Abstract
The anti-inflammatory activities of P53 in the vasculature have been associated with the enhancement of the endothelial barrier function. In the present study we employed human and bovine lung endothelial cells, to investigate whether P53 expression levels affect the redox status of pulmonary cells. Moreover, we tested the possibility that those events affect the endothelial integrity of the lung microvascular monolayers. Our observations suggest that P53 suppression by LPS, Pifithrin, or small interfering RNA increased the expression of the redox marker malondialdehyde. In contrast, P53 induction by Nutlin or the Hsp90 inhibitor AUY922 exerted the opposite effects, namely suppressed that lipid oxidation marker. The direct measurement of the reactive oxygen species by 2,7-Dichlorodihydrofluorescein diacetate; confirmed the anti-oxidant activity of P53 in the vasculature. Furthermore, the increased reactive oxygen species production due to P53 suppression was associated with lung hyper-permeability responses. In conclusion, P53 supports endothelial barrier function, at least in part, via the modulation of the reactive oxygen species.
Keywords: Acute Lung Injury, Inflammation, Barrier Function, Endothelium
Introduction
Acute Respiratory Distress Syndrome (ARDS) is the consequence of severe disruption in the lung microvasculature function; due to various cellular and environmental insults (i.e. inflammation). It affects 64.2 to 78.9 cases/100,000 persons per year[1]. Investigations on the signaling cascades streaming those events, may lead to the discovery of new therapeutic ways to oppose that syndrome. Twenty-five percent of ARDS cases are initially classified as mild and 75% as moderate or severe. A third of the mild cases progress to moderate or severe disease, and the overall mortality rate is still extremely high (43%)[2].
P53 is a transcription factor, associated with anti-inflammatory responses in the vasculature[3]. Recent evidence suggest that it regulates crucial “players” of the actomyosin architecture, to support endothelial integrity. It was recently suggested that P53 mediates the Rac1/RhoA signaling[4], suppresses the APE1/Ref1 inflammatory factor[5], and serves as the direct target of the unfolded protein response element[6]. To excel our studies, we investigated whether P53 affects the redox status of the lung microvasculature. Increases in the production of the reactive oxygen species, have been associated with hyper permeability responses[7]. Indeed, anti - inflammatory compounds such as Growth Hormone Releasing Hormone antagonists have been shown to exert the opposite effects, namely to support the structure of the endothelium[8].
In the present study we investigated the hypothesis that P53 enhances lung endothelial barrier function, by suppressing the production of the Reactive Oxygen Species (ROS). Our observations supports the protective role of P53 towards the lung vasculature, and demonstrates that the protective activities of this transcription factor towards the lung vasculature are associated with a reduction in ROS levels.
Materials and Methods
Reagents:
The Hsp90 inhibitor AUY922, Nutlin, Pifithrin, RIPA buffer, MDA, anti-mouse and anti-rabbit IgG HRP linked antibodies, 2,7-Dichlorodihydrofluorescein diacetate, as well as nitrocellulose membranes were obtained from VWR (Radnor, PA). P53 antibody was obtained from Cell Signaling Technology (Danvers, MA). B-actin antibody was purchased from Sigma-Aldrich (St Louis, MO).
Cell Culture:
Bovine Pulmonary Arterial Endothelial Cells (BPAEC) was purchased from Genlantis (San Diego, CA) and were maintained in DMEM medium supplemented with 10% FBS. Human Lung Microvascular Endothelium cells (HuLEC-5a) were purchased from ATCC (Manassas, VA) and were maintained in PromoCell Endothelial Cell Growth Medium MV. All cultures were maintained at 37°C in a humidified atmosphere of 5% CO2/ 95% air supplemented with medium containing 1X penicillin/streptomycin. The reagents were purchased from VWR (Radnor, PA).
Protein Isolation, Western Blot Analysis and Transfections:
Proteins were isolated using RIPA buffer according to manufacturer’s instructions. Protein-matched samples were separated by electrophoresis through 12% sodium dodecyl sulfate (SDS–PAGE) Tris-HCl gels and were subjected to Western Blot according to standard procedures[6]. All reagents were purchased from VWR (Radnor, PA). The transfections were performed as previously described[5].
Measurement of endothelial barrier function:
The barrier function of endothelial cell monolayers was estimated by electric cell-substrate impedance sensing (ECIS), utilizing ECIS model ZΘ (Applied Biophysics, Troy, NY, USA). All the experiments were conducted on confluent cells which had reached a steady-state resistance of at least 800 Ω[9].
Densitometry and Statistical Analysis:
Image J software (National Institute of Health) was used to perform densitometry of immunoblots. All data are expressed as mean values ± SEM (standard error of mean). A value of P<0.05 was considered significant. GraphPad Prism 5.01 from GraphPad (CA, USA) was used for data analysis. The letter n represents the number of experimental repeats.
Results
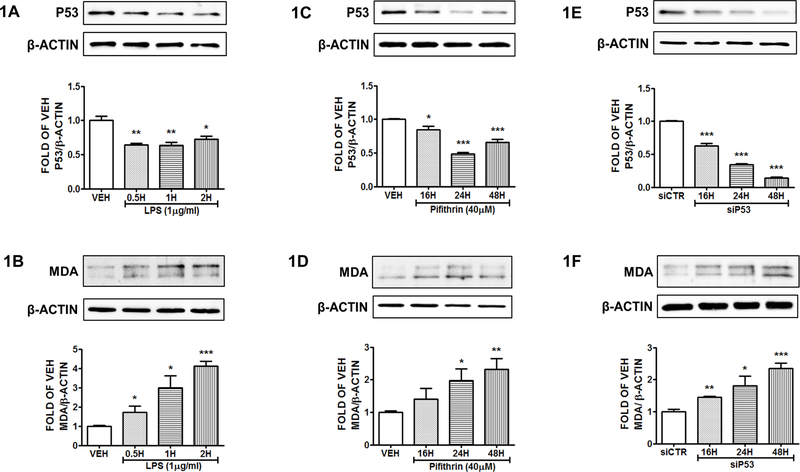
Reduction of P53 by LPS increases MDA expression levels in HuLEC-5a.
Human Lung Microvascular Endothelial Cells were treated with vehicle (PBS), or LPS (1μg/ml) for 0.5, 1 and 2 hours. The results depicted in Figure 1A demonstrate the suppression of P53 by LPS. That effect, was associated with an increase of the MDA expression levels (Fig. 1B).
Figure 1: P53 suppression induces MDA expression in HuLEC-5a.
Western Blot analysis of P53 and β actin (A), MDA and β actin (B) after treatment of HuLEC-5a with vehicle (PBS) or LPS (1μg/ml) for 0.5, 1 and 2 hours. Western Blot analysis of P53 and β actin (C), MDA and β actin (D) after treatment of HuLEC-5a with vehicle (DMSO) or Pifithrin (PTH) (40 μg/ml) for 16, 24 and 48 hours. Western Blot analysis of P53 and β actin (E), MDA and β actin (F) after treatment of HuLEC-5a with irrelevant siRNA (siCTR) or siRNA for P53 (siP53) (0.3 μM) for 16, 24 and 48 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and MDA bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM.
Suppression of P53 by Pifithrin induces MDA expression in HuLEC-5a.
Human lung cells were exposed to vehicle (DMSO) or Pifithrin (40μΜ) for 16, 24 or 48 hours. The results depicted in Figure 1C reveal the successful inhibition of P53 by this P53 inhibitor. Moreover, MDA levels were induced due to P53 suppression (Fig. 1D).
Silencing of P53 gene expression results to MDA induction.
HuLEC-5a were exposed to siRNA for P53 (0.3 μM) or irrelevant siRNA for 16, 24 and 48 hours. The successful suppression of P53 was confirmed by Western Blot (Fig. 1E). P53 silencing, resulted to the elevation of the intracellular MDA levels (Fig. 1F). The irrelevant siRNA did not affect the expression levels of P53.
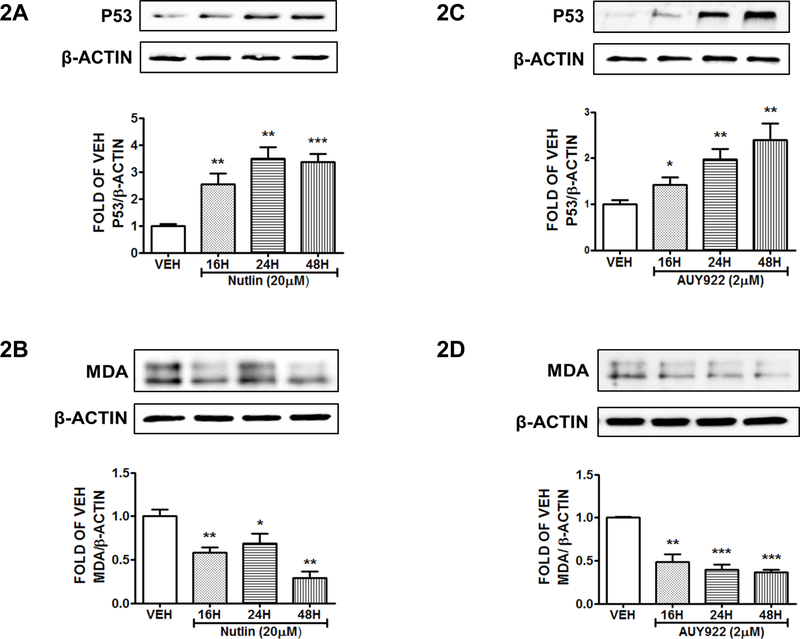
Induction of P53 by Nutlin suppresses MDA expression levels:
Human cells were treated with either vehicle (DMSO) or Nutlin (20 μΜ) for 16, 24, and 48 hours. Figure 2A shows the elevated P53 levels due to Nutlin treatment. This Nutlin-mediated effect was associated with a reduction in MDA levels (Fig. 2B).
Figure 2: P53 induction suppresses MDA expression in HuLEC-5a.
Western Blot analysis of P53 and β actin (A), MDA and β actin (B) after treatment of HuLEC-5a with vehicle (DMSO) or Nutlin (30 μM) for 16, 24 and 48 hours. Western Blot analysis of P53 and β actin (C), MDA and β actin (D) after treatment of HuLEC-5a with vehicle (DMSO) or AUY922 (2 μΜ) for 16, 24 and 48 hours. The blots shown are representative of 3 independent experiments. The signal intensity of the P53 and MDA bands were analyzed by densitometry. Protein levels were normalized to β actin. *P<0.05, **P<0.01, ***P<0.001 vs vehicle. Means ± SEM.
Hsp90 inhibition suppresses MDA levels:
HuLEC-5a were treated with either vehicle (DMSO) or AUY922 (2μΜ) for 16, 24 and 48 hours. Τhe induction of P53 appears in Figure 2C. Moreover, P53 increase resulted to MDA suppression (Fig. 2D)
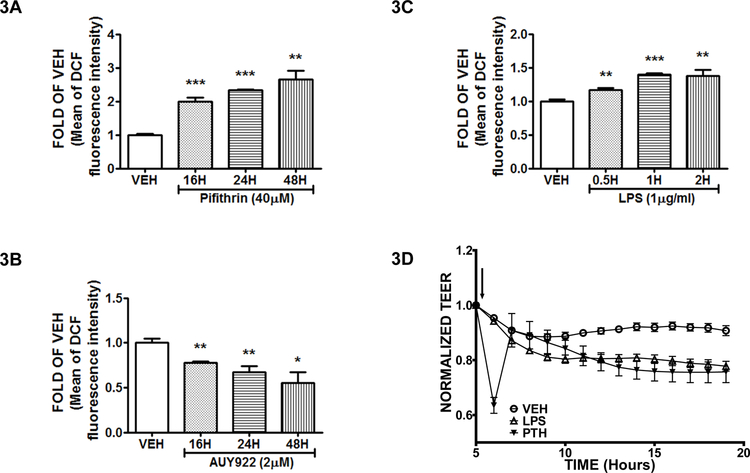
P53 induction reduces the production of Reactive Oxygen Species (ROS), while its suppression exerts the opposite effects:
Bovine Pulmonary Aortic Endothelial Cells (BPAEC) were exposed for 16, 24 and 48 hours to either vehicle (DMSO), or the P53 inducer AUY922 (2μΜ) (Fig. 3A), or the P53 suppressor Pifithrin (40μΜ) (Fig. 3B). Moreover, the bovine cells were treated with vehicle (PBS) or LPS (1μg/ml) for 0.5, 1 and 2 hours (Fig. 3C). The results indicate that AUY922 suppressed the production of ROS, and the P53 suppressors (Pifithrin, LPS) reduced it.
Figure 3: P53 enhances endothelial integrity by suppressing ROS levels.
ROS measurement was conducted in BPAEC treated with vehicle (DMSO), or Pifithrin (40 μΜ) for 16, 24 and 48 hours (A), or AUY922 (2 μΜ) for 16, 24 and 48 hours (B). ROS measurement in BPAEC treated with either vehicle (PBS) or LPS (1μg/ml) for 0.5, 1 and 2 hours (C). Pifithrin (PTH), or LPS, or vehicle (PBS) was added into the media of confluent BPAEC monolayers. The addition of the reagents is indicated by the black arrow. A gradual increase in the endothelial permeability (decreased TEER) was observed in the cells exposed to Pifithrin or LPS. n = 3 per group. Means ± S.E. (D).
Discussion
ARDS affects thousands of people nationwide, and the current therapies do not efficiently counteract the high mortality rates associated with that syndrome, which has been shown to be associated with increased production of ROS [10]. In our continuing efforts to delineate the molecular mechanisms involved in the progression of that disorder, we show that the endothelial defender P53 is strongly involved in the regulation of the redox status of lung cells. P53 efficiently suppresses the lipid oxidation levels of the lung microvasculature, as reflected in the MDA (redox marker) measurements[11]. P53 has also been shown to modulate ROS production in other tissues[12, 13]. P53 suppression by LPS, Pifithrin or siRNA for P53 induced the MDA levels (Fig.1), while P53 induction by AUY922 and Nutlin had the opposite effects (Fig. 2). Those events were reflected in the ROS production of the cells subjected to treatment with the previously mentioned P53 modulators (Fig. 3).
Furthermore, in order to evaluate whether the P53-mediated regulation of ROS production affects the permeability of the lung cells, we proceeded with measurement of endothelial resistance by ECIS means. The increased ROS production due to P53 suppression by both LPS and Pifithrin, resulted to a weakened endothelial function (Fig. 3). Furthermore, it was previously shown that AUY922 enhances the barrier function of human lung microvascular cells[5], and that Hsp90 inhibitors are associated with anti-oxidant activities[14]. Since P53 reduction induces ROS metabolism, we suggest the possibility that this mechanism is involved in the induction of the Unfolded Protein Response (UPR)-triggered repairing activities towards the lung endothelium[4, 15]. Those intercellular events, may involve the activities of the redox regulator APE1/Ref1. The latter molecule is suppressed by P53[5].
The present study did not examine the effects of P53 manipulation in the ROS production and lung function in vivo. Moreover, it did not delineate the exacts mechanisms which mediate the P53-induced ROS suppression in vitro. Future studies will address the corresponding topics, and will test whether the P53-mediated reduced ROS production is a necessary element towards the protective activities of UPR and P53 in the lungs. In conclusion, our results suggest that P53 regulates the redox status of the lung endothelial cells, and contribute to the further understanding of the protective role of P53 towards the lung microvasculature.
Acknowledgments
Funding: Dr. Barabutis research is supported by I) R&D, Research Competitiveness Subprogram (RCS) of the Louisiana Board of Regents through the Board of Regents Support Fund (LEQSF(2019-22)-RD-A-26) (P.I: N.B) II) National Institute of General Medical Sciences of the National Institutes of Health (5P20GM103424-15, 3P20GM103424-15S1) III) Malcolm Feist PAC Seed Program, Center for Cardiovascular Diseases and Sciences, LSU Health Shreveport, Shreveport, LA 71103 IV) Faculty Research Support Program award of the College of Pharmacy, University of Louisiana Monroe, Monroe LA 71201
Footnotes
Publisher's Disclaimer: This Author Accepted Manuscript is a PDF file of a an unedited peer-reviewed manuscript that has been accepted for publication but has not been copyedited or corrected. The official version of record that is published in the journal is kept up to date and so may therefore differ from this version.
Ethical approval: This article does not contain any studies with human participants or animals performed by any of the authors.
Disclosure of potential conflicts of interest: None
REFERENCES
- 1.Barabutis N, Unfolded Protein Response in Acute Respiratory Distress Syndrome. Lung, 2019. [DOI] [PubMed]
- 2.Matthay MA, et al. , Acute respiratory distress syndrome. Nat Rev Dis Primers, 2019. 5(1): p. 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Uddin MA and Barabutis N, P53: The endothelium defender. J Cell Biochem, 2019. [DOI] [PMC free article] [PubMed]
- 4.Barabutis N, Unfolded Protein Response supports endothelial barrier function. Biochimie, 2019. 165: p. 206–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Uddin MA, et al. , P53 supports endothelial barrier function via APE1/Ref1 suppression. Immunobiology, 2019. 224(4): p. 532–538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akhter MS, Uddin MA, and Barabutis N, Unfolded protein response regulates P53 expression in the pulmonary endothelium. J Biochem Mol Toxicol, 2019: p. e22380. [DOI] [PMC free article] [PubMed]
- 7.Mundi S, et al. , Endothelial permeability, LDL deposition, and cardiovascular risk factors-a review. Cardiovasc Res, 2018. 114(1): p. 35–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wang T, et al. , Particulate matter disrupts human lung endothelial cell barrier integrity via Rho-dependent pathways. Pulm Circ, 2017. 7(3): p. 617–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Barabutis N, et al. , p53 protects against LPS-induced lung endothelial barrier dysfunction. Am J Physiol Lung Cell Mol Physiol, 2015. 308(8): p. L776–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Karki P and Birukov KG, Rho and Reactive Oxygen Species at Crossroads of Endothelial Permeability and Inflammation. Antioxid Redox Signal, 2019. [DOI] [PMC free article] [PubMed]
- 11.Ortega MA, et al. , Patients with Incompetent Valves in Chronic Venous Insufficiency Show Increased Systematic Lipid Peroxidation and Cellular Oxidative Stress Markers. Oxid Med Cell Longev, 2019. 2019: p. 5164576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Song J, et al. , Stretching magnitude-dependent inactivation of AKT by ROS led to enhanced p53 mitochondrial translocation and myoblast apoptosis. Mol Biol Cell, 2019. 30(10): p. 1182–1197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang P, et al. , ROS -mediated p53 activation by juglone enhances apoptosis and autophagy in vivo and in vitro. Toxicol Appl Pharmacol, 2019. 379: p. 114647. [DOI] [PubMed] [Google Scholar]
- 14.Madrigal-Matute J, et al. , HSP90 inhibition by 17-DMAG attenuates oxidative stress in experimental atherosclerosis. Cardiovasc Res, 2012. 95(1): p. 116–23. [DOI] [PubMed] [Google Scholar]
- 15.Ozgur R, et al. , Interplay between the unfolded protein response and reactive oxygen species: a dynamic duo. J Exp Bot, 2018. 69(14): p. 3333–3345. [DOI] [PubMed] [Google Scholar]