Abstract
Objective
NOD-like receptor protein 1 (NLRP1) inflammasome complex has been recently associated with chronic unpredictable mild stress (CUMS) model of depression. Our aim was to investigate whether ketamine-induced antidepressant effect is associated with suppression of NLRP1.
Methods
Wistar albino rats were divided into control, CUMS, CUMS+acute ketamine (a single 10 mg/kg dose) and CUMS+chronic ketamine (daily 10 mg/kg injections for 3 weeks) groups (n=10 for each group). Sucrose preference test and forced swimming test were performed to assess anhedonia and immobility time respectively for the severety of depression symptoms. Brain tissues were dissected and prefrontal cortex and hippocampus regions were used for real-time polymerase chain reaction (PCR) and immunohistochemical analysis.
Results
CUMS procedure significantly induced depressive-like symptoms whereas both acute and chronic ketamine treatment ameliorated them. mRNA expression levels of NLRP1, caspase 1, apoptosis-associated speck-like protein containing a CARD (ASC), NF-κB, endothelial nitric oxide synthase, IL-1β, IL-6, toll-like receptor 4 (TLR-4) and purinergic 2×7 receptor (P2X7R) and numbers of Iba- 1+and GFAP+glial cells were reduced by acute and/or chronic ketamine treatment.
Conclusion
In the present study for the first time upstream and downstream elements of the NLRP1 inflammasome complex are shown to be suppressed by ketamine thus reinforcing the involvement of NLRP1 in the physiopathology of depression.
Keywords: Depression, Ketamine, Inflammasome, NLRP1, Glia
INTRODUCTION
Major depressive disorder (MDD) is a mental disorder characterized with low mood, reduced self-esteem, anhedonia, disturbed sleeping, eating habits, cognitive dysfunction and a high risk of suicide [1,2]. Although serotonergic and noradrenergic mechanisms have long been claimed in the pathophysiology of MDD, monoamine hypothesis is now believed to be insufficient to fully explain the pathogenesis of the disease [1-3]. In this context, factors such as disturbed synaptic plasticity, reduced neurogenesis and enhanced inflammation have been suggested to participate in MDD induction [1,4]. Chronic inflammatory diseases including rhematoid arthritis and diabetes have a high comorbidity of depression. MDD patients display increased proinflammatory cytokine (IL-1β, IL-6, TNF-α) levels, which are associated with mood status, dysphoria and anxiety. Moreover, IL-1β inhibition ameliorates depressive symptoms induced by chronic stress exposure [1]. Many major antidepressant medications have been shown to reduce proinflammatory cytokine levels and promote anti-inflammatory (e.g., IL-10) cytokine production [1].
Although serotonin and noradrenaline inhibitors are mainly used for treatment of depression [5], only a small fraction of the patients give significant response to antidepressant treatment [6]. Moreover, therapeutic effect can be elicited weeks to months after treatment initiation thus prompting innovation of more efficient treatment methods. Ketamine is a non-competitive N-methyl-D-aspartate (NMDA) receptor antagonist used for anesthesia and pain treatment [4]. Administration of a single sub-psychotomimetic dosage of ketamine induces notable anti-depressant effects, which may last as long as two weeks [7]. Treatment effect may also be observed in treatment resistant depression cases [4]. One of the mechanisms by which ketamine acts as a prompt anti-depressant medication might putatively be inhibition of inflammatory mechanisms.
In MDD, neuroinflammation is primarily mediated by enhanced glial activity [8,9], which may be induced by, among other factors, the purinergic 2×7 receptor (P2X7R)-inflammasome and toll-like receptor 4 (TLR-4)/NF-κB inflammatory signal pathways [10,11]. Activation of NOD-like receptor proteins (NLRP) by P2X7R results in formation of a multi-protein complex with apoptosis-associated speck-like protein containing a CARD (ASC) and pro-caspase-1, termed as the inflammasome, which relieves caspase-1, thereby leading to production of IL-1β and IL-18 [12]. On the other side, TLR activity is also involved in production of pro-inflammatory cytokines (e.g., IL-1β, IL-6) by microglia [13].
In a recent study, we showed for the first time that NLRP1-mediated inflammasome activation is significantly involved in chronic unpredictable mild stress (CUMS) model and inhibition of this complex via blockade of P2X7R ameliorates neuroinflammation and depressive symptoms [14]. In this study, to delineate whether ketamine administration has a similar mechanism of action, we investigated the impact of ketamine administration on the upstream and downstream elements of the NLRP1 inflammasome pathway and behavioral parameters in CUMS model of rats.
METHODS
Animals and housing
Adult male Wistar albino rats (8–10 weeks old) were obtained from Kocaeli University Experimental Medical Research and Application Center (DETAB, Kocaeli, Turkey) and were housed in groups of five per cage under standard laboratory conditions (22±2℃ room temperature; 12-hour light/dark cycle and relative humidity of 55–50%). Tap water and food pellets were provided ad libitum throughout the experiment. The Animal Research Ethics Committee of Marmara University granted ethical approval (July 14, 2015; Number 57.2015.mar) and all experiments were conducted in accordance with the regulations of animal research ethics committee. Before the experiments rats were allowed to habituate to the laboratory environment and the experimenters for 2 weeks.
Chronic unpredictable mild stress procedure and experimental design
Rats were divided into 4 experimental groups at day 0 (n=10 rats in each group) including the control (non-stressed naïve healthy rats), CUMS, CUMS+10 mg/kg i.p. acute ketamine (once at the sixth week of CUMS procedure; day 42) and CUMS+10 mg/kg i.p. chronic ketamine (once a day in the last three weeks of CUMS procedure; days 21–42) groups. Control and CUMS groups received i.p. physiological saline in the same volume of ketamine. CUMS procedure was applied for a total duration of 6 weeks (42 days), as described previously [15]. Briefly, rats were subjected to different types of stressors, as listed: cage tilting for 24 hours, wet bedding for 24 hours, swimming in 4℃ cold water for 5 minutes, swimming in 45℃ hot water for 5 minutes, pairing with another stressed animal for 48 hours, level shaking for 10 minutes, nip tail for 1 minute, and inversion of the light/dark cycle for 24 hours. These stressors were randomly applied for 6 weeks, and each stressor was applied 3 to 4 times during this period. Rats received only one of these stressors per day, and the same stressor was not applied on two consecutive days to prevent animals from predicting the occurrence of stimulation. The stress procedure did not involve any food or water deprivation. The control animals were kept in a separate experiment room and received no stress apart from saline injections and daily care. Body weights of rats were measured before and at the end of the 6-weeks (Table 1). The sucrose preference test (SPT) and forced swimming test were applied 12 hours after the final ketamine administration. Rats were sacrificed 12 hours after completion of these tests.
Table 1.
Body weight alterations (data are expressed as mean±standard error)
| Experimental groups | Day 0 measurements (g) | 6th week measurements (g) |
|---|---|---|
| Control | 310.5±10.4 | 339.3±10.5 |
| CUMS | 300.6±7.4 | 278.1±7.5* |
| CUMS+acute ketamine | 255.8±5.7 | 287.3±5.8 |
| CUMS+chronic ketamine | 260.4±5.3 | 284.2±5.7 |
p<0.05 compared with control group.
CUMS: chronic unpredictable mild stress
Sucrose Preference Test
Anhedonia-like behaviors were assessed by SPT [16]. In brief, each rat was placed in a test cage identical to home cage and was pre-exposed to the sucrose consumption test for 5 days (15 minutes each day) for the adaptation period. The SPT was carried out on the sixth day of testing. Rats were housed individually and were exposed to two bottles, one containing 100 mL of 20% sucrose and the other containing 100 mL tap water, for a period of 1 hour after 23 hours of food and water deprivation. Water and sucrose intake and preference (%) for sucrose [sucrose solution intake (g)/total fluid intake (g)×100] were calculated.
Forced Swimming Test
The forced swimming test apparatus was a cylinder (height, 40 cm; inside diameter, 38 cm) containing 30 cm of tap water maintained at 25±1℃. The procedure was designed as previously described [17]. The experimental session consisted of two trials; conditioning and the test. During the conditioning trial, rats were gently placed into the cylinder and left in the water for 15 minutes. After the conditioning trial, rats were dried and placed into a warm cage with paper towels for 10 to 15 minutes before being returned to their home cages. The test trial was carried out 24 hours after the conditioning trial. Rats were placed again into the cylinder and left in the water for a 5-minute test session. After the test session, rats were removed from the cylinder, and dried with a towel before being returned to their home cages. The immobility time, which was defined as the lack of motion of the whole body except for the small movements necessary to keep the animal’s head above the water, was recorded. An observer blind to the treatment conditions recorded the time spent immobile in the test session.
Real-time Polymerase Chain Reaction (PCR) analysis
Frozen prefrontal cortex tissues were homogenized and total RNA was extracted using a commercial RNAzol RT isolation kit (Molecular Research Center, Inc., Cincinnati, OH, USA). RNA concentrations were determined by spectrophotometry. The purification and the concentration of 1 μL RNA samples were assessed by 260/280 and 260/230 ratios. Complementary DNA (cDNA) synthesis from RNA samples were performed with commercial cDNA synthesis kit (Jena Bioscience, Jena, Germany). Two microliters of the cDNA sample was used along with qPCR GreenMaster kit (Jena Bioscience) for real-time reverse transcription PCR. The primers used in the study were obtained from DNA Technology (Moscow, Russia) (Table 2). Beta-actin (β-actin) was used as an internal control (housekeeping). The cycle of threshold (CT) of investigated primers was determined and normalized to housekeeping gene, β-actin. Relative quantitation was calculated with 2^-(ddCT) method and data are presented as relative changes to control group.
Table 2.
Primers used in real-time RT-PCR analysis
| Gene | Forward primer | Reverse primer |
|---|---|---|
| NLRP1 | GTTGCAAGTCCCTTCAGCTC | CATCTCTGTTTCCGAGCACA |
| ASC | GCAATGTGCTGACTGAAGGA | TGTTCCAGGTCTGTCACCAA |
| Caspase 1 | GCTTGAAAGACAAGCCCAAG | CCTTTCAGTGGTTGGCATCT |
| NF-ĸB | GGCTGACCTGAGTCTTCTGG | GATAAGGAGTGCTGCCTTGC |
| IL-6 | CCGGAGAGGAGACTTCACAG | ACAGTGCATCATCGCTGTTC |
| eNOS | CGAGATATCTTCAGTCCCAAGC | GTGGATTTGCTGCTCTCTAGG |
| TLR4 | AGCTTTGGTCAGTTGGCTCT | CAGGATGACACCATTGAAGC |
| P2X7R | GTGGAGACGGTGAAGGTGTT | AACGACACCTTTGGGTCTTG |
| β-actin | GCCCCCGGTTTCTATAAATTG | GTCGAACAGGAGGAGCAGAGA |
Immunohistochemistry
Due to the extensive panel of inflammation mediators, the prefrontal cortex was used for real time PCR analysis, whereas hippocampus, another brain region afflicted by depression, was used for immunohistochemistry studies. After perfusion fixation with 4% paraformaldehyde in 0.1 M phosphate-buffered saline (pH 7.4) and decapitation, brain tissues were incubated in the same fixative overnight at 4℃ and were embedded in paraffin. Five-micron-thick paraffin sections were obtained. After deparaffinization, ethanol treatment, antigen retrieval with sodium citrate buffer and incubation with 3% H2O2, sections were incubated with blocking solution and subsequently with anti-ionized calcium-binding adapter molecule-1 (Iba-1) (Abcam, Cambridge, UK) and anti-glial fibrillary acidic protein (GFAP) antibodies (Millipore, Burlington, MA, USA) at 37℃ for 1 hour. After incubation with biotinylated secondary antibody and horseradish peroxidase streptavidin (Invitrogen, Carlsbad, CA, USA), reaction was obtained by 3,3’-diaminobenzidine (Invitrogen). Mayer’s hematoxylin was used for counterstaining. Five areas/per mouse from the hippocampus were evaluated for Iba-1 and GFAP immunoreactive cells under a microscope by using 40×objective.
Statistical analysis
Results were expressed as mean±standard errors. Oneway analysis of variance (ANOVA) was used for statistical comparisons between groups. Tukey’s test was used for post hoc analysis. Baseline and post-treatment body weights of each study group were compared by Student’s t-test. A p value less than 0.05 was considered as a value of significance.
RESULTS
The effects of ketamine treatment on behavioral parameters
Body weights were increased in control, CUMS+acute ketamine and CUMS+chronic ketamine groups and there were no significant differences between the baseline vs. post-treatment values. By contrast, body weights of rats in the CUMS group was significantly reduced, as compared to their baseline values (p<0.05) (Table 1). Rats exposed to 6-week CUMS procedure developed anhedonia-like behavior as demonstrated by significantly lower sucrose preference compared to non-stressed control group (p<0.01) in sucrose preference test. On the other hand, both acute (p<0.01) and chronic treatment (p<0.001) with ketamine significantly increased sucrose preference compared to CUMS group, or in other words ketamine reversed CUMS-induced anhedonia-like behaviors in our study (Figure 1). In forced swimming test, the time of immobility was significantly elevated in CUMS group compared to control group (p<0.01). Rats with chronic, but not acute ketamine treatment, showed significantly shorter duration of immobility indicating that chronic ketamine administration was able to ameloriate CUMS-induced despair behaviors of rats (Figure 1).
Figure 1.
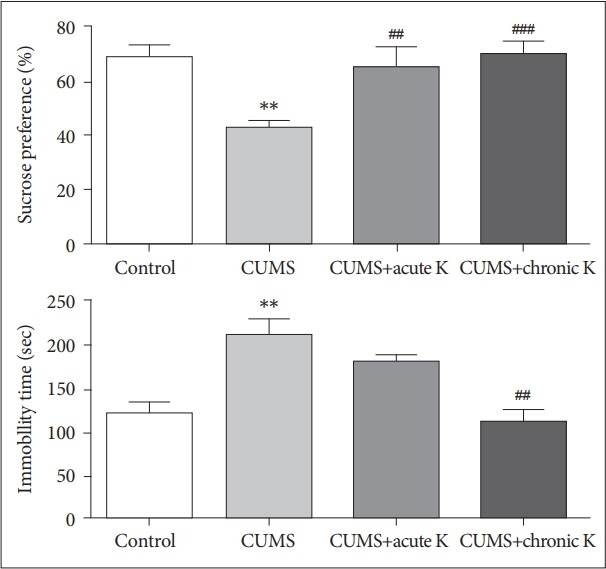
The effect of chronic unpredictable mild stress (CUMS) procedure and acute or chronic ketamine (K) treatment on anhedonia-like behaviors of rats in sucrose preference test (upper panel) and on despair-like behaviors of rats in forced swimming test (lower panel). Vertical bars indicate standard errors. **p<0.01 vs. control group, ##p<0.01 and ###p<0.001 vs. CUMS group.
The effects of ketamine treatment on gene expression levels of NLRP1 cascade and relevant neuroinflammatory components
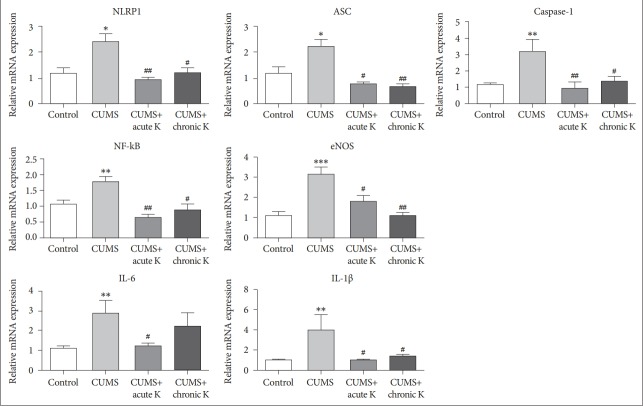
Expression analysis revealed that 6-week CUMS procedure caused a significant elevation in mRNA levels of inflammasome forming protein, NLRP1 (F=5.825, p<0.05). This effect was reversed by both single dose (p<0.01) and chronic intermittent (p<0.05) ketamine treatment (Figure 2). In addition to NLRP1, we investigated two other molecular components, caspase-1 and ASC that together participate in inflammasome formation and thereby play a crucial role in initiating inflammasome-mediated inflammatory responses. Relative mRNA levels of caspase-1 (F=7.790, p<0.01) and ASC (F=6.192, p<0.05) were significantly elevated in the prefrontal cortex of CUMS-treated rats compared to controls. This increase was attenuated by both acute (p<0.01 and p<0.05 for caspase-1 and ASC, respectively) and chronic ketamine (p<0.05 and p<0.01 for caspase-1 and ASC, respectively) treatment. CUMS-induced increase in expression levels of NF-κB (F=8.728, p<0.01) and endothelial nitric oxide synthase (eNOS) (F=8.982, p<0.001) was also reversed by both acute (p<0.01 and p<0.05 for NF-κB and eNOS, respectively) and chronic ketamine (p<0.05 and p<0.01 for NF-κB and eNOS, respectively) treatment. CUMS procedure also elevated relative gene expression of the major pro-inflammatory cytokines IL-6 (F=4.766, p<0.01) and IL-1β (F=4.482, p<0.01). Both acute (p<0.05) and chronic (p<0.05) ketamine administration significantly reduced the mRNA levels of IL-1β. Although IL-6 expression was reduced by acute ketamine treatment (p<0.05), no significant amelioration was observed in IL-6 levels by chronic ketamine treatment (p>0.05) (Figure 2).
Figure 2.
The effect of chronic unpredictable mild stress (CUMS) procedure and acute or chronic ketamine (K) treatment on mRNA levels of NOD-like receptor protein (NLRP) inflammasome components and relevant neuroinflammatory mediators in the rat brain. *p<0.05, **p <0.01 and ***p<0.001 vs. control group. #p <0.05 and ##p <0.01 vs. CUMS group. ASC: apoptosis speck like protein, IL: interleukin, NF-kB: nuclear factor kappa B, eNOS: endothelial nitric oxide synthase. Vertical bars indicate standard errors.
The effects of ketamine treatment on microglial and astroglial activation
Hippocampal Iba-1 immunoreactivity was found to be significantly increased in the CUMS group compared to the nonstressed control group (p<0.01). On the other side, both acute (p<0.01) and chronic intermittent (p<0.01) treatment with ketamine decreased the number of CUMS-induced hippocampal Iba-1+cells (Figure 3A and C). Likewise, the CUMS procedure caused significantly increased GFAP expression in the hippocampus (p<0.01), whereas acute ketamine treatment significantly decreased the number of GFAP+cells (p<0.01). Although rats with chronic intermittent ketamine administration showed trends towards exhibiting reduced numbers of GFAP+cells, this trend did not attain statistical significance (p>0.05) (Figure 3B and C).
Figure 3.
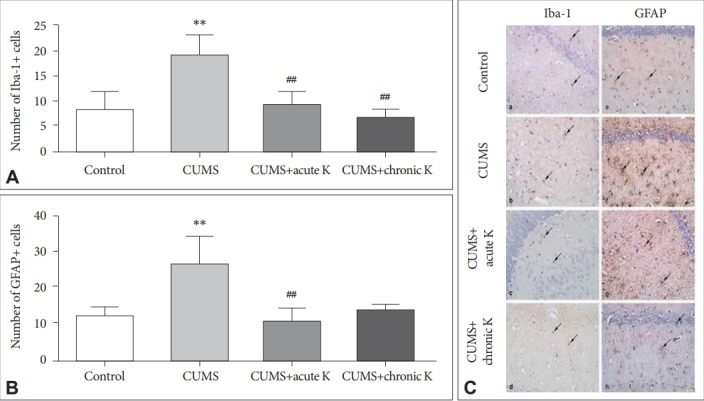
The effect of chronic unpredictable mild stress (CUMS) procedure and acute or chronic ketamine (K) treatment on microglia and astrocyte activation. (A) Number of Iba-1+ microglial cells in the hippocampus. (B) Number of GFAP+ astrocytes in the hippocampus. (C) Iba-1 (a-d) and GFAP (e-h) immunostaining of hippocampus: (a, e) control, (b, f) CUMS, (c, g) CUMS+acute K treatment, and (d, h) CUMS+chronic K treatment. Arrows, Iba-1 (a-d) or GFAP (e-h) immunoreactive microglia or astrocytes, respectively. Original magnification, ×400. **p <0.01 vs. control group, ##p <0.01 vs. CUMS group. Iba-1: ionized calcium-binding adapter molecule-1, GFAP: glial fibrillary acidic protein.
The effects of ketamine treatment on inflammasome-activating receptors
To find out the cell surface receptors by which ketamine alleviates NLRP1-mediated inflammation in the brain tissues of rats exposed to the CUMS model, expression levels of two major inflammasome-activating receptors TLR-4 and P2X7R were measured by real time PCR. The 6-week CUMS procedure caused a significant elevation in mRNA levels of TLR-4 (p<0.05) and P2X7R (p<0.05). CUMS-induced increase in P2X7R expression was reversed by acute (p<0.05) and chronic (p<0.01) ketamine treatment. Alternatively, TLR-4 expression increase was significantly decreased by acute (p<0.05) but not chronic (p>0.05) ketamine treatment (Figure 4).
Figure 4.
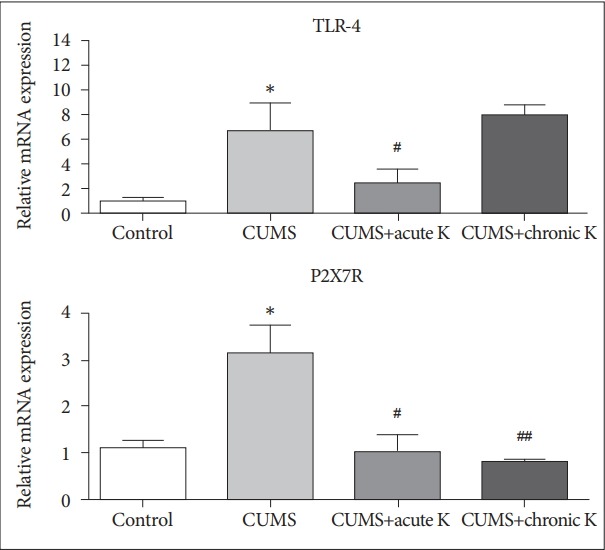
The effect of chronic unpredictable mild stress (CUMS) procedure and acute or chronic ketamine (K) treatment on mRNA levels of toll-like receptor 4 (TLR-4) and purinergic 2X7 receptor (P2X7R). Vertical bars indicate standard errors. *p<0.05 vs. control group, #p <0.05 and ##p <0.01 vs. CUMS group.
DISCUSSION
Chronic stress models of depression closely mimic etiological and clinical aspects of depression and thus are preferred in preclinical studies of MDD. CUMS is one of these chronic models and is characterized with certain aspects of depression such as anhedonia and despair-like behavior that are assessed by sucrose preference test and forced swimming test, respectively [18,19]. In our study, ketamine treatment effectively attenuated sucrose preference and forced swimming test results and also prevented CUMS-induced weight loss. These results are in accordance with a few more studies showing improved parameters of depression in ketaminetreated experimental animals [20-22]. Moreover, in our study, long-term continuous administration of ketamine inhibited both anhedonia and despair-like symptoms, whereas administration of a single dose of ketamine managed to reverse anhedonia but failed to ameliorate despair-like symptoms. Thus, chronic treatment regimen appears to be more effective in treatment of CUMS.
NLRP1 is primarily expressed by neurons and oligodendrocytes and its activation cleaves caspase-1 into its active subunits thereby eliciting IL-1β and IL-18 production [23,24]. Establishment of the CUMS model in rats resulted in enhanced NLRP1 expression in the prefrontal cortex, as reported previously [14]. Acute and chronic ketamine administration reduced expression levels of both NLRP1 and other components of the inflammasome complex, caspase-1 and ASC. These effects occurred in parallel to amelioration of behavioral symptoms by ketamine administration thus suggesting that at least one of the mechanisms by which ketamine alleviates depressive symptoms is inhibition of the NLRP1-caspase-1-ASC complex. Since cerebral caspase-1 and ASC expressions are both enhanced in depression [25,26], ketamine may serve as an effective neuroinflammation inhibiting agent in MDD patients. Our results also suggest that neurons might as well partake in neuroinflammation aspects of MDD, as proposed previously [23].
NF-κB is known to play an important role in the physiopathology of depression by enhancing the transcription of proinflammatory cytokines [27]. Ketamine has been found to inhibit lipopolysaccharide (LPS)-induced NF-κB activation in astrocytes, human glioma cells and mouse brain cells [28-31]. Ketamine has also been found to inhibit NF-κB activity enhanced by bacterial endotoxins in the cecal ligation and puncture model of sepsis [32,33]. Our results showing ketaminmediated reduction in the NF-κB activity of the prefrontal cortex further support the findings of previous reports. Since NF-κB is involved in production of both IL-1β and IL-6 [27], ketamin-mediated reduction of these two cytokines in brain tissues of rats exposed to CUMS may also be explained by reduced NF-κB expression.
IL-1β and eNOS, which are end products of the inflammasome cascade and are actively involved in physiopathology of depression, were both suppressed by acute and chronic ketamine administration [12,33-36]. IL-1β partakes in occurrence of depressive symptoms and anti-depressant medications including ketamine reduce production of IL-1β [1,37]. In a single study, ketamine has also been shown to inhibit eNOS activity in human umbilical vein endothelial cells [38]. Thus, our results further indicate that ketamine is effective in inhibition of both inflammasome complex and its downstream elements.
Given the significant impact of ketamine on the NLRP1 cascade, an important question was the mechanism of action by which ketamine inhibits the elements of this cascade. While ketamine is simply recognized as an NMDAR antagonist, it has diverse effects on other cell surface molecules such as those that belong to glutamatergic and GABAergic systems [39]. In this context, we investigated expression levels of two prominent cell surface receptors involved in neuroinflammation mechanisms, P2X7R and TLR-4. P2X7R is a well-established inflammasome activator and, as previously shown, the selective P2X7R antagonist brilliant blue G is capable of inhibiting CUMS-induced depressive symptoms, glial activity and NLRP1 inflammasome cascade in parallel [14]. We herein have shown that both acute and chronic ketamine treatments reduce prefrontal cortex P2X7R expression in CUMS model of depression and has thus emphasized this purinergic receptor as one of the potential targets of ketamine medication. Similarly, ketamine has been shown to reduce P2X7R expression in chronic restraint stress model of depression [40].
TLR-4/NF-κB/IL-6 is another inflammatory pathway implicated in depression [1,11]. IL-6 production is increased in MDD patients and is associated with dysphoria and anxiety [1]. Ketamine has been shown to reduce expression levels of LPS- or endotoxin-induced IL-6 without altering levels of other cytokines [41,42]. A similar effect was observed in prefrontal cortex samples of rats treated with a single dose of ketamine in our study. However, expression levels of neither IL-6 nor TLR-4 were significantly altered after chronic ketamine treatment. Nevertheless, this treatment regimen ameliorated CUMS-induced body weight loss and depressivelike symptoms. These results suggest that TLR-4 inhibition is not required for treatment of depressive-like symptoms. Thus, depression may putatively be treated through selective inhibition of ATP receptors without influencing TLR functions, which are crucially involved in innate immunity’s actions in first defense against pathogens. This assertion needs to be further analyzed by depression model treatment studies performed with selective TLR inhibitors.
Iba-1 and GFAP molecules are well-known markers of microglial and astrocytic activation, respectively and increased hippocampal glial activation has been shown in chronic stress models of depression including CUMS [43,44]. In a previous study, ketamine reversed enhanced expression levels of Iba-1, IL-1β and TNF-α and memory impairment in rats exposed to electroconvulsive shock [45]. Activated glial cells produce neurotoxic mediators leading to neuronal death and cognitive dysfunction [46]. Therefore, inhibition of glial activity by ketamine confers a neuroprotective role to this drug. Moreover, ketamine has been shown to inhibit LPS-induced astrocyte activation and enhanced GFAP expression by suppressing the TLR-4/NF-ĸB pathway [28]. Our experiments with acute ketamine treatment supports this mode of action of ketamine since astrocytic activity, TLR-4 and IL-6 were downregulated together. By contrast, none of these parameters showed a significant attenuation in rats treated with the chronic ketamine regimen. These results underline the requirement of effective TLR-4 downregulation for inhibition of astrocytic activity.
An intriguing point is that although the half-life of ketamine is less than one hour, the clinical and molecular effects of acute and chronic ketamine administration is observed in brain samples obtained several days after final ketamine administration. This indicates that the anti-depressant effect of ketamine is much long lasting than its anesthetic effects, as also demonstrated by some previous reports [21,47]. At least one of the mechanisms underlying this long lasting effect could be induction of new synapse formation [48].
In conclusion, ketamine treatment was shown to ameliorate depressive-like symptoms induced by chronic stress in a wellestablished and validated animal model. Moreover, our molecular and immunohistochemical studies suggest that this treatment effect is at least partially mediated by suppression of neuroinflammation through downregulation of the NLRP1 inflammasome complex. Our results also suggest that different treatment regimens with ketamine may have diverse effects on neuroinflammatory factors and thus precise details of ketamine treatment should be carefully determined in MDD patients during clinical trials. P2X7R stands out as a major receptor in mediation of anti-neuroinflammatory effects of ketamine. Selective inhibitors of this protein might result in more effective treatment methods for MDD.
Acknowledgments
This study was partially supported by the TÜBİTAK 1002 (SBAG-216S797) and Scientific Research Project Units of Marmara University (Grant numbers: SAG-C-DRP-110915-0417 and SAG-C-DRP-110913-0365).
Footnotes
The authors have no potential conflicts of interest to disclose.
Authors’ contribution
Conceptualization: Feyza Aricioğlu, Ceren Sahin Ozkartal. Data curation: Feyza Aricioğlu, Ceren Sahin Ozkartal, Canan Yalcinkaya, Erdem Tuzun. Formal analysis: Ceren Sahin Ozkartal, Erdem Tuzun. Funding acquisition: Feyza Aricioğlu. Investigation: Feyza Aricioğlu, Ceren Sahin Ozkartal, Canan Yalcinkaya, Tijen Utkan, Cem Ismail Kucukali. Methodology: Canan Yalcinkaya, Serap Sirvanci, Cem Ismail Kucukali. Supervision: Feyza Aricioğlu, Tijen Utkan, Erdem Tuzun. Writing—original draft: Ceren Sahin. Writing—review & editing: Feyza Aricioğlu, Erdem Tuzun.
REFERENCES
- 1.Iwata M, Ota KT, Duman RS. The inflammasome: pathways linking psychological stress, depression, and systemic illnesses. Brain Behav Immun. 2013;31:105–114. doi: 10.1016/j.bbi.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Palazidou E. The neurobiology of depression. Br Med Bull. 2012;101:127–145. doi: 10.1093/bmb/lds004. [DOI] [PubMed] [Google Scholar]
- 3.Sanacora G, Treccani G, Popoli M. Towards a glutamate hypothesis of depression: an emerging frontier of neuropsychopharmacology for mood disorders. Neuropharmacology. 2012;62:63–77. doi: 10.1016/j.neuropharm.2011.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Browne CA, Lucki I. Antidepressant effects of ketamine: mechanisms underlying fast-acting novel antidepressants. Front Pharmacol. 2013;4:161. doi: 10.3389/fphar.2013.00161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zarate C, Duman RS, Liu G, Sartori S, Quiroz J, Murck H. New paradigms for treatment-resistant depression. Ann N Y Acad Sci. 2013;1292:21–31. doi: 10.1111/nyas.12223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li M, Soczynska JK, Kennedy SH. Inflammatory biomarkers in depression: an opportunity for novel therapeutic interventions. Curr Psychiatry Rep. 2011;13:316–320. doi: 10.1007/s11920-011-0210-6. [DOI] [PubMed] [Google Scholar]
- 7.Kavalali ET, Monteggia LM. How does ketamine elicit a rapid antidepressant response? Curr Opin Pharmacol. 2015;20:35–39. doi: 10.1016/j.coph.2014.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sild M, Ruthazer ES, Booij L. Major depressive disorder and anxiety disorders from the glial perspective: Etiological mechanisms, intervention and monitoring. Neurosci Biobehav Rev. 2017;83:474–488. doi: 10.1016/j.neubiorev.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 9.Wang Q, Jie W, Liu JH, Yang JM, Gao TM. An astroglial basis of major depressive disorder? An overview. Glia. 2017;65:1227–1250. doi: 10.1002/glia.23143. [DOI] [PubMed] [Google Scholar]
- 10.Janks L, Sharma CVR, Egan TM. A central role for P2X7 receptors in human microglia. J Neuroinflammation. 2018;15:325. doi: 10.1186/s12974-018-1353-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang F, Zhang C. Rnf112 deletion protects brain against intracerebral hemorrhage [ICH] in mice by inhibiting TLR-4/NF-κB pathway. Biochem Biophys Res Commun. 2018;507:43–50. doi: 10.1016/j.bbrc.2018.10.141. [DOI] [PubMed] [Google Scholar]
- 12.Bhattacharya A, Jones DNC. Emerging role of the P2X7-NLRP3-IL1β pathway in mood disorders. Psychoneuroendocrinology. 2018;98:95–100. doi: 10.1016/j.psyneuen.2018.08.015. [DOI] [PubMed] [Google Scholar]
- 13.Alyu F, Dikmen M. Inflammatory aspects of epileptogenesis: contribution of molecular inflammatory mechanisms. Acta Neuropsychiatr. 2017;29:1–16. doi: 10.1017/neu.2016.47. [DOI] [PubMed] [Google Scholar]
- 14.Aricioglu F, Ozkartal CS, Bastaskin T, Tüzün E, Kandemir C, Sirvanci S, et al. Antidepressant-like effects induced by chronic blockade of the purinergic 2X7 receptor through inhibition of non-like receptor protein 1 inflammasome in chronic unpredictable mild stress model of depression in rats. Clin Psychopharmacol Neurosci. 2019;17:261–272. doi: 10.9758/cpn.2019.17.2.261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Willner P, Muscat R, Papp M. Chronic mild stress-induced anhedonia: a realistic animal model of depression. Neurosci Biobehav Rev. 1992;16:525–534. doi: 10.1016/s0149-7634(05)80194-0. [DOI] [PubMed] [Google Scholar]
- 16.Sarkisova KY, Kuznetsova GD, Kulikov MA, van Luijtelaar G. Spikewave discharges are necessary for the expression of behavioral depression-like symptoms. Epilepsia. 2010;51:146–160. doi: 10.1111/j.1528-1167.2009.02260.x. [DOI] [PubMed] [Google Scholar]
- 17.Porsolt RD, Le Pichon M, Jalfre M. Depression: a new animal model sensitive to antidepressant treatments. Nature. 1977;266:730–732. doi: 10.1038/266730a0. [DOI] [PubMed] [Google Scholar]
- 18.Gerhard DM, Wohleb ES, Duman RS. Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity. Drug Discov Today. 2016;21:454–464. doi: 10.1016/j.drudis.2016.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hill MN, Hellemans KG, Verma P, Gorzalka BB, Weinberg J. Neurobiology of chronic mild stress: parallels to major depression. Neurosci Biobehav Rev. 2012;36:2085–2117. doi: 10.1016/j.neubiorev.2012.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garcia LS, Comim CM, Valvassori SS, Réus GZ, Stertz L, Kapczinski F, et al. Ketamine treatment reverses behavioral and physiological alterations induced by chronic mild stress in rats. Prog Neuropsychopharmacol Biol Psychiatry. 2009;33:450–455. doi: 10.1016/j.pnpbp.2009.01.004. [DOI] [PubMed] [Google Scholar]
- 21.Tizabi Y, Bhatti BH, Manaye KF, Das JR, Akinfiresoye L. Antidepressant- like effects of low ketamine dose is associated with increased hippocampal AMPA/NMDA receptor density ratio in female Wistar-Kyoto rats. Neuroscience. 2012;213:72–80. doi: 10.1016/j.neuroscience.2012.03.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li N, Liu RJ, Dwyer JM, Banasr M, Lee B, Son H, et al. Glutamate Nmethyl- D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol Psychiatry. 2011;69:754–761. doi: 10.1016/j.biopsych.2010.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yap JKY, Pickard BS, Chan EWL, Gan SY. The Role of Neuronal NLRP1 Inflammasome in Alzheimer’s Disease: Bringing Neurons into the Neuroinflammation Game. Mol Neurobiol. 2019;56:7741–7753. doi: 10.1007/s12035-019-1638-7. [DOI] [PubMed] [Google Scholar]
- 24.Kaushal V, Dye R, Pakavathkumar P, Foveau B, Flores J, Hyman B, et al. Neuronal NLRP1 inflammasome activation of Caspase-1 coordinately regulates inflammatory interleukin-1-beta production and axonal degeneration-associated Caspase-6 activation. Cell Death Differ. 2015;22:1676–1686. doi: 10.1038/cdd.2015.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inserra A, Mastronardi CA, Rogers G, Licinio J, Wong ML. Neuroimmunomodulation in major depressive disorder: focus on caspase 1, inducible nitric oxide synthase, and interferon-gamma. Mol Neurobiol. 2019;56:4288–4305. doi: 10.1007/s12035-018-1359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dick MS, Sborgi L, Rühl S, Hiller S, Broz P. ASC filament formation serves as a signal amplification mechanism for inflammasomes. Nat Commun. 2016;7:11929. doi: 10.1038/ncomms11929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim KN, Ko SC, Ye BR, Kim MS, Kim J, Ko EY, et al. 5-Bromo-2-hydroxy- 4-methyl-benzaldehyde inhibited LPS-induced production of pro-inflammatory mediators through the inactivation of ERK, p38, and NF-κB pathways in RAW 264.7 macrophages. Chem Biol Interact. 2016;258:108–114. doi: 10.1016/j.cbi.2016.08.022. [DOI] [PubMed] [Google Scholar]
- 28.Wu Y, Li W, Zhou C, Lu F, Gao T, Liu Y, et al. Ketamine inhibits lipopolysaccharide- induced astrocytes activation by suppressing TLR4/NF-ĸB pathway. Cell Physiol Biochem. 2012;30:609–617. doi: 10.1159/000341442. [DOI] [PubMed] [Google Scholar]
- 29.Welters ID, Hafer G, Menzebach A, Mühling J, Neuhäuser C, Browning P, et al. Ketamine inhibits transcription factors activator protein 1 and nuclear factor-kappaB, interleukin-8 production, as well as CD11b and CD16 expression: studies in human leukocytes and leukocytic cell lines. Anesth Analg. 2010;110:934–941. doi: 10.1213/ANE.0b013e3181c95cfa. [DOI] [PubMed] [Google Scholar]
- 30.Yu M, Shao D, Yang J, Feng S, Xu J. Ketamine suppresses intestinal TLR4 expression and NF-kappaB activity in lipopolysaccharide-treated rats. Croat Med J. 2006;47:825–831. [PMC free article] [PubMed] [Google Scholar]
- 31.Sakai T, Ichiyama T, Whitten CW, Giesecke AH, Lipton JM. Ketamine suppresses endotoxin-induced NF-kappaB expression. Can J Anaesth. 2000;47:1019–1024. doi: 10.1007/BF03024876. [DOI] [PubMed] [Google Scholar]
- 32.Yu M, Shao D, Liu J, Zhu J, Zhang Z, Xu J. Effects of ketamine on levels of cytokines, NF-kappaB and TLRs in rat intestine during CLP-induced sepsis. Int Immunopharmacol. 2007;7:1076–1082. doi: 10.1016/j.intimp.2007.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Sun J, Zhou ZQ, Lv R, Li WY, Xu JG. Ketamine inhibits LPS-induced calcium elevation and NF-kappa B activation in monocytes. Inflamm Res. 2004;53:304–308. doi: 10.1007/s00011-004-1262-4. [DOI] [PubMed] [Google Scholar]
- 34.Yoshino Y, Ochi S, Yamazaki K, Nakata S, Iga JI, Ueno SI. Endothelial nitric oxide synthase in rat brain is downregulated by sub-chronic antidepressant treatment. Psychopharmacology [Berl] 2017;234:1663–1669. doi: 10.1007/s00213-017-4567-z. [DOI] [PubMed] [Google Scholar]
- 35.Liu W, Wang Y, Li H, Ji L. The role of nitric oxide in the antidepressant actions of 5-aminoimidazole-4-carboxamide-1-β-D-ribofuranoside in insulin-resistant mice. Psychosom Med. 2016;78:102–112. doi: 10.1097/PSY.0000000000000268. [DOI] [PubMed] [Google Scholar]
- 36.Deng X, Huang W, Peng J, Zhu TT, Sun XL, Zhou XY, et al. Irisin alleviates advanced glycation end products-induced inflammation and endothelial dysfunction via inhibiting ROS-NLRP3 inflammasome signaling. Inflammation. 2018;41:260–275. doi: 10.1007/s10753-017-0685-3. [DOI] [PubMed] [Google Scholar]
- 37.Tanaka T, Kai S, Matsuyama T, Adachi T, Fukuda K, Hirota K. General anesthetics inhibit LPS-induced IL-1β expression in glial cells. PLoS One. 2013;8:e82930. doi: 10.1371/journal.pone.0082930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen RM, Chen TL, Lin YL, Chen TG, Tai YT. Ketamine reduces nitric oxide biosynthesis in human umbilical vein endothelial cells by down-regulating endothelial nitric oxide synthase expression and intracellular calcium levels. Crit Care Med. 2005;33:1044–1049. doi: 10.1097/01.ccm.0000163246.33366.51. [DOI] [PubMed] [Google Scholar]
- 39.Weckmann K, Deery MJ, Howard JA, Feret R, Asara JM, Dethloff F, et al. Ketamine’s Effects on the Glutamatergic and GABAergic Systems: A Proteomics and Metabolomics Study in Mice. Mol Neuropsychiatry. 2019;5:42–51. doi: 10.1159/000493425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tan S, Wang Y, Chen K, Long Z, Zou J. Ketamine alleviates depressivelike behaviors via down-regulating inflammatory cytokines induced by chronic restraint stress in mice. Biol Pharm Bull. 2017;40:1260–1267. doi: 10.1248/bpb.b17-00131. [DOI] [PubMed] [Google Scholar]
- 41.Taniguchi T, Shibata K, Yamamoto K. Ketamine inhibits endotoxininduced shock in rats. Anesthesiology. 2001;95:928–932. doi: 10.1097/00000542-200110000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Helmer KS, Cui Y, Chang L, Dewan A, Mercer DW. Effects of ketamine/xylazine on expression of tumor necrosis factor-alpha, inducible nitric oxide synthase, and cyclo-oxygenase-2 in rat gastric mucosa during endotoxemia. Shock. 2003;20:63–69. doi: 10.1097/01.shk.0000065766.72937.cf. [DOI] [PubMed] [Google Scholar]
- 43.Calcia MA, Bonsall DR, Bloomfield PS, Selvaraj S, Barichello T, Howes OD. Stress and neuroinflammation: a systematic review of the effects of stress on microglia and the implications for mental illness. Psychopharmacology [Berl] 2016;233:1637–1650. doi: 10.1007/s00213-016-4218-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kreisel T, Frank MG, Licht T, Reshef R, Ben-Menachem-Zidon O, Baratta MV, et al. Dynamic microglial alterations underlie stress-induced depressive-like behavior and suppressed neurogenesis. Mol Psychiatry. 2014;19:699–709. doi: 10.1038/mp.2013.155. [DOI] [PubMed] [Google Scholar]
- 45.Zhu X, Li P, Hao X, Wei K, Min S, Luo J, Xie F, Jin J. Ketamine-mediated alleviation of electroconvulsive shock-induced memory impairment is associated with the regulation of neuroinflammation and soluble amyloid-beta peptide in depressive-like rats. Neurosci Lett. 2015;599:32–37. doi: 10.1016/j.neulet.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 46.Fehlings MG, Nguyen DH. Immunoglobulin G: a potential treatment to attenuate neuroinflammation following spinal cord injury. J Clin Immunol. 2010;30(Suppl 1):S109–S112. doi: 10.1007/s10875-010-9404-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhang GF, Liu WX, Qiu LL, Guo J, Wang XM, Sun HL, et al. Repeated ketamine administration redeems the time lag for citalopram’s antidepressant- like effects. Eur Psychiatry. 2015;30:504–510. doi: 10.1016/j.eurpsy.2014.11.007. [DOI] [PubMed] [Google Scholar]
- 48.Duman RS, Voleti B. Signaling pathways underlying the pathophysiology and treatment of depression: novel mechanisms for rapid-acting agents. Trends Neurosci. 2012;35:47–56. doi: 10.1016/j.tins.2011.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]