Abstract
It has been accepted that the force produced by a skeletal muscle myofibril depends on its cross-sectional area but not on the number of active sarcomeres because they are arranged in series. However, a previous study performed by our group showed that blocking actomyosin interactions within an activated myofibril and depleting the thick filaments in one sarcomere unexpectedly reduced force production. In this study, we examined in detail how consecutive depletion of thick filaments in individual sarcomeres within a myofibril affects force production. Myofibrils isolated from rabbit psoas were activated and relaxed using a perfusion system. An extra microperfusion needle filled with a high-ionic strength solution was used to erase thick filaments in individual sarcomeres in real time before myofibril activation. The isometric forces were measured upon activation. The force produced by myofibrils with intact sarcomeres was significantly higher than the force produced by myofibrils with one or more sarcomeres lacking thick filaments (p < 0.0001) irrespective of the number of contractions imposed on the myofibrils and their initial sarcomere length. Our results suggest that the myofibril force is affected by intersarcomere dynamics and the number of active sarcomeres in series.
Significance
The sarcomere is the most basic unit responsible for muscle contraction. Several sarcomeres in series form a myofibril, and several myofibrils form a muscle cell. It has been known that sarcomeres do not necessarily contract homogeneously during a contraction, but the intrinsic mechanisms are not known. In this study, we directly investigated the effects of sarcomere length nonuniformity on force production in isolated myofibrils. We used a novel system to deplete proteins of individual sarcomeres to check the effects on myofibril contraction.
Introduction
The sarcomere is the single, most basic unit of muscle. Within the sarcomere, the interaction of myosin and actin filaments causes myofibrils, and ultimately muscle fibers, to contract and produce force (1). Two theories dominate the way we think about contraction: the cross-bridge and the sliding filament theories (2, 3, 4). Together, these theories postulate that contraction occurs when actin filaments slide over myosin filaments through the creation and subsequent detachment of cross-bridges formed by the myosin heads, a process that is powered by ATP hydrolysis. One of the best evidences that these theories work is the relationship that exists between force and sarcomere length (SL). It shows that the active force generated by a sarcomere is proportional to the degree of filament overlap; maximal force is reached when the overlap between thick and thin filaments is optimal (4). It is now recognized that myosin and actin are not the only proteins that are important for contraction because they are part of an immense array of sarcomeric proteins (5, 6, 7) that are involved in structural stability and passive force production and that also modulate the active force produced by a myofibril.
Many studies have shown that sarcomeres exhibit a nonuniform behavior during myofibril activation, such that their length will not change uniformly. This phenomenon—referred to as SL nonuniformities (8,9)—affects force production through a complex intersarcomere dynamic that is not yet well understood. In a previous study performed in our laboratory (10), we found that extracting myosin filaments in individual sarcomeres before activation, which causes individual SL adjustments, reduced the force significantly. We interpreted the force reduction to be a result of changes in intersarcomere dynamics, which may dictate the levels of force produced by the myofibril.
In this study, we aimed to explore in more detail how depletion of thick myosin filaments affects intersarcomere dynamics and force production in single myofibrils. We tested the myofibril’s ability to continue to produce force after sequential extraction of myosin A-bands with the use of a microfluidic perfusion system used previously in our laboratory (10).
We observed that one A-band extraction was sufficient to decrease force production by the myofibrils and that successive extractions continued to decrease force independently of the initial SL (SLi) at which the myofibril was activated and the number of contractions imposed on the myofibril. Our results strengthen the hypothesis that intersarcomere dynamics and the number of active sarcomeres in series are important for myofibril force production, likely because of the transmission of forces through contractile and passive elements among sarcomeres.
Materials and Methods
Myofibril preparations
Small muscle bundles from rabbit psoas were dissected, rinsed in rigor solution, and tied to wooden sticks. The protocol was approved by the McGill University Animal Care Committee and complied with the guidelines of the Canadian Council on Animal Care. The samples were stored in rigor solution/glycerol (50:50) solution for 15 h at −20°C and then transferred to fresh rigor solution/glycerol (50:50) solution containing a mixture of protease inhibitors (Roche Diagnostics, Rotkreuz, Switzerland) for at least 7 days before experiments. On the day of the experiment, small muscle pieces were cut and defrosted in rigor solution at 4°C for 1 h. These pieces were then homogenized in rigor solution using a VWR 250 Homogenizer Unit (VWR International, Mississauga, Canada) with a 5-mm generator probe. The homogenate was transferred to an experimental temperature-controlled bath (4°C), where it was washed with rigor solution. Subsequently, the rigor solution was carefully replaced by relaxing solution.
Myofibrils were imaged with a phase contrast inverted microscope (Eclipse TE2000-U; Nikon Canada Inc, Canada). Under high magnification (Plan Fluor 100 oil objective plus 1.0× built-in microscope magnification), the contrast between the dark A-bands and the light I-bands provided a dark-light intensity pattern representing the striation pattern produced by the sarcomeres; based on these patterns, myofibrils were chosen and their average SL was set during experiments (Fig. 1, bottom). Myofibril contraction was induced using an activation solution (Video S3).
Figure 1.
(Top) Three-dimensional (3D) schematic representation of the experimental system. The myofibril is held between two precalibrated needles (in gray holding the fiber) that allow force measurement upon activation. Activation is triggered by rapid changes in solution from the double-barreled perfusion (large gray structure in the back). Extraction of individual myosin A-bands is done with the microperfusion pipette (blue), which is controlled by a pressure system. (Middle) One myofibril attached between two needles is held at a mean SLi = 2.9 μm, surrounded by a large perfusion (not visible). The six different frames show the myofibril at different time points during the experiment. (1) Myofibril is shown attached to the needles before the A-band extraction and activation. (2) The microperfusion is placed close to a sarcomere in the center of the myofibril. (3) The release of the HIS in the microperfusion causes the extraction of the A-band. (4) Microperfusion is pulled away. (5) Myofibril activation is shown here. (6) Myofibril relaxation is shown here. In (4)–(6), the asterisk indicates the position of the extracted A-band. (Bottom) A magnified picture of a myofibril shows the SL measurements. Double-headed arrows point to the Z-disks on the myofibril and the corresponding point on the gray intensity profile of the long axis. The profile represents different areas of the striated pattern of the myofibril: the high peaks represent the I-bands, the small valleys in-between represent the Z-disks, the big valleys represent the A-bands, and the small peaks in-between represent the M-line.
Experimental solutions
The rigor solution contained 50 mM Tris, 100 mM KCl, 4 mM MgCl2, and 10 mM EGTA (pH 7.0). The relaxing solution contained 70 mM KCl, 20 mM imidazole, 5 mM MgCl2, 5 mM ATP, 14.5 mM creatine phosphate, 7 mM EGTA, and (pCa2+ (−log[Ca2+]) 9.0 (pH 7.0)). The activation solution contained 50 mM KCl, 20 mM imidazole, 5 mM MgCl2, 5 mM ATP, 14.5 mM creatine phosphate, and 7.2 mM EGTA (pCa2+ 4.5 (pH 7.0)). The high ionic strength (HIS) solution used to extract the A-bands contained 900 mM KCl added to the relaxing solution.
Experimental system
We used two precalibrated needles to pierce the myofibrils parallel to the Z-disks. The stiffness of all needles (45.18 ± 2.14 nN/μm) was measured using an atomic force cantilever, as previously described (11). Both needles were deflectable. We used a glass micropipette to erase A-bands from the myofibrils (Fig. 1, top, middle). The micropipette tip diameter was adjusted to 1 μm to keep it smaller than the sarcomere A-bands (we refer to this micropipette hereupon as the microperfusion). Needles and micropipettes were pulled using a capillary puller (Kopf Model 720; David Kopf Instruments, Tujunga, CA) and controlled with micromanipulators (NT88-V3; Narishige Co., Ltd. Tokyo, Japan).
Individual sarcomeres within myofibrils were point depleted by the microperfusion system filled with a HIS solution, which was controlled by a pressure system. The sarcomere was treated with HIS solution for 3–5 s. A large multichannel perfusion, to avoid diffusion of the microperfusion HIS solution, was positioned at an angle from the needles and provided a constant flow of relaxing solution in the preparation, hence controlling the remaining sarcomeres in the preparation (Fig. 1, top; Video S2). The large perfusion system was computer controlled and was connected to two pipettes, allowing us to change between relaxing and activating solution. All procedures were imaged using a Hamamatsu Orca-ER digital camera (Hamamatsu Photonics, Japan). Videos were recorded at 43.3 frames per second. All experimental procedures were recorded and analyzed using the TrackMate plugin for ImageJ software (National Institutes of Health).
In this example, we also added food dye to detect the flow coming out of the micropipette by producing a darker flow, which can be detected in video A as a dark shadow that follows the movement of the micropipette. Video B shows the same experiment but it has been modified with the Threshold tool from Fiji which transforms an image into two or more classes of pixels (17). In video B the flow is easily detected by the class change of the pixels. We can observe there is no damage to the myofibril from the jet-fluid coming out of the microperfusion as shown by its regular structure.
Experimental procedure
At 100× magnification, myofibrils were pierced and lifted from the coverslip, and SL was adjusted to either 2.8 or 3.2 μm. The myofibrils were activated or relaxed by changing the flow of relaxing or activating solutions. A-bands within myofibrils were point depleted using the HIS solution flowed through the microperfusion system under relaxing solution, and once the A-band was extracted, the microperfusion was withdrawn from the experimental bath (to avoid the depletion of adjacent bands). In some experiments, we depleted more than one pair of A-bands during extraction because of small lateral displacement of the microperfusion system. We included these experiments in our analysis to study the effects of more than one extraction at a time.
Data analysis
Absolute force was measured by tracking the displacement in position of the calibrated needles. We compared the force production of intact myofibrils versus the force production of myofibrils lacking sequentially extracted A-bands. Because the forces produced by myofibrils are proportional to their cross-sectional areas and because we wanted to compare different conditions irrespective of this variable, all measured forces were normalized and reported in units of stress, assuming a circular geometry for the myofibril (nanonewtons per micrometer2). This procedure was done by taking the myofibril image, measuring the diameter at every A-band, and averaging those values. The individual SL of the myofibrils in each group was calculated separately, and the average SL was obtained. The myofibrils were adjusted during the experiment to a nominal SLi of 2.7μm (±0.02 μm) or 3.1 μm (±0.02 μm), the latter along the descending limb of the force-SL relationship.
Statistical analyses
A two-way analysis of variance (ANOVA) was used to compare the force among myofibrils considering the number of extractions and the SLi groups. We used Bonferroni’s test for post hoc analysis when significant changes were observed. p ≤ 0.05 was the level of significance set for all analyses. Results are presented as means ± standard error (SE).
Results
Force traces after extraction of A-bands
Myofibrils were divided into three groups: 1) controls (no A-band extractions), 2) SLi = 2.7 μm, and 3) SLi = 3.1 μm (both with A-band extractions). To demonstrate the appearance of a myofibril under myosin A-band extraction, the middle panel in Fig. 1 displays six frames collected at different time points in an experiment (see also Video S1). Frame 1 shows the myofibril under relaxed condition attached to the needles surrounded by a large perfusion, which provides a constant flow of relaxing solution to the sample. Frame 2 shows the moment the tip of the microperfusion system is placed close to the myofibril before releasing the HIS solution. Frame 3 displays the moment the myosin A-band close to the middle of the myofibril has been extracted. We can observe that the myofibril remains intact after extraction of one A-band on frame 4 after the microperfusion has been carefully pulled away from the myofibril. Frame 5 shows the myofibril during contraction, when the large perfusion flow has been switched from relaxing to activating solution. Frame 6 shows the time when the flow has been changed back to relaxing solution. Frames 4–6 summarize the moment in which the force was measured, as described in the Materials and Methods. A limitation in this study is that it is virtually impossible for us to know precisely if all structures were removed from the A-band. However, the images collected during our experiments follow those shown in previous experiments performed in our laboratory (10) and others (12,13).
This video is also summarized in 6 frames on Fig. 1. The myofibril is attached between two needles held at a mean SLi = 2.9 μm, surrounded by a large perfusion (not visible). We observe the myofibril attached to the needles before A-band extraction and activation. The microperfusion is placed close to a sarcomere in the center of the myofibril. Subsequently, the HIS is flown through the microperfusion and one A-band is extracted. The microperfusion is then pulled away and the myofibril is activated and relaxed.
We often observed SL nonuniformities that developed during activation of the myofibrils, as evident in Fig. 1. The degree of nonuniformity varied considerably between myofibrils and experiments. Importantly, the SL uniformity is fully reestablished when the myofibrils are deactivated (see Video S3).
Fig. 2 shows representative force traces produced by a control myofibril, without extracted A-bands. The force decreased slightly in each contraction. In this case, the force changed from 234.5 nN/μm2 during the first contraction to 154.6 nN/μm2 during the sixth contraction, which accounts for ∼70% of the original force production. This decline is commonly observed in experiments with myofibrils (10). Fig. 3 shows two myofibrils in which the A-bands were extracted sequentially and that have been activated at different average SLi. The force decreased more significantly than in control myofibrils. When activated at SLi = 2.6 μm, consecutive extraction of four A-bands reduced maximal force production during the plateau from 174.1 to 77.11 nN/μm2 (Fig. 3 A). This represents ∼40% of the original force production during the first contraction, when no bands had been extracted. When another myofibril was activated at SLi = 3.12 μm with four A-bands extracted (Fig. 3 B), the maximal force changed from 120.6 to 35.49 nN/μm2, which accounts for ∼30% of the original force production.
Figure 2.
Force traces of a representative control myofibril during six activation and relaxation cycles.
Figure 3.
(A and B) Force traces of a representative myofibril activated at 2.6 and 3.12 μm, respectively. The traces in both graphs show force development during five contractions after the extraction of myosin A-bands, as specified in the legend.
Force change after consecutive contractions and A-band extractions
The results from control myofibrils and myofibrils with A-band extractions are grouped in Fig. 4. Force changes rapidly during the second contraction after only one A-band extraction (Fig. 4 B). The mean force for all of the myofibrils that we tested in this group was 69.19 ± 2.36% of the initial force produced during the first contraction, whereas the control myofibrils still produced 86.80 ± 1.81% of the initial force. It required at least eight control contractions for the mean of the control group to fall below 50% of the original force production (mean = 44.13 ± 6.02%), whereas in the experimental group, four contractions (approximately three A-bands extracted) were enough for the mean force production to fall under 50% (mean = 48.03 ± 1.96%). Force dropped twice as fast in the group with extractions (p < 0.0001), highlighting the importance of the number of sarcomeres in series for force production.
Figure 4.
Normalized, maximal force obtained in different myofibrils as they are consecutively activated and relaxed. (A) In the control group, the force decreases as each myofibril is exposed to more contractions; however, the force remains relatively high for the first few contractions and does not decrease as rapidly as in myofibrils with extracted sarcomeres. The sample sizes are as follows: 1 (n = 29), 2 (n = 29), 3 (n = 24), 4 (n = 19), 5 (n = 12), 6 (n = 8), 7 (n = 7), 8 (n = 5), 9 (n = 3), 10 (n = 1), 11 (n = 1), and 12 (n = 1). (B) In the A-band extraction group, the force decreases significantly (p < 0.0001) as each myofibril is exposed to a contraction after each extraction. The sample sizes are as follows: 0 (n = 46), 1 (n = 33), 2 (n = 40), 3 (n = 32), 4 (n = 30), 5 (n = 26), 6 (n = 18), 7 (n = 19), 8 (n = 10), 9 (n = 10), 10 (n = 5), 11 (n = 1), 12 (n = 1), and 13 (n = 1).
Change in force after consecutive contractions in myofibrils of different lengths
Because the myofibrils used in these experiments had different lengths and therefore different numbers of sarcomeres, we evaluated how force changes after consecutive contractions while considering the length of the myofibril (Fig. 5). This is important because A-band loss could result in a greater relative enhancement of series compliance in the shorter myofibrils. We performed a two-way ANOVA using 1) number of sarcomeres and 2) number of contractions as repeated factors. We evaluated the first four contractions in all myofibrils without missing values. We chose this procedure because myofibrils with A-band extractions lose 50% of their contractile force after four contractions. We confirmed that the number of sarcomeres did not affect the force decrease (p = 0.39), although the number of contractions decreased force production (p < 0.0001). The percent change in control myofibrils from one contraction to the next is on average 12% for the second and third contraction and on average 6% for every contraction thereafter.
Figure 5.
(A) Data points show the mean ± SE of the maximal force obtained during the plateau in control myofibrils with different numbers of sarcomeres as they are exposed to successive contractions. The force decreases as each myofibril is exposed to more contractions (p < 0.0001). (B) shows the same data as (A), highlighting only the mean for the specific number of contractions at each point. The sample sizes are as follows: 7 (n = 1), 9 (n = 1), 12 (n = 3), 13 (n = 3), 14 (n = 3), 15 (n = 4), 16 (n = 1), 17 (n = 3), 18 (n = 1), 19 (n = 2), 20 (n = 1), 21 (n = 1), 22 (n = 1), 25 (n = 2), 28 (n = 1), and 30 (n = 1).
Change in force after extraction of myosin A-bands in myofibrils of different lengths and the corresponding contraction
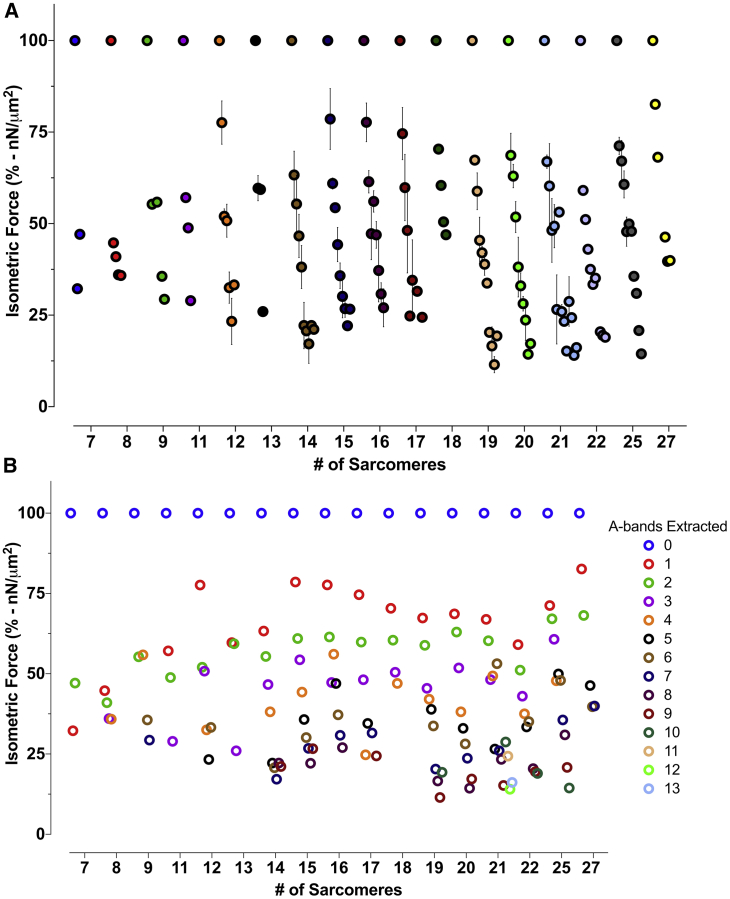
We considered how the length of the myofibril affected force changes in the myofibrils that had the A-bands extracted. Fig. 6 shows the change in mean force (%) of myofibrils of different lengths after successive individual extractions of A-bands. For all myofibrils, the successive extraction of individual myosin A-bands produced a reduction in total isometric force (Fig. 6). On average, the first A-band extraction reduced force production by 31%. The second and third extractions reduced force by approximately an extra 10% and 4% thereafter. In this group, shorter myofibrils showed a more pronounced force change than longer myofibrils, confirming results previously obtained at our lab (10).
Figure 6.
(A) Data points show the change (mean ± SE) in force for myofibrils of different lengths as individual A-bands are successively extracted. (B) shows the same data from (A), distinguishing the mean for the specific number of extractions at each point. The sample sizes are as follows: 7 (n = 1), 8 (n = 1), 9 (n = 1), 11 (n = 1), 12 (n = 6), 13 (n = 2), 14 (n = 4), 15 (n = 4), 16 (n = 4), 17 (n = 5), 18 (n = 1), 19 (n = 3), 20 (n = 4), 21 (n = 4), 22 (n = 1), 25 (n = 3), and 27 (n = 1).
Next, we considered the effects of the number of contractions imposed on a myofibril and the number of A-bands extracted from the same myofibril. Fig. 7 shows the isometric forces produced by myofibrils with different numbers of myosin A-bands extracted, while considering the number of contractions previously imposed on the myofibril, from the second to the 10th contraction. When we control for the number of contractions, before a particular number of extractions, myofibrils lacking a greater number of A-bands will produce less force. This pattern remains the same for all contractions and emphasizes the importance of the individual contributions of sarcomeres for myofibril activation and force production.
Figure 7.
Effect of the number of extractions for different number of contractions performed. For a particular contraction, myofibrils lacking a greater number of A-bands produced less force. The sample sizes are as follows: contraction no. (no. of extractions (n)) = second (0 (29), 1 (30), 2 (7), 4 (1)); third (0 (24), 1 (1), 2 (29), 3 (7), 4 (1), 5 (1)); fourth (0 (19), 1 (2), 2 (2), 3 (25), 4 (5), 5 (2), 7 (2)); fifth (0 (12), 2 (2), 4 (22), 5 (7), 6 (2), 7 (1), 9 (1)); sixth (0 (8), 3 (1), 4 (1), 5 (15), 6 (4), 7 (3), 9 (1)); seventh (0 (7), 6 (12), 7 (4), 8 (2), 10 (1)); eighth (0 (5), 7 (8), 8 (4), 9 (2)); ninth (0 (3), 8 (4), 9 (4), 10 (1)); and 10th (0 (1), 9 (2), 10 (1)).
Change in force and passive force
Finally, to test the effects of myofibril stiffness, and likely the involvement of titin and passive force in the transmission of force between sarcomeres, we compared control myofibrils and myofibrils with A-band extractions according to their SLi (Figs. 8 and 9, respectively). Myofibrils were grouped into either short or long mean SLi. The short SL myofibrils had a mean SLi = 2.70 ± 0.02 μm, and the long SL myofibrils had a mean SLi = 3.10 ± 0.02 μm. When we performed a two-way ANOVA, we observed that the SLi at which the myofibril was activated had a significant main effect in control myofibrils (p = 0.03) (Fig. 8). However, in myofibrils with successive myosin A-band extractions, the SLi had no main effect (p = 0.27) (Fig. 9).
Figure 8.
Mean isometric force (%) produced by myofibrils in two different experimental conditions: SLi = 2.7 μm and SLi = 3.1 μm during successive control contractions. The dotted line represents the normalized isometric force of the first contraction. The two conditions were significantly different (p = 0.03). The sample sizes for SLi = 2.7 μm are as follows: 1 (n = 19), 2 (n = 19), 3 (n = 16), 4 (n = 12), 5 (n = 8), 6 (n = 5), 7 (n = 4), 8 (n = 3), 9 (n = 2), 10 (n = 1), 11 (n = 1), and 12 (n = 1). The sample sizes for SLi = 3.1 μm are as follows: 1 (n = 10), 2 (n = 10), 3 (n = 8), 4 (n = 7), 5 (n = 4), 6 (n = 3), 7 (n = 3), 8 (n = 2), and 9 (n = 1).
Figure 9.
Mean isometric force (%) produced by myofibrils in two different experimental conditions: SLi = 2.7 μm and SLi = 3.1 μm after successive extractions of myosin A-bands. The dotted line represents the normalized isometric force before A-band extraction. The two conditions were not significantly different (p = 0.27). The sample sizes for SLi = 2.7 μm are as follows: 0 (n = 29), 1 (n = 17), 2 (n = 26), 3 (n = 19), 4 (n = 18), 5 (n = 15), 6 (n = 10), 7 (n = 12), 8 (n = 6), 9 (n = 6), 10 (n = 4), 11 (n = 1), 12 (n = 1), and 13 (n = 1). The sample sizes for SLi = 3.1 μm are as follows: 0 (n = 17), 1 (n = 16), 2 (n = 14), 3 (n = 13), 4 (n = 12), 5 (n = 11), 6 (n = 8), 7 (n = 7), 8 (n = 4), 9 (n = 4), and 10 (n = 1).
Discussion
The main findings of this study were that 1) extraction of thick filaments from an individual sarcomere decreased myofibril force significantly and 2) extraction of the first pair of myosin A-bands represented the most significant force loss when compared to subsequent extraction of myosin A-bands. Consequently, the first extraction severs a critical linkage between adjacent sarcomeres and disrupts myofibril force development. Myofibrils adjusted to longer SLi showed similar force loss levels when compared to myofibrils adjusted to shorter SLi.
There are studies looking at intersarcomere dynamics in isolated myofibrils (1,14). In a study using α-actinin to localize the Z-disk boundary, Telley et al. (1) showed that differences in active force produced in neighboring A-bands were responsible for A-band shifts that occur early during activation of a myofibril. This nonuniform sarcomere dynamic was linked to a different number of potential cross-bridges in each half-sarcomere (1). Shimamoto et al. (14) reported that adjacent sarcomeres influence each other through Z-disk structures. The authors observed active force drop ∼2-fold after antibody binding to α-actinin in the Z-disk under partial activation conditions. This study showed that intermediate structures such as the Z-disk not only have a role in structural organization but also actively regulate force transmission by participating in intersarcomere coordination. Taken together with our results, it is clear that sarcomeres have several levels of cross talk that go beyond the sliding of filaments (4), and that the modulation of the thick filament lattice in an individual sarcomere is able to disrupt efficient intersarcomere communication.
A possible explanation for the significant effects of myosin A-band extraction, when compared to our control group, can be found in the potential for cross-bridge formation of half-sarcomeres relayed through intermediate filaments. Initially, when all sarcomeres are intact, the potential cross-bridge formation is high. After the first A-band is extracted (when the most substantial reduction in force production occurs), a critical link involved in sarcomere dynamics is disengaged, and the potential for cross-bridge formation of neighboring sarcomeres decreases. This signal relay through intermediate lattice structures along the myofibril continues to drop with each A-band extraction, in parallel with the effects of consecutive contractions. This is further supported by the dissimilar force loss between short myofibrils (with fewer sarcomeres) and long myofibrils in the A-band extraction group, as previously reported (10). This interpretation is also in line with previous studies investigating sarcomere dynamics, suggesting that the shortening and the force produced by one sarcomere influence the work of adjacent sarcomeres. For example, for many years it has been shown that spontaneous oscillatory contractions (SPOCs) that happen during partial activation are associated with a cross talk among sarcomeres (14). During this phenomenon, each sarcomere repeats cycles of slow shortening and rapid lengthening sequentially. Most interestingly, the sarcomere lengthening phase propagates to adjacent sarcomeres along the axis of myofibrils, creating “waves” of activation (14). These SPOCs strongly suggest a cooperative behavior of sarcomeres not necessarily related to nonuniform activation, but rather associated with an intrinsic property of myofibrils. Very importantly in the context of this work, SPOCs happen in clustered groups of sarcomeres, not randomly through the myofibril.
It has also been shown that the relaxation of single sarcomeres propagates along individual myofibrils after a rapid removal of Ca2+ (15), strengthening the notion of cooperative behavior of individual sarcomeres. Telley et al. (1) evaluated the process of sequential half-sarcomere relaxation along a myofibril, and their results imply that cross-bridge detachments in adjacent half-sarcomeres are coupled (1). Our results agree with their conclusion because at any time we depleted the A-band on an individual sarcomere, we also impaired the capacity of lateral force transmission in the myofibrils, consequently decreasing force production.
It is important to note that, because our system measures force based on the deflection of the microneedles, the contractions developed by the myofibrils were never fully isometric. In our case, fully activated control myofibrils shortened 18.2 ± 4.2% per sarcomere. Myofibrils with depleted A-bands shortened less. After the first extraction, they shortened 12.4 ± 5.1%. In this case, sarcomeres from which the A-bands were removed extended by 7.4 ± 1.2%, which increased the SL nonuniformity in the preparation.
A previous study performed in our laboratory also demonstrated that blocking actomyosin interactions of a single sarcomere during contraction reduced force significantly. More importantly, it showed that stretching the myofibril after the extraction did not recover the force levels to those of intact myofibrils (10). In light of this, we tested myofibrils in different SLi. When activated at a long SL, the myofibril lies on the descending limb of the force-length relationship in which passive tension from titin is most predominant (16). In our study, there was no difference in force loss between myofibrils adjusted to short or long SLi after A-band extraction. Furthermore, we observed that in the control group, short myofibrils do not lose force production ability more steeply than long myofibrils (Fig. 5), suggesting that their cross-bridge formation potential remains relatively high because their intermediate filaments’ communication mechanism has not been disrupted (1,14). The possibility remains that passive force structures and intermediate filaments bear the loss of one myosin A-band, but the underlying mechanisms are unclear.
Conclusion
In summary, the number of sarcomeres in series of a myofibril has an important role in force production, which is dictated by the number of active sarcomeres in series and their ability to efficiently relay information to each other. Such cross talk between sarcomeres suggests a cooperative mechanism of myofibril activation and deactivation that remains to be fully understood.
Author Contributions
D.E.R. designed research. A.C.M. carried out all experiments and analyzed the data. A.C.M. and D.E.R. wrote the article.
Acknowledgments
We thank engineer David Hernández Viloria for his help with the three-dimensional representation of the system. D.E.R. is a Canada Research Chair in Muscle Biophysics. This work was supported by the Natural Sciences and Engineering Research Council of Canada.
Editor: David Warshaw.
Footnotes
Supporting Material can be found online at https://doi.org/10.1016/j.bpj.2020.03.007.
Supporting Citations
Reference (17) appears in the Supporting Material.
Supporting Material
References
- 1.Telley I.A., Denoth J., Stehle R. Half-sarcomere dynamics in myofibrils during activation and relaxation studied by tracking fluorescent markers. Biophys. J. 2006;90:514–530. doi: 10.1529/biophysj.105.070334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Huxley A.F., Niedergerke R. Structural changes in muscle during contraction; interference microscopy of living muscle fibres. Nature. 1954;173:971–973. doi: 10.1038/173971a0. [DOI] [PubMed] [Google Scholar]
- 3.Huxley H.E. The double array of filaments in cross-striated muscle. J. Biophys. Biochem. Cytol. 1957;3:631–648. doi: 10.1083/jcb.3.5.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gordon A.M., Huxley A.F., Julian F.J. The variation in isometric tension with sarcomere length in vertebrate muscle fibres. J. Physiol. 1966;184:170–192. doi: 10.1113/jphysiol.1966.sp007909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Maruyama K., Natori R., Nonomura Y. New elastic protein from muscle. Nature. 1976;262:58–60. doi: 10.1038/262058a0. [DOI] [PubMed] [Google Scholar]
- 6.Wang K., McClure J., Tu A. Titin: major myofibrillar components of striated muscle. Proc. Natl. Acad. Sci. USA. 1979;76:3698–3702. doi: 10.1073/pnas.76.8.3698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wang K., Ramirez-Mitchell R. A network of transverse and longitudinal intermediate filaments is associated with sarcomeres of adult vertebrate skeletal muscle. J. Cell Biol. 1983;96:562–570. doi: 10.1083/jcb.96.2.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Edman K.A., Reggiani C. Redistribution of sarcomere length during isometric contraction of frog muscle fibres and its relation to tension creep. J. Physiol. 1984;351:169–198. doi: 10.1113/jphysiol.1984.sp015240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Morgan D.L. New insights into the behavior of muscle during active lengthening. Biophys. J. 1990;57:209–221. doi: 10.1016/S0006-3495(90)82524-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Souza Leite F., Minozzo F.C., Rassier D.E. Microfluidic perfusion shows intersarcomere dynamics within single skeletal muscle myofibrils. Proc. Natl. Acad. Sci. USA. 2017;114:8794–8799. doi: 10.1073/pnas.1700615114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Leite F.S., Minozzo F.C., Rassier D.E. Reduced passive force in skeletal muscles lacking protein arginylation. Am. J. Physiol. Cell Physiol. 2016;310:C127–C135. doi: 10.1152/ajpcell.00269.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ishiwata S. Melting from both ends of an A-band in a myofibril. Observation with a phase-contrast microscope. J. Biochem. 1981;89:1647–1650. doi: 10.1093/oxfordjournals.jbchem.a133361. [DOI] [PubMed] [Google Scholar]
- 13.Ishiwata S., Muramatsu K., Higuchi H. Disassembly from both ends of thick filaments in rabbit skeletal muscle fibers. An optical diffraction study. Biophys. J. 1985;47:257–266. doi: 10.1016/S0006-3495(85)83915-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shimamoto Y., Suzuki M., Ishiwata S. Inter-sarcomere coordination in muscle revealed through individual sarcomere response to quick stretch. Proc. Natl. Acad. Sci. USA. 2009;106:11954–11959. doi: 10.1073/pnas.0813288106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stehle R., Krüger M., Pfitzer G. Force kinetics and individual sarcomere dynamics in cardiac myofibrils after rapid ca(2+) changes. Biophys. J. 2002;83:2152–2161. doi: 10.1016/S0006-3495(02)73975-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Labeit D., Watanabe K., Granzier H. Calcium-dependent molecular spring elements in the giant protein titin. Proc. Natl. Acad. Sci. USA. 2003;100:13716–13721. doi: 10.1073/pnas.2235652100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.ImageJ . 2020. Thresholding.https://imagej.net/Thresholding [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
In this example, we also added food dye to detect the flow coming out of the micropipette by producing a darker flow, which can be detected in video A as a dark shadow that follows the movement of the micropipette. Video B shows the same experiment but it has been modified with the Threshold tool from Fiji which transforms an image into two or more classes of pixels (17). In video B the flow is easily detected by the class change of the pixels. We can observe there is no damage to the myofibril from the jet-fluid coming out of the microperfusion as shown by its regular structure.
This video is also summarized in 6 frames on Fig. 1. The myofibril is attached between two needles held at a mean SLi = 2.9 μm, surrounded by a large perfusion (not visible). We observe the myofibril attached to the needles before A-band extraction and activation. The microperfusion is placed close to a sarcomere in the center of the myofibril. Subsequently, the HIS is flown through the microperfusion and one A-band is extracted. The microperfusion is then pulled away and the myofibril is activated and relaxed.