Abstract
This study aimed to evaluate the effect of self-assembling peptide P11-4 (SAP) in the therapy of initial smooth surface caries (white spot lesions, WSL) following orthodontic multibracket treatment. Twenty-three patients (13f/10m; average age 15.4 years) with at least two teeth with WSL were recruited for the randomised controlled clinical trial with split-mouth design. In opposite to the control teeth, the test teeth were treated with SAP on Day 0. The primary endpoint was the impedance measurement of WSL using customised tray to ensure reproducibility of the measurement location. The secondary endpoint was the morphometric measurement of WSL using a semi-automated approach to determine the WSL size in mm2. Treatment effects were adjusted for site-specific baseline values using mixed models adapted from the cross-over design. Test WSL showed a mean baseline impedance value of 46.7, which decreased to 21.1, 18.4, and 19.7 after 45, 90, and 180 days, respectively. Control WSL showed a mean baseline value of 42.0, which decreased to 35.0, 29.5, and 33.7, respectively. The overall treatment contrast was −13.7 (95% CI: −19.6 – −7.7; p < 0.001). For the secondary endpoint, the test WSL size decreased from 8.8 at baseline to 6.5 after 180 days. The control WSL decreased from 6.8 to 5.7, respectively. The related treatment contrast was −1.0 in favour of test WSL (95% CI: −1.6 – −0.5; p = 0.004). The treatment of initial carious lesions with self-assembling peptide P11−4 leads to superior remineralisation of the subsurface lesions compared with the control teeth.
Subject terms: Fixed appliances, Fixed appliances, Minimal intervention dentistry, Minimal intervention dentistry, Randomized controlled trials
Introduction
Orthodontic treatments with fixed multibracket appliances hindering oral hygiene, support plaque accumulation, and caries progression1,2. These orthodontic treatment-induced carious lesions are typically visible first as so-called white spot lesions (WSL) on the buccal surface of the tooth outlining the brackets3–6.
Modern treatment concepts for caries emphasise tooth preservation and remineralisation concepts especially for initial non-cavitated carious lesions, in order to hinder or to delay the first invasive intervention, meaning destruction of the natural tooth structure7.
Unique for buccal WSL is the addition of an aesthetic component to the cariological issue3,8. Fluorides prevent the formation of so-called white spot lesions (WSL) but have shown little effect on the reduction of existing WSL9–11. As their effect is restricted to the outer surface layer of the enamel (i.e. top 50 µm) and does not promote the remineralisation throughout the demineralised lesion body. The WSL persist visually almost unchanged12–14. Other remineralisation agents, often based on calcium phosphate, have been investigated, but could not show clinically significant advantage over fluoride10,11,15–17.
As a consequence, new treatment approaches have been called for and biomimetic mineralisation seems to be one promising possibility18–22. However, the only clinically available products at present are based on the self-assembling peptide P11-4 (SAP P11-4)21.
The mode of action for the treatment of initial caries with SAP P11-4 is as follows23,24. Monomeric P11-4 diffuses into the subsurface lesion, self-assembles into fibres to form a 3D-matrix and attracts calcium-ions from saliva and templates the formation of hydroxyapatite crystals, thus supporting the natural remineralisation mechanism driven by saliva.
Clinically, SAP P11-4 has previously been investigated on buccal lesions. A first-in-man trial, mostly on inactive lesions, demonstrated regression of the WSL size and a trend toward remineralisation25. Randomised clinical trials could show superior regression of the lesion as judged by morphometric analysis compared with both placebo26 and/or fluoride varnish26,27. As neither of the previous clinical trials investigated the effect of SAP P11-4 following orthodontic treatment, it can be assumed that the lesions investigated in those clinical trials were mostly inactive25–27.
Recently, the caries activity status of a lesion has become a new focus in cariology28.
Orthodontic WSL are of particular interest for clinical investigations of caries in this respect, as they are assumed to be active until debonding of the brackets, whereupon they become inactive4,8 and good accessible for several measurement methods16,29.
Therefore, the present split-mouth study investigated the effect of SAP P11-4 in addition to the conventional treatment of early carious lesions after debonding of orthodontic brackets. The primary endpoint was the impedance measurement29–31. Moreover, a semi-automated approach to measure the WSL size was used as done in other clinical studies on SAP P11-425–27.
Results
Baseline characteristics
The mean duration of the orthodontic treatment with fixed appliances was 27 months (min/max 13/39). Of the 23 recruited patients (13f 10 m; mean age 15.4 years), 21 could be analysed in mixed models (12f/9 m; mean age 15.3 years). One patient showed established dentin caries (primary endpoint = 100) on both teeth after 30 days and cavitated lesions after 90 days. The related four values of the primary endpoint after 90 and 180 days were set to 100. No further cavitated lesions were observed. The QHI at t0 was 2.2/2.2 (test/control teeth).
Impedance measurement of white spot lesion (primary endpoint)
Both test and control teeth exhibited a substantial decrease of impedance readings throughout the study period (Table 1). Control teeth showed a mean baseline value of 42.0 at day 0, which decreased to 35.0 at day 45, 29.5 at day 90, and 33.7 at day 180. Test teeth showed a mean baseline value of 46.7 and exhibited a markedly larger decrease to 21.1 (day 45), 18.4 (day 90), and 19.7 (day 180).
Table 1.
Primary endpoint: Impedance measurement in test and control teeth (N = 21, split-mouth design, 122 observations in 2 sites or teeth at 3 time points).
| Observed values | Estimated values (adjusted for baseline values) | |||||
|---|---|---|---|---|---|---|
| Test Group | Control Group | Test Group | Control Group | Treatment effect | ||
| Mean ± SE | Mean ± SE | Difference (95% CI) | p value | |||
| Baseline | ||||||
| Mean ± SD | 46.7 ± 16.9 | 42.0 ± 15.7 | ||||
| Median (IQR) | 50 (44–52) | 47 (35–50) | ||||
| Day 45 | ||||||
| Mean ± SD | 21.1 ± 23.2 | 35.0 ± 23.4 | 20.7 ± 5.1 | 35.4 ± 5.1 | −14.7 (−22.8 – −6.6) | 0.001 |
| Median (IQR) | 14 (7–23) | 32 (17–51) | ||||
| Day 90 | ||||||
| Mean ± SD | 18.4 ± 22.3 | 29.5 ± 25.0 | 18.2 ± 5.0 | 29.8 ± 5.0 | −11.7 (−19.4 – −3.9) | 0.005 |
| Median (IQR) | 10 (7–21) | 19 (9–47) | ||||
| Day 180 | ||||||
| Mean ± SD | 19.7 ± 24.2 | 33.7 ± 25.5 | 19.4 ± 5.4 | 34.0 ± 5.4 | −14.6 (−24.5 – −4.8) | 0.007 |
| Median (IQR) | 10 (5–27) | 35 (10–49) | ||||
| Baseline vs | ||||||
| Day 45 | ||||||
| Mean ± SD | 25.5 ± 23.3 | 7.0 ± 21.7 | ||||
| Median (IQR) | 30 (18–43) | 15 (−1–21) | ||||
| Day 90 | ||||||
| Mean ± SD | 28.1 ± 25.1 | 12.2 ± 22.9 | ||||
| Median (IQR) | 36 (16–43) | 14 (−4–32) | ||||
| Day 180 | ||||||
| Mean ± SD | 26.8 ± 28.0 | 8.0 ± 27.4 | ||||
| Median (IQR) | 36 (17–45) | 10 (1–28) | ||||
According to the manufacturer impedance values correspond to the following:
Sound: 0 = sound; 1–20 = Sound enamel, caries at the very outer enamel; Enamel caries 21–30 = caries in the outer 1/3 of the enamel; 31–50 = caries in the middle 1/3 of the enamel; 51–90 = caries in the inner 1/3 of the enamel; 91–99 = caries at the dentine enamel junction; 100 = established dentine caries.
Abbreviation: CI, confidence interval; IQR, interquartile range; SD, standard deviation; SE, standard error of the mean.
The treatment effect was statistically significant (overall treatment contrast, which is the mean of the three single contrasts in Table 1: -13.7, 95% CI: −19.6 – −7.7; p < 0.001). More importantly, the global F test of no treatment effect was rejected (p = 0.003 for the test including one term for treatment and two terms for the interaction between treatment and time). After treatment, the difference between test and control tooth changed only slightly over time (p = 0.623; Fig. 1), yielding a large treatment effect of 43% after 180 days (14.6/34.0 in Table 1), which is usually considered clinically relevant. Robust analyses using the means of the three time points after treatment confirmed results of the mixed model in observed and imputed data with and without excluding dropouts (20, 21, and 23 subjects, respectively; treatment differences −13.1 (95% CI: −18.8 – −7.4), −13.3 (95% CI: −20.2 – −6.3), and −12.8 (95% CI: −22.4 – −3.2), respectively; for the “missing not at random” scenario by adding 50 to the treatment site of the three dropouts: −9.7 (95% CI: −18.9 – −0.5)).
Figure 1.
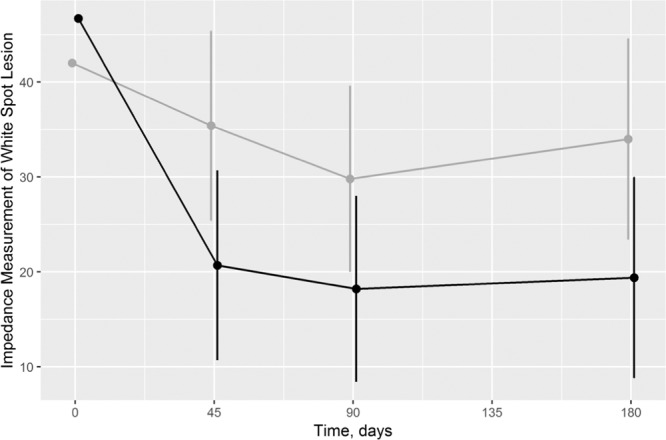
Impedance measurement of White Spot Lesion at different time points (black: test tooth/grey: control tooth). As “temporally and logically, a baseline cannot be a response to treatment, so baseline and response cannot be modeled in an integrated framework”43, baseline and response were graphed differently. Consequently, the response and the 95% CI are adjusted for baseline values43. p = 0.001, p = 0.005, and p = 0.007 for treatment differences after 45, 90, and 180 days, respectively.
Morphometric measurement of white spot lesion (secondary endpoint)
After treatment, the decrease in size according to semi-automated morphometric measurements was more pronounced in test teeth (p = 0.030 for the interaction between treatment and time; Fig. 2). After 180 days, the difference between test and control group was statistically significant in mixed model analysis although the 95% CI was also consistent with a weak to moderate effect (Table 2). The global test of no treatment effect was rejected (p = 0.013).
Figure 2.
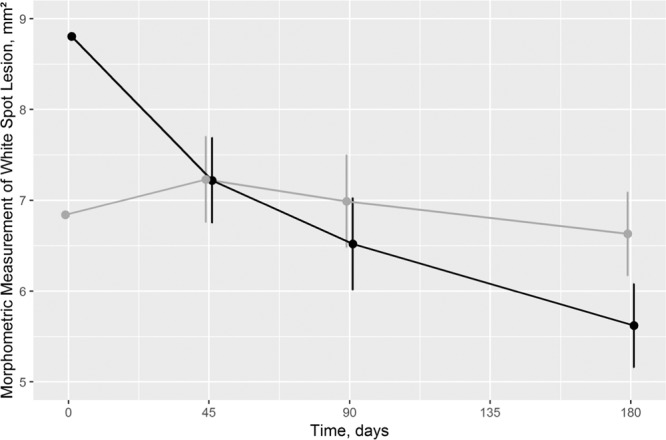
Morphometric measurement of White Spot Lesion Size in mm2 at different time points (black: test tooth/grey: control tooth). As “temporally and logically, a baseline cannot be a response to treatment, so baseline and response cannot be modeled in an integrated framework”43, baseline and response were graphed differently. Consequently, the response and the 95% CI are adjusted for baseline values43. p = 0.969, p = 0.137, and p = 0.004 for treatment differences after 45, 90, and 180 days, respectively.
Table 2.
Secondary endpoint: Morphometric measurement (mm2) in test and control teeth (N = 17, split-mouth design, 94 observations in 2 sites or teeth at 3 time points).
| Observed values | Estimated values (adjusted for baseline values) | |||||
|---|---|---|---|---|---|---|
| Test Group | Control Group | Test Group | Control Group | Treatment effect | ||
| Mean ± SE | Mean ± SE | Difference (95% CI) | p value | |||
| Baseline | ||||||
| Mean ± SD | 8.8 ± 7.8 | 6.8 ± 5.1 | ||||
| Median (IQR) | 8.6 (2.0–11) | 6.7 (2.8–9.6) | ||||
| Day 45 | ||||||
| Mean ± SD | 8.1 ± 7.3 | 6.2 ± 4.7 | 7.2 ± 0.24 | 7.2 ± 0.24 | 0.0 (−0.6 – 0.6) | 0.969 |
| Median (IQR) | 6.0 (1.9–11) | 5.9 (2.4–9.9) | ||||
| Day 90 | ||||||
| Mean ± SD | 7.5 ± 7.0 | 6.1 ± 4.6 | 6.5 ± 0.26 | 7.0 ± 0.26 | −0.5 (−1.1 – 0.2) | 0.137 |
| Median (IQR) | 5.4 (2.4–10) | 5.4 (2.5-9.1) | ||||
| Day 180 | ||||||
| Mean ± SD | 6.5 ± 6.5 | 5.7 ± 4.4 | 5.6 ± 0.24 | 6.6 ± 0.24 | −1.0 (−1.6 – −0.4) | 0.004 |
| Median (IQR) | 5.0 (0.5–11) | 4.9 (2.4–9.4) | ||||
| Baseline vs | ||||||
| Day 45 | ||||||
| Mean ± SD | 1.2 ± 1.0 | 0.9 ± 1.1 | ||||
| Median (IQR) | 0.9 (0.4–1.9) | 0.4 (0.1–1.3) | ||||
| Day 90 | ||||||
| Mean ± SD | 1.8 ± 1.4 | 1.1 ± 0.9 | ||||
| Median (IQR) | 1.7 (0.6–2.8) | 0.9 (0.3–1.7) | ||||
| Day 180 | ||||||
| Mean ± SD | 2.7 ± 1.7 | 1.5 ± 1.3 | ||||
| Median (IQR) | 2.5 (1.3–3.8) | 1.2 (0.4–2.5) | ||||
Missing values explain discrepancies between differences of observed values and calculated changes from baseline (see results).
Abbreviation: CI, confidence interval; IQR, interquartile range; SD, standard deviation; SE, standard error of the mean.
Missing values were also dealt with by multiple imputation, including sensitivity analysis for missing data when true values are systematically lower or higher (nonignorable non-response). Both WSL of one patient were too small to be measured semi-automatically. The two cavitated lesions were not measurable after 90 and 180 days. These paired nonignorable non-responses are levelled off by the split-mouth design. Missing values, for which ignorable non-responses were assumed, occurred for a total of four subjects. One of the two patients who were excluded in mixed models for missing baseline values could be re-included in robust analysis, resulting in 15 patients available for complete case analysis. Robust analysis of contrasts between the first and third time points (-t1 + t3 to model the interaction) confirmed the result of the mixed model by using complete and imputed data without dropouts (15 and 21 subjects, respectively). The treatment effect became uncertain if “ignorable non-response” did not hold in sensitivity analyses after multiple imputation and by including dropouts (23 subjects).
Discussion
Our trial raised comparable parameters as the previously reported trials25–27,32 but differs from the other trials on buccal caries, as active carious lesions (clinically visible as rough, chalky and matte surface) were treated, and not predominantly inactive ones (clinically visible as a white spot under a hard, shiny surface)25–27. Both the impedance values and morphometric measurements showed favourable WSL reduction results for the test group.
The greater difference between test and control lesions, however, occurred in the impedance values compared with that in the morphometric measurements, thereby confirming that SAP P11-4 acts predominantly by regenerating the mineral structure of the enamel of the lesion body and not just the surface23,24. Moreover, at the last study visit at day 180, the impedance values for test lesions indicated regression of caries into very outer enamel, whereas the impedance values for the control lesions indicated still caries throughout the whole enamel layer31,33, which agrees with previous data that fluoride (in the present study as fluoride-based professional prophylaxis paste and home care toothpaste) only acts in the top 30-50 µm of the enamel surface13,14,34.
In recent years, caries activity has become a central topic within cariology, as activity defines the clinical need and opportunity to intervene in the decay process. Active carious lesions, as treated in the present study, have open pores allowing communication between the subsurface carious lesion body and the oral cavity. From the lesion body, calcium and phosphate ions can diffuse out (demineralisation) or in (remineralisation). In a similar fashion, the open pores allow SAP P11-4 to diffuse into the lesion body to support remineralisation and thus regression of the tooth decay.
The active carious lesions in the conventional treatment control exhibited spontaneous regression of the measured WSL size over the study period until day 1803,8, which is comparable to literature data27,29. However, SAP P11-4 enhanced the remineralisation to a higher decrease of the measured WSL size.
The present clinical trial is not without shortcomings. The possibility of random error because of the relatively small sample size. Second, the trial was run within a university setting and the patients were Caucasians, thereby limiting the generalizability of the findings. Third, information bias could have influenced our results. The morphometric assessment represents only a part of the clinical situation because it is related only to the visible tooth surface. Critical clinical assessment factors such as the hardness and gloss of the lesion could not be taken into account as they could not be measured reliably within an in vivo study. It would be interesting to measure the enamel hardness induced by SAP P11-4, and compare those to the previously reported in vitro microhardness results34,35.
However, there are also several strengths. First, the source population and the chosen inclusion criteria ensured that active carious lesions, not inactive ones, were investigated. Second, the split-mouth design is appealing since “each participant acts as their own control”36. By this favourable control mechanism, the split-mouth design is related to counterfactual queries (would a subject have no caries had the subject been treated with SAP given that the subject has in fact caries and is not treated with SAP), whereas the usual randomised clinical trial in parallel-groups is merely related to intervention queries (would a subject have no caries if we make sure that the subject is treated with SAP)37. Therefore, the split-mouth design is clearly superior to a parallel group design (unless in identical twins) if the study design assumptions hold38. As SAP P11-4 acts solely on tooth level23,24, it is biologically well justified to assume the absence of any cross-contamination or carry-across effect from one site to another39. Note that the theory of causality37 has been rapidly developed since the split-mouth design was criticized for statistical reasons in 199040. Third, the split-mouth design was very efficient because the within-patient correlation was clearly different from zero (p = 0.61 for the primary endpoint, p = 0.72 for the secondary endpoint). For the primary and secondary endpoint, the sample sizes of n = 21 and n = 17 in our split-mouth design correspond to total sample sizes of 108 and 118 patients in a parallel group design, respectively39. Fourth, three measurements of the primary endpoint were taken to increase reliability. Fifth, the duration of the trial worked for both endpoints. It was long enough to gain important insights into the mechanism of the treatment, especially for the secondary endpoint. Finally, the statistical analysis is state-of-the-art39,41–43, follows rigorously the intention-to-treat principle by accounting for the uncertainty due to dropouts, and supports confidence intervals44.
Overall, our results not only agree with other clinical trials on the effectiveness of SAP P11-420,25–27 but also show that SAP P11-4 is effective in the treatment of active WSL26,27 supporting as already mentioned the proposed mechanism of action within the subsurface lesion body23,24. Thus, SAP P11-4 treatment gets one step closer to a regeneration ad integrum, that would be the ultimate goal in healing carious enamel.
One of the reasons that the treated enamel does not return to full translucency is seen in the fact that the SAP P11-4-induced fibres support the formation of de novo hydroxyapatite crystals in a fan-type non-prismatic arrangement around the fibres24,45, which have another refractive index than the prismatic hydroxyapatite crystals produced by the Tomes processes from ameloblasts in the final stage of enamel deposition.
Although buccal carious lesions chosen in order to quantitatively assess the effect of the treatment are rare outside the orthodontics, the clinical significance of the results relates to the treatment of caries in general. Thus, the aesthetic shortcomings of the SAP P11-4 treatment46 are neglectable for almost all of the initial carious lesions as most clinically relevant caries develops in proximal or occlusal sites and if buccal lesions occur, they are mostly positioned on premolars and molars.
Professionals are trained to identify initial caries in any location, either visually, with caries diagnostics or on x-rays. Based on those assessments the clinician will decide on whether invasive restorative treatments are needed. Regenerative procedures should be considered whenever possible, in order to conserve natural tooth structure and function and to avoid or at least to delay as far as possible the entry into the conventional filling approach leading to ever larger fillings47. Bröseler et al. have shown that the SAP P11-4 treatment can be transferred into a practice setting27.
Conclusion
The treatment of initial carious lesions with self-assembling peptide P11-4 leads to superior remineralisation of the subsurface lesions compared with the control teeth.
Material and Methods
Study design
The clinical trial was designed and performed as a split-mouth conventional treatment-controlled trial investigating the effect of the treatment of buccal carious lesions following orthodontic treatment by using SAP P11-4 as add-on with the conventional treatment control. Clinical study procedures were performed parallel to regular appointments at the Orthodontic Department of the University Medicine Greifswald (Fig. 3) according to the Declaration of Helsinki and in compliance with ISO 14155:2012. Approval for all clinical procedures and the trial was obtained by the ethical committee of the University of Greifswald (Code BB 99/12, date of approval: 28th August 2012).
Figure 3.
Patient Flow Chart.
The clinical trial was registered in the German Database for clinical trials (DRKS00016501, date of registry: 30th January 2019).
Patient and study tooth selection
Twenty-three patients (13f/10 m; average age 15.4 years) with at least two WSL after removal of fixed orthodontic appliances could be recruited into the trials from the Orthodontic Department of the University Medicine Greifswald and treated by the Restorative Department. The written informed consent was obtained from every participance or from a parent of every participance under 18 years prior to any study-related procedures two days before baseline (Fig. 3).
Patients had to fulfil all the following selection criteria:
Inclusion
At least two active carious lesions around the bracket area with a rough, chalky and matte surface;
Size and form of the active carious lesion: The carious lesion must be fully visible and assessable and accessible;
Able and willing to observe good oral hygiene throughout the study;
≥20 teeth and a BP score of <3;
Age ≥12 years and ≤18 years;
Willing and able to attend the on-study visits;
Willing and able to understand all study-related procedures;
-
Written informed consent before participation in the study.
Exclusion
Pre-treated WSL;
Tooth with another carious lesion apart from the ones developed during orthodontic treatment;
Evidence of tooth erosion; History of head and neck illnesses;
Any pathology or concomitant medication affecting salivary flow;
Concurrent participation in another clinical trial.
Randomisation and blinding
Within each subject, the treatment was randomly allocated to a site by flipping a coin. For three subjects having had test and control teeth in the same quadrant, the more anterior tooth was assigned to the opposite site. Based on our add-on study design, the investigator and patient were not blinded during applying SAP on the tooth, however, outcome assessor and statistician (except for sensitivity analysis after multiple imputation described below) were blinded.
Study treatment procedures (besides the regular orthodontic treatment)
At the Day 0 visit, all teeth were cleaned with a non-fluoridated tooth cleaning paste (Clean Polish, Kerr, Germany), (Fig. 3).
Further, the patients received an electrical tooth brush (Oral-B Pro 1000 Precision Clean, P&G, Germany) and an instruction in it. After that, general patient assessment was recorded prior to allocation of the treatment.
“Each participant acts as their own control”36. In opposite to the control teeth, which got no further treatment on Day 0, the test teeth were prepared according to the instruction of the use for the SAP P11-4 product (Curodont Repair, Credentis, Windisch, Switzerland). The test teeth were cleaned with 2–3% NaOCl, rinse with H2O; etched with 35% Phosphoric acid for 20 sec (Ultra-Etch, Ultradent Products Inc., USA), rinsed with H2O and air-dried. After cleaning, the SAP P11-4 solution was applied to the WSL and left in place for 5 minutes.
The patients were strongly encouraged to use fluoride toothpaste (at least 1450 ppm Fluoride) twice daily.
At every follow-up visits (Day 45, Day 90 and Day 180) parallel to regular orthodontic control appointments, all teeth (including test tooth and control tooth) were cleaned with a fluoridated prophylaxis paste (Flairesse 12.300 ppm Fluoride, DMG, Germany) as at 2 days before baseline (Day 0). Oral hygiene instructions were provided at every study visit (Fig. 3).
Outcomes
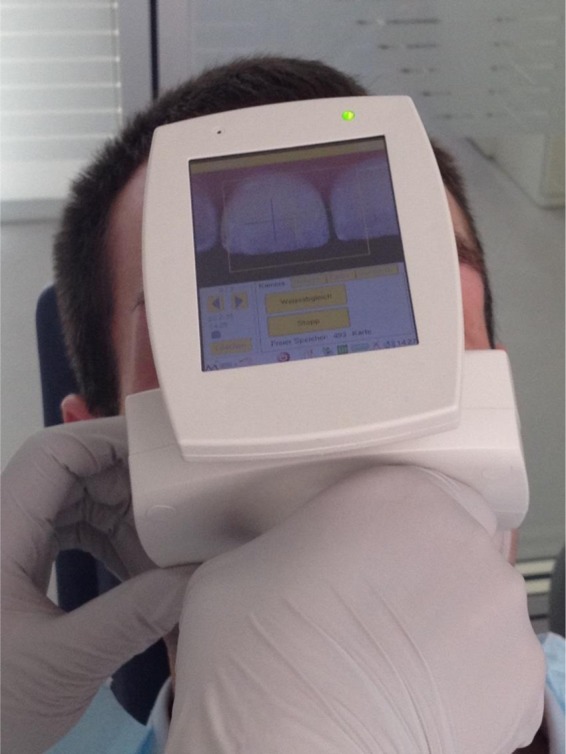
Primary endpoint of the study was initial carious lesions as measured by the impedance (CarieScan, Orange Dental, Biberach, Germany)29–31. Before measurements, the device was checked and handled according to the manufacturer’s instructions. The area was kept dry by optragate (Ivoclar Vivadent, Ellwangen, Germany) and the tooth surface air-dried for 6 seconds. The impedance measurements were taken three times and the internal CarieScan values were averaged for each time point. To ensure reproducibility of the location of the WSL throughout the study, customised trays were prepared, and holes were drilled at the place of the WSL fitted to the nozzle of the diagnostic. The measurements were performed with the tray in place to ensure that readings were taken at the same place (Fig. 4).
Figure 4.
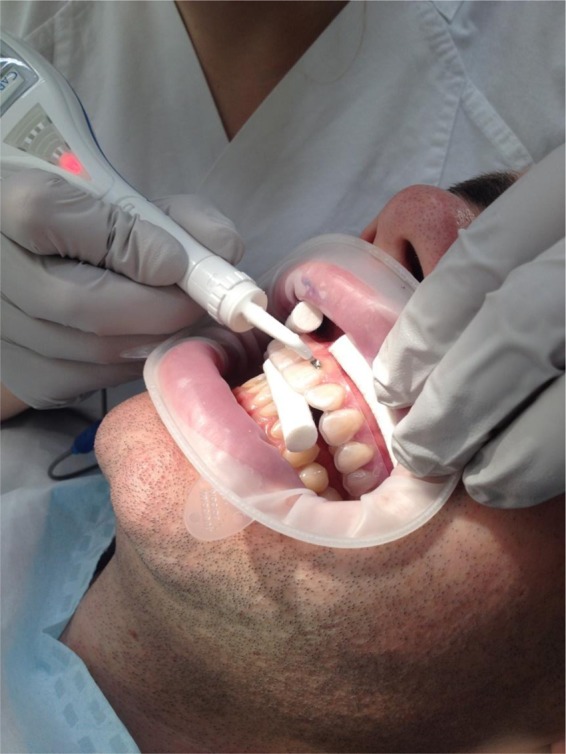
Image of Impedance measurement of WSL with CarieScan Pro (Orangedental/Biberach/Germany).
The secondary endpoint (WSL size) throughout the study were evaluated morphometrically via Shadepilot (DeguDent, Hanau, Germany) (Fig. 5). In order to ensure reproducibility of the measurement, a representative and isolated area of the WSL on the study tooth was selected and its size semi-automatically determined as follows: A buccal tooth area was selected on the measuring device, the WSL area within the area calculated by the device, and refined, if necessary, by the blinded assessor. For clarity: Not necessarily the whole affected area of the study tooth was taken into account but only clearly definable WSL (Fig. 6).
Figure 5.
Overview image of Morphometric measurement of WSL with Shadepilot (DeguDent/Hanau/Germany).
Figure 6.
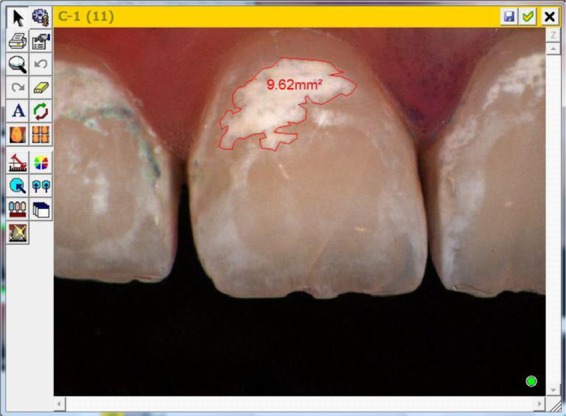
Computer Screen image of Morphometric measurement of WSL with Shadepilot (DeguDent/Hanau/Germany).
Sample size estimation
At the time of designing the study previous clinical data were not available whereas the SAP treatment was new. The experience over the last years supports that “In practice, for many trials, it is unlikely that there will be data to support a realistic estimate” of the within-subject correlation36. This is especially true in dentistry, where data from at least three levels (subject, jaw, and tooth) are required to account for within-subject variability. Thus, “it is a more sensible approach to sample-size determination to have a look at what sort of trial has been run in the past in a particular area and see what sort of inferences were possible rather than going through some complicated power calculation: often this is no more than a ritual”48. This is what was done; for the primary endpoint, which is continuous, at least 22 participants were aimed for49,50. Note that inferences from confidence intervals as presented herein are more appropriate than inferences from p-values44,51.
Statistical analysis
Replacing “period” by “site”, the split-mouth design was analysed as cross-over design39. The repeated measurements were examined in mixed models for random subject effects and in robust analyses for the two-treatment design52. Treatment, time, the interaction between treatment and time, site-specific baseline values using restricted cubic splines with 3 knots, site, and the interaction between site and time were fixed factors. In addition, subject and site were included as hierarchical random factors, and time was modeled as repeated factor by three variance and three unstructured covariance terms within site, yielding a total of seven random factors52. The Kenward-Roger method41 using the observed information matrix was applied to correct for small-sample inference. Mixed models can deal with imbalanced or missing data if the “missing at random” assumption holds43. The complex mixed models were analysed using Stata software (release 14.2, Stata Corporation, College Station, TX, USA) and checked using SAS software (release 9.4, Cary, NC, USA). In robust analyses of the direct treatment effect (not including baseline values), the two-sample t-test was used for differences in linear combinations of repeated measurements between groups defined by the site of treatment52. To cover the uncertainty due to dropouts, multiple imputations were generated by fully conditional specification using R software43. Scenarios other than “missing at random” were examined in sensitivity analysis after multiple imputation.
Acknowledgements
Our gratitude goes to our statisticians of the University Medicine Greifswald, Dr. P. Kolyschkow, Dr. C. Holtfreter for their help. Further, we would like to thank Dr. M. Müller for his clinical support and credentis ag, Procter & Gamble Germany GmbH & Co Operations oHG, DeguDent GmbH, orangedental GmbH & Co. KG for its material support of the study.
Author contributions
Conceived, designed and managed the clinical study: A.W.; treated the patients: A.R.; conducted measurements: M.R.; conducted statistical analysis: Ch.S.; wrote the manuscript: A.W.; reviewing the manuscript: Ch.S., A.R., M.R. and K.F.K.
Data availability
The datasets of the current study are available from the corresponding author on request.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Staudt CB, Lussi A, Jacquet J, Kiliaridis S. White spot lesions around brackets: in vitro detection by laser fluorescence. Eur J Oral Sci. 2004;112:237–243. doi: 10.1111/j.1600-0722.2004.00133.x. [DOI] [PubMed] [Google Scholar]
- 2.Mattousch TJ, van der Veen MH, Zentner A. Caries lesions after orthodontic treatment followed by quantitative light-induced fluorescence: a 2-year follow-up. Eur J Orthod. 2007;29:294–298. doi: 10.1093/ejo/cjm008. [DOI] [PubMed] [Google Scholar]
- 3.Höchli D, Hersberger-Zurfluh M, Papageorgiou SN, Eliades T. Interventions for orthodontically induced white spot lesions: a systematic review and meta-analysis. Eur J Orthod. 2017;39:122–133. doi: 10.1093/ejo/cjw065. [DOI] [PubMed] [Google Scholar]
- 4.Ren Y, Jongsma MA, Mei L, van der Mei HC, Busscher HJ. Orthodontic treatment with fixed appliances and biofilm formation–a potential public health threat? Clin Oral Investig. 2014;18:1711–1718. doi: 10.1007/s00784-014-1240-3. [DOI] [PubMed] [Google Scholar]
- 5.Gorelick L, Geiger AM, Gwinnett AJ. Incidence of white spot formation after bonding and banding. Am J Orthod. 1982;81:93–98. doi: 10.1016/0002-9416(82)90032-X. [DOI] [PubMed] [Google Scholar]
- 6.Richter AE, Arruda AO, Peters MC, Sohn W. Incidence of caries lesions among patients treated with comprehensive orthodontics. Am J Orthod Dentofacial Orthop. 2011;139:657–664. doi: 10.1016/j.ajodo.2009.06.037. [DOI] [PubMed] [Google Scholar]
- 7.Featherstone JDB, Chaffee BW. The Evidence for Caries Management by Risk Assessment (CAMBRA(R)) Adv Dent Res. 2018;29:9–14. doi: 10.1177/0022034517736500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gao SS, Zhang S, Mei ML, Lo EC, Chu CH. Caries remineralisation and arresting effect in children by professionally applied fluoride treatment - a systematic review. BMC Oral Health. 2016;16:12. doi: 10.1186/s12903-016-0171-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Petersson LG, Twetman S, Pakhomov GN. The efficiency of semiannual silane fluoride varnish applications: a two-year clinical study in preschool children. J Public Health Dent. 1998;58:57–60. doi: 10.1111/j.1752-7325.1998.tb02991.x. [DOI] [PubMed] [Google Scholar]
- 10.de Oliveira BH, Dos Santos AP. Semiannual Fluoride Applications in Low-Risk Toddlers May Not Be More Effective Than Toothbrushing Instruction and Dietary Counseling in Controlling Dental Caries. J Evid Based Dent Pract. 2016;16:246–248. doi: 10.1016/j.jebdp.2016.11.006. [DOI] [PubMed] [Google Scholar]
- 11.de Oliveira, P. R., Fonseca, A. B., Silva, E. M., Coutinho, T. C. & Tostes, M. A. Remineralizing potential of CPP-ACP cremes with and without fluoride in artificial enamel lesions. Aust Dent J, 10.1111/adj.12305 (2015). [DOI] [PubMed]
- 12.Muller F, et al. Elemental depth profiling of fluoridated hydroxyapatite: saving your dentition by the skin of your teeth? Langmuir. 2010;26:18750–18759. doi: 10.1021/la102325e. [DOI] [PubMed] [Google Scholar]
- 13.Bergman G, Lind PO. A quantitative microradiographic study of incipient enamel caries. J Dent Res. 1966;45:1477–1484. doi: 10.1177/00220345660450053701. [DOI] [PubMed] [Google Scholar]
- 14.Pandya M, Diekwisch TGH. Enamel biomimetics-fiction or future of dentistry. Int J Oral Sci. 2019;11:8. doi: 10.1038/s41368-018-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rechmann P, Chaffee BW, Rechmann BMT, Featherstone JDB. Changes in Caries Risk in a Practice-Based Randomized Controlled Trial. Adv Dent Res. 2018;29:15–23. doi: 10.1177/0022034517737022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Urquhart, O. et al. Nonrestorative Treatments for Caries: Systematic Review and Network Meta-analysis. J Dent Res, 22034518800014, 10.1177/0022034518800014 (2018). [DOI] [PMC free article] [PubMed]
- 17.Fernandez-Ferrer L, et al. Enamel remineralization therapies for treating postorthodontic white-spot lesions: A systematic review. J Am Dent Assoc. 2018;149:778–786 e772. doi: 10.1016/j.adaj.2018.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Jablonski-Momeni A, Heinzel-Gutenbrunner M. Efficacy of the self-assembling peptide P11-4 in constructing a remineralization scaffold on artificially-induced enamel lesions on smooth surfaces. J Orofac Orthop. 2014;75:175–190. doi: 10.1007/s00056-014-0211-2. [DOI] [PubMed] [Google Scholar]
- 19.Amaechi BT. Remineralisation - the buzzword for early MI caries management. Br Dent J. 2017;223:173–182. doi: 10.1038/sj.bdj.2017.663. [DOI] [PubMed] [Google Scholar]
- 20.Alkilzy M, Santamaria RM, Schmoeckel J, Splieth CH. Treatment of Carious Lesions Using Self-Assembling Peptides. Adv Dent Res. 2018;29:42–47. doi: 10.1177/0022034517737025. [DOI] [PubMed] [Google Scholar]
- 21.Philip N. State of the Art Enamel Remineralization Systems: The Next Frontier in Caries Management. Caries Res. 2018;53:284–295. doi: 10.1159/000493031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jablonski-Momeni A, et al. Randomised in situ clinical trial investigating self-assembling peptide matrix P11-4 in the prevention of artificial caries lesions. Sci Rep. 2019;9:269. doi: 10.1038/s41598-018-36536-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kind L, et al. Biomimetic Remineralization of Carious Lesions by Self-Assembling Peptide. J Dent Res. 2017;96:790–797. doi: 10.1177/0022034517698419. [DOI] [PubMed] [Google Scholar]
- 24.Kirkham, J. et al. Self-assembling peptide scaffolds promote enamel remineralization. J Dent Res86, 426–430, 86/5/426 (2007). [DOI] [PubMed]
- 25.Brunton PA, et al. Treatment of early caries lesions using biomimetic self-assembling peptides - a clinical safety trial. Br Dent J. 2013;215:E6. doi: 10.1038/sj.bdj.2013.741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mannaa, A., Sedlakova, P., Bommer, C., di Bella, E. & Krejci, I. In IADR Vol. 96th General Session (London, GB, 2018).
- 27.Bröseler, F. et al. Randomised clinical trial investigating self-assembling peptide P11-4 in the treatment of early caries. Clin Oral Investig, 10.1007/s00784-019-02901-4 (2019). [DOI] [PubMed]
- 28.Nyvad B, Baelum V. Nyvad Criteria for Caries Lesion Activity and Severity Assessment: A Validated Approach for Clinical Management and Research. Caries Res. 2018;52:397–405. doi: 10.1159/000480522. [DOI] [PubMed] [Google Scholar]
- 29.Mortensen, D., Gizani, S., Salamara, O., Sifakakis, I. & Twetman, S. Monitoring regression of post-orthodontic lesions with impedance spectroscopy: a pilot study. Eur J Orthod, 10.1093/ejo/cjy075 (2018). [DOI] [PubMed]
- 30.Longbottom C, Huysmans MC, Pitts NB, Los P, Bruce PG. Detection of dental decay and its extent using a.c. impedance spectroscopy. Nat Med. 1996;2:235–237. doi: 10.1038/nm0296-235. [DOI] [PubMed] [Google Scholar]
- 31.Cohen, J. E. The association between CarieScan Pro readings and histologic depth of caries in non cavitated occlusal lesion in vitro. MS (Master of Science) thesis, University of Iowa, (2013).
- 32.Alkilzy M, Tarabaih A, Santamaria RM, Splieth CH. Self-assembling Peptide P11-4 and Fluoride for Regenerating Enamel. J Dent Res. 2018;97:148–154. doi: 10.1177/0022034517730531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tassery H, et al. Use of new minimum intervention dentistry technologies in caries management. Aust Dent J. 2013;58(Suppl 1):40–59. doi: 10.1111/adj.12049. [DOI] [PubMed] [Google Scholar]
- 34.Schmidlin P, Zobrist K, Attin T, Wegehaupt F. In vitro re-hardening of artificial enamel caries lesions using enamel matrix proteins or self-assembling peptides. J Appl Oral Sci. 2016;24:31–36. doi: 10.1590/1678-775720150352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kamal D, Hassanein H, Elkassas D, Hamza H. Comparative evaluation of remineralizing efficacy of biomimetic self-assembling peptide on artificially induced enamel lesions: An in vitro study. J Conserv Dent. 2018;21:536–541. doi: 10.4103/JCD.JCD_123_18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pandis N, Chung B, Scherer RW, Elbourne D, Altman DG. CONSORT 2010 statement: extension checklist for reporting within person randomised trials. BMJ. 2017;357:j2835. doi: 10.1136/bmj.j2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Pearl, J. Causality. Models, Reasoning, and Inference. 2nd edn, (Cambridge University Press, 2009).
- 38.Harrell, F. E., Jr. & Slaughter, J. C. In Biostatistics for Biomedical Research (2001–2019).
- 39.Lesaffre E, Philstrom B, Needleman I, Worthington H. The design and analysis of split-mouth studies: what statisticians and clinicians should know. Stat Med. 2009;28:3470–3482. doi: 10.1002/sim.3634. [DOI] [PubMed] [Google Scholar]
- 40.Hujoel PP, Loesche WJ. Efficiency of split-mouth designs. J Clin Periodontol. 1990;17:722–728. doi: 10.1111/j.1600-051x.1990.tb01060.x. [DOI] [PubMed] [Google Scholar]
- 41.Kenward MG, Roger JH. Small sample inference for fixed effects from restricted maximum likelihood. Biometrics. 1997;53:983–997. doi: 10.2307/2533558. [DOI] [PubMed] [Google Scholar]
- 42.Committee for Proprietary Medicinal Products (CPMP). Points to consider on adjustment for baseline covariates. Statistics in Medicine23, 701–709, 10.1002/sim.1647 (2004). [DOI] [PubMed]
- 43.Harrell, F. E., Jr. Regression modeling strategies. With applications to linear models, logistic and ordinal regression, and survival analysis. 2nd edn, ix, 5, 19, 104, (Springer, 2015).
- 44.Wasserstein LR, Lazar NA. The ASA’s Statement on p-Values: Context, Process, and Purpose. The American Statistician. 2016;70:129–133. doi: 10.1080/00031305.2016.1154108. [DOI] [Google Scholar]
- 45.Takahashi F, et al. Ultrasonic assessment of the effects of self-assembling peptide scaffolds on preventing enamel demineralization. Acta Odontol Scand. 2016;74:142–147. doi: 10.3109/00016357.2015.1066850. [DOI] [PubMed] [Google Scholar]
- 46.Wierichs RJ, Kogel J, Lausch J, Esteves-Oliveira M, Meyer-Lueckel H. Effects of Self-Assembling Peptide P11-4, Fluorides, and Caries Infiltration on Artificial Enamel Caries Lesions in vitro. Caries Res. 2017;51:451–459. doi: 10.1159/000477215. [DOI] [PubMed] [Google Scholar]
- 47.Krejci I, Lieber CM, Lutz F. Time required to remove totally bonded tooth-colored posterior restorations and related tooth substance loss. Dent Mater. 1995;11:34–40. doi: 10.1016/0109-5641(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 48.Senn, S. Cross-over Trials in Clinical Research. 2nd edn, (John Wiley & Sons, Ltd, 2002).
- 49.Boersma JG, van der Veen MH, Lagerweij MD, Bokhout B, Prahl-Andersen B. Caries prevalence measured with QLF after treatment with fixed orthodontic appliances: influencing factors. Caries Res. 2005;39:41–47. doi: 10.1159/000081655. [DOI] [PubMed] [Google Scholar]
- 50.van der Veen MH, Attin R, Schwestka-Polly R, Wiechmann D. Caries outcomes after orthodontic treatment with fixed appliances: do lingual brackets make a difference? Eur J Oral Sci. 2010;118:298–303. doi: 10.1111/j.1600-0722.2010.00733.xEOS733. [DOI] [PubMed] [Google Scholar]
- 51.Greenland S, et al. Statistical tests, P values, confidence intervals, and power: a guide to misinterpretations. Eur J Epidemiol. 2016;31:337–350. doi: 10.1007/s10654-016-0149-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jones, B. & Kenward, M. G. Design and Analysis of Cross-Over Trials. 3rd edn, (CRC Press, 2015).
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets of the current study are available from the corresponding author on request.