Abstract
Purpose
Stability of dental implants is an important factor for evaluation of osseointegration. The aim of this study was to evaluate the effect of combined use of low-level laser (LLL) and light-emitting diode (LED) therapy on the stability of dental implants during the healing phase.
Materials and Methods
This was a randomized clinical trial. Patients were assigned to two groups: In group 1, patients received LLL and LED 20 min/day for 10 days after implant insertion. Patients in group 2 (controls) did not undergo LLL and LED. The implant stability quotient (ISQ) was measured at 0 (time 0), 10 (time 1), 21 (time 2), 42 (time 3) and 63 days (time 4) after implant placement. Independent t test was used to compare the ISQs between the two groups.
Results
Fifty-eight patients were studied in two groups (n = 28). The mean ISQ did not differ immediately after insertion (P > 0.05). The mean ISQ differed significantly between the two groups on days 10, 21, 42 and 63 (P < 0.05). Results demonstrated an increase in the amount of ISQ in group 1 (intervention) at times 1, 2, 3 and 4. In the control group, the amount of ISQ decreased on days 10 and 21 following implant insertion, but increased afterward on days 42 and 63.
Conclusion
The results of this study showed that simultaneous use of LLL and LED increased the stability of the implants after 9 weeks of follow-up.
Keywords: Low-level laser therapy, Osseointegration, Lasers, Dental implants
Introduction
Healing time of dental implants after placement is an important factor for shortening of the treatment period. Several methods have been recommended to shorten the healing time of dental implants, namely use of surface treatments, bone morphogenetic proteins and platelet-rich plasma [1, 2]. Low-level laser (LLL) has been recommended to enhance the bone healing process [3]. Laser light has been used to improve wound healing, fibroblast proliferation and collagen synthesis [4–6]. Infrared wavelengths increase collagen deposition, osteoblastic proliferation and bone deposition compared to non-irradiated bone [7]. Light-emitting diode (LED) beam is a noninvasive and effective therapeutic method to stimulate wound healing in various tissues such as bone, skin and nerve tissue [8]. There are few studies that assessed the effect of LLL combined with LED on dental implant stability during the healing phase [9]. The aim of this study was to see if combined LLL and LED promotes stability of dental implants during the healing phase and provide possibility for early loading.
Materials and Methods
Authors designed a randomized clinical trial. The study sample was derived from the population of patients who presented to the Department of Oral and Maxillofacial Implants of…Dental School from January 1, 2013 through December 31, 2014. The research was approved by the medical ethics committee of….University of Medical Sciences (NCT03362307).
Patients eligible for inclusion had an edentulous area at the first molar site of the mandible and needed a dental implant. Patients were excluded from the study enrollment if they had any diseases that affect bone, were smokers, had insufficient bone in the edentulous area requiring bone augmentation, failure to return for follow-up or refused to enroll.
All implants were placed at least 3 months after tooth extraction. The size of the implants was 4.8 × 10 mm (Zimmer, USA).
Patients were divided into two groups based on computer randomization. In group 1 (intervention group), patients received LLL and LED after implant placement, and in group 2 (controls), the same device was used while it was turned off.
A portable device was used for laser irradiation in the study group, which irradiated 830 nm laser (λ 830 nm, 10 mW, ϕ ~ 0.0015 cm2) combined with 632-nm LED in four points around the implants. Patients underwent LLL (15 mw/cm2) and LED (10 mw/cm2) for 20 min every day for 10 days.
Implant Stability Measurements
The stability of the implants was evaluated with resonance frequency analysis (RFA). The measurements were made with the Osstell device (Osstell, Sweden) by connecting the transducer (SmartPeg) to the fixture. The mesiodistal and buccolingual directions were measured, and the mean implant stability quotient (ISQ) was determined. The RFA measurements were made immediately after insertion (time 0), and 10 days (time 1), 3 weeks (time 2), 6 weeks (time 3) and 9 weeks (time 4) after implant placement (Figs. 1, 2).
Fig. 1.
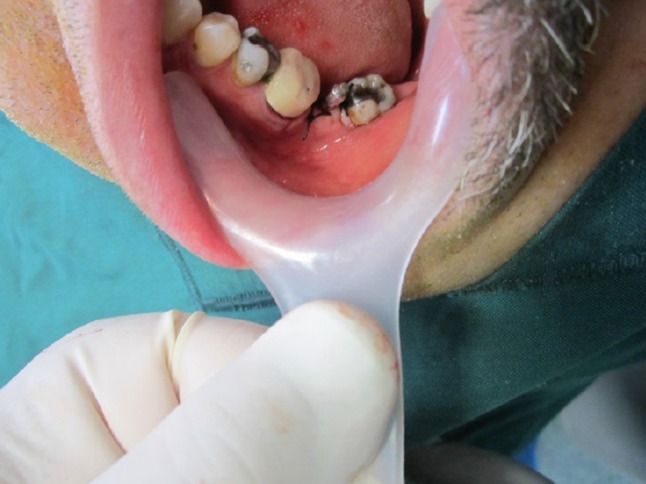
SmartPeg was placed after a fixture insertion
Fig. 2.
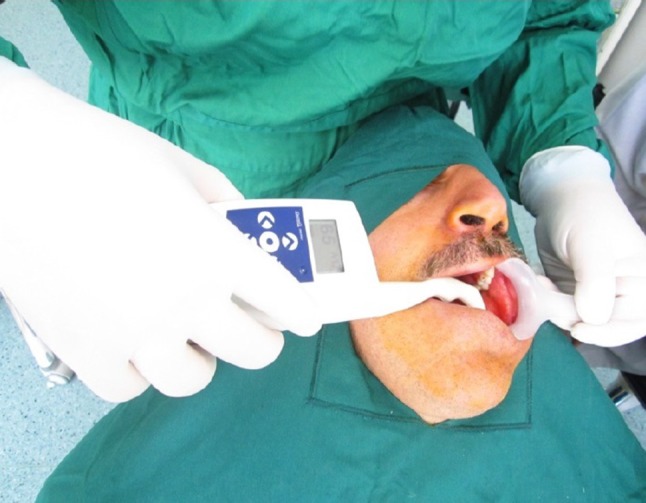
Osstell mentor was used to assess ISQ at different times
Age and gender were the variables of the study. Use of LLL with LED was the study predictive factor, and the amount of ISQ at different time points was the study outcome (Fig. 3).
Fig. 3.
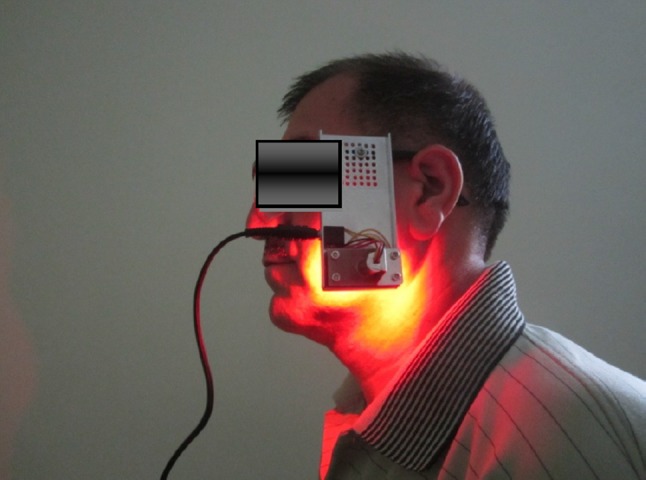
LLL and LED were applied 20 min/day for 10 days
Statistical Analysis
The statistical analyses were performed using SPSS version 19 (SPSS Inc., IL, USA). Repeated Measures ANOVA was used to compare ISQs at various measurement times. Independent t test was used to assess the mean age between the two groups. Chi-square test was applied for comparison of gender in the two groups.
Results
Fifty-eight patients were studied in two groups (28 patients in each group). The mean age of patients was 36.57 ± 11.08 years in group 1 and 39.34 ± 9.31 years in group 2. There was no significant difference between the two groups with regard to age (P = 0.31). Fourteen males and 15 females were in group 1 and 18 males, and 11 females were in group 2 (P = 0.42, Table 1).
Table 1.
Comparison of variables between the two groups
| Variables | Group 1 | Group 2 | P value |
|---|---|---|---|
| Age | 36.57 ± 11.08 | 39.34 ± 9.31 | P = 0.31* |
| Gender | 14 Males, 15 females | 18 Males, 11 females | P = 0.42** |
*Independent t test
**Chi-square test
The mean ISQ immediately after insertion was 67.26 ± 6.8 in group 1 and 68.00 ± 6.42 in group 2. There was no difference in ISQ immediately after insertion (P = 0.70). The mean ISQ at time 1 (10 days after implant insertion) was 68.24 ± 6.51 in group 1 and 64.71 ± 6.91 in group 2. There was a significant difference for ISQ at 10 days after implant insertion between the two groups (P = 0.02). The mean ISQ at time 2 (21 days after implant insertion) was 68.56 ± 6.55 in group 1 and 63.26 ± 6.21 in group 2. Analysis of the data demonstrated a significant difference between the two groups for ISQ at 3 weeks after insertion (P = 0.02). The mean ISQ at time 3 (6 weeks after implant insertion) was 70.52 ± 6.14 and 64.92 ± 6.34 in groups 1 and 2, respectively. A significant difference was seen between the two groups for ISQ at 6 weeks after insertion (P = 0.01). The mean ISQ was 71.18 ± 5.75 in group 1 at time 4 (9 weeks after insertion) and 66.50 ± 6.73 in group 2. There was a significant difference between the two groups for ISQ at time 4 (P = 0.01, Table 2).
Table 2.
Comparison of ISQ between groups 1 and 2 in various assessment times
| Time | Group 1 | Group 2 | Independent t test |
|---|---|---|---|
| Time 0 | 67.26 ± 6.8 | 68.00 ± 6.42 | P = 0.70 |
| Time 1 | 68.24 ± 6.51 | 64.71 ± 6.91 | P = 0.02 |
| Time 2 | 68.56 ± 6.55 | 63.26 ± 6.21 | P = 0.02 |
| Time 3 | 70.52 ± 6.14 | 64.92 ± 6.34 | P = 0.01 |
| Time 4 | 71.18 ± 5.75 | 66.50 ± 6.73 | P = 0.01 |
The results demonstrated an increase in the amount of ISQ in group 1 at times 1, 2, 3 and 4. In the control group, the amount of ISQ decreased at 10 days and 21 days after implant insertion and then increased at days 42 and 63.
Discussion
Application of LLL varies in different medical fields, and it is used to control pain, edema and inflammation and to enhance wound and nerve tissue healing. There are also recommendations for using LLL therapy for bone healing as in fractures and osseointegration of dental implants [3, 10–12]. LLL therapy modulates cell proliferation and migration through its effects on ATP production, reactive oxygen species and transcription factors. This process leads to higher potential for bone regeneration and callus formation [3, 10]. According to a recent systematic review, LLL therapy can enhance bone density and promote anti-inflammatory and analgesic effects in maxillofacial bony defects [13]. There are several studies reporting enhancement of bone formation following the application of LLL. Kocyigit et al. [14] reported higher bone mineral density of the newly formed bone through distraction osteogenesis by LLL application. Valiati et al. [15] reported successful application of LLL in a block allograft for bone defect regeneration.
Wound healing contains three phases: first, inflammatory phase, second, cells proliferation and third, tissue remodeling. It was showed that laser biostimulation affects primarily cell proliferation phase of the wound healing process [16]. Mitochondria are sensitive to monochromatic near-infrared light and that laser light likely enhances respiratory metabolism of certain cells [17, 18]. Laser light treatment affects processes such as fibroblast proliferation, synthesis of procollagen and collagen and, growth factor production (including transforming growth factor [TGF], keratinocyte growth factor [KGF], and platelet-derived growth factor [PDGF]), macrophage stimulation, lymphocyte stimulation [19] and greater rate of extracellular matrix production [20].
Since the quality of osseointegration and the shortest possible loading time after dental implant insertion are common concerns in dental implantology, many modalities such as different surface treatments (e.g., surface roughness, osteoconductive coatings, etc.) and surgical procedures have been used to achieve these goals [2]. Obradović et al. [21] in a systematic review suggested using LLL therapy as an auxiliary bone osseointegration stimulating factor in dental implants when factors adversely affecting osseointegration were present.
In this study, LLL was used for 10 days. Previous studies applied LLL between 7 and 15 days [9, 22]. There are controversies regarding the positive effect of LLL therapy on dental implant stability, especially in human and animal studies. In a double-blind randomized clinical trial by García-Morales et al. [23], LLL therapy every 48 h for 14 days had no significant macroscopic impact on dental implant stability in the posterior mandible. Mandić et al. conducted another study regarding the effect of LLL therapy on low-density bone in the posterior maxilla.
In their study, daily use of LLL during the first postoperative week had no significant effect on osseointegration [24]. In animal models, Mayer at el. reported higher peri-implant bone formation and higher osseointegration following the use of LLL therapy [25]. In another in vivo study, application of LLL significantly enhanced osseointegration of implants with initially poor stability [26]. Gomes et al. [27] also reported higher peri-implant bone repair and stability in animal models by using LLL therapy. Despite the paucity of human studies regarding the effect of LLL application on dental implant stability and osseointegration, a systematic review by Prados-Frutos et al. [12] suggested improvement of osseointegration according to experimental results.
Another study by photobiomodulation suggested enhancement of dental implant stability using LED. Similar to LLL, the exact biological effects of LED are still unclear. But, the possible mechanisms are similar to those of LLL [28]. In vitro studies suggest that LED light could enhance osteogenic differentiation of mesenchymal stem cells in an osteogenic medium [29]. Gokmenoglu et al. reported a positive effect on osseointegration and stability of dental implants using LED stimulation. In the treatment group, LED was applied for 20 min three times a week for 3 weeks, and the stability was maintained for 12 weeks postoperatively. However, in the control group, a significant reduction in stability occurred from 2 to 12 weeks postoperatively [30].
Our study aimed to evaluate the effect of both LLL and LED lights on dental implant stability. Our study results revealed positive effect of combined application of LLL and LED on stability of dental implants. In treatment group, the mean of ISQ was 70.52 ± 6.14 in 6 weeks and 71.18 ± 5.75 in 9 weeks. According to the literature, a minimal ISQ 60–65 is needed to early loading. It seems using LLL and LED provides loading condition in 6–9 weeks [31]. Further studies should be performed to confirm the effectiveness of photobiomodulation for improving dental implant stability following LLL and LED application.
Conclusion
The results of this study showed that simultaneous use of LLL and LED resulted in increased stability of implants after 9 weeks of follow-up.
Funding
The funding was provided by Shahid Beheshti University of Medical Sciences (1391-3456).
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Weibrich G, Hansen T, Kleis W, Buch R, Hitzler W. Effect of platelet concentration in platelet-rich plasma on peri-implant bone regeneration. Bone. 2004;34(4):665–671. doi: 10.1016/j.bone.2003.12.010. [DOI] [PubMed] [Google Scholar]
- 2.Le Guéhennec L, Soueidan A, Layrolle P, Amouriq Y. Surface treatments of titanium dental implants for rapid osseointegration. Dent Mater. 2007;23(7):844–854. doi: 10.1016/j.dental.2006.06.025. [DOI] [PubMed] [Google Scholar]
- 3.Kazem Shakouri S, Soleimanpour J, Salekzamani Y, Oskuie MR. Effect of low-level laser therapy on the fracture healing process. Lasers Med Sci. 2010;25(1):73–77. doi: 10.1007/s10103-009-0670-7. [DOI] [PubMed] [Google Scholar]
- 4.Forney R, Mauro T. Using lasers in diabetic wound healing. Diabetes Technol Ther. 1999;1(2):189–192. doi: 10.1089/152091599317404. [DOI] [PubMed] [Google Scholar]
- 5.Pourzarandian A, Watanabe H, Ruwanpura SM, Aoki A, Ishikawa I. Effect of low-level Er:YAG laser irradiation on cultured human gingival fibroblasts. J Periodontol. 2005;76(2):187–193. doi: 10.1902/jop.2005.76.2.187. [DOI] [PubMed] [Google Scholar]
- 6.Sculean A, Schwarz F, Berakdar M, Windisch P, Arweiler NB, Romanos GE. Healing of intrabony defects following surgical treatment with or without an Er: YAG laser. J Clin Periodontol. 2004;31(8):604–608. doi: 10.1111/j.1600-051X.2004.00525.x. [DOI] [PubMed] [Google Scholar]
- 7.da Silva RV, Camilli JA. Repair of bone defects treated with autogenous bone graft and low-power laser. J Craniofac Surg. 2006;17(2):297–301. doi: 10.1097/00001665-200603000-00017. [DOI] [PubMed] [Google Scholar]
- 8.Mohajerani SH, Tabeie F, Bemanali M, Tabrizi R. Effect of low-level laser and light-emitting diode on inferior alveolar nerve recovery after sagittal split osteotomy of the mandible: a randomized clinical trial study. J Craniofac Surg. 2017;28(4):e408–e411. doi: 10.1097/SCS.0000000000002929. [DOI] [PubMed] [Google Scholar]
- 9.Lopes CB, Pinheiro AL, Sathaiah S, Duarte J, Cristinamartins M. Infrared laser light reduces loading time of dental implants: a Raman spectroscopic study. Photomed Laser Surg. 2005;23(1):27–31. doi: 10.1089/pho.2005.23.27. [DOI] [PubMed] [Google Scholar]
- 10.Farivar S, Malekshahabi T, Shiari R. Biological effects of low level laser therapy. J Lasers Med Sci. 2014;5(2):58–62. [PMC free article] [PubMed] [Google Scholar]
- 11.Andrade Fdo S, Clark RM, Ferreira ML. Effects of low-level laser therapy on wound healing. Revista do Colegio Brasileiro de Cirurgioes. 2014;41(2):129–133. doi: 10.1590/S0100-69912014000200010. [DOI] [PubMed] [Google Scholar]
- 12.Prados-Frutos JC, Rodriguez-Molinero J, Prados-Privado M, Torres JH, Rojo R. Lack of clinical evidence on low-level laser therapy (LLLT) on dental titanium implant: a systematic review. Lasers Med Sci. 2016;31(2):383–392. doi: 10.1007/s10103-015-1860-0. [DOI] [PubMed] [Google Scholar]
- 13.Santinoni CD, Oliveira HF, Batista VE, Lemos CA, Verri FR. Influence of low-level laser therapy on the healing of human bone maxillofacial defects: a systematic review. J Photochem Photobiol B. 2017;169:83–89. doi: 10.1016/j.jphotobiol.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Kocyigit ID, Coskunses FM, Pala E, Tugcu F, Onder E, Mocan A. A comparison of the low-level laser versus low intensity pulsed ultrasound on new bone formed through distraction osteogenesis. Photomed Laser Surg. 2012;30(8):438–443. doi: 10.1089/pho.2012.3263. [DOI] [PubMed] [Google Scholar]
- 15.Valiati R, Paes JV, de Moraes AN, Gava A, Agostini M, Masiero AV, et al. Effect of low-level laser therapy on incorporation of block allografts. Int J Med Sci. 2012;9(10):853. doi: 10.7150/ijms.4440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Whelan HT, Smits RL, Jr, Buchman EV, Whelan NT, Turner SG, Margolis DA, et al. Effect of NASA light-emitting diode irradiation on wound healing. J Clin Laser Med Surg. 2001;19(6):305–314. doi: 10.1089/104454701753342758. [DOI] [PubMed] [Google Scholar]
- 17.Tamura M. Non-invasive monitoring of the redox state of cytochrome oxidase in living tissue using near-infrared laser lights. Jpn Circ J. 1993;57(8):817–824. doi: 10.1253/jcj.57.817. [DOI] [PubMed] [Google Scholar]
- 18.Beauvoit B, Evans SM, Jenkins TW, Miller EE, Chance B. Correlation between the light scattering and the mitochondrial content of normal tissues and transplantable rodent tumors. Anal Biochem. 1995;226(1):167–174. doi: 10.1006/abio.1995.1205. [DOI] [PubMed] [Google Scholar]
- 19.Mester A, Nagylucskay S, Mako E, Hoffmann G, Serenyi M. Experimental imunological study with radiological application of low power laser. Laser in der Medizin Laser in Medicine. Berlin: Springer; 1998. pp. 509–512. [Google Scholar]
- 20.Sommer AP, Pinheiro AL, Mester AR, Franke R-P, Whelan HT. Biostimulatory windows in low-intensity laser activation: lasers, scanners, and NASA’s light-emitting diode array system. J Clin Laser Med Surg. 2001;19(1):29–33. doi: 10.1089/104454701750066910. [DOI] [PubMed] [Google Scholar]
- 21.Obradovic RR, Kesic LG, Pesevska S. Influence of low-level laser therapy on biomaterial osseointegration: a mini-review. Lasers Med Sci. 2009;24(3):447–451. doi: 10.1007/s10103-008-0573-z. [DOI] [PubMed] [Google Scholar]
- 22.Cotler HB, Chow RT, Hamblin MR, Carroll J. The use of low level laser therapy (LLLT) for musculoskeletal pain. MOJ Orthop Rheumatol. 2015;2(5):1–16. doi: 10.15406/mojor.2015.02.00068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Garcia-Morales JM, Tortamano-Neto P, Todescan FF, de Andrade JC, Jr, Marotti J, Zezell DM. Stability of dental implants after irradiation with an 830-nm low-level laser: a double-blind randomized clinical study. Lasers Med Sci. 2012;27(4):703–711. doi: 10.1007/s10103-011-0948-4. [DOI] [PubMed] [Google Scholar]
- 24.Mandic B, Lazic Z, Markovic A, Mandic B, Mandic M, Djinic A, et al. Influence of postoperative low-level laser therapy on the osseointegration of self-tapping implants in the posterior maxilla: a 6-week split-mouth clinical study. Vojnosanit Pregl. 2015;72(3):233–240. doi: 10.2298/VSP131202075M. [DOI] [PubMed] [Google Scholar]
- 25.Mayer L, Gomes FV, de Oliveira MG, de Moraes JF, Carlsson L. Peri-implant osseointegration after low-level laser therapy: micro-computed tomography and resonance frequency analysis in an animal model. Lasers Med Sci. 2016;31(9):1789–1795. doi: 10.1007/s10103-016-2051-3. [DOI] [PubMed] [Google Scholar]
- 26.Campanha BP, Gallina C, Geremia T, Loro RC, Valiati R, Hubler R, et al. Low-level laser therapy for implants without initial stability. Photomed Laser Surg. 2010;28(3):365–369. doi: 10.1089/pho.2008.2429. [DOI] [PubMed] [Google Scholar]
- 27.Gomes FV, Mayer L, Massotti FP, Baraldi CE, Ponzoni D, Webber JB, et al. Low-level laser therapy improves peri-implant bone formation: resonance frequency, electron microscopy, and stereology findings in a rabbit model. Int J Oral Maxillofac Surg. 2015;44(2):245–251. doi: 10.1016/j.ijom.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 28.Eells JT, Wong-Riley MT, VerHoeve J, Henry M, Buchman EV, Kane MP, et al. Mitochondrial signal transduction in accelerated wound and retinal healing by near-infrared light therapy. Mitochondrion. 2004;4(5–6):559–567. doi: 10.1016/j.mito.2004.07.033. [DOI] [PubMed] [Google Scholar]
- 29.Kim HK, Kim JH, Abbas AA, Kim DO, Park SJ, Chung JY, et al. Red light of 647 nm enhances osteogenic differentiation in mesenchymal stem cells. Lasers Med Sci. 2009;24(2):214–222. doi: 10.1007/s10103-008-0550-6. [DOI] [PubMed] [Google Scholar]
- 30.Gokmenoglu C, Ozmeric N, Erguder I, Elgun S. The effect of light-emitting diode photobiomodulation on implant stability and biochemical markers in peri-implant crevicular fluid. Photomed Laser Surg. 2014;32(3):138–145. doi: 10.1089/pho.2012.3473. [DOI] [PubMed] [Google Scholar]
- 31.Gallucci GO, Benic GI, Eckert SE, Papaspyridakos P, Schimmel M, Schrott A, et al. Consensus statements and clinical recommendations for implant loading protocols. Int J Oral Maxillofac Implants. 2014;29(Suppl):287–290. doi: 10.11607/jomi.2013.g4. [DOI] [PubMed] [Google Scholar]


