Abstract
The arcuate nucleus (ARH) is an important hypothalamic area for the homeostatic control of feeding and other metabolic functions. In the ARH, proopiomelanocortin- (POMC) and agouti-related peptide (AgRP)-expressing neurons play a key role in the central regulation of metabolism. These neurons are influenced by circulating factors, such as leptin and growth hormone (GH). The objective of the present study was to determine whether a direct action of GH on ARH neurons regulates the density of POMC and AgRP axonal projections to major postsynaptic targets. We studied POMC and AgRP axonal projections to the hypothalamic paraventricular (PVH), lateral (LHA) and dorsomedial (DMH) nuclei in leptin receptor (LepR)-deficient mice (Leprdb/db), GH-deficient mice (Ghrhrlit/lit) and in mice carrying specific ablations of GH receptor (GHR) either in LepR- or AgRP-expressing cells. Leprdb/db mice presented reduction in the density of POMC innervation to the PVH compared to wild-type and Ghrhrlit/lit mice. Additionally, both Leprdb/db and Ghrhrlit/lit mice showed reduced AgRP fiber density in the PVH, LHA and DMH. LepR GHR knockout mice showed decreased density of POMC innervation in the PVH and DMH, compared to control mice, whereas a reduction in the density of AgRP innervation was observed in all areas analyzed. Conversely, AgRP-specific ablation of GHR led to a significant reduction in AgRP projections to the PVH, LHA and DMH, without affecting POMC innervation. Our findings indicate that GH has direct trophic effects on the formation of POMC and AgRP axonal projections and provide additional evidence that GH regulates hypothalamic neurocircuits controlling energy homeostasis.
Keywords: AgRP, development, food intake, GH, melanocortin system, POMC
INTRODUCTION
Feeding behavior is regulated by complex neurocircuits that integrate homeostatic and decision-making/reward information. The neurons that control feeding are distributed in different areas of the central nervous system, including the hypothalamus, amygdala, cerebral cortex and brainstem (Andermann and Lowell, 2017; Livneh et al., 2017; Ramos-Lobo and Donato, 2017). Although it is evident that food intake is controlled by neurocircuits and not by isolated neural populations, the arcuate nucleus of the hypothalamus (ARH) plays a particularly important role regulating not only feeding, but different aspects of metabolism. This occurs because ARH neurons are able to detect changes in systemic levels of different hormones, nutrients and metabolites proving critical homeostatic information to the brain (Andermann and Lowell, 2017; Livneh et al., 2017; Ramos-Lobo and Donato, 2017). In this sense, the alterations in food intake induced by different hormones, including leptin (Xu et al., 2018), ghrelin (Wang et al., 2014), insulin (Loh et al., 2017) or glucagon-like peptide-1 (He et al., 2019), depend on ARH neurons.
“Novel” neuronal populations in the ARH have been described to regulate food intake, including tyrosine hydroxylase- or somatostatin-expressing neurons (Zhang and van den Pol, 2016; Campbell et al., 2017; Luo et al., 2018). However, neurons that express either the proopiomelanocortin (POMC) or the agouti-related peptide (AgRP) are classically involved in the control of food intake and energy balance (Andermann and Lowell, 2017; Livneh et al., 2017; Ramos-Lobo and Donato, 2017). Activation of AgRP neurons induces feeding (Luquet et al., 2005; Aponte et al., 2011; Krashes et al., 2011), whereas the release of α-melanocyte-stimulating hormone by POMC neurons and the consequent activation of melanocortin receptor 3 (MC3R) and 4 (MC4R) inhibit food intake and are required for energy homeostasis (Fan et al., 1997; Huszar et al., 1997; Ollmann et al., 1997).
A pronounced expression of GH receptor (GHR) has been described in the ARH (Walsh et al., 1990; Burton et al., 1992; Kamegai et al., 1996; Furigo et al., 2017). In addition, recent studies indicate that GH action in both AgRP and POMC neurons control different aspects of metabolism (Bohlen et al., 2019; Furigo et al., 2019b; Quaresma et al., 2019; Teixeira et al., 2019). For example, Furigo et al. (2019b) showed that GH activates AgRP neurons and increases food intake, whereas AgRP-specific GHR ablation impairs the neuroendocrine adaptions to weight loss. On the other hand, GHR deletion in POMC cells blunts the glucoprivic hyperphagia induced by 2-deoxy-D-glucose administration (Quaresma et al., 2019). Thus, GH emerges as a new circulating factor that regulates metabolism via ARH neurons.
Some hormones that regulate food intake, such as leptin, also exhibit trophic effects on ARH neurons by regulating axonal development (Bouret et al., 2004b; Bouret et al., 2004a) and synaptic plasticity (Pinto et al., 2004). Interestingly, GH deficient or GHR knockout (KO) mice also present reduced formation of AgRP and POMC projections to postsynaptic targets (Sadagurski et al., 2015). However, these mouse models present multiple confounding factors, such as dwarfism, changes in leptin and insulin-like growth factor-1 (IGF-1) levels, increased insulin sensitivity, endocrine defects and developmental alterations (Young et al., 2016; Basu et al., 2018). Thus, whether the formation of ARH neural projections requires a direct action of GH on AgRP and POMC cells or is indirectly influenced by the endocrine abnormalities caused by the complete deficiency of GH action remains to be determined. Thus, the objective of the present study was to investigate if GHR expression in ARH neurons regulates the density of axonal terminals to major hypothalamic projection areas. To investigate the neurotrophic effects of leptin and GH, we studied POMC and AgRP axonal projections in leptin receptor (LepR)-deficient (Leprdb/db) and GH-deficient (Ghrhrlit/lit) mice, as well as in mice carrying specific ablations of GHR in both LepR- and AgRP-expressing cells.
EXPERIMENTAL PROCEDURES
Mice
All experiments were conducted in 4-month-old male mice in accordance with the Ethics Committee on the Use of Animals of the Institute of Biomedical Sciences at the University of São Paulo (protocol #73/2017). C57BL/6 wild-type (WT) mice, Leprdb/db mice (Stock No: 000697; The Jackson Laboratory) and Ghrhrlit/lit mice (Stock No: 000533; The Jackson Laboratory) were used in the experiments. Adult Leprdb/db and Ghrhrlit/lit mice exhibit, respectively, morbid obesity and dwarfism. Genetic deletion of the Ghr gene either in AgRP cells or LepR-expressing cells were obtained through the breeding of AgRP-Cre mouse (Stock No: 012899; The Jackson Laboratory) or LepR-Cre mouse (Stock No: 008320; The Jackson Laboratory) with animals carrying loxP-flanked Ghr alleles (List et al., 2013). AgRP GHR KO and LepR GHR KO mice carried the Cre transgene and were homozygous for the loxP- flanked alleles, whereas their respective controls were littermates negative for the Cre transgene. To visualize AgRP- or LepR-expressing neurons, a group of control, AgRP GHR KO and LepR GHR KO mice also carried a Cre-dependent expression of the tdTomato reporter protein (Stock No: 007909, The Jackson Laboratory). Animals were weaned at 21 days of age and tail tips were collected for DNA extraction. Genomic DNA was amplified by PCR, and PCR products were submitted to electrophoresis in agarose gel for the identification of their mutations. Mice were maintained in standard conditions of 12h light/dark cycles and controlled temperature (21–23 °C), and received potable water and regular chow ad libitum (2.99 kcal/g; 9.4% calories from fat; Quimtia, Brazil).
Evaluation of axonal projections from ARH neurons
Adult (4-month-old) male mice were anesthetized with isoflurane and transcardially perfused with saline followed by 10% buffered formalin solution (~180 mL/mouse). Collected brains were maintained for 1 hour in formalin for post-fixation and were transferred to 20% sucrose solution overnight. Brains were cut in a freezing microtome in 30 μm thickness sections and maintained in cryoprotection buffer at −20°C. Brain sections were subjected to immunofluorescence staining to evaluate the innervation of POMC or AgRP fibers in the paraventricular nucleus of the hypothalamus (PVH), lateral hypothalamic area (LHA) and dorsomedial nucleus of the hypothalamus (DMH). Brain sections were rinsed in 0.02 M potassium PBS, pH 7.4 (KPBS), followed by incubation in 3% normal donkey serum for 1 hour. Next, sections were incubated overnight in anti-AgRP antisera (1:2,000, Phoenix Pharmaceuticals, Inc.; Cat# H-003–53) or anti-β-endorphin antisera (1:2,000; Phoenix Pharmaceuticals; Burlingame, CA; Cat# H-022–33), since β-endorphin is a POMC-derived peptide and presents a complete overlapping expression compared to α-melanocyte-stimulating hormone (Lima et al., 2016). Subsequently, sections were incubated for 90 min in Alexa Fluor488-conjugated secondary antibody (Jackson ImmunoResearch Laboratories; Cambridge, MA). After rinses in KPBS, sections were mounted onto gelatin-coated slides and covered with Fluoromount G mounting medium (Electron Microscopic Sciences, Hatfield, PA). One representative rostrocaudal level of the LHA (Bregma −1.22) and DMH (Bregma −1.82), and two levels of the PVH (Bregma −0.70 and −0.94) were analyzed. In the case of PVH, we considered the mean values obtained in the two rostrocaudal levels. Initially, epifluorescence photomicrographs were acquired with an Axio Imager A1 microscope (Carl Zeiss, Munich, Germany), as demonstrated in the representative pictures. Using the ImageJ software (http://rsb.info.nih.gov/ij), we selected the area corresponding to each brain structure (PVH: 0.03 mm2; LHA: 0.2 mm2; DMH: 0.08 mm2). Then, we used the Measure tool to obtain the integrated optical density (IOD), which represents the average staining intensity in the selected area. The IOD obtained in the PVH, LHA and DMH were subtracted from the IOD determined in adjacent nuclei with low staining (background) in each photomicrograph. In the first set of experiments, we studied WT (n = 5), Leprdb/db (n = 6) and Ghrhrlit/lit (n = 6) mice. In the second experiment, LepR GHR KO mice (n = 7) and control littermates (n = 7) were compared. In the third set of experiments, AgRP GHR KO mice (n = 6) and control littermates (n = 6) were evaluated.
Evaluation of GH responsive neurons
Control (n = 4–5), AgRP GHR KO (n = 3) and LepR GHR KO (n = 3) mice received an intraperitoneal injection of a saline solution containing GH extracted from porcine pituitary (from Dr. A.F. Parlow, National Institute of Diabetes and Digestive and Kidney Diseases, National Hormone and Pituitary Program, Los Angeles, CA) at a final dose of 20 μg/g body weight. PBS-injected mice (n = 4) were used as a negative control of pSTAT5 staining. Mice were perfused 90 minutes later. To detect the phosphorylated form of the signal transducer and activator of transcription-5 (pSTAT5), brain slices were rinsed in KPBS, followed by pretreatment in water solution containing 1% hydrogen peroxide and 1% sodium hydroxide for 20 min. After rinsing in KPBS, sections were incubated in 0.3% glycine and 0.03% lauryl sulfate for 10 min each. Next, slices were blocked in 3% normal donkey serum for 1 h, followed by incubation in anti-pSTAT5Tyr694 primary antibody (1:1000; Cell Signaling Technology; Beverly, MA; Cat# 9351) for 40 h. After that, sections were rinsed in KPBS and incubated for 90 min in AlexaFluor488-conjugated secondary antibody (1:500, Jackson ImmunoResearch Laboratories). The visualization of tdTomato fluorescence does not require any reaction. Sections were mounted onto gelatin-coated slides and covered with Fluoromount G (Electron Microscopic Sciences). Photomicrographs were acquired with an Axio Imager A1 microscope (Carl Zeiss).
Gene expression analysis
For the gene expression analysis, total RNA from the whole hypothalamus was extracted with TRIzol (Invitrogen). Assessment of RNA quantity and quality was determined using an Epoch Microplate Spectrophotometer (Biotek). Total RNA was incubated in DNase I RNase-free (Roche Applied Science), followed by reverse transcription using 2 μg of total RNA with SuperScript II Reverse Transcriptase (Invitrogen) and random primers p(dN)6 (Roche Applied Science). Real-time polymerase chain reaction was performed using the 7500TM Real-Time PCR System (Applied Biosystems) and Power SYBR Green PCR Master Mix (Applied Biosystems). Relative quantification of mRNA was calculated by 2−ΔΔCt. Data were normalized to the expression of Actb and reported as fold changes compared to values obtained from the control group (set at 1.0). The following primers were used: Actb (forward: gctccggcatgtgcaaag; reverse: catcacaccctggtgccta), Mc3r (forward: ttgatgaaaacctgctcgca; reverse: tatccgacgctgcctaacct) and Mc4r (forward: cttccccagagactcgctggca; reverse: acccaccaccatggcatgta).
Statistical analysis
Data were expressed as mean ± s.e.m. and analyzed with GraphPad Prism software (San Diego, CA). Comparisons between two groups were performed using two-tailed Student’s t-test. When three groups were compared simultaneously, the analysis was performed by one-way ANOVA with Tukey’s test for multiple comparisons. Differences were considered significant if P ≤ 0.05.
RESULTS
Development of ARH projections requires GH and leptin signaling
Previous studies indicate that the absence of leptin or GH signaling leads to defects in the development of ARH neuronal projections (Bouret et al., 2004b; Bouret et al., 2004a; Sadagurski et al., 2015; Ramos-Lobo et al., 2019). To confirm the importance of the neurotrophic effects of leptin and GH, AgRP and POMC projections were compared among WT, Leprdb/db and Ghrhrlit/lit mice. As expected, 4-month-old Leprdb/db mice are morbidly obese, whereas the dwarfism of Ghrhrlit/lit mice was confirmed by their reduced body weight, compared to WT animals (Fig. 1A). The number of POMC-expressing cells in the ARH was reduced in Leprdb/db mice, which is in accordance with the stimulatory effect of leptin on ARH POMC neurons that is missing in Leprdb/db mice (Schwartz et al., 1997; Thornton et al., 1997). In contrast, Ghrhrlit/lit mice exhibited a similar number of POMC neurons compared to WT animals (Fig. 1B). Next, ARH projections were evaluated. Leprdb/db mice presented reduced density of POMC innervation in the PVH, compared to both WT and Ghrhrlit/lit mice (Fig. 1C and Fig. 2). However, Leprdb/db mice exhibited no changes in POMC fiber density in the LHA and DMH (Fig. 1D–E and Fig. 2). Additionally, POMC innervation in Ghrhrlit/lit mice was similar to that observed in WT mice (Fig. 1C–E and Fig. 2). Regarding the innervation of AgRP, both Leprdb/db and Ghrhrlit/lit mice showed reduced AgRP fiber density in the PVH, LHA and DMH, compared to WT animals (Fig. 1F–H and Fig. 3).
Fig. 1. Axonal projections of POMC and AgRP neurons require GH and leptin signaling.
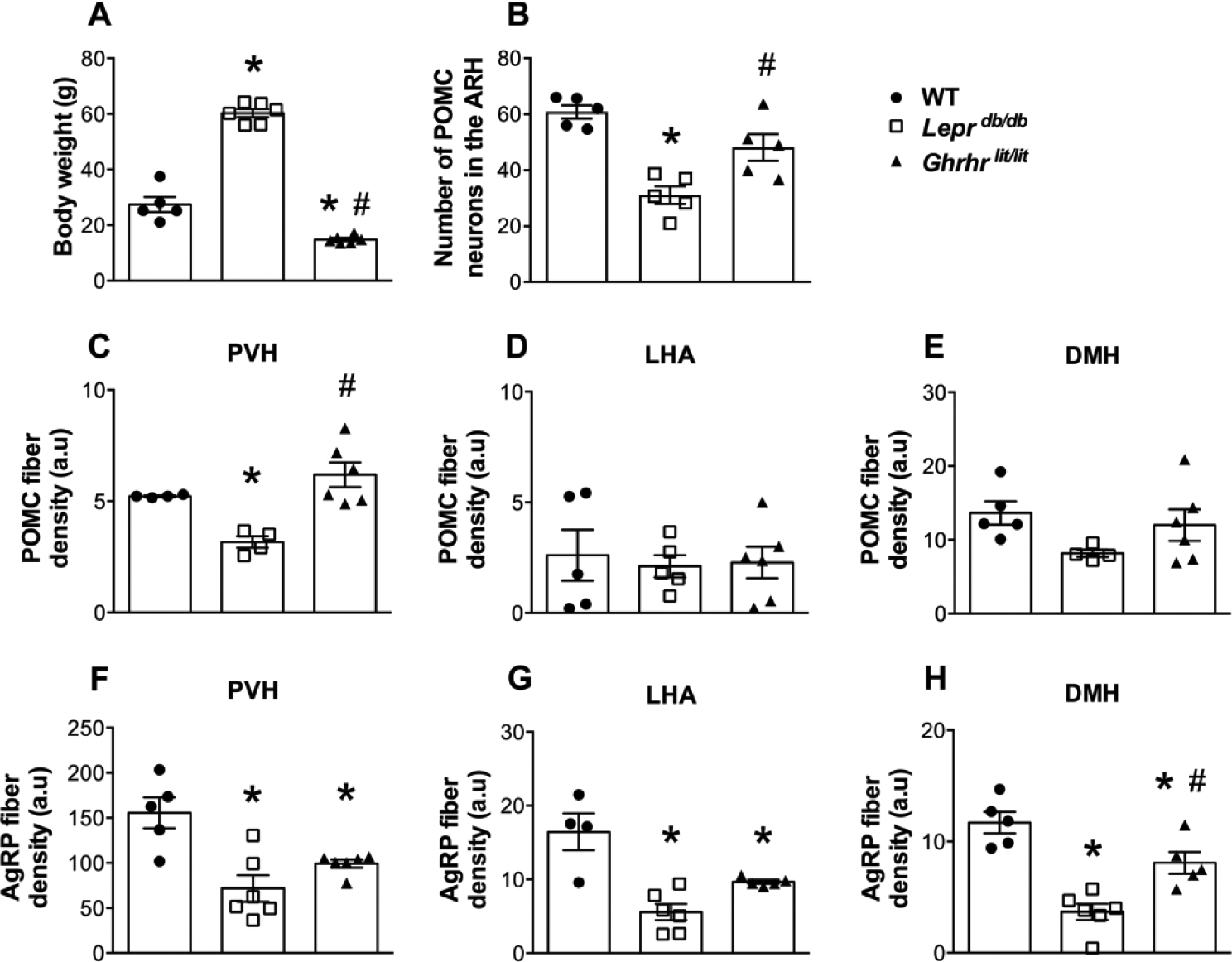
A. Body weight of 4 month-old control (WT, n = 5), Leprdb/db (n = 6) and Ghrhrlit/lit (n = 6) male mice. B. Number of POMC-expressing cells in the ARH of WT (n = 5), Leprdb/db (n = 5) and Ghrhrlit/lit (n = 5) mice. C-E. Quantification of POMC fiber density in the PVH (B), LHA (C) and DMH (D) of WT (n = 4–5), Leprdb/db (n = 4–5) and Ghrhrlit/lit (n = 6) mice. F-H. Quantification of AgRP fiber density in the PVH (E), LHA (F) and DMH (G) of WT (n = 4–5), Leprdb/db (n = 6) and Ghrhrlit/lit (n = 5–6) mice. Mean ± s.e.m. One-way ANOVA followed by Tukey’s post-hoc test. * P < 0.05 vs. WT. # P < 0.05 vs. Leprdb/db.
Fig. 2. Projections of POMC neurons in mice deficient of leptin receptor and GH.
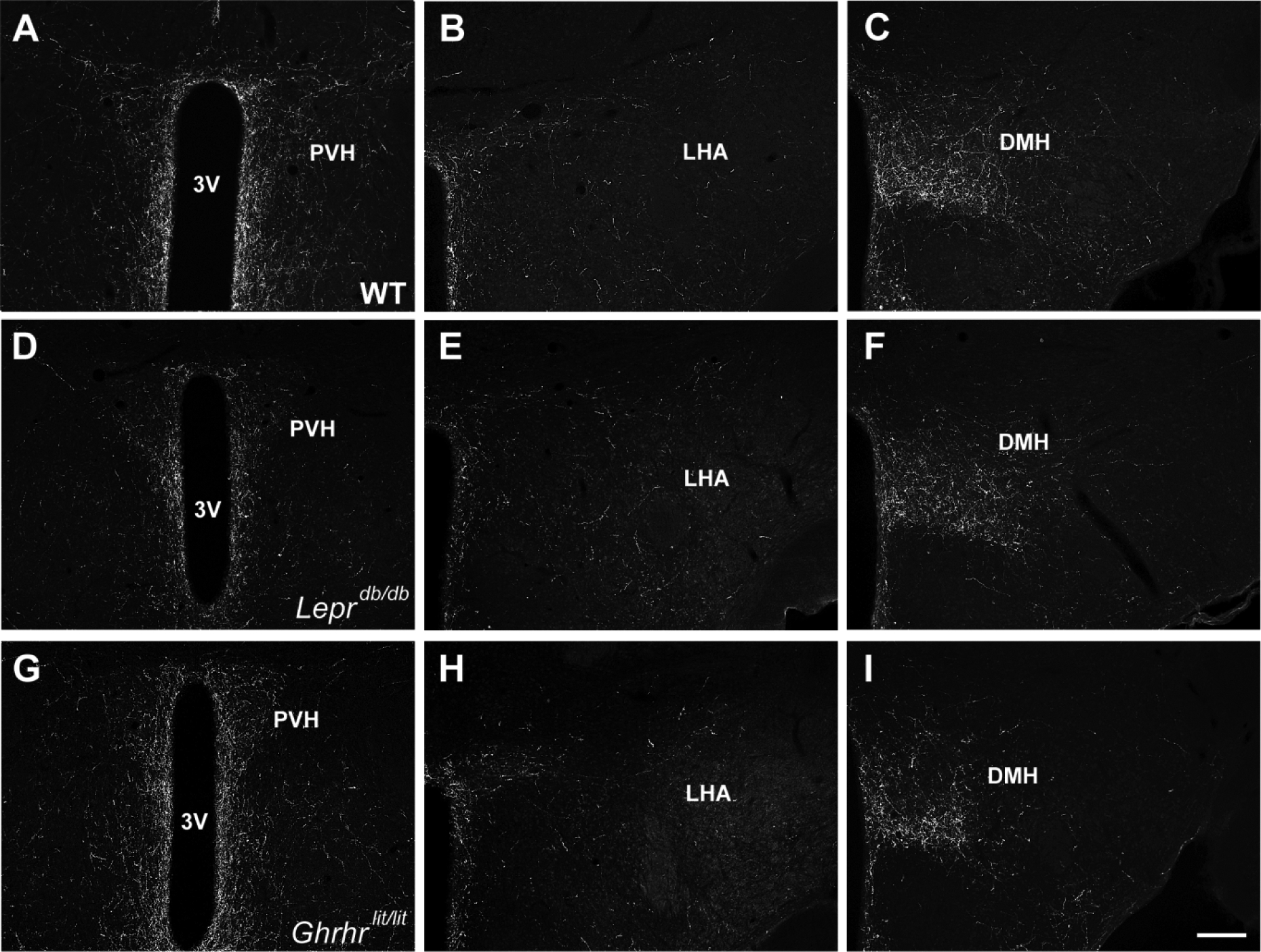
A-C. Representative photomicrographs showing POMC innervation in WT mice. D-F. Representative photomicrographs showing POMC innervation in Leprdb/db mice. G-I. Representative photomicrographs showing POMC innervation in Ghrhrlit/lit mice. Abbreviations: 3V, third ventricle; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus. Scale bar = 200 μm.
Fig. 3. Projections of AgRP neurons in mice deficient of leptin receptor and GH.
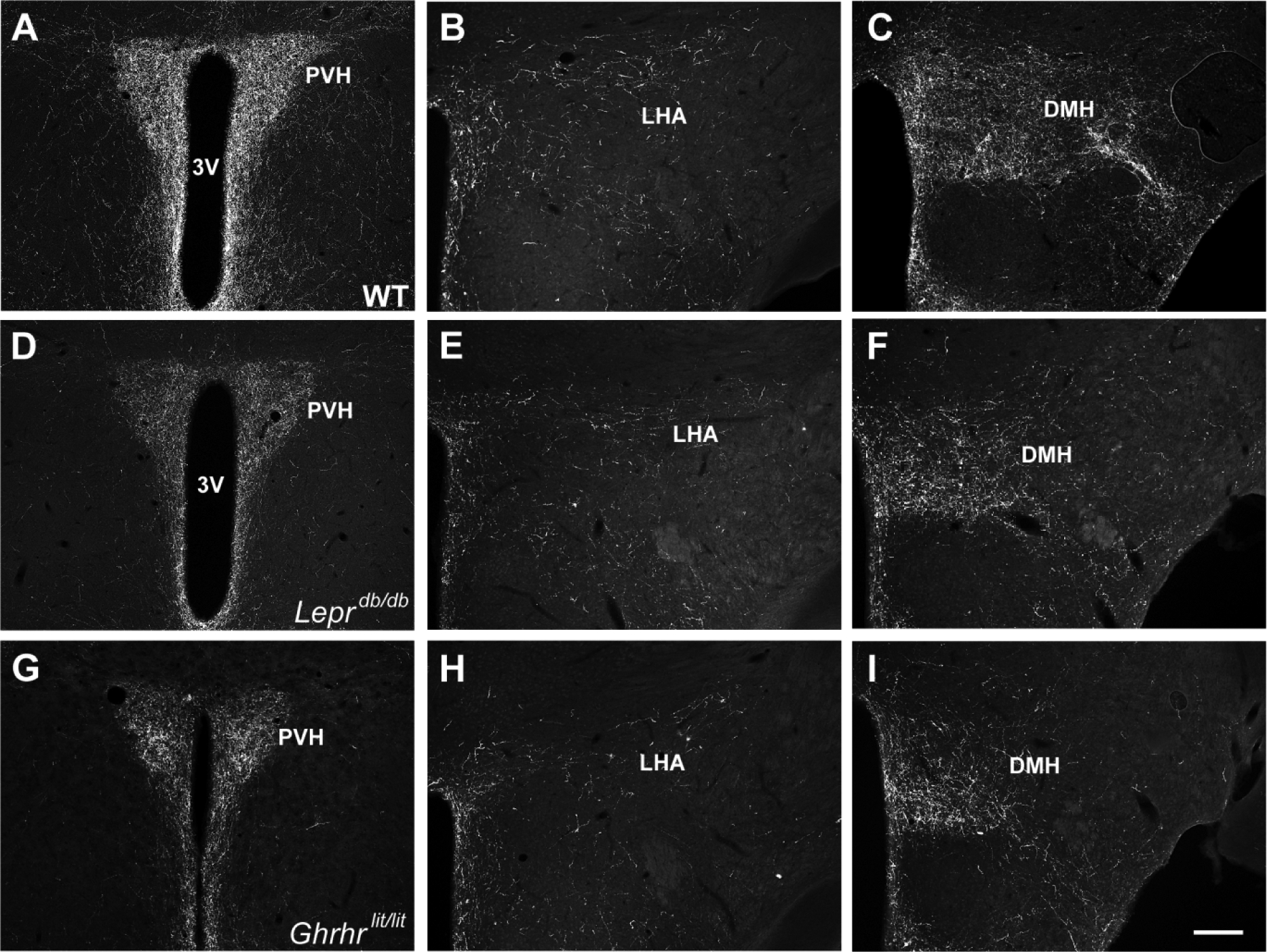
A-C. Representative photomicrographs showing AgRP innervation in WT mice. D-F. Representative photomicrographs showing AgRP innervation in Leprdb/db mice. G-I. Representative photomicrographs showing AgRP innervation in Ghrhrlit/lit mice. Abbreviations: 3V, third ventricle; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus. Scale bar = 200 μm.
Axonal projections of POMC and AgRP neurons are affected by LepR-specific GHR disruption
To determine whether a direct action of GH on ARH neurons is necessary for the formation of POMC and AgRP axonal projections, mice carrying ablation of GHR only in LepR-expressing cells, which include the majority of POMC and AgRP neurons (Lima et al., 2016; Xu et al., 2018), were produced. We determined the expression of pSTAT5 as a marker of GH responsive cells (Furigo et al., 2017; Silveira et al., 2019). PBS-injected mice showed very few pSTAT5 cells in the ARH and PVH and no co-localization with LepR-expressing cells (Fig. 4A–B). In accordance with previous studies (Furigo et al., 2019a; Furigo et al., 2019b; Teixeira et al., 2019), 93.2 ± 0.8% of ARH LepR-expressing neurons co-localized with GH-induced pSTAT5 in control animals (Fig. 4C,F). In contrast, 11.0 ± 2.6% of LepR-expressing cells in the ARH of LepR GHR KO mice remained responsive to GH (Fig. 4D,F), demonstrating the efficacy of our gene deletion. Importantly, other areas that exhibit low LepR expression, such as the PVH, still exhibited a normal distribution of GH responsive cells in LepR GHR KO mice (Fig. 4E). LepR GHR KO mice tended to have more LepR-expressing cells in the ARH compared to control animals (P = 0.0578; Fig. 5A), although the number of POMC neurons was similar (P = 0.1549; Fig. 5B). Additionally, the hypothalamic expression of Mc3r and Mc4r were not affected by GHR ablation in LepR-expressing cells (Fig. 5C). Regarding the innervation of ARH neurons, LepR GHR KO mice exhibited a decreased density of POMC innervation in the PVH and DMH compared to control mice (Fig. 5D,F and Fig. 6A–F), whereas no significant differences were observed in POMC innervation of the LHA (Fig. 5E). A significant decrease in the density of AgRP innervation in the PVH, LHA and DMH was also observed in LepR GHR KO mice (Fig. 5G–I and Fig. 6G–L). Thus, GH signaling specifically in LepR neurons is required for the development of ARH axonal projections.
Fig. 4. LepR-expressing neurons in the arcuate nucleus are responsive to GH.
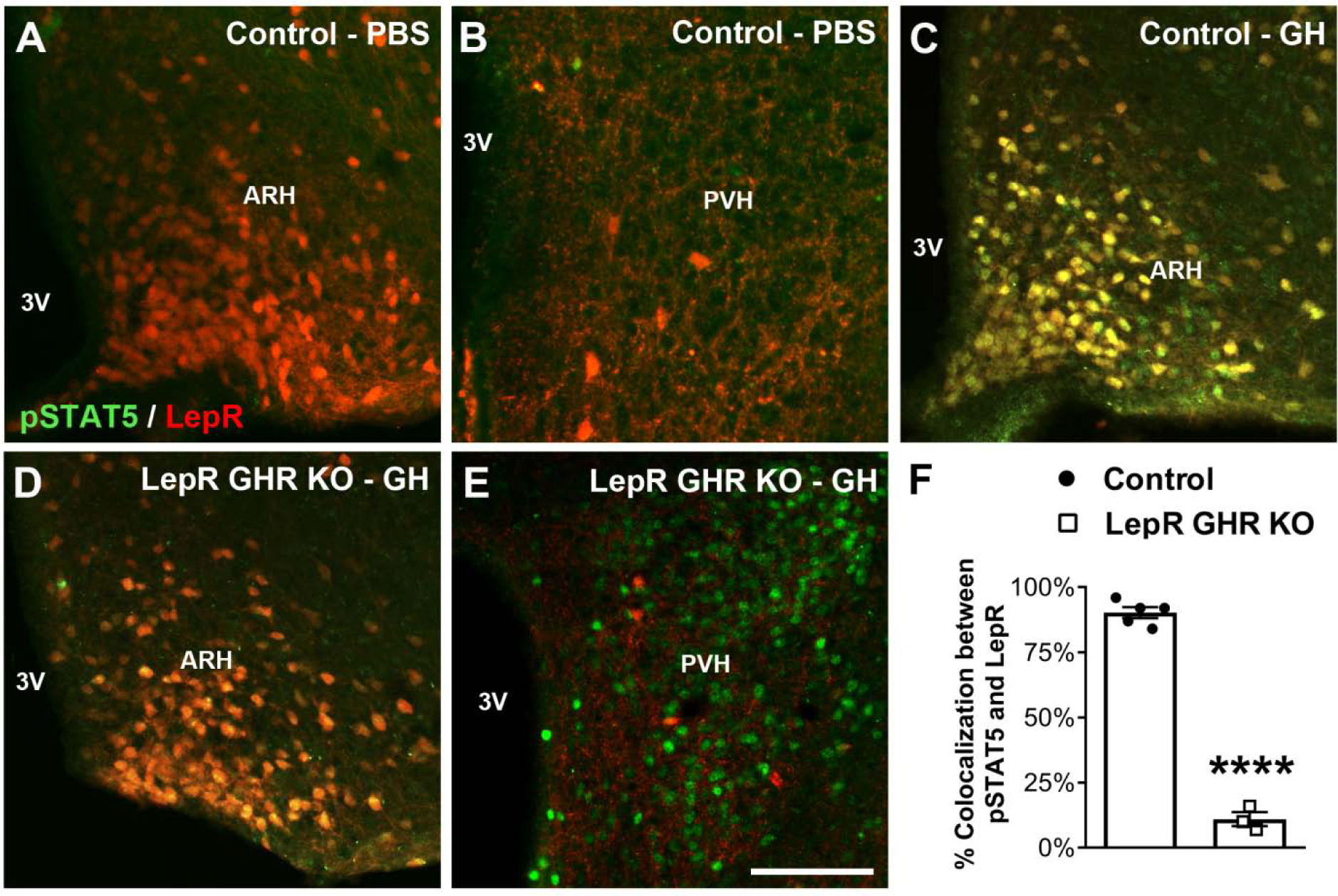
A-E. Epifluorescence photomicrographs showing double-labeling immunofluorescence staining of pSTAT5 (green) and LepR (red tdTomato protein) in the arcuate nucleus (ARH) or paraventricular nucleus of the hypothalamus (PVH) of PBS-injected control mice (A-B), GH-injected control mice (C) and GH-injected LepR GHR KO mice (D-E). Yellow represents double-labeled cells. F. Percentage of colocalization between pSTAT5 and LepR in the ARH of GH-injected control mice (n = 5) and GH-injected LepR GHR KO mice (n = 3). Mean ± s.e.m. Abbreviation: 3V, third ventricle. Scale bar = 100 μm. **** P < 0.0001 vs. LepR GHR KO.
Fig. 5. GHR ablation in LepR-expressing neurons affects the neuronal projections of the arcuate nucleus.
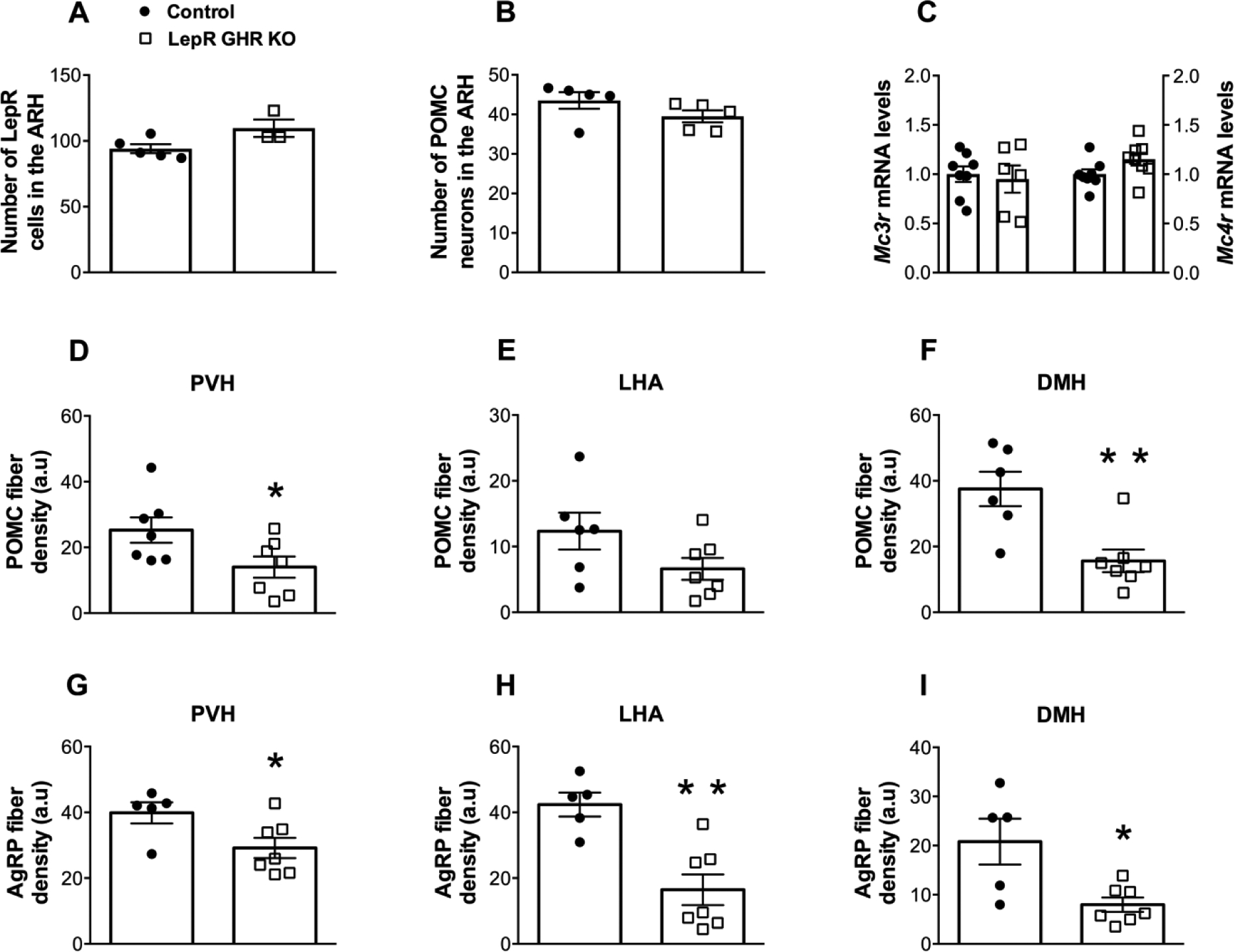
A. Number of LepR-expressing cells in the ARH of control (n = 5) and LepR GHR KO (n = 3) mice. B. Number of POMC neurons in the ARH of control (n = 5) and LepR GHR KO (n = 5) mice. C. Hypothalamic mRNA levels of Mc3r and Mc4r in control (n = 8) and LepR GHR KO (n = 6–8) mice. D-F. Quantification of POMC fiber density in the PVH (A), LHA (B) and DMH (C) of control (n = 6–7) and LepR GHR KO (n = 7) mice. G-I. Quantification of AgRP fiber density in the PVH (D), LHA (E) and DMH (F) of control (n = 5) and LepR GHR KO (n = 7) mice. Mean ± s.e.m. Two-tailed Student’s t-test. * P < 0.05, ** P < 0.01 vs. LepR GHR KO.
Fig. 6. GHR ablation in LepR-expressing neurons affects the projections of POMC and AgRP neurons.
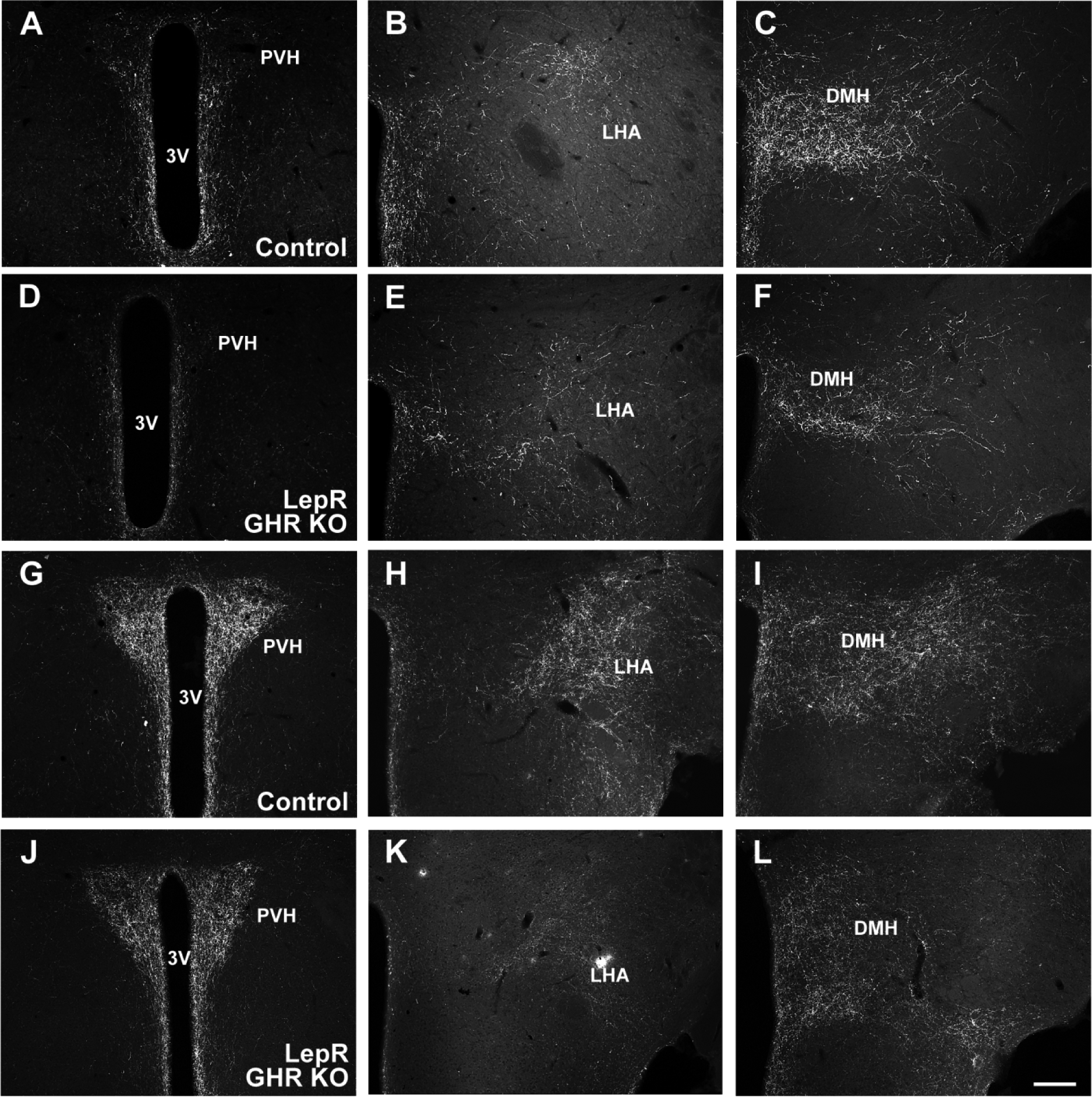
A-C. Representative photomicrographs showing POMC innervation in control mice. D-F. Representative photomicrographs showing POMC innervation in LepR GHR KO mice. G-I. Representative photomicrographs showing AgRP innervation in control mice. J-L. Representative photomicrographs showing AgRP innervation in LepR GHR KO mice. Abbreviations: 3V, third ventricle; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus. Scale bar = 200 μm.
AgRP innervation is impaired in mice carrying AgRP-specific GHR ablation
While PBS-injected control mice showed virtually no pSTAT5 in the ARH or other brain areas (Fig. 7A), we observed that 91.7 ± 0.7% of AgRP neurons in GH-injected control animals contained pSTAT5 (Fig. 7B). As expected, AgRP-specific GHR ablation significantly reduced the expression of pSTAT5 in AgRP neurons (12.3 ± 2.2%), whereas GH responsive cells were still observed in the lateral ARH and surrounding areas (Fig. 7C–D). AgRP GHR KO mice exhibited a similar number of AgRP or POMC neurons compared to control animals (Fig. 8A–B). In addition, AgRP-specific GHR ablation did not cause changes in Mc3r and Mc4r mRNA levels in the hypothalamus (Fig. 8C). The innervation of POMC neurons was initially investigated and AgRP GHR KO mice showed similar density of POMC fibers in the PVH, LHA and DMH, compared to control animals (Fig. 8D–F and Fig. 9A–F). In contrast, the density of AgRP innervation in these brain areas was significantly reduced in AgRP GHR KO mice (Fig. 8G–I and Fig. 9G–L). Thus, GHR ablation produces a cell-specific effect on the development of ARH neuronal projections.
Fig. 7. AgRP-expressing neurons are responsive to GH.
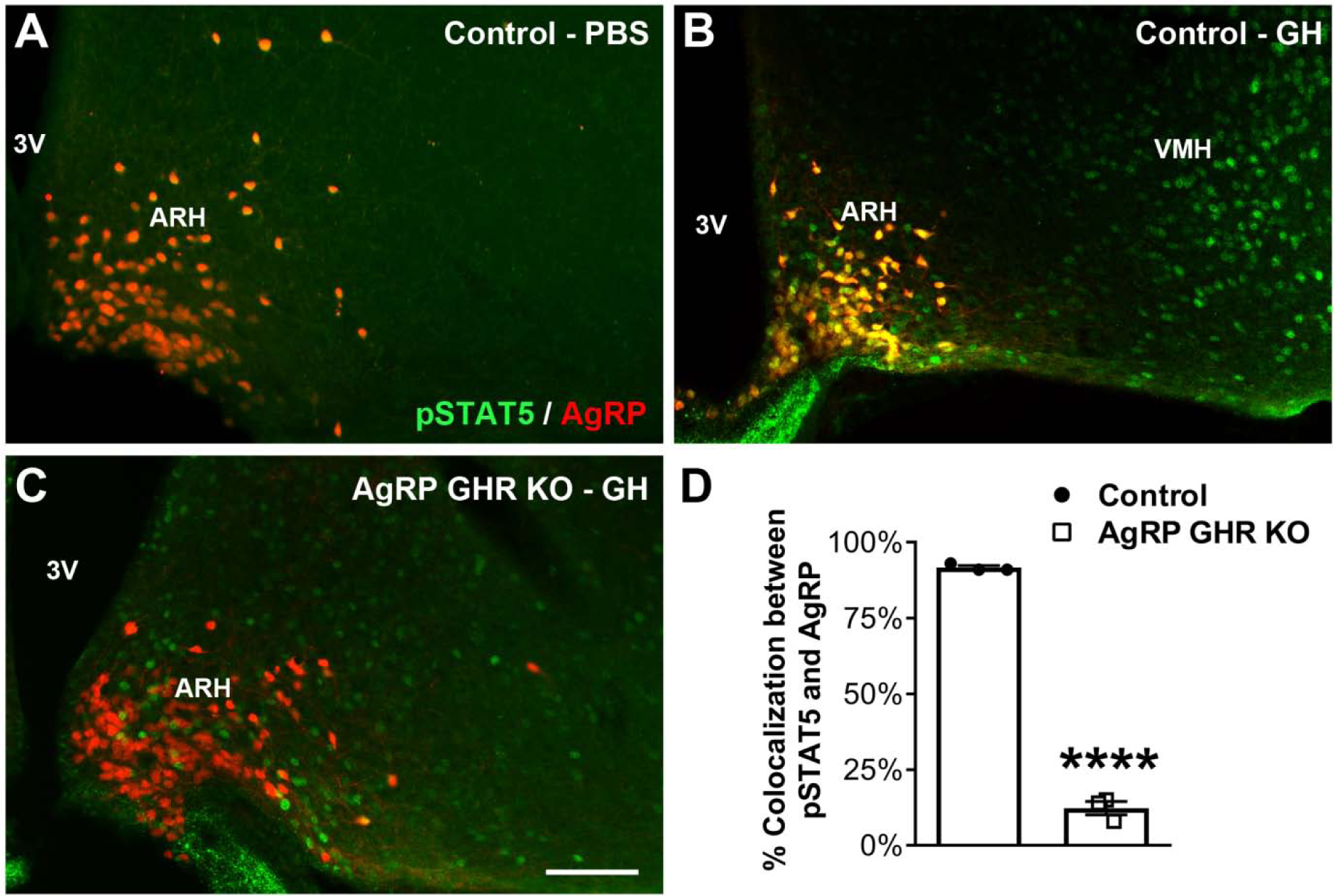
A-C. Epifluorescence photomicrographs showing double-labeling immunofluorescence staining of pSTAT5 (green) and AgRP (red tdTomato protein) in the arcuate nucleus of the hypothalamus (ARH) of PBS-injected control mice (A), GH-injected control mice (B) and GH-injected AgRP GHR KO mice (C). Yellow represents double-labeled cells. D. Percentage of colocalization between pSTAT5 and AgRP in the ARH of GH-injected control mice (n = 3) and GH-injected AgRP GHR KO mice (n = 3). Mean ± s.e.m. Abbreviations: 3V, third ventricle; VMH, ventromedial nucleus of the hypothalamus. Scale bar = 100 μm. **** P < 0.0001 vs. AgRP GHR KO.
Fig. 8. GHR ablation in AgRP-expressing cells reduces the projections of AgRP neurons without affecting POMC projections.
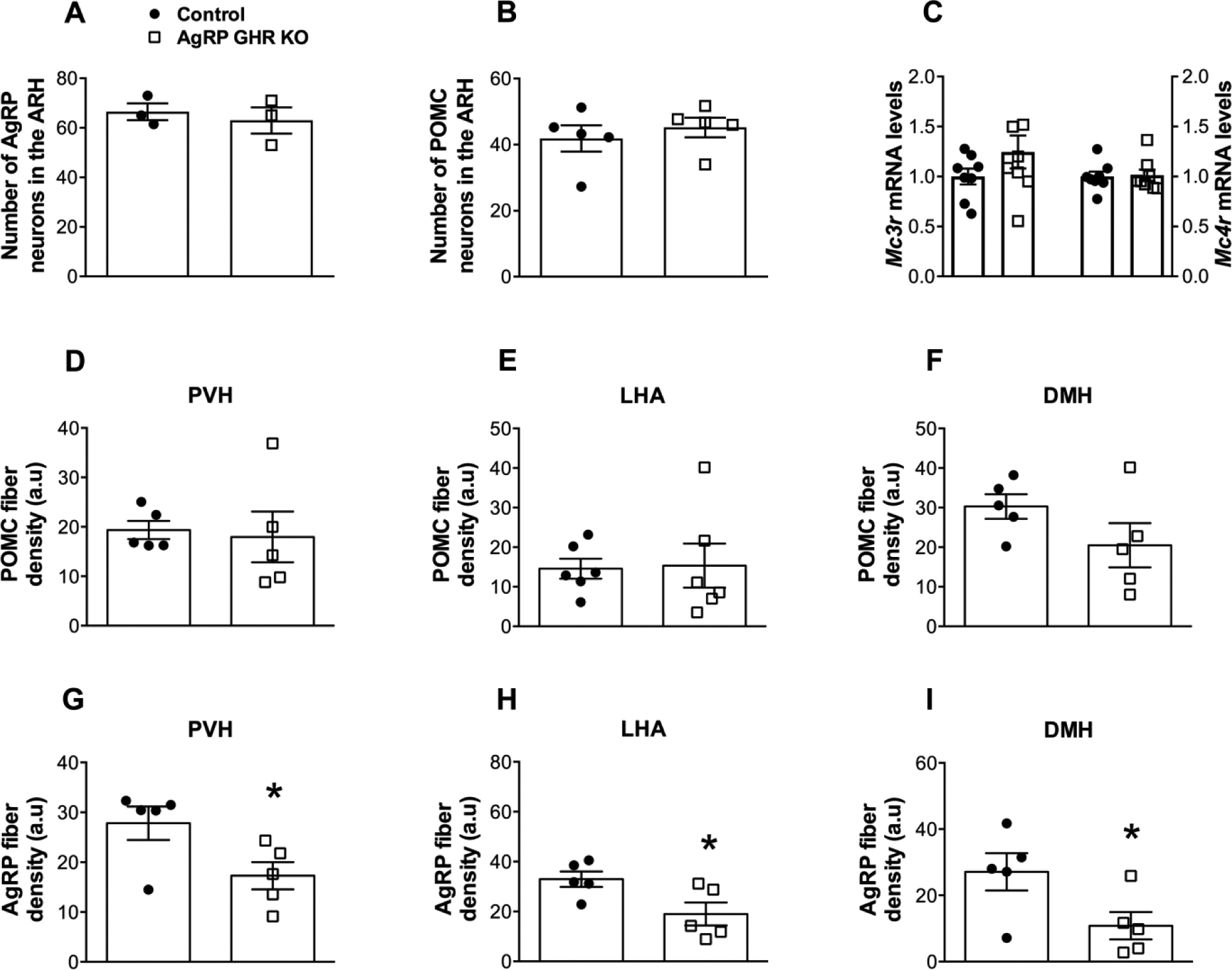
A. Number of AgRP neurons in the ARH of control (n = 3) and AgRP GHR KO (n = 3) mice. B. Number of POMC neurons in the ARH of control (n = 5) and AgRP GHR KO (n = 5) mice. C. Hypothalamic mRNA levels of Mc3r and Mc4r in control (n = 8) and AgRP GHR KO (n = 8) mice. D-F. Quantification of POMC fiber density in the PVH (A), LHA (B) and DMH (C) of control (n = 5–6) and AgRP GHR KO (n = 5–6) mice. G-I. Quantification of AgRP fiber density in the PVH (D), LHA (E) and DMH (F) of control (n = 5) and AgRP GHR KO (n = 5) mice. Mean ± s.e.m. Two-tailed Student’s t-test. * P < 0.05 vs. AgRP GHR KO.
Fig. 9. GHR ablation in AgRP-expressing cells reduces the projections of AgRP neurons.
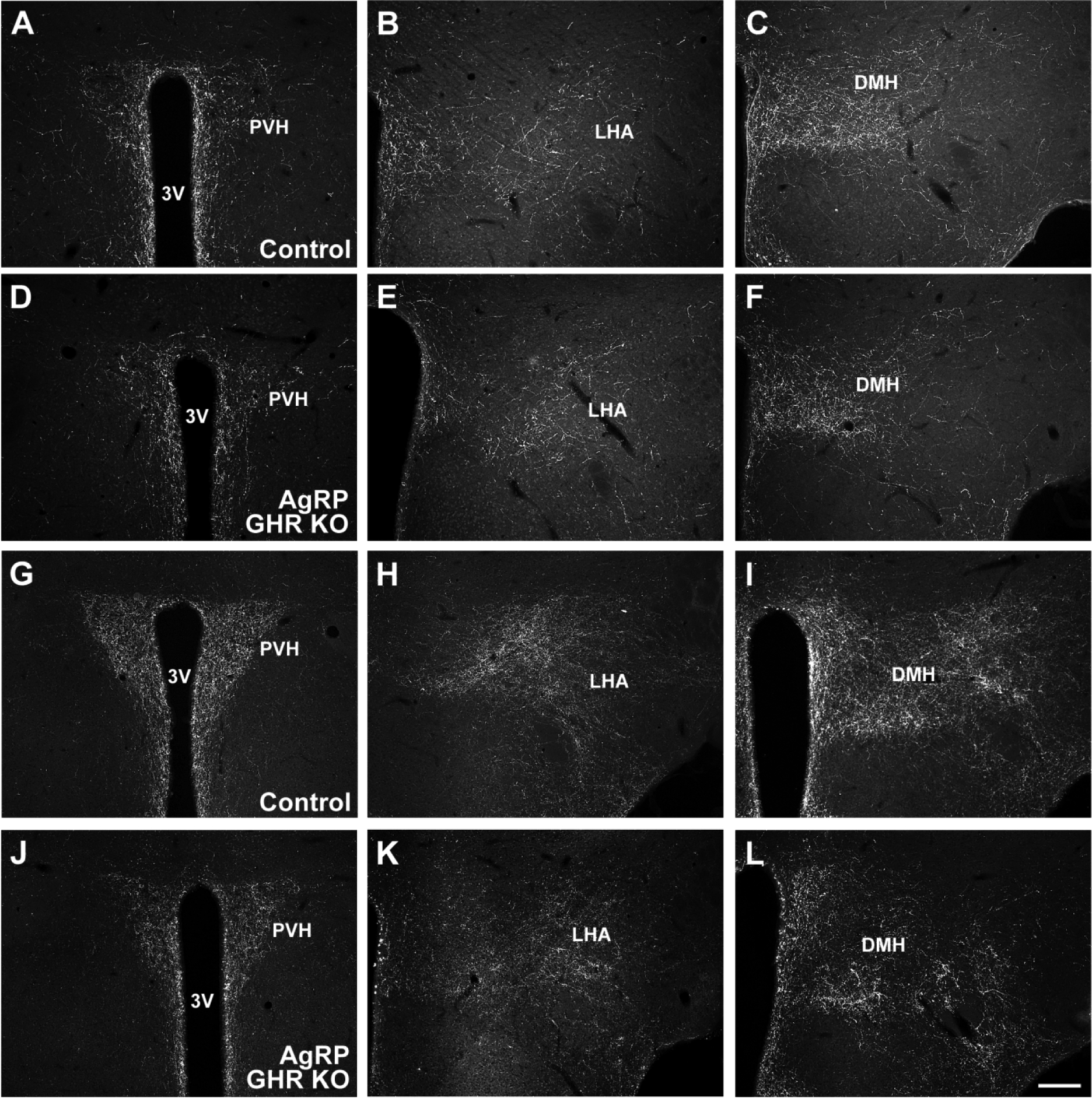
A-C. Representative photomicrographs showing POMC innervation in control mice. D-F. Representative photomicrographs showing POMC innervation in AgRP GHR KO mice. G-I. Representative photomicrographs showing AgRP innervation in control mice. J-L. Representative photomicrographs showing AgRP innervation in AgRP GHR KO mice. Abbreviations: 3V, third ventricle; DMH, dorsomedial nucleus of the hypothalamus; LHA, lateral hypothalamic area; PVH, paraventricular nucleus of the hypothalamus. Scale bar = 200 μm.
DISCUSSION
Although the expression of GHR in the ARH was first reported almost 30 years ago (Walsh et al., 1990; Burton et al., 1992; Lobie et al., 1993; Minami et al., 1993; Bennett et al., 1995; Burton et al., 1995; Chan et al., 1996; Kamegai et al., 1996; Pellegrini et al., 1996), the physiological function of GH signaling on ARH neurons is only beginning to be described. Our previous studies have demonstrated that GH is able to regulate food intake, energy expenditure and glucose homeostasis via ARH neurons (Bohlen et al., 2019; Furigo et al., 2019b; Quaresma et al., 2019; Teixeira et al., 2019). Several distinct neurochemically-defined neuronal populations can be found in the ARH (Campbell et al., 2017). Among the different neural populations located in the ARH, we know that GH responsive cells are composed of AgRP- (Furigo et al., 2019b; Teixeira et al., 2019) and POMC-expressing cells (Quaresma et al., 2019). In contrast, ARH kisspeptin-expressing neurons do not contain GH-induced pSTAT5 (Bohlen et al., 2019; Silveira et al., 2019). It is worth mentioning that the majority of AgRP and POMC neurons are also responsive to leptin (Lima et al., 2016; Xu et al., 2018), confirming the large percentage of GH responsive cells in the ARH that co-localize with LepR (Bohlen et al., 2019; Furigo et al., 2019a; Furigo et al., 2019b; Teixeira et al., 2019). In the present study, we revealed that, in addition to regulating metabolism, GH action on ARH neurons leads to direct trophic effects controlling the formation of axonal projections from POMC and AgRP neurons to important postsynaptic targets, including the PVH, DMH and LHA. In this aspect, GH shares with leptin neurotrophic effects on the neurocircuits that regulate energy homeostasis.
In the present study, the axonal projections of AgRP and POMC neurons were investigated through the detection of immunoreactive fibers using antibodies against these peptides. Although this method has been widely used for this purpose (Bouret et al., 2004b; Bouret et al., 2004a; Bouret et al., 2008; Kirk et al., 2009; Vogt et al., 2014; Sadagurski et al., 2015; Kamitakahara et al., 2018; Ramos-Lobo et al., 2018; Ramos-Lobo et al., 2019), the use of neurotracers could produce a more reliable way to detect and visualize axonal projections. In this regard, we cannot guarantee that the peptide content is uniformly distributed along the axon or if its expression is not different between the experimental groups, regardless of the innervation. However, in a previous study we compared AgRP axonal projections visualized by immunofluorescence staining or tdTomato expression using AgRP-specific reporter mice and the distribution of AgRP fibers in the brain was similar (Lima et al., 2019). In addition, LepR-deficient mice exhibit increased hypothalamic AgRP expression (Ramos-Lobo et al., 2019), even though AgRP innervation is drastically reduced, compared to WT mice, indicating that the method we used is efficient to detect axonal terminals, independently of changes in peptide expression. Thus, although our data may be interpreted with caution, our findings are consistent with the literature and indicate that both leptin (Bouret et al., 2004b; Bouret et al., 2004a; Kamitakahara et al., 2018; Ramos-Lobo et al., 2019) and GH (Sadagurski et al., 2015) are required for the normal formation of POMC and AgRP axonal projections.
Although a reduced density of POMC fibers was observed in the PVH of Leprdb/db mice, we were unable to detect significant changes in POMC innervation in the LHA and DMH of Leprdb/db or Ghrhrlit/lit mice. In contrast, AgRP innervation was reduced in all areas analyzed in both Leprdb/db and Ghrhrlit/lit mouse models. Rather than a specific effect of leptin or GH signaling on AgRP neurons, we believe that the lower amount of POMC fibers, in comparison with the density of AgRP innervation, reduced the sensitivity of our IOD quantification. Thus, the analysis of AgRP fibers seems to be more accurate than the quantification of POMC fibers. Nevertheless, a reduced density of POMC innervation in the PVH and DMH was detected in LepR GHR KO mice.
After comparing the density of axonal projections of ARH neurons between Leprdb/db and Ghrhrlit/lit mice, our findings indicate that the absence of leptin signaling caused a greater reduction in fiber density compared to GH-deficient animals, although statistically significant changes were only observed in POMC innervation in the PVH and AgRP innervation in the DMH. Although these findings may indicate that leptin has a dominant neurotrophic role, compared to GH, Ghrhrlit/lit mice are not entirely deficient of GH (Godfrey et al., 1993; Cecchi et al., 2014). On the other hand, the long form of LepR is completely non-functional in Leprdb/db mouse, which makes this animal irresponsive to leptin (Chen et al., 1996). Thus, the comparison in the density of POMC and AgRP innervation between these models does not allow us to determine whether the neurotrophic action of one hormone prevails over the other.
The first study describing that GH possesses trophic effects on ARH neurons investigated these projections in two models of GH-deficient dwarf mice and in whole-body GHR KO dwarf mice (Sadagurski et al., 2015). In this study, the authors showed that early-life GH treatment rescues POMC and AgRP projections in GH-deficient mice. Furthermore, they also demonstrated that liver-specific GHR KO mice show normal POMC and AgRP innervation (Sadagurski et al., 2015), despite a drastic reduction in serum IGF-1 levels (List et al., 2014). Thus, these previous studies suggest that GH rather than IGF-1 is required for the formation of POMC and AgRP projections. In the present study, we provided further evidence of a direct neurotrophic effect of GH on ARH neurons. Although LepR GHR KO mice have normal body growth and no gross hormonal dysfunctions (Furigo et al., 2019a; Furigo et al., 2019b; Teixeira et al., 2019), ablation of GHR from LepR-expressing cells decreased both POMC and AgRP innervation to major hypothalamic projection areas. Importantly, GHR ablation exclusively from AgRP-expressing cells led to a reduction in AgRP innervation without affecting the projections of POMC neurons. These findings indicate that only cells affected by the conditional GHR deletion exhibit defects in the development of ARH neuronal projections.
We described in a recent study the consequences of inactivating GHR in POMC cells (Quaresma et al., 2019). The density of POMC and AgRP innervation was also determined, but the lack of GH action on POMC cells caused no apparent changes in the projections of ARH neurons (Quaresma et al., 2019). Several possibilities can explain this contrasting result compared to our findings. First, approximately 60% of POMC neurons are responsive to GH (Quaresma et al., 2019), compared to 90 to 95% of AgRP neurons (Furigo et al., 2019b). Thus, only a subset of POMC neurons is affected by GHR ablation. Second, as previously mentioned, the reduced density of POMC innervation, compared to AgRP fibers, decreases the sensitivity to detect slight changes. Third, a small number of POMC-expressing neurons are also found in the nucleus of the solitary tract (NTS), contributing to the POMC innervation observed in the brain (Huo et al., 2006). However, differently than ARH neurons, POMC neurons in the NTS are not responsive to GH (Quaresma et al., 2019). Additionally, NTS POMC neurons send axons predominantly to the brainstem, although they also innervate the hypothalamus and particularly the PVH (Wang et al., 2015). Finally, POMC neurons may present a distinct plasticity capacity to compensate the absence of the trophic effects of GH.
Some additional points deserve to be discussed. In the present study, we studied exclusively male mice, so we cannot assure that the present findings are also observed in females. Of note, males and females exhibit important differences in GH secretion (Jansson et al., 1985; Maiter et al., 1991; Painson and Tannenbaum, 1991; Chowen et al., 1996; van den Berg et al., 1996; Jaffe et al., 1998). However, male and female mice present similar responsiveness to GH in ARH LepR-expressing cells or AgRP neurons (Bohlen et al., 2019; Furigo et al., 2019b; Teixeira et al., 2019; Furigo et al., 2020). Thus, it is likely that both genders may exhibit similar neurotrophic actions of GH, despite sexually dimorphic changes in the pattern of GH secretion. Additionally, AgRP neurons co-express other neurotransmitters, in special neuropeptide Y (NPY) (Hahn et al., 1998; Cowley et al., 1999). In this sense, NPY transmission is also important for the metabolic effects of ARH AgRP/NPY neurons (Erickson et al., 1996; Hahn et al., 1998). We decided to investigate AgRP rather than NPY innervation because NPY is expressed in numerous brain areas (Morris, 1989), whereas AgRP neurons are exclusively found in the ARH (Hahn et al., 1998). Consequently, while AgRP staining represents a reliable marker of ARH AgRP/NPY-expressing cells, NPY immunoreactive fibers may originate from several neural populations. Nevertheless, defects in the development of AgRP fibers can also lead to changes in NPY neurotransmission of ARH neurons with all associated physiological repercussions.
Until now, several studies described situations in which the innervation of POMC or AgRP neurons to postsynaptic targets is reduced. In this regard, these projections are impaired by the lack of leptin signaling (Bouret et al., 2004a; Bouret et al., 2008; Kamitakahara et al., 2018; Ramos-Lobo et al., 2019) or GH action (Sadagurski et al., 2015), by maternal obesity (Bouret et al., 2008; Kirk et al., 2009; Vogt et al., 2014) and by alterations in gestational metabolic adaptations (Ramos-Lobo et al., 2018). Although defects in these projections may indicate a reduced capacity of ARH neurons to influence the neurocircuits that control energy and glucose homeostasis, which presumably leads to metabolic imbalances, no study to our knowledge demonstrated that the reduced innervation of ARH neurons is the primary cause of these dysfunctions. Regarding GH action on ARH neurons, both AgRP- and POMC-specific GHR KO mice exhibit normal body weight, energy expenditure and food intake in ad libitum fed conditions or during refeeding (Furigo et al., 2019b; Quaresma et al., 2019). Therefore, it is essential that future studies investigate the importance of changes in the innervation of POMC or AgRP neurons for the central regulation of metabolism. Nevertheless, our findings clearly demonstrate that ARH neurons are directly affected by GH action. Thus, GH should be included in the set of hormones that modify neurocircuits that control feeding, body weight and glucose homeostasis.
HIGHLIGHTS.
Formation of POMC and AgRP projections requires GH and leptin signaling.
POMC and AgRP projections are affected by LepR-specific GH receptor disruption.
AgRP innervation is impaired in mice carrying AgRP-specific GH receptor ablation.
ACKNOWLEDGEMENTS
We thank Ana M.P. Campos for the technical assistance. This work was supported by Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP-Brazil; grant numbers: 14/11752-6 to A.M.R.L.; 16/20897-3 to F.W., 16/09679-4 to I.F. and 17/02983-2 to J.D.) and NIH (NIA; grant number: R01AG059779 to E.O.L. and J.J.K.).
Abbreviations:
- 3
third ventricle
- AgRP
agouti-related peptide
- ARH
arcuate nucleus of the hypothalamus
- DMH
dorsomedial nucleus of the hypothalamus
- GH
growth hormone
- GHR
growth hormone receptor
- IGF-1
insulin-like growth factor-1
- IOD
integrated optical density
- KO
knockout
- LepR
leptin receptor
- LHA
lateral hypothalamic area
- MC3R
melanocortin receptor 3
- MC4R
melanocortin receptor 4
- NPY
neuropeptide Y
- NTS
nucleus of the solitary tract
- POMC
proopiomelanocortin
- PVH
paraventricular nucleus of the hypothalamus
- STAT5
signal transducer and activator of transcription-5
- WT
wild-type
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
COMPETING INTERESTS
The authors declare no conflicts of interest.
REFERENCES
- Andermann ML, Lowell BB (2017) Toward a Wiring Diagram Understanding of Appetite Control. Neuron 95:757–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aponte Y, Atasoy D, Sternson SM (2011) AGRP neurons are sufficient to orchestrate feeding behavior rapidly and without training. Nat Neurosci 14:351–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basu R, Qian Y, Kopchick JJ (2018) MECHANISMS IN ENDOCRINOLOGY: Lessons from growth hormone receptor gene-disrupted mice: are there benefits of endocrine defects? Eur J Endocrinol 178:R155–R181. [DOI] [PubMed] [Google Scholar]
- Bennett PA, Levy A, Sophokleous S, Robinson IC, Lightman SL (1995) Hypothalamic GH receptor gene expression in the rat: effects of altered GH status. J Endocrinol 147:225–234. [DOI] [PubMed] [Google Scholar]
- Bohlen TM, Zampieri TT, Furigo IC, Teixeira PD, List EO, Kopchick J, Donato J Jr., Frazao R (2019) Central growth hormone signaling is not required for the timing of puberty. J Endocrinol 243:161–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB (2004a) Formation of projection pathways from the arcuate nucleus of the hypothalamus to hypothalamic regions implicated in the neural control of feeding behavior in mice. J Neurosci 24:2797–2805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bouret SG, Draper SJ, Simerly RB (2004b) Trophic Action of Leptin on Hypothalamic Neurons That Regulate Feeding. Science 304:108–110. [DOI] [PubMed] [Google Scholar]
- Bouret SG, Gorski JN, Patterson CM, Chen S, Levin BE, Simerly RB (2008) Hypothalamic neural projections are permanently disrupted in diet-induced obese rats. Cell Metab 7:179–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KA, Kabigting EB, Clifton DK, Steiner RA (1992) Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology 131:958–963. [DOI] [PubMed] [Google Scholar]
- Burton KA, Kabigting EB, Steiner RA, Clifton DK (1995) Identification of target cells for growth hormone’s action in the arcuate nucleus. Am J Physiol 269:E716–722. [DOI] [PubMed] [Google Scholar]
- Campbell JN, Macosko EZ, Fenselau H, Pers TH, Lyubetskaya A, Tenen D, Goldman M, Verstegen AM, Resch JM, McCarroll SA, Rosen ED, Lowell BB, Tsai LT (2017) A molecular census of arcuate hypothalamus and median eminence cell types. Nat Neurosci 20:484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cecchi CR, Higuti E, Oliveira NA, Lima ER, Jakobsen M, Dagnaes-Hansen F, Gissel H, Aagaard L, Jensen TG, Jorge AA, Bartolini P, Peroni CN (2014) A novel homologous model for gene therapy of dwarfism by non-viral transfer of the mouse growth hormone gene into immunocompetent dwarf mice. Curr Gene Ther 14:44–51. [DOI] [PubMed] [Google Scholar]
- Chan Y, Steiner R, Clifton D (1996) Regulation of hypothalamic neuropeptide-Y neurons by growth hormone in the rat. Endocrinology 137:1319–1325. [DOI] [PubMed] [Google Scholar]
- Chen H, Charlat O, Tartaglia LA, Woolf EA, Weng X, Ellis SJ, Lakey ND, Culpepper J, Moore KJ, Breitbart RE, Duyk GM, Tepper RI, Morgenstern JP (1996) Evidence that the diabetes gene encodes the leptin receptor: Identification of a mutation in the leptin receptor gene in db/db mice. Cell 84:491–495. [DOI] [PubMed] [Google Scholar]
- Chowen JA, Garcia-Segura LM, Gonzalez-Parra S, Argente J (1996) Sex steroid effects on the development and functioning of the growth hormone axis. Cell Mol Neurobiol 16:297–310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowley MA, Pronchuk N, Fan W, Dinulescu DM, Colmers WF, Cone RD (1999) Integration of NPY, AGRP, and melanocortin signals in the hypothalamic paraventricular nucleus: evidence of a cellular basis for the adipostat. Neuron 24:155–163. [DOI] [PubMed] [Google Scholar]
- Erickson JC, Hollopeter G, Palmiter RD (1996) Attenuation of the obesity syndrome of ob/ob mice by the loss of neuropeptide Y. Science 274:1704–1707. [DOI] [PubMed] [Google Scholar]
- Fan W, Boston BA, Kesterson RA, Hruby VJ, Cone RD (1997) Role of melanocortinergic neurons in feeding and the agouti obesity syndrome. Nature 385:165–168. [DOI] [PubMed] [Google Scholar]
- Furigo IC, de Souza GO, Teixeira PDS, Guadagnini D, Frazao R, List EO, Kopchick JJ, Prada PO, Donato J Jr. (2019a) Growth hormone enhances the recovery of hypoglycemia via ventromedial hypothalamic neurons. FASEB J 33:11909–11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furigo IC, Metzger M, Teixeira PD, Soares CR, Donato J Jr. (2017) Distribution of growth hormone-responsive cells in the mouse brain. Brain Struct Funct 222:341–363. [DOI] [PubMed] [Google Scholar]
- Furigo IC, Teixeira PD, Quaresma PGF, Mansano NS, Frazao R, Donato J (2020) STAT5 ablation in AgRP neurons increases female adiposity and blunts food restriction adaptations. J Mol Endocrinol 64:13–27. [DOI] [PubMed] [Google Scholar]
- Furigo IC, Teixeira PDS, de Souza GO, Couto GCL, Romero GG, Perello M, Frazao R, Elias LL, Metzger M, List EO, Kopchick JJ, Donato J Jr. (2019b) Growth hormone regulates neuroendocrine responses to weight loss via AgRP neurons. Nat Commun 10:662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Godfrey P, Rahal JO, Beamer WG, Copeland NG, Jenkins NA, Mayo KE (1993) GHRH receptor of little mice contains a missense mutation in the extracellular domain that disrupts receptor function. Nat Genet 4:227–232. [DOI] [PubMed] [Google Scholar]
- Hahn TM, Breininger JF, Baskin DG, Schwartz MW (1998) Coexpression of Agrp and NPY in fasting-activated hypothalamic neurons. Nat Neurosci 1:271–272. [DOI] [PubMed] [Google Scholar]
- He Z, Gao Y, Lieu L, Afrin S, Cao J, Michael NJ, Dong Y, Sun J, Guo H, Williams KW (2019) Direct and indirect effects of liraglutide on hypothalamic POMC and NPY/AgRP neurons - Implications for energy balance and glucose control. Mol Metab 28:120–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huo L, Grill HJ, Bjorbaek C (2006) Divergent regulation of proopiomelanocortin neurons by leptin in the nucleus of the solitary tract and in the arcuate hypothalamic nucleus. Diabetes 55:567–573. [DOI] [PubMed] [Google Scholar]
- Huszar D, Lynch CA, Fairchild-Huntress V, Dunmore JH, Fang Q, Berkemeier LR, Gu W, Kesterson RA, Boston BA, Cone RD, Smith FJ, Campfield LA, Burn P, Lee F (1997) Targeted disruption of the melanocortin-4 receptor results in obesity in mice. Cell 88:131–141. [DOI] [PubMed] [Google Scholar]
- Jaffe CA, Ocampo-Lim B, Guo W, Krueger K, Sugahara I, DeMott-Friberg R, Bermann M, Barkan AL (1998) Regulatory mechanisms of growth hormone secretion are sexually dimorphic. J Clin Invest 102:153–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansson JO, Eden S, Isaksson O (1985) Sexual dimorphism in the control of growth hormone secretion. Endocr Rev 6:128–150. [DOI] [PubMed] [Google Scholar]
- Kamegai J, Minami S, Sugihara H, Hasegawa O, Higuchi H, Wakabayashi I (1996) Growth hormone receptor gene is expressed in neuropeptide Y neurons in hypothalamic arcuate nucleus of rats. Endocrinology 137:2109–2112. [DOI] [PubMed] [Google Scholar]
- Kamitakahara A, Bouyer K, Wang CH, Simerly R (2018) A critical period for the trophic actions of leptin on AgRP neurons in the arcuate nucleus of the hypothalamus. J Comp Neurol 526:133–145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirk SL, Samuelsson AM, Argenton M, Dhonye H, Kalamatianos T, Poston L, Taylor PD, Coen CW (2009) Maternal obesity induced by diet in rats permanently influences central processes regulating food intake in offspring. PLoS One 4:e5870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krashes MJ, Koda S, Ye C, Rogan SC, Adams AC, Cusher DS, Maratos-Flier E, Roth BL, Lowell BB (2011) Rapid, reversible activation of AgRP neurons drives feeding behavior in mice. J Clin Invest 121:1424–1428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lima LB, Metzger M, Furigo IC, Donato J Jr. (2016) Leptin receptor-positive and leptin receptor-negative proopiomelanocortin neurons innervate an identical set of brain structures. Brain Res 1646:366–376. [DOI] [PubMed] [Google Scholar]
- Lima LB, Pedroso JAB, Metzger M, Gautron L, Donato J Jr. (2019) Relationship of alpha-MSH and AgRP axons to the perikarya of melanocortin-4 receptor neurons. Brain Res 1717:136–146. [DOI] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Gosney ES, Jara A, Kelder B, Wang X, Kutz L, Troike K, Lozier N, Mikula V, Lubbers ER, Zhang H, Vesel C, Junnila RK, Frank SJ, Masternak MM, Bartke A, Kopchick JJ (2013) The role of GH in adipose tissue: lessons from adipose-specific GH receptor gene-disrupted mice. Mol Endocrinol 27:524–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- List EO, Berryman DE, Funk K, Jara A, Kelder B, Wang F, Stout MB, Zhi X, Sun L, White TA, LeBrasseur NK, Pirtskhalava T, Tchkonia T, Jensen EA, Zhang W, Masternak MM, Kirkland JL, Miller RA, Bartke A, Kopchick JJ (2014) Liver-specific GH receptor gene-disrupted (LiGHRKO) mice have decreased endocrine IGF-I, increased local IGF-I, and altered body size, body composition, and adipokine profiles. Endocrinology 155:1793–1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livneh Y, Ramesh RN, Burgess CR, Levandowski KM, Madara JC, Fenselau H, Goldey GJ, Diaz VE, Jikomes N, Resch JM, Lowell BB, Andermann ML (2017) Homeostatic circuits selectively gate food cue responses in insular cortex. Nature 546:611–616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobie PE, Garcia-Aragon J, Lincoln DT, Barnard R, Wilcox JN, Waters MJ (1993) Localization and ontogeny of growth hormone receptor gene expression in the central nervous system. Brain Res Dev Brain Res 74:225–233. [DOI] [PubMed] [Google Scholar]
- Loh K, Zhang L, Brandon A, Wang Q, Begg D, Qi Y, Fu M, Kulkarni R, Teo J, Baldock P, Bruning JC, Cooney G, Neely GG, Herzog H (2017) Insulin controls food intake and energy balance via NPY neurons. Mol Metab 6:574–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo SX, Huang J, Li Q, Mohammad H, Lee CY, Krishna K, Kok AM, Tan YL, Lim JY, Li H, Yeow LY, Sun J, He M, Grandjean J, Sajikumar S, Han W, Fu Y (2018) Regulation of feeding by somatostatin neurons in the tuberal nucleus. Science 361:76–81. [DOI] [PubMed] [Google Scholar]
- Luquet S, Perez FA, Hnasko TS, Palmiter RD (2005) NPY/AgRP neurons are essential for feeding in adult mice but can be ablated in neonates. Science 310:683–685. [DOI] [PubMed] [Google Scholar]
- Maiter D, Koenig JI, Kaplan LM (1991) Sexually dimorphic expression of the growth hormone-releasing hormone gene is not mediated by circulating gonadal hormones in the adult rat. Endocrinology 128:1709–1716. [DOI] [PubMed] [Google Scholar]
- Minami S, Kamegai J, Hasegawa O, Sugihara H, Okada K, Wakabayashi I (1993) Expression of growth hormone receptor gene in rat hypothalamus. J Neuroendocrinol 5:691–696. [DOI] [PubMed] [Google Scholar]
- Morris BJ (1989) Neuronal localisation of neuropeptide Y gene expression in rat brain. J Comp Neurol 290:358–368. [DOI] [PubMed] [Google Scholar]
- Ollmann MM, Wilson BD, Yang YK, Kerns JA, Chen Y, Gantz I, Barsh GS (1997) Antagonism of central melanocortin receptors in vitro and in vivo by agouti-related protein. Science 278:135–138. [DOI] [PubMed] [Google Scholar]
- Painson JC, Tannenbaum GS (1991) Sexual dimorphism of somatostatin and growth hormone-releasing factor signaling in the control of pulsatile growth hormone secretion in the rat. Endocrinology 128:2858–2866. [DOI] [PubMed] [Google Scholar]
- Pellegrini E, Bluet-Pajot MT, Mounier F, Bennett P, Kordon C, Epelbaum J (1996) Central administration of a growth hormone (GH) receptor mRNA antisense increases GH pulsatility and decreases hypothalamic somatostatin expression in rats. J Neurosci 16:8140–8148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinto S, Roseberry AG, Liu H, Diano S, Shanabrough M, Cai X, Friedman JM, Horvath TL (2004) Rapid rewiring of arcuate nucleus feeding circuits by leptin. Science 304:110–115. [DOI] [PubMed] [Google Scholar]
- Quaresma PGF, Teixeira PDS, Furigo IC, Wasinski F, Couto GC, Frazao R, List EO, Kopchick JJ, Donato J Jr. (2019) Growth hormone/STAT5 signaling in proopiomelanocortin neurons regulates glucoprivic hyperphagia. Mol Cell Endocrinol 498:110574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lobo AM, Donato J Jr. (2017) The role of leptin in health and disease. Temperature 4:258–291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lobo AM, Furigo IC, Teixeira PDS, Zampieri TT, Wasinski F, Buonfiglio DC, Donato J Jr. (2018) Maternal metabolic adaptations are necessary for normal offspring growth and brain development. Physiol Rep 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramos-Lobo AM, Teixeira PD, Furigo IC, Melo HM, de Lyra E. Silva NM, De Felice FG, Donato J Jr. (2019) Long-term consequences of the absence of leptin signaling in early life. eLife 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sadagurski M, Landeryou T, Cady G, Kopchick JJ, List EO, Berryman DE, Bartke A, Miller RA (2015) Growth hormone modulates hypothalamic inflammation in long-lived pituitary dwarf mice. Aging cell 14:1045–1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz MW, Seeley RJ, Woods SC, Weigle DS, Campfield LA, Burn P, Baskin DG (1997) Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 46:2119–2123. [DOI] [PubMed] [Google Scholar]
- Silveira MA, Zampieri TT, Furigo IC, Abdulkader F, Donato J Jr., Frazao R (2019) Acute effects of somatomammotropin hormones on neuronal components of the hypothalamic-pituitary-gonadal axis. Brain Res 1714:210–217. [DOI] [PubMed] [Google Scholar]
- Teixeira PDS, Couto GC, Furigo IC, List EO, Kopchick JJ, Donato J Jr. (2019) Central growth hormone action regulates metabolism during pregnancy. Am J Physiol Endocrinol Metab 317:E925–E940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornton JE, Cheung CC, Clifton DK, Steiner RA (1997) Regulation of hypothalamic proopiomelanocortin mRNA by leptin in ob/ob mice. Endocrinology 138:5063–5066. [DOI] [PubMed] [Google Scholar]
- van den Berg G, Veldhuis JD, Frolich M, Roelfsema F (1996) An amplitude-specific divergence in the pulsatile mode of growth hormone (GH) secretion underlies the gender difference in mean GH concentrations in men and premenopausal women. J Clin Endocrinol Metab 81:2460–2467. [DOI] [PubMed] [Google Scholar]
- Vogt MC, Paeger L, Hess S, Steculorum SM, Awazawa M, Hampel B, Neupert S, Nicholls HT, Mauer J, Hausen AC, Predel R, Kloppenburg P, Horvath TL, Bruning JC (2014) Neonatal insulin action impairs hypothalamic neurocircuit formation in response to maternal high-fat feeding. Cell 156:495–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh RJ, Mangurian LP, Posner BI (1990) The distribution of lactogen receptors in the mammalian hypothalamus: an in vitro autoradiographic analysis of the rabbit and rat. Brain Res 530:1–11. [DOI] [PubMed] [Google Scholar]
- Wang D, He X, Zhao Z, Feng Q, Lin R, Sun Y, Ding T, Xu F, Luo M, Zhan C (2015) Whole-brain mapping of the direct inputs and axonal projections of POMC and AgRP neurons. Front Neuroanat 9:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Liu C, Uchida A, Chuang JC, Walker A, Liu T, Osborne-Lawrence S, Mason BL, Mosher C, Berglund ED, Elmquist JK, Zigman JM (2014) Arcuate AgRP neurons mediate orexigenic and glucoregulatory actions of ghrelin. Mol Metab 3:64–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Bartolome CL, Low CS, Yi X, Chien CH, Wang P, Kong D (2018) Genetic identification of leptin neural circuits in energy and glucose homeostases. Nature 556:505–509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JA, List EO, Kopchick JJ (2016) Deconstructing the Growth Hormone Receptor(GHR): Physical and Metabolic Phenotypes of Tissue-Specific GHR Gene-Disrupted Mice. Prog Mol Biol Transl Sci 138:27–39. [DOI] [PubMed] [Google Scholar]
- Zhang X, van den Pol AN (2016) Hypothalamic arcuate nucleus tyrosine hydroxylase neurons play orexigenic role in energy homeostasis. Nat Neurosci 19:1341–1347. [DOI] [PMC free article] [PubMed] [Google Scholar]


