Abstract
Objective:
To determine factors associated with rectal cancer surgery performed at high-volume hospitals (HVH) and by high-volume surgeons (HVS), including the roles of rurality and diagnostic colonoscopy provider characteristics.
Summary Background Data:
Although higher-volume hospitals/surgeons often achieve superior surgical outcomes, many rectal cancer resections are performed by lower-volume hospitals/surgeons, especially among rural populations.
Methods:
Patients age 66+ diagnosed from 2007–2011 with stage II/III primary rectal adenocarcinoma were selected from Surveillance, Epidemiology, and End Results-Medicare data. Patient ZIP codes were used to classify rural status. Hierarchical logistic regression was used to determine factors associated with surgery by HVH and HVS.
Results:
Of 1601 patients, 22% were rural and 78% were urban. Fewer rural patients received surgery at a HVH compared to urban patients (44% vs. 65%; p<0.0001). Compared to urban patients, rural patients more often had colonoscopies performed by general surgeons (and less often from gastroenterologists or colorectal surgeons), and lived substantially further from HVHs; these factors were both associated with lower odds of surgery at a HVH or by a HVS. In addition, while over half of both rural and urban patients received their colonoscopy and surgery at the same hospital, rural patients who stayed at the same hospital were significantly less likely to receive surgery at a HVH or by a HVS compared to urban patients.
Conclusions:
Rural rectal cancer patients are less likely to receive surgery from a HVH/HVS. The role of the colonoscopy provider has important implications for referral patterns and initiatives seeking to increase centralization.
Introduction
Over 44,000 rectal cancer cases are expected in 2019 in the United States.1 Rectal cancer surgery is technically challenging given its anatomical constraints, and studies have demonstrated that higher hospital volume, surgical volume, and surgeon specialization improve adherence to guideline-recommended care and oncologic outcomes.2–9 Consequently, many countries around the world have centralized rectal cancer care, which has also been associated with improved outcomes.10, 11 However, in the US, efforts to centralize rectal cancer care have been challenging,6, 8, 9, 12 especially in rural areas.13
Access to a high-volume hospital (HVH) or specialized surgeon is particularly problematic for rural patients as these hospitals are often located in urban areas.13, 14 Compared to urban patients, rural patients travel longer to reach HVHs and high-volume surgeons (HVSs).13–15 A qualitative study of Iowa rectal cancer patients found many rural patients sought treatment at local lower volume hospitals because it was familiar or recommended by a trusted source, usually a physician.16 In contrast, only a few patients considered driving distance to be a barrier to receiving care at HVHs.16 These findings suggest that, at least for Iowa rectal cancer patients, referral patterns may be more of a determinant of treatment at a HVH than travel time. Whether this is true empirically for rural patients across the US requires further investigation.
The goal of this analysis is to examine travel time and rurality in the context of other potentially important factors that contribute to rectal cancer patients’ decisions on where to receive surgical treatment. SEER-Medicare data were used to construct episodes of care spanning from cancer diagnosis to primary resection. The primary objective of this study was to determine factors associated with receipt of rectal cancer surgery at a 1) HVH and 2) by HVS or colorectal surgeon (CRS). We also aimed to explore the role of the type of colonoscopy provider and facility in receiving surgery at HVHs and by HVSs. Since the National Comprehensive Cancer Network (NCCN) recommends radical resection for all patients with stage II/III disease, we specifically focused on these patients.17
Methods
Data Sources
SEER-Medicare data were used to conduct a retrospective analysis of rectal cancer patients. SEER data contain demographic, tumor, and survival information for cancer cases from 18 population-based cancer registries.18 Medicare data contain enrollment and claims files that can be used to derive detailed treatment information and timelines, along with information about providers and hospitals. Medicare provides health insurance to 97% of the US population aged 65 and above.18 SEER and Medicare files19 are linked by the National Cancer Institute (NCI) and the Centers for Medicare and Medicaid Services.
Timeline for Episodes of Care
We constructed episodes of care that began with the colonoscopy that identified the cancer through time of surgery (Figure 1). SEER provides only month and year of cancer diagnosis, so an algorithm derived from previous research was developed to impute rectal cancer diagnosis date based on the first colonoscopy claim20, 21 with an associated colorectal cancer ICD-9 diagnosis code (1530–1541, 1548, 2113, 2114, 2119, 2302, 2304, 2352, 2355, 2390) in the same month and year as the SEER diagnosis date (colon cancer diagnoses were included to account for potential misclassification of codes). The primary surgical resection date was identified by the first rectal cancer resection CPT or ICD-9 procedure codes adapted from a previously described list.12
Figure 1.
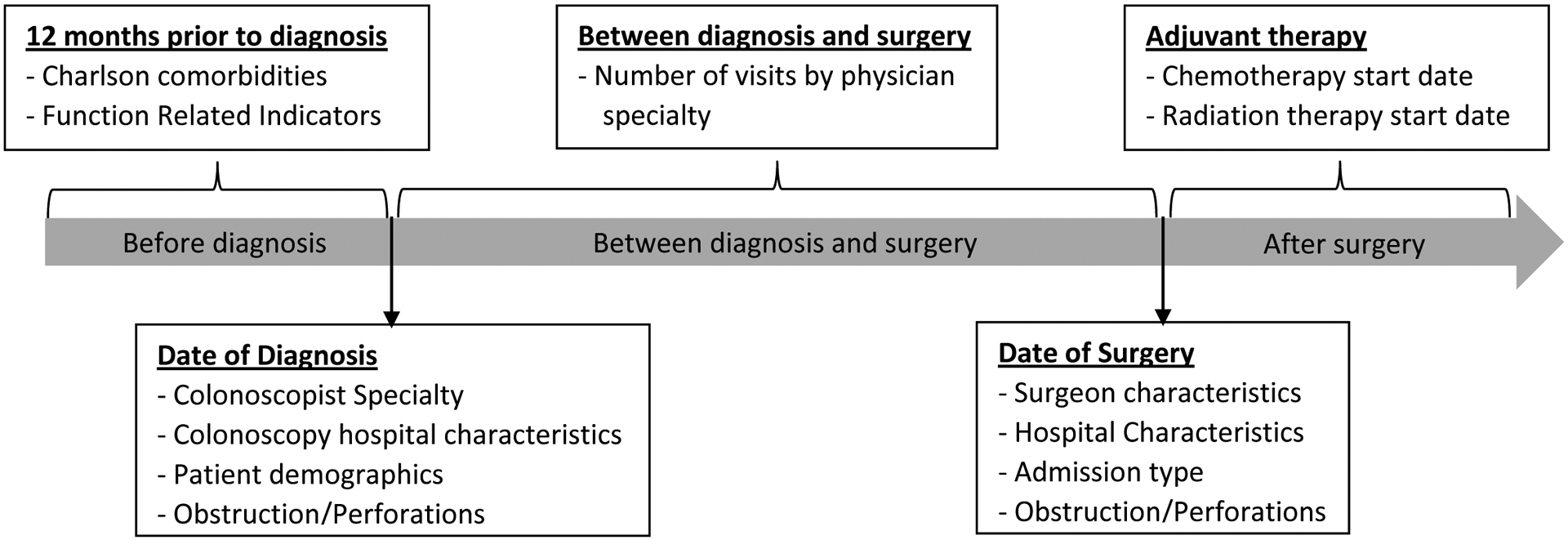
Episodes of care timeline and variables analyzed at each time point
Study Population
Patients diagnosed between 2007 and 2011 with AJCC 6th edition stage II/III primary rectal (ICD-O-3 site: C209) adenocarcinoma (histology: 8140–8571) and not diagnosed at death or autopsy were included in the study (Figure 2). Patients with unknown month of diagnosis or unknown rectal cancer surgery date/hospital were excluded. Additionally, patients who had prior cancer(s), another cancer diagnosed within 6 months of diagnosis, did not have continuous fee-for-service Medicare parts A and B coverage or died within 3 months of diagnosis were also excluded. Patients who received surgery during an inpatient admission that began on the date of diagnosis were considered to have had immediate surgery and were also excluded because they may not have had the opportunity to choose their hospital or surgeon. The 2006 Rural–Urban Commuting Area (RUCA) classification system22 was used to define rurality based on patient ZIP codes from the SEER-Medicare PEDSF files.19 Patients whose ZIP code did not map to the RUCA rural/urban classification were excluded from the analysis.
Figure 2.
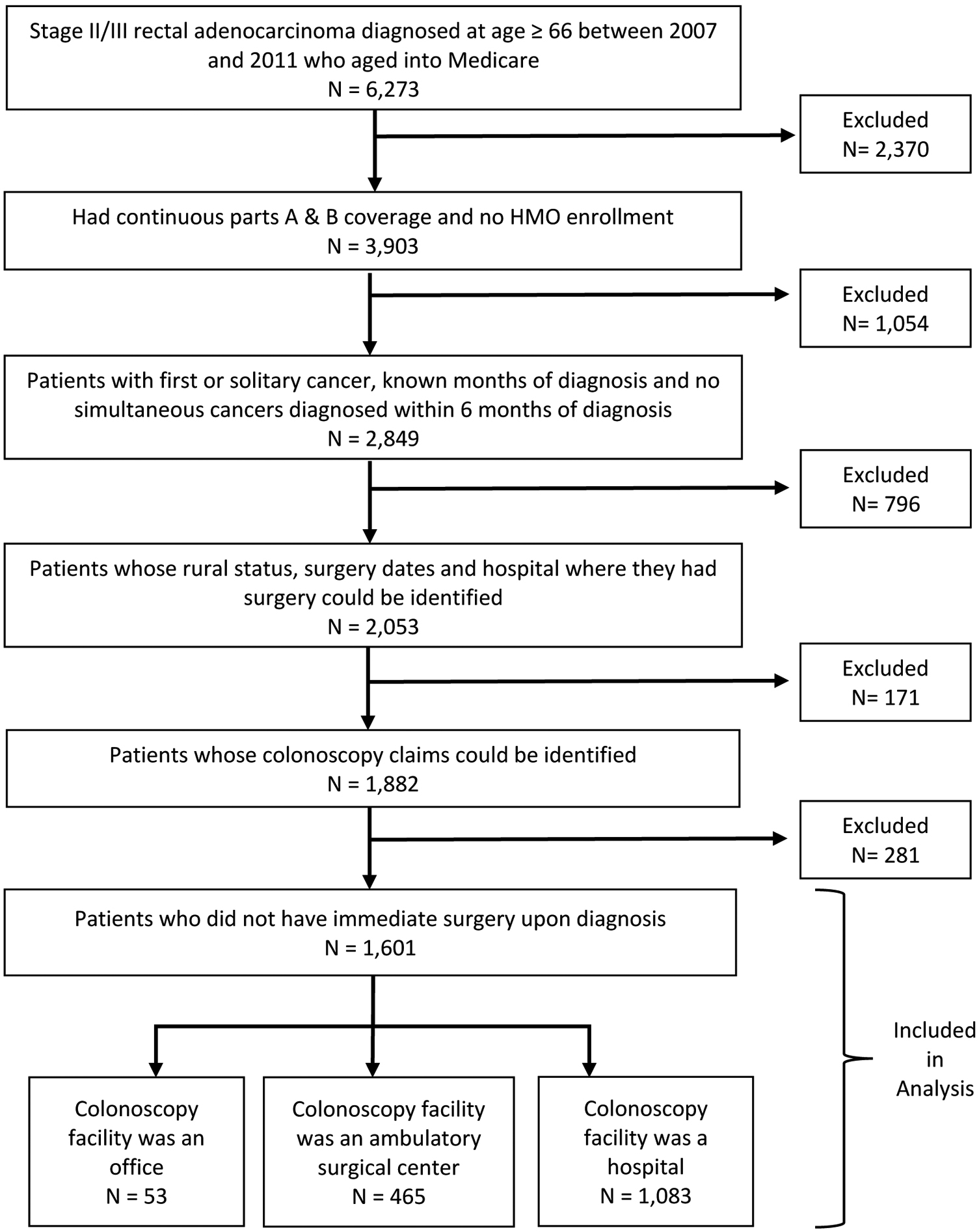
Flow chart of study population selection
Primary Outcome
Primary outcomes were receipt of rectal cancer resection at a HVH and by a HVS/CRS. A secondary outcome was receipt of colonoscopy at a HVH. MEDPAR and Outpatient files19 were used to identify rectal cancer surgery hospital volume. These volumes only reflect total procedures performed on Medicare patients >65 years old who resided in SEER Registry catchment areas, but they have been shown to correlate well with total volume in previous research.23–25 Hospital volume was defined as the number of rectal cancer surgeries performed on patients within 6 months of their cancer diagnosis between 2007 and 2011. Hospital volume over the entire 2007–2011 time period was categorized into quartiles: very low (1–3), low (4–7), medium (8–15) and HVH (16+). NCI-designated comprehensive cancer centers were also classified as HVH because they have become a measure of quality cancer care and are associated with better survival.15, 26 Hospital facility type was categorized into hospital vs. freestanding ambulatory surgical center (ASC). ASCs do not have the capability to perform rectal cancer resections, while hospitals have varying levels of capability based on the facility and surgeon expertise.27
Physician specialty was obtained from the National Claims History (NCH) and American Medical Association (AMA) files.19, 28 Physicians were categorized as CRS (10% were surgical oncologists who were included in this category due to small numbers and their focus on cancer resections), general surgeon (GS), medical oncologist, radiation oncologist, gastroenterologist and primary care physician (PCP) by decreasing hierarchical order; the higher specialization was assigned when specialty variables did not match in NCH and AMA files. Surgeon rectal cancer surgery volume over the entire 2007–2011 time period was defined using NCH files in a similar manner to hospital volume defined above. Surgeon volume was categorized into the following quartiles: very low (1–2), low (3–4), medium (5–10) and HVS (11+). Since CRSs receive advanced training in rectal cancer surgery, and are typically associated with high rectal cancer surgery volumes and better outcomes,3, 4 CRS were also considered HVSs.
Predictor Variables
Patient age, sex, marital status, race, stage and registry location at time of diagnosis were derived from the SEER Patient Entitlement and Diagnosis Summary File (PEDSF) file. State buy-in, an indicator that the beneficiary was dual-enrolled in Medicaid or received another type of state-based healthcare assistance, was extracted from PEDSF. The Charlson comorbidity count and Function Related Indicators (FRIs) were calculated based on claims occurring one year prior to diagnosis. FRIs were developed to reflect frailty and are based on elements such as dementia, limited mobility, recent pneumonia, respiratory failure, oxygen use at home, blood transfusion, chronic skin ulcers, malnutrition, unintended weight loss and home-based supplemental nutrition.29
Median household income, percentage of people who finished high school, percentage of people who finished 4-year college and percentage living below the poverty level were derived from the Tract census file. Percentage of people who finished high school was categorized into low (0–80), medium (>80–90) and high (>90), while median household income was categorized into low (0-$20,000), medium (>$20,000-$30,000) and high (>$30,000). The percentage of people who finished 4-year college and percentage below poverty level were categorized as follows: low (0–10%), medium (>10–20%) and high (>20%). Patients who had missing census tract level information (n<11) had their socioeconomic status information derived from the ZIP code census file.
Hospital ZIP codes were extracted from MEDPAR files. Hospital and patient ZIP codes were used to determine travel times (in minutes) from the patient’s ZIP code to: 1) surgery hospital, and 2) nearest HVH. Microsoft MapPoint was used to calculate driving time between two ZIP code’s centroids while factoring geographic topology, structures and impact of speed limits on travel time.30 Patients who resided in the same ZIP code as their admitting hospital were assigned an arbitrary travel time of 5 minutes; sensitivity analysis using 10 or 15 minutes showed similar associations between drive time and outcomes.
Statistical Analysis
Pearson Chi-square tests and Kruskal-Wallis tests were used in bi-variate analyses. Kappa tests were used to ascertain agreement between colonoscopy and surgery hospital volume for patients who had colonoscopy at a hospital. Multivariate logistic regression models were fitted to assess factors associated with receipt of colonoscopy at HVHs, receipt of surgery at HVHs and surgery performed by HVS/CRS. The models were stratified by hospital facility type, and interaction terms were used to evaluate the interaction between rural status and hospital characteristics. The University of Iowa Institutional Review Board approved this study. Statistical analyses were performed using SAS 9.4 and Stata/SE 13.1.
Results
Overall study population
There were 1,601 rectal cancer patients eligible for final analyses (Figure 2). The majority of patients resided in urban areas (78%). Compared to urban patients, rural patients had significantly lower median age (73 vs. 75 years), were more frequently White (94% vs. 86%), and married (62% vs. 55%). Rural census tracts had significantly higher poverty, lower median incomes, and lower education levels compared to urban census tracts (Table 1). Clinical characteristics including stage and Charlson score were not significantly different between rural and urban patients.
Table 1.
Patient demographic and clinical characteristics by rurality
| Urban (n=1,248) | Rural (n=353) | |||||
|---|---|---|---|---|---|---|
| Patient characteristics | n | (%) | n | (%) | P-value | |
| Age | Median (IQR) | 75 | (70–80) | 73 | (70–78) | 0.04 * |
| 66–69 | 297 | (24%) | 86 | (24%) | 0.01 * | |
| 70–74 | 327 | (26%) | 121 | (34%) | ||
| 75–79 | 272 | (22%) | 70 | (20%) | ||
| 80+ | 352 | (28%) | 76 | (22%) | ||
| Gender | Female | 561 | (45%) | 153 | (43%) | 0.59 |
| Male | 687 | (55%) | 200 | (57%) | ||
| Race | Non-white | 174 | (14%) | 21 | (6%) | <0.0001 * |
| White | 1074 | (86%) | 332 | (94%) | ||
| Marital status | Married | 686 | (55%) | 218 | (62%) | 0.02 * |
| Divorceda | 562 | (45%) | 135 | (38%) | ||
| State buy-in | No | 1034 | (83%) | 298 | (84%) | 0.49 |
| Yes | 214 | (17%) | 55 | (16%) | ||
| Median income (thousands) | Median (IQR) | 31 | (24–40) | 21 | (18–26) | <0.0001 * |
| Low | 151 | (12%) | 143 | (41%) | <0.0001 * | |
| Medium | 418 | (33%) | 171 | (48%) | ||
| High | 679 | (54%) | 39 | (11%) | ||
| Poverty indicator | Low | 685 | (55%) | 71 | (20%) | <0.0001 * |
| Medium | 345 | (28%) | 139 | (39%) | ||
| High | 218 | (17%) | 143 | (41%) | ||
| % High school | Low | 253 | (20%) | 149 | (42%) | <0.0001 * |
| Medium | 347 | (28%) | 117 | (33%) | ||
| High | 648 | (52%) | 87 | (25%) | ||
| % 4 year college | Low | 113 | (9%) | 87 | (25%) | <0.0001 * |
| Medium | 276 | (22%) | 165 | (47%) | ||
| High | 859 | (69%) | 101 | (29%) | ||
| Year of diagnosis | 2007 | 309 | (25%) | 67 | (19%) | 0.02 * |
| 2008 | 262 | (21%) | 67 | (19%) | ||
| 2009 | 229 | (18%) | 60 | (17%) | ||
| 2010 | 227 | (18%) | 73 | (21%) | ||
| 2011 | 221 | (18%) | 86 | (24%) | ||
| Stage | II | 576 | (46%) | 160 | (45%) | 0.78 |
| III | 672 | (54%) | 193 | (55%) | ||
| Charlson score | 0 | 727 | (58%) | 199 | (56%) | 0.82 |
| 1 | 328 | (26%) | 97 | (27%) | ||
| 2+ | 193 | (15%) | 57 | (16%) | ||
| Function Related Indicators | 0 | 834 | (67%) | 219 | (62%) | 0.25 |
| 1 | 250 | (20%) | 81 | (23%) | ||
| 2+ | 164 | (13%) | 53 | (15%) | ||
Includes widowed or separated patients
IQR=Interquartile range
Significant at α=0.05
Colonoscopy
Compared to urban patients, a higher proportion of rural patients had their colonoscopies performed at a hospital (84% vs. 63%), and correspondingly a lower proportion of rural patients had their colonoscopies performed at a freestanding ASC (16% vs. 37%). Compared to urban patients, more rural patients received colonoscopy from a GS (31% vs. 7%), while fewer rural patients received colonoscopy from a gastroenterologist (50% vs. 75%) or CRS (7% vs. 14%, Table 2).
Table 2.
Physician and hospital characteristics by rurality
| Urban (n=1,248) | Rural (n=353) | P-value | ||||
|---|---|---|---|---|---|---|
| Colonoscopy hospital and colonoscopist specialty | n | (%) | n | (%) | ||
| Colonoscopy facility type | Ambulatory Surgical Centera | 460 | (37%) | 58 | (16%) | <0.0001 * |
| Hospital facility | 788 | (63%) | 295 | (84%) | ||
| Colonoscopist specialty | Gastroenterologist | 938 | (75%) | 175 | (50%) | <0.0001 * |
| General surgeon | 90 | (7%) | 109 | (31%) | ||
| Colorectal surgeon | 170 | (14%) | 25 | (7%) | ||
| Primary care provider/other | 50 | (4%) | 44 | (12%) | ||
| Surgeon and surgery hospital characteristics | ||||||
| Surgeon volume b | Very Low | 259 | (26%) | 86 | (32%) | 0.05 |
| Low | 145 | (21%) | 51 | (24%) | ||
| Medium | 99 | (12%) | 30 | (15%) | ||
| High | 696 | (56%) | 166 | (47%) | ||
| Unknown | 49 | (4%) | 20 | (6%) | ||
| Surgeon specialty | General surgeon | 467 | (37%) | 157 | (44%) | 0.05 * |
| Colorectal surgeon | 670 | (54%) | 165 | (47%) | ||
| Unknown | 111 | (9%) | 31 | (9%) | ||
| Surgery hospital volume b | Median (IQR) | 20 | (10–32) | 11 | (4–30) | <0.0001 * |
| Very Low | 78 | (6%) | 67 | (19%) | ||
| Low | 106 | (8%) | 67 | (19%) | ||
| Medium | 256 | (21%) | 62 | (18%) | ||
| High | 808 | (65%) | 157 | (44%) | <0.0001 * | |
| Drive time to hospital in minutes | ||||||
| Drive time to hospital for surgery | Median (IQR) | 18 | (11–28) | 55 | (32–96) | <0.0001 * |
| 0–15 | 502 | (40%) | 54 | (15%) | <0.0001 * | |
| >15–30 | 469 | (38%) | 32 | (9%) | ||
| >30–45 | 132 | (11%) | 63 | (18%) | ||
| >45 | 145 | (12%) | 204 | (58%) | ||
| Drive time to hospital for surgery at HVHd | Median (IQR) | 18 | (12–29) | 83 | (60–132) | <0.0001 * |
| Drive time to hospital for surgery at non-HVHe | Median (IQR) | 17 | (10–27) | 35 | (5–61) | <0.0001 * |
| Minutes between patient residence and nearest HVH | Median (IQR) | 16 | (11–27) | 78 | (52–104) | <0.0001 * |
| 0–15 | 541 | (43%) | S | S | <0.0001 * | |
| >15–30 | 437 | (35%) | S | S | ||
| >30–45 | 126 | (10%) | S | S | ||
| >45 | 144 | (12%) | 287 | (81%) | ||
Includes 53 patients who received colonoscopy in an office setting
Volume refers to the SEER-Medicare rectal cancer resection volume of surgeon or hospital between 2007 and 2011
S=Numbers suppressed; HVH=High rectal cancer resection volume hospital; IQR=Interquartile range
Significant at α=0.05
Among patients who had their colonoscopy at a hospital facility (n=1,083), a higher proportion of rural vs. urban patients had their colonoscopy performed at hospitals with very low (24% vs. 9%) or low (17% vs. 11%) rectal cancer surgery volume (results not shown). In contrast, a substantially lower proportion of rural vs. urban patients had their colonoscopy performed at high-volume hospitals (14% vs. 48%). The majority of both rural (53%) and urban (69%) patients subsequently received surgery at the same hospital as their colonoscopy.
Care received between colonoscopy and surgery
Between colonoscopy and surgery, a significantly smaller proportion of rural (77%) vs. urban (84%) patients visited a PCP. However, there was no significant variation by rurality in visits to medical oncologists (60% vs. 63%) or radiation oncologists (54% vs. 54%), and visits to more than one surgeon (33% vs. 31%, results not shown).
Surgery
A significantly smaller proportion of rural patients received surgery at a HVH compared to urban patients (44% vs. 65%; Table 2). A larger proportion of rural vs. urban patients would have had to travel more than 45 minutes to reach the nearest HVH (81% vs. 12%). Among patients who received surgery at a HVH, approximately half of both rural (55%) and urban (49%) patients did not go to the nearest HVH but rather drove to one further away. Rural patients who received surgery at a HVH traveled a median of 83 minutes for surgery, whereas rural patients who received surgery at a non-HVH traveled a median of 35 minutes. In contrast, the median drive time was almost the same between urban patients who went to HVHs vs non-HVHs (18 vs. 17 minutes, respectively). There was a nearly significant difference in surgeon volumes between rural and urban patients (p=0.05); a greater proportion of rural patients received surgery from a very low volume surgeon (32% vs. 26%). Additionally, rural patients were less likely to receive their surgery from a colorectal surgeon than urban patients (47% vs. 54%).
Hospital and surgeon referral patterns
Among patients who had colonscopies performed by a surgeon, most received their surgery from the same surgical specialty that performed their colonoscopy (Figure 3A): 84% of patients whose colonoscopy was performed by a CRS had surgery performed by a CRS, and 65% of patients whose colonoscopy was performed by a GS had surgery performed by a GS. Only 28% of patients whose colonoscopy was performed by a GS had surgery performed by a CRS. In contrast, among those who had colonoscopies performed by a gastroenterologist or PCP, 51% subsequently had surgery performed by a CRS. Similarly, 55% of patients who had colonoscopies performed by a gastroenterologist or PCP subsequently had surgery at a high-volume hospital (Figure 3B). Almost all patients (87%) who had colonoscopies by a CRS went on to have surgery at a high-volume hospital, whereas only 30% of patients who had colonoscopies by a GS went on to have surgery at a high-volume hospital; the majority of these patients (57%) had surgery at a low/very low volume hospital.
Figure 3. Colonoscopist specialty and characteristics of subsequent cancer surgeon.
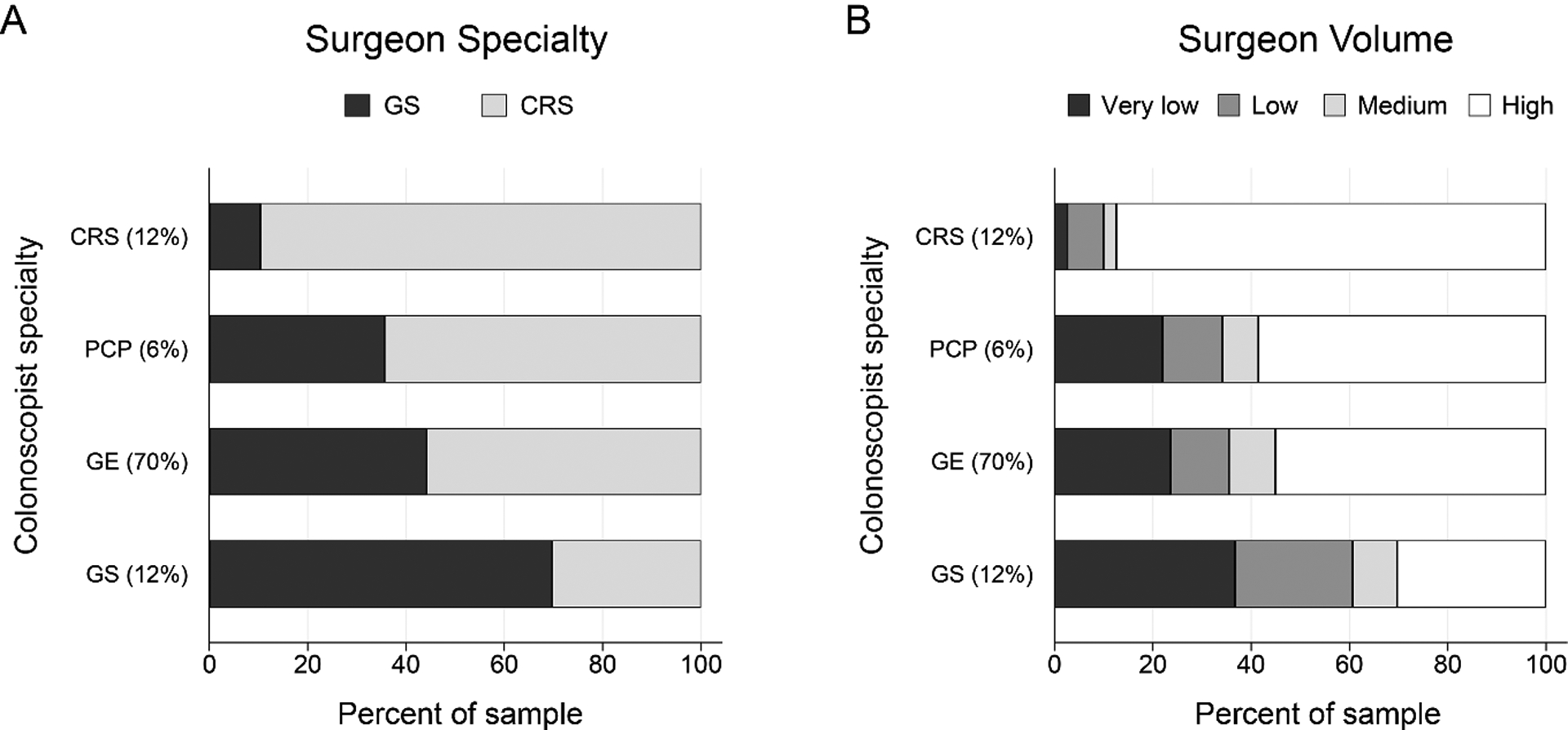
Panel A: the percent of patients treated by a general surgeon (GS) or colorectal surgeon (CRS). Panel B: the percent of patients treated by very low, low, medium, and high volume surgeons. Colonoscopist specialties include CRS, gastroenterologist (GE), GS and primary care provider (PCP). Distribution of the study cohort by colonoscopist specialty is reported in parentheses.
Multivariate analysis
Predictors of surgery at a HVH included: no Charlson comorbidities (OR=1.82; 1.18–2.80), colonoscopy by colorectal surgeon (OR=3.75; 2.18–6.45), living ≤15 minutes from nearest HVH (OR=7.50; 4.37–12.9) and 16–30 minutes from nearest HVH (OR=3.11; 1.83–2.80) (Table 3, Model 1). Predictors of receipt of surgery from HVS/CRS were: living in census tracts with higher percentages of 4-year college completion (OR=1.83; 1.11–3.01) and colonoscopy by CRS (vs. gastroenterologist) (OR=7.89; 4.41–14.1) (Table 3, Model 2). Having colonoscopy performed by a GS (vs. gastroenterologist) was associated with lower odds of receiving surgery from a HVS/CRS (OR=0.34; 0.22–0.54). The interaction term for rural status and colonoscopy and surgery at the same hospital was statistically significant in both Models 1 and 2, demonstrating that rural patients who stay at the same hospital for both colonoscopy and surgery are the least likely to receive surgery from a HVS/CRS or at a HVH, but rural patients who went to different hospitals for colonscopy and surgery are the most likely to receive surgery from a HVS/CRS or at a HVH, with urban patients having the middle likelihood.For patients who received their colonoscopy at an ASC, predictors of surgery at HVH were: more recent year of diagnosis (OR=1.23; 1.05–1.44), state buy-in (i.e., state-based healthcare assistance; OR=2.72; 1.26–5.87), colonoscopy from CRS (OR=3.15; 1.30–7.58), living 15 minutes or less from nearest HVH (OR=5.53; 2.59–11.8) and between 16 and 30 minutes from nearest HVH (OR=2.78; 1.31–5.91) (both vs. 45+ minutes) (Table 3, Model 3). Similarly, predictors of surgery by HVS/CRS were: more recent year of diagnosis (OR=1.17; 1.00–1.36), higher stage (OR=1.59; 1.03–2.48), and colonoscopy from a CRS (OR=5.06; 2.01–12.7) (Table 3, Model 4). Conversely, colonoscopy from a GS was associated with lower odds of receiving surgery from a HVS/CRS (OR=0.31; 0.10–0.94).
Table 3.
Factors predicting: 1) surgery at HVH and 2) surgery from HVS; stratified by colonoscopy facility type.
| Demographic characteristics | Surgery at HVHab | Surgery by HVS/CRSab | |||
|---|---|---|---|---|---|
| Received colonoscopy at a hospital | Model 1 (n=1,059) | Model 2 (n=1,015) | |||
| OR | CI | OR | CI | ||
| Age (years) | 0.99 | (0.97–1.01) | 0.99 | (0.97–1.01) | |
| Year of diagnosis | 0.99 | (0.89–1.09) | 1.06 | (0.96–1.17) | |
| % 4 year college | Low | Ref. | Ref. | ||
| Medium | 1.26 | (0.77–2.08) | 1.55 | (0.95–2.53) | |
| High | 1.62 | (0.96–2.71) | 1.83 | (1.11–3.01)* | |
| State buy-in | Yes | Ref. | Ref. | ||
| No | 1.29 | (0.84–1.97) | 1.44 | (0.96–2.15) | |
| Stage | II | Ref. | Ref. | ||
| III | 1.20 | (0.89–1.62) | 0.92 | (0.70–1.23) | |
| Charlson score | 0 | 1.82 | (1.18–2.80)* | 0.91 | (0.60–1.39) |
| 1 | 1.50 | (0.95–2.39) | 0.75 | (0.47–1.18) | |
| 2+ | Ref. | Ref. | |||
| Colonoscopist specialty | GE | Ref. | Ref. | ||
| CRS | 3.75 | (2.18–6.45)* | 7.89 | (4.41–14.1)* | |
| GS | 0.79 | (0.51–1.24) | 0.34 | (0.22–0.54)* | |
| PCP/Other | 1.19 | (0.65–2.16) | 0.77 | (0.42–1.41) | |
| Drive time to nearest | 0–15 | 7.50 | (4.37–12.9)* | 1.22 | (0.75–2.00) |
| HVH (minutes) | >15–30 | 3.11 | (1.83–2.80)* | 1.21 | (0.73–2.01) |
| >30–45 | 1.69 | (0.99–2.89) | 1.00 | (0.59–1.70) | |
| 45+ | Ref. | Ref. | |||
| Colonoscopy and surgery at same hospital X Rural status | Same hospital/urban | Ref. | Ref. | ||
| Same hospital/rural | 0.65 | (0.35–1.20) | 0.51 | (0.28–0.92)* | |
| Different hospital/urban | 2.54 | (1.70–3.78)* | 1.93 | (1.35–2.76)* | |
| Different hospital/rural | 6.25 | (3.36–11.62)* | 4.96 | (2.71–9.10)* | |
| Received colonoscopy at an Ambulatory Surgical Center | Model 3 (n=518) | Model 4 (n=496) | |||
| OR | CI | OR | CI | ||
| Age (years) | 1.00 | (0.96–1.03) | 1.00 | (0.96–1.03) | |
| Year of diagnosis | 1.23 | (1.05–1.44)* | 1.17 | (1.00–1.36)* | |
| % 4 year college | Low | Ref. | Ref. | ||
| Medium | 1.00 | (0.38–2.85) | 1.37 | (0.51–3.66) | |
| High | 2.04 | (0.74–5.58) | 1.77 | (0.64–4.84) | |
| State buy-in | Yes | Ref. | Ref. | ||
| No | 2.72 | (1.26–5.87)* | 1.82 | (0.87–3.78) | |
| Stage | II | Ref. | Ref. | ||
| III | 1.57 | (0.99–2.48) | 1.59 | (1.03–2.48)* | |
| Charlson score | 0 | Ref. | Ref. | ||
| 1 | 1.81 | (0.92–3.60) | 0.86 | (0.43–1.74) | |
| 2+ | 1.87 | (0.89–3.91) | 0.77 | (0.37–1.62) | |
| Colonoscopist specialty | GE | Ref. | Ref. | ||
| CRS | 3.15 | (1.30–7.58)* | 5.06 | (2.01–12.7)* | |
| GS | 0.53 | (0.17–1.60) | 0.31 | (0.10–0.94)* | |
| PCP/Other | 0.68 | (0.20–2.35) | 1.14 | (0.33–3.91) | |
| Rural status | Urban | Ref. | Ref. | ||
| Rural | 1.57 | (0.70–3.49) | 1.69 | (0.76–3.73) | |
| Drive time to nearest | 0–15 | 5.53 | (2.59–11.8)* | 1.96 | (0.95–4.02) |
| HVH (minutes) | >15–30 | 2.78 | (1.31–5.91)* | 1.48 | (0.72–3.07) |
| >30–45 | 1.51 | (0.67–3.41) | 1.40 | (0.63–3.13) | |
| 45+ | Ref. | Ref. | |||
In addition to all variables in the table, all models were also adjusted for gender, race, marital status, function related indicators and census tract percentage below poverty indicator
Models did not include patients with missing independent variable information
GE=Gastroenterologist; CRS=Colorectal surgeon; GS=General surgeon; PCP=Primary care provider; HVH=High rectal cancer resection volume hospital; HVS=High rectal cancer resection volume surgeon
Significant at α=0.05
We constructed an additional model to examine predictors of receipt of colonoscopy at a HVH among patients who received their colonoscopy at a hospital facility (results not shown). Distance to nearest HVH was the only significant predictor, with the odds of colonoscopy at a HVH decreasing substantially with increasing distance (≤15 minutes: OR=12.7; 16–30 minutes: OR=5.50; 31–45 minutes: OR=2.90; all ORs relative to those living 45+ minutes from nearest HVH).
Discussion
In this study of stage II/III rectal adenocarcinoma patients across the US, we found rural (vs. urban) patients were less likely to receive surgery from a HVH or by HVS/CRS, and the place of colonoscopy and colonoscopist specialty heavily influenced the place of surgery and surgeon specialty. Compared to urban patients, rural patients more often had colonoscopies performed by general surgeons and less often from gastroenterologists or colorectal surgeons, which was in turn associated with lower odds of surgery at a HVH and by a HVS/CRS. Rural patients also had substantially longer driving times to HVHs which was associated with lower odds of surgery at a HVH or by a HVS. Furthermore, driving time was a significant predictor of receiving colonoscopy at a HVH, with a distance-response relationship suggesting that locality was an important factor for patients when choosing their colonoscopy provider/site. The majority of both rural and urban patients received their colonoscopy and surgery at the same hospital, but rural patients who stayed at the same hospital were significantly less likely to receive surgery at a HVH or by a HVS compared to urban patients. To our knowledge, this is the first study that analyzes how factors upon diagnosis such as place of colonoscopy, colonoscopy specialty and drive time impact stage II/III rectal cancer patient’s navigation of the US health care system.
The role of the place of colonoscopy found in this study has important implications for initiatives that seek to improve rural rectal cancer outcomes. Studies have found significant associations between surgical specialization and improved outcomes,4, 31–34 and rectal cancer patients at HVHs have superior lymph node yield, and better neoadjuvant treatment adherence and survival than those at LVHs.35 Efforts to improve rectal cancer treatment through centralization should consider rural practice and referral patterns, especially the role of the colonoscopist. Efforts should also address patient-level barriers, such as drive time.
Drive time was an important predictor for receipt of surgery at HVH; this has been observed across multiple diseases/specialties,35–38 especially in rural areas.39, 40 A recent study reported superior lymph node yield, neoadjuvant treatment adherence and survival for rectal cancer patients who travelled longer distances to HVHs compared to patients who travelled shorter distances to low volume hospitals.35 Our results also support findings from Birkmeyer at al., who reported many cancer patients tend to travel past a HVH; this suggests efforts to centralize cancer surgery could potentially be implemented without imposing excessive travel burdens on patients, though perhaps not feasible for all rural people who live great distances from HVSs.41 HVS/CRSs could potentially travel to rural areas to ensure access to high quality care in regions with limited access to cancer care; this has been shown to be effective in prior research.42, 43
Alternatively, interventions could focus on improving the quality of rectal cancer management in hospitals across the U.S. One program that aims to improve the quality of rectal cancer care on a national scale is the National Accreditation Program for Rectal Cancer (NAPRC), which was designed by the American College of Surgeons and other national societies, with input from the Consortium for Optimizing the Surgical Treatment of Rectal Cancer (OSTRICH). A recent survey of OSTRICH member hospitals showed that only 3% of hospitals were projected to meet all 22 NAPRC standards and that high-volume centers were more likely to be compliant compared to low volume centers.44 Similarly, in another recent study of 1,135 CoC-accredited hospitals, only 3% met all five NAPRC process standards, and 17% were able to meet four of the standards.45 Given that the majority of community hospitals where most rectal cancer patients receive care are not well positionend to achieve NAPRC accreditation, it may be important to target interventions that could be more readily implemented in lower volume hospitals to facilitate broader access to guideline-recommended care.
Care received between colonoscopy and surgery offers additional insight into how rectal cancer patients may navigate the healthcare system. Rural patients were less likely to see a PCP during this time period than urban patients, but no difference was found for other specialties such as medical or radiation oncologists. This finding is reassuring in that there does not appear to be an urban-rural disparity in receiving care from non-surgical treatment specialists. It also suggests the PCP may play a role in surgical referrals that is different between rural and urban areas. Further studies on the role and accessibility of PCPs in rectal cancer diagnosis and referrals may be important for interventions seeking to address patient navigation patterns.
For patients who received their colonoscopy at an ASC, more recent year of diagnosis, no state buy-in (healthcare assistance) and higher cancer stage were significant predictors of receipt of surgery at HVHs or by HVS/CRS, but this was not seen in patients who received their colonoscopy at a hospital. This highlights system-level differences between ASC and hospital referral patterns. Since 94% of freestanding ASCs are located in urban areas27, the variation in referral patterns could be attributed to rural-urban differences in access to HVHs and HVS/CRS. In addition, radical resections must be performed in an inpatient hospital setting, so patients cannot stay at the same facility for surgery when they start at an ASC.
Our study has several limitations. Although we adjusted for several patient, physician and hospital characteristics, we were not able to adjust for other factors that may affect surgical decision making such as history of previous surgery or radiation. Also, we included cases diagnosed from 2007 to 2011 which may not fully reflect more recent referral patterns. However, our recent analysis of SEER Patterns of Care showed that the proportion of stage II/III rectal cancer patients receiving surgery at high-volume hospitals only increased from 23% to 27% from 2010 to 2015.46 No other studies have demonstrated a steep increase in centralization during this time period. Finally, we excluded beneficiaries without contiuous fee-for-service Medicare part A and B because we could not observe their utilization. Thus our results would not apply to these excluded patients.
A major strength of this study is the development and use of an algorithm that was used to determine the rectal cancer date of diagnosis in SEER-Medicare data, which enabled the evaluation of health care episodes upon diagnosis. Furthermore, SEER-Medicare provides a large population-based dataset with information about rectal cancer episodes of care in older patients, and SEER data are known to be highly accurate.18
Rural rectal cancer patients are less likely to receive surgery from a HVH, HVS or surgical specialist which is likely mediated by where patients are receiving their colonoscopy. Initiatives that target referral patterns rather than patient-level factors may be more effective in efforts to improve rectal cancer outcomes by centralization. Any intervention aimed at facilitiating centralization would need to address our findings that suggest referrals, particularly in rural areas, often remain within the same institution. Therefore, forming new referral patterns to outside institutions (e.g., NAPRC hospitals) would be necessary but challenging. Alternatively, if patients ultimately want to have surgery in a local hospital they are more familiar with, more work is needed to understand whether high-volume surgeons can achieve the same outcomes outside of their high-volume institutions and, if so, how to promote outreach services to rural areas from high-volume surgeons or to engage lower-volume surgeons in low volume hospitals in quality improvement efforts.
Acknowledgements:
This study used the linked SEER-Medicare database. The interpretation and reporting of these data are the sole responsibility of the authors. The authors acknowledge the efforts of the National Cancer Institute; the Office of Research, Development and Information, Medicare and Medicaid Services; Information Management Services (IMS), Inc.; and the Surveillance, Epidemiology, and End Results (SEER) Program tumor registries in the creation of the SEER-Medicare database.
Grant Support: This work was supported by NIH/NCI K07 Cancer Prevention, Control, Behavioral Sciences and Population Sciences Career Development Award: 1K07CA197067 and in part by NIH/NCI contract number HHSN261201300020I.
Footnotes
Publisher's Disclaimer: Disclaimers: This manuscript is original and neither published, accepted, or submitted for publication elsewhere.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2019. CA Cancer J Clin 2019; 69(1):7–34. [DOI] [PubMed] [Google Scholar]
- 2.Chioreso C, Del Vecchio N, Schweizer ML, et al. Association Between Hospital and Surgeon Volume and Rectal Cancer Surgery Outcomes in Patients With Rectal Cancer Treated Since 2000: Systematic Literature Review and Meta-analysis. Dis Colon Rectum 2018; 61(11):1320–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smith JA, King PM, Lane RH, et al. Evidence of the effect of ‘specialization’ on the management, surgical outcome and survival from colorectal cancer in Wessex. Br J Surg 2003; 90(5):583–92. [DOI] [PubMed] [Google Scholar]
- 4.Read TE, Myerson RJ, Fleshman JW, et al. Surgeon specialty is associated with outcome in rectal cancer treatment. Dis Col Rectum 2002; 45(7):904–914. [DOI] [PubMed] [Google Scholar]
- 5.Monson JR, Probst CP, Wexner SD, et al. Failure of evidence-based cancer care in the United States: the association between rectal cancer treatment, cancer center volume, and geography. Ann Surg 2014; 260(4):625–31; discussion 631–2. [DOI] [PubMed] [Google Scholar]
- 6.Etzioni DA, Young-Fadok TM, Cima RR, et al. Patient survival after surgical treatment of rectal cancer: impact of surgeon and hospital characteristics. Cancer 2014; 120(16):2472–81. [DOI] [PubMed] [Google Scholar]
- 7.Ptok H, Marusch F, Kuhn R, et al. Influence of hospital volume on the frequency of abdominoperineal resection and long-term oncological outcomes in low rectal cancer. Eur J Surg Oncol 2007; 33(7):854–61. [DOI] [PubMed] [Google Scholar]
- 8.Baek J-H, Alrubaie A, Guzman EA, et al. The association of hospital volume with rectal cancer surgery outcomes. Int J Colorectal Dis 2013; 28(2):191–196. [DOI] [PubMed] [Google Scholar]
- 9.Aquina CT, Probst CP, Becerra AZ, et al. High volume improves outcomes: the argument for centralization of rectal cancer surgery. Surgery 2016; 159(3):736–748. [DOI] [PubMed] [Google Scholar]
- 10.Khani MH, Smedh K. Centralization of rectal cancer surgery improves long-term survival. Colorectal Disease 2010; 12(9):874–879. [DOI] [PubMed] [Google Scholar]
- 11.Manchon-Walsh P, Aliste L, Espinas JA, et al. Improving survival and local control in rectal cancer in Catalonia (Spain) in the context of centralisation: A full cycle audit assessment. Eur J Surg Oncol 2016; 42(12):1873–1880. [DOI] [PubMed] [Google Scholar]
- 12.Charlton ME, Hrabe JE, Wright KB, et al. Hospital Characteristics Associated with Stage II/III Rectal Cancer Guideline Concordant Care: Analysis of Surveillance, Epidemiology and End Results-Medicare Data. J Gastrointest Surg 2016; 20(5):1002–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cirocco WC, Steele SR, Buie WD. Advancing standards of rectal cancer care: lessons from Europe adapted to the vast expanse of North America. Dis Colon Rectum 2014; 57(2):260–6. [DOI] [PubMed] [Google Scholar]
- 14.Charlton M, Schlichting J, Chioreso C, et al. Challenges of Rural Cancer Care in the United States. Oncology (Williston Park) 2015; 29(9):633–40. [PubMed] [Google Scholar]
- 15.Onega T, Duell EJ, Shi X, et al. Influence of NCI cancer center attendance on mortality in lung, breast, colorectal, and prostate cancer patients. Med Care Res Rev 2009; 66(5):542–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Charlton ME, Shahnazi AF, Gribovskaja-Rupp I, et al. Determinants of Rectal Cancer Patients’ Decisions on Where to Receive Surgery: a Qualitative Analysis. J Gastrointest Surg 2019; 23(7):1461–1473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benson AB 3rd, Venook AP, Al-Hawary MM, et al. Rectal Cancer, Version 2.2018, NCCN Clinical Practice Guidelines in Oncology. J Natl Compr Canc Netw 2018; 16(7):874–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Warren JL, Klabunde CN, Schrag D, et al. Overview of the SEER-Medicare data: content, research applications, and generalizability to the United States elderly population. Med Care 2002; 40(8 Suppl):IV-3–18. [DOI] [PubMed] [Google Scholar]
- 19.Institute NC. SEER-Medicare Linked Database. Available at: http://appliedresearch.cancer.gov/seermedicare/.AccessedMarch 12, 2019.
- 20.Charlton ME, Matthews KA, Gaglioti A, et al. Is Travel Time to Colonoscopy Associated With Late-Stage Colorectal Cancer Among Medicare Beneficiaries in Iowa? J Rural Health 2016; 32(4):363–373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lai SM, Jungk J, Garimella S. Colorectal Cancer Identification Methods Among Kansas Medicare Beneficiaries, 2008–2010. Prev Chronic Dis 2015; 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hart LG, Larson EH, Lishner DM. Rural definitions for health policy and research. Am J Public Health 2005; 95(7):1149–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Begg CB, Cramer LD, Hoskins WJ, et al. Impact of hospital volume on operative mortality for major cancer surgery. JAMA 1998; 280(20):1747–51. [DOI] [PubMed] [Google Scholar]
- 24.Bach PB, Cramer LD, Schrag D, et al. The influence of hospital volume on survival after resection for lung cancer. N Engl J Med 2001; 345(3):181–8. [DOI] [PubMed] [Google Scholar]
- 25.Bianco FJ, Riedel ER, Begg CB, et al. Variations among high volume surgeons in the rate of complications after radical prostatectomy: Further evidence that technique matters. J Urol 2005; 173(6):2099–2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Laliberte L, Fennell ML, Papandonatos G. The relationship of membership in research networks to compliance with treatment guidelines for early-stage breast cancer. Med Care 2005:471–479. [DOI] [PubMed] [Google Scholar]
- 27.Medicare Payment Advisory Commission (U.S.). Healthcare spending and the Medicare program : a data book. Washington, DC: Medicare Payment Advsory Commission, 2012. pp. v. [Google Scholar]
- 28.(AMA) AMA. AMA Physician Masterfile. (June262019).
- 29.Chrischilles EA, Schneider KM, Schroeder MC, et al. Association Between Preadmission Functional Status and Use and Effectiveness of Secondary Prevention Medications in Elderly Survivors of Acute Myocardial Infarction. J Am Geriatr Soc 2016; 64(3):526–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schroeder MC, Chapman CG, Nattinger MC, et al. Variation in geographic access to chemotherapy by definitions of providers and service locations: a population-based observational study. BMC Health Serv Res 2016; 16:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Garcia-Granero E, Marti-Obiol R, Gomez-Barbadillo J, et al. Impact of surgeon organization and specialization in rectal cancer outcome. Colorectal Dis 2001; 3(3):179–84. [DOI] [PubMed] [Google Scholar]
- 32.Hall GM, Shanmugan S, Bleier JI, et al. Colorectal specialization and survival in colorectal cancer. Colorectal Dis 2016; 18(2):O51–60. [DOI] [PubMed] [Google Scholar]
- 33.Anwar S, Fraser S, Hill J. Surgical specialization and training - its relation to clinical outcome for colorectal cancer surgery. J Eval Clin Pract 2012; 18(1):5–11. [DOI] [PubMed] [Google Scholar]
- 34.Oliphant R, Nicholson GA, Horgan PG, et al. Contribution of surgical specialization to improved colorectal cancer survival. Br J Surg 2013; 100(10):1388–95. [DOI] [PubMed] [Google Scholar]
- 35.Xu Z, Becerra AZ, Justiniano CF, et al. Is the Distance Worth It? Patients With Rectal Cancer Traveling to High-Volume Centers Experience Improved Outcomes. Dis Colon Rectum 2017; 60(12):1250–1259. [DOI] [PubMed] [Google Scholar]
- 36.Ambroggi M, Biasini C, Del Giovane C, et al. Distance as a Barrier to Cancer Diagnosis and Treatment: Review of the Literature. Oncologist 2015; 20(12):1378–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Loree JM, Javaheri KR, Lefresne SV, et al. Impact of Travel Distance and Urban-Rural Status on the Multidisciplinary Management of Rectal Cancer. J Rural Health 2017; 33(4):393–401. [DOI] [PubMed] [Google Scholar]
- 38.Hogan C Patterns of travel for rural individuals hospitalized in New York State: relationships between distance, destination, and case mix. J Rural Health 1988; 4(2):29–41. [DOI] [PubMed] [Google Scholar]
- 39.Onega T, Duell EJ, Shi X, et al. Geographic access to cancer care in the U.S. Cancer 2008; 112(4):909–18. [DOI] [PubMed] [Google Scholar]
- 40.Lin CC, Bruinooge SS, Kirkwood MK, et al. Association Between Geographic Access to Cancer Care and Receipt of Radiation Therapy for Rectal Cancer. Int J Radiat Oncol Biol Phys 2016; 94(4):719–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Birkmeyer JD, Siewers AE, Marth NJ, et al. Regionalization of high-risk surgery and implications for patient travel times. JAMA 2003; 290(20):2703–8. [DOI] [PubMed] [Google Scholar]
- 42.O’Sullivan BG, McGrail MR, Stoelwinder JU. Reasons why specialist doctors undertake rural outreach services: an Australian cross-sectional study. Hum Resour Health 2017; 15(1):3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruca TS, Pyo TH, Nelson GC. Improving Rural Access to Orthopaedic Care Through Visiting Consultant Clinics. J Bone Joint Surg Am 2016; 98(9):768–74. [DOI] [PubMed] [Google Scholar]
- 44.Lee L, Dietz DW, Fleming FJ, et al. Accreditation Readiness in US Multidisciplinary Rectal Cancer Care: A Survey of OSTRICH Member Institutions. JAMA Surg 2018; 153(4):388–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Antunez AG, Kanters AE, Regenbogen SE. Evaluation of Access to Hospitals Most Ready to Achieve National Accreditation for Rectal Cancer Treatment. JAMA surgery 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Del Vecchio N SJ, Chioreso C, et al. Guideline-recommended chemoradiation for rectal cancer patients at large hospitals: a trend in the right direction. Dis Col Rectum 2019; 62(10):1186–1194. [DOI] [PMC free article] [PubMed] [Google Scholar]


