Abstract
BACKGROUND
Ankylosing spondylitis (AS) frequently occurs in people aged 30-45 years, and its prevalence is generally believed to be between 0.1% and 1.4% globally. At present, the “gold standard” for diagnosis of AS requires the provision of pelvic X-rays, which makes it more difficult to perform in population-based epidemiological studies. Therefore, the identification of serological indicators related to the diagnosis, treatment, and prognosis of AS patients is of great significance.
AIM
To analyze the therapeutic, diagnostic significance and prognostic value of dickkopf-related protein-1 (DKK-1) and tumor necrosis factor-α (TNF-α) in AS.
METHODS
A total of 113 patients with active AS were selected as the research group, and 100 healthy subjects who underwent physical examination were selected as the control group. The levels of DKK-1 and TNF-α in peripheral blood in the two groups were compared. The diagnostic and predictive values of DKK-1 and TNF-α for AS were analyzed with ROC curves, and the factors influencing AS recurrence were analyzed with COX regression.
RESULTS
Before treatment, the research group showed lower DKK-1 levels but higher TNF-α levels than the control group (both aP < 0.05). In the research group, DKK-1 was up-regulated and TNF-α was down-regulated after 12 wk of treatment (aP < 0.05). The area under the curve, sensitivity and specificity of DKK-1 combined with TNF-α for diagnosing AS were 0.934, 82.30% and 97.00%, respectively. Before treatment, the area under the curve, cutoff value, sensitivity and specificity of DKK-1 for predicting the curative effect were 0.825, 68.42 pg/mL, 73.68% and 80.00%, respectively, and those of TNF-α were 0.863, 32.79 ng/L, 92.11% and 77.33%, respectively. DKK-1 and TNF-α levels after treatment were closely related to the curative effect (aP < 0.05). C-reactive protein, the Bath Ankylosing Spondylitis Disease Activity Index, DKK-1, and TNF-α were risk factors for AS recurrence (aP < 0.05).
CONCLUSION
DKK-1 and TNF-α are effective in the diagnosis and treatment of AS and are risk factors for its recurrence. In addition, DKK-1 may be a potential target for the diagnosis of AS.
Keywords: Dickkopf-related protein-1, Tumor necrosis factor-α, Ankylosing spondylitis, Diagnosis, Prognosis, Peripheral blood
Core tip: In this study, we demonstrated that dickkopf-related protein-1 and tumor necrosis factor-α are effective in the diagnosis and treatment of ankylosing spondylitis and are risk factors for its recurrence. In addition, dickkopf-related protein-1 may be a potential target for future treatment of ankylosing spondylitis.
INTRODUCTION
Ankylosing spondylitis (AS) is an inflammatory arthritis within a family of disorders including reactive arthritis, inflammatory bowel disease-associated spondyloarthritis (SpA), and undifferentiated spondyloarthritis (uSpA)[1], which is common in people aged 30-45 years old. The prevalence of AS is generally believed to be between 0.1% and 1.4% globally[2]. At present, the “gold standard” for diagnosis of AS is the modified New York criteria[3]. However, these criteria require the provision of pelvic X-rays, which are more difficult to perform in population-based epidemiological studies. In addition, the prognosis of patients with AS is variable and closely related to extraspinal manifestations (such as uveitis, psoriasis and inflammatory bowel disease), age at diagnosis and treatment. AS often severely impairs spinal mobility and body function, and affects the quality of life of patients[4,5]. Therefore, the identification of serological indicators related to the treatment, diagnosis and prognosis is of great significance for AS screening.
Dickkopf-related protein-1 (DKK-1) is a natural inhibitor of the Wnt signaling pathway, and Wnt controls osteoblastogenesis and is the key to ectopic bone formation[6]. Increased DKK-1 level is related to bone resorption, while a decreased level is related to new bone formation[7,8]. There is evidence that dysfunctional DKK-1 in AS reduces its inhibition on the Wnt signaling pathway, and is related to the functional dominance of circulating bone formation–promoting factors[9]. The correlation between tumor necrosis factor α (TNF-α) and AS has been widely studied, and anti-TNF-α therapy has also been reported to play a role in the treatment of AS[10,11]. Therefore, DKK-1 and TNF-α may have potential in the diagnosis and treatment of AS, providing an objective and convenient index. This study examined the therapeutic, diagnostic significance and prognostic value of DKK-1 and TNF-α in AS to provide references for the diagnosis and treatment of AS.
MATERIALS AND METHODS
Research subjects
A total of 113 patients with active AS admitted to Lishui People’s Hospital from October 2015 to July 2017 were selected as the research group, and 100 healthy subjects who underwent physical examination in Lishui People’s Hospital during the same period were enrolled as the control group. Patients in both groups were aged 30-45 years. Inclusion criteria were as follows: Patients in the research group meeting the modified New York criteria for AS revised in 1984[3], and treated according to the guidelines[12] formulated by the American College of Rheumatology, the American Spondylitis Association, and the Spondyloarthritis Research and Treatment Network in 2015; patients with complete medical records and follow-up data; controls with no obvious clinical symptoms related to spinal or joint lesions. The study followed the principle of the “Helsinki Declaration”. Exclusion criteria were as follows: Patients with non-radiographic Axial SpA; patients in the research group with comorbid rheumatoid diseases, intervertebral disc herniation, peripheral arthritis and other joint diseases; patients with mental diseases; pregnant or lactating women. This study was approved by the Lishui People’s Hospital Ethics Committee. Patients and their families were consulted by telephone or letter and signed an informed consent form.
Outcome measures
Basic information such as sex and age were collected from clinical medical records. After 12 wk of treatment, the therapeutic effect was assessed by the Assessment in Ankylosing Spondylitis 20% (ASAS20) criteria developed by the ASAS International Society[13]. The levels of DKK-1 and TNF-α in peripheral blood in the two groups were compared, and the correlations of DKK-1 and TNF-α with AS-related indices were analyzed. ROC analysis was used to analyze the diagnostic and predictive value of DKK-1 and TNF-α in AS, and COX regression was used to analyze the factors influencing AS recurrence. During follow-up, patients with a Bath Ankylosing Spondylitis Disease Activity Index (BASDAI) ≥ 3.5 and BASDAI scores exceeding 60% of the baseline value were considered to have recurrence.
Detection methods
Peripheral blood from all subjects was obtained by nurses in Lishui People’s Hospital before and 12 wk after treatment, and DKK-1 and TNF-α were quantified by enzyme linked immunosorbent assay (ELISA). The specific method used was in accordance with the kit instructions. The detection kits were purchased from Abcam Company, United States, with the article numbers ab100501 and ab181421.
Statistical analysis
Statistical analysis was carried out with SPSS 19.0 (Asian Analytics Formerly SPSS China). The counting data were expressed as percentage (%), and the comparison adopted χ2 test. The measurement data were expressed as mean ± SD and analyzed by the Students' t test. The ROC curve was applied to analyze the diagnostic and predictive value of DKK-1 and TNF-α in AS, and COX regression was used to analyze the factors influencing the recurrence of AS. A correlation analysis was performed with Spearman and Pearson tests. A value of aP < 0.05 indicated statistical significance.
RESULTS
General information
There were 113 patients in the research group, including 76 males and 37 females, aged (36.7 ± 4.1) years, and 100 subjects in the control group, including 76 males and 24 females, aged (37.3 ± 4.6) years. There were no significant differences in sex ratio and age between the two groups (Table 1).
Table 1.
General information
| Study group (n = 113) | Control group (n = 100) | P value | |
| Sex, n (%) | 0.159 | ||
| Male | 76 (67.26) | 76 (76.00) | |
| Female | 37 (32.74) | 24 (24.00) | |
| Age (yr) | 36.7 ± 4.1 | 37.3 ± 4.6 | 0.315 |
| BMI (kg/m2) | 22.25 ± 1.83 | 23.49 ± 2.16 | 0.381 |
| Course of disease (yr) | 1.5 ± 0.4 | ||
| CRP (ng/L) | |||
| Before treatment | 8.61 ± 0.72 | 0.50 ± 0.00 | < 0.001 |
| After treatment | 1.32 ± 0.121 | ||
| IgG (g/L) | |||
| Before treatment | 16.00 ± 1.75 | ||
| After treatment | 2.89 ± 0.451 | ||
| IgA (g/L) | |||
| Before treatment | 4.88 ± 0.91 | ||
| After treatment | 0.82 ± 0.211 | ||
| IgM (g/L) | |||
| Before treatment | 1.49 ± 0.48 | ||
| After treatment | 0.24 ± 0.081 | ||
| Rheumatoid factor | (-) | ||
| HLA-B27 (+), n (%) | |||
| Before treatment | 85 (75.00) | 0 (0.00) | < 0.001 |
| After treatment | 15 (13.27)1 | ||
| ESR (mm/h) | |||
| Before treatment | 48.83 ± 7.79 | 12.29 ± 2.73 | < 0.001 |
| After treatment | 28.14 ± 4.321 | ||
| BASDAI | |||
| Before treatment | 4.98 ± 1.61 | ||
| After treatment | 0.93 ± 0.241 | ||
| BASMI | |||
| Before treatment | 4.26 ± 1.96 | ||
| After treatment | 0.71 ± 0.311 |
indicates compared with the research group before treatment, P < 0.05. BMI : Body mass index; CRP: C-reactive protein; IgG: Immunoglobulin G; IgA: Immunoglobulin A; IgM: Immunoglobulin M; HLA-B27: Human leukocyte antigen-B27; ESR: Erythrocyte sedimentation rate; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASMI: Bath Ankylosing Spondylitis Metrology Index.
Difference in DKK-1 expression between the two groups
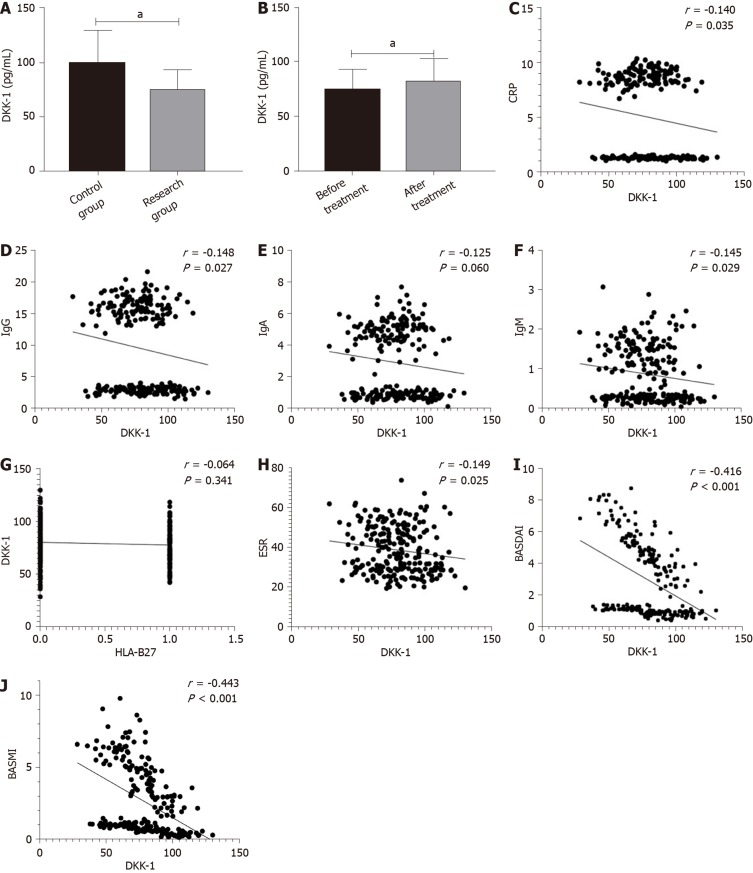
Before treatment, the research group showed lower DKK-1 levels than the control group (aP < 0.05). After treatment, the level of DKK-1 in the research group was higher than that before treatment (aP < 0.05). Correlation analysis showed that the expression of DKK-1 was significantly correlated with C-reactive protein (CRP), Immunoglobulin G (IgG), IgA, IgM, Human leukocyte antigen B27 (HLA-B27), erythrocyte sedimentation rate (ESR), BASDAI and Bath Ankylosing Spondylitis Metrology Index (BASMI) (aP < 0.05), but was not correlated with human leukocyte antigen-B27 (HLA-B27) (Figure 1).
Figure 1.
Difference in Dickkopf-related protein-1 expression in the two groups. A: Difference in dickkopf-related protein-1 (DKK-1) expression between the control group and the research group before treatment; B: Difference in DKK-1 expression before and after treatment in the research group; C: Correlation between DKK-1 and C-reactive protein; D: Correlation between DKK-1 and Immunoglobulin G; E: Correlation between DKK-1 and Immunoglobulin A; F: Correlation between DKK-1 and Immunoglobulin M; G: Correlation between DKK-1 and HLA-B27; H: Correlation between DKK-1 and erythrocyte sedimentation rate; I: Correlation between DKK-1 and the Bath Ankylosing Spondylitis Disease Activity Index; J: Correlation between DKK-1 and the Bath Ankylosing Spondylitis Metrology Index. aP < 0.05.
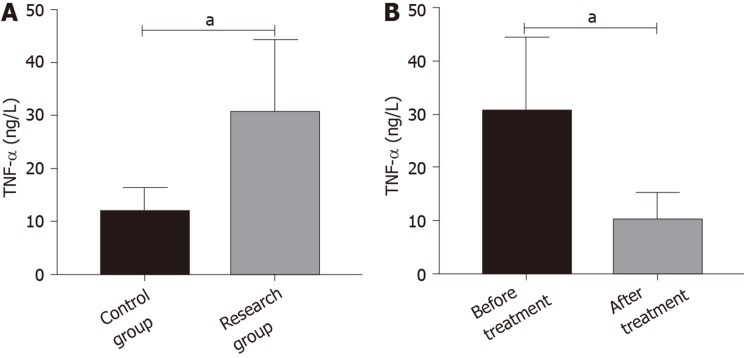
Difference in TNF-α expression in the two groups
Before treatment, the research group showed higher TNF-α levels than the control group (aP < 0.05). After treatment, the level of TNF-α in the research group was lower than that before treatment (aP < 0.05) (Figure 2).
Figure 2.
Difference in tumor necrosis factor-α expression in the two groups. A: Difference in tumor necrosis factor-αexpression between the control group and the research group before treatment; B: Difference in tumor necrosis factor-α expression before and after treatment in the research group. aP < 0.05. TNF-α: Tumor necrosis factor-α.
Diagnostic value of DKK-1 and TNF-α in AS
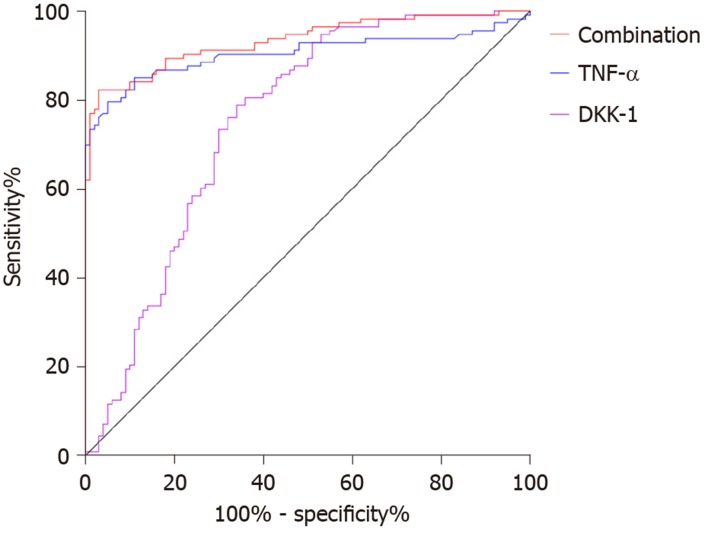
The area under the curve (AUC), cutoff value, sensitivity and specificity of DKK-1 for diagnosing AS were 0.752, 87.02 pg/mL, 78.76% and 66.00%, respectively, and those of TNF-α were 0.904, 18.79 ng/L, 79.65% and 95.00%. The AUC, sensitivity and specificity of combined diagnosis were 0.934, 82.30% and 97.00%, respectively (Table 2, Figure 3).
Table 2.
Diagnostic value of dickkopf-related protein-1 and tumor necrosis factor-α in ankylosing spondylitis by receiver operating characteristic curve
| DKK-1 | TNF-α | Combination | |
| AUC | 0.752 | 0.904 | 0.934 |
| 95%CI | 0.684-0.820 | 0.859-0.950 | 0.900-0.967 |
| Cutoff value | 87.02 pg/mL | 18.79 ng/L | |
| Sensitivity | 78.76% | 79.65% | 82.30% |
| Specificity | 66.00% | 95.00% | 97.00% |
TNF-α: Tumor necrosis factor-α; DKK: Dickkopf-related protein-1; AUC: Area under the curve.
Figure 3.
Diagnostic value of dickkopf-related protein-1 and tumor necrosis factor-α in ankylosing spondylitis. The area under the curve, cutoff value, sensitivity and specificity of dickkopf-related protein-1 for diagnosing ankylosing spondylitis were 0.752, 87.02 pg/mL, 78.76% and 66.00% respectively, and those of tumor necrosis factor-α were 0.904, 18.79 ng/L, 79.65% and 95.00%. The area under the curve, sensitivity and specificity of combined diagnosis were 0.934, 82.30% and 97.00% respectively. TNF-α: Tumor necrosis factor-α; DKK: dickkopf-related protein-1.
Relationship between DKK-1, TNF-α and curative effect in AS
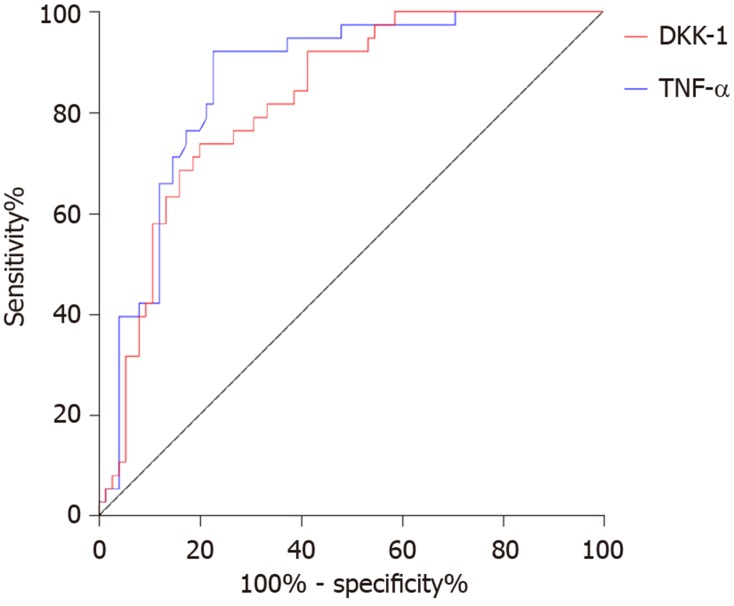
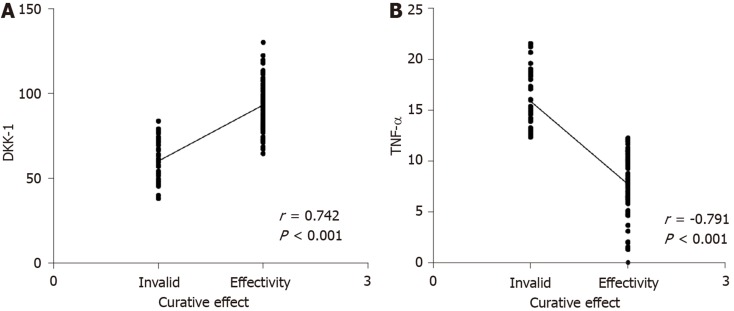
Seventy-five patients met the ASASAS20 criteria (= 2) and 35 patients did not (= 1). The AUC, cutoff value, sensitivity and specificity of DKK-1 in predicting the curative effect in AS before treatment were 0.825, 68.42 pg/mL, 73.68%, and 80.00%, respectively, and those of TNF-α were 0.863, 32.79 ng/L, 92.11% and 77.33%, respectively (Table 3, Figure 4). After treatment, DKK-1 was positively correlated with the curative effect, while TNF-α was negatively correlated with the curative effect (aP < 0.05) (Figure 5).
Table 3.
Predictive value of dickkopf-related protein-1 and tumor necrosis factor-α on the curative effect in ankylosing spondylitis by receiver operating characteristic curve
| DKK-1 | TNF-α | |
| AUC | 0.825 | 0.863 |
| 95%CI | 0.749-0.901 | 0.794-0.932 |
| Cutoff value | 68.42 pg/mL | 32.79 ng/L |
| Sensitivity | 73.68% | 92.11% |
| Specificity | 80.00% | 77.33% |
TNF-α: Tumor necrosis factor-α; DKK: Dickkopf-related protein-1; AUC: Area under the curve.
Figure 4.
Predictive value of dickkopf-related protein-1 and tumor necrosis factor-α in the curative effect in ankylosing spondylitis. The area under the curve, cutoff value, sensitivity and specificity of dickkopf-related protein-1 for predicting the curative effect before treatment were 0.825, 68.42 pg/mL, 73.68% and 80.00% respectively, and those of tumor necrosis factor-α were 0.863, 32.79 ng/L, 92.11% and 77.33%, respectively. TNF-α: Tumor necrosis factor-α; DKK: Dickkopf-related protein-1.
Figure 5.
Correlation between dickkopf-related protein-1, tumor necrosis factor-α and the curative effect in ankylosing spondylitis. A: Correlation between dickkopf-related protein-1 and curative effect in ankylosing spondylitis; B: Correlation between tumor necrosis factor-α and curative effect in ankylosing spondylitis. TNF-α: Tumor necrosis factor-α; DKK: Dickkopf-related protein-1.
Influencing factors for AS recurrence
The patients in the research group were followed up for 9 mo, 80 of whom had recurrent AS, with a recurrence rate of 70.80%. COX analysis showed that CRP, BASDAI, DKK-1, and TNF-α were risk factors for recurrence (aP < 0.05) (Table 4).
Table 4.
Multivariate analysis of recurrence of ankylosing spondylitis
| B | SE | Wald | df | Sig. | Exp (B) |
95%CI |
||
| Lower part | Upper part | |||||||
| TNF-α | -0.450 | 0.362 | 1.549 | 1 | 0.013 | 0.637 | 0.314 | 1.296 |
| CRP | 3.871 | 2.473 | 2.450 | 1 | 0.018 | 47.992 | 0.377 | 61.152 |
| IgG | 0.162 | 0.089 | 3.325 | 1 | 0.168 | 1.176 | 0.988 | 1.445 |
| HLA-B27 | -13.651 | 139.432 | 0.010 | 1 | 0.062 | 1.325 | 0.745 | 3.306 |
| BASDAI | 0.305 | 0.094 | 10.524 | 1 | 0.001 | 1.357 | 1.128 | 1.631 |
| BASMI | -0.063 | 0.071 | 0.79 | 1 | 0.074 | 0.939 | 0.816 | 1.079 |
| DKK-1 | 0.020 | 0.008 | 6.877 | 1 | 0.009 | 1.021 | 1.005 | 1.036 |
TNF-α: Tumor necrosis factor-α; BASDAI: Bath Ankylosing Spondylitis Disease Activity Index; BASMI: Bath Ankylosing Spondylitis Metrology Index; CRP: C-reactive protein; IgG: Immunoglobulin G; DKK: Dickkopf-related protein-1.
DISCUSSION
AS is a chronic inflammatory disease characterized by new bone formation, which gradually leads to spinal dysfunction[14]. At present, there is no cure for AS, only early diagnosis can improve the prognosis; thus, early diagnosis and prognosis evaluation are of great significance for patients and clinical treatment. This study revealed the high diagnostic and therapeutic value of DKK-1 and TNF-α in AS, and these parameters were found to be associated with AS recurrence.
Our findings suggested that DKK-1 expression decreased in the peripheral blood of patients with AS, while TNF-α expression increased. Moreover, after treatment, DKK-1 correspondingly increased and TNF-α decreased. At present, the relationship between TNF-α and AS is relatively clear, and TNF-α inhibitors are widely applied in the clinical treatment of AS[15,16]. DKK-1 was reported to have decreased expression and disordered function in AS patients, suggesting that DKK-1 is closely related to AS[17,18], which is similar to our results. DKK-1 is a natural inhibitor of the Wnt pathway and inhibits bone formation by inhibiting this pathway. Decreased or dysfunctional DKK-1 may reduce the inhibitory effect on the Wnt pathway, resulting in increased bone formation, which is currently considered to be one of the mechanisms of DKK-1 in AS[19,20]. The diagnostic value of DKK-1 and TNF-α in AS was further analyzed in this study, and it was found that the AUC of DKK-1 in the diagnosis of AS was 0.752, that of TNF-α was 0.904, and that of combined diagnosis reached 0.934, which indicates that DKK-1 combined with TNF-α is effective in the diagnosis of AS. Bal et al[21] stated that TNF-α could be used for AS diagnosis, but the specific diagnostic value was not verified. There are few reports on the diagnostic value of DKK-1 and TNF-α in AS; however, DKK-1 has been found to be closely related to the occurrence and development of AS. Wu et al[22] reported that DKK-1 was a biomarker of inflammation and radiographic damage in AS. We also found that DKK-1 was significantly linearly correlated with CRP, IgG, IgM, ESR, BASDAI, and BASMI, but the correlation coefficient was insignificant; therefore, further validation in more cases is required. Liao et al[23] reported that DKK-1 expression was significantly correlated with disease activity, radiological and functional indices in AS patients, which is similar to our findings. The correlation between DKK-1, TNF-α and the therapeutic effect in AS was also analyzed in this study. Before treatment, the AUC of DKK-1 level for predicting the curative effect was 0.825, and that of TNF-α was 0.863, suggesting that both DKK-1 and TNF-α have good predictive value for curative effect assessment, which may contribute to the development and improvement of treatment regimens. Moreover, a correlation was observed between DKK-1, TNF-α levels and curative effect after treatment, which verified the relationship between them in AS. Further analysis also found that DKK-1, TNF-α, CRP, and BASDAI were risk factors for AS recurrence. Deng et al[24] found that the patient global assessment and CRP can be regarded as predictive factors for the recurrence of AS. Baraliakos et al[25] reported that BASDAI, CRP, age and course of disease were related to the recurrence time of AS, which supports our findings. Therefore, DKK-1 and TNF-α are predictors of curative effect and prognostic risk factors of AS.
However, this study also had some shortcomings. Firstly, although we observed a significant correlation between DKK-1 and CRP and ESR in AS patients, it is not known whether the difference in the expression of DKK-1 in AS is affected by inflammation and ESR; thus, this will be investigated in our next study. Secondly, the relationship between DKK-1 and the severity of AS also requires further analysis.
In conclusion, DKK-1 and TNF-α are effective in the diagnosis and treatment of AS and are risk factors for its recurrence. In addition, DKK-1 may be a potential target for future treatment of AS.
ARTICLE HIGHLIGHTS
Research background
Ankylosing spondylitis (AS) frequently occurs in people aged 30-45 years, and its prevalence is generally believed to be between 0.1% and 1.4% globally. At present, the “gold standard” for diagnosis of AS requires the provision of pelvic X-rays, which makes it more difficult to perform in population-based epidemiological studies. Therefore, the identification of serological indicators related to the diagnosis, treatment, and prognosis of AS patients is of great significance.
Research motivation
The “gold standard” for ankylosing spondylitis depends on X-rays, and the prognosis of patients with ankylosing spondylitis is variable and closely related to the patient’s extraspinal manifestations (such as uveitis, psoriasis, and IBD), age at diagnosis, and treatment. The identification of convenient serological indicators related to patient treatment, diagnosis and prognosis is of great significance.
Research objectives
This study aimed to analyze the therapeutic, diagnostic significance and prognostic value of Dickkopf-related protein-1 (DKK-1) and tumor necrosis factor-α (TNF-α) in AS.
Research methods
A total of 113 patients with active AS were selected as the research group, and 100 healthy subjects who underwent physical examination were selected as the control group. The levels of DKK-1 and TNF-α in peripheral blood in the two groups were compared. The diagnostic and predictive value of DKK-1 and TNF-α for AS were analyzed with ROC curves, and the factors influencing AS recurrence were analyzed with COX regression.
Research results
Before treatment, the research group showed lower DKK-1 levels but higher TNF-α levels than the control group (both aP < 0.05). In the research group, DKK-1 was up-regulated and TNF-α was down-regulated after 12 wk of treatment (aP < 0.05). The (area under the curve) AUC, sensitivity and specificity of DKK-1 combined with TNF-α for diagnosing AS were 0.934, 82.30% and 97.00%, respectively. Before treatment, the AUC, cutoff value, sensitivity and specificity of DKK-1 for predicting the curative effect were 0.825, 68.42 pg/mL, 73.68% and 80.00%, respectively, and those of TNF-α were 0.863, 32.79 ng/L, 92.11% and 77.33%, respectively. DKK-1 and TNF-α levels after treatment were closely related to the curative effect (aP < 0.05). C-reactive protein, the Bath Ankylosing Spondylitis Disease Activity Index, DKK-1, and TNF-α were risk factors for AS recurrence (aP < 0.05).
Research conclusions
DKK-1 and TNF-α are effective in the diagnosis and treatment of AS and are risk factors for its recurrence. In addition, DKK-1 may be a potential target for the diagnosis of AS.
Research perspectives
Further analysis of the differential diagnostic value of DKK-1 and TNF-α in AS and suspected AS (such as lumbosacral joint strain, tuberculous spondylitis, Forestier disease, etc.), and the provision of more evidence for the clinical diagnosis of AS are required.
Footnotes
Institutional review board statement: This study was reviewed and approved by the Lishui People’s Hospital Ethics Committee.
Informed consent statement: All patients in our study provided informed consent.
Conflict-of-interest statement: The authors declare no conflict of interest.
STROBE statement: The authors have read the STROBE Statement-checklist of items, and the manuscript was prepared and revised according to the STROBE Statement-checklist of items.
Manuscript source: Unsolicited manuscript
Peer-review started: December 25, 2019
First decision: February 20, 2020
Article in press: March 19, 2020
Specialty type: Medicine, research and experimental
Country/Territory of origin: China
Peer-review report’s scientific quality classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C, C
Grade D (Fair): 0
Grade E (Poor): 0
P-Reviewer: Cure E, Soriano ER S-Editor: Zhang L L-Editor: Webster JR E-Editor: Liu JH
Contributor Information
Jun-Hui Xiong, Department of Rheumatology, Lishui People’s Hospital, Lishui 323000, Zhejiang Province, China.
Jian Liu, Department of Rheumatology, Lishui People’s Hospital, Lishui 323000, Zhejiang Province, China.
Jian Chen, Department of Rheumatology, Lishui People’s Hospital, Lishui 323000, Zhejiang Province, China. cairuke99@163.com.
Data sharing statement
No additional data are available.
References
- 1.Dean LE, Jones GT, MacDonald AG, Downham C, Sturrock RD, Macfarlane GJ. Global prevalence of ankylosing spondylitis. Rheumatology (Oxford) 2014;53:650–657. doi: 10.1093/rheumatology/ket387. [DOI] [PubMed] [Google Scholar]
- 2.Akkoc N. Are spondyloarthropathies as common as rheumatoid arthritis worldwide? A review. Curr Rheumatol Rep. 2008;10:371–378. doi: 10.1007/s11926-008-0060-3. [DOI] [PubMed] [Google Scholar]
- 3.Reveille JD. Epidemiology of spondyloarthritis in North America. Am J Med Sci. 2011;341:284–286. doi: 10.1097/MAJ.0b013e31820f8c99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boonen A, van der Linden SM. The burden of ankylosing spondylitis. J Rheumatol Suppl. 2006;78:4–11. [PubMed] [Google Scholar]
- 5.Olivieri I, van Tubergen A, Salvarani C, van der Linden S. Seronegative spondyloarthritides. Best Pract Res Clin Rheumatol. 2002;16:723–739. doi: 10.1053/berh.2002.0263. [DOI] [PubMed] [Google Scholar]
- 6.Diarra D, Stolina M, Polzer K, Zwerina J, Ominsky MS, Dwyer D, Korb A, Smolen J, Hoffmann M, Scheinecker C, van der Heide D, Landewe R, Lacey D, Richards WG, Schett G. Dickkopf-1 is a master regulator of joint remodeling. Nat Med. 2007;13:156–163. doi: 10.1038/nm1538. [DOI] [PubMed] [Google Scholar]
- 7.MacDonald BT, Joiner DM, Oyserman SM, Sharma P, Goldstein SA, He X, Hauschka PV. Bone mass is inversely proportional to Dkk1 levels in mice. Bone. 2007;41:331–339. doi: 10.1016/j.bone.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Zhu Ke H, Li X, Richards WG. Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone. 2006;39:754–766. doi: 10.1016/j.bone.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 9.Daoussis D, Liossis SN, Solomou EE, Tsanaktsi A, Bounia K, Karampetsou M, Yiannopoulos G, Andonopoulos AP. Evidence that Dkk-1 is dysfunctional in ankylosing spondylitis. Arthritis Rheum. 2010;62:150–158. doi: 10.1002/art.27231. [DOI] [PubMed] [Google Scholar]
- 10.Poddubnyy D, Fedorova A, Listing J, Haibel H, Baraliakos X, Braun J, Sieper J. Physical Function and Spinal Mobility Remain Stable Despite Radiographic Spinal Progression in Patients with Ankylosing Spondylitis Treated with TNF-α Inhibitors for Up to 10 Years. J Rheumatol. 2016;43:2142–2148. doi: 10.3899/jrheum.160594. [DOI] [PubMed] [Google Scholar]
- 11.Carbo MJG, Spoorenberg A, Maas F, Brouwer E, Bos R, Bootsma H, van der Veer E, Wink F, Arends S. Ankylosing spondylitis disease activity score is related to NSAID use, especially in patients treated with TNF-α inhibitors. PLoS One. 2018;13:e0196281. doi: 10.1371/journal.pone.0196281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ward MM, Deodhar A, Akl EA, Lui A, Ermann J, Gensler LS, Smith JA, Borenstein D, Hiratzka J, Weiss PF, Inman RD, Majithia V, Haroon N, Maksymowych WP, Joyce J, Clark BM, Colbert RA, Figgie MP, Hallegua DS, Prete PE, Rosenbaum JT, Stebulis JA, van den Bosch F, Yu DT, Miller AS, Reveille JD, Caplan L. American College of Rheumatology/Spondylitis Association of America/Spondyloarthritis Research and Treatment Network 2015 Recommendations for the Treatment of Ankylosing Spondylitis and Nonradiographic Axial Spondyloarthritis. Arthritis Rheumatol. 2016;68:282–298. doi: 10.1002/art.39298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sieper J, Deodhar A, Marzo-Ortega H, Aelion JA, Blanco R, Jui-Cheng T, Andersson M, Porter B, Richards HB MEASURE 2 Study Group. Secukinumab efficacy in anti-TNF-naive and anti-TNF-experienced subjects with active ankylosing spondylitis: results from the MEASURE 2 Study. Ann Rheum Dis. 2017;76:571–592. doi: 10.1136/annrheumdis-2016-210023. [DOI] [PubMed] [Google Scholar]
- 14.Pelechas E, Kaltsonoudis E, Voulgari PV, Drosos AA. Illustrated Handbook of Rheumatic and Musculo-Skeletal Diseases. Springer Nature Switzerland AG: Springer, 2019: 121-140. [Google Scholar]
- 15.Maas F, Arends S, Wink FR, Bos R, Bootsma H, Brouwer E, Spoorenberg A. Ankylosing spondylitis patients at risk of poor radiographic outcome show diminishing spinal radiographic progression during long-term treatment with TNF-α inhibitors. PLoS One. 2017;12:e0177231. doi: 10.1371/journal.pone.0177231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Maas F, Spoorenberg A, Brouwer E, Bos R, Efde M, Chaudhry RN, Veeger NJ, van Ooijen PM, Wolf R, Bootsma H, van der Veer E, Arends S. Spinal radiographic progression in patients with ankylosing spondylitis treated with TNF-α blocking therapy: a prospective longitudinal observational cohort study. PLoS One. 2015;10:e0122693. doi: 10.1371/journal.pone.0122693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang J, Song G, Yin Z, Fu Z, Zhang L. Altered expression of microRNAs targeting Dkk-1 in peripheral blood mononuclear cells of patients with ankylosing spondylitis. Cent Eur J Immunol. 2019;44:59–64. doi: 10.5114/ceji.2019.84018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang L, Ouyang H, Xie Z, Liang ZH, Wu XW. Serum DKK-1 level in the development of ankylosing spondylitis and rheumatic arthritis: a meta-analysis. Exp Mol Med. 2016;48:e228. doi: 10.1038/emm.2016.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sun W, Tian L, Jiang L, Zhang S, Zhou M, Zhu J, Xue J. Sclerostin rather than Dickkopf-1 is associated with mSASSS but not with disease activity score in patients with ankylosing spondylitis. Clin Rheumatol. 2019;38:989–995. doi: 10.1007/s10067-018-4356-z. [DOI] [PubMed] [Google Scholar]
- 20.Orsolini G, Adami G, Rossini M, Ghellere F, Caimmi C, Fassio A, Idolazzi L, Gatti D, Viapiana O. Parathyroid hormone is a determinant of serum Dickkopf-1 levels in ankylosing spondylitis. Clin Rheumatol. 2018;37:3093–3098. doi: 10.1007/s10067-018-4205-0. [DOI] [PubMed] [Google Scholar]
- 21.Bal A, Unlu E, Bahar G, Aydog E, Eksioglu E, Yorgancioglu R. Comparison of serum IL-1 beta, sIL-2R, IL-6, and TNF-alpha levels with disease activity parameters in ankylosing spondylitis. Clin Rheumatol. 2007;26:211–215. doi: 10.1007/s10067-006-0283-5. [DOI] [PubMed] [Google Scholar]
- 22.Wu M, Chen M, Ma Y, Yang J, Han R, Yuan Y, Hu X, Wang M, Zhang X, Xu S, Liu R, Jiang G, Xu J, Shuai Z, Zou Y, Pan G, Pan F. Dickkopf-1 in ankylosing spondylitis: Review and meta-analysis. Clin Chim Acta. 2018;481:177–183. doi: 10.1016/j.cca.2018.03.010. [DOI] [PubMed] [Google Scholar]
- 23.Liao HT, Lin YF, Tsai CY, Chou TC. Bone morphogenetic proteins and Dickkopf-1 in ankylosing spondylitis. Scand J Rheumatol. 2018;47:56–61. doi: 10.1080/03009742.2017.1287305. [DOI] [PubMed] [Google Scholar]
- 24.Deng X, Zhang J, Zhang J, Huang F. Thalidomide reduces recurrence of ankylosing spondylitis in patients following discontinuation of etanercept. Rheumatol Int. 2013;33:1409–1413. doi: 10.1007/s00296-012-2571-5. [DOI] [PubMed] [Google Scholar]
- 25.Baraliakos X, Listing J, Brandt J, Zink A, Alten R, Burmester G, Gromnica-Ihle E, Kellner H, Schneider M, Sörensen H, Zeidler H, Rudwaleit M, Sieper J, Braun J. Clinical response to discontinuation of anti-TNF therapy in patients with ankylosing spondylitis after 3 years of continuous treatment with infliximab. Arthritis Res Ther. 2005;7:R439–R444. doi: 10.1186/ar1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No additional data are available.