Scholar]; * Established interactions of the ventilatory centers with ascending tracts activated by straining.
- 18.Miller AD, Wilson VJ. ‘Vomiting center’ reanalyzed: an electrical stimulation study. Brain Res. 1983;270(1):154–158. [DOI] [PubMed] [Google Scholar]
- 19.Miller AD, Leslie RA. The area postrema and vomiting. Front Neuroendocrinol. 1994;15:301–320. [DOI] [PubMed] [Google Scholar]
- 20.Larnon AMM, Hewitt GP. The Evolution of Human Speech: The Role of Enhanced Breathing Control. American Journal of physical anthropology. 1999;109:341–363. [DOI] [PubMed] [Google Scholar]
- 21.Holstege G, Subramanian HH. Two different motor systems are needed to generate human speech. J Comp Neurol. 2016. June 1;524(8):1558–77. doi: 10.1002/cne.23898. [DOI] [PubMed] [Google Scholar]
- 22.Conrad B, Schonle P. Speech and respiration. Arch Psychiatr Nervenkr (1970). 1979. April 12;226(4):251–68. [DOI] [PubMed] [Google Scholar]
- 23.Enad JG, Fournier M, Sieck GC. Oxidative capacity and capillary density of diaphragm motor units. J Appl Physiol. 1989;67((2)):620–627. [DOI] [PubMed] [Google Scholar]
- 24.Sieck GC. Diaphragm muscle: Structural and functional organization. Clin Chest Med. 1988;9(2):195–210. [PubMed] [Google Scholar]
- 25.Sieck GC, Fournier M, Enad JG. Fiber type composition of muscle units in the cat diaphragm. Neuroscience Letters. 1989. February 13;97(1–2):29–34. [DOI] [PubMed] [Google Scholar]
- 26.Sieck GC. Neural control of the inspiratory pump. NIPS. 1991;6:260–264. [Google Scholar]
- 27.Fogarty MJ, Mantilla CB, Sieck GC. Impact of sarcopenia on diaphragm muscle fatigue. Exp Physiol. 2019. July;104(7):1090–1099. doi: 10.1113/EP087558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fournier M, Sieck GC. Somatotopy in the segmental innervation of the cat diaphragm. J Appl Physiol (1985). 1988. January;64(1):291–8. [DOI] [PubMed] [Google Scholar]
- 29.Burke RE, Levine DN, Zajac FE, 3rd. Mammalian motor units: physiological-histochemical correlation in three types in cat gastrocnemius. Science. 1971;174(10):709–12. [DOI] [PubMed] [Google Scholar]; * Early description of motor unit types.
- 30.Geiger PC, Cody MJ, Macken RL, et al. Maximum specific force depends on myosin heavy chain content in rat diaphragm muscle fibers. J Appl Physiol. 2000;89(2):695–703. [DOI] [PubMed] [Google Scholar]; * Detailed single fiber analysis of the force-generation and cross-sectional areas of muscle fibers from different types of motor units present in diaphragm muscle.
- 31.Fogarty MJ, Marin Mathieu N, Mantilla CB, et al. Aging Reduces Succinate Dehydrogenase Activity in Rat Type IIx/IIb Diaphragm Muscle Fibers. J Appl Physiol (1985). 2019. November 27. doi: 10.1152/japplphysiol.00644.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sieck GC, Fournier M, Prakash YS, et al. Myosin phenotype and SDH enzyme variability among motor unit fibers. J Appl Physiol. 1996. June;80(6):2179–2189. [DOI] [PubMed] [Google Scholar]
- 33.Dukkipati SS, Garrett TL, Elbasiouny SM. The vulnerability of spinal motoneurons and soma size plasticity in a mouse model of amyotrophic lateral sclerosis. J Physiol. 2018. May 1;596(9):1723–1745. doi: 10.1113/JP275498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fogarty MJ, Omar TS, Zhan WZ, et al. Phrenic motor neuron loss in aged rats. J Neurophysiol. 2018. May 1;119(5):1852–1862. doi: 10.1152/jn.00868.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fogarty MJ, Mu EW, Lavidis NA, et al. Size-dependent vulnerability of lumbar motor neuron dendritic degeneration in SOD1G93A mice. Anat Rec (Hoboken). 2019;In Press. [DOI] [PubMed] [Google Scholar]
- 36.Sieck GC, Lewis MI, Blanco CE. Effects of undernutrition on diaphragm fiber size, SDH activity, and fatigue resistance. J Appl Physiol. 1989;66:2196–2205. [DOI] [PubMed] [Google Scholar]
- 37.Rana S, Mantilla CB, Sieck GC. Glutamatergic Input Varies with Phrenic Motor Neuron Size. J Neurophysiol. 2019. August 7. doi: 10.1152/jn.00430.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Henneman E Relation between size of neurons and their susceptibility to discharge. Science. 1957. December 27;126(3287):1345–7.; eng. [DOI] [PubMed] [Google Scholar]
- 39.Henneman E, Somjen G, Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965. May;28(3):560–580. [DOI] [PubMed] [Google Scholar]
- 40.Dick TE, Kong FJ, Berger AJ. Correlation of recruitment order with axonal conduction velocity for supraspinally driven diaphragmatic motor units. J Neurophysiol. 1987;57(1):245–259. [DOI] [PubMed] [Google Scholar]
- 41.Cullheim S, Fleshman JW, Glenn LL, et al. Three-dimensional architecture of dendritic trees in type-identified alpha-motoneurons. J Comp Neurol. 1987;255(1):82–96. [DOI] [PubMed] [Google Scholar]
- 42.Cullheim S, Fleshman JW, Glenn LL, et al. Membrane area and dendritic structure in type-identified triceps surae alpha motoneurons. J Comp Neurol. 1987;255(1):68–81. [DOI] [PubMed] [Google Scholar]
- 43.Cullheim S, Ulfhake B. Relation between cell body size, axon diameter and axon conduction velocity of triceps surae alpha motoneurons during postnatal development in the cat. J Comp Neurol. 1979;188:679–686. [DOI] [PubMed] [Google Scholar]
- 44.Leroy F, Lamotte d’Incamps B, Imhoff-Manuel RD, et al. Early intrinsic hyperexcitability does not contribute to motoneuron degeneration in amyotrophic lateral sclerosis. Elife. 2014. October 14;3. doi: 10.7554/eLife.04046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Manuel M, Chardon M, Tysseling V, et al. Scaling of Motor Output, From Mouse to Humans. Physiology (Bethesda). 2019. January 1;34(1):5–13. doi: 10.1152/physiol.00021.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Seven YB, Mantilla CB, Sieck GC. Recruitment of Rat Diaphragm Motor Units Across Motor Behaviors with Different Levels of Diaphragm Activation. J Appl Physiol. 2014. December 1;117(11):1308–16. doi: 10.1152/japplphysiol.01395.2013.; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jodkowski JS, Viana F, Dick TE, et al. Electrical properties of phrenic motoneurons in the cat: correlation with inspiratory drive. J Neurophysiol. 1987;58(1):105–124. [DOI] [PubMed] [Google Scholar]
- 48.Smith JC, Ellenberger HH, Ballanyi K, et al. Pre-Botzinger complex: a brainstem region that may generate respiratory rhythm in mammals Science. 1991. November 1;254(5032):726–9. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Firsr description of the central pattern generator for ventilatory activity.
- 49.Ellenberger HH, Feldman JL. Monosynaptic transmission of respiratory drive to phrenic motoneurons from brainstem bulbospinal neurons in rats. J Comp Neurol. 1988;269:47–57. [DOI] [PubMed] [Google Scholar]
- 50.Ellenberger HH, Feldman JL. Brainstem connections of the rostral ventral respiratory group of the rat. Brain Res. 1990;513:35–42 [DOI] [PubMed] [Google Scholar]
- 51.Vinit S, Gauthier P, Stamegna JC, et al. High cervical lateral spinal cord injury results in long-term ipsilateral hemidiaphragm paralysis. J Neurotrauma. 2006. July;23(7):1137–46. [DOI] [PubMed] [Google Scholar]
- 52.Goshgarian HG, Ellenberger HH, Feldman JL. Decussation of bulbospinal respiratory axons at the level of the phrenic nuclei: a possible substrate for the crossed-phrenic phenomenon. Exp Neurol. 1991;111:135–139. [DOI] [PubMed] [Google Scholar]
- 53.Davies JG, Kirkwood PA, Sears TA. The distribution of monosynaptic connexions from inspiratory bulbospinal neurones to inspiratory motoneurones in the cat. J Physiol. 1985. November;368:63–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Feldman JL, Loewy AD, Speck DF. Projections from the ventral respiratory group to phrenic and intercostal motoneurons in cat: an autoradiographic study. J Neurosci. 1985;5(8):1993–2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lois JH, Rice CD, Yates BJ. Neural circuits controlling diaphragm function in the cat revealed by transneuronal tracing. J Appl Physiol. 2009. January;106(1):138–52.; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rana S, Sieck GC, Mantilla CB. Heterogeneous glutamatergic receptor mRNA expression across phrenic motor neurons in rats. J Neurochem. 2019. September 28. doi: 10.1111/jnc.14881. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Study showing that smaller phrenic motor neurons can be distinguished from larger phrenic motor neurons by the increased mRNA transcripts of glutamatergic receptor subtype expressions.
- 57.Bellingham MC, Lipski J. Respiratory interneurons in the C5 segment of the spinal cord of the cat. Brain Res. 1990. November 12;533(1):141–6. [DOI] [PubMed] [Google Scholar]; * These local semental interneurons are considered prime sources of the latent sources of inputs that facilitate recovery following cervical spinal cord injury.
- 58.Duffin J, Iscoe S. The possible role of C5 segment inspiratory interneurons investigated by cross-correlation with phrenic motoneurons in decerebrate cats. Exp Brain Res. 1996. November;112(1):35–40. [DOI] [PubMed] [Google Scholar]
- 59.Gandevia SC, Rothwell JC. Activation of the human diaphragm from the motor cortex. J Physiol. 1987;384:109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Macefeild G, Gandevia SC. The cortical drive to human respiratory muscles in the awake state assessed by premotor cerebral potentials. J Physiol. 1991:1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Holstege G The periaqueductal gray controls brainstem emotional motor systems including respiration. Prog Brain Res. 2014;209:379–405. doi: 10.1016/B978-0-444-63274-6.00020-5. [DOI] [PubMed] [Google Scholar]
- 62.Li P, Janczewski WA, Yackle K, et al. The peptidergic control circuit for sighing. Nature. 2016. February 18;530(7590):293–7. doi: 10.1038/nature16964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dick TE, Oku Y, Romaniuk JR, et al. Interaction between central pattern generators for breathing and swallowing in the cat. J Physiol. 1993. June;465:715–30. doi: 10.1113/jphysiol.1993.sp019702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brown TG. On the nature of the fundamental activity of the nervous centres; together with an analysis of the conditioning of rhythmic activity in progression, and a theory of the evolution of function in the nervous system. The Journal of physiology. 1914. March 31;48(1):18–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vizzard MA, Erickson VL, Card JP, et al. Transneuronal labeling of neurons in the adult rat brainstem and spinal cord after injection of pseudorabies virus into the urethra. J Comp Neurol. 1995. May 15;355(4):629–40. doi: 10.1002/cne.903550411. [DOI] [PubMed] [Google Scholar]
- 66.Guertin PA. Preclinical evidence supporting the clinical development of central pattern generator-modulating therapies for chronic spinal cord-injured patients. Front Hum Neurosci. 2014;8:272. doi: 10.3389/fnhum.2014.00272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Truitt WA, Coolen LM. Identification of a potential ejaculation generator in the spinal cord. Science. 2002. August 30;297(5586):1566–9. doi: 10.1126/science.1073885. [DOI] [PubMed] [Google Scholar]
- 68.Fukuda H, Fukai K. Ascending and descending pathways of reflex straining in the dog. Jpn J Physiol. 1986;36(5):905–20. [DOI] [PubMed] [Google Scholar]
- 69.Maggi CA, Giuliani S, Santicioli P, et al. Neural pathways and pharmacological modulation of defecation reflex in rats. Gen Pharmacol. 1988;19(4):517–23. [DOI] [PubMed] [Google Scholar]
- 70.Vizzard MA, Brisson M, de Groat WC. Transneuronal labeling of neurons in the adult rat central nervous system following inoculation of pseudorabies virus into the colon. Cell and Tissue Research. 2000. January;299(1):9–26. [DOI] [PubMed] [Google Scholar]
- 71.Miller AD, Ruggiero DA. Emetic reflex arc revealed by expression of the immediate-early gene c-fos in the cat. J Neurosci. 1994. February;14(2):871–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Hackett DA, Chow CM. The Valsalva maneuver: its effect on intra-abdominal pressure and safety issues during resistance exercise. J Strength Cond Res. 2013. August;27(8):2338–45. doi: 10.1519/JSC.0b013e31827de07d. [DOI] [PubMed] [Google Scholar]; * Review with a compilation of quantifications of human intra-abdominal pressures (kPa) while performing Valsalva maneuvers and various exercises.
- 73.Rada CC, Pierce SL, Grotegut CA, et al. Intrauterine telemetry to measure mouse contractile pressure in vivo. J Vis Exp. 2015. April 6(98):e52541. doi: 10.3791/52541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bellemare F, Bigland-Ritchie B. Assessment of human diaphragm strength and activation using phrenic nerve stimulation. Respir Physiol Neurobiol. 1984;58:263–277. [DOI] [PubMed] [Google Scholar]; * Human study with early observation that Pdimax was >200 cm H2O, far in excess of ventilatory requirements.
- 75.Iqbal A, Haider M, Stadlhuber RJ, et al. A study of intragastric and intravesicular pressure changes during rest, coughing, weight lifting, retching, and vomiting. Surg Endosc. 2008. December;22(12):2571–5. doi: 10.1007/s00464-008-0080-0. [DOI] [PubMed] [Google Scholar]
- 76.Winslow C, Rozovsky J. Effect of spinal cord injury on the respiratory system. Am J Phys Med Rehabil. 2003. October;82(10):803–14. [DOI] [PubMed] [Google Scholar]
- 77.Brown R, DiMarco AF, Hoit JD, et al. Respiratory dysfunction and management in spinal cord injury. Respir Care. 2006. August;51(8):853–68;discussion 869–70. [PMC free article] [PubMed] [Google Scholar]
- 78.Linn WS, Adkins RH, Gong H Jr., et al. Pulmonary function in chronic spinal cord injury: a cross-sectional survey of 222 southern California adult outpatients. Archives Of Physical Medicine and Rehabilitation. 2000. June;81(6):757–63. [DOI] [PubMed] [Google Scholar]
- 79.White NH. Facts and Figures at a Glance In: Alabama Uo, editor. National Spinal Cord Injury Statistical Center. Birmingham: National Spinal Cord Injury Statistical Center; 2019. [Google Scholar]
- 80.Anke A, Aksnes AK, Stanghelle JK, et al. Lung volumes in tetraplegic patients according to cervical spinal cord injury level. Scandinavian journal of rehabilitation medicine. 1993. June;25(2):73–7. [PubMed] [Google Scholar]
- 81.Bluechardt MH, Wiens M, Thomas SG, et al. Repeated measurements of pulmonary function following spinal cord injury. Paraplegia. 1992. November;30(11):768–74. doi: 10.1038/sc.1992.148. [DOI] [PubMed] [Google Scholar]
- 82.Berlowitz DJ, Wadsworth B, Ross J. Respiratory problems and management in people with spinal cord injury. Breathe (Sheff). 2016. December;12(4):328–340. doi: 10.1183/20734735.012616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Lane MA, Lee KZ, Salazar K, et al. Respiratory function following bilateral mid-cervical contusion injury in the adult rat. Exp Neurol. 2012. May;235(1):197–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Golder FJ, Fuller DD, Lovett-Barr MR, et al. Breathing patterns after mid-cervical spinal contusion in rats. Exp Neurol. 2011. September;231(1):97–103. doi: S0014-4886(11)00217-2 [pii] 10.1016/j.expneurol.2011.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Khurram OU, Fogarty MJ, Rana S, et al. Diaphragm muscle function following midcervical contusion injury in rats. J Appl Physiol (1985). 2019. January 1;126(1):221–230. doi: 10.1152/japplphysiol.00481.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Study characterising the Pdimax in the cervical contusion model. Notably this is the only Pdi deficit present in this model.
- 86.Rana S, Sieck GC, Mantilla CB. Diaphragm electromyographic activity following unilateral midcervical contusion injury in rats. J Neurophysiol. 2017. February 01;117(2):545–555. doi: 10.1152/jn.00727.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.el-Bohy AA, Schrimsher GW, Reier PJ, et al. Quantitative assessment of respiratory function following contusion injury of the cervical spinal cord. Exp Neurol. 1998. March;150(1):143–52. [DOI] [PubMed] [Google Scholar]
- 88.Nicaise C, Hala TJ, Frank DM, et al. Phrenic motor neuron degeneration compromises phrenic axonal circuitry and diaphragm activity in a unilateral cervical contusion model of spinal cord injury. Exp Neurol. 2012. June;235(2):539–52. doi: S0014-4886(12)00105-7 [pii] 10.1016/j.expneurol.2012.03.007. [DOI] [PubMed] [Google Scholar]
- 89.Nicaise C, Putatunda R, Hala TJ, et al. Degeneration of phrenic motor neurons induces long-term diaphragm deficits following mid-cervical spinal contusion in mice J Neurotrauma. 2012. December 10;29(18):2748–60. doi: 10.1089/neu.2012.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Nicaise C, Frank DM, Hala TJ, et al. Early phrenic motor neuron loss and transient respiratory abnormalities following unilateral cervical spinal cord contusion. J Neurotrauma. 2013. March 27;30(12):1092–1099. doi: 10.1089/neu.2012.2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Jones LL, Margolis RU, Tuszynski MH. The chondroitin sulfate proteoglycans neurocan, brevican, phosphacan, and versican are differentially regulated following spinal cord injury. Exp Neurol. 2003. August;182(2):399–411. [DOI] [PubMed] [Google Scholar]
- 92.Choi H, Liao WL, Newton KM, et al. Respiratory abnormalities resulting from midcervical spinal cord injury and their reversal by serotonin 1A agonists in conscious rats. J Neurosci. 2005. May 4;25(18):4550–9. doi: 10.1523/JNEUROSCI.5135-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kramer JL, Lammertse DP, Schubert M, et al. Relationship between motor recovery and independence after sensorimotor-complete cervical spinal cord injury. Neurorehabil Neural Repair. 2012. Nov-Dec;26(9):1064–71. doi: 10.1177/1545968312447306. [DOI] [PubMed] [Google Scholar]
- 94.Alvarez-Argote S, Gransee HM, Mora JC, et al. The Impact of Midcervical Contusion Injury on Diaphragm Muscle Function. J Neurotrauma. 2016. March 1;33(5):500–9. doi: 10.1089/neu.2015.4054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Khurram OU, Sieck GC, Mantilla CB. Compensatory effects following unilateral diaphragm paralysis. Respir Physiol Neurobiol. 2017. August 05;246:39–46. doi: 10.1016/j.resp.2017.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Porter WT. The Path of the Respiratory Impulse from the Bulb to the Phrenic Nuclei. J Physiol. 1895. April 6;17(6):455–85.; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lane MA. Spinal respiratory motoneurons and interneurons. Respir Physiol Neurobiol. 2011. October 15;179(1):3–13. doi: 10.1016/j.resp.2011.07.004.; eng. [DOI] [PubMed] [Google Scholar]
- 98.Lane MA, White TE, Coutts MA, et al. Cervical prephrenic interneurons in the normal and lesioned spinal cord of the adult rat. J Comp Neurol. 2008. December 10;511(5):692–709.; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fuller DD, Golder FJ, Olson EB, Jr., et al. Recovery of phrenic activity and ventilation after cervical spinal hemisection in rats. J Appl Physiol. 2006. March;100(3):800–6. [DOI] [PubMed] [Google Scholar]
- 100.Goshgarian HG. Plasticity in Respiratory Motor Control: Invited Review: The crossed phrenic phenomenon: a model for plasticity in the respiratory pathways following spinal cord injury. J Appl Physiol. 2003;94(2):795–810. [DOI] [PubMed] [Google Scholar]
- 101.Gransee HM, Zhan WZ, Sieck GC, et al. Targeted Delivery of TrkB Receptor to Phrenic Motoneurons Enhances Functional Recovery of Rhythmic Phrenic Activity after Cervical Spinal Hemisection. PloS one. 2013;8(5):e64755. doi: 10.1371/journal.pone.0064755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gransee HM, Zhan WZ, Sieck GC, et al. Localized Delivery of Brain-Derived Neurotrophic Factor-Expressing Mesenchymal Stem Cells Enhances Functional Recovery following Cervical Spinal Cord Injury. J Neurotrauma. 2015. February 1;32(3):185–93. doi: 10.1089/neu.2014.3464.; eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Mantilla CB, Gransee HM, Zhan WZ, et al. Motoneuron BDNF/TrkB signaling enhances functional recovery after cervical spinal cord injury. Exp Neurol. 2013. April 10;247C:101–109. doi: 10.1016/j.expneurol.2013.04.002.; Eng. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mantilla CB, Greising SM, Zhan WZ, et al. Prolonged C2 spinal hemisection-induced inactivity reduces diaphragm muscle specific force with modest, selective atrophy of type IIx and/or IIb fibers. J Appl Physiol. 2013. November 29;114(3): 380–386. doi: 10.1152/japplphysiol.01122.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Miyata H, Zhan WZ, Prakash YS, et al. Myoneural interactions affect diaphragm muscle adaptations to inactivity. J Appl Physiol. 1995;79:1640–1649. [DOI] [PubMed] [Google Scholar]
- 106.Prakash YS, Miyata H, Zhan WZ, et al. Inactivity-induced remodeling of neuromuscular junctions in rat diaphragmatic muscle. Muscle Nerve. 1999;22(3):307–19. [DOI] [PubMed] [Google Scholar]
- 107.Zhan WZ, Miyata H, Prakash YS, et al. Metabolic and phenotypic adaptations of diaphragm muscle fibers with inactivation. J Appl Physiol. 1997;82(4):1145–53. [DOI] [PubMed] [Google Scholar]
- 108.Prakash YS, Zhan WZ, Miyata H, et al. Adaptations of diaphragm neuromuscular junction following inactivity. Acta Anatomica. 1995;154(2):147–161. [DOI] [PubMed] [Google Scholar]
- 109.Martinez-Galvez G, Zambrano JM, Diaz Soto JC, et al. TrkB gene therapy by adeno-associated virus enhances recovery after cervical spinal cord injury. Exp Neurol. 2016. February;276:31–40. doi: 10.1016/j.expneurol.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Mantilla CB, Greising SM, Stowe JM, et al. TrkB Kinase Activity is Critical for Recovery of Respiratory Function after Cervical Spinal Cord Hemisection. Exp Neurol. 2014;261:190–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hernandez-Torres V, Gransee HM, Mantilla CB, et al. BDNF effects on functional recovery across motor behaviors after cervical spinal cord injury. J Neurophysiol. 2017. February 01;117(2):537–544. doi: 10.1152/jn.00654.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.McBride RL, Feringa ER, Garver MK, et al. Retrograde transport of fluoro-gold in corticospinal and rubrospinal neurons 10 and 20 weeks after T-9 spinal cord transection. Exp Neurol. 1990. April;108(1):83–5. doi: 10.1016/0014-4886(90)90011-g. [DOI] [PubMed] [Google Scholar]
- 113.Nielson JL, Sears-Kraxberger I, Strong MK, et al. Unexpected survival of neurons of origin of the pyramidal tract after spinal cord injury. J Neurosci. 2010. August 25;30(34):11516–28. doi: 10.1523/JNEUROSCI.1433-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Nielson JL, Strong MK, Steward O. A reassessment of whether cortical motor neurons die following spinal cord injury. J Comp Neurol. 2011. October 1;519(14):2852–69. doi: 10.1002/cne.22661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wannier T, Schmidlin E, Bloch J, et al. A unilateral section of the corticospinal tract at cervical level in primate does not lead to measurable cell loss in motor cortex. J Neurotrauma. 2005. June;22(6):703–17. doi: 10.1089/neu.2005.22.703. [DOI] [PubMed] [Google Scholar]
- 116.Devivo MJ. Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord. 2012. May;50(5):365–72. doi: 10.1038/sc.2011.178sc2011178 [pii]. [DOI] [PubMed] [Google Scholar]
- 117.Fuller DD, Doperalski NJ, Dougherty BJ, et al. Modest spontaneous recovery of ventilation following chronic high cervical hemisection in rats. Exp Neurol. 2008. May;211(1):97–106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Moreno DE, Yu XJ, Goshgarian HG. Identification of the axon pathways which mediate functional recovery of a paralyzed hemidiaphragm following spinal cord hemisection in the adult rat. Exp Neurol. 1992;116:219–228. [DOI] [PubMed] [Google Scholar]
- 119.Gransee HM, Gonzalez Porras MA, Zhan WZ, et al. Motoneuron glutamatergic receptor expression following recovery from cervical spinal hemisection. J Comp Neurol. 2017. April 01;525(5):1192–1205. doi: 10.1002/cne.24125. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Details the importance of postsynaptic glutamate receptor neuroplasticity of phrenic motor neurons in the recovery of eupnic behaviour post cervical spinal hemisection.
- 120.Tai Q, Goshgarian HG. Ultrastructural quantitative analysis of glutamatergic and GABAergic synaptic terminals in the phrenic nucleus after spinal cord injury. J Comp Neurol. 1996;372(3):343–55. [DOI] [PubMed] [Google Scholar]
- 121.Mantilla CB, Zhan WZ, Gransee HM, et al. Phrenic motoneuron structural plasticity across models of diaphragm muscle paralysis. J Comp Neurol. 2018. November 8. doi: 10.1002/cne.24503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Fogarty MJ. Driven to Decay: Excitability and Synaptic Abnormalities in Amyotrophic Lateral Sclerosis. Brain Res Bull. 2018. June 2;140:318–333. doi: 10.1016/j.brainresbull.2018.05.023. [DOI] [PubMed] [Google Scholar]
- 123.Goshgarian HG, Yu XJ, Rafols JA. Neuronal and glial changes in the rat phrenic nucleus occurring within hours after spinal cord injury. J Comp Neurol. 1989;284:519–533. [DOI] [PubMed] [Google Scholar]
- 124.Hadley SD, Walker PD, Goshgarian HG. Effects of serotonin inhibition on neuronal and astrocyte plasticity in the phrenic nucleus 4 h following C2 spinal cord hemisection. Exp Neurol. 1999;160(2):433–45. [DOI] [PubMed] [Google Scholar]
- 125.Mantilla CB, Gransee HM, Zhan WZ, et al. Impact of glutamatergic and serotonergic neurotransmission on diaphragm muscle activity after cervical spinal hemisection. J Neurophysiol. 2017. September 01;118(3):1732–1738. doi: 10.1152/jn.00345.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kinkead R, Zhan WZ, Prakash YS, et al. Cervical dorsal rhizotomy enhances serotonergic innervation of phrenic motoneurons and serotonin-dependent long-term facilitation of respiratory motor output in rats. J Neurosci. 1998;18(20):8436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.McGuire M, Zhang Y, White DP, et al. Serotonin receptor subtypes required for ventilatory long-term facilitation and its enhancement after chronic intermittent hypoxia in awake rats. Am J Physiol Regul Integr Comp Physiol. 2004. February;286(2):R334–41. [DOI] [PubMed] [Google Scholar]
- 128.Zhou SY, Basura GJ, Goshgarian HG. Serotonin(2) receptors mediate respiratory recovery after cervical spinal cord hemisection in adult rats. J Appl Physiol. 2001;91(6):2665–73. [DOI] [PubMed] [Google Scholar]
- 129.Fuller DD, Baker-Herman TL, Golder FJ, et al. Cervical spinal cord injury upregulates ventral spinal 5-HT2A receptors. J Neurotrauma. 2005. February;22(2):203–13. [DOI] [PubMed] [Google Scholar]; * Highlights the important role that non-monosynaptic inputs to phrenic motor neurons have to recovery from cervical spinal cord injury.
- 130.Legg Ditterline BE, Aslan SC, Randall DC, et al. Baroreceptor reflex during forced expiratory maneuvers in individuals with chronic spinal cord injury. Respir Physiol Neurobiol. 2016. July 15;229:65–70. doi: 10.1016/j.resp.2016.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]; * Descriptions of spinal cord injury patients with impaired Valsalva co-contraction maneuvers.
- 131.Estenne M, De Troyer A. Cough in tetraplegic subjects: an active process. Ann Intern Med. 1990. January 1;112(1):22–8. doi: 10.7326/0003-4819-112-1-22. [DOI] [PubMed] [Google Scholar]
- 132.Estenne M, Gorini M. Action of the diaphragm during cough in tetraplegic subjects. J Appl Physiol (1985). 1992. March;72(3):1074–80. doi: 10.1152/jappl.1992.72.3.1074. [DOI] [PubMed] [Google Scholar]
- 133.De Troyer A, Estenne M, Vincken W. Rib cage motion and muscle use in high tetraplegics. Am Rev Respir Dis. 1986;133:1115–1119. [DOI] [PubMed] [Google Scholar]
- 134.Bolser DC, Jefferson SC, Rose MJ, et al. Recovery of airway defensive behaviors after spinal cord injury. Respir Physiol Neurobiol. 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.De Looze D, Van Laere M, De Muynck M, et al. Constipation and other chronic gastrointestinal problems in spinal cord injury patients. Spinal Cord. 1998. January;36(1):63–6. doi: 10.1038/sj.sc.3100531. [DOI] [PubMed] [Google Scholar]; * Effort to catalogue the expulsive deficits following spinal cord injury with paricular attention and discrimination of evacuation difficulty (constipation, transit times) compared to sphincter laxity (incontinence).
- 136.Hughes M Bowel management in spinal cord injury patients. Clin Colon Rectal Surg. 2014. September;27(3):113–5. doi: 10.1055/s-0034-1383904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Krassioukov A, Eng JJ, Claxton G, et al. Neurogenic bowel management after spinal cord injury: a systematic review of the evidence. Spinal Cord. 2010. October;48(10):718–33. doi: 10.1038/sc.2010.14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cross LL, Meythaler JM, Tuel SM, et al. Pregnancy, labor and delivery post spinal cord injury. Paraplegia. 1992. December;30(12):890–902. doi: 10.1038/sc.1992.166. [DOI] [PubMed] [Google Scholar]; * One of very few quantitative studies examining parturition complications in a spinal cord injury cohort.
- 139.Gill LC, Ross HH, Lee KZ, et al. Rapid diaphragm atrophy following cervical spinal cord hemisection. Respir Physiol Neurobiol. 2014. February 1;192:66–73. doi: 10.1016/j.resp.2013.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Golder FJ, Fuller DD, Davenport PW, et al. Respiratory motor recovery after unilateral spinal cord injury: eliminating crossed phrenic activity decreases tidal volume and increases contralateral respiratory motor output. J Neurosci. 2003;23(6):2494–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Lee KZ, Dougherty BJ, Sandhu MS, et al. Phrenic motoneuron discharge patterns following chronic cervical spinal cord injury. Exp Neurol. 2013. November;249:20–32. doi: 10.1016/j.expneurol.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Bae H, Nantwi KD, Goshgarian HG. Recovery of respiratory function following C2 hemi and carotid body denervation in adult rats: influence of peripheral adenosine receptors. Exp Neurol. 2005. January;191(1):94–103. [DOI] [PubMed] [Google Scholar]
- 143.Nantwi KD, El-Bohy A, Schrimsher GW, et al. Spontaneous functional recovery in a paralyzed hemidiaphragm following upper cervical spinal cord injury in adult rats. Neurorehab Neural Repair. 1999;13:225–234. [Google Scholar]
- 144.Dougherty BJ, Lee KZ, Lane MA, et al. Contribution of the spontaneous crossed-phrenic phenomenon to inspiratory tidal volume in spontaneously breathing rats. J Appl Physiol. 2012. January;112(1):96–105. doi: japplphysiol.00690.2011 [pii] 10.1152/japplphysiol.00690.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bhaskar KR, Brown R, O’Sullivan DD, et al. Bronchial mucus hypersecretion in acute quadriplegia. Macromolecular yields and glycoconjugate composition. Am Rev Respir Dis. 1991. March;143(3):640–8. doi: 10.1164/ajrccm/143.3.640. [DOI] [PubMed] [Google Scholar]
- 146.Reid WD, Brown JA, Konnyu KJ, et al. Physiotherapy Secretion Removal Techniques in People With Spinal Cord Injury: A Systematic Review. Journal of Spinal Cord Medicine. 2010. October;33(4):353–370. doi: Doi 10.1080/10790268.2010.11689714.; English. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Schilero GJ, Spungen AM, Bauman WA, et al. Pulmonary function and spinal cord injury. Respir Physiol Neurobiol. 2009. May 15;166(3):129–41. doi: 10.1016/j.resp.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 148.Raurich JM, Rialp G, Llompart-Pou JA, et al. Respiratory CO(2) response in acute cervical spinal cord injury (CO(2) response in spinal cord injury). Spinal Cord. 2014. January;52(1):39–43. doi: 10.1038/sc.2013.115. [DOI] [PubMed] [Google Scholar]
- 149.Sinha RP, Ducker TB, Perot PL Jr. Arterial oxygenation. Findings and its significance in central nervous system trauma patients. Journal of the American Medical Association. 1973. May 28;224(9):1258–60. doi: 10.1001/jama.224.9.1258. [DOI] [PubMed] [Google Scholar]
- 150.Bach JR. Mechanical insufflation-exsufflation. Comparison of peak expiratory flows with manually assisted and unassisted coughing techniques. Chest. 1993. November;104(5):1553–62. doi: 10.1378/chest.104.5.1553. [DOI] [PubMed] [Google Scholar]
- 151.Kirby NA, Barnerias MJ, Siebens AA. An evaluation of assisted cough in quadriparetic patients. Archives Of Physical Medicine and Rehabilitation. 1966. November;47(11):705–10. [PubMed] [Google Scholar]
- 152.Bach JR, Ishikawa Y, Kim H. Prevention of pulmonary morbidity for patients with Duchenne muscular dystrophy. Chest. 1997. October;112(4):1024–8. doi: 10.1378/chest.112.4.1024. [DOI] [PubMed] [Google Scholar]
- 153.DiMarco AF, Kowalski KE, Geertman RT, et al. Spinal cord stimulation: a new method to produce an effective cough in patients with spinal cord injury. Am J Respir Crit Care Med. 2006. June 15;173(12):1386–9. doi: 10.1164/rccm.200601-097CR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.DiMarco AF, Kowalski KE, Geertman RT, et al. Lower thoracic spinal cord stimulation to restore cough in patients with spinal cord injury: results of a National Institutes of Health-sponsored clinical trial. Part I: methodology and effectiveness of expiratory muscle activation. Archives Of Physical Medicine and Rehabilitation. 2009. May;90(5):717–25. doi: 10.1016/j.apmr.2008.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.McBain RA, Boswell-Ruys CL, Lee BB, et al. Abdominal muscle training can enhance cough after spinal cord injury. Neurorehabil Neural Repair. 2013. Nov-Dec;27(9):834–43. doi: 10.1177/1545968313496324. [DOI] [PubMed] [Google Scholar]
- 156.Kang SW, Bach JR. Maximum insufflation capacity: vital capacity and cough flows in neuromuscular disease. Am J Phys Med Rehabil. 2000. May-Jun;79(3):222–7. doi: 10.1097/00002060-200005000-00002. [DOI] [PubMed] [Google Scholar]
- 157.Smith PE, Calverley PM, Edwards RH, et al. Practical problems in the respiratory care of patients with muscular dystrophy. New England Journal of Medicine. 1987. May 7;316(19):1197–205. doi: 10.1056/NEJM198705073161906. [DOI] [PubMed] [Google Scholar]
- 158.Polkey MI, Harris ML, Hughes PD, et al. The contractile properties of the elderly human diaphragm. Am J Respir Crit Care Med. 1997. May;155(5):1560–4. [DOI] [PubMed] [Google Scholar]
- 159.Steier J, Kaul S, Seymour J, et al. The value of multiple tests of respiratory muscle strength. Thorax. 2007. November;62(11):975–80. doi: thx.2006.072884 [pii] 10.1136/thx.2006.072884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.McCaughey EJ, Butler JE, McBain RA, et al. Abdominal Functional Electrical Stimulation to Augment Respiratory Function in Spinal Cord Injury. Top Spinal Cord Inj Rehabil. 2019. Spring;25(2):105–111. doi: 10.1310/sci2502-105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Lim J, Gorman RB, Saboisky JP, et al. Optimal electrode placement for noninvasive electrical stimulation of human abdominal muscles. J Appl Physiol (1985). 2007. April;102(4):1612–7. doi: 10.1152/japplphysiol.00865.2006. [DOI] [PubMed] [Google Scholar]
- 162.Groah SL, Charlifue S, Tate D, et al. Spinal cord injury and aging: challenges and recommendations for future research. Am J Phys Med Rehabil. 2012. January;91(1):80–93. doi: 10.1097/PHM.0b013e31821f70bc. [DOI] [PubMed] [Google Scholar]
- 163.Fogarty MJ, Gonzalez Porras MA, Mantilla CB, et al. Diaphragm neuromuscular transmission failure in aged rats. J Neurophysiol. 2019. July 1;122(1):93–104. doi: 10.1152/jn.00061.2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Mantilla CB, Sieck GC. Neuromotor Control in Chronic Obstructive Pulmonary Disease. J Appl Physiol. 2013. January 17;114:1246–52. doi: japplphysiol.01212.2012 [pii] 10.1152/japplphysiol.01212.2012. [DOI] [PubMed] [Google Scholar]


