Abstract
We introduce the use of laser ablation to develop a multi-drug encapsulating theranostic nanoformulation for HIV-1 antiretroviral therapy. Laser ablated nanoformulations of ritonavir, atazanavir, and curcumin, a natural product that has both optical imaging and pharmacologic properties, were produced in an aqueous media containing Pluronic® F127. Cellular uptake was confirmed with the curcumin fluorescence signal localized in the cytoplasm. Formulations produced with F127 had improved water dispersibility, are ultrasmall in size (20–25 nm), exhibit enhanced cellular uptake in microglia, improve blood-brain barrier (BBB) crossing in an in vitro BBB model, and reduce viral p24 by 36 fold compared to formulations made without F127. This work demonstrates that these ultrasmall femtosecond laser-ablated nanoparticles are effective in delivering drugs across the BBB for brain therapy and show promises of our approach as an effective method to formulate nanoparticles for brain theranostics, reducing the need for organic solvents during preparation.
Keywords: laser ablation, multi-drug ablation, multi-drug encapsulation, antiretroviral therapy, blood-brain barrier crossing
Graphical Abstract
Ultrasmall femtosecond laser-ablated nanoparticles were synthesized in F127 from a cast film containing atazanavir, ritonavir, and curcumin. These ultrasmall nanoparticles effectively suppress HIV replication and are capable of crossing the blood-brain barrier.
Background
There is an inherent need to improve bioavailability, pharmacology, cytotoxicity, and interval dosing of antiretroviral medications to treat human immunodeficiency virus (HIV) infection.1–3 The central nervous system (CNS) is a major target and reservoir for HIV-1 infection which is responsible for neurotoxicity and neurodegeneration resulting in a spectrum of mild to moderate neurological disorders, designated as HIV associated neurological disorders (HAND). HIV-1 infection of the CNS occurs in the early stages of infection, where various resident cell populations can serve as reservoirs for HIV-1.4–6 Macrophage, microglia, and astrocytes are the primary sources of HIV-1 replication in CNS.7 There is a clinical need to attenuate viral replication in tissue reservoirs, specifically the CNS.8 Therefore, developing strategies that treat HIV infection and eliminate persistent viral reservoirs with the ability to cross the blood brain barrier (BBB) are needed.
Multi-drug combinations capable of effectively crossing the BBB have emerged as a therapeutic possibility for the elimination of HIV-1.9–12 Currently, a nanoformulation composed of antiretroviral drug crystals of ritonavir (RTV) and atazanavir (ATV) termed NanoART is being developed.13 Conventionally, micron-sized drug particles are prepared in water dispersion using different methods (e.g., high pressure homogenization) and toxic organic solvents.14–16 Ideally, nanoparticles (NPs) for biological applications should have the following characteristics: (i) must be an aqueous dispersion; (ii) must be small (10–50 nm) with narrow size dispersion 17; (iii) must be free of toxic impurities; (iv) must be stable in biological environments; (v) must have a long circulation time in the blood stream; and if necessary, (vi) must cross the BBB following systemic administration.18 To overcome typical NP drug delivery shortcomings, we hypothesized that femtosecond laser-ablated ultrasmall ART NPs could be designed to optimize cell uptake, prolong stability, improve antiretroviral efficacy, minimize cytotoxicity or adverse events, and reduce the need for organic solvents during the manufacturing process.
Laser ablation in water has been utilized to fabricate NPs using poorly water-soluble drugs, and can be produced at a clinical scale.19–20 A common laser ablation experimental setup (Figure 1a) uses an intense laser to ablate a solid drug target in an ultrapure liquid environment, yielding production of NPs without any contamination.21 LASER ABLATION is now more widely used and is an irreplaceable novel physical technique to produce nanomaterials capable of controlling NP size while conserving the stoichiometry of complex formulations. In contrast to chemical methods, laser-ablation in aqueous media can provide ideal physical and biological properties; spherical, stable, and monodispersed NPs of co-crystalized materials, which also promises a solution to reducing residual solvent toxicity and offer breakthroughs in biomedical applications.22–24 Therefore, this technique has emerged as a ‘green’ physical alternative to the conventional chemical or electrochemical routes for synthesis of NPs.
Figure 1.
Preparation of aqueous laser ablated nanoparticles. (a) experimental setup; (b) schematic of NP obtained after aqueous laser ablation
Laser ablated NPs made from organic molecules have been the center of attention of research activities in nanomedicine, profiting from their size, shape, and physicochemical properties. Laser ablated NPs can have unique surface chemistry compared to conventional colloidal NPs.25 Laser ablated NPs can bind, adsorb, encapsulate, and carry compounds such as small-molecule drugs, DNA, RNA, proteins, and biological probes with high efficiency, which makes them extremely promising for various biological applications including cancer theranostics, and treatment of neurological diseases.26–27 Laser ablated NPs can be functionalized during laser ablation in solution with various biocompatible molecules including F127, PEG, biopolymers, or oligosaccharides, to achieve dispersions with increased bioavailability that open up novel approaches in biomedical research. Recent efforts in our laboratories have focused on developing novel drug delivery systems that are capable of crossing the BBB.28–29 Additionally, the specificity of the resulting NPs and their corresponding formation of a molecular hard corona should be of high interest for biocompatibility, targeting, biological transport, and delivery.
In this interdisciplinary study, we introduce the fabrication of multi-drug (ATV/RTV/Cur) NPs by femtosecond aqueous laser ablation to produce a novel theranostic nanoformulation for the treatment of HIV, particularly in relation to neuro-AIDS. Femtosecond laser ablated nanoformulations of RTV, ATV, and Cur were characterized for size, charge, cellular uptake, cytotoxicity, ability to cross an in vitro BBB model, and suppression of HIV-1 in CHME microglial cells. We advance current ART therapies by: 1) co-crystalizing ATV and RTV enabling a single cell to uptake both drugs simultaneously in a clinically used combination; 2) adding curcumin to ART, which reduces HIV viral replication; and 3) creating small nanoparticles with F127 that are capable of crossing the BBB. Combined with the relatively low cost of the laser irradiation needed for the ablation process, this technique appears to be a promising candidate for scaling up laser-ablative synthesis with reduced cost of the final product.
Methods
Aqueous laser ablation:
F127 and Cur were purchased from Sigma-Aldrich (St. Louis, MO). ATV sulfate and RTV were a generous donation from Gyma Laboratories of America Inc. (Westbury, NY). RTV and Cur were used as obtained, without any further purifications. ATV sulfate was converted to the free base as follows: 200 mg ATV was dissolved in water (50 ml), precipitated using 0.5 M NaOH, and dried overnight under vacuum. ATV, RTV, Cur, or the mixture of all three drugs (ARC) in a weight ratio of 6:3:0.2 were dissolved in dichloromethane, cast on glass slides, and dried under vacuum overnight. NPs were produced by laser ablation in either (i) distilled water or (ii) a 5% (w/v) F127 solution in water. Ablation was conducted in 3 ml of distilled water by focusing a CPA-2010 femtosecond laser (800 nm, Clark-MXR, Inc. Dexter MI) onto a drug cast glass slide for 30 minutes at a power of 100 mW with the following laser parameters: ~160 fs pulse duration, 1 kHz repetition rate, 0.4 mJ pulse energy, divergence angle 0.3 mrad. The beam was focused on the surface of the glass slide using a lens with a focal length of 10 cm. The F127 laser ablated ART NPs were washed by centrifugation (17,000 × g for 20 minutes followed by re-dispersion in deionized water) to remove excess F127.
Physical Characterization:
The size and zeta potential of the nanoparticles were determined by dynamic light scattering (DLS) using a Brookhaven 90 Plus Particle Analyzer (Brookhaven Instruments, Holtsville, NY) with measurements conducted at an angle of 90°. Each measurement was performed by mixing 500 μl of NPs with 500 μl of distilled water in a semi-micro disposable cuvette at 25°C in triplicate. The size and morphology of the drug laser ablated ART NPs were further evaluated by transmission electron microscopy (TEM) using a JEM-2010 microscope (JEOL USA, Inc., Peabody, MA) at an acceleration voltage of 200 kV. To evaluate the size of the nanoformulations, a negative staining technique was employed to aid in visualization due to low electron density. To prepare the nanoparticles for TEM, 10 μL of the nanoparticle suspension was dropped onto the surface of a 200-mesh fomvar coated copper grid and allowed to sit for 2 mins. The nanoparticle suspension was then absorbed using the corner of a piece of 125mm Whatman™ filter paper (GE Healthcare, Chicago, IL) cut in to triangles. Subsequently, 10 μL of a 2% phosphotungstic acid solution (pH 7, Fluka Chemical, Ronkonkoma, NY), was dropped on to the grid and allowed to sit for 3 mins. This was subsequently absorbed and the grid was allowed to dry overnight before visualization. The absorption and fluorescence spectra were acquired using a spectrophotometer (LAMBDA 750 UV/VIS/NIR, PerkinElmer) and spectrofluorometer (Fluorolog-3.1.1, Horiba, Jobin Yvon, Edison, NJ)., with a slit width defining a spectral resolution of 5 nm.
Differential Scanning Calorimetry (DSC) and X-Ray Diffraction (XRD) analysis:
DSC analysis was performed using a Q20 DSC (TA Instruments, Lindon, UT), from 35 to 220°C at a 10°C/min scan speed and an N2 purge. X-ray diffraction patterns were recorded by an Ultima IV X-Ray Diffractometer (Rigaku, Tokyo, Japan) using Cu Kα radiation. The 2θ angle of the XRD spectra was recorded 5 to 50 degrees at a scanning rate of 5°/min. ATV, RTV and Cur were tested as either individual material, physically mixed material, or cast and ground material. Cast and ground material was prepared by dissolving the compound(s) in MC, casting them on a glass slide, and subsequently grinding the cast film into a powder.
Cell Culture:
CHME and CHME-5/HIV cells:
Both the CHME and the CHME-5/HIV cells are primary human microglial cell lines that were generously provided by Dr. Jonathan Karn, Case Western Reserve University (Cleveland, OH). CHME-5/HIV-1 are primary human microglial cells immortalized with SV40 T antigen (CHME-5 cells) that were co-transfected with an HIV LTR reporter and the HIV Tat gene 30 and is used as the experimental control. Both cell lines were maintained in DMEM, high glucose supplemented with 5% heat inactivated fetal bovine serum (FBS; ThermoFisher Scientific, Grand Island, NY) and 1% penicillin/streptomycin (ThermoFisher Scientific). CHME-5/HIV were grown to 80% confluency, cells were subsequently treated with TNF-α (50 ng/ml; Cat # P01375, Recombinant Human TNF-α, R&D Systems, Minneapolis, MN) overnight for stimulation and activation of HIV transcription in CHME-5/HIV cells as previously described.31 Cells are then treated with the nanoformulation as described and at the end of the incubation period, cells were washed in phosphate buffer saline (PBS), harvested, and RNA was extracted and used for gene expression studies. The untreated CHME-5/HIV cells were used as controls.
Monocytic THP-1 cells:
THP-1 cells (American Type Culture Collection [ATCC], Manassas, VA) were maintained at 37°C, 5% CO2 in RPMI 1640 media supplemented with 10% heat-inactivated FBS, penicillin (100 units/ml), streptomycin (100 mg/ml), and L-glutamine (2 mM). THP-1 cells are cultured as single-cell suspension cultures and were split at a ratio of 1:4 once a week.
Cellular uptake of LAW NPs:
We studied the ability of CHME-5/HIV cells to phagocytose Cur loaded laser ablated ART NPs. CHME-5/HIV cells were incubated with laser ablated ART NPs for 2 h. Cellular images were obtained using an EVOS XL Core Cell Imaging System (Thermo Fisher Scientific, Waltman, MA) equipped with a GFP Light Cube (Ex: 470/22, Em: 525/50) to obtain curcumin fluorescence images and a DAPI light cube (Ex: 357/44, Em: 447/60) to obtain DAPI fluorescence images.
Cytotoxicity:
Cell viability was measured using the 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenyl tetrazoliumbromide (MTT) colorimetric assay. Microglia cell lines (CHME and CHME-5/HIV cells) were seeded in 96-well plates (10,000 cells/well) and cultured at 37°C, 5% CO2 for 24 h. Subsequently, 10 μg of each formulation (ATV/RTV/Cur/ARC and FATV/FRTV/FCur/FARC) were added to the culture media and cells were incubated for 24 h. MTT reagent was dissolved in PBS (5 mg/ml) and added to each well (50 μL) and the plates were incubated for 3 h at 37°C. After the 3 h incubation, the media containing MTT was removed and 150 μL of dimethyl sulfoxide was added to each well to dissolve the MTT formazan crystals. Finally, the plates were shaken and MTT efficacy was measured by reading the 96-well plate on a plate spectrophotometer at 490 nm with a reference wavelength of 630. The results were plotted as percent survival compared with the corresponding control experiment results.
In vitro BBB crossing:
Our 2D in vitro BBB model consists of a two-chamber Transwell system with the upper (luminal) compartment separated from the lower (abluminal) by a semipermeable membrane (polyethylene terephthalate, PET) insert. Human brain microvascular endothelial cells (BMVECs) were grown to confluency on the upper side while a confluent layer of normal human astrocytes (NHAs) were grown on the lower side. After tight BBB formation was confirmed by transendothelial electrical resistance (TEER) measurement, dispersed NPs (400 μL media with NPs) were added to the upper chamber and incubated at 37°C, 5% CO2. Media from the lower chamber was collected at different time points (0.25, 0.5, 1, 8, 16, 24, 32, 40, and 48 h) and the absorbance was measured at λabs = 400 nm to assess transmigration. TEER was measured again after laser ablated ART NPs exposure to make sure that the transmigration was not due to the compromise of the BBB.
HIV-1 infection of THP-1 monocyte derived macrophages:
Monocytic cells (THP-1 cells) were treated with 100 ng/ml phorbol 12-myristate 13-acetate (PMA) overnight and then cultured for 7 days until they have transformed to adherent macrophage. The differentiated THP-1 cells, now macrophage were infected overnight with HIV-1 Ba-L (NIH AIDS Research and Reference Reagent Program) at a concentration equivalent to 10 ng viral isolate/ml of culture media. The infected macrophages were then washed and reconstituted in RPMI media supplemented with 10% FBS and incubated at 37°C, 5% CO2 for 7 days, and were used for evaluating the anti-HIV-1 efficacy of the nanoformulation.
HIV-1 p24 ELISA:
HIV-1 infection was monitored by the viral p24 concentration in harvested cell culture supernatants. Monocytic cells (THP-1 cells) were treated with 100 ng/ml PMA overnight and then cultured for a period of 7 days until they were transformed to macrophage. After cells were transformed to macrophage they were infected with HIV-1 Ba-L (Cat # 510; NIH AIDS Reagent Program) overnight, at multiplicity of infection (MOI) of 0.05 or at a concentration, equivalent to 10 ng viral isolate/ml of culture media, and then virus washed out and macrophage returned to culture after which they were treated with and without laser ablated ART NPs for 48 h, followed by change in media. The concentration of HIV p24 antigen in culture supernatants was measured after 7 days using enzyme-linked immunosorbent assay (ELISA) plates (Zeptometrix, Buffalo, NY) 7 days post-infection. The results are expressed in pg/mL as the mean and standard deviation (SD) of 3 separate experiments.
Real-time quantitative PCR (RT-qPCR):
Cytoplasmic RNA was extracted by an acid guanidinium-thiocyanate-phenol-chloroform method using Trizol reagent (Invitrogen, Carlsbad, CA). Briefly, 100,000 cells/ml were seeded in a 12-well plate, viral activation was initiated by treating cells with 50 ng/ml TNF-α for 24 h, followed by treatment with laser ablated ART NPs and appropriate nanoformulation and antiviral drug controls for 48 h, following which cells were harvested, RNA extracted and HIV-1 LTR gene expression quantitated by RT-qPCR. The amount of RNA was quantified using a spectrophotometer (NanoDrop ND-1000; Thermo Scientific, Wilmington, DE) and the isolated RNA was stored at −80°C until quantified. The LTR-R/U5 region represents early stages of reverse transcription of HIV-1. Following conversion of RNA to cDNA using reverse transcription, relative abundance of mRNA species was quantified by RT-qPCR using LTR/RU5-specific primers and Brilliant SYBR green QPCR master mix (Stratagene, La Jolla, CA). The primer sequences used for LTR/RU5 are as follows: Forward primer 5′-TCTCTCTGGTTAGACCAGATCTG-3′; and Reverse primer 5′-ACTGCTAGAGATTTTCCACACTG-3′. The relative expression of mRNA species was calculated using the comparative CT method. All data were controlled for quantity of RNA input by performing measurements on an endogenous reference gene, β-actin. In addition, results obtained on RNA from treated samples were normalized to results obtained on RNA from the control, untreated sample. Results were expressed as transcript accumulation index (TAI). This calculation assumes that all PCR reactions occurred with 100% efficiency.
Results
NPs preparation and characterization
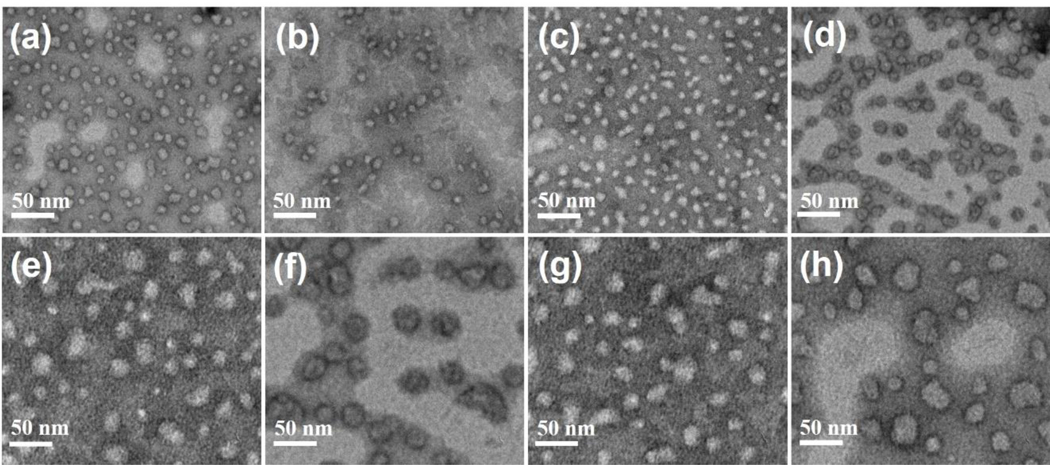
Femtosecond (fs) laser ablation was performed on pre-cast drugs (ATV, RTV, Cur, or their combination [ARC], Figure 1). The concentration of NPs increased as a function of laser ablation time, leveling off after 30 minutes. After 15 minutes, the continued laser treatment was able to refine the mean diameter of the nanoparticles to as small as 10 nm (Figures 2 and 3). The size of the resulting laser ablated colloids of ATV, RTV, Cur, and ARC have a number-weighted hydrodynamic diameter of 12 ± 2.8 nm when measured by dynamic light scattering (DLS) (Figure 2). When F127 was present in the surrounding water, the size increased to 20 ± 5 nm. The zeta potential of all nanoparticles is negative. The size and zeta potential of these laser ablated NPs are tabulated in Table 1.
Figure 2.
Particle size distribution of laser ablated ART NPs determined by DLS.
Figure 3.
Representative TEM images of laser ablated NPs: Produced in water; (a) ATV; (b) RTV; (c) Cur, (d) ARC. Produced in water containing F127; (e) FATV; (f) FRTV; (g) FCur; (h) FARC.
Table 1.
Size and zeta potential of the ablated nanoparticles.
| Samples (Ablated NPs) | Size (nm) | Zeta Potential (mV) |
|---|---|---|
| ATV | 12.2 | −29.74 |
| RTV | 11.7 | −35.48 |
| Cur | 12.4 | −25.86 |
| ARC | 13.5 | −27.38 |
| FATV | 30.6 | −10.92 |
| FRTV | 32.3 | −08.90 |
| FCur | 31.8 | −07.54 |
| FARC | 39.2 | −09.66 |
TEM images demonstrate that the dispersed colloids are spherical NPs with an average diameter of 10 ± 2.3 nm (Figure 3a–d). Ablation in polymeric amphiphile (F127) surfactant increases the size to 15 ± 1.8 nm (Figure 3e–h). No apparent precipitates were observed in the dispersion.
Assessment of Co-encapsulation
To prove the co-assembly of multiple drugs in one nanoformulation, we measured changes to differential scanning calorimetry endotherm and exotherm peaks, X-ray diffraction patterns, as well as used a previously reported anthracene-based aggregation induced emission fluorescent dye (ANT), which exhibits a spectral shift when molecular stacking is impeded, to assess crystallinity and homogeneity of materials prior to laser ablation.18, 32
The DSC spectrum of RTV shows an endotherm with a minimum at 126.13°C (Peak 1), Hydrophobic ATV shows an exotherm with a maximum at 165.27°C (Peak 2) followed by and endotherm with a minimum at 206.71°C (Peak 3), and Cur shows an endotherm with a minimum at 176.59°C (Peak 4). When ATV and RTV are combined through physical mixing Peaks 1, 2, and 3 are all visible on the DSC spectrum. Additionally, when ATV, RTV, and Cur are combined through physical mixing Peaks 1 and 3 are visible while Peaks 2 and 4 seem to combine ultimately resulting in an exotherm that is left shifted with respect to that seen in the DSC spectrum of hydrophobic ATV (Figure S1).
The DSC spectrum of cast and ground RTV has an endotherm with a minimum at 123.16°C (Peak 1) which is consistent with that seen in the DSC spectrum of the raw powder. Cast and ground hydrophobic ATV has an exotherm with a maximum at 178.27°C (Peak 2) followed by and endotherm with a minimum at 206.87°C (Peak 3); the exotherm is right shifted while the exotherm is consistent with that seen in the spectrum of the raw powder. The DSC spectrum of cast and ground Cur has an endotherm with a minimum at 177.86°C (Peak 4) which is also consistent with the raw powder DSC spectrum (Figure S2). When ATV and RTV are co-dissolved in MC, cast as a film, and ground, Peaks 1, 2, and 3 are no longer visible, while an endotherm (Peak 5) with a minimum at 190.69°C appears. Additionally, when ATV, RTV and Cur are co-dissolved, cast, and ground, Peaks 1, 2, 3, and 4 are no longer visible, while the same endotherm (Peak 5) as was seen in the ATV+RTV cast and ground sample is observed (Figure S2).
The X-ray diffraction patterns of the powder and “cast and ground” samples support the findings from the DSC (Figure S3). The crystallinity of RTV and Cur are not affected by the cast and grind procedure. However, when RTV is cast and ground with amorphous hydrophobic ATV, the resulting X-ray diffraction patterns become amorphous.
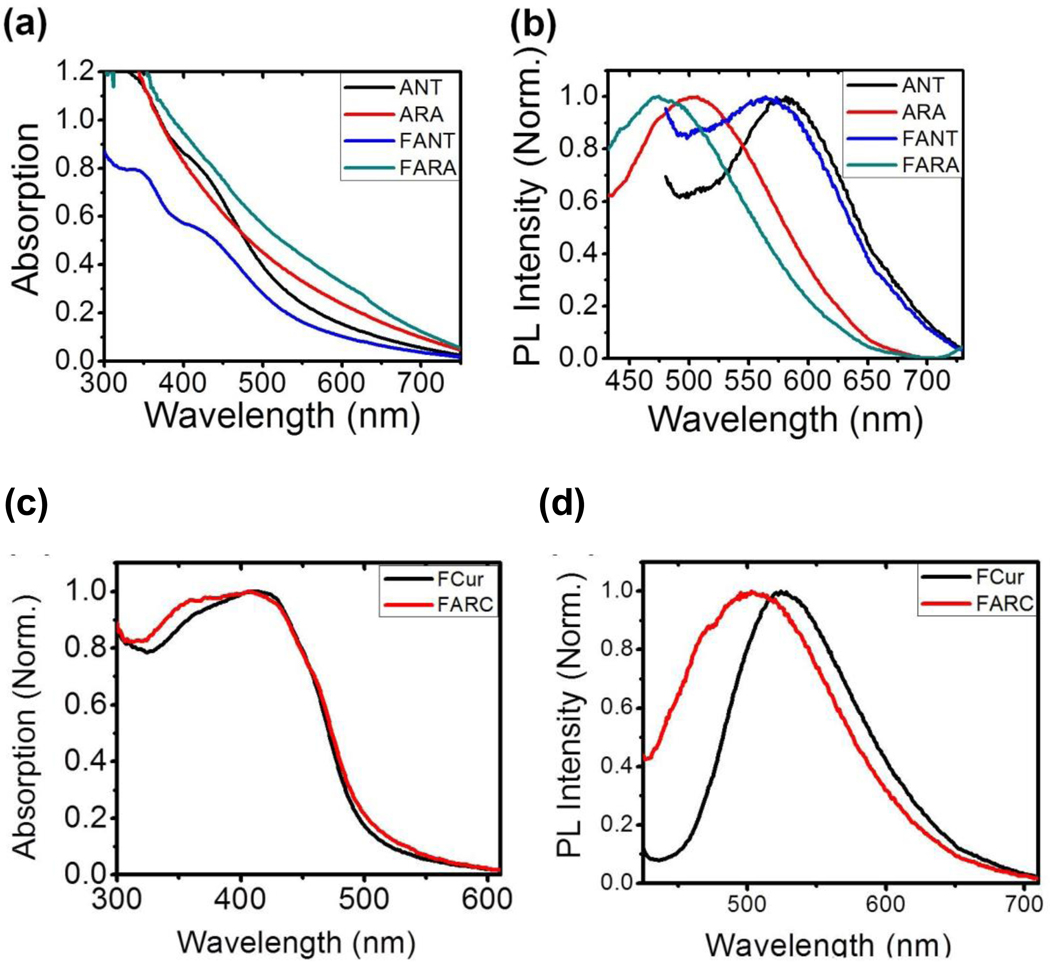
Co-assembly was further confirmed by co-encapsulation of an anthracene-based aggregation induced emission fluorescent dye (ANT). Upon excitation, laser ablated ANT NPs exhibited an intense red photoluminescence (PL) (λmaxPL = ~590 nm), which is the characteristic spectra of ANT as a pure nano-aggregate (Figure 4b). When a ternary mixture of ATV/RTV/ANT (ARA) underwent aqueous laser ablation to produce water dispersed NPs, the PL spectrum of ARA NPs and F127 functionalized nano-aggregates, F127-ARA (FARA) NPs exhibited a hypsochromic shift (λmaxPL = 520 nm and 460 nm, respectively) compared to that of ANT and F127-ANT (FANT) NPs (λmaxPL = 590 nm and 580 nm, respectively) (Figure 4 c&d).
Figure 4.
Absorption (a) and emission (b) spectra of laser ablated ANT, ARA, FANT and FARA NPs in water. Absorption (c) and emission (d) spectra of laser ablated FCur and FARC NPs in water.
Cellular uptake and Toxicity
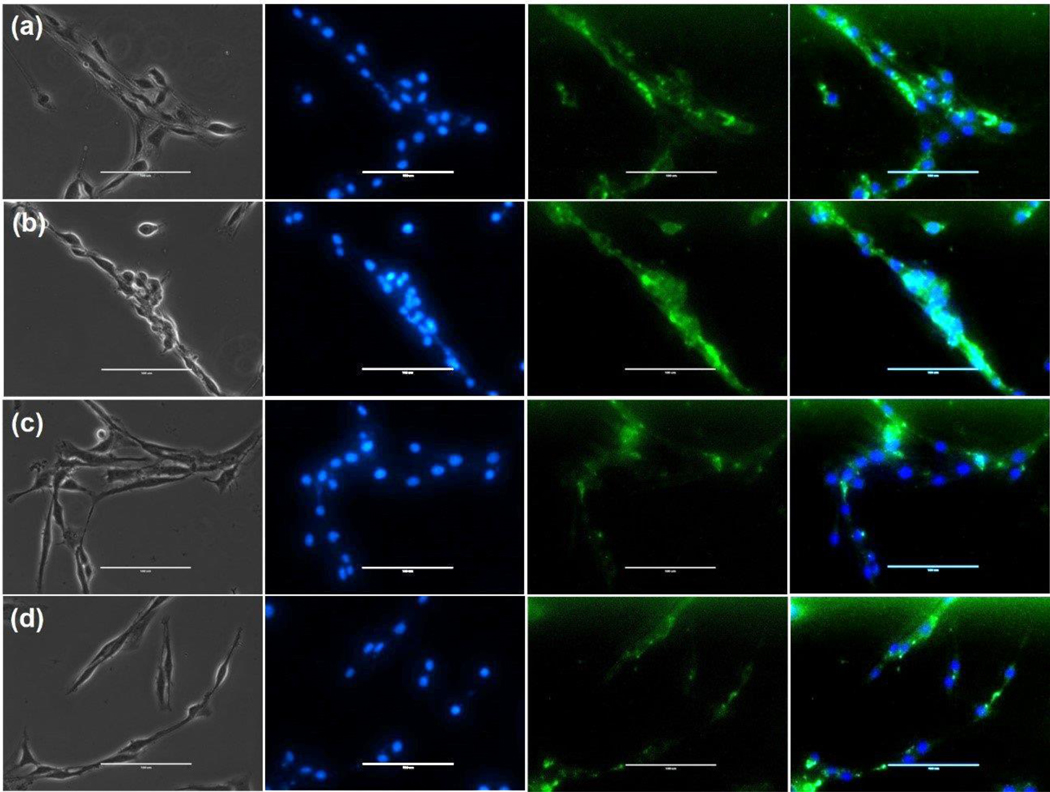
The uptake and localization of laser ablated ART NPs, which also contained Cur as a fluorescent tracer, was observed in CHME-5/HIV cells. The Cur fluorescence signal was localized in the cytoplasm of the cells (Figure 5). Laser ablated NPs prepared in water containing F127 (Figure 5a&b) exhibit higher cellular uptake than NPs prepared without F127 (Figure 5c&d).
Figure 5.
Cellular uptake of laser ablated ART NPs in CHME-5/HIV cells. (a) FCur; (b) FARC; (c) Cur; and (d) ARC. The cells were incubated for 2 h at 37°C. Left to right: bright field; blue is DAPI staining; green is curcumin channel; image overlay. Scale bar represents 100μm.
Cytotoxicity
An MTT assay was performed to evaluate the cell viability of CHME and CHME-5/HIV cells treated with laser ablated ART NPs. Cell viability was measured after 24 h treatment with laser ablated-ART NPs. This study demonstrates that laser ablated ART NPs are not cytotoxic (>90% viability) in CHME cells exposed for 24 h, while <60% cell viability is observed in CHME-5/HIV cells treated with FARC NPs (Figure 6).
Figure 6.
Cell viability of laser ablated ART NPs in (a) CHME cells and (b) CHME-5/HIV cells.
In vitro BBB Crossing
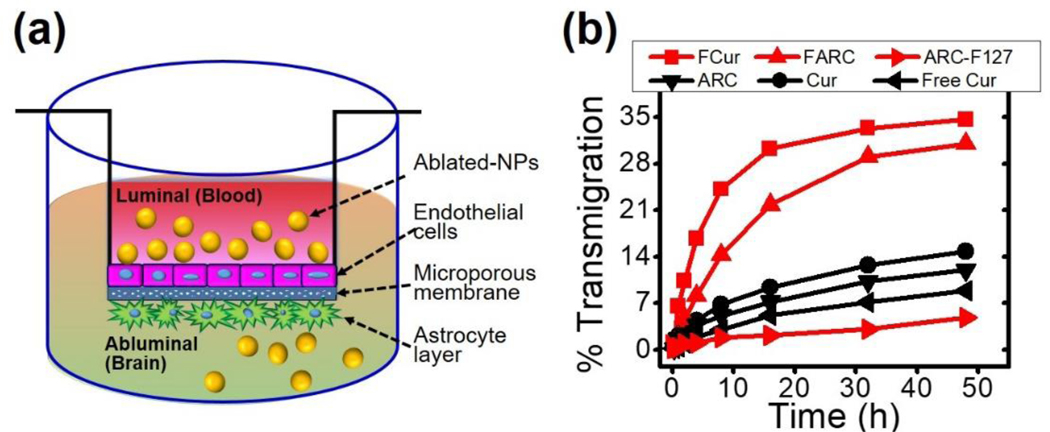
We prepared and validated a cell-based model of the BBB to examine quantitative permeability and transendothelial migration of NPs.28–29 Cur and FCur NPs are able to cross the BBB more readily than non-formulated free Cur (Figure 7b). Furthermore, F127 enhances the ability of the NPs to cross the BBB. At 48 h, the concentration of FCur NPs in the lower well is 5 fold higher than Cur NPs. A similar trend is seen with FARC NPs when compared to ARC NPs and non-formulated free ARC with F127 added to the media (ARC-F127).
Figure 7.
(a) Schematic of in vitro BBB model (b) Ability of laser ablated ART NPs to cross the BBB.
Therapeutic Effect
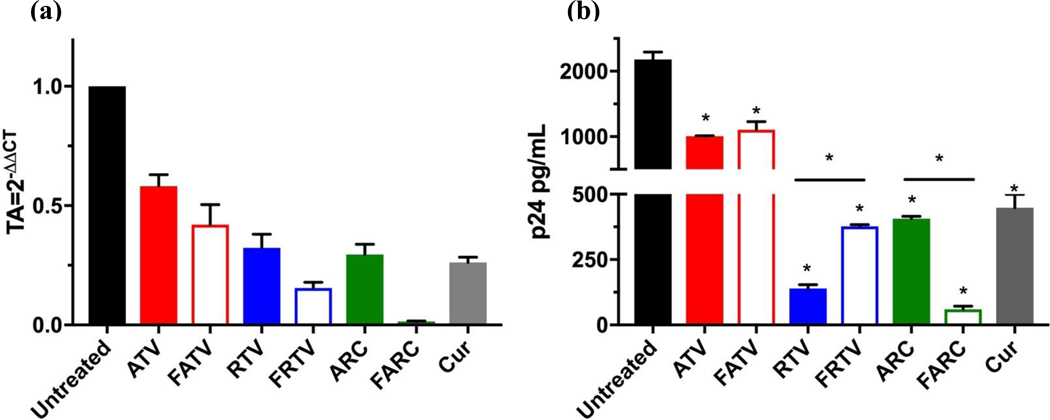
In the CNS, macrophages, microglia, and astrocytes all serve as vehicles for viral transport, and reservoirs for ongoing HIV-1 replication. In the current study, latently infected CHME microglial cells (CHME-5/HIV) were treated with laser ablated ART NPs and the antiviral activity was measured using HIV-1 LTR gene expression. HIV-1 LTR RNA was decreased ~66 fold when treated with laser ablated FARC NPs when compared to untreated controls (Figure 8A). Latently infected THP-1 macrophages (THP-1/HIV) were also treated with laser ablated ART NPs and the antiviral activity was evaluated by determining p24 antigen levels. There was a significant decrease in p24 antigen production in THP-1/HIV macrophages when treated with FARC NPs as compared to untreated controls as well as treatment with ATV, RTV or Cur alone. Additionally, FARC NPs decrease the production of p24 in HIV-1-infected macrophages ~10-fold when compared to ARC NPs (Figure 8B). The relative HIV-1 LTR gene expression, and the p24 antigen levels, including the mean and standard deviation, can be found in table S3 and S4 respectively.
Figure 8.
(a) Effect of NPs on LTR gene expression. Cells infected with HIV-1 were treated with the NPs. RNA was extracted and quantified by RT-qPCR using primers specific for the LTR region of the HIV-1 genome. (b) Effect of laser ablated ART NPs on HIV-1 replication in THP-1/HIV cells. FARC NPs suppress HIV-1 replication.
Discussion
NP preparation and characterization
It is important that organic NPs can be easily prepared by purely physical synthesis methods using laser ablation in a well-controlled, biologically compatible environment (e.g., deionized water). Such an approach is unique in avoiding any residual toxic organic solvents, which is a huge advantage over conventional electrochemical or colloidal chemistry routes for the fabrication of colloidal nanomaterials.
The laser ablation process resulted in a homogeneously dispersed colloidal suspension, that was spontaneously formed by the self-assembly behavior of the hydrophobic drugs in water (Figure 1b). Initially, larger fragments of the pre-cast crystals became suspended in the surrounding water followed by subsequent fragmentation of these crystals into smaller ones. The observed increase in nanoparticle concentration over time is consistent with this observation. The subsequent leveling off of the NP concentration after 30 minutes suggest that after this amount of time, the suspension became excessively turbid, which ultimately decreased the ablation efficiency. This is thought to be a result of excessive beam scattering that greatly attenuates the energy available to ablate the remaining cast drug. When observed using TEM, no apparent precipitates were observed in the dispersion, suggesting that these water insoluble drugs were stabilized in the hydrophobic interior of the self-assembled nanostructure of F127.
Assessment of Co-encapsulation
The endotherms observed in the DSC spectra of RTV and Cur are indicative of a crystalline to amorphous melt suggesting that in its raw powder form RTV and Cur are crystalline. The DSC spectrum of hydrophobic ATV, is indicative of crystallization followed by crystal melting, which demonstrates that hydrophobic ATV in its raw powder form is amorphous. The DSC spectrum of ATV/RTV that physical mixing does not alter crystallinity of either compound, as the peaks characteristic of the individual powers are all observed. However, upon co-casting ATV/RTV/Cur the resulting DSC spectrum combines Peaks 2 and 4, resulting in a leftward shift of the (which I think we use a different word) The DSC spectrum of ATV/RTV/Cur suggest the combination of Peaks 2 and 4 which results in the apparent leftward shift in the exotherm associated with ATV and the disappearance of the endotherm associated with Cur. Due to the 6:0.2 ratio of ATV:Cur it would be expected that the ATV exotherm would dampen the Cur endotherm resulting in the observed disappearance of Peak 4. This data demonstrates the physical mixing of raw powders of ATV, RTV, and Cur does not alter the crystalline properties of these drugs. This suggests that physical mixing results in heterogeneous mixture made up of intermittent pockets of each individual compound.
The “cast and grind” procedure used to prepare the cast and ground samples for DSC was meant to mimic the laser ablation process. The endotherms observed in the DSC spectra of cast and ground RTV and Cur, which are consistent with those observed in the spectra of the raw powders, indicate that the cast and grind procedure does not alter the crystallinity of RTV or Cur. The rightward shift of the exotherm in the DSC spectrum of cast and ground ATV, suggests a change in the crystallization temperature as a result of the cast and grind process, however the spectrum is still indicative of an amorphous solid. The disappearance of Peaks 1, 2, and 3, and the appearance of Peak 5, in the DSC spectrum of cast and ground ATV/RTV indicates the cast and grind method does alter the crystallinity of the compounds when co-dissolved. The disappearance of Peaks 1, 2, 3, and 4 and the appearance of Peak 5 is also observed in the DSC spectrum of cast and ground ATV/RTV/Cur, which indicates that when co-dissolved, cast, and ground, these compounds do not retain their individual properties and no longer exist in a heterogeneous mixture. This suggest there are no longer individual pockets of each compound, rather they exist in a homogeneous co-crystal when cast in MC. This is evidence that following laser ablation, individual nanoparticles should contain a mixture of all three compounds.
The agreement of the X-ray diffraction patterns with the result from the DSC supports the conclusion that when cast together as a film the compounds form a homogenous mixture, not just pockets of each individual compound. This also reinforces the inference that upon formation of the nanoparticles each compound is present, suggesting co-delivery of ATV, RTV, and Cur through the use of F127 laser ablated nanoparticles.
Further supporting this claim, the hypsochromic shift observed in in FARA NPs when compared to FANT NPs implies loose molecular stacking (i.e., neither closely packed nor well-ordered), indicating that ATV, RTV, and ANT are molecularly mixed within the pre-cast material prior to aqueous laser ablation. FANT NPs also have a hypsochromic shifted spectra compared to ANT NPs, again indicating that the ANT molecules are loosely packed in the polymeric matrix. Similarly, the broadened absorption and blue shifted emission spectra in FARC compared to FCur, reveals the homogenous intermixing of all three drugs in the nanoformulation (Figure 4 c&d).
In vitro BBB Crossing
As a reservoir of HIV, it is important to improve the brain penetration of ARTs. The cellular pharmacokinetics of many ARTs are dependent on membrane bound protein efflux pumps, which are highly expressed in the BBB limiting brain penetration. F127 enhances the in vivo efficacy of ARTs by influencing their biodistribution to sites protected by efflux mechanisms. This can facilitate an increase in brain accumulation of these compounds resulting in suppression of viral replication. The improvement in crossing observed by FCur and FARC NPs indicates that the addition of F127 to the formulation significantly enhances BBB penetration, which correlates with our previous findings.29. This suggest the laser ablated ART NPs will have improved brain accumulation over non-formulated free ATV and RTV, indicating this nanoformulation as a possible option to overcome limitations currently associated with the treatment of HIV in relation to neuro-AIDS.
Therapeutic Effect
HIV-1 RNA and HIV-1 capsid protein (p24 antigen) are two of the main viral markers used to detect and monitor the progression of HIV infections. The p24 antigen in particular appears within the first two weeks of infection and is associated with high levels of viremia, while HIV-1 is used to determine the risk of disease progression to AIDS or death. 4, 33–34 Upon exposure of CHME-5/HIV cells to NPs prepared in the presence of F127, there is a significant reduction in both HIV-1 gene expression and HIV p24 antigen levels as compared to those prepared without F127. These results confirm that the F127-NPs are more effective in both delivering ARTS intracellularly and suppressing HIV-1 viral replication. This combined with the ability of the FARC NPs to traverse the BBB, suggests this ability of this nanoformulation to inhibit the progression of HIV infections and decrease the risk of AIDS, specifically neuro-AIDS, or death in those diagnosed with the disease.
In Summary, we demonstrate the use of femtosecond laser ablation to prepare small (10–20 nm) monodisperse NPs in a biocompatible environment. NPs were produced by laser ablation of pre-cast drugs (ATV, RTV, Cur), which eliminates contamination by organic solvents that may interfere with future biological studies or cause in vivo toxicity. We demonstrate that laser ablation significantly improves ATV/RTV cellular bioavailability. Laser-ablated NPs form a stable dispersion in water. These lasers ablated ART NPs demonstrate limited cytotoxicity with high cellular uptake in CHME cells.
FARC NPs, prepared in water, appear as promising effective nanocarriers for diagnosis and therapeutic applications. This physico-chemical, optical, and pharmacological interdisciplinary approach and the convergence of the methods used allowed us to better understand the basic identity, biological behavior, and properties of F127-NPs under pharmacological conditions. This unique NP fabrication method provides novel opportunities for the synthesis and functionalization of nanomaterials for biosensing, bioimaging, and therapeutic applications.
Supplementary Material
Acknowledgments
Sources of Funding:
Research reported in this publication was supported by University of Rochester Center for AIDS Research (CFAR) grant P30AI078498 (NIH/NIAID); Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant 1T32GM099607; and the National Center for Advancing Translational Sciences of the National Institutes of Health under award number UL1TR001412; National Institute of Allergy and Infectious Disease of the National Institutes of Health under award number R01AI129649.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Broder S, The development of antiretroviral therapy and its impact on the HIV-1/AIDS pandemic. Antiviral Res 2010, 85 (1), 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Este JA; Cihlar T, Current status and challenges of antiretroviral research and therapy. Antiviral Res 2010, 85 (1), 25–33. [DOI] [PubMed] [Google Scholar]
- 3.Moreno S; Lopez Aldeguer J; Arribas JR; Domingo P; Iribarren JA; Ribera E; Rivero A; Pulido F; Project HIV, The future of antiretroviral therapy: challenges and needs. J Antimicrob Chemother 2010, 65 (5), 827–35. [DOI] [PubMed] [Google Scholar]
- 4.Alexaki A; Liu Y; Wigdahl B, Cellular reservoirs of HIV-1 and their role in viral persistence. Curr HIV Res 2008, 6 (5), 388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Eisele E; Siliciano RF, Redefining the viral reservoirs that prevent HIV-1 eradication. Immunity 2012, 37 (3), 377–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gray LR; Roche M; Flynn JK; Wesselingh SL; Gorry PR; Churchill MJ, Is the central nervous system a reservoir of HIV-1? Curr Opin HIV AIDS 2014, 9 (6), 552–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Koppensteiner H; Brack-Werner R; Schindler M, Macrophages and their relevance in Human Immunodeficiency Virus Type I infection. Retrovirology 2012, 9, 82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Reynolds A; Laurie C; Mosley RL; Gendelman HE, Oxidative stress and the pathogenesis of neurodegenerative disorders. Int Rev Neurobiol 2007, 82, 297–325. [DOI] [PubMed] [Google Scholar]
- 9.Chow YK; Hirsch MS; Merrill DP; Bechtel LJ; Eron JJ; Kaplan JC; D’Aquila RT, Use of evolutionary limitations of HIV-1 multidrug resistance to optimize therapy. Nature 1993, 361 (6413), 650–4. [DOI] [PubMed] [Google Scholar]
- 10.Iyidogan P; Anderson KS, Current perspectives on HIV-1 antiretroviral drug resistance. Viruses 2014, 6 (10), 4095–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mazzulli T; Rusconi S; Merrill DP; D’Aquila RT; Moonis M; Chou TC; Hirsch MS, Alternating versus continuous drug regimens in combination chemotherapy of human immunodeficiency virus type 1 infection in vitro. Antimicrob Agents Chemother 1994, 38 (4), 656–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menendez-Arias L, Targeting HIV: antiretroviral therapy and development of drug resistance. Trends Pharmacol Sci 2002, 23 (8), 381–8. [DOI] [PubMed] [Google Scholar]
- 13.Nowacek AS; Miller RL; McMillan J; Kanmogne G; Kanmogne M; Mosley RL; Ma Z; Graham S;Chaubal M; Werling J; Rabinow B; Dou H; Gendelman HE, NanoART synthesis, characterization, uptake, release and toxicology for human monocyte-macrophage drug delivery. Nanomedicine (Lond) 2009, 4 (8), 903–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chaowanachan T; Krogstad E; Ball C; Woodrow KA, Drug synergy of tenofovir and nanoparticle-based antiretrovirals for HIV prophylaxis. PLoS One 2013, 8 (4), e61416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Coelho JF; Ferreira PC; Alves P; Cordeiro R; Fonseca AC; Gois JR; Gil MH, Drug delivery systems: Advanced technologies potentially applicable in personalized treatments. EPMA J 2010, 1 (1), 164–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Govender T; Ojewole E; Naidoo P; Mackraj I, Polymeric nanoparticles for enhancing antiretroviral drug therapy. Drug Deliv 2008, 15 (8), 493–501. [DOI] [PubMed] [Google Scholar]
- 17.Singh A; Lim CK; Lee YD; Maeng JH; Lee S; Koh J; Kim S, Tuning solid-state fluorescence to the near-infrared: a combinatorial approach to discovering molecular nanoprobes for biomedical imaging. ACS Appl Mater Interfaces 2013, 5 (18), 8881–8. [DOI] [PubMed] [Google Scholar]
- 18.Singh A; Seo YH; Lim CK; Koh J; Jang WD; Kwon IC; Kim S, Biolighted Nanotorch Capable of Systemic Self-Delivery and Diagnostic Imaging. ACS Nano 2015, 9 (10), 9906–11. [DOI] [PubMed] [Google Scholar]
- 19.Ding W; Sylvestre J-P; Bouvier E; Leclair G; Meunier M, Ultrafast laser processing of drug particles in water for pharmaceutical discovery. Applied Physics A 2014, 114 (1), 267–276. [Google Scholar]
- 20.Kenth S; Sylvestre JP; Fuhrmann K; Meunier M; Leroux JC, Fabrication of paclitaxel nanocrystals by femtosecond laser ablation and fragmentation. J Pharm Sci 2011, 100 (3), 1022–30. [DOI] [PubMed] [Google Scholar]
- 21.Baati T; Al-Kattan A; Esteve MA; Njim L; Ryabchikov Y; Chaspoul F; Hammami M; Sentis M;Kabashin AV; Braguer D, Ultrapure laser-synthesized Si-based nanomaterials for biomedical applications: in vivo assessment of safety and biodistribution. Sci Rep 2016, 6, 25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Al-Kattan A; Ryabchikov YV; Baati T; Chirvony V; Sanchez-Royo JF; Sentis M; Braguer D; Timoshenko VY; Esteve M-A; Kabashin AV, Ultrapure laser-synthesized Si nanoparticles with variable oxidation states for biomedical applications. Journal of Materials Chemistry B 2016, 4 (48), 7852–7858. [DOI] [PubMed] [Google Scholar]
- 23.Correard F; Maximova K; Esteve MA; Villard C; Roy M; Al-Kattan A; Sentis M; Gingras M;Kabashin AV; Braguer D, Gold nanoparticles prepared by laser ablation in aqueous biocompatible solutions: assessment of safety and biological identity for nanomedicine applications. Int J Nanomedicine 2014, 9, 5415–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kucherik AO; Ryabchikov YV; Kutrovskaya SV; Al-Kattan A; Arakelyan SM; Itina TE; Kabashin AV, Cavitation-Free Continuous-Wave Laser Ablation from a Solid Target to Synthesize Low-Size-Dispersed Gold Nanoparticles. Chemphyschem 2017, 18 (9), 1185–1191. [DOI] [PubMed] [Google Scholar]
- 25.Asahi T; Sugiyama T; Masuhara H, Laser fabrication and spectroscopy of organic nanoparticles. Acc Chem Res 2008, 41 (12), 1790–8. [DOI] [PubMed] [Google Scholar]
- 26.Gongalsky MB; Osminkina LA; Pereira A; Manankov AA; Fedorenko AA; Vasiliev AN; Solovyev VV; Kudryavtsev AA; Sentis M; Kabashin AV; Timoshenko VY, Laser-synthesized oxide-passivated bright Si quantum dots for bioimaging. Sci Rep 2016, 6, 24732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tamarov KP; Osminkina LA; Zinovyev SV; Maximova KA; Kargina JV; Gongalsky MB;Ryabchikov Y; Al-Kattan A; Sviridov AP; Sentis M; Ivanov AV; Nikiforov VN; Kabashin AV; Timoshenko VY, Radio frequency radiation-induced hyperthermia using Si nanoparticle-based sensitizers for mild cancer therapy. Sci Rep 2014, 4, 7034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aalinkeel R; Kutscher HL; Singh A; Cwiklinski K; Khechen N; Schwartz SA; Prasad PN; Mahajan SD, Neuroprotective effects of a biodegradable poly(lactic-co-glycolic acid)-ginsenoside Rg3 nanoformulation: a potential nanotherapy for Alzheimer’s disease? J Drug Target 2018, 26 (2), 182–193. [DOI] [PubMed] [Google Scholar]
- 29.Singh A; Kim W; Kim Y; Jeong K; Kang CS; Kim Y; Koh J; Mahajan SD; Prasad PN; Kim S, Multifunctional Photonics Nanoparticles for Crossing the Blood-Brain Barrier and Effecting Optically Trackable Brain Theranostics. Adv Funct Mater 2016, 26 (39), 7057–7066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Janabi N; Peudenier S; Heron B; Ng KH; Tardieu M, Establishment of human microglial cell lines after transfection of primary cultures of embryonic microglial cells with the SV40 large T antigen. Neurosci Lett 1995, 195 (2), 105–8. [DOI] [PubMed] [Google Scholar]
- 31.Wires ES; Alvarez D; Dobrowolski C; Wang Y; Morales M; Karn J; Harvey BK, Methamphetamine activates nuclear factor kappa-light-chain-enhancer of activated B cells (NF-kappaB) and induces human immunodeficiency virus (HIV) transcription in human microglial cells. J Neurovirol 2012, 18 (5), 400–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee YD; Lim CK; Singh A; Koh J; Kim J; Kwon IC; Kim S, Dye/peroxalate aggregated nanoparticles with enhanced and tunable chemiluminescence for biomedical imaging of hydrogen peroxide. ACS Nano 2012, 6 (8), 6759–66. [DOI] [PubMed] [Google Scholar]
- 33.Bystryak S; Acharya C, Detection of HIV-1 p24 antigen in patients with varying degrees of viremia using anELISA with a photochemical signal amplification system. Clin Chim Acta 2016, 456, 128–136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Staszewski S; DeMasi R; Hill AM; Dawson D, HIV-1 RNA, CD4 cell count and the risk of progression toAIDS and death during treatment with HIV-1 reverse transcriptase inhibitors. AIDS 1998, 12 (15), 1991–1997. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.