Abstract
Doxorubicin is a potent anticancer drug with cardiotoxicity hampering its use. Neuropeptide Y (NPY) is the most abundant neuropeptide in the heart and a co-transmitter of the sympathetic nervous system that plays a role in cardiac diseases. The aim of this work was to study the impact of NPY on doxorubicin-induced cardiotoxicity. Transgenic mice overexpressing NPY in noradrenergic neurons (NPY-OEDβH) and wild-type mice were treated with a single dose of doxorubicin. Doxorubicin caused cardiotoxicity in both genotypes as demonstrated by decreased weight gain, tendency to reduced ejection fraction, and changes in the expression of several genes relevant to cardiac pathology. Doxorubicin resulted in a tendency to lower ejection fraction in NPY-OEDβH mice more than in wild-type mice. In addition, gain in the whole body lean mass gain was decreased only in NPY-OEDβH mice, suggesting a more severe impact of doxorubicin in this genotype. The effects of doxorubicin on genes expressed in the heart were similar between NPY-OEDβH and wild-type mice. The results demonstrate that doxorubicin at a relatively low dose caused significant cardiotoxicity. There were differences between NPY-OEDβH and wild-type mice in their responses to doxorubicin that suggest NPY to increase susceptibility to cardiotoxicity. This may point to the therapeutic implications as suggested for NPY system in other cardiovascular diseases.
Electronic supplementary material
The online version of this article (10.1007/s12012-019-09557-2) contains supplementary material, which is available to authorized users.
Keywords: Doxorubicin, Neuropeptide Y, Cardiotoxicity model, Mouse model
Introduction
Doxorubicin (DOX) is a member in the anthracycline family and is one of the most effective anticancer drugs. Its use is severely limited by cardiotoxicity that can lead to cardiomyopathy and heart failure [1]. Among cancer survivors, cancer therapy-related heart disease induced by anthracyclines has been shown to be a major cause of morbidity and mortality [2]. In order to develop ways to treat and prevent the adverse cardiac reactions, the mechanisms contributing to DOX-induced cardiotoxicity need to be defined in detail.
Increased sympathetic nervous system (SNS) activity has been shown to play a role in DOX-induced cardiomyopathy [3, 4], and similar to other forms of heart failure, β-blockers significantly improve cardiac function in DOX-induced heart failure [5, 6]. However, sympathetic nerves not only excrete noradrenaline, but also other neurotransmitters that could play a role in the pathogenesis of DOX-induced cardiomyopathy. Neuropeptide Y (NPY) is a co-transmitter in sympathetic nerves, and is the most abundant neuropeptide in the heart [7, 8]. It exerts its effects via Gi-protein-coupled Y-receptors, of which Y1-, Y2- and Y5-receptors are expressed in the heart. The demonstrated actions of NPY in the heart are extensive and affect the contractility of ventricular cardiomyocytes and the excitation–contraction coupling as well as cellular growth, blood supply, and neuronal control [9, 10]. There is evidence linking NPY to different types of cardiovascular diseases with differential, even opposing, effects via diverse mechanisms [8, 9, 11]. We hypothesized that it may also play a role in DOX-induced cardiotoxicity.
Disturbed calcium cycling plays a major role in the pathogenesis of DOX-induced cardiomyopathy [12]. DOX alters deleteriously the expression of many genes specific for cardiac calcium handling including ryanodine receptor (RyR2), sarcoplasmic reticulum Ca2+ ATPase (Serca2a), and phospholamban (Pln), a SERCA2a inhibitor [12–14]. DOX leads to decreased Serca2a expression thus inhibiting SERCA2a pump, decreases RyR2 expression, and induces inappropriate opening of the ryanodine receptors [12, 15, 16]. In the failing heart, a decrease in SERCA2a expression and activity results in myocardial dysfunction due to diminished calcium uptake and release by sarcoplasmic reticulum [17]. Y-receptor activation inhibits adenylate cyclase and decreases cAMP/PKA stimulation of L-type Ca2+ currents. On the other hand, Y1-receptor has been shown to couple also to Gq protein to modulate calcium transients [18] and increase intracellular Ca2+ level [19, 20] in cardiomyocytes. Thus, NPY could have an impact on the disturbed calcium handling induced by DOX.
DOX alters cardiac function also via other mechanisms than calcium handling to induce contractile dysfunction and pathological remodeling. It has been shown to upregulate matrix metalloproteinase 2 (Mmp2) [16, 21] which along with matrix metalloproteinase 9 (Mmp9) participates in the degradation of sarcomeric and cytoskeletal proteins [12, 22]. DOX has also been reported to increase the β-myosin heavy chain (Mhc-β) levels [23–25], which together with changes in α-myosin heavy chain (Mhc-α) associates to altered contractile performance in different cardiomyopathies [24–26]. Moreover, DOX-induced cardiotoxicity involves the inflammatory responses and oxidative stress as evidenced by changes in cytokines, mitochondrial gene, and protein expression [12, 27]. NPY has been linked to the remodeling of myocardial tissue that could be beneficial in short-term, but can lead to cardiac hypertrophy and pathological remodeling [8] in the long run. The demonstrated effects of NPY include reduced degradation and stimulated synthesis of proteins [28, 29], increased survival of myocytes and decreased fibrosis [30] or activation of fibroblasts [31]. NPY may have proinflammatory effects in atherosclerosis [32], but the inflammatory effects have not been studied in the context of heart diseases. However, studies on the effects of NPY on cardiomyocyte mitochondria have demonstrated impaired mitochondrial function and energy metabolism with changes in the levels of PGC-1α, a key regulator of mitochondrial biogenesis [33, 34]. Thus, NPY could impact DOX-induced cardiotoxicity via various mechanisms.
In order to address the question whether NPY has an impact on DOX-induced cardiotoxicity, we treated mice overexpressing NPY in noradrenergic neurons (NPY-OEDβH) with DOX and studied the effects on body composition, cardiac structure, and function as well as explored the potential mechanisms. The NPY-OEDβH mouse model was previously generated and was verified to have about twofold increased Npy expression in noradrenergic neurons including adrenal gland and brain stem [35, 36]. The level of overexpression is relevant in terms of NPY excess in chronic mild stress and gain-of-function polymorphisms of NPY in humans as the NPY-OEDβH model recapitulates findings in these situations [37]. The metabolic phenotype of NPY-OEDβH mouse has been extensively characterized and includes adult-onset obesity, impaired glucose tolerance, and dyslipidemia [35, 36, 38]. The cardiovascular phenotype has not been studied in detail, but NPY-OEDβH mice are more sensitive to endothelial damage-induced vascular wall hypertrophy, and neointima formation [39]. The aim of the current study was to use the NPY-OEDβH mouse model to elucidate the effects of excess NPY on DOX-induced cardiotoxicity.
Methods
Animals
Adult, 8–10 weeks old male homozygous transgenic OE-NPYDβH from homozygous breeders and wild-type C57BL/6N mice (WT) from WT breeders originating from the same heterozygous breedings maintained on a C57BL/6N inbred background were used. The young age was selected to avoid the full metabolic phenotype of OE-NPYDβH. Mice were housed individually in a Ventilated Cage System (Scanbur) at 22 ± 1 °C, 55 ± 5% humidity, and on a 12 h dark/light cycle with free access to mouse chow food and tap water ad libitum. All animal work was done with authorization from the National Animal Experiment Board (ELLA), license number: ESAVI/1256/04.10.07/2015.
Doxorubicin Administration
Cardiotoxicity was induced by administrating DOX (Caelyx 2 mg/ml, at a dose of 20 mg/kg, Janssen Pharmaceutica NV, Belgium) or PBS to mice (n = 7–9/group) as a single intraperitoneal injection. The DOX protocol was based on earlier studies including our recent paper where we used the same DOX administration protocol to induce cardiotoxicity [40–42].
Body Weight and Composition
In order to follow general well-being, the mice were weighted weekly, and the body composition (fat and lean mass) of the mice was measured at week 0 and week 6 by quantitative nuclear magnetic resonance (NMR) scanning with EchoMRI-700 (Echo Medical Systems, Houston, Texas, USA).
Echocardiography
Cardiac structure and function was analyzed at week six by echocardiography that was conducted with VisualSonics Vevo 2100 ultrasound system (VisualSonics, Toronto, Canada) equipped with a 30-MHz transducer. Mice were anesthetized by inhalation of 4.5% isoflurane. Anesthesia was maintained by isoflurane gating the heart rate between 400 and 500 beats per minute for imaging the heart. The chests of the mice were shaved from the hairs with a chemical hair remover (Veet; Reckitt Benckiser) and gel (Eco Supergel; Ceracarta, Forlì, Italy) was applied to the chest before the placement of the probe. Left ventricular function, dimensions, and heart rate were measured among other parameters in short-axis view.
Samples and Histology
Mice were sacrificed at 6 weeks after DOX or saline administration, and heart, lungs, and liver of each mouse was weighed. The tibia was collected and its length was recorded. Hearts were cut in half after which the apex of the heart was frozen in liquid nitrogen immediately after collection, stored at − 70 °C, and used later for RNA extraction, and the base of the heart was fixed for 24 h in 4% paraformaldehyde and subsequently transferred to 70% ethanol and embedded in paraffin. Paraffin Sections (5 μm) were prepared and the sections were collected onto Superfrost plus slides (O. Kindler GmbH, Germany). The paraffin sections were stained with hematoxylin and eosin as well as with Van Gieson’s staining for histological analysis. The sections were scanned using Pannoramic 250F Flash III SlideScanner (3dHistech, Hungary). Myocardial degeneration, inflammation, fibrosis, and nuclear atypia were analyzed and scored by two observers, an experienced pathologist (MSö), and the primary investigator (MM). Histological scoring was carried out blinded as to the other investigator and to the treatment status of the mice. Myocardial degeneration, inflammation, fibrosis, and nuclear atypia were scored on a scale from 0 to 3. In case of discrepancy, investigators evaluated the samples together to gain consensus.
Quantitative Reverse Transcriptase PCR
RNA was extracted from the frozen heart tissue with RNeasy mini kit including DNase treatment (Qiagen, Germany). RNA was transcribed to cDNA with High Capacity RNA-to-cDNA Kit (Applied Biosystems, USA). Quantitative reverse transcriptase PCR was performed using SYBR Green method with Kapa Sybr Fast qPCR Kit (Kapa Biosystems, Woburn, MA, USA) and Applied Biosystems 7300 Real-Time PCR system. Target gene expression was normalized to S29 housekeeping gene, and primers were fwd 5′-ATGGGTCACCAGCAGCTCTA-3′ and rev 5′-AGCCTATGTCCTTCGCGTACT-3′. The fold induction was calculated using the comparative ΔCt method and presented as relative transcript levels (2−ΔΔCt). Primers were for Anp fwd 5′-GCTTCCAGGCCATATTGGAG-3′, rev 5′-GGGGGCATGACCTCATCTT-3′; for Mhc-β fwd 5′-AGGGTGGCAAAGTCACTGCT-3′, rev 5′-CATCACCTGGTCCTCCTTCA-3′; for Mmp2 fwd 5′-GATGTCGCCCCTAAAACAGAC-3′, rev 5′-CAGCCATAGAAAGTGTTCAGGT-3′; for Mmp9 fwd 5′-CTGGACAGCCAGACACTAAAG-3′, rev 5′-CTGGCGGCAAGTCTTCAGAG-3′; for Npy fwd 5′-CTCCGCTCTGCGACACTAC-3′, rev 5′-GGAAGGGTCTTCAAGCCTTGT-3′; for Pln fwd 5′-CACTGTGACGATCACCGAAG-3′, rev 5′-CAGCATCTCGTTTCGCATTA-3′; for RyR2 fwd 5′-CAGCATCTCGTTTCGCATTA-3′, rev 5′-GGCTGTGTTCCACCTTCAAT-3′; for Serca2a fwd 5′-GAGAACGCTCACACAAAGACC-3′, rev 5′-CTTCTTCAGCCGGCAATTCGTTG-3′; and for Th fwd 5′-CCCAAGGGCTTCAGAAGAG-3′, rev 5′-GGGCATCCTCGATGAGACT-3′. Additionally, α-Sma, Beta1R, Bnp, Il-1β, Mmp13, Npy1R, Npy5R, Pgc-1α, Tgf-β, and Tnf-α gene expression were studied (sequences available upon request).
Statistical Analysis
GraphPad Prism 6 software (La Jolla, USA) was used for statistical analyses. Statistical significance was accepted at the level of p < 0.05. Statistical significances were determined with unpaired Student’s t test when comparing two groups, or with two-way ANOVA using NPY overexpression and DOX as independent variables. In two-way ANOVA, multiple comparisons were analyzed and corrected with Tukey post hoc test when p-value for interaction of genotype and treatment effect was < 0.1. Data are presented as mean of absolute values ± standard error mean (SEM). Quantitative PCR values are presented as means ± SEM in relation to the housekeeping gene S29 mRNA level. Formula 2−ΔΔCT was used to calculate the gene expression relative to the expression level of WT saline mice.
Results
Weight and Body Composition
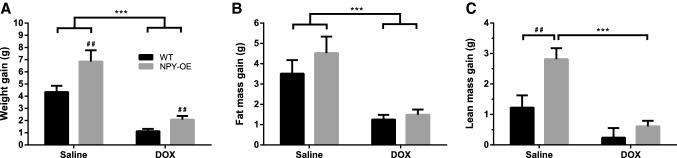
In the beginning of experiment, the weight of the animals (WT saline 24.8 ± 1.0 g, NPY-OEDβH saline 24.2 ± 0.7 g, WT DOX 24.9 ± 0.8 g, NPY-OEDβH DOX 25.0 ± 0.5 g) did not differ between the groups (two-way ANOVA genotype effect p = 0.76, treatment effect p = 0.52). DOX decreased gain in body weight (Fig. 1a) and fat mass (Fig. 1b), leading to significant difference between saline and DOX-treated mice. Moreover, in the NPY-OEDβH mice, weight gain was significantly increased compared to WT mice in both saline and DOX-treated groups (Fig. 1a), an effect which was not seen in fat mass gain (Fig. 1b). DOX decreased lean mass gain, which was statistically significant only in the NPY-OEDβH mice, which showed higher gain on the control treatment (Fig. 1c).
Fig. 1.
Body weight and composition in saline and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 7–9/group), a body weight gain in 6 weeks, b fat mass gain, and c lean mass gain. Values are presented as means ± SEM. *** p < 0.001 DOX vs. saline, ## p < 0.01 NPY vs. WT. a Two-way ANOVA genotype and treatment effects, b two-way ANOVA treatment effect, c Tukey post hoc test following two-way ANOVA
Echocardiography Analysis
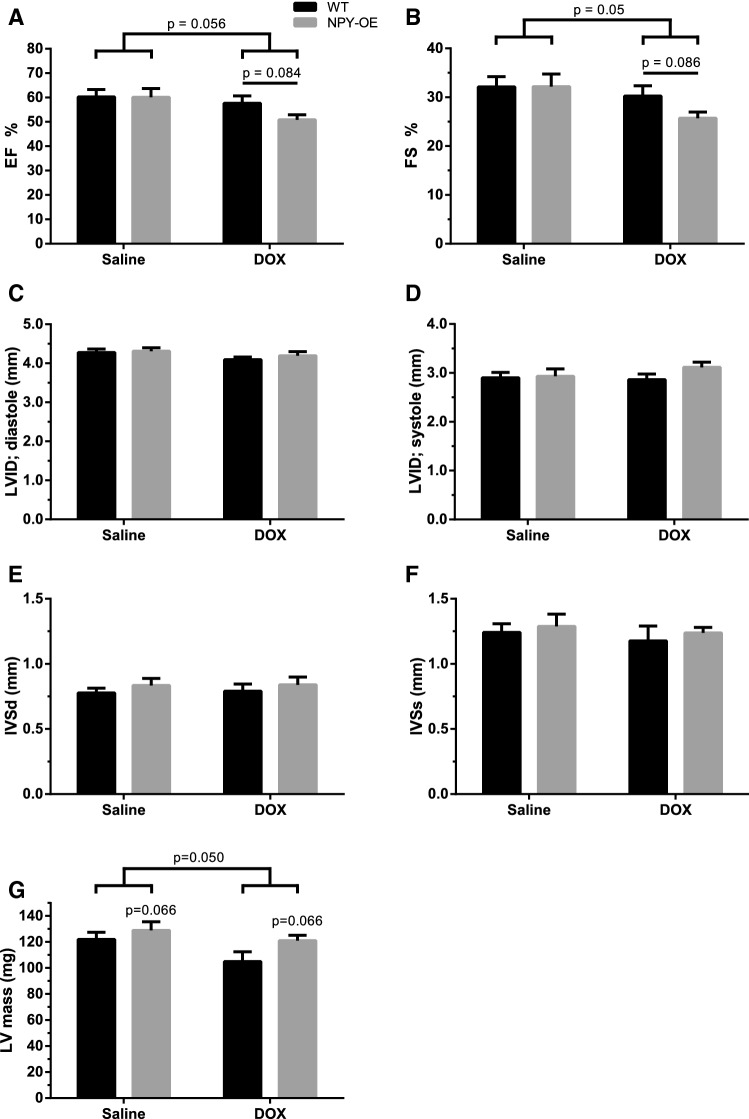
Echocardiography was conducted at week six. DOX treatment tended to decrease ejection fraction (NPY-OEDβH DOX 50.8 ± 2.1% and WT DOX 57.6 ± 3.0% compared to NPY-OEDβH saline 60.1 ± 3.6% and WT saline 60.2 ± 3.1%, p = 0.056) (Fig. 2a). In addition, the decrease in ejection fraction tended to be larger in NPY-OEDβH mice compared to DOX-treated WT mice (p = 0.084) (Fig. 2a). Fractional shortening follows these tendencies of DOX treatment (NPY-OEDβH DOX 25.7 ± 1.3% and WT DOX 30.2 ± 2.1% compared to NPY-OEDβH saline 32.2 ± 2.6% and WT saline 32.1 ± 2.1%, p = 0.050) and comparing DOX-treated NPY-OEDβH mice to WT mice (p = 0.086) (Fig. 2b), there were no differences in the left ventricle internal diameter in diastole or systole (Fig. 2c, d) or in interventricular septum in systole and diastole (Fig. 2e, f). Moreover, DOX tended to decrease the mass of the left ventricle (NPY-OEDβH DOX 121.1 ± 4.1 mg and WT DOX 104.9 ± 7.5 mg compared to NPY-OEDβH saline 129.0 ± 6.6 mg and WT saline 121.9 ± 5.6 mg, p = 0.050) and at the same time the mass of the left ventricle tended to be decreased in NPY-OEDβH mice (p = 0.066) (Fig. 2g).
Fig. 2.
Echocardiography data in saline- and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 7–8/group), a Ejection fraction (EF), b fractional shortening (FS), c left ventricle internal diameter, diastole (LVID, diastole), d left ventricle internal diameter, systole (LVID, systole), e interventricular septum, diastole (IVSd), f interventricular septum, systole (IVSs), g left ventricle mass (LV mass). Values are presented as means ± SEM. DOX vs. saline, two-way ANOVA treatment effect p = 0.056, p = 0.050; NPY-OE vs. WT, two-way ANOVA genotype effect p = 0.066; DOX-treated NPY-OE vs WT, t-test p = 0.084, p = 0.086
Heart Pathology
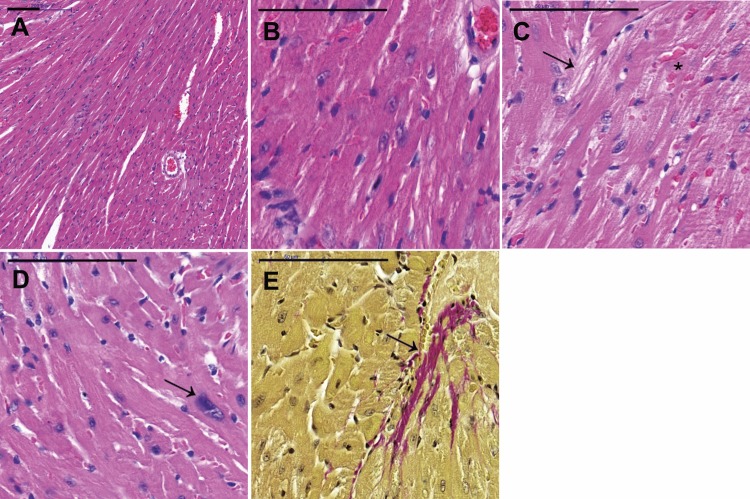
In histological analysis, saline-treated hearts were histologically normal as no nuclear atypia, changes in nuclear size, fibrosis, or perivascular inflammation were seen (Fig. 3a, b). As an example of findings, in DOX-treated NPY-OEDβH hearts, there was seen some cytoplasmic degeneration (Fig. 3c, arrow) and hypertrophy of the myocardial cell nuclei (Fig. 3d, arrow). In addition, some extravasated red blood cells (Fig. 3c, asterisk) and mild fibrosis were seen (Fig. 3e, arrow). In DOX-treated WT hearts, there were changes in nuclear size and hyperchromacy, as well as in cytoplasmic degeneration of myocardial cells. The histological findings were scored and the results were compared between the different treatment groups, but in statistical analysis the histological scoring failed to show any statistical significance. DOX treatment tended to decrease the weight of the heart (p = 0.061) and heart/tibia ratio (p = 0.073) compared to saline controls (Table 1). The weight of the liver or the lungs did not differ between the groups.
Fig. 3.
Examples of histological findings in the paraffin sections of the heart stained with hematoxylin/eosin and Van Gieson’s staining, a normal, saline-treated mouse; 10× zoom, b normal, saline-treated mouse; 40× zoom, c–e doxorubicin-treated mouse; 40× zoom. Scale bar indicates 100 µm length
Table 1.
Organ weights and tibia lengths of the saline- and doxorubicin -treated wild-type and NPY-OEDβH mice
| WT saline | NPY-OE saline | WT DOX | NPY-OE DOX | |
|---|---|---|---|---|
| Heart (mg) | 148.5 ± 6.2 | 147.7 ± 4.1 | 133.6 ± 3.1 | 142.9 ± 5.4 |
| Lungs (mg) | 138.1 ± 8.0 | 144.9 ± 3.9 | 154.3 ± 8.8 | 157.0 ± 7.0 |
| Liver (g) | 1.45 ± 0.12 | 1.57 ± 0.09 | 1.47 ± 0.06 | 1.47 ± 0.04 |
| Tibia (mm) | 17.6 ± 0.05 | 17.5 ± 0.09 | 17.6 ± 0.04 | 17.6 ± 0.02 |
| Heart/Tibia | 8.39 ± 0.39 | 8.44 ± 0.25 | 7.60 ± 0.16 | 8.11 ± 0.31 |
WT wild-type mice, NPY-OE neuropeptide Y overexpressive mice, saline saline treatment, DOX doxorubicin treatment
Values are presented as mean ± SEM. Statistics were analyzed with two-way ANOVA (n = 6–8/group)
Expression of Npy-Related Genes in the Heart
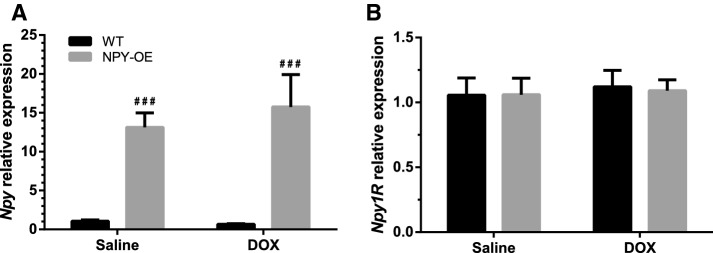
In order to analyze the expression level of NPY-related genes in the heart, Npy-, Npy1R-, and Npy5R-relative mRNA levels were quantified with qPCR. Npy expression in the heart was low, but it was found to be over ten times higher in NPY-OEDβH mice than in normal WT mice (Fig. 4a). DOX treatment did not alter Npy expression. There were no differences in neuropeptide Y 1 receptor expression levels (Fig. 4b) and the expression level of neuropeptide Y 5 receptor was under the reliable detection level by qPCR.
Fig. 4.
Relative mRNA expression level in the heart in saline and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 7–8/group), a neuropeptide Y (Npy) level and b neuropeptide Y 1 receptor (Npy1R) level. Values are presented as means ± SEM, ### p < 0.001, NPY-OE vs. WT, two-way ANOVA genotype effect
Effect of Doxorubicin on Genes Modifying Calcium Cycling System
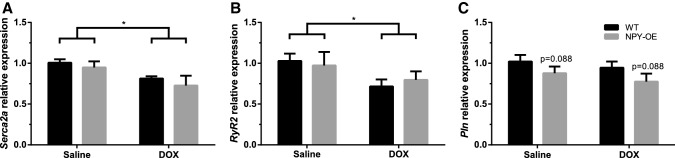
DOX treatment resulted in lower Serca2a (Fig. 5a) and RyR2 (Fig. 5b) levels compared to saline-treated mice. In Serca2a and RyR2 expression, there were no differences between the NPY-OEDβH and WT genotypes. In the NPY-OEDβH genotype, Pln expression tended to be decreased compared to WT mice (Fig. 5c), but the expression was not affected by DOX treatment.
Fig. 5.
Relative mRNA expression levels of genes modifying calcium cycling system in the heart in saline- and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 5–8/group), a sarcoplasmic reticulum Ca2+ ATPase (Serca2a) level, b ryanodine receptor (RyR2) level, c phospholamban (Pln) level. Values are presented as means ± SEM, * p < 0.05 for DOX vs. Saline, two-way ANOVA treatment effect; p = 0.088 for NPY-OE vs WT, two-way ANOVA genotype effect
Effect of Doxorubicin on Marker Genes of Cardiomyopathy
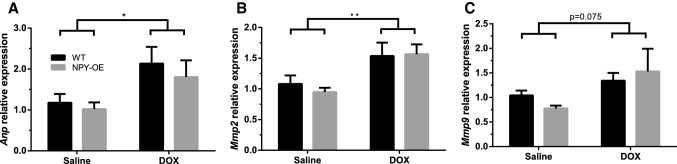
Anp gene expression levels were significantly higher in DOX-treated mice compared to saline-treated mice (Fig. 6a). DOX altered also the expression of two genes involved in the degradation of proteins. Mmp2 expression was increased (Fig. 6b) and Mmp9 tended to be increased (Fig. 6c) by DOX. There were no differences in Bnp and Mmp13 expression (Online Resource 1a, b) or in the expression levels of cytokines measured including IL-1β, Tgf-β ,and Tnf-α (Online Resource 1c–e). Regarding fibrosis, we saw no significant changes in myofibroblast marker α-Sma (Online Resource 1f).
Fig. 6.
Relative mRNA expression levels of cardiomyopathy marker genes in the heart in saline- and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 5–8/group), a atrial natriuretic peptide (Anp) level, b matrix metalloproteinase 2 (Mmp2) level, c matrix metalloproteinase 9 (Mmp9) level. Values are presented as means ± SEM, * p < 0.05, ** p < 0.01, p = 0.075, DOX vs. saline, two-way ANOVA treatment effect
Effect of Doxorubicin on Genes Modifying Sympathetic Activity and Contractility
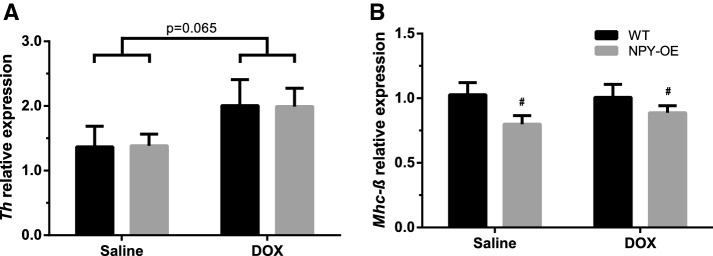
DOX treatment tended to increase the expression of tyrosine hydroxylase, Th (Fig. 7a), the rate-limiting enzyme in noradrenaline synthesis, while there were no differences in beta-1 adrenergic receptor expression, Beta1R (Online Resource 1g). Mhc-β expression was decreased in the NPY-OEDβH genotype compared to WT mice while DOX had no effect (Fig. 7b). There were no differences in Mhc-α (Online Resource 1h) or mitochondrial biogenesis regulator Pgc-1α (Online Resource 1i) expression.
Fig. 7.
Relative mRNA expression levels of a tyrosine hydroxylase (Th), b β-myosin heavy chain (Mhc-β) in the hearts in saline- and doxorubicin (DOX) -treated wild-type (WT) and NPY-OEDβH (NPY-OE) mice (n = 6–8/group). Values are presented as means ± SEM, p = 0.065 DOX vs. saline, two-way ANOVA treatment effect; # p < 0.05, NPY-OE vs. WT, two-way ANOVA genotype effect
Discussion
This is the first study analyzing the role of NPY in DOX-induced cardiotoxicity. To this end, a previously developed mouse model overexpressing NPY in the sympathetic nervous system was treated with DOX using a protocol that led to a decrease in heart function and clear signs of cardiac toxicity. There were only small differences between NPY-OEDβH and WT mice in their response to DOX, but these tendencies suggest that NPY overexpression increased the susceptibility to cardiotoxicity.
DOX treatment with the dosing regimen used in the study, consisting of a single intraperitoneal injection at a dose of 20 mg/kg, induced a tendency of decrease in ejection fraction suggesting impaired heart function, but did not lead to severe heart failure as indicated by e.g., unchanged lung and liver weights. However, it affected the general well-being of the animals as weight gain and fat mass gain were significantly hampered, which fits with the previous reports using different doses of DOX [40–45]. Furthermore, higher expression of the rate-limiting enzyme of noradrenaline synthesis (Th) suggested that cardiac SNS activity was increased in DOX-treated mice, and the result fits with previous work showing DOX to upregulate TH protein level and linking DOX-induced cardiomyopathy to increased SNS activity [3, 4, 46]. On molecular level, DOX affected several previously well-described mechanisms of DOX-induced cardiotoxicity including decreases in the expression of genes involved in intracellular calcium metabolism, Serca2a and RyR2 [13, 14, 16] and increases in Anp and Mmp2 levels, consistent with observations in the human heart failure [47–50]. DOX-treated mice tended to have lower left ventricular mass in echocardiography and this was supported by the heart weights. Fitting with this, increased Mmp2 expression points to cellular level activation of degradation of structural proteins. Although heart weights or histology did not reveal myocyte hypertrophy, increased Anp indicates that the cellular mechanisms driving hypertrophy were recruited. The histology revealed hypertrophy of the myocardial cell nuclei, cytoplasmic degeneration, and mild fibrosis in DOX-treated animals, even though the statistical significance was lacking. However, these findings are supported by previous reports of DOX treatment leading to cardiomyocyte disorganization and myofibrillar loss [40, 43, 44, 51, 52]. Thus, the DOX administration protocol used in the current study did not induce severe heart failure during the study period, but led to significant cardiotoxicity, resulting in a decline in the general condition of the mice, a slight decline in the myocardial function and mass, and affecting several well-described components of DOX-induced cardiotoxicity.
In this study, we asked if NPY has an effect on DOX-induced cardiotoxicity. Previous studies have shown a diverse role for NPY in different types of cardiomyopathies, including an increase in NPY levels and effects on several pathological processes involved also in DOX-induced cardiotoxicity [11, 30, 53–56]. Transgenic NPY overexpression had some effects on DOX-induced toxicity. First, NPY affected body composition of DOX-treated mice. Whereas body weight and fat mass gain were decreased in DOX-treated mice of both genotypes, lean mass gain was compromised significantly only in the NPY-OEDβH mice. Ejection fraction tended to be decreased in DOX-treated mice, and the effect seemed to be larger in NPY-OEDβH mice. These findings suggest that the NPY-OEDβH mice were more severely affected by DOX. Cardiac histology or markers of calcium handling, hypertrophy, and fibrosis were not differently affected by DOX in WT and NPY-OEDβH mice. PGC-1a, marker of mitochondrial biogenesis, was previously shown to be disturbed by DOX and NPY, but was not changed in the current study [27, 33, 34]. NPY overexpression genotype caused some changes that were evident in both saline- and DOX -treated mice. LV ventricular mass tended to be increased, while genes involved in the heart contractile function, Mhc-β and Pln, were downregulated [26, 57, 58]. The increased mass could be contributed by the known mitogenic effect of NPY on (vascular) smooth muscle cells that was evident also in NPY-OEDβH mice after endothelial damage [39]. The reduction in Mhc-β and Pln has been associated with improved contractility [24–26, 59, 60], which could fit with the known effects of NPY on cardiac contractility [8], but is not supported by the EF results. Thus, they may rather be a sign of compensation to changes caused by NPY overexpression that render the mice more susceptible to the DOX-induced cardiotoxicity.
DOX-induced cardiotoxicity has been associated with increased SNS activity [3, 4]. Since NPY is a co-transmitter, which is released in a more prolonged manner than noradrenaline, it is likely that NPY release is also increased in this chronic stress condition. Our study did not address this question as quantitating neurotransmitter release to target tissues is demanding and requires a special experimental setting. Npy expression in the heart was measured, and was not changed by DOX. Interestingly, it was upregulated in the transgenic mice. This is the only non-neuronal tissue which overexpresses NPY in NPY-OEDβH mice, and it is likely that the expression represents expression in the intracardiac noradrenergic neurons rather than in cardiac myocytes [8]. However, the expression level was still very low and the SNS-derived NPY is likely responsible for any direct effects on cardiac tissue. In the transgenic mouse model used, Npy expression is driven under the dopamine-β-hydroxylase promoter (DβH) targeting the transgene expression to noradrenergic neurons with very little ectopic expression shown [35]. Npy expression is almost twice as high in noradrenergic regions of the brain and significantly higher in adrenal glands compared to WT mice leading to increased protein levels in these tissues [36]. This fairly modest increase may represent the increase in NPY during mild chronic stress or due to genetic variants, but not the high levels that have been associated with poor survival in heart failure patients [61].
NPY-OEDβH mice have previously been shown to present with a metabolic syndrome -like phenotype (37), and fitting with this NPY-OEDβH mice gained more weight compared with WT mice in the current experiment. The metabolic changes seem to be mediated mostly via inhibition of adrenergic tone to the metabolic tissues rather than direct effects of NPY on adipose tissue and the liver. This has been evidenced by decreased Th expression in the brain noradrenergic nuclei and the adrenal gland, decreased urinary adrenaline levels, and relevant changes in the target tissue beta receptor expression [36, 62]. In the current experiment, the neuronal Th was not studied but Npy had no effect on cardiac Th or beta1-receptor. These findings do not exclude the possibility that changes in the autonomic nervous system control of the heart could play a role. Furthermore, it is possible that the effects of NPY overexpression on the vascular system (including increased blood pressure) could play a role.
In summary, in our model, DOX at a relatively low dose caused significant cardiotoxicity. There were differences between NPY-OEDβH and WT mice in their responses to DOX suggesting increased susceptibility to cardiotoxicity associated with NPY overexpression. This may point to therapeutic implications as suggested for NPY system in other cardiovascular diseases.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Acknowledgement
Open access funding provided by University of Turku (UTU) including Turku University Central Hospital. We would like to acknowledge histocore (Institute of Biomedicine, University of Turku, Turku, Finland) for staining the paraffin sections. The work was supported by Drug Research Doctoral Programme in the University of Turku Graduate School, Turku University Hospital Research Fund, Turku University Foundation, Finnish Cultural Foundation Varsinais-Suomi Regional fund, Finnish Foundation for Cardiovascular Research, Orion Research Foundation, and Aarne Koskelo Foundation.
Author Contributions
MM, MS, and ES designed the study. LA contributed to the experimental set-up and conducting the study. MM was responsible for producing and analyzing the data. The histopathology was analyzed together with MSö. MM, MS, ES, and MSö wrote the paper.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflicts of interest.
Ethical Approval
No human studies were carried out by the authors for this article. All institutional and national guidelines for the care and use of laboratory animals were followed and approved by the appropriate institutional committees.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Chatterjee K, Zhang J, Honbo N, Karliner J. Doxorubicin cardiomyopathy. Cardiology. 2010;115:155–162. doi: 10.1159/000265166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghigo A, Li M, Hirsch E. New signal transduction paradigms in anthracycline-induced cardiotoxicity. Biochimica et Biophysica Acta. 2016;1863:1916–1925. doi: 10.1016/j.bbamcr.2016.01.021. [DOI] [PubMed] [Google Scholar]
- 3.Tong J, Ganguly PK, Singal PK. Myocardial adrenergic changes at two stages of heart failure due to adriamycin treatment in rats. American Journal of Physiology. 1991;260:H909–H916. doi: 10.1152/ajpheart.1991.260.3.H909. [DOI] [PubMed] [Google Scholar]
- 4.Bartoli C, Brittian K, Giridharan G, Koenig S, Hamid T, Prabhu S. Bovine model of doxorubicin-induced cardiomyopathy. Journal of Biomedicine and Biotechnology. 2011;2011:758736–758736. doi: 10.1155/2011/758736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hjalmarson, A., Waagstein, F. (1994). The role of beta-blockers in the treatment of cardiomyopathy and ischaemic heart failure. Drugs, 47(Suppl 4), 31–9; discussion 39 [DOI] [PubMed]
- 6.Kalay N, Basar E, Ozdogru I, Er O, Cetinkaya Y, Dogan A, et al. Protective effects of carvedilol against anthracycline-induced cardiomyopathy. Journal of the American College of Cardiology. 2006;48:2258–2262. doi: 10.1016/j.jacc.2006.07.052. [DOI] [PubMed] [Google Scholar]
- 7.Ekblad E, Edvinsson L, Wahlestedt C, Uddman R, Håkanson R, Sundler F. Neuropeptide Y co-exists and co-operates with noradrenaline in perivascular nerve fibers. Regulatory Peptides. 1984;8:225–235. doi: 10.1016/0167-0115(84)90064-8. [DOI] [PubMed] [Google Scholar]
- 8.McDermott BJ, Bell D. NPY and cardiac diseases. Current Topics in Medicinal Chemistry. 2007;7:1692–1703. doi: 10.2174/156802607782340939. [DOI] [PubMed] [Google Scholar]
- 9.Dvorakova M, Kruzliak P, Rabkin S. Role of neuropeptides in cardiomyopathies. Peptides. 2014;61:1–6. doi: 10.1016/j.peptides.2014.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Abdel Samad D, Jacques D, Perreault C, Provost C. NPY regulates human endocardial endothelial cell function. Peptides. 2007;28:281–287. doi: 10.1016/j.peptides.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 11.Tan CMJ, Green P, Tapoulal N, Lewandowski A, Leeson P, Herring N. The role of neuropeptide Y in cardiovascular health and disease. Frontiers in Physiology. 2018;9:1281–1281. doi: 10.3389/fphys.2018.01281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Octavia Y, Tocchetti C, Gabrielson K, Janssens S, Crijns H, Moens A. Doxorubicin-induced cardiomyopathy: from molecular mechanisms to therapeutic strategies. Journal of Molecular and Cellular Cardiology. 2012;52:1213–1225. doi: 10.1016/j.yjmcc.2012.03.006. [DOI] [PubMed] [Google Scholar]
- 13.Boucek RJ, Miracle A, Anderson M, Engelman R, Atkinson J, Dodd DA. Persistent effects of doxorubicin on cardiac gene expression. Journal of Molecular and Cellular Cardiology. 1999;31:1435–1446. doi: 10.1006/jmcc.1999.0972. [DOI] [PubMed] [Google Scholar]
- 14.Hanna A, Lam A, Tham S, Dulhunty A, Beard N. Adverse effects of doxorubicin and its metabolic product on cardiac RyR2 and SERCA2A. Molecular Pharmacology. 2014;86:438–449. doi: 10.1124/mol.114.093849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gambliel H, Burke B, Cusack B, Walsh G, Zhang Y, Mushlin P, et al. Doxorubicin and C-13 deoxydoxorubicin effects on ryanodine receptor gene expression. Biochemical and Biophysical Research Communications. 2002;291:433–438. doi: 10.1006/bbrc.2002.6380. [DOI] [PubMed] [Google Scholar]
- 16.Tocchetti C, Carpi A, Coppola C, Quintavalle C, Rea D, Campesan M, et al. Ranolazine protects from doxorubicin-induced oxidative stress and cardiac dysfunction. European Journal of Heart Failure. 2014;16:358–366. doi: 10.1002/ejhf.50. [DOI] [PubMed] [Google Scholar]
- 17.Olson E. A decade of discoveries in cardiac biology. Nature Medicine. 2004;10:467–474. doi: 10.1038/nm0504-467. [DOI] [PubMed] [Google Scholar]
- 18.Jacques D, Abdel Samad D. Neuropeptide Y (NPY) and NPY receptors in the cardiovascular system: implication in the regulation of intracellular calcium. Canadian Journal of Physiology and Pharmacology. 2007;85:43–53. doi: 10.1139/Y06-106. [DOI] [PubMed] [Google Scholar]
- 19.Chen M, Li X, Dong Q, Li Y, Liang W. Neuropeptide Y induces cardiomyocyte hypertrophy via calcineurin signaling in rats. Regulatory Peptides. 2005;125:9–15. doi: 10.1016/j.regpep.2004.07.034. [DOI] [PubMed] [Google Scholar]
- 20.Heredia MDP, Delgado C, Pereira L, Perrier R, Richard S, Vassort G, et al. Neuropeptide Y rapidly enhances [Ca2 +]i transients and Ca2 + sparks in adult rat ventricular myocytes through Y1 receptor and PLC activation. Journal of Molecular and Cellular Cardiology. 2005;38:205–212. doi: 10.1016/j.yjmcc.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 21.Chen X, Guo Z, Wang P, Xu M. Erythropoietin modulates imbalance of matrix metalloproteinase-2 and tissue inhibitor of metalloproteinase-2 in doxorubicin-induced cardiotoxicity. Heart, Lung and Circulation. 2014;23:772–777. doi: 10.1016/j.hlc.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 22.Polegato B, Minicucci M, Azevedo P, Carvalho R, Chiuso Minicucci F, Pereira E, et al. Acute doxorubicin-induced cardiotoxicity is associated with matrix metalloproteinase-2 alterations in rats. Cellular Physiology and Biochemistry. 2015;35:1924–1933. doi: 10.1159/000374001. [DOI] [PubMed] [Google Scholar]
- 23.Umlauf J, Horký M. Molecular biology of doxorubicin-induced cardiomyopathy. Experimental and Clinical Cardiology. 2002;7:35–39. [PMC free article] [PubMed] [Google Scholar]
- 24.Kucerova D, Doka G, Kruzliak P, Turcekova K, Kmecova J, Brnoliakova Z, et al. Unbalanced upregulation of ryanodine receptor 2 plays a particular role in early development of daunorubicin cardiomyopathy. American Journal of Translational Research. 2015;7:1280–1294. [PMC free article] [PubMed] [Google Scholar]
- 25.Doka G, Malikova E, Galkova K, La Rocca G, Kruzliak P, Adamek M, et al. Downregulation of myogenic microRNAs in sub-chronic but not in sub-acute model of daunorubicin-induced cardiomyopathy. Molecular and Cellular Biochemistry. 2017;432:79–89. doi: 10.1007/s11010-017-2999-8. [DOI] [PubMed] [Google Scholar]
- 26.Abraham WT, Gilbert EM, Lowes BD, Minobe WA, Larrabee P, Roden RL, et al. Coordinate changes in Myosin heavy chain isoform gene expression are selectively associated with alterations in dilated cardiomyopathy phenotype. Molecular Medicine. 2002;8:750–760. [PMC free article] [PubMed] [Google Scholar]
- 27.Yin J, Guo J, Zhang Q, Cui L, Zhang L, Zhang T, et al. Doxorubicin-induced mitophagy and mitochondrial damage is associated with dysregulation of the PINK1/parkin pathway. Toxicology in Vitro. 2018;51:1–10. doi: 10.1016/j.tiv.2018.05.001. [DOI] [PubMed] [Google Scholar]
- 28.Millar BC, Schlüter KD, Zhou XJ, McDermott BJ, Piper HM. Neuropeptide Y stimulates hypertrophy of adult ventricular cardiomyocytes. American Journal of Physiology. 1994;266:C1271–C1277. doi: 10.1152/ajpcell.1994.266.5.C1271. [DOI] [PubMed] [Google Scholar]
- 29.Bell D, Allen A, Kelso E, Balasubramaniam A, McDermott B. Induction of hypertrophic responsiveness of cardiomyocytes to neuropeptide Y in response to pressure overload. The Journal of Pharmacology and Experimental Therapeutics. 2002;303:581–591. doi: 10.1124/jpet.102.038448. [DOI] [PubMed] [Google Scholar]
- 30.Matyal R, Sakamuri S, Wang A, Mahmood E, Robich M, Khabbaz K, et al. Local infiltration of neuropeptide Y as a potential therapeutic agent against apoptosis and fibrosis in a swine model of hypercholesterolemia and chronic myocardial ischemia. European Journal of Pharmacology. 2013;718:261–270. doi: 10.1016/j.ejphar.2013.08.023. [DOI] [PubMed] [Google Scholar]
- 31.Zhu X, Gillespie D, Jackson E. NPY1-36 and PYY1-36 activate cardiac fibroblasts: an effect enhanced by genetic hypertension and inhibition of dipeptidyl peptidase 4. AJP-Heart and Circulatory Physiology. 2015;309:H1528–H1542. doi: 10.1152/ajpheart.00070.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhou J, Zhang L, Wei H, Wang X, Ni H, Yang F, et al. Neuropeptide Y induces secretion of high-mobility group box 1 protein in mouse macrophage via PKC/ERK dependent pathway. Journal of Neuroimmunology. 2013;260:55–59. doi: 10.1016/j.jneuroim.2013.04.005. [DOI] [PubMed] [Google Scholar]
- 33.Luo G, Xu X, Guo W, Luo C, Wang H, Meng X, et al. Neuropeptide Y damages the integrity of mitochondrial structure and disrupts energy metabolism in cultured neonatal rat cardiomyocytes. Peptides. 2015;71:162–169. doi: 10.1016/j.peptides.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 34.Hu J, Xu X, Zuo Y, Gao X, Wang Y, Xiong C, et al. NPY impairs cell viability and mitochondrial membrane potential through Ca2 + and p38 signaling pathways in neonatal rat cardiomyocytes. Journal of Cardiovascular Pharmacology. 2017;70:52–59. doi: 10.1097/FJC.0000000000000493. [DOI] [PubMed] [Google Scholar]
- 35.Ruohonen S, Pesonen U, Moritz N, Kaipio K, Röyttä M, Koulu M, et al. Transgenic mice overexpressing neuropeptide Y in noradrenergic neurons: a novel model of increased adiposity and impaired glucose tolerance. Diabetes. 2008;57:1517–1525. doi: 10.2337/db07-0722. [DOI] [PubMed] [Google Scholar]
- 36.Vähätalo LH, Ruohonen ST, Mäkelä S, Kovalainen M, Huotari A, Mäkelä KA, et al. Neuropeptide Y in the noradrenergic neurones induces obesity and inhibits sympathetic tone in mice. Acta Physiologica. 2015;213:902–919. doi: 10.1111/apha.12436. [DOI] [PubMed] [Google Scholar]
- 37.Ruohonen S, Pesonen U, Savontaus E. Neuropeptide Y in the noradrenergic neurons induces the development of cardiometabolic diseases in a transgenic mouse model. Indian Journal of Endocrinology and Metabolism. 2012;16:S569–S576. doi: 10.4103/2230-8210.105574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vähätalo L, Ruohonen S, Ailanen L, Savontaus E. Neuropeptide Y in noradrenergic neurons induces obesity in transgenic mouse models. Neuropeptides. 2016;55:31–37. doi: 10.1016/j.npep.2015.11.088. [DOI] [PubMed] [Google Scholar]
- 39.Ruohonen S, Abe K, Kero M, Toukola L, Röyttä M, Koulu M, et al. Sympathetic nervous system-targeted neuropeptide Y overexpression in mice enhances neointimal formation in response to vascular injury. Peptides. 2009;30:715–720. doi: 10.1016/j.peptides.2008.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li K, Sung RYT, Huang W, Yang M, Pong N, Lee S, et al. Thrombopoietin protects against in vitro and in vivo cardiotoxicity induced by doxorubicin. Circulation. 2006;113:2211–2220. doi: 10.1161/CIRCULATIONAHA.105.560250. [DOI] [PubMed] [Google Scholar]
- 41.Neilan T, Jassal D, Perez Sanz T, Raher M, Pradhan A, Buys E, et al. Tissue Doppler imaging predicts left ventricular dysfunction and mortality in a murine model of cardiac injury. European Heart Journal. 2006;27:1868–1875. doi: 10.1093/eurheartj/ehl013. [DOI] [PubMed] [Google Scholar]
- 42.Mattila M, Koskenvuo J, Söderström M, Eerola K, Savontaus M. Intramyocardial injection of SERCA2a-expressing lentivirus improves myocardial function in doxorubicin-induced heart failure. Journal of Gene Medicine. 2016;18:124–133. doi: 10.1002/jgm.2885. [DOI] [PubMed] [Google Scholar]
- 43.Arola OJ, Saraste A, Pulkki K, Kallajoki M, Parvinen M, Voipio Pulkki LM. Acute doxorubicin cardiotoxicity involves cardiomyocyte apoptosis. Cancer Research. 2000;60:1789–1792. [PubMed] [Google Scholar]
- 44.Zhu J, Zhang J, Xiang D, Zhang Z, Zhang L, Wu M, et al. Recombinant human interleukin-1 receptor antagonist protects mice against acute doxorubicin-induced cardiotoxicity. European Journal of Pharmacology. 2010;643:247–253. doi: 10.1016/j.ejphar.2010.06.024. [DOI] [PubMed] [Google Scholar]
- 45.Zhang W, Deng J, Sunkara M, Morris A, Wang C, St Clair D, et al. Loss of multidrug resistance-associated protein 1 potentiates chronic doxorubicin-induced cardiac dysfunction in mice. The Journal of Pharmacology and Experimental Therapeutics. 2015;355:280–287. doi: 10.1124/jpet.115.225581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jin W, Qiao Z, Zheng C, Li S, Chen H. Protein interacting with kinase Cα mediates the down-regulation of myocardial norepinephrine transporter expression in mice with adriamycin-induced congestive heart failure. 中华心血管病杂志. 2014;42:219–224. [PubMed] [Google Scholar]
- 47.Meyer M, Schillinger W, Pieske B, Holubarsch C, Heilmann C, Posival H, et al. Alterations of sarcoplasmic reticulum proteins in failing human dilated cardiomyopathy. Circulation. 1995;92:778–784. doi: 10.1161/01.cir.92.4.778. [DOI] [PubMed] [Google Scholar]
- 48.Polyakova V, Loeffler I, Hein S, Miyagawa S, Piotrowska I, Dammer S, et al. Fibrosis in endstage human heart failure: severe changes in collagen metabolism and MMP/TIMP profiles. International Journal of Cardiology. 2011;151:18–33. doi: 10.1016/j.ijcard.2010.04.053. [DOI] [PubMed] [Google Scholar]
- 49.Volpe M, Rubattu S, Burnett J. Natriuretic peptides in cardiovascular diseases: current use and perspectives. European Heart Journal. 2014;35:419–425. doi: 10.1093/eurheartj/eht466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acsai K, Ördög B, Varró A, Nánási P. Role of the dysfunctional ryanodine receptor—Na(+)-Ca(2 +)exchanger axis in progression of cardiovascular diseases: What we can learn from pharmacological studies? European Journal of Pharmacology. 2016;779:91–101. doi: 10.1016/j.ejphar.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 51.Sahu B, Kumar J, Kuncha M, Borkar R, Srinivas R, Sistla R. Baicalein alleviates doxorubicin-induced cardiotoxicity via suppression of myocardial oxidative stress and apoptosis in mice. Life Sciences. 2016;144:8–18. doi: 10.1016/j.lfs.2015.11.018. [DOI] [PubMed] [Google Scholar]
- 52.Zhu J, Zhang J, Zhang L, Du R, Xiang D, Wu M, et al. Interleukin-1 signaling mediates acute doxorubicin-induced cardiotoxicity. Biomedicine & Pharmacotherapy. 2011;65:481–485. doi: 10.1016/j.biopha.2011.06.005. [DOI] [PubMed] [Google Scholar]
- 53.Ananda S, Wang Y, Zhu S, Wang R, Zhou X, Zhuo L, et al. Role of neuropeptide Y and peroxisome proliferator-activated receptor γ coactivator-1α in stress cardiomyopathy. Journal of Huazhong University of Science and Technology (Medical Science) 2012;32:823–828. doi: 10.1007/s11596-012-1041-3. [DOI] [PubMed] [Google Scholar]
- 54.Shanks J, Herring N. Peripheral cardiac sympathetic hyperactivity in cardiovascular disease: role of neuropeptides. American Journal of Physiology. Regulatory Integrative and Comparative Physiology. 2013;305:R1411–R1420. doi: 10.1152/ajpregu.00118.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Herring N. Autonomic control of the heart: going beyond the classical neurotransmitters. Experimental Physiology. 2015;100:354–358. doi: 10.1113/expphysiol.2014.080184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Özkaramanli Gür D, Sagbas M, Akyüz A, Güzel S, Alpsoy S, Güler N. Role of sympathetic cotransmitter galanin on autonomic balance in heart failure: an active player or a bystander? Anatolian Journal of Cardiology. 2017;18:281–288. doi: 10.14744/AnatolJCardiol.2017.7831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.MacLennan D, Kranias E. Phospholamban: A crucial regulator of cardiac contractility. Nature Reviews Molecular Cell Biology. 2003;4:566–577. doi: 10.1038/nrm1151. [DOI] [PubMed] [Google Scholar]
- 58.Haghighi K, Bidwell P, Kranias E. Phospholamban interactome in cardiac contractility and survival: A new vision of an old friend. Journal of Molecular and Cellular Cardiology. 2014;77:160–167. doi: 10.1016/j.yjmcc.2014.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Iwanaga Y, Hoshijima M, Gu Y, Iwatate M, Dieterle T, Ikeda Y, et al. Chronic phospholamban inhibition prevents progressive cardiac dysfunction and pathological remodeling after infarction in rats. Journal of Clinical Investigation. 2004;113:727–736. doi: 10.1172/JCI18716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tsuji T, Del Monte F, Yoshikawa Y, Abe T, Shimizu J, Nakajima Takenaka C, et al. Rescue of Ca2 + overload-induced left ventricular dysfunction by targeted ablation of phospholamban. AJP-Heart and Circulatory Physiology. 2009;296:H310–H317. doi: 10.1152/ajpheart.00975.2008. [DOI] [PubMed] [Google Scholar]
- 61.Hulting J, Sollevi A, Ullman B, Franco Cereceda A, Lundberg JM. Plasma neuropeptide Y on admission to a coronary care unit: raised levels in patients with left heart failure. Cardiovascular Research. 1990;24:102–108. doi: 10.1093/cvr/24.2.102. [DOI] [PubMed] [Google Scholar]
- 62.Ailanen L, Ruohonen S, Vähätalo L, Tuomainen K, Eerola K, Salomäki Myftari H, et al. The metabolic syndrome in mice overexpressing neuropeptide Y in noradrenergic neurons. Journal of Endocrinology. 2017;234:57–72. doi: 10.1530/JOE-16-0223. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.