Abstract
Five different weed plants viz. Convulvulus arvensis, Chenopodium murale, Tribulus terrestris, Trianthema portulacastrum, and Achyranthes aspera were investigated for their entomocidal and genotoxic effects against Culex quinquefasciatus mosquitoes. High mortality was observed at 72 hours in a dose dependent manner. Among all the tested plants, A. aspera was found highly significant which showed 100% mortality at 250 ppm after 72 hours with LC50 of 87.46, 39.08 and 9.22 ppm at 24, 48, respectively. In combination with Bacillus thuringiensis israelensis (Bti); A. aspera also caused 100% mortality at 250 ppm concentration after 72 hours (LC50 8.29 ppm). Phytochemical analysis of all the tested weed plants showed the presence of flavonoids, saponins, tannins, steroids, cardiac glycosides, alkaloids, anthrequinones and terpenoids. Random Amplification of Polymorphic DNA-Polymerase chain reaction (RAPD-PCR) and comet assay were performed to assess the genotoxic effect of A. aspera but no change in DNA profile was observed. Furthermore, FTIR showed the presence of phenolic compounds in A. aspera extract. It is suggested that certain phenolic compounds such as flavonoids modulate the enzymatic activity and, hence, cause the death of larvae of Cx. quinquefasciatus. Altogether, current study would serve as an initial step towards replacement of synthetic insecticides to plant-microbe based biopesticide against Culex mosquitoes in future.
Subject terms: Agricultural genetics, Entomology
Introduction
Mosquitoes are reported to cause nuisance to humans and transmit several viral and protozoan diseases of public health concern worldwide. These are female mosquitoes which make a bite during their search for blood meal before oviposition which thus, increases their tendency to transmit several diseases including malaria, filariasis, dengue fever, japanese encephalitis, chikungunya, zika virus and yellow fever. These diseases make life at risk of millions of people particularly in subtropical/tropical world1,2. Of various mosquito species, Cx. quinquefasciatus transmits various diseases i.e., West Nile virus, Japanese encephalitis, filariasis, bancroftian filariasis (Wuchereria bancrofti), St. Louis encephalitis, and avian malaria3. In southern United States, St. Louis virus and West Nile virus (WNV) were transmitted by Cx. quinquefasciatus4,5. Almost 120 million people are affected annually only by lymphatic filariasis, whereas 1.3 billion are at risk resulting in nearly $1.3 billion loss of productivity per year6. Similarly, three billion individuals are at risk of being infected by Japanese encephalitis with 30,000–50,000 reported cases every year in disease endemic areas7. Besides disease transmission in humans; Cx. quinquefasciatus is also responsible for transmitting several diseases to livestock and companion animals viz. Rift Valley fever, canine dirofilariasis (dog heartworm), avian malaria, avian pox, and West Nile encephalitis which lead to high mortalities or decreased productivity8.
To avoid proliferation of mosquito borne diseases; mosquito control is necessary which is essentially performed through using chemical insecticides. Many synthetic agents such as organochlorine and organophosphate compounds have been developed and employed with a considerable success despite of therein including toxicity to non-targeted organisms and fostered sometimes severe environmental and human health concerns9. The continuous use of synthetic insecticide results in the development of resistance in mosquitoes. Furthermore non-degradable nature of synthetic insecticide causes biomagnification which makes the situation overall more worst10–12. Collectively, the current status situation urges to find out environment friendly, cost-effective, biodegradable and target specific insecticides against mosquitoes. Although, an eco-friendly alternative approach such as biological control became the central focus to exploiting certain natural enemies including predatory and parasitic species; however, mosquito control always remained a very serious issue.
Plant extracts have been reported for the control of mosquitoes13,14 and recently, weed plant extracts are being investigated15. Nevertheless, the plant extracts are biodegradable, non-hazardous and have been found active against a number of insect pests16. Previously, it was also focused on the commercial use of plant extracts as potent insect-control agents17,18. Subsequently, plants derived secondary metabolites are responsible for defense to survive against selection pressure of herbivore predators and different environmental factors. Numerous phytochemical groups like alkaloids, terpenoids, steroids, phenolics and essential oils extracted from various plants are reported as potent insecticides16,19. For instance, Salvia ballotiflora contains 37 different compounds with β-caryophyllene and caryophyllene oxide as main components and resulted in 80% larval mortality of Cx. quinquefasciatus20. The phytochemical analysis showed the presence of aromadendrene, naphthalene, α-humulene, caryophyllene oxide, caryophyllene, phenol, 4-(3,7-dimethyl-3-ethenylocta-1,6- dienyl) and methyl hexadecanoate compounds in Psoralea corylifolia and caused DNA damage in Cx. quinquefasciatus21. Though, first report of genomic alterations using RAPD-PCR fingerprinting was reported by Lalrotluanga & Gurusubramanian22 in mosquito larvae treated with various plant extracts. Variations in DNA band were observed when P. ferulacea essential oil-treated larvae of E. kuehniella and were subjected to DNA damage analysis by RAPD assay23. A significant DNA damage has been reported in Aedes aegypti due to A. aspera24. Similarly, DNA damage was also shown in Cx. quinquefasciatus larvae treated with Curcuma longa and Melia azedarach plant extracts21.
It has also been described that the enzymatic profiles are modulated in response to natural oils from plants25. Esterases, a major detoxifying enzyme in insects, are involved in detoxification of insecticides26. Plant extracts are described as AChE inhibitors10,27. Subsequent changes in Phosphatases enzyme activity are also reported in insects25,26. Additionally, the discovery of highly toxic bacterial strains against dipteran larvae such as Bacillus thuringiensis israelensis (Bti) and Bacillus sphaericus Neide (Bs) provided an option to incorporate in mosquito control programs as a potent biolarvicides around the world28–30.
Weeds are undesired flora which compete with crop plants for food space and nutrients and provide the alternative food for pests. In attempting to eradicate these weeds; why not this is appropriate to use them for insect control and to develop an environmentally safe insect-control agent in future15. Therefore, the current study was designed to evaluate the entomocidal impact of different weed plant extracts individually and in combination with microbial strains along with the genotoxic effect of weed plant extracts against Cx. quinquefasciatus mosquitoes.
Materials and methods
Collection and Rearing of Culex quinquefasciatus
The adults and larvae of Cx. quinquefasciatus were collected from main drain of Government College University, Faisalabad. The mosquitoes were then reared in plastic and enamel tray with tap water under standard conditions (26 ± 1 °C, 60 ± 10% RH with 12 hours day/night cycle) in Lab, Department of Zoology in Government College University Faisalabad. The newly emerged larvae were fed on grounded Fish Food and 5–8 days old larvae were fed on two tablets of Purina Cat Food daily. The grounded Cat Food was added after 8 days. Tray was kept in insect cage after the formation of pupae. After 2 days, adults were emerged from the pupae. A beaker having cotton soaked in 10% sugar solution was kept to provide sugar contents to adult mosquitoes. For blood feeding to female Cx. quinquefasciatus mosquitoes, an albino rat in a small cage was left overnight in the rearing cage31.
Collection of microbes
The Psuedomonas aeruginosa isolate was procured from Department of Microbiology whereas Bacillus thuringiensis israelensis (Bti) was purchased from Summit®, USA. P. aeruginosa was isolated from burn wound samples using Psuedomonas agar (oxoid, UK). The bacterial count was adjusted to 1 × 108 colony forming unit/ml (CFU/ml). This stock solution was used to prepare different ppm solutions. Similarly, 100% solution was prepared by dissolving 10% dunk of Bti in 10 ml distilled water. Then 1000 ppm stock solution was prepared by dissolving 10 µl of this Bti solution in 90 µl distilled water. This stock solution was used for further dilutions.
Collection and identification of weed plants
Weed plants were collected from different areas of District Faisalabad and identified from Department of Botany, Government College University Faisalabad and Department of Botany, University of Agriculture Faisalabad, Pakistan. The used weed plants for extraction are listed in Table 1.
Table 1.
List of weed plants used for oil extraction.
| Sr # | Common name | Scientific Name | Collection Area | GPS Coordinates |
|---|---|---|---|---|
| 1 | Lilly | Convulvulus arvensis | Sammundri | 31.0691°N, 72.9361°E |
| 2 | Krund | Chenopodium murale | Sammundri | 31.0691°N, 72.9361°E |
| 3 | Bhakhra | Tribulus terrestris | Satyana | 31.2047°N, 73.1711°E |
| 4 | Itsit | Trianthema portulacastrum | Satyana | 31.2047°N, 73.1711°E |
| 5 | Puthkanda | Achyrnathes aspera | Tandlianwala | 31.0368°N, 73.1379°E |
Petroleum ether extraction of weed plants
Weed plants were cleaned by washing with clean water and shade-dried in Lab for a week. Dried plants were crushed in small pieces for further grinding. Crushed dried plants were again oven dried at 60 °C for 20 minutes and grinded in electrical grinder to obtain powder form. Extracts were obtained from 15 g of powder in 150 ml of petroleum ether of each weed plant using Soxhlet apparatus after several rotations in 8 hours. The extracts were then stored in clean and air tight bottles at 4 °C15.
Mortality bioassay test
Different concentrations of each weed plant extract from 10 ppm to 250 ppm were used to perform the bioassay test following WHO protocol32. Twenty larvae of Cx. quinquefasciatus were treated with different concentrations of each extract individually and in combination with microbes (1:1 volume); P. aeruginosa and Bacillus thuringiensis israelensis (Bti) along with control group and group treated with different concentrations of permethrin (10, 20, 30, 50, 100, 150 and 250 ppm) in water. Three replicates were performed for each test and the mortality data was recorded at 24, 48 and 72 hours of post treatment16. Immovable larvae were considered as dead and removed to prevent decomposition which might cause the mortality of other intact alive larvae. The dead larvae were stored in ethanol in 1.5 ml eppendorf tubes to further determine the DNA damage by RAPD PCR and comet assay.
Esterases and phosphatases enzyme assay
The larvae of Cx. quinquefasciatus mosquitoes were thoroughly washed with distilled water and adhering water was removed using blotting paper. The larvae were homogenized using ice-cold Sodium Phosphate buffer (20 mM. pH 7.0) with the help of Teflon hand homogenizer. The homogenate was centrifuged at 8000 × g and 4 °C for 20 minutes using centrifuge machine, SIGMA, Germany. The supernatant was used for the estimation of Esterases and Phosphatases (AChE = acetylcholinesterase, AcP = acid phosphatases, AkP = alkaline phosphatases, α-Carboxyl = α-Carboxylesterases and β-Carboxyl = β-Carboxylesterases). All the solutions and glassware used for homogenization were kept at 4 °C prior to use and the homogenates were held on ice until used for assays. The protocols for enzymatic assays as already described Younes et al.33 and Sultana et al.15 were followed.
Phytochemical analysis of weeds extracts
Phytochemical analysis of five tested weed plant extracts were performed in order to detect the chemical constituents as described by Harborne34, Trease and Evans35 and Sofowara36.
Fourier transform infrared spectroscopy (FTIR) analysis
The functional groups of active components in the extracts of weed plants were identified using FTIR spectrometer (Bruker Tensor II) on the basis of vibrational frequencies between atomic bonds. The extracts were chilled at −80 °C followed by lyophilization to obtain the IR spectrum of lyophilized extract (Alpha, Bruker, California, USA). FTIR spectra were measured in the frequency ranges from 400–4000 cm−1 sample by scanning the sample. The samples were run in triplet form37.
DNA extraction
The stored samples of mosquito larvae were homogenized in 300 µl lysis buffer (0.4 M NaCl, 2 mM EDTA, and 10 mM Tris-HCL pH 8.0), 100 µl Proteinase K (100 mg/µl) of BIOSHOP, Canada and 20% sodium dodecyl sulphate (SDS). The homogenate was incubated at 55 °C for one hour, then, 300 µl of 5 M NaCl was added and vortexed for few seconds. The mixture was centrifuged at 13,000 rpm for 10 minutes. The DNA from supernatant was precipitated by adding ice cold ethanol in equal volume, and kept at −20 °C for 1 hour and afterwards recovered by centrifugation. The DNA pellet was air dried and resuspended in D3H2O38–40.
The optical absorbance of each sample was calculated by measuring the absorption at 260 nm wavelength of UV light using spectrophotometer of HITACHI, Japan. DNA Concentration was calculated as:
RAPD-PCR amplification
A total of five RAPD primers (GenLink: A-03, A-04, A-06, A-18 and C-04; Supplementary Table) were selected to amplify the mosquitoes genomic DNA following the PCR conditions as described by Bibi et al.39 and Zahoor et al.41.
Haemocyte collection
Cx. quinquefasciatus haemocytes were collected according to Irving et al.42. The collected larvae from trail beaker were first washed with distilled water, sterilized in 5% bleach and dried. The cuticle was removed with two fine forceps. The haemolymph and haemocytes were taken in microcentrifuge tubes. The pooled haemolymph was centrifuged at 300 × g and 4 °C for 10 minutes, the supernatant was discarded and the pellet was resuspended in 20 µL of cold PBS.
Comet assay
The comet assay was performed according to Singh et al.43 with minor modifications. The cell samples were carefully suspended in 140 µL of 0.75% LMA (Low melting agar) and then layered onto microscope slides coated with 150 µL of 1% NMA (Normal melting agar) and dried at room temperature. The two gels on each slide were mounted, covered with a coverslip and put at 4 °C for10 minutes to let solidify the gel. The coverslip was immediately removed after agarose solidification. The slides were immersed in a cold fresh lysis solution (2.5 M NaCl, 100 mM EDTA pH 10, 10 mM Tris, 1% Triton X-100 and 5% DMSO) for 2 hours at 4 °C in a dark chamber. The slides were placed in a horizontal gel electrophoresis tank filled with cold electrophoretic buffer (1 mM Na2EDTA and 300 mM NaOH, pH 13) for 25 minutes for DNA unwinding. The electrophoresis was performed in the same buffer for 20 min at 25 V and 300 mA (0.73 V/cm). After electrophoresis, the slide was washed twice with 0.4 mM Tris (pH 7.5) for 5 minutes to neutralize the slides. The slides were stained with 20 µL of DAPI (1 µg/mL) per gel and examined at 400× magnification with Komet 5.5 Image Analysis System fitted to an Olympus BX50 fluorescence microscope equipped with 590 nm barrier filter and 480–550 nm wide band excitation filter. One hundred randomly selected cells (50 cells per two replicate slides) per treatment were analyzed21,44,45.
Data analysis
Probit Analysis program (version 1.5) was used to determine the LC50 between concentration and percent mortality at various concentrations of plant extract and bacteria46. Abbot’s formula was used to analyze the data of mortality obtained through bioassay tests. The corrected mortality data was subjected to ANOVA using Statistica 13.0 for Windows15. The means were separated using Tuckey’s HSD (Honest Significant Difference) test at a significance level of 0.05. A value of p < 0.05 was considered statistically significant14,47,48. PCR products were analyzed using gel electrophoresis and the genetic data were analyzed using POPGENE software40. For comet assay, the DNA damage in cells of Cx. quinquefasciatus larvae was assessed by two distinct types of DNA damage measurements: the length of DNA comet tail and the percentage of fragmented DNA present in the tail after electrophoresis21.
Results
Mortality assay of culex quinquefasciatus using weed plant extracts at various concentrations and different time intervals
The comparison of insecticidal activity of five different weed plant extracts against larvae of Cx. quinquefasciatus using different concentrations at various exposure intervals is shown in Table 2. High mortality was obtained by all the weed plant extracts after 72 hours exposure time compared to Azadirachta indica (Neem) extract at 250 ppm concentration. But as compare to synthetic pesticide (Permethrin) only T. terrestris and C. murale showed the low mortality among all weed plants extracts. The highest mean mortality was shown by Achyrathes aspera (42.76, 61.28, 66.93, 78.11, 88.22, 93.43 and 100%) followed by C. arvensis (34.34, 42.76, 44.67, 49.50, 62.96, 71.85 and 88.22%) and T. portulacastrum (32.66, 44.44, 49.86, 62.96, 74.75, 79.06% and 88.22%) at 10, 20, 30, 50, 100, 150 and 250 ppm concentrations, respectively. T. terrestris showed low mean mortality (15.82, 29.29, 31.78, 34.34, 52.86, 59.32 and 73.06%) whereas, lowest mortality among all the five extracts was observed with C. murale (15.82, 20.88, 22.68, 27.61, 42.76, 49.82 and 64.65%) at 10, 20, 30, 50, 100, 150 and 250 ppm concentrations, respectively. Permethrin showed (13.51, 18.01, 39.28, 43.36, 51.95, 67.14 and 76.46%) mortality at the same concentrations, respectively. A. indica (Neem) extract showed lowest mortality when compared to all the tested plants and Permethrin (Table 2). The overall results indicated that mortality was increased with increased extract concentrations.
Table 2.
Mean mortality of Culex quinquefasciatus by different weed extracts at various concentration and different time intervals.
| Treatment | Conc. | F Value |
df | P value |
Mean mortality with different time intervals | ||
|---|---|---|---|---|---|---|---|
| 24 hours | 48 hours | 72 hours | |||||
| Z1 | 10 ppm | 41.91 | 2 | <0.05 | 6.67 ± 1.67c | 16.67 ± 1.67b | 34.34 ± 2.92a |
| 20 ppm | 88.23 | 2 | <0.05 | 11.67 ± 1.67c | 23.33 ± 1.67b | 42.76 ± 1.68a | |
| 30 ppm | 68.49 | 2 | <0.05 | 15.68 ± 1.68c | 28.34 ± 1.68b | 44.67 ± 1.67a | |
| 50 ppm | 59.05 | 2 | <0.05 | 20.00 ± 2.89c | 36.67 ± 1.67b | 49.50 ± 0.00a | |
| 100 ppm | 78.87 | 2 | <0.05 | 25.00 ± 2.89c | 48.33 ± 1.67b | 62.96 ± 1.68a | |
| 150 ppm | 92.58 | 2 | <0.05 | 29.88 ± 2.88c | 55.67 ± 1.67b | 71.85 ± 1.68a | |
| 250 ppm | 134.03 | 2 | <0.05 | 38.33 ± 1.67c | 65.00 ± 2.89b | 88.22 ± 1.68a | |
| 10 ppm | 14.13 | 2 | <0.05 | 3.33 ± 1.67b | 8.33 ± 1.67b | 15.82 ± 1.68a | |
| 20 ppm | 8.61 | 2 | <0.05 | 11.67 ± 1.67b | 13.33 ± 1.67b | 20.88 ± 1.68a | |
| 30 ppm | 7.79 | 2 | <0.05 | 12.38 ± 1.67b | 15.67 ± 1.67b | 22.68 ± 1.68a | |
| Z2 | 50 ppm | 27.27 | 2 | <0.05 | 13.33 ± 1.67c | 20.00 ± 0.00b | 27.61 ± 1.68a |
| 100 ppm | 61.98 | 2 | <0.05 | 16.67 ± 1.67c | 26.67 ± 1.67b | 42.76 ± 1.68a | |
| 150 ppm | 89.46 | 2 | <0.05 | 17.58 ± 1.67c | 29.78 ± 1.67b | 49.82 ± 1.68a | |
| 250 ppm | 146.34 | 2 | <0.05 | 18.33 ± 1.67c | 35.00 ± 0.00b | 64.65 ± 2.92a | |
| 10 ppm | 7.52 | 2 | <0.05 | 6.67 ± 1.67b | 11.67 ± 1.67ab | 15.82 ± 1.68a | |
| 20 ppm | 13.88 | 2 | <0.05 | 13.33 ± 1.67b | 23.33 ± 1.67a | 29.29 ± 2.92a | |
| 30 ppm | 28.95 | 2 | <0.05 | 14.86 ± 1.67b | 24.33 ± 1.67a | 31.78 ± 1.68a | |
| Z3 | 50 ppm | 34.62 | 2 | <0.05 | 18.33 ± 1.67c | 26.67 ± 1.67b | 34.34 ± 0.00a |
| 100 ppm | 116.09 | 2 | <0.05 | 18.33 ± 1.67c | 26.67 ± 1.67b | 52.86 ± 1.68a | |
| 150 ppm | 189.23 | 2 | <0.05 | 20.67 ± 1.67c | 28.33 ± 1.67b | 59.32 ± 1.68a | |
| 250 ppm | 247.44 | 2 | <0.05 | 23.33 ± 1.67c | 33.33 ± 1.67b | 73.06 ± 1.68a | |
| 10 ppm | 39.43 | 2 | <0.05 | 11.67 ± 1.67c | 21.67 ± 1.67b | 32.66 ± 1.68a | |
| 20 ppm | 93.72 | 2 | <0.05 | 18.33 ± 1.67c | 28.33 ± 1.67b | 44.44 ± 0.00a | |
| 30 ppm | 67.43 | 2 | <0.05 | 22.88 ± 1.67c | 32.67 ± 1.67b | 49.86 ± 1.68a | |
| Z4 | 50 ppm | 89.45 | 2 | <0.05 | 31.67 ± 1.67c | 43.33 ± 1.67b | 62.96 ± 1.68a |
| 100 ppm | 42.37 | 2 | <0.05 | 41.67 ± 1.67c | 55.00 ± 2.89b | 74.75 ± 2.92a | |
| 150 ppm | 89.75 | 2 | <0.05 | 43.33 ± 1.67c | 59.67 ± 1.67b | 79.06 ± 1.68a | |
| 250 ppm | 109.47 | 2 | <0.05 | 53.33 ± 1.67c | 68.33 ± 1.67b | 88.22 ± 1.68a | |
| 10 ppm | 53.49 | 2 | <0.05 | 18.33 ± 1.67c | 31.67 ± 1.67b | 42.76 ± 1.68a | |
| 20 ppm | 39.00 | 2 | <0.05 | 31.67 ± 1.67c | 43.33 ± 1.67b | 61.28 ± 1.68a | |
| 30 ppm | 63.06 | 2 | <0.05 | 34.67 ± 1.67c | 57.78 ± 1.67b | 66.93 ± 1.68a | |
| Z5 | 50 ppm | 108.82 | 2 | <0.05 | 43.33 ± 1.67c | 58.33 ± 1.67b | 78.11 ± 1.68a |
| 100 ppm | 35.34 | 2 | <0.05 | 68.33 ± 1.67c | 78.33 ± 1.67b | 88.22 ± 1.68a | |
| 150 ppm | 83.49 | 2 | <0.05 | 72.86 ± 1.82c | 82.96 ± 1.87b | 93.43 ± 1.68a | |
| 250 ppm | 45.50 | 2 | <0.05 | 81.67 ± 1.67c | 91.67 ± 1.67b | 100.00 ± 0.00a | |
| 10 ppm | 12.43 | 2 | <0.05 | 2.34 ± 1.67b | 3.02 ± 1.67a | 3.89 ± 1.67a | |
| 20 ppm | 16.02 | 2 | <0.05 | 2.89 ± 1.67b | 3.98 ± 1.67a | 4.25 ± 1.67a | |
| Azadirachta indica | 30 ppm | 17.87 | 2 | <0.05 | 3.56 ± 1.67b | 4.08 ± 1.67a | 4.78 ± 1.67a |
| 50 ppm | 8.98 | 2 | <0.05 | 6.06 ± 1.67b | 8.21 ± 1.67a | 9.32 ± 1.68a | |
| 100 ppm | 44.21 | 2 | <0.05 | 8.23 ± 1.67b | 8.95 ± 1.67b | 11.63 ± 1.67a | |
| 150 ppm | 62.80. | 2 | <0.05 | 9.78 ± 1.67b | 10.67 ± 1.67b | 13.84 ± 1.68a | |
| 250 ppm | 78.27 | 2 | <0.05 | 12.36 ± 1.67c | 14.01 ± 1.67b | 16.17 ± 1.68a | |
| Permethrin | 10 ppm | 7.56 | 2 | <0.05 | 2.32 ± 1.78c | 5.67 ± 1.29b | 13.51 ± 1.57a |
| 20 ppm | 16.67 | 2 | <0.05 | 3.85 ± 1.25c | 8.92 ± 2.18b | 18.01 ± 1.92a | |
| 30 ppm | 23.02 | 2 | <0.05 | 9.43 ± 3.62c | 22.46 ± 2.57b | 39.28 ± 1.98a | |
| 50 ppm | 28.77 | 2 | <0.05 | 12 ± 2.48c | 29.84 ± 1.89b | 43.36 ± 0.2.16a | |
| 100 ppm | 37.53 | 2 | <0.05 | 19.27 ± 2.38c | 35.17 ± 2.61b | 51.95 ± 2.58a | |
| 150 ppm | 127.05 | 2 | <0.05 | 23.63 ± 1.58c | 41.21 ± 1.68b | 67.14 ± 2.18a | |
| 250 ppm | 273.38 | 2 | <0.05 | 29.32 ± 1.68c | 52.89 ± 1.49b | 76.46 ± 2.47a | |
*Convulvulus arvensis (Z1), Chenopodium murale (Z2), Tribulus terrestris (Z3), Trianthema portulacastrum (Z4), Achyranthes aspera (Z5), Azadirachta indica (Neem) and Permethrin.
LC50 of weed plant extracts against Culex quinquefasciatus mosquitoes
A. aspera showed lowest LC50 (87, 39 and 9 ppm; p < 0.05) at 24, 48 and 72 hours, Similarly, low LC50 (205, 123.65 and 34.64; p < 0.05) was found with T. portulacas at 24, 48 and 72 hours, respectively; followed by C. arvensis (LC50 304, 151, and 57; p < 0.05). C. murale showed highest LC50 (>250 ppm) at 24, 48 hours among all five extracts. High LC50 was observed with T. terrestris (>250 ppm) at 24, 48 hours, while it showed low LC50 (126 ppm; p < 0.05) but higher than LC50 (98 ppm; p < 0.05) of Permethrin at 72 hours. The LC50 of Permethrin was LC50 > 250 ppm and 247 ppm at 24 and 48 hours, respectively. Overall, it was found that all the weeds extracts showed significant results at 72 hours (p < 0.05) as compare to A. indica (Neem) extract, which showed LC50 (>>250 ppm) after all exposure time (p > 0.05; Table 3).
Table 3.
Toxicity of weed plant extracts against Culex quinquefasciatus.
| Treatment | Observation | N | LC50 (ppm) (Upper + lower values) |
Slope ± SE | X2 ± | df | SE | P |
|---|---|---|---|---|---|---|---|---|
| Z1 | 24 | 100 | LC50 > 250 ppm | 0.0039830 ± 0.0006982 | 5.296 | 5 | 16.82 | 0.00 |
| 48 | 100 | 151.0515 (125.8312 ± 185.6088) | 0.0049704 ± 0.0006737 | 8.383 | 5 | 14.52 | 0.00 | |
| 72 | 100 | 57.1057 (37.0145 ± 75.4342) | 0.0063345 ± 0.0007791 | 1.319 | 5 | 9.50 | 0.00 | |
| Z2 | 24 | 100 | LC50 >> 250 ppm | 0.0018983 ± 0.0007777 | 6.174 | 5 | 246.23 | 0.32 |
| 48 | 100 | LC50 > 250 ppm | 0.0018983 ± 0.0007777 | 6.174 | 5 | 53.40 | 0.10 | |
| 72 | 100 | 164.9892 (140.0672 ± 199.3816) | 0.0053894 ± 0.0006789 | 3.451 | 5 | 14.47 | 0.05 | |
| Z3 | 24 | 100 | LC50 >> 250 ppm | 0.0020102 ± 0.0007382 | 4.355 | 5 | 184.87 | 0.22 |
| 48 | 100 | LC50 >> 250 ppm | 0.0019029 ± 0.0006802 | 6.097 | 5 | 133.47 | 0.10 | |
| 72 | 100 | 126.5443 (106.1374 ± 151.5917) | 0.0058605 ± 0.0006952 | 8.582 | 5 | 11.22 | 0.03 | |
| Z4 | 24 | 100 | 205.0356 (169.5248 ± 262.854) | 0.0043157 ± 0.0006678 | 12.09 | 5 | 22.03 | 0.03 |
| 48 | 100 | 123.6593 (99.0462 154.7968) | 0.0046799 ± 0.000674 | 9.695 | 5 | 13.55 | 0.00 | |
| 72 | 100 | 34.6413 (13.5864 ± 51.9514) | 0.0069505 ± 0.0008466 | 11.74 | 5 | 9.45 | 0.00 | |
| Z5 | 24 | 100 | 87.4652 (70.6095 ± 105.3030) | 0.0069648 ± 0.0007523 | 17.60 | 5 | 8.64 | 0.01 |
| 48 | 100 | 39.0875 (21.3783 ± 54.3348) | 0.0078991 ± 0.0009073 | 10.63 | 5 | 8.1687 | 0.00 | |
| 72 | 100 | 9.2235 (6.3036 ± 19.7265) | 0.014552 ± 0.002114 | 5.608 | 5 | 6.2459 | 0.00 | |
| 24 | 100 | 616.028(429.372 ± 1279.82) | 0.0029602 ± 0.0008561 | 2.577 | 5 | 147.95 | 0.75 | |
| A. indica Neem | 48 | 100 | 600.278(420.494 ± 1220.25) | 0.0028745 ± 0.0008160 | 2.54713 | 5 | 141.535 | 0.76 |
| 72 | 100 | 546.163(394.025 ± 1005.21) | 0.0029899 ± 0.0007771 | 3.86339 | 5 | 116.025 | 0.56 | |
| Permethrin | 24 | 100 | LC50 > 250 ppm | 0.03432162 ± 0.0065762 | 14.2512 | 6 | 15.7262 | 0.03 |
| 48 | 100 | 247.0248 (179.6136 ± 298.7321) | 0.0042325 ± 0.00071697 | 8.1245 | 6 | 13.23 | 0.02 | |
| 72 | 100 | 98.2859 (86.7189 ± 145.4137) | 0.0068465 ± 0.00082418 | 5.82 | 6 | 7.49 | 0.01 |
*Convulvulus arvensis (Z1), Chenopodium murale (Z2), Tribulus terrestris (Z3), Trianthema portulacastrum (Z4), Achyranthes aspera (Z5), Azadirachta indica (Neem) and Permethrin.
Mortality Assay of Culex quinquefasciatus mosquitoes using Achyrathes aspera in-combination with microbes at various concentration and different time intervals
A. aspera showed significant results for mortality and thus, it was selected for combinatorial trials with Bti and P. aeruginosa. The mean mortality induced by Bti and Pseudomonas individually and in combinations with A. aspera extract at different concentrations and time intervals is shown in Table 4. It was observed that mortality increased with increase in concentration and exposure time. High mortality was observed at 250 ppm concentration after 72 hours, whereas, highest mortality (100%) was shown by of A. aspera with Bti followed by combination of Pseudomonas and A. aspera (72.79%) at 250ppm concentration as compared to control treatment (Table 4).
Table 4.
Mean mortality of Culex quinquefasciatus using Achyranthes aspera with microbes at various concentration and different time intervals.
| Treatment | Conc. | F Value |
df | P value |
Mean mortality with different time intervals | ||
|---|---|---|---|---|---|---|---|
| 24 hours | 48 hours | 72 hours | |||||
| 10 ppm | 27.81 | 2 | <0.05 | 12.46 ± 1.68c | 20.07 ± 1.70b | 30.27 ± 1.70a | |
| 20 ppm | 16.90 | 2 | <0.05 | 22.56 ± 1.68c | 33.67 ± 2.95b | 43.88 ± 2.95a | |
| 30 ppm | 30.67 | 2 | <0.05 | 24.86 ± 1.68c | 35.48 ± 1.70b | 45.63 ± 1.70a | |
| Bti | 50 ppm | 39.90 | 2 | <0.05 | 30.98 ± 1.68c | 42.18 ± 1.70b | 52.38 ± 1.70a |
| 100 ppm | 35.34 | 2 | <0.05 | 42.76 ± 1.68c | 50.68 ± 1.70b | 59.18 ± 0.00a | |
| 150 ppm | 31.61 | 2 | <0.05 | 45.24 ± 1.68c | 53.67 ± 1.70b | 63.58 ± 1.70a | |
| 250 ppm | 34.51 | 2 | <0.05 | 51.18 ± 1.68c | 60.88 ± 1.70b | 71.09 ± 1.70a | |
| Bti + Z5 | 10 ppm | 77.64 | 2 | <0.05 | 17.51 ± 1.68c | 30.27 ± 1.70b | 47.28 ± 1.70a |
| 20 ppm | 46.87 | 2 | <0.05 | 30.98 ± 1.68c | 43.88 ± 2.95b | 60.88 ± 1.70a | |
| 30 ppm | 43.58 | 2 | <0.05 | 35.28 ± 1.68c | 48.33 ± 2.57b | 65.45 ± 1.70a | |
| 50 ppm | 39.33 | 2 | <0.05 | 42.76 ± 1.68c | 55.78 ± 3.40b | 72.79 ± 1.70a | |
| 100 ppm | 54.70 | 2 | <0.05 | 54.55 ± 2.92c | 69.39 ± 2.95b | 89.80 ± 0.00a | |
| 150 ppm | 98.19 | 2 | <0.05 | 57.72 ± 1.68c | 74.68 ± 1.70b | 92.53 ± 1.70a | |
| 250 ppm | 183.90 | 2 | <0.05 | 62.96 ± 1.68c | 86.39 ± 1.70b | 100.00 ± 0.00a | |
| Pseudomonas | 10 ppm | 7.25 | 2 | <0.05 | 1.35 ± 1.35b | 4.76 ± 1.70ab | 9.86 ± 1.70a |
| 20 ppm | 41.06 | 2 | <0.05 | 4.04 ± 0.00c | 11.56 ± 1.70b | 21.77 ± 1.70a | |
| 30 ppm | 78.54 | 2 | <0.05 | 4.89 ± 0.00c | 11.93 ± 1.70b | 23.91 ± 1.70a | |
| 50 ppm | 52.23 | 2 | <0.05 | 7.41 ± 1.68c | 13.27 ± 0.00b | 26.87 ± 1.70a | |
| 100 ppm | 41.51 | 2 | <0.05 | 14.14 ± 0.00c | 21.77 ± 1.70b | 31.97 ± 1.70a | |
| 150 ppm | 63.46 | 2 | <0.05 | 17.26 ± 1.68c | 23.45 ± 1.70b | 36.62 ± 1.70a | |
| 250 ppm | 83.41 | 2 | <0.05 | 22.56 ± 1.68c | 30.27 ± 1.70b | 52.38 ± 1.70a | |
| Pseudomonas + Bti | 10 ppm | 15.50 | 2 | <0.05 | 4.04 ± 0.00c | 9.86 ± 1.70b | 14.97 ± 1.70a |
| 20 ppm | 55.10 | 2 | <0.05 | 7.41 ± 1.68c | 14.97 ± 1.70b | 31.97 ± 1.70a | |
| 30 ppm | 86.98 | 2 | <0.05 | 9.04 ± 1.68c | 16.63 ± 1.70b | 33.47 ± 1.70a | |
| 50 ppm | 46.23 | 2 | <0.05 | 12.46 ± 1.68c | 21.77 ± 1.70b | 35.37 ± 1.70a | |
| 100 ppm | 33.51 | 2 | <0.05 | 22.56 ± 1.68c | 31.97 ± 1.70b | 42.18 ± 1.70a | |
| 150 ppm | 98.34 | 2 | <0.05 | 26.77 ± 1.68c | 36.52 ± 1.70b | 49.32 ± 1.70a | |
| 250 ppm | 150.66 | 2 | <0.05 | 32.66 ± 1.68c | 43.88 ± 0.00b | 65.99 ± 1.70a | |
| Pseudomonas + Z5 | 10 ppm | 39.25 | 2 | <0.05 | 2.69 ± 1.35c | 9.86 ± 1.70b | 18.37 ± 0.00a |
| 20 ppm | 46.04 | 2 | <0.05 | 7.41 ± 1.68c | 16.67 ± 1.70b | 30.27 ± 1.70a | |
| 30 ppm | 28.92 | 2 | <0.05 | 10.63 ± 1.68c | 21.34 ± 1.70b | 36.82 ± 1.70a | |
| 50 ppm | 36.17 | 2 | <0.05 | 15.82 ± 1.68c | 28.57 ± 2.95b | 42.18 ± 1.70a | |
| 100 ppm | 52.50 | 2 | <0.05 | 27.61 ± 1.68c | 40.48 ± 1.70b | 59.18 ± 2.95a | |
| 150 ppm | 47.48 | 2 | <0.05 | 31.23 ± 1.68c | 46.59 ± 1.70b | 64.34 ± 2.95a | |
| 250 ppm | 58.84 | 2 | <0.05 | 36.03 ± 1.68c | 52.38 ± 1.70b | 72.79 ± 3.40a | |
*Achyranthes aspera (Z5), Psuedomonas, Bacillus thuringiens isisraeliensis (Bti).
LC50 of Achyrathes aspera extract in-combination with microbes against Culex quinquefasciatus mosquitoes
The combination of A. aspera and Bti showed low LC50 (137, 49 and 8 ppm; p < 0.05) at 24, 48 and 72 h, respectively. However, the combination of Pseudomonas and A. aspera showed very high LC50 (>250 ppm) at 24 h and, moderate to high LC50 (213 and 111 ppm; p > 0.05) was found at 48 and 72 h, respectively. For control treatment, the LC50 of Bti (213.45, 145.80 and 76.12 ppm; p < 0.05) was found lower than Pseudomonas (>250 ppm) at 24, 48 and 72 h, respectively. The LC50 of combination of Pseudomonas and Bti is shown in Table 5.
Table 5.
Toxicity of Achyranthes aspera extract in combination with microbes against Culex quinquefasciatus.
| Treatment | Observation | N | LC50 (ppm) (Upper ± lower values) |
Slope ± SE | X2 ± | df | SE | P value |
|---|---|---|---|---|---|---|---|---|
| Bti | 24 | 100 | 213.4507 (172.9759 ± 284.6938) | 0.0038483 ± 0.0006629 | 11.840 | 3 | 25.789 | 0.00 |
| 48 | 100 | 145.8052 (113.6218 ± 194.4482) | 0.0036579 ± 0.0006575 | 10.203 | 3 | 18.893 | 0.01 | |
| 72 | 100 | 76.1231 (42.6566 ± 107.7533) | 0.0036931 ± 0.0006723 | 7.694 | 3 | 15.507 | 0.05 | |
| Bti ± Z5 | 24 | 100 | 137.0498 (109.2724 ± 175.2752) | 0.0042117 ± 0.0006644 | 16.361 | 3 | 15.810 | 0.00 |
| 48 | 100 | 49.6853 (27.9094 ± 68.5992) | 0.0061320 ± 0.0007723 | 8.902 | 3 | 10.034 | 0.00 | |
| 72 | 100 | 8.2924 (−7.7614 ± 18.9967) | 0.014400 ± 0.002129 | 1.475 | 3 | 6.4015 | 0.00 | |
| Pseudomonas | 24 | 100 | LC50 > 250 ppm | 0.0044863 ± 0.0008278 | 5.935 | 3 | 53.815 | 0.11 |
| 48 | 100 | LC50 > 250 ppm | 0.0034591 ± 0.0007267 | 5.432 | 3 | 61.388 | 0.14 | |
| 72 | 100 | 226.9449 (187.1139 ± 294.4419) | 0.0042459 ± 0.0006707 | 6.336 | 3 | 25.155 | 0.09 | |
| Pseudomonas + Bti | 24 | 100 | LC50 > 250 ppm | 0.0044517 ± 0.0007379 | 7.302 | 3 | 39.260 | 0.06 |
| 48 | 100 | LC50 > 250 ppm | 0.0039967 ± 0.0006806 | 5.565 | 3 | 32.651 | 0.13 | |
| 72 | 100 | 153.6023 (127.0986 ± 190.8331) | 0.0047483 ± 0.0006711 | 7.794 | 3 | 15.399 | 0.05 | |
| Pseudomonas + Z5 | 24 | 100 | LC50 > 250 ppm | 0.0047247 ± 0.0007258 | 16.513 | 3 | 32.242 | 0.00 |
| 48 | 100 | 213.0870 (177.4763 ± 270.5488) | 0.0044985 ± 0.0006722 | 11.693 | 3 | 22.067 | 0.03 | |
| 72 | 100 | 111.5831 (90.7470 ± 135.9624) | 0.0055370 ± 0.0006938 | 13.540 | 3 | 11.147 | 0.68 |
*Achyranthes aspera (Z5), Psuedomonas, Bacillus thuringiens isisraeliensis (Bti).
Enzyme inhibitory effect of weed plant extracts in Culex quinquefasciatus mosquitoes
Maximum inhibition activity of AChE, AcP, AkP, α-Carboxyl and β-Carboxyl was found for A. aspera as 54.68, 34.28, 24.58, 71.08 and 59.96 at 250 ppm, respectively. The mean value for inhibition activity for aforementioned mentioned enzymes by C. arvensis was found 52.94, 32.02, 21.01, 63.88 and 46.91%, respectively; followed by C. murale as 59.95, 29.98, 26.08, 45.04 and 50.01% at 250 ppm concentration. Similalry, T. terrestris showed inhibition of AChE, AcP, AkP, α-Carboxyl and β-Carboxyl as 48.78, 28.16, 25.02, 47.13 and 47.04, respectively; likewise, T. portulacastrum showed as 51.14, 29.08, 24.98, 47.68 and 49.69, respectively at 250 ppm. Overall, the enzyme assay showed that the inhibition of enzyme activity increased with increase of concentration (Table 6).
Table 6.
Percent inhibition of enzyme activity in Culex quinquefasciatus larvae using different concentrations of Bti at 30% concentrations.
| Plants | Concentration | AChE | AcP | AkP | α-Carboxyl | β-Carboxyl |
|---|---|---|---|---|---|---|
| Z1 | 50 ppm | 14.85 ± 2.83 a | 14.90 ± 1.75 a | 8.35 ± 1.08 a | 30.01 ± 2.02 a | 26.65 ± 2.23 a |
| 100 ppm | 31.14 ± 3.36 b | 21.07 ± 1.43 b | 12.95 ± 0.78 a | 52.80 ± 3.01 b | 43.87 ± 3.34 b | |
| 250 ppm | 52.94 ± 2.43 c | 32.02 ± 1.14 c | 21.01 ± 1.57 b | 63.88 ± 1.67 c | 46.91 ± 3.08 b | |
| F, df and P value | (F = 92.99; df = 2; P < 0.05) | (F = 32.03; df = 2; P < 0.05) | (F = 24.56 df = 2; P < 0.05) | (F = 78.92; df = 2; P < 0.05) | (F = 23.02; df = 2; P < 0.05) | |
| Z2 | 50 ppm | 17.83 ± 2.3 a | 15.77 ± 1.43 a | 8.87 ± 1.16 a | 24.08 ± 2.13 a | 26.13 ± 3.38 a |
| 100 ppm | 31.12 ± 3.32 b | 20.97 ± 1.03 b | 12.91 ± 0.93 b | 33.10 ± 4.11 a | 39.78 ± 3.03 b | |
| 250 ppm | 59.95 ± 2.47 c | 29.98 ± 1.23 c | 26.08 ± 0.69 c | 45.04 ± 3.513b | 51.01 ± 2.57 b | |
| F, d.f and P value | (F = 90.86; df = 2; P < 0.05) | (F = 38.79; df = 2; P < 0.05) | (F = 128.87; df = 2; P < 0.05) | (F = 13.05; df = 2; P < 0.05) | (F = 14.03; df = 2; P < 0.05) | |
| Z3 | 50 ppm | 11.93 ± 2.69 a | 19.61 ± 1.38 a | 9.49 ± 1.19 a | 22.93 ± 2.82 a | 16.18 ± 3.74 a |
| 100 ppm | 24.03 ± 2.59 b | 21.83 ± 1.68 a | 16.09 ± 1.39 b | 36.15 ± 3.08 b | 36.24 ± 3.49 b | |
| 250 ppm | 48.78 ± 1.82 c | 28.16 ± 1.24 b | 25.02 ± 1.29 c | 47.13 ± 3.39 c | 47.04 ± 2.52 b | |
| F, d.f and P value | (F = 59.57; df = 2; P < 0.05) | (F = 12.89; df = 2; P < 0.05) | (F = 39.94; df = 2; P < 0.05) | (F = 23.29; df = 2; P < 0.05) | (F = 23.31; df = 2; P < 0.05) | |
| Z4 | 50 ppm | 20.67 ± 2.87 a | 19.96 ± 1.49 a | 11.03 ± 1.49 a | 28.49 ± 2.59 a | 22.03 ± 4.87 a |
| 100 ppm | 27.79 ± 2.39 a | 23.21 ± 1.54 b | 13.48 ± 1.58 a | 45.08 ± 2.78 b | 35.03 ± 3.61 ab | |
| 250 ppm | 51.14 ± 2.78 b | 29.08 ± 1.29 c | 24.98 ± 1.38 b | 47.68 ± 3.71 b | 49.69 ± 3.89 b | |
| F, d.f and P value | (F = 45.89; df = 2; P < 0.05) | (F = 42.39; df = 2; P < 0.05) | (F = 33.54; df = 2; P < 0.05) | (F = 12.89; df = 2; P < 0.05) | (F = 11.23; df = 2; P < 0.05) | |
| Z5 | 50 ppm | 20.69 ± 2.03 a | 18.07 ± 1.08 a | 10.17 ± 0.74 a | 37.93 ± 2.11 a | 31.01 ± 2.93 a |
| 100 ppm | 38.76 ± 3.06 b | 23.29 ± 1.39 b | 15.34 ± 0.74 b | 58.17 ± 2.89 b | 48.16 ± 3.04 b | |
| 250 ppm | 54.68 ± 1.35 c | 34.28 ± 1.13 c | 24.58 ± 1.14 c | 71.08 ± 3.03 c | 59.96 ± 1.68 c | |
| F, d.f and P value | (F = 59.24; df = 2; P < 0.05) | (F = 47.87; df = 2; P < 0.05) | (F = 51.02; df = 2; P < 0.05) | (F = 52.23; df = 2; P < 0.05) | (F = 31.07; df = 2; P < 0.05) |
Convulvulus arvensis (Z1), Chenopodium murale (Z2), Tribulus terrestris (Z3), Trianthema portulacastrum (Z4) and Achyranthes aspera (Z5).
AChE = acetylcholinesterase, AcP = acid phosphatases, AkP = alkaline phosphatases, α-Carboxyl = α-Carboxylesterases and β-Carboxyl = β-Carboxylesterases.
Means sharing the same letter within each treatment is not statistically different.
Phytochemical constituents in weed extracts
Phytochemical analysis revealed the presence of flavonoids, saponins, tannins, steroids, cardiac glycosides, alkaloids, anthrequinones and terpenoids in all the five weeds extracts used against Cx. quiquefasciatus larvae (Table 7).
Table 7.
The chemical constituents present in five weeds extract.
| Sr. No. | Code# | Weed Plants | Chemical Constituents | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Fl | Sa | Tn | St | CG | Al | Anth | Ter | |||
| 1 | Z1 | C. arvensis | + | + | + | − | + | + | + | + |
| 2 | Z2 | C.muale | + | + | + | + | + | + | − | − |
| 3 | Z3 | T. terrestris | + | + | + | + | − | + | + | + |
| 4 | Z4 | T. portulacastrum | + | + | + | + | + | + | + | + |
| 5 | Z5 | A.aspera | + | + | + | + | + | + | + | + |
*Convulvulus arvensis (Z1), Chenopodium murale (Z2), Tribulus terrestris (Z3), Trianthema portulacastrum (Z4) and Achyranthes aspera (Z5).
*Fl = flavonoids, Sa = saponins, Tn= tannins, St = steroids, CG = Cardiac glycosides, Al= alkaloids, Anth= anthrequinones and Ter = terpenoids.
Fourier transform infrared spectroscopy (FTIR) analysis of Achyranthes aspera extract
The Infra-red spectrum of A. aspera extract revealed peak at 3338. 23 cm−1 which corresponds to –OH group for phenols. Moreover, peak at around 1317.85 cm−1 and 1377.38 cm−1 was assigned to C=H group and 1077.88 cm−1 for C-O Stretching. The peak at 779.00 cm−1 shows the stretching vibration of plane C-H bending. In addition, the peak at 669.12 cm−1 corresponds to aromatic ring having phosphate group (Fig. 1). Hence, the FTIR results showed the presence of phenolic compound in extract of A. aspera.
Figure 1.
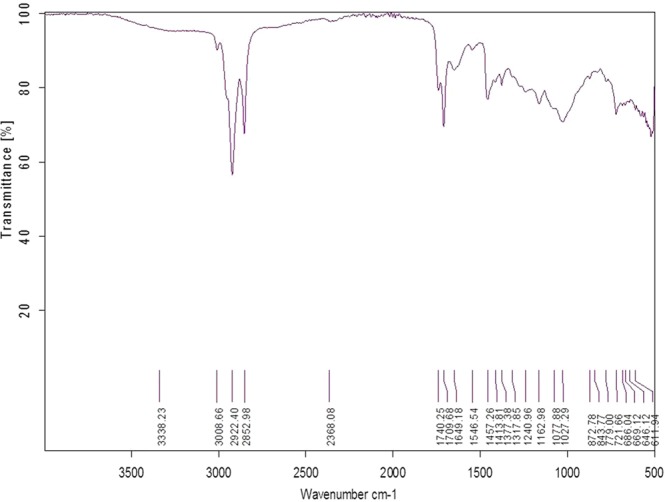
Full FTIR spectrum of Achyranthes aspera. The spectrum shows a range of 4000 to 400 cm−1 wave number (along X-axis) the function of percent (%) transmittance (along Y-axis). The peaks observed at 669.12 cm−1 correspond to aromatic ring having phosphate group, 779.00 cm−1 out of plane CH bending, 1077.88 cm−1 C-O Stretching, 1317.85 cm−1 and 1377.38 cm−1 C=H group, 3008.66 cm−1 alkyl C-H group stretching and 3338.23 cm−1–OH group for phenols.
Random amplified polymerase DNA polymerase chain reaction (RAPD-PCR) analysis
The extracted DNA from larvae of Cx. quiquefasciatus (treated with Achyrathes aspera and its combination with Bti) was amplified using RAPD-PCR. The banding profile showed that no DNA damage had occurred due to application of A. aspera individually and in combination with Bti compared to control (Fig. 2).
Figure 2.
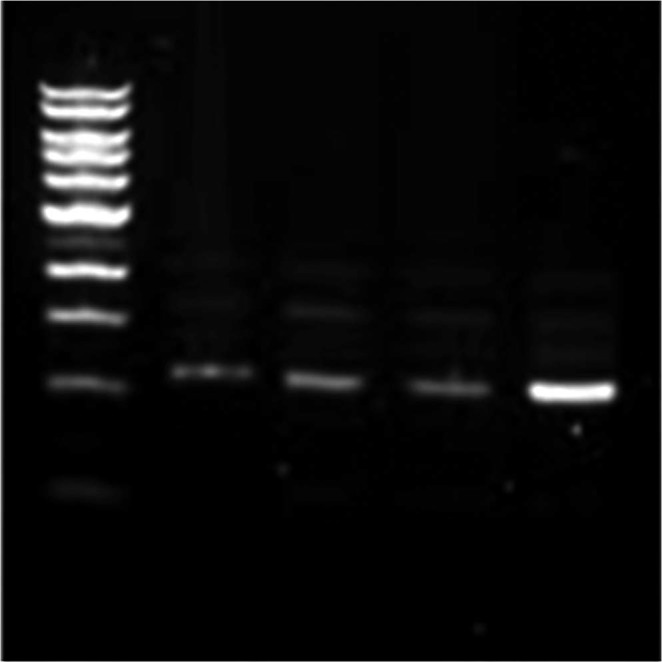
Comparison of RAPD profile for genotoxicity using C-04 primer. (1) Control group (Non-treated Cx. quiquefasciatus larvae). (2) Treated larvae with A. aspera extract. (3) Treated larvae with Bti. (4) The combination of A. aspera with Bti.
Comet assay
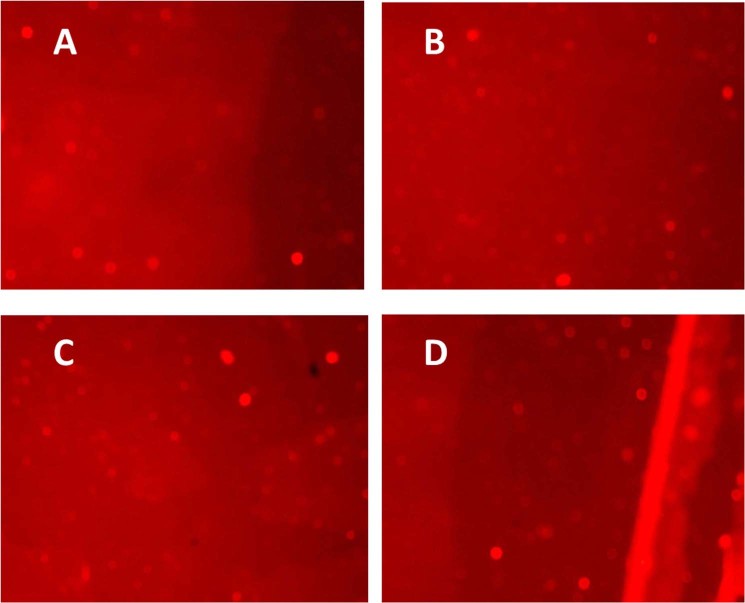
The comet assay was performed to detect the DNA damage in cells of treated larvae of Cx. quiquefasciatus with significant plant extract A. aspera and its combination with Bti. It was found that no DNA damage had occurred in treatment group compared to control (Fig. 3).
Figure 3.
Comparison of Comet assay profile for genotoxicity in larvae of Culex quiquefasciatus. (A) Control. (B) A. aspera. (C) Bti. (D) A. aspera + Bti.
Discussions
During the present study, weed plant extracts were exploited for their insecticidal potential in Cx. quiquefasciatus. Five different weed plants extracts C. arvensis, C. murale, T. terrestris, T. portulacastrum and A. aspera were employed against larvae of Cx. quiquefasciatus. The extracts of three weed plants viz. A. aspera, T. portulacastrum and C. arvensis showed significant results as compare to synthetic pesticide Permethrin, and in comparison with A. indica (neem) extract all plants showed high mortality. A. indica extract had already been reported by Sagheer et al.14 which showed 16.11% mortality at 15% concentration. Recently, Sultana49 and Sultana et al.15 described that weed plant extracts have more insecticidal activity as compared to A. indica extracts. Subsequently, A. aspera revealed LC50 value 9.22 ppm with 100% mortality which is lower than that of Clausena dentate (LC50 28.60 ppm) against Cx. quinquefasciatus larvae as described by Sakthivadivel et al.50. Similarly, it was also revealed that A. aspera had more entomocidal potential when compared the LC50 89.03 ppm of Croton rhamnifolioides as reported by Santos et al.51.
In current study, all the plant extracts showed high mortality at 72 hours compared to 24 and 48 hours. And no LC50 was found more than 250 ppm (LC50 < 250 ppm) at 72 hours exposure time. Thus, the insecticidal activity of weed plant extracts is time dependent15. It was thus found that the mortality had increased with increased concentrations of weed plant extracts. Hence, the percent mortality and toxicity data is in accordance to the previous findings of Odeyemi and Ashamo52, Sultana et al.15 and Sagheer et al.14 that the plant extracts become more toxic with increased dose and exposure time. Interestingly, few LC50 values in the current findings were extrapolated which are in line with study of Prabakar and Jebanesan53 who reported LC50 of Benincasa cerifera (LC50 = 1189.30 ppm) and Citrullus vulgaris (LC50 = 1636.04 ppm) and found higher LC50 than 1000 ppm (maximum concentration)53.
Plant extracts have also been used in combination with certain microbes. Kumar et al.54 described that B. thuringiensis in combination with Solanum xanthocarpum showed higher larval mortality54. Recently, B. thuringiensis was reported as very effective causing high mortality when used in combination with weed plant extracts49. In addition, Kupferschmied et al.28 extensively reviewed the root-associated Pseudomonas with their insecticidal activities against various insect pests. Consistently, the current findings showed that A. aspera in combination with B. thuringiensis had caused 100% mortality against larvae of Cx. quinquefasciatus.
Phytochemical analysis of the weed plant extracts used in present study indicated the presence of flavonoids. It has been reported by Gautam et al.55 that flavonoid extracted from aerial parts of Androgrpahis paniculata was inactive at 600 ppm against Ae. aegypti larvae, but it caused 70% mortality against An. stephensi at 200 ppm concentration. However, flavonoid extracted from flower-buds showed 100% mortality for Ae. aegypti and An. stephensi at 600 & 200 ppm concentration, respectively. A. aspera weed plant contains flavonoids, saponins, tannins, steroids, cardiac glycosides, alkaloids, anthrequinones and terpenoids. These compounds are also present in other tested plants, except steroids and cardiac glycosides which were found absent in C. arvensis and T. terrestris. Similarly, anthrequinones and terpenoids were also found absent in C. muale. Low mortality was shown by C. muale in the current study which might be due to the absence of anthrequinones and terpenoids, and absence of steroids in case of T. terrestris.
Subsequent studies by Cárdenas-Ortega et al.20 reported 37 different compounds with β-caryophyllene and caryophyllene oxide as main components in Salvia ballotiflora resulted in 80% larval mortality. Evans56 reported that alkaloids cause the death of treated organisms due to their ability to bind DNA of organisms and affecting the replication process and synthesis of molecules. Alkaloids compound were identified in all of our used weed plants extracts but no DNA damage was observed in the treated larvae of Cx. quinquefasciatus mosquiotes. The enzymatic profiles are also modulated in response to natural oils from plants25. For instance, Esterases, a major detoxifying enzyme in insects and have been reported to involve in detoxification of insecticides26. Plant extracts have been reported as AChE inhibitors27. The death in insects due to treatment with plant extracts suggested that the molecules present therein possibly interfere at the cholinergic synapse and destroyed the communication network from one exonic end to another; thereby, blocking the nerve impulse transmission. Thus, the lethal effect may also be due to the accumulation of acetylcholine (ACh), a neurotransmitter, at synaptic junctions, which interrupts the coordination between the nervous and muscular junctions (neurotoxicity)27. Subsequent changes in enzyme activity are also reported for Phosphatases in insects. The hydrolysis of acid phosphatase (ACP) and alkaline phosphatase (ALP) phosphomonoesters under acid or alkaline conditions, respectively25. Alkaline phosphatase (ALP) is used as a membrane marker enzyme, active in intestinal epithelial cells, malpighian tubules and hemolymph of insects25,57. A decrease in ACP levels due to plant extract could be attributed to reduced phosphorous liberation for energy metabolism, decreased rate of metabolism as well as decreased rate of transport of metabolites25,26. The enzyme activity of AChE, AcP, AkP, α-Carboxyl and β-Carboxyl was inhibited with increase of concentration of all the plant extracts which is also in agreement to Santos et al.51 who also reported that essential oil of Croton rhamnifolioides showed the inhibitory effect on a digestive enzyme (Trypsin) from larvae of Ae. aegypti.
The genotoxicity and carcinogenicity in the cell genome are caused by genotoxic agents having some lethal or sub-lethal effects which are induced by some xenobiotic substances22,26.
Thus, the DNA damage due to exposure of an organism to plant extracts21,22 may result from the formation of covalently bound adducts between metabolites and DNA; and the faulty repair of these adducts often results in mutations and sometimes cytogenetic changes. Recently A. aspera was found genotoxic against Ae. aegypti with significant changes in the RAPD profiles. These changes suggested that certain phytocomponents in A. aspera caused the probable DNA damage and mutations in the larval g-DNA which could be the possible reason of larval mortality24. In contrast, no DNA damage was found in the current findings. Moreover, FTIR analysis of A. aspera indicated the presence of phytochemicals composed of hydrogen bonded –OH functional group. Mostly phenolic phytochemicals such as tannins and flavonoids are composed of –OH functional group58. FTIR spectrum of C. arvensis showed the presence of alkaloids. These all compounds are reported as toxic to insects and produced insecticidal activities. It has already been reported that phenolic compounds can be potentially used for the control of insect pests of various crops59.
Hence, it is suggested that the mortality in larvae of Cx. quinquefasciatus cannot be attributed due to genotoxicity. Rather, perhaps it is caused by the presence of certain phenolic phytochemicals such as flavonoids which modulate the enzymatic activity and thus, cause the death of larvae of Cx. quinquefasciatus. Thus, it is suggested that A. aspera weed plant can be further exploited for extraction and purification of phenolic compounds to use against mosquitoes. In addition, the current study which is the first one performed in Pakistan using weed plants extracts; also suggests that weed plants can be explored for their insecticidal activity against other insect pests.
Conclusions
The petroleum ether extracts of five weed plants were used against the larvae of Cx. quinquefasciatus. A. aspera extract showed highest mortality. Thus, based on LC50 values (p-values), Achyrathes aspera weed plant extract was used along with Bti and Pseudomonas bacteria for further trials. The highest mortality of C. quinquefasciatus was found using A. aspera with Bti. Enzyme inhibition activity of AChE, AcP, AkP, α-Carboxyl and β-Carboxyl was found in tested weed plant extracts. Phytochemical analysis showed the presence of flavonoids, saponins, tannins, steroids, cardiac glycosides, alkaloids, anthrequinones and terpenoids. Moreover, FTIR analysis showed that A. aspera contains phenolic compounds which have been reported to show insecticidal activity. Genotoxic activity was also observed using RAPD-PCR and comet assay. It was found that no DNA damage had been occurred due to either A. aspera extractor using the extract in combination with Bti. It is suggested that certain phenolic compounds such as flavonoids which modulate the enzymatic activity and, causes the death of larvae of Cx. quinquefasciatus. A. aspera plant is easily available in Pakistan and its extract could be very used to control Culex mosquitoes. In future, further studies are needed to extract and characterize the particular potential of phenolic compound found in A. aspera to use in mosquito control programs.
Supplementary information
Acknowledgements
The facilities provided by Department of Zoology, Government College University Faisalabad (GCUF) are highly acknowledged to conduct this research work. The authors are also thankful to Department of Microbiology for kind gift of bacteria. The authors declare that there is no conflict of interest.
Author contributions
M.K.Z. designed and supervised the work. M.Z. performed experiments. H.N.M., A.R. and K.R. did collection, extraction and helped in chemical as well as data analyses. A.A., M.A.Z. and F.J. gave their input during write up. All authors read and approved the final version of the manuscript.
Competing interests
The authors declare no competing interests.
Footnotes
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
is available for this paper at 10.1038/s41598-020-63815-w.
References
- 1.Auguste AJ, et al. Yellow fever virus maintenance in trinidad and its dispersal throughout the Americas. J. Virol. 2010;84:9967–9977. doi: 10.1128/JVI.00588-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Korgaonkar NS, Kumar A, Yadav RS, Kabadi D, Dash AP. Mosquito biting activity on humans & detection of Plasmodium falciparum infection in Anopheles stephensi in Goa, India. Ind. J. Med. Res. 2012;135:120–126. doi: 10.4103/0971-5916.93434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Paily KP, Hoti SL, Balaraman K. Development of lymphatic filarial parasite Wuchereria bancrofti (Spirurida: Onchocercidae) in mosquito species (Diptera: Culicidae) fed artificially on microfilaremic blood. J. Med. Entomo. 2006;43:1222–1226. doi: 10.1093/jmedent/43.6.1222. [DOI] [PubMed] [Google Scholar]
- 4.Mitchell, C. J., Francy, D. B., & Monath, T. P. Arthropod vectors. St. Louis encephalitis. Washington. APHA, 313–79 (1980).
- 5.Richards SL, Lord CC, Pesko KN, Tabachnick WJ. Environmental and biological factors influencing Culex pipiens quinquefasciatus (Diptera: Culicidae) vector competence for West Nile Virus. Amer J. Trop. Med. Hyg. 2010;83:126–134. doi: 10.4269/ajtmh.2010.09-0776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Conteh L, Engels T, Molyneux DH. Socioeconomic aspects of neglected tropical diseases. Lancet. 2010;375:239–247. doi: 10.1016/S0140-6736(09)61422-7. [DOI] [PubMed] [Google Scholar]
- 7.Solomon T. Control of Japanese encephalitis-within our grasp? N. Engl. J. Med. 2007;355:869–871. doi: 10.1056/NEJMp058263. [DOI] [PubMed] [Google Scholar]
- 8.Subra R. Biology and control of Culex quinquefasciatus Say, 1823 (Diptera, Culicidae) with special reference to Africa. Int. J. Trop. Insect Sci. 1981;1:319–338. doi: 10.1017/S1742758400000618. [DOI] [Google Scholar]
- 9.Lee SE, Kim JE, Lee HS. Insecticide resistance in increasing interest. Agric. Chem. Biotech. 2001;44:105–112. [Google Scholar]
- 10.Yousuf MJ, Anjum SI, Faiz R. Toxicological attributes of plant chemicals and their biochemical impacts on cholinesterase and protein levels in relation with conventional insecticides against mosquito larvae of Karachi city. Toxicol. Envir Chem. 2014;96(7):1088–1095. doi: 10.1080/02772248.2015.1008789. [DOI] [Google Scholar]
- 11.Brausch JM, Smith PN. Pesticide resistance from historical agricultural chemical exposure in Thamnocephalus platyurus (Crustacea: Anostraca) Envir Poll. 2009;157:481–487. doi: 10.1016/j.envpol.2008.09.010. [DOI] [PubMed] [Google Scholar]
- 12.Thakur JS, et al. Adverse reproductive and child health outcomes among people living near highly toxic waste water drains in Punjab. J. Epidem Comm. Health. 2010;64:148–154. doi: 10.1136/jech.2008.078568. [DOI] [PubMed] [Google Scholar]
- 13.Schmutterer H. Properties and potential of natural pesticides from the neem tree, Azadirachta indica. Ann. Rev. Ent. 1990;35:271–297. doi: 10.1146/annurev.en.35.010190.001415. [DOI] [PubMed] [Google Scholar]
- 14.Sagheer M, Mansoor-ul-Hasan H-U-R, Ahmad FZ, Tarar A. Screening of some medicinal plant extracts for toxic and repellent potential against adult stage of rust red flour beetle, Tribolium castaneum (Herbst) (Coleoptera: Tenebrionidae) Int. J. Biosci. 2013;3:273–279. [Google Scholar]
- 15.Sultana K, et al. Insecticidal activity of weed plants, Euphorbia prostrata and Chenopodiastrum murale against stored grain insect pest Trogoderma granarium Everts, 1898 (Coleoptera: Dermestidae) Turk. J. Ent. 2016;40(3):291–301. [Google Scholar]
- 16.Aydin, T., Bayrak, N., Baran, E. & Cakir, A. Insecticidal effects of extracts of Humulus lupulus (hops) L. cones and its principal component, xanthohumol. Bull Entomol Res. 1–7 (2017). [DOI] [PubMed]
- 17.Tang, W., & Eisenbrand, G. Panax ginseng CA Mey. In: Chinese drugs of plant origin. Springer Berlin Heidelberg. pp. 711–737 (1992).
- 18.Namba, T. The Encyclopedia of Wakan-Yaku”. Hoikusha, Osaka. 165 (1993).
- 19.Shaalan EAS, Canyon D, Younes MWF, Abdel-Wahab H, Mansour AH. A review of botanical phytochemicals with mosquitocidal potential. Env. Int. 2005;31:1149–1166. doi: 10.1016/j.envint.2005.03.003. [DOI] [PubMed] [Google Scholar]
- 20.Cárdenas-Ortega NC, et al. Composition of the essential oil of Salvia ballotiflora (Lamiaceae) and its insecticidal activity. Molecule. 2015;20(5):8048–8059. doi: 10.3390/molecules20058048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dua VK, Kumar A, Pandey AC, Kumar S. Insecticidal and genotoxic activity of Psoralea corylifolia Linn.(Fabaceae) against Culex quinquefasciatus Say, 1823. Parasites vectors. 2013;6(1):30. doi: 10.1186/1756-3305-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lalrotluanga N, Gurusubramanian G. Evaluation of the random amplified polymorphic DNA (RAPD) assay for the detection of DNA damage in mosquito larvae treated with plant extracts. Sci. Vis. 2011;11(3):155–158. [Google Scholar]
- 23.Ercan FS. Use of random amplified polymorphic DNA (RAPD) to detect DNA damage induced by Prangos ferulacea (Umbelliferae) essential oil against the Mediterranean flour moth Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Arch. Biol. Sci. 2015;67(1):235–239. doi: 10.2298/ABS140903029E. [DOI] [Google Scholar]
- 24.Sharma, A., Kumar, S., & Tripathi, P. Assessment of Achyranthes aspera induced toxicity and molecular analysis of RAPD-PCR profiles of larval genomic DNA of Aedes aegypti L. (Diptera: Culicidae). J Parasatic Dis. 1–8 (2017). [DOI] [PMC free article] [PubMed]
- 25.Nathan SS, Kalaivani K, Murugan K, Chung PG. The toxicity and physiological effect of neem limonoids on Cnaphalocrocis medinalis (Guenee) the rice leaf folder. Pest. Biochem. Physiol. 2005;81:113–122. doi: 10.1016/j.pestbp.2004.10.004. [DOI] [Google Scholar]
- 26.Sameer HQ, Abdel-Fattah NAH, Shehawy AA. Assessment of DNA damage and biochemical responses in Rhyzopertha dominica exposed to some plant volatile oils. J. Pharm. Toxicol. 2017;12:87–96. doi: 10.3923/jpt.2017.87.96. [DOI] [Google Scholar]
- 27.Begum N, Sharma B, Pandey RS. Toxicity potential and anti AchE activity of some plant extracts in Musca domestica. J. Biofert Biopest. 2010;2:108. [Google Scholar]
- 28.Kupferschmied P, Maurhofer M, Keel C. Promise for plant pest control: root-associated pseudomonads with insecticidal activities. Front. Plant. Sci. 2013;4:287. doi: 10.3389/fpls.2013.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Poopathi S, Tyagi BK. Studies on Bacillus sphaericus toxicity-related resistance development and biology in the filariasis vector, Culex quinquefasciatus (Diptera: Culicidae) from South India. Appl. Ent Zool. 2002;37:365–371. doi: 10.1303/aez.2002.365. [DOI] [Google Scholar]
- 30.Poopathi S, Tyag BK. Mosquitocidal toxins of spore forming bacteria: recent advancement. Afr. J. Biotech. 2004;3:643–650. [Google Scholar]
- 31.Gerberg EJ, Barnard DR, Ward RA. Manual for Mosquito Rearing and Experimental Techniques. Amer Mosq. Cont. Assoc. Bull. 1994;5:61–62. [Google Scholar]
- 32.WHO. Report of the WHO informal consultation on the evaluation and testing of insecticides”. CTD/WHOPES/IC/961996, 1, 69 (1996).
- 33.Younes A, et al. Mocetinostat for relapsed classical Hodgkin’s lymphoma: an open-label, single-arm, phase 2 trial. Lancet Oncol. 2011;12(13):1222–1228. doi: 10.1016/S1470-2045(11)70265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Harborne JB. Phytochemical methods, London. Chapman Hall, Ltd. 1973;1973:49–188. [Google Scholar]
- 35.Trease, G. E. & Evans, W. C. Pharmacognsy”. 11th Edition. Brailliar Tiridel Can. Macmillian publishers (1989).
- 36.Sofowara, A. Medicinal plants and Traditional medicine in Africa. Spectrum Books Ltd, Ibadan, Nigeria. p. 289 (1993).
- 37.Binac SS, Afshan F, Gulzar T, Sultana R. Tetracyclic triterpenoids from the leaves of Azadirachta indica and their insecticidal activities. Chem. Pharm. Bull. 2003;51(4):415–417. doi: 10.1248/cpb.51.415. [DOI] [PubMed] [Google Scholar]
- 38.Zahoor MK, Suhail A, Zahoor S, Iqbal A, Awan FS. Molecular characterization of Scarab beetles (Scarabaeidae: Coleoptera) using RAPD markers. Pak. J. Life Soc. Sci. 2013;11:238–243. [Google Scholar]
- 39.Bibi M, et al. Genetic analysis of mosquitoes from rural and urban areas of Sialkot, Pakistan. Int. J. Agric. Biol. 2015;17(4):809–814. doi: 10.17957/IJAB/14.0027. [DOI] [Google Scholar]
- 40.Ashraf HM, Zahoor MK, Nasir S, Majeed HN, Zahoor S. Genetic Analysis of Aedes aegypti Using Random Amplified Polymorphic DNA (RAPD) Markers from Dengue Outbreaks in Pakistan. J. Arthr-Borne Dis. 2016;10(4):546–559. [PMC free article] [PubMed] [Google Scholar]
- 41.Zahoor MK, et al. Population dynamics and genetic homogeneity in natural populations of Drosophila melanogaster from Faisalabad, Pakistan. Iran. J. Sci. Techn. Trans. A: Sci. 2017;41(2):277–285. doi: 10.1007/s40995-017-0267-0. [DOI] [Google Scholar]
- 42.Irving P, et al. New insights into Drosophila larval haemocyte functions through genome-wide analysis. Cell Microbiol. 2005;7:335–350. doi: 10.1111/j.1462-5822.2004.00462.x. [DOI] [PubMed] [Google Scholar]
- 43.Singh NP, McCoy MT, Tice RR, Schneider EL. A simple technique for quantitation of low levels of DNA damage in individual cells. Exper Cell Res. 1988;175:184–191. doi: 10.1016/0014-4827(88)90265-0. [DOI] [PubMed] [Google Scholar]
- 44.Kumaravel TS, Jha AN. Reliable Comet assay measurements for detecting DNA damage induced by ionising radiation and chemicals. Mutat. Res. 2006;605:7–16. doi: 10.1016/j.mrgentox.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 45.Lovell DP, Omori T. Statistical issues in the use of the comet assay. Mutagen. 2008;23:171–182. doi: 10.1093/mutage/gen015. [DOI] [PubMed] [Google Scholar]
- 46.Finney, D. J. Probit Analysis. Editor. D J Finney, Volume 60. 3rd edition. 32 E. 57thSt. New York: Cambridge University Press (1971).
- 47.Tapondjou, L. A., Adler, CLAC, Bouda, H. D. & Fontem, A. Efficacy of powder and essential oil from Chenopodium ambrosioides leaves as post-harvest grain protectants against six-stored product beetles. J. Stored Products Res. 38, 395–402 (2002).
- 48.Pandir D, Hatice BAŞ. Compositional analysis and toxicity of four plant essential oils to different stages of Mediterranean flour moth, Ephestia kuehniella Zeller (Lepidoptera: Pyralidae) Turk. J. Entom. 2016;40(2):185–195. [Google Scholar]
- 49.Sultana, K. Entomocidal, Repellent and Growth regulatory impact of indigenous plant extracts and Bacillus thuringiensis against four stored product insect pests”. PhD Thesis. Department of Zoology, Government College University Faisalabad, Pakistan (2017).
- 50.Sakthivadivel M, Eapen A, Dash AP. Evaluation of toxicity of plant extracts against vector of lymphatic filariasis, Culex quinquefasciatus. Ind. J. Med. Res. 2012;135:397. [PMC free article] [PubMed] [Google Scholar]
- 51.Santos GK, et al. Effects of Croton rhamnifolioides essential oil on Aedes aegypti oviposition, larval toxicity and trypsin activity. Molecules. 2014;19(10):16573–16587. doi: 10.3390/molecules191016573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Odeyemi OO, Ashamo MO. Efficacy of neem plant (Azadirachta indica) extracts in the control of Trogoderma granarium, a pest of stored groundnuts. J. Plant. Dis. Prot. 2005;112(6):586–593. doi: 10.1007/BF03356156. [DOI] [Google Scholar]
- 53.Prabakar K, Jebanesan A. Larvicidal efficacy of some Cucurbitacious plant leaf extracts against Culex quinquefasciatus (Say) Biores Technol. 2004;95(1):113–114. doi: 10.1016/j.biortech.2003.05.001. [DOI] [PubMed] [Google Scholar]
- 54.Kumar PM, Murugan K, Kovendan K, Subramaniam J, Amaresan D. Mosquito larvicidal and pupicidal efficacy of Solanum xanthocarpum (Family: Solanaceae) leaf extract and bacterial insecticide, Bacillus thuringiensis, against Culex quinquefasciatus Say (Diptera: Culicidae) Parasite Res. 2012;110(6):2541–2550. doi: 10.1007/s00436-011-2797-2. [DOI] [PubMed] [Google Scholar]
- 55.Gautam K, Kumar P, Poonia S. Larvicidal activity and GC-MS analysis of flavonoids of Vitex negundo and Andrographis paniculata against two vector mosquitoes Anopheles stephensi and Aedesaegypti. J. Vector Borne Dis. 2013;50:171–178. [PubMed] [Google Scholar]
- 56.Evans WT. Pharmacognosy: Baillence Tindall, Eastborne, London. 1992;1992:243–351. [Google Scholar]
- 57.Etebari K, Matindoost L. Effects of hypervitaminosis of vitamin B3 on silkworm biology. J. Biosc. 2004;29:417–422. doi: 10.1007/BF02712113. [DOI] [PubMed] [Google Scholar]
- 58.Poojary MM, Vishnumurthy KA, Adhikari AV. Extraction, characterization and biological studies of phytochemicals from Mammea suriga. J. Pharm. Anal. 2015;5:182–189. doi: 10.1016/j.jpha.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Alves APC, et al. Toxicity of the phenolic extract from jabuticabeira (Myrciaria cauliflora (Mart.) O. Berg) fruit skins on Spodoptera frugiperda. Chil. J. Agric. Res. 2014;74:200–204. doi: 10.4067/S0718-58392014000200011. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.