Abstract
Children residing in mining towns are potentially disproportionately exposed to metal(loid)s via ingestion and dust inhalation, thus, increasing their exposure when engaging in school or home gardening or playing outside. This citizen science study assessed preschool children’s potential arsenic (As), cadmium (Cd), and lead (Pb) exposure via homegrown produce, water, incidental soil ingestion, and dust inhalation at four sites. Participants were trained to properly collect water, soil, and vegetable samples from their preschools in Nevada County, California. As, Cd, and Pb concentrations in irrigation sources did not exceed the U.S. EPA’s maximum contaminant and action levels. In general, garden and playground As and Pb soil concentrations exceeded the U.S. EPA Regional Screening Level, CalEPA Human Health Screening Level, and California Department of Toxic Substances Control Screening Level. In contrast, all Cd concentrations were below these recommended screening levels. Dust samples (< 10 μm diameter) were generated from surface garden and playground soil collected at the preschools by a technique that simulated windblown dust. Soil and dust samples were then analyzed by in-vitro bioaccessibility assays using synthetic lung and gastric fluids to estimate the bioaccessible fraction of As, Cd, and Pb in the body. Metal(loid) exposure via homegrown produce revealed that lettuce, carrot, and cabbage grown in the preschool gardens accumulated a higher concentration of metal(loid) than those store-bought nation-wide. None of the vegetables exceeded the respective recommendation maximum levels for Cd and Pb set by the World Health Organization Codex Alimentarius Commission. The results of this study indicate that consumption of preschool-grown produce and incidental soil ingestion were major contributors to preschool-aged children’s exposure to As, Cd, and Pb. Traditionally, this level of site- and age-specific assessment and analyses does not occur at contaminated sites. The results of this holistic risk assessment can inform future risk assessment and public health interventions related to childhood metal(loid) exposures.
Keywords: children exposure assessment, risk characterization, arsenic, lead, cadmium, incidental soil ingestion, inhalation, plant uptake, mining waste, preschool gardening bioaccessibility
1. Introduction
The Sierra Nevada foothills area of Nevada County has a rich history of gold mining including the California Mother Lode zone containing hundreds of now abandoned mines (Smith, 1999). Nevada County, CA is impacted with metal(loid) contamination from legacy Gold Rush mining which began in 1848 by the toxic byproduct of legacy mining techniques (Alpers, 2017). Nevada County hosted a majority of the most productive placer, hydraulic, and hard rock mining techniques to date (Koshman and Bergendahl, 1968; Craig and Rimstidt, 1998), where more than 3,634 U.S. tons of gold (> US$165B in 2019 US$) has been extracted from the Sierra Nevada foothills (Solnit, 2006). Consequently, in addition to the persistent contamination from byproduct metal(loid)s released into the environment, this region has been shown to have significantly high concentrations of As (up to 5,000 mg kg−1) in mine tailings left behind from mining activity (Smith, 1999). These mine tailings were used for residential development, which led to health risks for residents and workers in the area (Behrshing et al., 2009). The impacts of contamination by active and legacy resource extraction sites are of increasing concern for neighboring communities. This is particularly worrisome for communities that rely on foods locally grown in community and school gardens. Active and legacy mining and smelting operations commonly release metal(loid)s such as arsenic (As) cadmium (Cd), and lead (Pb), which can be transported by wind and water erosion to nearby communities and contaminate groundwater via leaching (Csavina et al., 2011; and references therein).
Toxic contamination of metal(loid)s including As, Cd, and Pb has persisted in the region’s soil, surface water, and groundwater as a result of legacy gold mining. These metal(loid)s are of special concern as they are within the top 10 of the Agency for Toxic Substances and Disease Registry (ATSDR)’s 2017 Substance Priority List due to their frequent and likely exposure and potential toxicity (ATSDR, 2017). Arsenic is recognized as a human carcinogen by the International Agency for Research on Cancer (IARC) and U.S. Environmental Protection Agency (EPA), while Cd and Pb are classified as probable carcinogens by the U.S. EPA (U.S. EPA, 2018b; U.S. EPA, 2006a). Adverse health effects from an acute exposure to high levels of As include gastrointestinal, hepatic, renal, and cardiovascular damage, while chronic exposures have been shown to lead to severe peripheral neuropathy and multi-organ cancers (ATSDR, 2007a). Exposures to Cd have been associated with anemia and neurological impairments from acute and chronic exposures, respectively (ATSDR, 2008). High Pb levels pose a serious risk to children by causing neurobehavioral development delays (ATSDR, 2007b). These heavy metal(loid)s have also been shown to be endocrine-disrupting agents associated with the increase risk of cancers (Vaiserman, 2014). Children have an air and caloric intake that is 2 to 3 times greater than adults and undergo more rapid and complex body system development, which make them more susceptible to adverse health effects of environmental toxicants (Landrigan et al., 2011). Children residing in communities at-risk of pollution are principally exposed to metal(oid)s via incidental ingestion of dust and soil as a result of hand-to-mouth transfer (Huang et al., 1997; Beamer et al., 2012).
In efforts to increase public health prevention and informal science education activities, more schools have incorporated gardening programs in their curriculum (Turner et al., 2016) potentially increasing the risk of exposure to these contaminants in the area. Furthermore, gardening has become a more prevalent hobby in the United States, providing families with essential fruits, vegetables, and herbs, but also presenting a potential dietary exposure if soil or irrigation water is contaminated or garden produce accumulates these metal(loids) (Bian et al., 2015; Ramirez-Andreotta et al., 2013a & 2013b). Plants with metal(loid)-accumulating capacity are known as accumulators, while those with increased capacity are known as hyperaccumulators (Ávila et al., 2016; Tangahu et al., 2011). This ability is useful in phytoremediation but can pose a threat when consumed regularly by children. Members of the Apiaceae (Stilwell et al., 2008), Brassicaceae, and Asteraceae families accumulated more As (Ramirez-Andreotta et al., 2013a) and Cd in the edible portion of the plant than plants from other families. Further, it is suggested that members of the same plant family have the same metal(loid) up-taking properties (Ramirez-Andreotta et al., 2013a). Thus, members of these families are crucial to consider when assessing possible exposure to metal(loid)s by ingestion of garden plants.
Soil and dust can cause significant exposures to metal(loid)s including As, Cd, and Pb if ingested or inhaled through play and gardening activities. Most human risk assessments do not consider the bioavailability of a metal(loid), which may over- or under-estimate exposure. The bioavailability of a metal(loid) is the absorbed fraction of the metal(loid) that enters systemic circulation and may reach target organs, typically determined by in-vivo animal models (U.S. EPA, 2007c). Bioaccessibility is, therefore, the fraction of the total metal(loid) concentration released from the environmental matrix (e.g. dust, soil, food, water) into the biofluid and becomes available for absorption (Thomas et al., 2018; Roussel et al., 2010; Oomen et al., 2002). The bioavailability is, therefore, influenced by the bioaccessibility of that metal(loid) since solubilization is required for crossing membranes in the body (U.S. EPA, 2007c). The bioaccessible fraction determined by in-vitro assays are a useful and inexpensive tool that represents the maximum amount of metal(loid) that can be absorbed (Oomen et al., 2002).
Given the possible association between metal(loid) exposures through gardening and adverse health effects, the present study was designed to evaluate a child’s potential exposure to As, Cd, and Pb at preschools located in Nevada County, CA. Building upon the traditional U.S. EPA exposure assessment guidance (U.S. EPA, 1992), this study employs site-specific analysis to predict a preschool-aged child’s exposure to As, Cd, and Pb in a legacy mining community. Using a citizen-science approach, a child’s dietary assessment was conducted in tandem with the collection of irrigation water, soil and dust (garden and playground), and preschool-grown plant samples. With site-specific As, Cd, and Pb concentrations, a comprehensive exposure assessment was then completed to account for ingestion (locally grown food, soil, and water) and inhalation using gastric and lung fluid assays to evaluate bioaccessibility (Figure 1).
Figure 1.
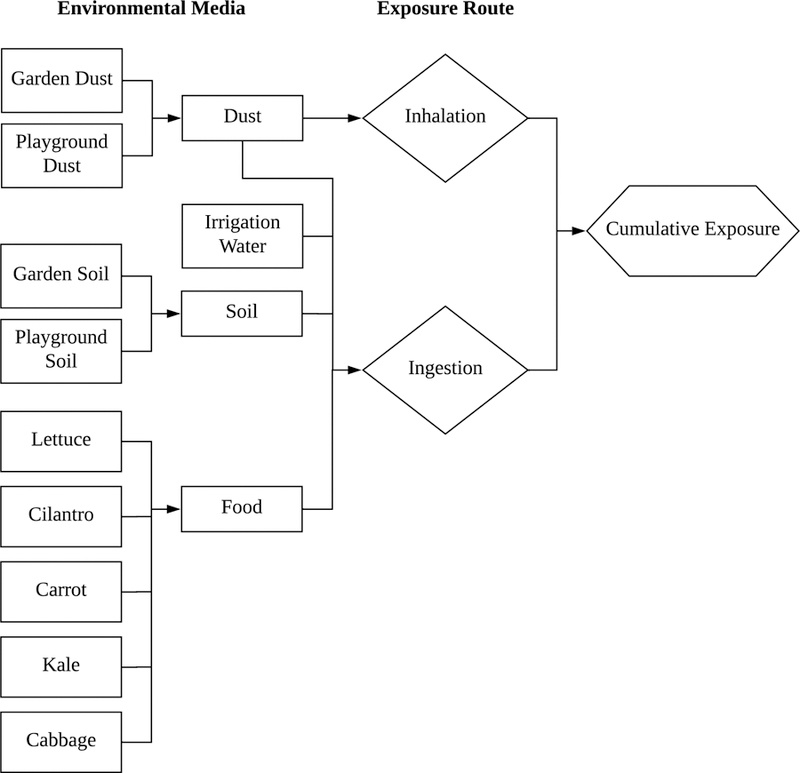
Schematic of a child’s multi-route exposure considered in this study.
2. Materials and Methods
2.1. Site description
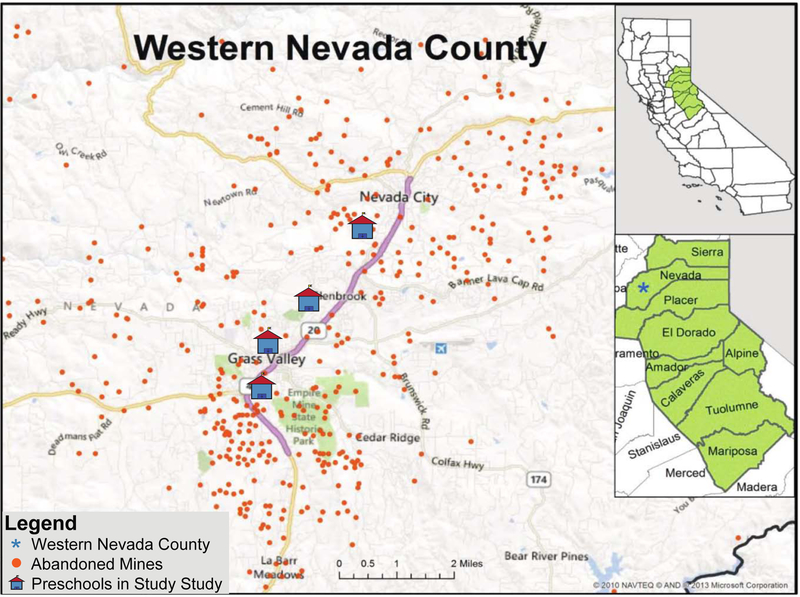
Four preschools throughout Grass Valley (95945) and Nevada City (95959), CA were selected as sampling sites (Figure 2) for Gardenroots: The Nevada County, CA Garden Project (referred to as Gardenroots hereafter). The two rural cities are located in the foothills of the Sierra Nevada mountain range in Northern California. As of 2010, Grass Valley comprised of 12.28 square kilometers and had 12,860 residents, while Nevada City has a population of less than 5,000 residents (U.S. Census Bureau, 2010).
Figure 2.
Map of abandoned gold mines in Western Nevada County, CA. (Original map source: US Geological Survey). Approximate location of preschools are indicated.
The gardens at all four schools were established before 2015 and have been used exclusively for vegetable gardening. Site 1 (S1) and site 3 (S3) have grown produce for > 8 years and 6–8 years, respectively. Sites 2 (S1) and 4 (S4) have been vegetable gardening for only 3–5 years. Site 1, S2, and S3 have previously used some garden produce for school meals, and S2 and S3 report that children take home produce from the school gardens. The mean soil pH at S1, S2, S3, and S4 gardens were 5.4, 5.8, 7.0, and 5.2, respectively. According to the U.S. Department of Agriculture (USDA) Web Soil Survey, the dominant soil type for the Nevada County area is classified as a Josephine-mariposa complex composed of a gravelly loam within the first 30.48 cm from the surface (USDA, 2018).
2.2. Dietary assessment and gardening description survey
A dietary assessment including a food frequency questionnaire (FFQ) and a gardening description survey was administered to school administrators and parents at each site (Manjon and Ramirez-Andreotta, unpublished results). Each respondent was consented under the University of California, San Francisco Institutional Review Board (IRB). The site administrators’ responses (N = 4) to the gardening description survey provided insight on possible contamination sources, child behavior, gardening activities, and time spent outside in the gardens. Parents’ responses (N = 10) to the FFQ questions listed below were used to calculate the ingestion rates of selected crops (Table 4).
Table 4.
Hazard quotient for As, Cd, and Pb per exposure age group and exposure media.
| Exposure Age Group | ||||
|---|---|---|---|---|
| Exposure Media | 1 to < 2 yrs | 2 to < 3 yrs | 3 to < 6 yrs | |
| As | Garden soil | 3.5 × 10−3 | 2.1 × 10−3 | 1.6 × 10−3 |
| Playground soil | 3.1 × 10−3 | 1.9 × 10−3 | 1.4 × 10−3 | |
| Lettuce | 6.0 × 10−2 | 3.7 × 10−1 | 3.0 × 10−3 | |
| Cilantro | 1.1 × 10−1 | 1.2 × 10−2 | 7.6 × 10−4 | |
| Carrot | 4.7 × 10−3 | 1.8 × 10−2 | 1.9 × 10−3 | |
| Kale | 8.1 × 10−2 | 3.4 × 10−2 | 2.5 × 10−2 | |
| Cabbage | 2.3 × 10−4 | 1.1 × 10−4 | 6.4 × 10−5 | |
| Garden dust | 1.5 × 10−4 | 1.5 × 10−4 | 1.2 × 10−4 | |
| Playground dust | 1.2 × 10−4 | 1.2 × 10−4 | 9.4 × 10−5 | |
| Water | 1 7 × 10−3 | 1.9 × 10−3 | 1.5 × 10−3 | |
| Cumulative | 2.6 × 10−1 | 4.4 × 10−1 | 3.5 × 10−2 | |
| Cd | Garden soil | 2.8 × 10−2 | 1.7 × 10−2 | 1.3 × 10−2 |
| Playground soil | 2.0 × 10−2 | 1.3 × 10−2 | 9.3 × 10−3 | |
| Lettuce | 1.3 × 10−1 | 7.7 × 10−1 | 6.4 × 10−3 | |
| Cilantro | 2.2 × 10−2 | 2.4 × 10−3 | 1.5 × 10−4 | |
| Carrot | 8.1 × 10−1 | 3.1* | 3.3 × 10−1 | |
| Kale | 2.1 × 10−2 | 8.5 × 10−3 | 6.3 × 10−3 | |
| Cabbage | 9.5 × 10−3 | 4.4 × 10−3 | 2.6 × 10−3 | |
| Garden dust | 4.4 × 10−4 | 4.3 × 10−4 | 3.4 × 10−4 | |
| Playground dust | 2.7 × 10−4 | 2.6 × 10−4 | 2.1 × 10−4 | |
| Water | 1.2 × 10−3 | 1.3 × 10−3 | 1.0 × 10−3 | |
| Cumulative | 1.0* | 3.9* | 3.7 × 10−1 | |
| Pb | Garden soil | 1.4 × 10−2 | 8.9 × 10−3 | 6.6 × 10−3 |
| Playground soil | 3.7 × 10−3 | 2.3 × 10−3 | 1.7 × 10−3 | |
| Lettuce | 2.8 × 10−4 | 1.7 × 10−3 | 1.4 × 10−5 | |
| Cilantro | 8.8 × 10−6 | 9.7 × 10−7 | 6.1 × 10−8 | |
| Carrot | 2.9 × 10−3 | 1.1 × 10−2 | 1.2 × 10−3 | |
| Kale | 3.1 × 10−6 | 1.3 × 10−6 | 9.4 × 10−7 | |
| Cabbage | 3.2 × 10−6 | 1.5 × 10−6 | 8.7 × 10−7 | |
| Garden dust | 4.5 × 10−7 | 4.4 × 10−7 | 3.4 × 10−7 | |
| Playground dust | 3.7 × 10−7 | 3.6 × 10−7 | 2.8 × 10−7 | |
| Water | 8.5 × 10−5 | 9.9 × 10−5 | 7.5 × 10−5 | |
| Cumulative | 2.1 × 10−2 | 2.4 × 10−2 | 9.6 × 10−3 | |
Indicates HQ ≥ 1
In the last 12 months, did your child eat [food]?
How often did your child eat [food]?
What was your child’s usual serving size of [food]?
In May 2018, Gardenroots participants (N = 33) including school administrators, teachers, parents, and community members were trained by the research group on how to properly collect irrigation water, soil, and plant samples at each preschool. Participants were given a sample-collection kit, as well as an easy-to-follow instruction manual written in English and Spanish to facilitate sample collection. The sample-collection kit contained all the necessary materials for sample collection, documentation, and personal protection equipment. Given their own insight regarding the preschool’s gardening practices and their students’ regular play areas, the participants decided on specific sampling locations for soil and irrigation water sources. Irrigation water and soil samples were collected during the sample collection trainings in May 2018, while the plant samples were collected from July to October 2018.
2.3. Water sampling, preparation, and analysis
Participants collected one water sample from the irrigation source for the garden as well as a field blank at each preschool. The samples and field blanks (N = 8) were collected in trace metal-free 50 mL tubes. To collect the field blank, a tube was filled with nanopure water, and then transferred into a clean tube next to the source of irrigation water where the sample was collected at each site. Samples and field blanks were refrigerated upon collection and promptly shipped on ice within two days to the University of Arizona (UA). Upon arrival at UA, the samples were processed and analyzed for total metal(loid) concentration including As, Cd, and Pb by Inductively Coupled Plasma-Mass Spectrometry (ICP-MS) (Agilent 7700 ICP-MS, Santa Clara, CA) per U.S. EPA Method 6020B (SW-846) (U.S. EPA, 2014) at the Arizona Laboratory for Emerging Contaminants (ALEC). The ICP-MS instrument quantifiable detection limits were 0.0018 – 0.033 μg L−1 for As, 0.00064 μg L−1 for Cd, and 0.00047 μg L−1 for Pb. The QA/QC protocol was adapted from U.S. EPA Method 200.8 (U.S. EPA, 1994). Calibration standards for the ICP-MS were prepared from multi-element stock solutions (SPEX Certiprep, Metuchen, NJ). Calibration curves include at least seven points with correlation coefficients > 0.995. The QC protocol includes a continuing calibration blank (CCB); a continuing calibration verification (CCV) solution; and at least one quality control sample (QCS) to be analyzed just after calibration, again after every 12 samples, and at the completion of the run. The QCS solutions are from an independent source, such as NIST SRM 1643e Trace Elements in Water. Acceptable QC responses must be between 90 and 110% of the certified value. Lastly, a suitable internal standard (usually Rh, In, Ga or Ge) is added using on-line addition into the sample line and mixing tee.
2.4. Soil sampling, preparation, and analysis
The research group and Gardenroots participants collected soil samples from the garden (4 to 8 samples) and playground (native, unamended, 3 to 4 samples) areas at each preschool for a total of 38 soil samples. For each garden, participants were instructed to select six sampling spots within a grid pattern drawn in each garden bed. Participants then collected the top 15 cm of soil from each of the six spots and homogenized it within a five-gallon plastic bucket. From the homogenized bulk sample, up to four samples were separated into individual brown paper bags. For the playground soil samples, participants selected an area in the surrounding playground areas where children often play. When appropriate, wood chips and other ground cover elements were removed before collecting the samples. All soil samples were immediately refrigerated and shipped on ice to UA within two days.
Similar to the garden and playground soil samples, participants and the research group collected a separate set of garden and playground soil samples at each preschool to be used for dust generation. For these garden samples, a scrape of approximately the top 2 cm of the garden soil was collected and homogenized into a five-gallon plastic bucket. This was repeated for a larger area of the playground. These five-gallon plastic buckets (N = 8) were also immediately refrigerated and shipped on ice to UA within two days.
Samples of garden amendments presumed to be similar to what was originally applied when the gardens were first built were collected from the same local businesses (Supplemental Material, Table 1). The samples were collected separately to evaluate their individual contribution to metal(loid) concentrations in garden soils. These garden amendment samples were promptly shipped to UA upon collection and stored in a laboratory refrigerator upon arrival before chemical analysis.
All garden and playground soil and amendment samples were air dried for 24–96 hours, sieved to ≤ 2 mm diameter and then oven dried at 105 °C to a constant mass. Given that the concentration of As and Cd may be higher in the smaller size fraction and to ensure we analyzed the particles in the size range that adheres to a human hand (Beamer et al., 2012), dried, garden and playground soil samples (excluding those used for dust generation) and the amendment samples were additionally sieved to ≤ 63 µm diameter using a standard stainless-steel mesh sieve (W.S. Tyler™, ASTM E-11 standard No. 230). These garden and playground soil samples were also sieved to 75–106 µm diameter using similar sieves and analyzed for comparison to the ≤ 63 µm soil samples (data not reported here). Soil and plant (described below) samples were stored in a desiccator prior to measuring mass.
Samples were digested for ICP-MS analysis by microwave-assisted acid digestion (modified from U.S. EPA Method 3051) with 0.1 g sample reacted with 1 mL concentrated nitric acid (Omni-trace HNO3, EMD Chemicals) at room temperature for 1 hr, followed by the addition of 1 mL ultrapure water (18 Mohm), then capped and subjected to high pressure and temperature via microwave digestion, (CEM Model MARS6 microwave, Matthews, North Carolina). A NIST sample of similar matrix (NIST SRM 2711a Montana II soil, NIST SRM 2584 Trace Elements in Indoor Dust, or NIST SRM 1515 Apple Leaves) was included in each batch. Soil and plant samples were analyzed for total metal(loid) concentrations via ICP-MS, with quantifiable detection limits of 0.041 – 0.0032 μg g−1 for As, 0.0018 μg g−1 for Cd, and 0.0016 μg g−1 for Pb.
2.5. Dust sample generation and calculations
The laboratory dust generator used in this study was developed to produce a mass of several grams of dust samples from bulk soil and tailing samples (Gonzales et al., 2014). The dust generator is comprised of a 208 L steel drum (57 cm diameter) with built-in baffles, which gently rotate to mix and resuspend the bulk soil sample. Clean air supplied by an air compressor flows at a rate of 28.3 L min−1 through a fixed 3.5 cm diameter PVC pipe threaded horizontally through the drum. The re-suspended particles within the drum flow through the pipe and enter a cyclone separator with a cut-point diameter of 10 µm (model URG-2000–30 EA, URG Corp. Chapel Hill, NC). Subsamples of the < 10 µm particulate matter (PM10) samples were acid-digested with microwave assistance and analyzed for total elemental concentrations as described for soil samples. These dust samples were later used to determine the particle size distribution and the lung in-vitro bioaccessibility assay. The generated dust simulates the particle size and composition of windblown fine atmospheric dust (Thomas et al., 2018; Gonzales et al., 2014).
Atmospheric PM10 metal(loid) concentrations (µg m−3) were estimated using the measured concentration (µg g−1) in generated dust samples and the highest PM10 concentrations (0.339 mg m−3) measured in 2018 by a local U.S. EPA AirData Air Quality Monitor approximately 30 mi away from the site locations (U.S. EPA, 2018a). This calculation was made to conservatively estimate the ambient metal(loid) concentration (Cma) at the preschools using the following equation:
where Cmd is the concentration of the metal(loid) measured in the generated dust samples (< 10 µm), CF is the unit conversion factor of 10−3, and CPM10 is the highest recorded PM10 concentration measured by the U.S. EPA air monitor in 2018 (U.S. EPA, 2018a).
Particle size distribution of the generated dust was determined by micrographs from environmental scanning electron microscopy (ESEM) (Inspect S, FEI Company, Hillsboro, OR). Samples were gold-coated and visualized at 1,300× and 2,500× magnification. Particle size and size-distribution were determined using the ImageJ software package (Version 2.0.0-rc69/1.52i, 2018). Imaged particle diameter was assumed to be spherical with a two-dimensional area of 𝐴 = 𝜋𝑟2. A limitation to this analysis was the inability to distinguish between large particles and multiple small particles aggregated together (See Figure 3).
Figure 3.
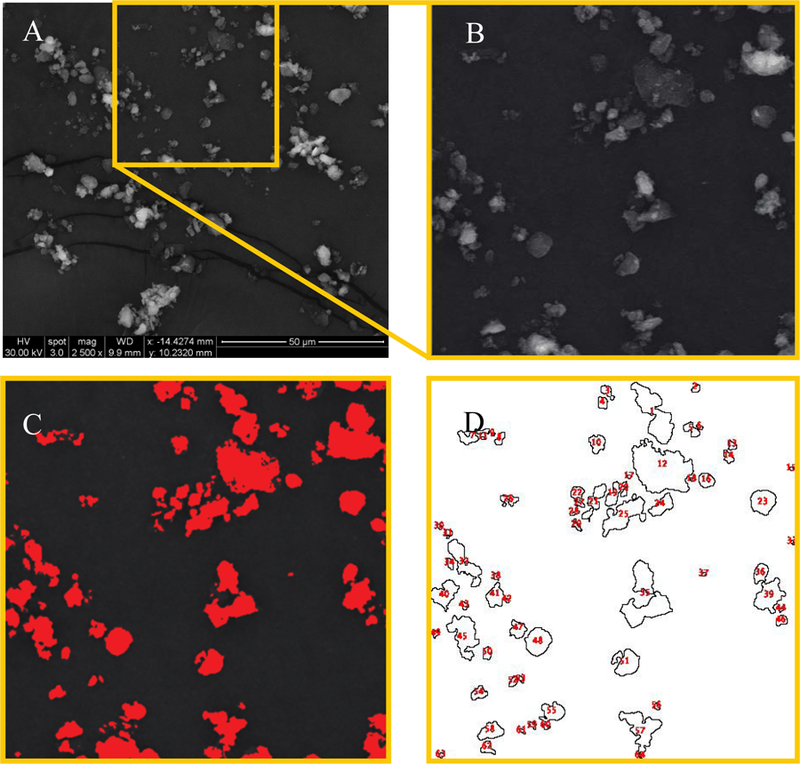
Process of determining particle size distribution by ImageJ. (A) Environmental Scanning Electron Microscopy image of S1 playground dust sample viewed at 2,500× magnification. Yellow box indicates analyzed area. (B) Contrast and brightness adjustment for selected area. (C) Particle area analyzed. (D) Outlines of analyzed particles.
2.6. Plant sampling, preparation, and analysis
A thorough literature review was conducted to select plants for the dietary assessment and to grow in each preschool garden (Manjon & Ramirez-Andreotta, unpublished results). Similar to the criteria previously used in a Gardenroots greenhouse study (Ramirez-Andreotta et al., 2013a), the following criteria was used to select plants for this study:
Shown to translocate As and/or Cd in previous studies
Commonly grown in the area
Regionally, culturally, ethnically, and age-appropriate for the target population
Based on these criteria, the following plants were sowed in each preschool garden between June and August 2018:
Santo cilantro (also known as coriander) (Apiaceae Coriandrum sativum)
Spearmint (Lamiaceae Mentha spicate)
Lacinato kale (Brassicaceae Brassica oleracea ‘Lacinato’)
Nantes organic carrot (Apiaceae Daucus carota)
Early Jersey Wakefield cabbage (Brassicaceae Brassica oleracea)
Ridgeline Romaine lettuce (Asteraceae Lactuca sativa)
The seeds were planted randomly throughout the gardens to account for variation and inconsistencies with shade, irrigation regime, and soil. A local farmer was contracted to tend to each garden and implement changes to the gardens and irrigation systems when necessary to aid plant growth. Due to low crop production, each garden was amended with 1.2 cm (S1, S2, S3) to 2.5 cm (S4) of an organic dairy compost. The plants were also replanted as needed using the same seeds and/or plant starts of the same cultivar.
Between July and October 2018, the research group and Gardenroots participants collected garden plant samples. They were instructed to collect three samples (replicates) of carrot, lettuce, and either cabbage or kale, and also mint or cilantro for a total of three samples per species. However, mint samples were unattainable due to plant bolting at each site before the collection period. A visual description of what each plant sample should look like (e.g. how many carrots should be included in a single sample) was included in the instruction manual to ensure enough plant material was collected per plant species. Participants were instructed to select portions of the plant that were representative of what the children would consume. Plant samples were placed in sterile sample bags (Whirl-Pak® or VWR®) and refrigerated upon collection.
Once in the laboratory, the inedible portions of each plant sample were removed unless noted otherwise and only the edible portion of the plant was analyzed. In order to simulate the average consumer at their home, samples were washed for 30 seconds with deionized water (at home, participants would be using tap water; however, deionized water was used to not introduce contaminants from the tap source). The samples were then oven-dried at 60 °C in brown paper bags until they reached a constant mass. Dried samples were then finely ground using a mortar and pestle or dedicated coffee grinder and shipped to UA for analysis. Due to inconsistencies in final dry weights for cilantro only, the fresh weight was calculated using the mean moisture content ratio of 0.8771 for parsley as reported by the U.S. EPA Exposure Factor Handbook (cilantro was not provided; U.S. EPA, 2018b).
Plant samples were microwave acid-digested and analyzed for total metal(loid) concentrations as described above. The metal(loid) concentrations in plants were compared to recommended maximum levels set by the World Health Organization (WHO) Codex Alimentarius Commission (referred to as Codex hereafter) (Joint FAO/WHO Codex Alimentarius Commission, 2018). These internationally recommended maximum levels were established to protect consumer health and ensure food safety. The metal(loid) concentrations measured from the present study were also compared to mean concentrations reported in the U.S. Food and Drug Administration (U.S. FDA) Total Diet Study (Market Baskets 2006–2013) (U.S. FDA, 2014) to determine how store-bought produce compares to the produce grown at the preschools. In the Total Diet Study, mean concentrations calculated with samples less than the limit of detections are reported a value of zero (U.S. FDA, 2014). For these non-detects, a more conservative, modified average (MA) was calculated with the metal(loid) limit of detection (LOD) using the following expression (Ramirez-Andreotta et al., 2013b; U.S. EPA, 2000): 𝑀𝐴 = 𝐿𝑂𝐷/2. Similarly, metal(loid) concentrations for all samples in this study that were at or below the detection limit are also reported as ½ the detection limit concentration. Lastly, a one-way ANOVA analysis and Tukey-Kramer Honest Significant Difference test was performed to compare the mean metal(loid) concentrations between plant types using JMP Pro Software version 13.0. For all comparisons, a probability of p < 0.05 was considered statistically significant.
2.7. Bioconcentration factor (BCF) calculation
The bioconcentration factor (BCF) is the ratio of metal(loid) concentration (dry weight) in the plant to the concentration in garden soil (75–106 µm diameter), expressed as 𝐵𝐶𝐹 = 𝐶𝑝𝑙𝑎𝑛𝑡/𝐶𝑠𝑜𝑖𝑙 (Ramirez-Andreotta et al, 2013a; Ávila et al., 2016; Alam et al., 2003). The BCF was calculated for each plant type grown in each preschool garden.
2.8. In-vitro bioaccessibility assay (IVBA)
A validated in-vitro bioaccessibility assay (IVBA) for As and Pb in soil was used to determine the bioaccessible fraction (BAF) of the ingested and inhaled dose of As, Cd, and Pb that would become available to target organs (U.S. EPA, 2017b). Due to limited dust samples and garden and playground soil within the ≤ 63 µm diameter size fraction from each site, 0.01 g of soil and dust samples to 1 mL of extraction fluid was used for the EPA Method 1340 (U.S. EPA, 2017b). Samples were ran in triplicates (unless otherwise noted) for both gastric and lung assays.
2.9. Gastric in-vitro bioaccessibility assay
The garden and playground soil samples (previously sieved to ≤ 63 µm diameter) from each preschool that contained the highest concentration of As, Cd, and Pb were selected for the gastric IVBA. Triplicates (with the exception of one soil sample) weighing 0.01 ± 0.0038 g were prepared for these soil samples (n = 20) and placed in black 1.5 mL polypropylene microcentrifuge tubes to prevent photocatalysis (Thomas et al., 2018). The gastric extraction fluid used was 0.4 M glycine adjusted to pH 1.51–1.71 using reagent-grade HCl. Within an anoxic chamber (2% H2(g) and 98% N2(g)), 0.01 g of soil sample was reacted with 1 mL of the extraction fluid in an end-over-end rotator (7 rpm) inside of an incubator (Bioexpress Genemate Mini Incubator Shaker, UT) at 37 °C for 1 h as described by Thomas et al. (2018). After an hour of agitation, the samples were centrifuged at 2,700 RCF for 1 min and the supernatant was then filtered through an Acrodisc® 0.2 μm trace metal-free hydrophilic polypropylene syringe filter to terminate the reaction. Lastly, 0.5 g of the filtered supernatant was acid preserved using 9.5 g of 0.01M HNO3 for ICP-MS analysis.
2.10. Lung in-vitro bioaccessibility assay
An IVBA simulating an inhalation exposure was conducted using the generated garden and playground dust samples for each site (N = 24). For this assay, a synthetic lung fluid composed of inorganic salts, surfactants, buffers, and humectant was used as described by Thomas et al. (2018) and originally proposed by the Solubility/Bioavailability Research Consortium (Kelley et al., 2002) and Boisa et al. (2014). Under oxic conditions, the same modified standard operating procedure and preservation as the gastric IVBA was used. Metal(loid) concentrations were then measured by ICP-MS.
2.11. Bioaccessible fraction (BAF) calculation
The bioaccessible fraction (BAF) here is defined as the fraction of metal(loid) concentration extracted by the synthetic gastric and lung fluid from the soil and dust samples, respectively. The BAF for As, Cd, and Pb was calculated using the total metal(loid) concentrations measured in garden and playground soil (< 63 µm diameter) (Cs) and dust (Cd) samples and metal(loid) concentrations measured in the IVBA extracts (Cext). BAFs were calculated using the expression:
where Vext is the extraction solution volume; Ms is the mass of soil sample used in the gastric IVBA; Md is the mass of dust sample used in the lung IVBA; and df is the dilution factor (U.S. EPA, 2017b).
2.12. Exposure Assessment
The estimated average daily dose (ADD, milligrams per kilogram of body weight per day) and lifetime average daily dose (LADD) of As, Cd, and Pb from ingested water, incidental soil, and plants grown in the gardens, as well as inhalation of dust was calculated using site-specific ingestion rates combined with environmental monitoring data. The As, Cd, and Pb concentration in plants (Cp), soil (Cs), dust (Cd), and water (Cw) was used to calculate the ADD for each age group using the expressions (Ramirez-Andreotta et al., 2013b; U.S. EPA. 2011):
Where: IR = ingestion rate; BAF = bioaccessible fraction; CF = conversion factor (only for soil and plant calculations); EF = exposure frequency; ED = exposure duration; BW = average body weight (child); AT-C = average time – cancer; and AT-NC = average time – non-cancer. Values used for these parameters for each child age group are presented in Supplemental Material, Tables 2–4. When not estimated from this study’s FFQ and gardening description survey, values for these parameters were obtained from the U.S. EPA Exposure Factors Handbook and Children’s Exposure Factors Handbook (U.S. EPA, 2019a, 2017a, 2011, 2008). The EF for incidental soil ingestion is defined as the frequency over which a child (of a defined age group) is exposed to the metal(loid) in question. Here, 181 days (26 weeks) was used to account for the child’s time at the preschool over one year (U.S. Department of Education, 2008). The ADD was modified to compare to values reported in the literature using the expression below as described by Ramirez-Andreotta et al. (2013b):
The cumulative ADD (CADD) and cumulative LADD (CLADD) describes the total exposure to As, Cd, or Pb by combined ingestion (of plant, soil, and water) and inhalation (of dust). The CADD and CLADD of each individual metal(loid) was calculated for each child age group using the expression below.
2.13. Risk Characterization
The incremental excess lifetime cancer risk (IELCR) was calculated for As per exposure age group. The IELCR characterizes the risk of cancer from a lifetime exposure to carcinogens beyond one’s natural risk. The IELCR from As exposures was calculated using the expression:
where the CSF is the cancer slope factor for As, which is the upper bound increased risk of cancer from exposure to As over a lifetime (Ramirez-Andreotta et al., 2013b; U.S. EPA, 2006a). The IELCR was calculated for ingestion of water, plants, (incidental) soil, and inhalation of dust using a CSF of 1.5 (mg kg−1 d−1)−1 (Supplemental Material, Table 5).
The cumulative hazard quotient (HQ) was calculated to characterize a child’s cumulative risk of non-carcinogenic effects for each exposure age group per metal(loid). The HQ was calculated using the expression:
where the reference dose (RfD) is the ratio extrapolated at which no-observable adverse effects are expected. When available, a child-specific RfD was used. The CalEPA and federal EPA recommended RfDs for As, Cd, and Pb, used for this calculation are shown in Supplemental Material, Table 5. An HQ ≤ 1 indicates no adverse health effects expected from an exposure.
3. Results
3.1. Irrigation Water
The total concentrations in irrigation water samples ranged from 0.041 to 0.18 µg L−1 As, 0.0013 to 0.0035 µg L−1 Cd, and 0.00066 to 0.53 µg L−1 Pb, respectively. The sample taken at S2 had the highest concentration of As (0.18 µg L−1) and Pb (0.53 µg L−1); however, these concentrations were two orders of magnitude below the U.S. EPA primary maximum contaminant level (MCL) of 10 µg L−1 for As and the Lead and Copper Rule action level (AL) of 15 µg L−1 for Pb for drinking water (Figure 4). Furthermore, the highest Cd concentration measured (S4) was three orders of magnitude less than the MCL of 5 µg L−1 for Cd. Tap water from the City of Grass Valley and Nevada City is currently the irrigation water source for all preschool gardens, as reported by all site administrators in the gardening description survey.
Figure 4.
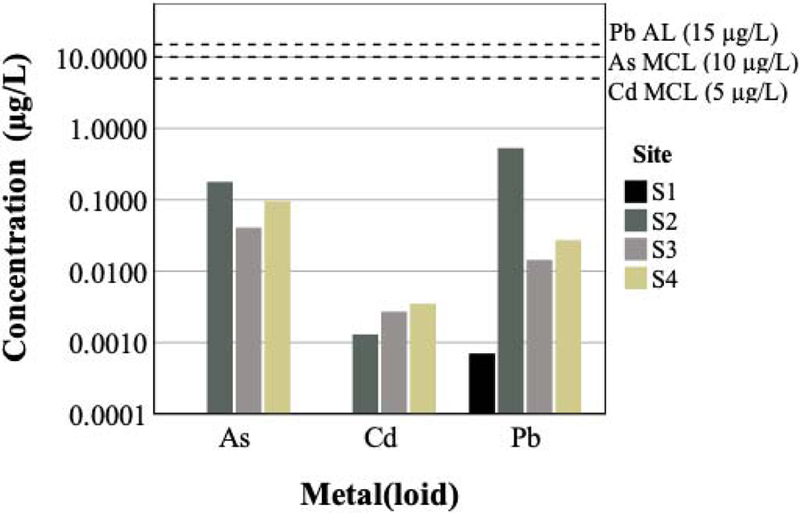
Garden irrigation water metal(loid) concentrations (μg L−1).
3.2. Soil
The metal(loid) concentrations for garden and playground soil samples sieved to < 63 µm diameter are reported in Figure 5. Concentrations of each metal(loid) are compared to soil screening levels recommended by federal and California state agencies (Supplemental Material, Table 6). These screening levels are based on the noncarcinogenic and carcinogenic effects of long-term exposures to metal(loid)s by multiple pathways with a target risk of 10−6 unless specified otherwise (U.S. EPA, 2019b; CalEPA DTSC 2018; OEHHA, 2010). Arsenic concentration in garden and playground soil collected from each school ranged from 1.2 to 19 µg g−1 and 0.90 to 15 µg g−1, respectively (Figure 5). All As concentrations in soil samples exceeded the recommended California Human Health Screening Level (CHHSL) (0.07 µg g−1), California Department of Toxic Substances Control Screening Level (DTSC-SL) of 0.11 µg g−1 and the U.S. EPA Regional Screening Level (RSL) of 0.68 µg g−1. Further, all soil samples exceeded the EPA RSL specific to exposures by ingestion (0.77 µg g−1). All garden amendment samples had median As concentrations that also exceeded these recommended screening levels. All median As concentrations in garden and playground samples and amendments were similar to CA background concentrations for As (Kearney Foundation of Soil Science, 1996).
Figure 5.
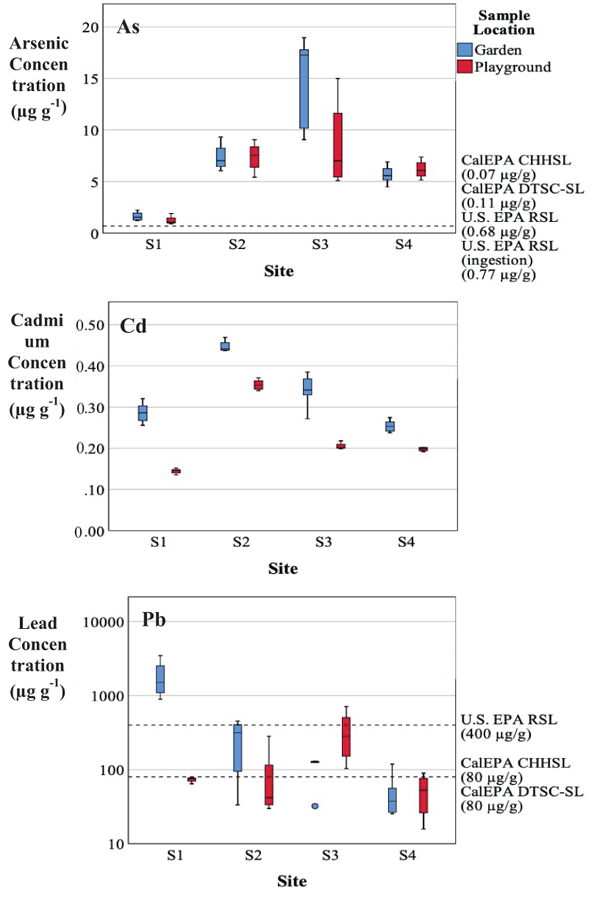
Metal(loid) concentrations (µg g−1) measured from garden and playground soil samples (< 63 μm). Note: The box represents the 25% to 75% range, the line in the box is the median, the whiskers are the 5% and 95%, and outliners are represented with circles.
Cadmium concentration in gardens and playgrounds ranged from 0.24 to 0.44 µg g−1, and 0.14 to 0.37 µg g−1, respectively (Figure 5). All garden and playground soil samples collected from all sites did not exceed the corresponding CHHSL, DTSC-SL, and EPA RSLs for Cd. The Cd concentrations in garden amendments also did not exceed these recommended levels. Median garden and playground samples and amendments from all sites were similar to CA Cd background levels (Kearney Foundation of Soil Science, 1996).
All garden soil samples (n = 6) from S1 exceeded all three recommended screening levels, with a median Pb concentration approximately 19 times higher than the California state screening levels (Figure 5). Likewise, the median Pb concentration in garden soil from S2 (3.2 × 102 µg g−1) and garden and playground soil from S3 (1.3 × 102 µg g−1 for garden and 2.9 × 102 µg g−1 for playground) exceeded the CHHSL and DTSC-SL. All garden and playground soil samples exceeded background Pb levels found throughout CA (Kearney Foundation of Soil Science, 1996). Lead concentrations were much greater in soil samples than in the amendments. This suggests that the garden amendments did not have a significant contribution to the elevated Pb levels measured in the soil.
3.3. Dust
The ambient metal(loid) concentration extrapolated from dust (< 10 µm diameter) samples produced from garden and playground surface soils are reported in Figure 6. The median ambient Pb concentrations estimated from garden and playground dust (0.14 and 0.011 µg m−3, respectively) were lower than the U.S. EPA National Ambient Air Quality Standard of 0.15 µg m−3 for Pb (U.S. EPA, 2006b). However, these concentrations were similar to concentrations in a smelter impacted site in Hayden, AZ of 0.023 µg m−3 reported by Csavina et al. (2011). Metal(loid) concentration in dust (< 10 µm diameter) samples were used to determine ADD and LADD for exposure age group. There is currently no federal or state standard for As or Cd concentration.
Figure 6.
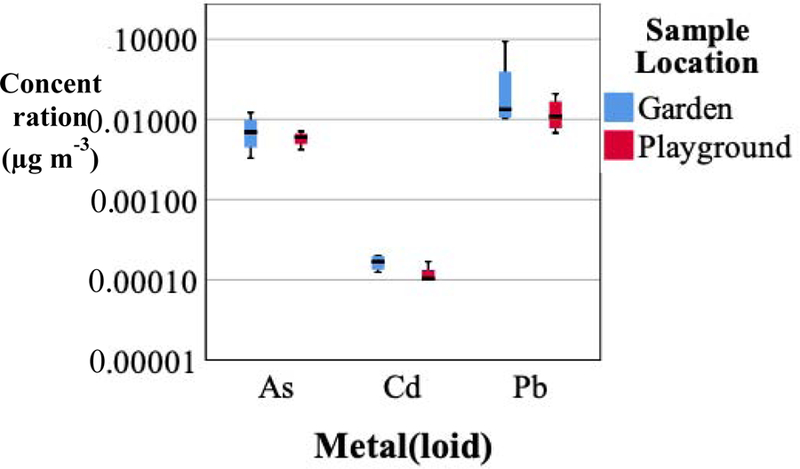
Atmospheric metal(loid) concentrations (µg m−3) estimated from garden and playground dust samples (< 10 μm effective diameter).
The particle size distribution of generated dust samples was analyzed using ImageJ. The particle diameter ranged from 0.80 to 26 µm and 0.41 to 17 µm, respectively. The percentage of particles with a diameter of ≤ 10 µm was 95–98%. The majority of particles were within the 0.40 to approximately 2.0 µm diameter size fraction.
3.4. Plants
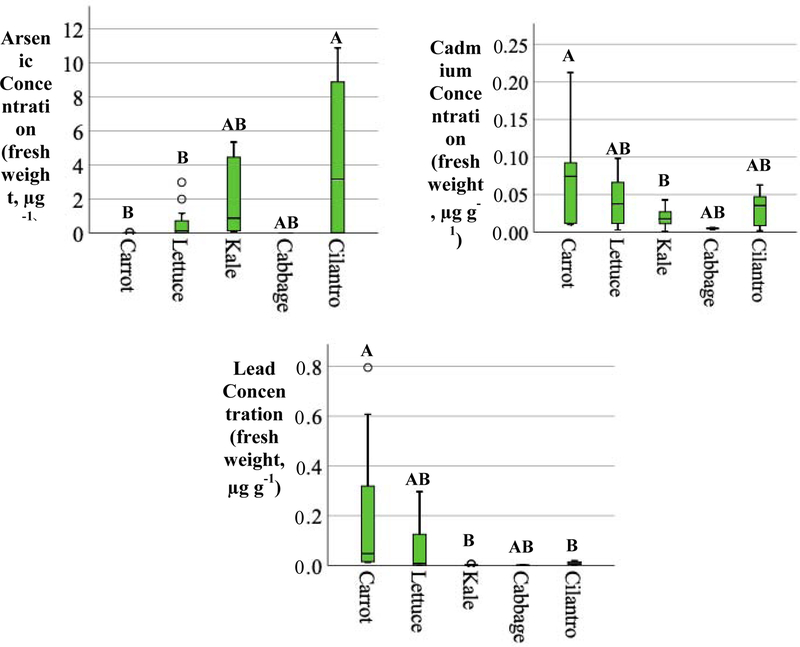
Apiaceae plants (carrot and cilantro) had the greatest median concentrations across all metal(loid)s (Figure 7). Significant differences (p-value < 0.05) in As, Cd, and Pb concentrations were observed between the different plant types shown in Figure 7.
Figure 7.
As, Cd, and Pb concentrations in preschool garden produce (fresh weight – FW, µg g−1), Significant differences (p < 0.05) in mean metal(loid) concentrations between plants are denoted by different letters (e.g. mean As concentration in cilantro was only significantly different to that in carrot and lettuce).
The median metal(loid) concentrations of As, Cd, and Pb observed in the plant samples were compared to the U.S. Food and Drug Administration Total Diet Study (U.S. FDA, 2014) to determine how store-bought produce compares to the produce grown at the preschools. Preschool-grown carrots had a higher concentration of each of the measured metal(loid)s than those store-bought. Preschool-grown lettuce accumulated more As and Pb while cabbage accumulated more As and Cd than store-bought lettuce and cabbage, respectively. Trends of decreasing mean metal(loid) concentrations in plant families grown at the preschools decreased in the order of:
As: Apiaceae (cilantro, carrot) > Brassicaceae (cabbage, kale) > Asteraceae (lettuce)
Cd: Apiaceae (cilantro, carrot) > Asteraceae (lettuce) > Brassicaceae (cabbage, kale)
Pb: Apiaceae (cilantro, carrot) > Asteraceae (lettuce) > Brassicaceae (cabbage, kale)
Median Cd and Pb concentrations in preschool-grown plants were below the respective recommendation maximum level set by WHO Codex in Table 1.
Table 1.
Metal(loid) concentrations and range (min-max) (fresh weight, mg kg−1), and mean metal(loid) concentrations reported in the U.S. FDA Total Diet Study and WHO Codex Recommended Maximum Levels (FW, mg kg−1).
| Plant (Family) | As | Cd | Pb | |
|---|---|---|---|---|
| Lettuce (Asteraceae) | Median (n = 12)b | 0.14* | 0.04 | 0.01* |
| Range (min – max) | (0.0014 – 3.0) | (0.0030 – 0.098) | (0.0010 – 0.30) | |
| U.S. FDA (leaf, raw) | 0.002 | 0.066 | 0.005 | |
| Codex (leafy vegetables) | N/A | 0.2 | 0.3 | |
| Cilantro (Apiaceae) | Median (n = 12)b | 3.2 | 0.035 | 0.0071 |
| Range (min – max) | (0.0025 – 11) | (0.0018 – 0.063) | (0.0031 – 0.020) | |
| U.S. FDA | N/A | N/A | N/A | |
| Codex (leafy vegetables) | N/A | 0.2 | 0.3 | |
| Carrot (Apiaceae) | Median (n = 9)b | 0.0079* | 0.074* | 0.048* |
| Range (min – max) | (0.0058 – 0.038) | (0.010 – 0.21) | (0.013 – 0.80) | |
| U.S. FDA (fresh, peeled, boiled) | 0.005a | 0.019 | 0.002 | |
| Codex (root and tuber vegetables) | N/A | 0.1 | 0.1 | |
| Kale (Brassicaceae) | Median (n = 9)b | .87 | 0.018 | 0.0017 |
| Range (min – max) | (0.068 – 5.3) | (0.0012 – 0.04) | (0.0003 – 0.0064) | |
| U.S. FDA | N/A | N/A | N/A | |
| Codex (leafy vegetables) | N/A | 0.2 | 0.3 | |
| Cabbage (Brassicaceae) | Median (n = 3)b | 0.0036*c | 0.0051* | 0.0013 |
| Range (min – max) | (0.0037 – 0.0061) | (0.0011 – 0.0015) | ||
| U.S. FDA (fresh, boiled) | 0.004a | 0.005 | 0.0025a | |
| Codex (Brassica vegetables – head cabbages) | N/A | 0.05 | 0.1 | |
Denotes a measured median concentration larger than the respective mean reported by the U.S. FDA Total Diet Study (U.S. FDA, 2014).
More conservative value calculated when a mean value of 0 is reported by the U.S. FDA. This value is calculated as ½ the detection limit (Ramirez-Andreotta et al., 2013b).
This study
Values reported are ½ the detection limit due to As concentrations of cabbage being at or below the detection limit (U.S. EPA, 2000).
The bioconcentration factor (BCF) of plants varied by site and metal(loid). The calculated BCF of each metal(loid) for each plant type per preschool is shown in Figure 8. The following categories were used to identify metal accumulators and hyperaccumulators: BCF < 1 – no metal(loid) accumulation; 1 ≤ BCF < 10 – metal(loid) accumulation; and BCF ≥ 10 – metal(loid) hyperaccumulation (Ávila et al., 2016; and reference therein). All plants (lettuce, cilantro, and kale) at S4 hyperaccumulated As, and nearly all accumulated Cd. Further, S2 cilantro and kale hyperaccumulated and accumulated As, respectively. The S1 and S2 cilantro also accumulated Cd. No plant accumulated or hyperaccumulated Pb (Figure 8). The median BCF was also calculated across all preschool locations to show a general accumulation trend across sites (Figure 9). In general, cilantro was an accumulator of As and Cd, and kale only accumulated As.
Figure 8.
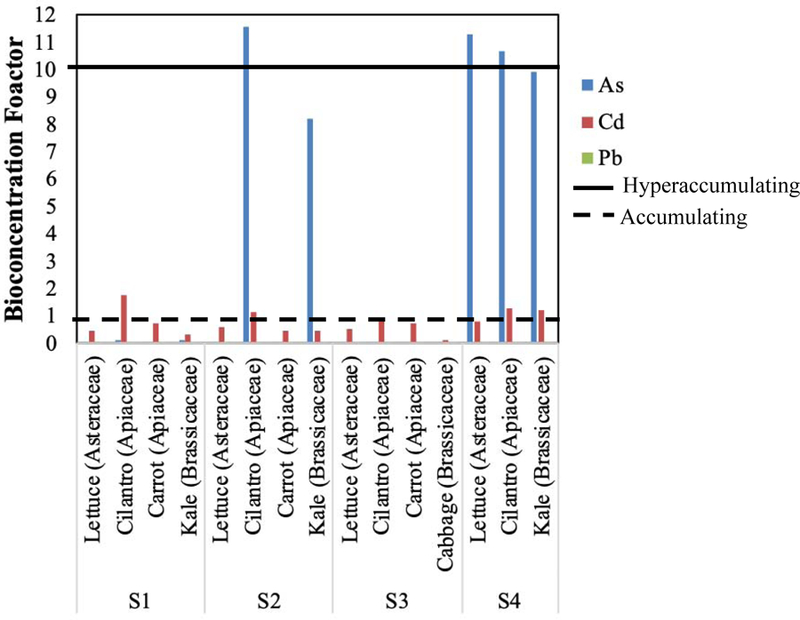
Site-specific median Bioconcentration factors of metal(loid) per plant type.
Figure 9.
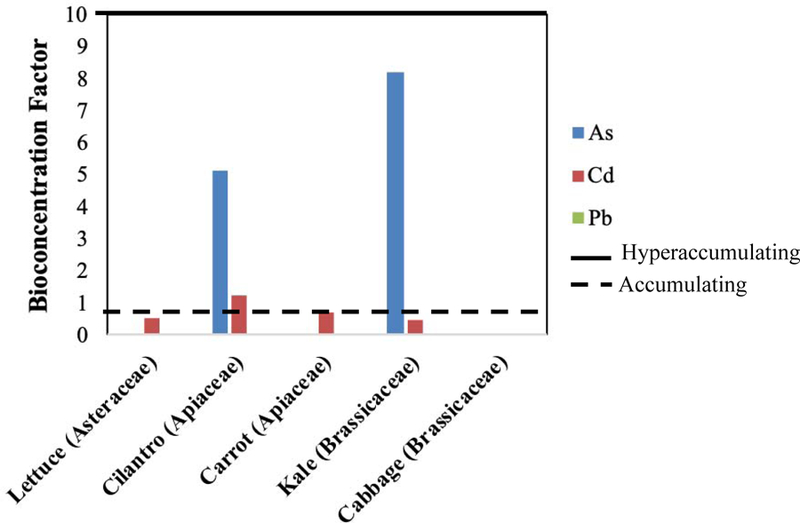
Median bioconcentration factor of metal(loid) per plant type (across all sites).
3.5. Bioaccessible fraction of As, Cd, Pb
The gastric and lung BAF varied by metal(loid) and sample location (garden vs. playground). The BAF of As, Cd, and Pb determined by the gastric and lung IVBA are shown in Figure 10. When considering sample location, the median of As, Cd, and Pb extracted by the synthetic gastric fluid were 12 and 7.4%, 62 and 58%, and 8.4 and 8.0%, respectively for garden and playground soils. Generally, the BAF of As, Cd, and Pb determined by gastric IVBA were greater than those by lung IVBA.
Figure 10.
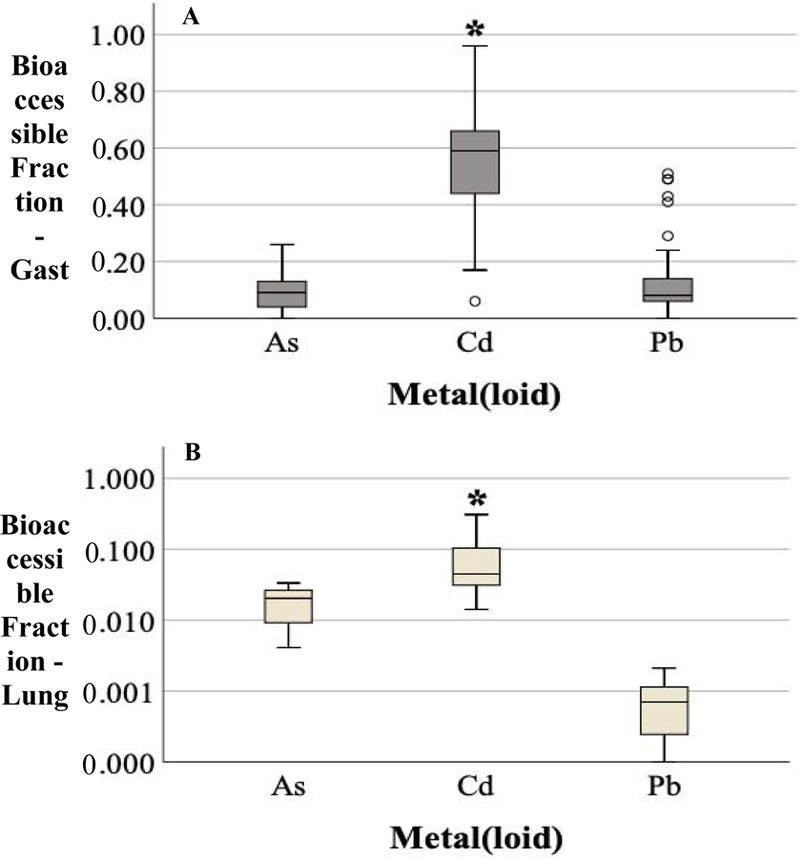
Bioaccessible fraction (BAF) of metal(loid) determined by gastric (A) and lung (B, logarithmic scale) IVBA. Asterisk signifies significant difference at p-value < 0.05.
3.6. Exposure Assessment
The percent contribution of As, Cd, and Pb from different environmental media, for children of each exposure age group is shown in Figure 11. Consumption of preschool-grown cilantro (41%), lettuce (84%), and kale (70%) accounted for the highest percent contribution to the cumulative exposure to As for children aged 1 to < 2 years, 2 to < 3 years, and 3 to < 6 years, respectively (Figure 11). Carrot contributed the most to a child’s cumulative exposure to Cd for each age group: 78%, 79%, and 90% for 1 to < 2 years, 2 to < 3 years, and 3 to < 6 years, respectively. Carrot contributed the most (37%) to a 2 to < 3 year-old child’s cumulative Pb exposure; however, incidental garden soil ingestion contributed the most Pb for children 1 to < 2 (67%) years and 3 to < 6 years of age (69%). The age-specific ADD and LADD of As, Cd, and Pb by exposure media are reported in Supplemental Material, Tables 7–10.
Figure 11.
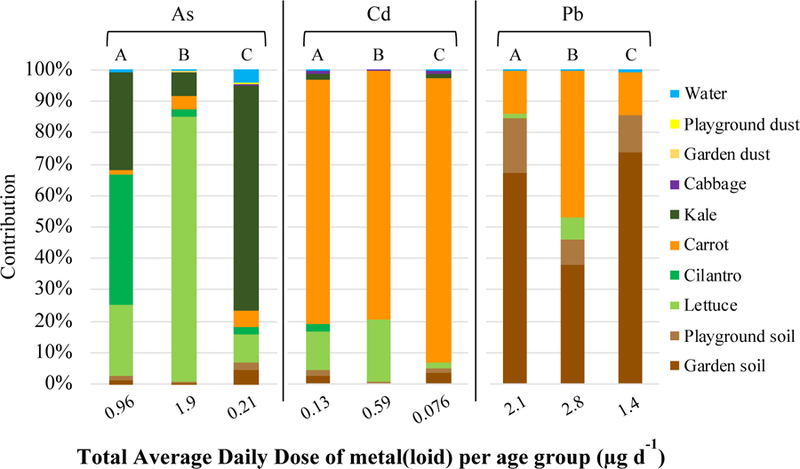
Percent contribution of exposure media to average daily dose (ADD) of As, Cd, and Pb for children of different exposure age groups: (A) 1 to < 2 yrs, (B) 2 to < 3 yrs, and (C) 3 to < 6 yrs. The value below each bar is the cumulative exposure to As, Cd, and Pb (mg kg−1 d−1) for a child of that age group.
The age-specific cumulative ADD and LADD of As, Cd, and Pb is shown in Table 2. Cumulative exposure (CADD) to As, Cd, and Pb ranged from 1.1 × 10−5 to 1.4 × 10−4 mg kg−1 d−1, 4.1 × 10−6 to 4.3 × 10−5 mg kg−1 d−1, and 8.2 × 10−5 to 2.0 × 10−4 mg kg−1 d−1, respectively. In general, a child 2 to < 3 years of age has a higher cumulative exposure to As, Cd, Pb than those of 1 to < 2 years and 3 to < 6 years of age. The CADD of Cd for children of ages 1 to < 2 (1.2 × 10−5 mg kg−1 d−1) and 2 to < 3 years (4.3 × 10−5 mg kg−1 d−1) was greater than the more conservative child-specific RfD for Cd of 1.1 × 10−5 mg kg−1 d−1 (CalEPA OEHHA, 2003).
Table 2.
Age-specific cumulative average daily dose and lifetime average daily dose of As, Cd, and Pb.
| Exposure Age Group | Element | Cumulative ADD (mg kg−1 d−1) | Cumulative ADDm (μg d−1) | Cumulative LADD (mg kg−1 d−1) | Cumulative LADDm (μg d−1) |
|---|---|---|---|---|---|
| 1 to < 2 yrs | As | 8.5 × 10−5 | 9.6 × 10−1 | 1.1 × 10−6 | 1.2 × 10−2 |
| Cd | 1.2 × 10−5* | 1.3 × 10−1 | 1.5 × 10−7 | 1.7 × 10−3 | |
| Pb | 1.8 × 10−4 | 2.1 | 2.3 × 10−6 | 2.7 × 10−2 | |
| 2 to < 3 yrs | As | 1.4 × 10−4 | 1.9 | 1.8 × 10−6 | 2.5 × 10−2 |
| Cd | 4.3 × 10−5* | 5.9 × 10−1 | 5.5 × 10−7 | 7.5 × 10−3 | |
| Pb | 2.0 × 10−4 | 2.8 | 2.6 × 10−6 | 3.3 × 10−2 | |
| 3 to < 6 yrs | As | 1.1 × 10−5 | 2.1 × 10−1 | 1 × 10−7 | 2.6 × 10−3 |
| Cd | 4.1 × 10−6 | 7.6 × 10−2 | 5.3 × 10−8 | 9.6 × 10−4 | |
| Pb | 8.2 × 10−5 | 1.4 | 1.0 × 10−6 | 1.3 × 10−2 | |
indicates that cumulative exposure value is greater than respective oral reference dose (RfD) listed in Table 5.
3.7. Risk Assessment
The IELCR for As per exposure media is shown in Table 3. The IELCR for 2 to 3-year-old children from As in lettuce met a target risk of 10−6; however, no IELCR from any exposure media exceeded a target risk of 10−5. Overall, IELCR from the exposure media decreased in an order of preschool plant ingestion > incidental soil ingestion > irrigation water ingestion > dust inhalation.
Table 3.
Age-specific incremental excess lifetime cancer risk from As per exposure media.
| Exposure Media | Exposure Age Group |
||
|---|---|---|---|
| 1 to < 2 yrs | 2 to < 3 yrs | 3 to < 6 yrs | |
| Garden soil | 9.4 × 10−9 | 5.9 × 10−9 | 4.3 × 10−9 |
| Playground soil | 8.5 × 10−9 | 5.2 × 10−9 | 3.9 × 10−9 |
| Lettuce (Asteraceae) | 1.7 × 10−7 | 1.0 × 10−6 | 8.3 × 10−9 |
| Cilantro (Apiaceae) | 3.0 × 10−7 | 3.3 × 10−8 | 2.1 × 10−9 |
| Carrot (Apiaceae) | 1.3 × 10−8 | 4.9 × 10−8 | 5.3 × 10−9 |
| Kale (Brassicaceae) | 2.2 × 10−7 | 9.2 × 10−8 | 6.8 × 10−8 |
| Cabbage (Brassicaceae) | 6.4 × 10−10 | 3.0 × 10−10 | 1.7 × 10−10 |
| Garden dust | 4.1 × 10−10 | 4.1 × 10−10 | 3.2 × 10−10 |
| Playground dust | 3.4 × 10−10 | 3.3 × 10−10 | 2.6 × 10−10 |
| Irrigation water | 4.6 × 10−9 | 5.3 × 10−9 | 4.0 × 10−9 |
The HQ characterizes risk from exposure to noncarcinogens. An HQ ≤ 1 indicates an acceptable risk (no adverse health effects expected). The only exposure media that exceeded this acceptable risk was carrot with an HQ of 3.1 for an exposure to Cd for 2 to < 3-year-olds (Table 10). Cumulatively, the HQ of Cd from all exposure media also exceeded the acceptable risk threshold of 1 for 1 to < 2-year-olds and 2 to 3 year-olds (Table 4).
4. Discussion
4.1. Cumulative exposure to arsenic, cadmium, and lead
The results of this study suggest children 1 to 3 years of age are potentially exposed to Cd levels that exceed the child-specific RfD of 1.1 × 10−5 mg kg−1 d−1 recommended by the CalEPA (OEHHA, 2003). The results also suggest that a child’s exposure to As, Cd, and Pb varies considerably by age and exposure pathway. The garden plants considered were selected based on their As and Cd accumulation properties, thus, they were expected to have a considerable contribution to the cumulative exposure to As and Cd, but not Pb. Since a child’s consumption of 4 of the 5 plants studied had major contributions to metal(loid) exposures, further studies are recommended to determine the bioaccessible fraction of metal(loid)s from plants to improve ADD and LADD estimations.
Children in this study consumed approximately 1 teaspoon to 0.5 cup (3,100 to 68,000 mg) of carrots and 1 tablespoon to 1 cup (4,200 to 32,000 mg) of lettuce per day (Manjon & Ramirez-Andreotta, unpublished results). Preschool-grown lettuce and carrot accumulated more As and Cd than store-bought lettuce and carrots analyzed by the U.S. FDA Total Diet Study (U.S. FDA, 2014). Since 100% and 90% of the participants reported that their child consumed carrot and lettuce, respectively, over the past 12 months, special consideration must be taken to ensure that children at these preschools limit the consumption of carrots grown in these preschool gardens. In contrast, children only consumed approximately 270 to 810 mg of cilantro and 570 to 10,000 mg of kale per day and only 70%, and 50% of the children reported consuming cilantro and kale, respectively, over the past 12 months. Thus, although cilantro and kale plants had a high contribution to a child’s As exposure, more caution should be taken with carrot and lettuce due to their higher ingestion rates. In contrast, relative As exposure contributions in this study differed from a previous Gardenroots study in the mining town of Dewey-Humboldt, AZ (Ramirez-Andreotta et al., 2013b). In general, water contributed the most to As exposures in Dewey-Humboldt, contrasting this study. The ADDs of As from lettuce (9.7 × 10−7 – 1.2 × 10−4 mg kg−1 d−1) and kale (8.0 × 10−6 – 2.6 × 10−5 mg kg−1 d−1) were generally greater than those estimated from home gardens reported Ramirez-Andreotta et al. (2013b). However, it is important to note that this study’s estimated ADDs of As from lettuce were below the reported ADD ranges of greenhouse-grown lettuce (Ramirez-Andreotta et al. 2013b) (kale was not among greenhouse plants analyzed). Further, similar to this study, Ramirez-Andreotta et al. (2013b) used a BAF of 80%.
The As, Cd, and Pb exposures from foods estimated in this study varied compared to those reported in the literature. For example, children less than 3 years of age have an estimated 2 to 3-fold higher dietary exposure to inorganic As when compared to adults due to their increased intake of foods per body weight (European Food Safety Authority (EFSA), 2009). A previous study estimated that a child’s (1–6 years) average dietary intake of inorganic As was 3.2 µg d−1 based on an analysis of 38 foods (Yost et al., 2004). The major sources of As in that study decreased in the order of grains (and grain products), fruits and fruit juices, rice (and rice products), and milk (Yost et al., 2004). In comparison, the current study estimates a cumulative median intake of 0.88 µg d−1 of As for children 1–6 years of age based on only the preschool grown plants analyzed. Further, a study done in China determined that the median Pb and Cd intake (normalized to body weight) was 1.74 and 0.23 µg kg−1 d−1 for children of 3–6 years of age based on a dietary assessment (Liu et al., 2010). In this study, the cumulative median exposure to Pb and Cd from the preschool-grown plants were 0.027 and 0.011 µg kg−1 d−1, respectively.
Elevated levels of Pb in garden and playground soils at the preschools may explain the large contribution to a child’s cumulative Pb exposure by incidental soil ingestion. Specific sampling locations at each preschool were informed by participants’ knowledge of where children play the most. Currently, there is no RfD for Pb (U.S. EPA, 2006a), nor safe levels identified for Pb due to potential irreversible neurological damage (Centers for Disease Control and Prevention (CDC), 2019). Therefore, it was recommended that these sites limit children’s access until further remediation action be taken (further details provided in section 4.5.2 below).
4.2. Critique of risk assessment model parameters
In this study, we reduced the amount of assumptions by providing site-specific analyses including metal(loid) concentration in four environmental media, bioaccessible fraction of metal(loid), exposure frequency, exposure duration, and average time (non-cancer) estimations. Performing site-specific assessments better estimates exposures and informs practical decisions to mitigate potential risks in communities impacted by pollution. The site-specific estimations reported herein can also inform future risk assessments for similar populations and sites.
Using traditional default values of 350 days and 6 years, respectively for exposure frequency (EF) and exposure duration (ED) would have overestimated a child’s ADD and LADD of As, Cd, and Pb and consequently, the IELCR and HQ estimations as well. Thus, more realistic values of 181 days and 1 year for the EF and ED parameters better estimates the potential exposure for a child attending a single CA preschool year and consuming from the preschool gardens regularly. If the child consumes these plants from local gardens with similar metal(loid) accumulation, then the default parameters would be appropriately conservative and capture the possible exposure outside of preschool.
Exposures to metal(loid) mixtures was not investigated in this study. This remains a challenge for establishment of regulatory standards and current risk assessors as this evaluation requires interdisciplinary integration of toxicology and epidemiology data (Hernández & Tsatsakis, 2017). Realistically, children (and adults) are exposed to As, Cd, Pb, and other harmful metal(loid)s in combination through environmental media. Bae et al. (2001) investigated the cytotoxic interactions of As, Cd, Pb, and Cr in human epidermal cells. It has been generally assumed that at low dose levels, the combined mixture is additive; however, the group found that the type of cytotoxicity (additivity, synergism, or antagonism) was highly dependent on the cell strain and dose of the mixture (Bae et al., 2001). Due to the mechanistic complexity of chemical interactions in the human body, future work is needed to understand the additive, synergistic, and/or antagonistic behaviors of these metal(loid)s in other target organs for more accurate risk assessments.
4.3. Critical issues in traditional bioavailability estimations
When site-specific metal(loid) bioavailability is unavailable, the bioavailability is often assumed in risk calculations. In-vivo validations of the in-vitro IVBA methodology for As and Pb used in this study suggest that BAFs determined by this method is an inexpensive, adequate alternative for predicting the maximum bioavailability of the metal(loid) for risk assessment calculations (U.S. EPA, 2017b; Bradham et al., 2011). Currently, the U.S. EPA Integrated Risk Information System (IRIS) toxicity values for As are estimated based on a relative bioavailability of 100% (U.S. EPA, 2012), which can overestimate an actual exposure (Bradham et al., 2011). Thus, based on more recent studies, a default value of 60% for As in soil is recommended when site-specific values are unavailable (U.S. EPA, 2012). The U.S. EPA Integrated Exposure Uptake and Biokinetic (IEUBK) model for Pb in children assumes a relative bioavailability of 60% for Pb in soils or dust (U.S. EPA., 2007c). Based on the BAFs reported here, an assumption of 60% for Pb and As greatly overestimates both an incidental soil ingestion and dust inhalation exposure to As and Pb. However, this is the opposite for Cd. The Cd bioavailability default value of 2.5% is used for foods (U.S. EPA., 2007c) and is significantly lower than the median soil BAFs of 62% (garden) and 58% (playground). This default value would drastically underestimate the simulated gastric exposure and lung exposure for children at the preschools. It is not uncommon to see a wide range for the Cd bioavailability (5–99%) depending on the soil properties and experimental design (Oomen et al., 2002). The higher Cd BAF (62% for garden soil, and 58% for playground soil) determined in the present study may also be explained by the low extraction fluid pH (1.51–1.71) since the IVBA only simulated the gastric stage of the human body and not the intestines, which naturally have a higher pH, and thus, extracts less metal from the soil over the same reaction time (Oomen et al., 2002). For risk calculations, in contrast to As and Pb, inputting a higher Cd BAF instead of the lower U.S. EPA guidance value, could increase the estimated exposure significantly.
4.4. Limitations to estimating BAFs from in-vitro bioaccessibility assays
Children are a sensitive population and current IVBAs may not be suited to simulate metal(loid) exposures for children. For example, children have a lower alveolar density and lower lung volume than adults (Balk et al., 2012). They also have increased inhalation rates and inhale more airborne pollutants per body weight than adults (Balk et al., 2012). Furthermore, children’s increased water and caloric intake requires increased gastrointestinal absorption of nutrients (Sly & Flack, 2008). When considering Pb, toddlers absorb 40–70%, while adults only absorb 5–20% of their ingested dose (Sly & Flack, 2008). In addition, As absorption increases with iron deficiencies (Balk et al., 2012), which is common in growing children (Sly & Flack, 2008). Once absorbed, As methylation is less effective in children than adults, resulting in higher concentrations of bioaccumulated As in the body (Balk et al., 2012).
It is widely accepted that children incidentally ingest settled dust and soil at higher rates than adults given their increased hand-to-mouth behavior (Ikegami et al., 2014). A recent study by Cao et al. (2018) determined that children were exposed more to metal(loid)s in settled ground dust than adults due to their behavior patterns, smaller size, and more frequent contact with the ground (Cao et al., 2018). In this study, we observed child behavior at each of the preschools and children were more actively engaging in the playgrounds and gardens than the adults. Thus, if dust is produced during outdoor activity, potential exposures may result from routes including contact with deposited dust on the ground, indoor and outdoor surfaces (e.g. playground equipment and playing indoor on floors), and adherence of atmospheric particles onto the skin (Cao et al. 2018; Hu et al., 2012). These exposure pathways more common to children may affect a child’s ingested or inhaled dose of metal(loid) and are not concurrently assessed during IVBAs, which may lead to underestimating a child’s actual exposure. With that said, a limitation to the methodology used in this study was exclusion of exposures by ingestion of settled dust by hand-to-mouth behavior (Cao et al., 2018), as well as ingestion of the dust deposited in the extrathoracic region (Kastury et al., 2017). Although this IVBA methodology has been previously validated with in-vivo gastric models for As and Pb, this and others commonly reported in the literature are still subject to limitations.
Another limitation of the present study is that soil texture analysis was not conducted for each site. In general, gastric BAFs are affected by site-specific factors such as soil texture and soil properties (Cai et al., 2016; Andrade & Vega, 2014; Roussel et al., 2010; Oomen et al., 2002). A previous study showed that increasing As bioaccessibility was of the same order of increasing sand content and decreasing clay content (Warren et al., 2003). Similarly, mobility of Cd is increased in sandy soils with low clay content, which increases its bioaccessibility (National Industrial Chemicals Notification, 2017). Other soil properties such as organic matter, iron and carbonate content, and soil pH also influence the BAF of metal(loid)s (Cai et al., 2016; Andrade & Vega, 2014; Roussel et al., 2010; Oomen et al., 2002).
The current literature lacks a general consensus on a standard IVBA methodology to simulate inhalation exposures (Kastury et al., 2017). In general, these IVBAs are affected by the metal(loid) extraction kinetics, composition of the biofluid, dust size fraction, and the ratio of solid to biofluid used (Katsury et al., 2018; Katsury et al., 2017; Boisa et al., 2014). The lung median As and Pb BAFs in this study were, in general, lower than those described by Thomas et al. (2018) and Boisa et al. (2014) who performed the IVBA with < 10 µm diameter dust originating from tailings and tailings/surface soils, respectively. This may have been due to the shorter IVBA leaching duration used in this study (1 h) and the fact that Thomas et al. (2018) used mine tailings with much greater As and Pb concentrations. Previous work has shown that metal(loid) extraction occurs rapidly within the first 0.5–5 h and then reaches equilibrium between 24 and 48 h (Kastury et al., 2017), which has resulted in using 24 h in studies that followed. Thomas et al. (2018) observed a similar trend where surface soils (0–52 cm) displayed a rapid fractional release of As during the first 4 h before reaching equilibrium after 4–24 h. Thus, future studies evaluating soils collected from topsoil or root-zone soil should consider using an extraction time of 24 h.
The BAF determined by lung IVBA is also dependent on the dust size fraction analyzed (Kastury et al., 2017; Hu et al., 2012). In this study, based on the hypothesis that the BAF of a metal(loid) increases with decreasing particle size (Csavina et al., 2011), dust was fractionated to < 10 µm instead of using all total suspended particulate (TSP). Further, finer particles have greater surface area to react with the lung biofluid; resulting in higher dissolution rates (as opposed to larger particles) (Csavina et al., 2012). Although Hu et al. (2012) used a nearly identical IVBA as our gastric IVBA to simulate inhalation exposures, the group determined no significant difference in the As, Cd, and Pb BAF determined from TSP and PM2.5.
Simple lung IVBAs ignore how particle deposition site and efficiency affect bioaccessibility. The respiratory system is comprised of the extrathoracic (nose, oral cavity, pharynx, and larynx), tracheobronchial, and alveolar region (Olumayede et al., 2018; Kastury et al., 2017; Csavina et al., 2012; Krombach et al., 1997). Course particles (PM10) deposit efficiently by inertial deposition in the extrathoracic region, where they are ultimately swallowed or cleared (Kastury et al., 2017; Csavina et al., 2012; Krombach et al., 1997). Therefore, a gastric IVBA rather than lung IVBA is more appropriate when assessing the bioaccessibility of course dust size fraction. Accumulation mode particles (0.1–1 μm) tend to be exhaled (approximately 80%) because they do not deposit efficiently in any region due to their relatively high inertia and low diffusivity (Kastury et al., 2017; Csavina et al., 2012). Lastly, fine (PM2.5) and ultrafine (< 0.1 μm) particles can deposit in the alveolar region and pose a great risk as they can enter blood circulation by diffusion across the alveoli sac (Kastury et al., 2018; Csavina et al., 2012). Olumayede et al. (2018) determined that the greatest dose and deposition of Pb and Cd generally occurs within this alveoli region in adults. Since macrophages within the lung create an acidic environment, clearing particles by phagocytosis may increase the bioavailability of metal(loid)s in the deep lung (Boisa et al., 2014; Csavina et al., 2012). Thus, it is necessary to understand the size distribution of the dust analyzed since the bioaccessibility (and therefore, bioavailability) of a metal will depend on where the dust particles deposit within the respiratory system. Future lung IVBA work should consider different regions of the respiratory system described above to comprehensively assess the bioaccessibility of metal(loid)s within the different size fractions.
4.5. Soil
All soil samples from all preschools exceeded As CalEPA and U.S. EPA recommended screening levels. These levels are conservative with a target risk of one in a million based on carcinogenic effects and do not consider background metal(loid) levels. Here, As and Cd concentrations in soil were relatively comparable to background levels measured throughout 50 benchmark locations in CA (Kearney Foundation of Soil Science, 1996) and a nearby island not impacted by legacy Gold Rush mining (Behrsing et al., 2009). The As concentrations across all preschools ranged from 0.90 to 19.0 µg g−1, which were lower than those in residential gardens (2.35–374 µg g−1) and yards (3.07–322 µg g−1) near the Iron King Mine and Humboldt Smelter Superfund site in Dewey-Humboldt, AZ (Ramirez-Andreotta et al., 2013a). Here, the majority of sites (S2, S3, S4) had As concentration in the garden that were above those reported for samples collected at a 100 mm depth approximately 4,000 m (2.5 mi) and 1,300 m (0.8 mi) from the operating Hayden smelter residing in Hayden, AZ (Félix et al., 2015). However, it is important to note that the garden soil samples in this study are comprised of the top 15 cm of soil, and not sectioned by depth like Félix et al. (2015).
Counter to previous studies in the area, soil Pb concentrations in all preschool gardens and S2 and S3 playgrounds were elevated relative to CA background concentrations (Kearney Foundation of Soil Science, 1996). The median Pb concentrations measured in this study were comparable to soil Pb concentrations in towns located near a mining smelter (approximately 330 µg g−1, Félix et al., 2015). The median Pb concentration in S1 garden (1,524 µg g−1) were about 4–5 times higher than the highest concentration observed at 0.4 mi from the smelter in Hayden (Félix et al., 2015). Since this study’s Pb levels were both comparable to other mining communities and above background non-impacted lands, these finding suggest that Pb enrichment could have originated from an anthropogenic source such as nearby tailings, mining waste, or industrial facility.
Currently, there are no industrial or federal facilities tracked by the U.S. EPA Toxic Release Inventory (TRI) in the cities of Grass Valley (95945) and Nevada City (95959) (U.S. EPA., 2017c). However, preschool site administrators provided valuable insight about their preschools and surrounding areas. Each site administrator was unaware of any Pb-producing industrial facility within 5 mi from their preschool, as well as any facility that manufactures and/or applies phosphate fertilizers that is recognized as a potential contributor to Cd contamination (Alam et al., 2003). Each administrator reported being aware of an active or legacy mining site within 10 mi, which may contribute to anthropogenic As, Cd, and Pb contamination. Fine particles containing As and Pb are thought to result from smelting operations, while course particles originate from natural sources (topsoil), mine tailings, or fugitive dust emissions (Csavina et al., 2014). Further, S3 administrator reported being within two blocks of a major highway and past commercial agriculture. Administrators at S2 and S3 also reported being within 10 mi from a waste incineration and disposal facility. Site administrators at S3 and S4 reported observable deteriorated paint on outside fences, garages, play structures, railings, building siding, windows, trims, and/or mailboxes on the property. Although the administrators’ responses could have been prone to reporting error, it is important to recall that elevated levels of Pb in soils were measured, while Pb was not in the garden amendments presumably added to the soil. Thus, we can assume that elevated Pb concentrations were not caused by the garden amendments, but rather historical past land use or potential anthropogenic enrichment from nearby contaminated point sources as reported by the site administrators.
4.6. Plant
The plants grown at each preschool were selected based on their As and Cd translocating properties. In the present study, in general, mean As uptake decreased in the order of Apiaceae > Brassicaceae > Asteraceae, which did not match the order of Asteraceae > Brassicaceae > Amaranthaceae > Cucurbitaceae > Liliaceae > Solanaceae > Fabaceae, reported previously by Ramirez-Andreotta et al. (2013a). Note that Ramirez-Andreotta et al., (2013a) did not report plants in the Apiaceae family due to low sample number.
The results of this study contradict the suggestion that plants from the same families can be characterized by the same As uptake capabilities (Ramirez-Andreotta, 2013a). This is apparent when comparing the median As uptake and BCF of cilantro and carrot (Apiaceae) and kale and cabbage (Brassicaceae). When considering all kale and cabbage grown across all sites, kale accumulated As (BCF = 8.2), while cabbage (BCF = 0.0015) did not (Figure 9), although they are both Brassicaceae plants. However, the BCF for kale was determined by using 9 samples, while all 3 cabbage samples had As concentrations below the limit of detection (Figures 8 and 9). Similarly, overall cilantro accumulated As (BCF = 5.2), while carrot did not, even though both belong to the Apiaceae family (Figure 9). Leafy vegetables within a plant family have been shown to accumulate more than root species within the same family (Alam et al., 2003). This may explain why cilantro accumulated As and Cd, while carrot did not.
These data support the hypothesis that some members of the Asteraceae and Brassicaceae family can hyperaccumulate As (Ramirez-Andreotta, 2013a; and references therein). When considering site-specific BCFs per plant type (Figure 8), S4 lettuce and cilantro hyperaccumulated As (both BCF = 11), while cilantro also hyperaccumulated As (BCF = 12) when grown at S2. Further, kale accumulated As at S2 (BCF = 8.2) and S4 (BCF = 9.9). Cilantro accumulated Cd at the majority of the preschools (Figure 8), while kale only accumulated Cd (BCF = 1.2) at S4. Overall, no preschool-grown plant accumulated or hyperaccumulated Pb despite the elevated levels in the gardens; however, these plants were not selected based on this capability. The median concentration of Cd was generally higher than Pb in the leafy vegetables, which contradicts the findings of Alam et al. (2003). It is recommended that gastric IBVA calculations be completed with food products to assess the BAF of metal(loid)s from foods, particularly those from the Apiaceae and Brassicaceae families which accumulated and hyperaccumulated As and/or Cd.
Lastly, in contrast to Ramirez-Andreotta et al. (2013a) and Alam et al. (2003), there was no observed trend between plant As, Cd, and Pb concentration and those in soil. Thus, suggesting that plant metal(loid) uptake was not dependent on soil metal(loid) concentrations alone, but rather implies that soil characteristics, fertility, and possibly contaminant speciation may play a larger role in accumulation. Variability in metal(loid) uptake among the different sites may be explained by differences in soil properties including clay content, pH, organic matter and content, and soil texture as previous studies have shown that As uptake in plants is higher in sands and sandy loams, while lower in clays and silts (Warren et al., 2003; and references therein). Further, Pb uptake is affected by soil pH, redox potential, organic matter, and phosphorus content (Tangahu et al., 2011).
5. Conclusion
This study highlights the importance of a site-specific analysis when assessing a child’s multi-route exposure to metal(loid)s, and the limitations associated with the risk assessment model. We determined that consumption of preschool-grown cilantro, lettuce, kale, and carrot, and incidental ingestion of garden soil were major contributors to the cumulative exposure of As, Cd, and/or Pb. Based on the cumulative exposure (ingestion of preschool-grown plants, incidental soil, and irrigation water, and dust inhalation over the course of a year), it was determined that a child 2 to 3 years of age would have an IELCR of one in a million from As exposure through the consumption of lettuce grown at the preschools and a child of 1 to < 3 years of age would also be at risk to non-cancerous effects of Cd. Furthermore, if a child 2 to < 3 years of age consumed more than one leaf of preschool-grown lettuce or a third of a small (5½ in long) preschool-grown carrot daily, they would be at risk of adverse non-cancerous health effects from Cd exposures. The results of this study also suggest that further investigation of the As and Cd accumulating/hyperaccumulating capabilities of other Apiaceae plants is warranted. Special consideration must be taken to avoid growing contaminant-hyperaccumulating plants in preschool gardens to reduce the risk of exposure, even if soil concentrations are low. We also observed that site-specific bioaccessibility of As, Cd, and Pb from soil and dust differed from those recommended by traditional risk assessment guidance: emphasizing the importance of site-specific analysis for future assessments.
Most importantly, this study provided the necessary framework to improve future exposure and risk assessments. This was done through the use of a co-created citizen science environmental monitoring program to measure metal(loid) concentrations in environmental media; implementation of a novel dietary assessment that included As and Cd accumulating foods to understand the preschool child consumption patterns, and site-specific IVBA analysis. By doing so, a child’s multi-route exposure to As, Cd, and Pb and potential risk to adverse health effects from these exposures at preschools can be best estimated. This site-specific evaluation is essential to protect vulnerable populations including children living in legacy mining communities.
Supplementary Material
A site- and age-specific child exposure and risk assessment was conducted.
Incidental soil ingestion was a major contributor to child Pb exposure.
Kale, lettuce, and cilantro were major contributors to child As exposure.
Carrots were major contributor to child Cd exposure.
Cumulative dose of Cd from all pathways exceeded child-specific reference dose.
Acknowledgments
This study was funded by the California Breast Cancer Research Program (Grant 23AB-1301A) and the National Institute of Environmental Health Sciences Superfund Research Program (P42ES04940). The authors are grateful for the time and effort that Gardenroots participants and the CHIME Community Advisory Board dedicated to this study. We would also like to give a special thank you to our colleagues in the Integrated Environmental Science and Health Risk Laboratory and the Arizona Laboratory for Emerging Contaminants. Lastly, we would like to thank Peggy Reynolds and Barbara Moore for their contributions to this study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declarations of interest: None
References
- Abadin H, Ashizawa A, Stevens Y-W, Llados F, Diamond G, Sage G, … Swarts SG (2007). Toxicological Profile for Lead. Agency for Toxic Substances and Disease Registry (ATSDR). Atlanta, Georgia: 10.1201/9781420061888_ch106 [DOI] [PubMed] [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR). 2008. Toxicological Profile for Cadmium (Draft for Public Comment). Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- Alam MGM, Snow ET, & Tanaka A (2003). Arsenic and heavy metal contamination of vegetables grown in Samta village, Bangladesh. The Science of the Total Environment, 308(308), 83–96. [DOI] [PubMed] [Google Scholar]
- Andrade ML, & Vega FA (2014). Geoderma Sequential extraction of heavy metals in soils from a copper mine: Distribution in geochemical fractions. Geoderma, 230–231, 108–118. 10.1016/j.geoderma.2014.04.011 [DOI] [Google Scholar]
- Alpers CN (2017). Arsenic and mercury contamination related to historical gold mining in the Sierra Nevada, California. Geochemistry: Exploration, Environment, Analysis, 17(2), 92–100. 10.1144/geochem2016-018 [DOI] [Google Scholar]
- ATSDR. (2007a). Toxicological profile for Arsenic. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- ATSDR. (2007b). Toxicological profile for Lead. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- ATSDR. (2008). Toxicological Profile for Cadmium. Atlanta, GA: U.S. Department of Health and Human Services, Public Health Service. [Google Scholar]
- ATSDR. (2017). Substance Priority List. Retrieved April 10, 2019, from https://www.atsdr.cdc.gov/spl/#2017spl.
- Ávila PF, Ferreira da Silva E, & Candeias C (2016). Health risk assessment through consumption of vegetables rich in heavy metals: the case study of the surrounding villages from Panasqueira mine, Central Portugal. Environmental Geochemistry and Health, 39(3), 565–589. 10.1007/s10653-016-9834-0 [DOI] [PubMed] [Google Scholar]
- Bae D, Gennings C, Carter WH, Yang RSH, & Campain JA (2001). Toxicological Interactions among Arsenic, Cadmium, Chromium, and Lead in Human Keratinocytes. Toxicological Sciences, 63, 132–142. 10.1093/toxsci/63.1.132 [DOI] [PubMed] [Google Scholar]
- Balk SJ, Etzel RA, & American Academy of Pediatrics. (2012). Pediatric Environmental Health (Vol. Third edition). [Elk Grove Village, IL: ]: American Academy of Pediatrics; Retrieved from https://search-ebscohost-com.ezproxy2.library.arizona.edu/login.aspx?direct=true&db=nlebk&AN=567176&site=ehost-live [Google Scholar]
- Beamer PI, Elish CA, Roe DJ, Loh M, Layton DW. (2012). Differences in Metal Concentration by Particle Size in House Dust and Soil. Journal of environmental monitoring: JEM;14(3):839–844. doi: 10.1039/c2em10740f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Behrshing et al. , (2009). https://www.dtsc.ca.gov/AssessingRisk/DocsLib.cfm
- Bian B, Zhou LJ, Li L, Lv L, & Fan YM (2015). Risk assessment of heavy metals in air, water, vegetables, grains, and related soils irrigated with biogas slurry in Taihu Basin, China. Environmental Science and Pollution Research. 10.1007/s11356-015-4292-2 [DOI] [PubMed] [Google Scholar]
- Boisa N, Elom N, Dean JR, Deary ME, Bird G, & Entwistle JA (2014). Development and application of an inhalation bioaccessibility method (IBM) for lead in the PM10 size fraction of soil. Environment International, 70, 132–142. 10.1016/j.envint.2014.05.021 [DOI] [PubMed] [Google Scholar]
- Bradham KD, Scheckel KG, Nelson CM, Seales PE, Lee GE, Hughes MF, … Thomas DJ (2011). Relative bioavailability and bioaccessibility and speciation of arsenic in contaminated soils. Environmental health perspectives, 119(11), 1629–1634. doi: 10.1289/ehp.1003352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai M, McBride MB, & Li K (2016). Bioaccessibility of Ba, Cu, Pb, and Zn in urban garden and orchard soils. Environmental Pollution, 208, 145–152. 10.1016/j.envpol.2015.09.050 [DOI] [PubMed] [Google Scholar]
- CalEPA Office of Environmental Health Hazard Assessment (OEHHA). (2003). Chemical Database. Sacramento, CA: https://oehha.ca.gov/chemicals. [Google Scholar]
- CalEPA OEHHA. (2010). California Human Health Screening Levels (CHHSLs). https://oehha.ca.gov/risk-assessment/california-human-health-screening-levels-chhsls
- CalEPA Department of Toxic Substances Control (DTSC). (2018). Human and Ecological Risk Office Human Health Risk Assessment Note 3: DTSC-modified Screening Levels (DTSC-SLs).
- Cao Z, Wang M, Chen Q, Zhang Y, Dong W, Yang T, Guangxuan Y, Xin Z, Yunqing P, Benye X, Bu Q (2018). Preliminary assessment on exposure of four typical populations to potentially toxic metals by means of skin wipes under the influence of haze pollution. Science of the Total Environment. 10.1016/j.scitotenv.2017.09.181 [DOI] [PubMed] [Google Scholar]
- Centers for Disease Control and Prevention (CDC) (2019). National Center for Environmental Health, Division of Emergency and Environmental Health Services. Atlanta, GA: https://www.cdc.gov/nceh/lead/default.htm [Google Scholar]
- Craig J, Rimstidt J, (1998). Gold production history of the United States. Ore Geology Reviews. 13, 407–64. [Google Scholar]
- Csavina J, Field J, Taylor MP, Gao S, Landázuri A, Betterton EA, & Sáez AE (2012). A review on the importance of metals and metalloids in atmospheric dust and aerosol from mining operations. Science of the Total Environment, The, 433, 58–73. 10.1016/j.scitotenv.2012.06.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Landázuri A, Wonaschütz A, Rine K, Rheinheimer P, Barbaris B, … Betterton EA (2011). Metal and Metalloid Contaminants in Atmospheric Aerosols from Mining Operations. Water, Air, & Soil Pollution, 221(1–4), 145–157. 10.1007/s11270-011-0777-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Csavina J, Taylor MP, Félix O, Rine KP, Eduardo Sáez A, & Betterton EA (2014). Size-resolved dust and aerosol contaminants associated with copper and lead smelting emissions: Implications for emission management and human health. Science of the Total Environment. 10.1016/j.scitotenv.2014.06.031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- European Food Safety Authority (EFSA). (2009). Scientific Opinion on Arsenic in Food. EFSA Journal, 7(10), 1351. [Google Scholar]
- Félix OI, Csavina J, Field J, Rine KP, Sáez AE, & Betterton EA (2015). Use of lead isotopes to identify sources of metal and metalloid contaminants in atmospheric aerosol from mining operations. Chemosphere. 10.1016/j.chemosphere.2014.11.057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzales P, Felix O, Alexander C, Lutz E, Ela W, & Eduardo Sáez A (2014). Laboratory dust generation and size-dependent characterization of metal and metalloid-contaminated mine tailings deposits. Journal of Hazardous Materials. 10.1016/j.jhazmat.2014.09.002 [DOI] [PubMed] [Google Scholar]
- Hu X, Zhang Y, Ding Z, Wang T, Lian H, Sun Y, & Wu J (2012). Bioaccessibility and health risk of arsenic and heavy metals (Cd, Co, Cr, Cu, Ni, Pb, Zn and Mn) in TSP and PM2.5 in Nanjing, China. Atmospheric Environment, 57, 146–152. 10.1016/j.atmosenv.2012.04.056 [DOI] [Google Scholar]
- Huang YH, Bornschein RL, Grote J, Menrath W, Roda S. (1997) Environmental arsenic exposure of children around a former copper smelter site. Environ. Res.; 72: 72–81. [DOI] [PubMed] [Google Scholar]
- Ikegami M, Yoneda M, Tsuji T, Bannai O, & Morisawa S (2014). Effect of Particle Size on Risk Assessment of Direct Soil Ingestion and Metals Adhered to Children’s Hands at Playgrounds. Risk Analysis, 34(9). 10.1111/risa.12215 [DOI] [PubMed] [Google Scholar]
- Joint FAO/WHO Codex Alimentarius Commission. (2018). General Standard for Contaminants and Toxins in Food and Feed Codex alimentarius. Rome: Food and Agriculture Organization of the United Nations: World Health Organization; Retrieved from http://www.fao.org/fao-who-codexalimentarius/thematic-areas/contaminants/en/ [Google Scholar]
- Kastury F, Smith E, Karna RR, Scheckel KG, & Juhasz AL (2018). Science of the Total Environment An inhalation-ingestion bioaccessibility assay (IIBA) for the assessment of exposure to metal (loid) s in PM 10. Science of the Total Environment, 631–632, 92–104. 10.1016/j.scitotenv.2018.02.337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastury F, Smith E, & Juhasz AL (2017). A critical review of approaches and limitations of inhalation bioavailability and bioaccessibility of metal(loid)s from ambient particulate matter or dust. Science of the Total Environment. 10.1016/j.scitotenv.2016.09.056 [DOI] [PubMed] [Google Scholar]
- Kearney Foundation of Soil Science, Division of Agriculture and Natural Resources, University of California. (1996). Background Concentrations of Trace and Major Elements in California Soils (Rep.). Retrieved on April 9, 2019, from: https://envisci.ucr.edu/downloads/chang/kearney_special_report_1996.pdf
- Kelley ME, Grauning SE, Schoof RA, & Ruby MV (2002). Assessing oral bioavailability of metals in soil. Columbus, OH: Battelle Press [Google Scholar]
- Kortenkamp BA, & Faust M (2018). Regulate to reduce chemical mixture risk. Science, 361(6399), 224–226. 10.1126/science.aat9219 ARTICLE [DOI] [PubMed] [Google Scholar]
- Koshman AH, Bergendahl MH, (1968). Prinicipal Gold-Producing Districts of the United States Geological Survey Professional Paper 610. United States Department of the Interior, Washington DC. [Google Scholar]
- Krombach F, Münzing S, Allmeling AM, Gerlach JT, Behr J, & Dörger M (1997). Cell size of alveolar macrophages: an interspecies comparison. Environmental health perspectives, 105 Suppl 5(Suppl 5), 1261–1263. doi: 10.1289/ehp.97105s51261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Landrigan BPJ, & Goldman LR (2011). Children’s Vulnerability To Toxic Chemicals: A Challenge And Opportunity To Strengthen Health And Environmental Policy, 5(5), 842–850. [DOI] [PubMed] [Google Scholar]
- Liu P, Wang C, Song X, & Wu Y (2010). International Journal of Hygiene and Dietary intake of lead and cadmium by children and adults – Result calculated from dietary recall and available lead / cadmium level in food in comparison to result from food duplicate diet method. International Journal of Hygiene and Environmental Health, 213(6), 450–457. 10.1016/j.ijheh.2010.07.002 [DOI] [PubMed] [Google Scholar]
- Manjon I & Ramirez-Andreotta MD (unpublished results). A Dietary Assessment Tool to Estimate Arsenic and Cadmium Exposures from Locally-Grown Foods. Submitted to the International Journal of Hygiene and Environmental Health. [DOI] [PMC free article] [PubMed]
- National Industrial Chemicals Notification. (2017). Water soluble cadmium (2+) salts: Environment tier II assessment. Retrieved April 13, 2019, from https://www.nicnas.gov.au/chemical-information/imap-assessments/imap-assessments/tier-ii-environment-assessments/water-soluble-cadmium-ii-salts.
- Olumayede EG, Oguntimehin I, Ediagbonya FT, Ojiodu C, & Sodipe GO (2018). Data set on concentrations, bioavailability, dose and lung deposition of labile metals bound to inhalable and respirable fractions of ambient particulate matters in Akure suburbs. Data in brief, 19, 2146–2154. doi: 10.1016/j.dib.2018.06.092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oomen AG, Hack A, Minekus M, Zeijdner E, Cornelis C, Schoeters G, et al. (2002). Comparison of Five In Vitro Digestion Models To Study the Bioaccessibility of Soil Contaminants. Environ Sci Technol 2002;36(15):3326–34. [DOI] [PubMed] [Google Scholar]
- Ramirez-Andreotta MD, Brusseau ML, Artiola JF, & Maier RM (2013a). A greenhouse and field-based study to determine the accumulation of arsenic in common homegrown vegetables grown in mining-affected soils. Science of the Total Environment, 443, 299–306. 10.1016/j.scitotenv.2012.10.095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Andreotta MD, Brusseau ML, Beamer P, & Maier RM (2013b). Home gardening near a mining site in an arsenic-endemic region of Arizona: Assessing arsenic exposure dose and risk via ingestion of home garden vegetables, soils, and water. Science of the Total Environment, 454–455, 373–382. 10.1016/j.scitotenv.2013.02.063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel H, Waterlot C, Pelfrene A, Pruvot C, Mazzuca M, & Douay F (2010). Cd, Pb and Zn Oral Bioaccessibility of Urban Soils Contaminated in the Past by Atmospheric Emissions from Two Lead and Zinc Smelters. Arch Environ Contam Toxicol, 945–954. 10.1007/s00244-009-9425-5 [DOI] [PubMed] [Google Scholar]
- Sly PD, & Flack F (2008). Susceptibility of Children to Environmental Pollutants. Annals of the New York Academy of Sciences, 1140(1), 163–183. 10.1196/annals.1454.017 [DOI] [PubMed] [Google Scholar]
- Smith Joseph V. (1999). Colloquium on geology, mineralogy, and human welfare (National Academy of Sciences colloquium series). Washington, D.C.: National Academy of Sciences. [PMC free article] [PubMed] [Google Scholar]
- Solnit R (2006). Winged Mercury and the Golden Calf. Retrieved February 20, 2019, from https://orionmagazine.org/article/winged-mercury-and-the-golden-calf/
- Stilwell DE, Rathier TM, & Musante CL (2008). Comparison of heavy metals in community garden produce versus store-bought produce. The Connecticut Agricultural Experiment Station, New Haven, 1020(August), 1–12. [Google Scholar]
- Tangahu BV, Sheikh Abdullah SR, Basri H, Idris M, Anuar N, & Mukhlisin M (2011). A review on heavy metals (As, Pb, and Hg) uptake by plants through phytoremediation. International Journal of Chemical Engineering, 2011. 10.1155/2011/939161 [DOI] [Google Scholar]
- Thomas AN, Root RA, Lantz RC, Sáez AE, & Chorover J (2018). Oxidative weathering decreases bioaccessibility of toxic metal(loid)s in PM10 emissions from sulfide mine tailings. GeoHealth, 2, 118–138. 10.1002/2017GH000118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner L, Eliason M, Sandoval A, & Chaloupka FJ (2016). Increasing Prevalence of US Elementary School Gardens, but Disparities Reduce. Journal of School Health, 86(12), 906–912. [DOI] [PubMed] [Google Scholar]
- U.S. Census Bureau, Population Division. (2010). QuickFacts. Nevada County, California; Grass Valley city, California; Retrieved from: https://www.census.gov/quickfacts/fact/table/nevadacountycalifornia,grassvalleycitycalifornia/PST045218 [Google Scholar]
- U.S. Department of Education. (2008). National Center for Education Statistics, Schools and Staffing Survey (SASS), “Public School Data File,” 2007–08. Retrieved from: https://nces.ed.gov/surveys/sass/tables/sass0708_035_s1s.asp
- U.S. EPA (1992). Guidelines for Exposure Assessment (Vol.57). Washington, DC: Retrieved from https://www.epa.gov/sites/production/files/2014-11/documents/guidelines_exp_assessment.pdf [Google Scholar]
- U.S. EPA. (1994). “Method 200.8: Determination of Trace Elements in Waters and Wastes by Inductively Coupled Plasma-Mass Spectrometry,” Revision 5.4. Cincinnati, OH. [Google Scholar]
- U.S. EPA (2000). Assigning Values to Non-Detected/Non-Quantified Pesticide Residues in Human Health Food Exposure Assessments. Washington, DC. [Google Scholar]
- U.S. EPA. (2006a). Integrated Risk Information System (IRIS). Office of Research and Development, National Center for Environmental Assessment; Washington, DC: http://www.epa.gov/iris/. [Google Scholar]
- U.S. EPA (2006b). National Ambient Air Quality Standards for Lead; Final Rule. Washington, D.C.: GPO; Web. Retrieved on 8 April. 2019. [Google Scholar]
- U.S. EPA. (2007a). “Method 3051A (SW-846): Microwave Assisted Acid Digestion of Sediments, Sludges, and Oils,” Revision 1. Washington, DC. [Google Scholar]
- U.S. EPA. (2007b). “Method 3015A (SW-846): Microwave Assisted Acid Digestion of Aqueous Samples and Extracts,” Revision 1. Washington, DC. [Google Scholar]
- U.S. EPA. (2007c). “Guidance for Evaluating the Oral Bioavailability of Metals in Soils for Use in Human Health Risk Assessment.” Washington, DC. [Google Scholar]
- U.S. EPA. (2008). Child-Specific Exposure Factors Handbook (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-06/096F, 2008. [Google Scholar]
- U.S. EPA. (2011). Exposure Factors Handbook 2011 Edition (Final Report). U.S. Environmental Protection Agency, Washington, DC, EPA/600/R-09/052F, 2011. [Google Scholar]
- U.S. EPA. (2012). Recommendations for Default Value for Relative Bioavailability of Arsenic in Soil. Washington, DC: https://semspub.epa.gov/work/HQ/175338.pdf [Google Scholar]
- U.S. EPA. (2014). “Method 6020B (SW-846): Inductively Coupled Plasma-Mass Spectrometry,” Revision 2. Washington, DC. [Google Scholar]
- U.S. EPA. (2017a). Exposure Factors Handbook Chapter 5 (Update): Soil and Dust Ingestion. U.S. EPA Office of Research and Development, Washington, DC, EPA/600/R-17/384F, 2017. [Google Scholar]
- U.S. EPA. (2017b). “Standard Operating Procedure for an In Vitro Bioaccessibility Assay for Lead and Arsenic in Soil.” Washington, DC. [Google Scholar]
- U.S. EPA. (2017c). TRI Explorer (2015 Dataset (released March 2017) (updated June 2, 2017)) [Internet database]. Retrieved from https://www.epa.gov/triexplorer.
- U.S. EPA. (2018a). AirData Air Quality Monitors. [Air Quality System database] available at https://epa.maps.arcgis.com/apps/webappviewer/index.html?id=5f239fd3e72f424f98ef3d5def547eb5&extent=-146.2334,13.1913,-46.3896,56.5319. Accessed May 1, 2019.
- U.S. EPA. (2018b). Exposure Factors Handbook Chapter 9 (Update): Intake of Fruits and Vegetables. U.S. EPA Office of Research and Development, Washington, DC, EPA/600/R-18/098F. [Google Scholar]
- U.S. EPA. (2019a). Exposure Factors Handbook Chapter 3 (Update): Ingestion of Water and Other Select Liquids. U.S. EPA Office of Research and Development, Washington, DC. [Google Scholar]
- U.S. EPA. (2019b). Regional Screening Levels for Chemical Contaminants at Superfund Sites. Washington, DC. [Google Scholar]
- U.S. Food and Drug Administration (FDA). (2014). Total Diet Study Elements Results Summary Statistics – Market Baskets 2006 through 2013. College Park, MD: Retrieved from https://www.fda.gov/food/science-research-food/total-diet-study [Google Scholar]
- United States Department of Agriculture (USDA), Natural Resources Conservation Service. (2018). Web Soil Survey. Soil Survey Area: Nevada County Area, California, Survey Area Data: Version 11, Sep 12, 2018. Accessed at: http://websoilsurvey.nrcs.usda.gov
- Vaiserman A (2014). Early-life Exposure to Endocrine Disrupting Chemicals and Later-life Health Outcomes: An Epigenetic Bridge? Aging and disease, 5(6), 419–429. doi: 10.14336/AD.2014.0500419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren GP, Alloway BJ, Lepp NW, Singh B, Bochereau FJM, & Penny C (2003). Field trials to assess the uptake of arsenic by vegetables from contaminated soils and soil remediation with iron oxides. Science of the Total Environment, 311(1–3), 19–33. 10.1016/S0048-9697(03)00096-2 [DOI] [PubMed] [Google Scholar]
- Yost LJ, Tao SH, Egan SK, Barraj LM, Smith KM, Tsuji JS, … Rachman NJ (2004). Estimation of dietary intake of inorganic arsenic in U.S. children. Human and Ecological Risk Assessment. 10.1080/10807030490452151 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.