Abstract
Introduction
New technological advances have revolutionized the field of oral and maxillofacial surgery. The three-dimensional (3D) models are new technological breakthroughs which have equipped the oral and maxillofacial surgeon to effectively reproduce or improve preoperative form and function. They have also allowed the surgeon to achieve minimal operative and postoperative morbidity.
Materials and methods
In this case series, we present the clinical application and benefits of 3D models that are used for the surgical planning and execution of surgery in treating a case of mid-face deficiency and for the treatment of extensive jaw pathologies using reconstruction plate bent preoperatively, which contributed to improved surgical outcomes and patient satisfaction.
Keywords: 3D models, Reconstruction plate, Bent preoperatively
Introduction
The primary objective of any surgical procedure is the restitution of preoperative form and function and enhancement of dysmorphology, disease and the deformity caused during surgery [1, 2].
Surgeons have always relied on their training, and coupled with their invaluable education, radiographic aids are employed in the planning of surgical processes. Often, due to the anatomical complexity of the operative site, two-dimensional or virtual images have not been sufficient to successfully convey the structural details. To address the limitations in virtual image analysis, 3D printing is now being used to create physical models of patient anatomy for surgical simulation and personalized preoperative planning [3]. The 3D models have enabled the oral and maxillofacial surgeon to successfully accomplish the goal of surgeries with ideal outcomes. These models are being used very effectively for multiple indications and in diverse clinical scenarios.
3D model is an unparalleled adjunct to traditional methods of planning reconstructive surgeries following resection of tumors, developmental abnormalities, complex temporomandibular joint reconstruction or debilitating trauma [2, 4, 5]. It utilizes computer technology and medical image processing techniques to generate a complex physical 3D model from computed tomography (CT) scans out of acrylic resin [2].
Reconstruction poses a challenge for the maxillofacial surgeon for a number of reasons, such as the complicated geometry of the mandible, muscles attached to the mandible which act in different directions, form and position of condyles in the glenoid fossa, and lastly, the occlusion, the importance of which cannot be overstated [3].
In this case series, we present three cases of mandibular reconstruction using a titanium reconstruction plate which was bent before the surgery, and one case of midface deficiency addressed using 3D models. It discusses how these 3D models were helpful in surgical planning before and during surgery and its impact on treatment feasibility and treatment outcome.
Case: Mandibular Tumor Surgery
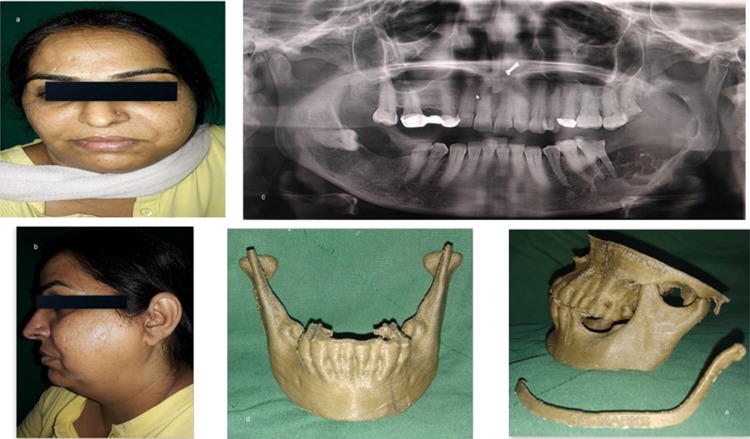
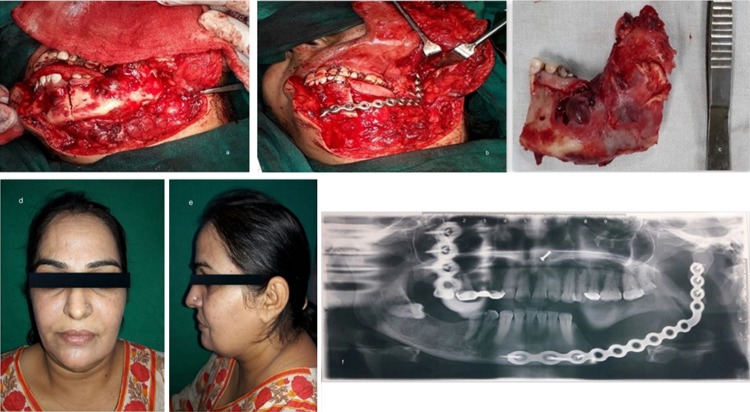
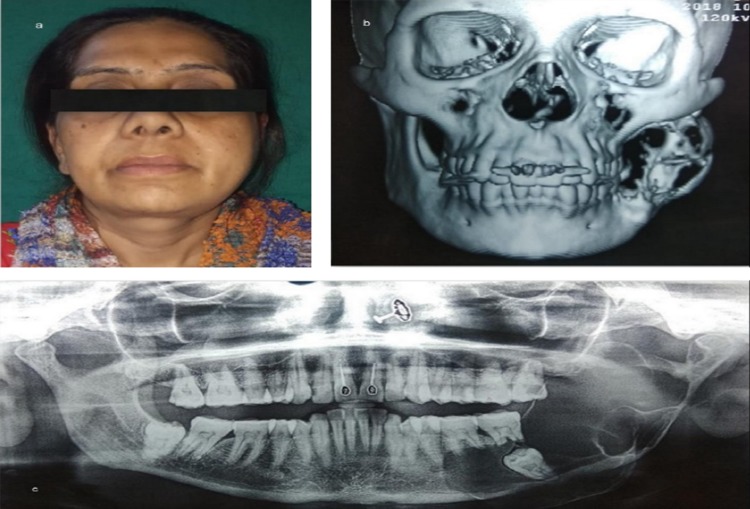
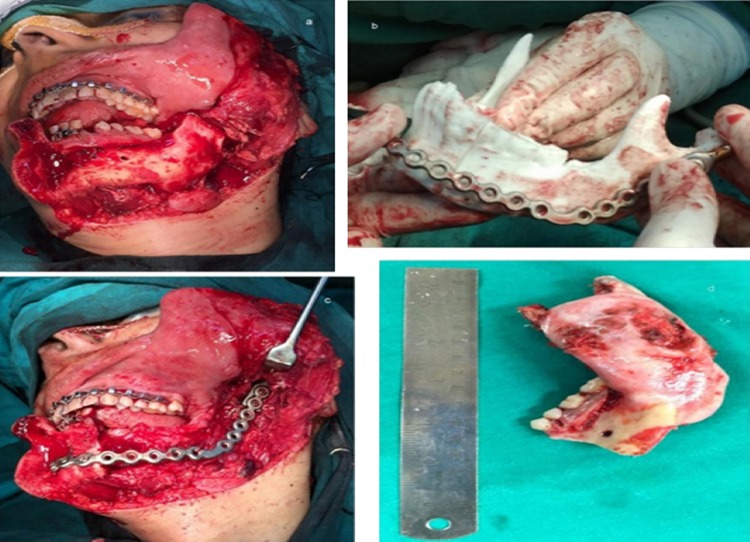
The first case was a 44-year-old lady with a large expansile lesion of the left mandible. Panoramic examination showed a multilocular radiolucent lesion located at the left mandible extending from the premolar, through the angle-ramus region and up the sigmoid notch (Fig. 1a–c). Incisional biopsy proved this to be an ameloblastoma. A CT scan was done which was used to construct the 3D model. The mirror image of the normal side of the mandible was reproduced onto the 3D model to mimic the premorbid anatomy of the left side (Fig. 1d, e). The resection margins were tentatively marked based on the radiographic and clinical judgment to be distal to the left second bicuspid with the plan to preserve the native condyle. The mandibular reconstruction plate was bent preoperatively to span the planned resection using the 3D model as a reference. Recording finer details such as screw placement and even their respective lengths were made possible due to the 3D model [6]. The resection was completed using a combined transoral and transcutaneous approach. Minimal adjustments were made intraoperatively on the plate because only minor corrections were incorporated into the tentative resection margins planned (Fig. 2a–c). This significantly reduced the operative time. Surgical sites were sutured in layers. The 3D model made it possible to design, obtain and adapt stock hardware into custom hardware meeting the unique anatomical and functional variations of the patient (Fig. 2d–f).
Fig. 1.
a, b Preoperative frontal view and left facial profile showing asymmetry and swelling of face in the left posterior region of mandible; c OPG showing multilocular radiolucent lesion associated with an impacted third molar extending from the left body region up to the sigmoid notch involving the lower border of mandible and anterior border of ramus; d, e 3D model obtained to facilitate plate adaptation prior to resection
Fig. 2.
a Intraoperative image showing the extent of the lesion; b custom prebent plate in position after resection; c specimen obtained after resection; d, e postoperative improved facial form; f postoperative panoramic radiograph showing reconstruction plate in place
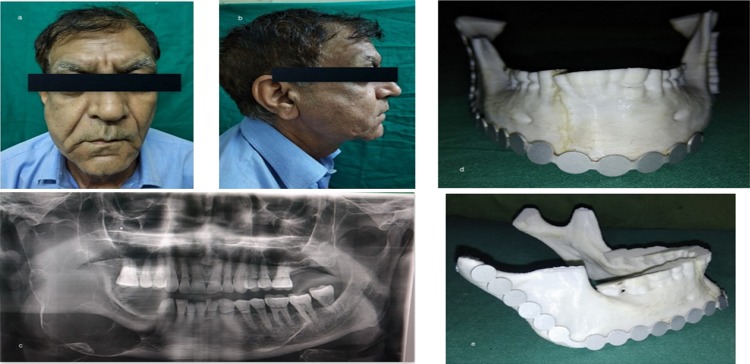
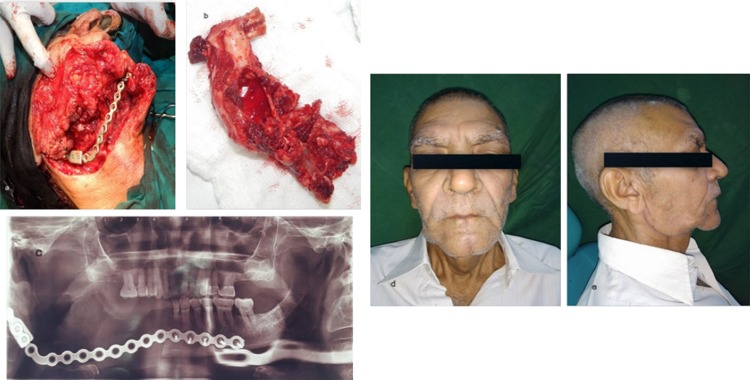
The second case was a 66-year-old male with a history of a month’s old expansile lesion of the right posterior mandible with associated paresthesia of the right lower lip. He was a diagnosed case of ameloblastoma which was enucleated few years back. The patient noticed a significant facial asymmetry recently, with deviation of the face toward the right side. Panoramic radiographic examination revealed an extensive radiolucent lesion of the mandible extending from the right mandibular canine up to the condyle through the posterior border of mandible (Fig. 3a–c). A high-resolution CT scan was obtained followed by the construction of the 3D model (Fig. 3d, e). The resection margins were provisionally defined to be distal to the left central incisor with disarticulation of the right mandibular condyle. A reconstruction plate was bent preoperatively with condylar prosthesis on the 3D model and was sterilized. The resection was completed as planned via combined extended submandibular and transoral approaches (Fig. 4a, b). Intraoperatively, the plate needed only minor modifications which significantly reduced the duration of the anesthesia. In the present scenario, the 3D-printed model helped in adapting the implant to exactly match the contour of the normal contra-lateral side to achieve satisfactory esthetic and functional results postoperatively (Fig. 4c–e).
Fig. 3.
a, b Preoperative frontal view and right facial profile showing asymmetry and swelling of face in the left posterior region of mandible; c OPG showing multilocular radiolucent lesion extending from the distal aspect of right first premolar up to the sigmoid notch involving the condyle, coronoid, anterior, posterior border of ramus and lower border of mandible; d, e 3D model obtained to facilitate plate adaptation prior to resection
Fig. 4.
a Custom prebent plate in position after resection; b specimen obtained after resection; c postoperative panoramic radiograph showing reconstruction plate in place; d, e postoperative improved facial form
The third case was a 42-year-old lady referred for evaluation of an expanding lesion involving the left body and angle of the mandible (Fig. 5a). OPG revealed a multilocular radiolucent lesion involving the left side of mandible extending from the second premolar, reaching up to the condyle. The lesion approximated the inferior border of the mandible and showed cortical expansion. Biopsy of this lesion yielded the diagnosis of ameloblastoma. A high-resolution CT scan was obtained for construction of a 3D model (Fig. 5b, c). A reconstruction plate was snugly adapted to the surface of the model. Combined transoral and transcutaneous approach was used. The resection was performed as planned from the left mandibular condyle to the first premolar region (Fig. 6a, b). Following hemimandibulectomy and unhinging of the condyle on the respective side, the reconstruction plate with the condylar prosthesis was swapped with the resected mandible. Only minute modifications to the custom bent plate were necessary (Fig. 6c, d).
Fig. 5.
a Preoperative frontal view showing asymmetry and swelling of face in the left posterior region of mandible; b 3D CT scan showing a large multilocular expansile lesion on the left mandible; c OPG showing multilocular radiolucent lesion associated with an impacted third molar extending from the left body region up to the sigmoid notch involving the lower border of mandible
Fig. 6.
a Intraoperative image showing the extent of the lesion; b preoperatively custom bent reconstruction plate being checked intraoperatively for minute adjustments on the 3D model before resection; c custom prebent plate in position after resection; d specimen obtained after resection
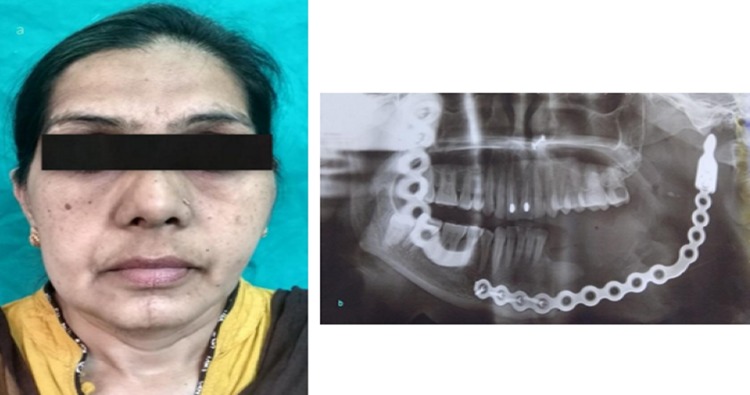
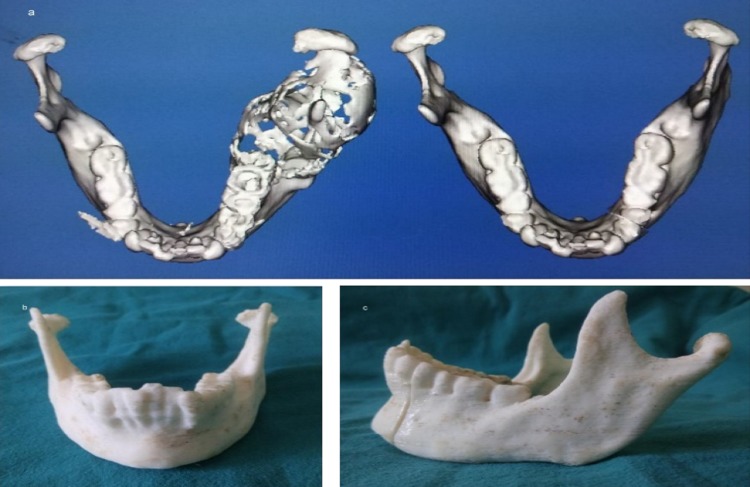
In all these three cases, the decision was made to create a 3D model of the mandible for three reasons: primarily, due to the destruction of the native mandible with the loss of normal anatomic contour, secondarily, to assist in planning of reconstruction and lastly, to meet the high esthetic concerns of the patients. Prebent reconstruction plate showed improved mandibular contour compared to other patients undergoing conventional mandibular reconstruction [7] (Fig. 7a, b). Application of this technology resulted in the maintenance of proper esthetics and symmetry of the face and the achievement of good functional result and enhanced quality of life. These models were outsourced for the purpose of fabrication. The mirror image of the contra-lateral side was superimposed onto to the diseased site, so that the end result of the 3D model is close to the pre-deformed form of the mandible [8, 9] (Fig. 8a–c). The use of the 3D model in these cases therefore helped to determine the required length of plate with the number and dimension of screws which are to be used intraoperatively before resection and also gave an accurately fitted and contoured reconstruction plate ready for placement. The nonsurgical aspect of the patient’s treatment could be performed preoperatively which allowed for acute precision which is often not achievable during the operative procedure [10, 11]. Plate handling was minimal in the operating theater, thus preserving its strength, reducing metal fatigue and risk of premature plate fracture [12–14]. A noteworthy advantage which cannot be overlooked is the remarkable reduction in operating time due to the bending of the reconstruction plate preoperatively [11, 13].
Fig. 7.
a Postoperative improved facial form; b postoperative panoramic radiograph showing reconstruction plate in place
Fig. 8.
a Mirror image of the contra-lateral side superimposed onto to the diseased site; b, c 3D model obtained to facilitate plate adaptation prior to resection
Case: Midface Deficiency
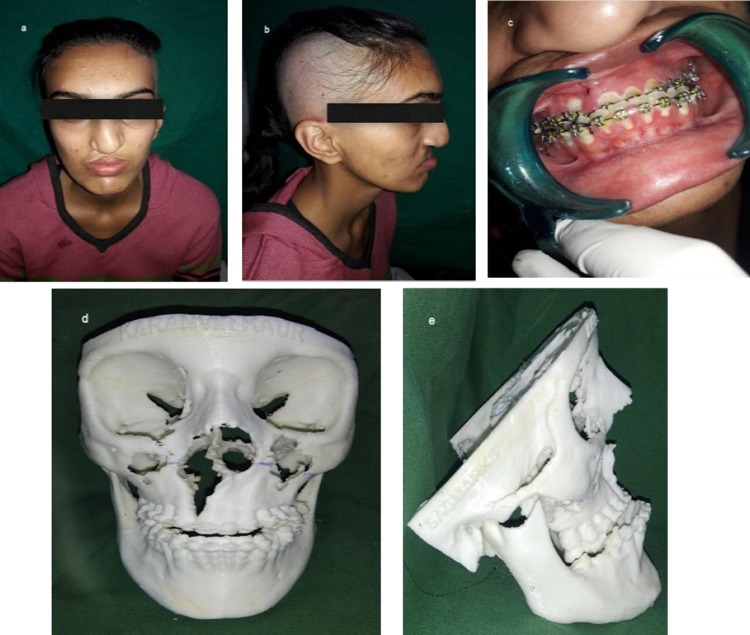
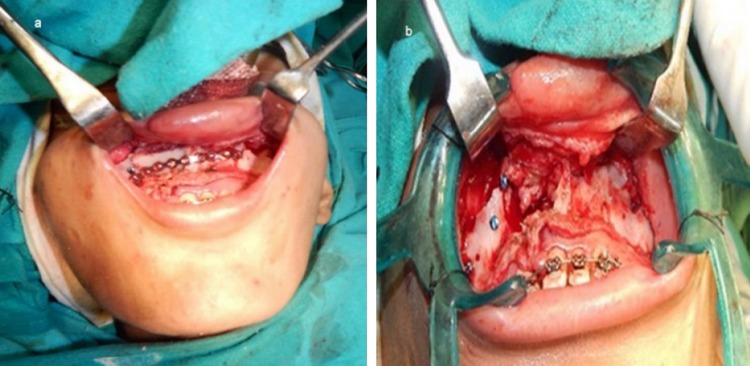
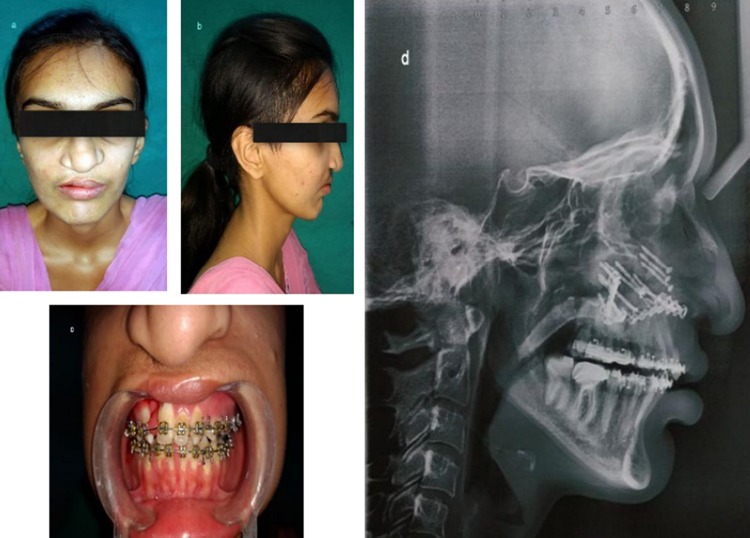
A 20-year-old lady presented with chief complaints of poor facial appearance and nasal blockage. She had undergone cheiloplasty and palatoplasty under general anesthesia 18 years ago, to correct her cleft lip and palate. She was on fixed orthodontic treatment since 4 years. Extra-oral examination revealed a concave facial profile with anterior facial divergence and deviation of nasal septum to the right side (Fig. 9a, b). No facial asymmetry was noted. The upper and middle facial thirds were 52 mm as opposed to the lower facial third which measured out to be 62 mm. Intra-oral examination revealed a class III occlusion and reverse overjet of ~ 10 mm and palatal scar of palatoplasty (Fig. 9c). Le-fort I osteotomy with maxillary advancement and iliac crest grafting under general anesthesia was done as the treatment. The surgeons had planned for rapid extra-oral distraction, but during surgery since the maxilla could be easily mobilized in a forward direction the plan was shifted from distracting the maxilla to plating the le-fort I osteotomy fragments. A 14-hole 1.5-mm miniplate was pre-bent on the 3D model which was secured to stabilize the maxilla which was in two segments. In this case, the 3D model gave a detailed presentation of the craniofacial complex which included the ability to know the extent of orthognathic movements possible with greater precision than with traditional model surgery prediction techniques [15] (Fig. 9d, e). It helped in enhanced analysis of surgical planning. The decision was made to stabilize the two piece maxilla due to the cleft, into one unit, and the plate was pre-adapted on the model which helped in saving intraoperative time (Fig. 10a). The 3D models resulted in enhanced collaboration between the orthodontist and the surgeon and also served as an excellent patient education tool. It also helped in anticipating the size and form of bony defect for grafting, thus minimizing the amount of graft material harvested and donor site morbidity [13, 15] (Fig. 10b). In addition to enabling the surgeon in planning the minute details of the procedure, the 3D model helped the surgeon and the patient to envisage the improved postoperative results (Fig. 11a–d).
Fig. 9.
a–c Preoperative frontal view, facial profile and occlusion; d, e 3D model showing midface deficiency and concave facial profile
Fig. 10.
a 14-hole miniplate custom bent preoperatively on 3D model secured to stabilize the maxilla; b bony defect created after le-fort 1 osteotomy which was grafted with iliac crest bone graft
Fig. 11.
a–c Postoperative improved frontal view, facial profile and occlusion; d postoperative lateral cephalogram showing the maxillary advancement and 14-hole custom bent miniplate and bone grafts secured with lag screws
Discussion
The craniofacial region is complex, with interrelationship between the topographic compartments of various anatomic sites. The treatments using 3D models offer a significantly higher accuracy in predicting clinical outcomes as opposed to similar treatments which have excluded 3D models. The use of 3D models has remarkably improved reconstruction of form and function, decreased operating time and facilitated the development and application of more precise and accurate surgical procedures [4, 8, 13].
Charles Hull is credited for inventing 3D models in 1986 [4]. 3D models have been used in oral and maxillofacial surgery to create models for diagnosis, treatment planning and surgical simulation. This technique was first used in oral and maxillofacial surgery by Brix and Lambrecht in 1987 [16]. Computer technology and medical image processing techniques are used to generate a complex plastic 3D model from CT scans [2].
In our center, these models were outsourced from Sagmarks private limited located in Ghaziabad, Uttar Pradesh. They use Dolphin software to fabricate these 3D models, and the material used is polylactic acid, acrylonitrile butadiene styrene or acrylic resin. These models can be sterilized by ethylene oxide or can be kept in formalin chamber.
3D models assist in various scenarios such as [4–8, 11–13, 17–22]:
Diagnosis and treatment planning.
Direct visualization of anatomic structures.
Formulating surgical guides/templates.
Adaptation of metallic implants.
Determining the dimensions of the required implant plates and screws prior to surgery.
Strategize osteotomies and designing incisions through surgical rehearsals.
Measurement of the displacement of all bone segments.
Precise anticipation of the size and the form of bone grafts.
Fabricating custom prostheses and distraction devices.
Reducing surgery and anesthesia time and wound exposure duration.
Amplifies the predictability of results.
Improves inter-personal communications and serves as an educational tool for patients.
Preoperative informed consent.
Chow and Cheung [23] conducted a study to assess the usefulness of 3D models on different subjects by comparing their applications in different treatment categories. As per their study, the surgeons found the models to be particularly useful for simulating surgeries, prosthesis fabrication, formulating intraoperative guide for reconstructive surgery. Furthermore, such models were potentially used as a teaching tool for medical students and for enhancing communication with patients. The postgraduate students found that the stereo-models improved their understanding of the disease from three dimensions, thereby facilitating treatment planning. The patients viewed stereo-models as a necessary tool for successful treatment of their conditions. Being satisfied with their surgical results, they would highly recommend stereo-models to other patients, particularly to those undergoing reconstructive surgery.
Traditional treatment planning for orthognathic surgery depends on clinical examination, two-dimensional (2D) cephalometric analysis and the study of plaster dental models. The development of 3D-printed models in orthognathic surgery has improved surgical planning, and the transition of the surgical plan into the operating room was smooth for a better surgical result with improved intraoperative orientation and bending of miniplates [24].
These synthetic models are a boon to the postgraduate student. It is one approach to aid in teaching and competent demonstration of invasive oral surgical procedures to the residents in surgery. It represents an anatomically accurate model which might be seen as a cost-effective, standardized alternative for teaching hard tissue oral surgical procedures [25].
Furthermore, extensive research is being conducted on the usage of generated models made from materials that are biocompatible and possess osteoconduction and induction characteristics. Successful application of this technology for jaw bone regeneration based on tissue engineering techniques offers exciting new prospects for the future [2, 26].
At variance to the mentioned advantages, the 3D models have a certain number of drawbacks. Of the drawbacks, the most inconvenient is the time interval involved in the process of fabrication of these models [2, 17, 20]. Due to this time lag, the 3D models cannot be used in the restitution of the extensive facial defect fractures [18]. Another feature which makes these models unsuitable to the average patient is the cost involved [2, 7, 10, 17, 20, 21]. Patients with either extensive tumors of the jaw or mutilated trauma will often have multidisciplinary involvement, a prolonged stay in the hospital post-surgery and various procedures for rehabilitation. In light of these issues, the nominal patient will more often than not find it taxing on their monetary reserves.
Conclusion
Based on our clinical experience, we found that the models were appropriate for every reconstructive and orthognathic case. The use of 3D models has contributed immensely to improved reconstruction of form and function and thereby rehabilitating the patient and meeting their esthetic demands. Due to the models, the surgeons were capable of planning the minute nitty-gritties of the surgery ahead in time and thereby reducing the duration and mental fatigue of the surgeon in the theater.
The models also assisted the postgraduate students in forming a mental picture of the 3D anatomy of the defect/deformity, and moreover, the technique of bending the reconstruction plate in various planes is better visualized. It was understood that all the patients were not ideal for 3D models due to the cost and time lag involved.
Finally, 3D models can be considered to be educational tools for novice surgeons. The success of any surgery hinges on the surgeon’s previous training and past experiences. Despite many advantages of this 3D model, it cannot replace a surgeon’s clinical judgment and skill. The 3D models prove to be a valuable adjunct for enhancing the efficiency, accuracy and creativity by decreasing the mental fatigue of a surgeon.
Compliance with Ethical Standards
Conflict of interest
All authors declare that they have no conflict of interest.
Informed Consent
Informed consent was obtained from all individual participants included in the study. Additional informed consent was obtained from all individual participants for whom identifying information is included in this article.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Nancy Mathew, Email: drnancyomfs@gmail.com.
Sumir Gandhi, Email: sumirgandhi@gmail.com.
Inderjot Singh, Email: drinderjotsingh@yahoo.co.in.
Manisha Solanki, Email: drmanisha80@gmail.com.
Navpreet Singh Bedi, Email: osnavpreetbedi@gmail.com.
References
- 1.Steinbacher DM. Three-dimensional analysis and surgical planning in craniomaxillofacial surgery. J Oral Maxillofac Surg. 2015;73(12):S40–S56. doi: 10.1016/j.joms.2015.04.038. [DOI] [PubMed] [Google Scholar]
- 2.Cunningham LL, Madsen MJ, Peterson G. Stereolithographic modeling technology applied to tumor resection. J Oral Maxillofac Surg. 2005;63(6):873–878. doi: 10.1016/j.joms.2005.02.027. [DOI] [PubMed] [Google Scholar]
- 3.Ganguli A, Pagan-Diaz GJ, Grant L, Cvetkovic C, Bramlet M, Vozenilek J, Kesavadas T, Bashir R. 3D printing for preoperative planning and surgical training: a review. Biomed Microdevices. 2018;20(3):65. doi: 10.1007/s10544-018-0301-9. [DOI] [PubMed] [Google Scholar]
- 4.Mehra P, Miner J, D’Innocenzo R, Nadershah M. Use of 3-d stereolithographic models in oral and maxillofacial surgery. J Maxillofac Oral Surg. 2011;10(1):6–13. doi: 10.1007/s12663-011-0183-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fry RR, Gargya I, Goyal S, Pal J, Chawla S, Pandher PK, Dhaliwal G, Singh P. Additive manufacturing-an enigma: the future of oral and maxillofacial surgery. IOSR J Dent Med Sci. 2016;15(9):78–83. [Google Scholar]
- 6.Robiony M, Salvo I, Costa F, Zerman N, Bandera C, Filippi S, Felice M, Politi M. Accuracy of virtual reality and stereolithographic models in maxillo-facial surgical planning. J Craniofac Surg. 2008;19(2):482–489. doi: 10.1097/SCS.0b013e31814fb5c1. [DOI] [PubMed] [Google Scholar]
- 7.Azuma M, Yanagawa T, Ishibashi-Kanno N, Uchida F, Ito T, Yamagata K, Hasegawa S, Sasaki K, Adachi K, Tabuchi K, Sekido M. Mandibular reconstruction using plates prebent to fit rapid prototyping 3-dimensional printing models ameliorates contour deformity. Head Face Med. 2014;10(1):45. doi: 10.1186/1746-160X-10-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Santler G, Kärcher H, Ruda C. Indications and limitations of three-dimensional models in cranio-maxillofacial surgery. J Craniomaxillofac Surg. 1998;26(1):11–16. doi: 10.1016/S1010-5182(98)80029-2. [DOI] [PubMed] [Google Scholar]
- 9.Hannen EJ. Recreating the original contour in tumor deformed mandibles for plate adapting. Int J Oral Maxillofac Surg. 2006;35(2):183–185. doi: 10.1016/j.ijom.2005.07.012. [DOI] [PubMed] [Google Scholar]
- 10.Yun PY. The application of three-dimensional printing techniques in the fi eld of oral and maxillofacial surgery. J Korean Assoc Oral Maxillofac Surg. 2015;41(4):169–170. doi: 10.5125/jkaoms.2015.41.4.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kernan BT, Wimsatt JA. Use of a stereolithography model for accurate, preoperative adaptation of a reconstruction plate. J Oral Maxillofac Surg. 2000;58(3):349–351. doi: 10.1016/S0278-2391(00)90071-5. [DOI] [PubMed] [Google Scholar]
- 12.Salgueiro MI, Stevens MR. Experience with the use of prebent plates for the reconstruction of mandibular defects. Craniomaxillofac Trauma Reconstr. 2010;3(04):201–208. doi: 10.1055/s-0030-1268520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cohen A, Laviv A, Berman P, Nashef R, Abu-Tair J. Mandibular reconstruction using stereolithographic 3-dimensional printing modeling technology. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;108(5):661–666. doi: 10.1016/j.tripleo.2009.05.023. [DOI] [PubMed] [Google Scholar]
- 14.Kamali P, Dean D, Skoracki R, Koolen PG, Paul MA, Ibrahim A, Lin SJ. The current role of three-dimensional printing in plastic surgery. Plast Reconstr Surg. 2016;137(3):1045–1055. doi: 10.1097/01.prs.0000479977.37428.8e. [DOI] [PubMed] [Google Scholar]
- 15.Erickson DM, Chance D, Schmitt S, Mathts J. An opinion survey of reported benefits from the use of stereolithographic models. J Oral Maxillofac Surg. 1999;57(9):1040–1043. doi: 10.1016/S0278-2391(99)90322-1. [DOI] [PubMed] [Google Scholar]
- 16.Morris CL, Barber RF, Day R. Orofacial prosthesis design and fabrication using stereolithography. Aust Dent J. 2000;45(4):250–253. doi: 10.1111/j.1834-7819.2000.tb00259.x. [DOI] [PubMed] [Google Scholar]
- 17.Chopra K, Gastman BR, Manson PN. Stereolithographic modeling in reconstructive surgery of the craniofacial skeleton after tumor resection. Plast Reconstr Surg. 2012;129(4):743e–745e. doi: 10.1097/PRS.0b013e318245e765. [DOI] [PubMed] [Google Scholar]
- 18.James WJ, Slabbekoorn MA, Edgin WA, Hardin CK. Correction of congenital malar hypoplasia using stereolithography for presurgical planning. J Oral Maxillofac Surg. 1998;56(4):512–517. doi: 10.1016/S0278-2391(98)90726-1. [DOI] [PubMed] [Google Scholar]
- 19.Hrusak D, Bolek M, Bolek L. On site 3D printing in oral and maxilofacial surgery for trauma and oncological bone reconstruction. Int J Oral Maxillofac Surg. 2015;44:e225–e226. doi: 10.1016/j.ijom.2015.08.128. [DOI] [Google Scholar]
- 20.Holck DE, Boyd EM, Jr, Ng J, Mauffray RO. Benefits of stereolithography in orbital reconstruction. Ophthalmology. 1999;106(6):1214–1218. doi: 10.1016/S0161-6420(99)90254-3. [DOI] [PubMed] [Google Scholar]
- 21.Louvrier A, Marty P, Barrabé A, Euvrard E, Chatelain B, Weber E, Meyer C. How useful is 3D printing in maxillofacial surgery? J Stomatol Oral Maxillofac Surg. 2017;118(4):206–212. doi: 10.1016/j.jormas.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 22.Toro C, Robiony M, Costa F, Zerman N, Politi M. Feasibility of preoperative planning using anatomical facsimile models for mandibular reconstruction. Head Face Med. 2007;3(1):5. doi: 10.1186/1746-160X-3-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chow LK, Cheung LK. The usefulness of stereomodels in maxillofacial surgical management. J Oral Maxillofac Surg. 2007;65(11):2260–2268. doi: 10.1016/j.joms.2006.11.041. [DOI] [PubMed] [Google Scholar]
- 24.Lin HH, Lonic D, Lo LJ. 3D printing in orthognathic surgery: a literature review. J Formos Med Assoc. 2018;117(7):547–558. doi: 10.1016/j.jfma.2018.01.008. [DOI] [PubMed] [Google Scholar]
- 25.Shepherd S, Macluskey M, Napier A, Jackson R. Oral surgery simulated teaching; 3D model printing. Oral Surg. 2017;10(2):80–85. doi: 10.1111/ors.12228. [DOI] [Google Scholar]
- 26.Zopf DA, Mitsak AG, Flanagan CL, Wheeler M, Green GE, Hollister SJ. Computer aided-designed, 3-dimensionally printed porous tissue bioscaffolds for craniofacial soft tissue reconstruction. Otolaryngol Head Neck Surg. 2015;152(1):57–62. doi: 10.1177/0194599814552065. [DOI] [PMC free article] [PubMed] [Google Scholar]