Abstract
Background
Wnt1-inducible signaling pathway protein 1, or cellular communication network factor 4 (CCN4), a member of CCN family of secreted, extracellular matrix associated signaling proteins, recently was validated as a novel adipose tissue derived cytokine.
Objective
To assess the relationships between circulating CCN4, adipose tissue distribution and function, and chronic low-grade inflammation in subjects with type 2 diabetes.
Methods
We observed 156 patients with type 2 diabetes and 24 healthy controls. Serum levels of CCN4, hsCRP and alpha1-acid glycoprotein (alpha1-AGP) were measured by ELISA. Serum concentrations of leptin, resistin, visfatin, adipsin, adiponectin, IL-6, IL-8, IL-18 and TNF-alpha were determined by multiplex analysis. Fat mass and distribution was assessed by DEXA. Mean diameter of adipocytes was estimated in samples of subcutaneous adipose tissue.
Results
Patients with diabetes had higher levels of circulating CCN4, leptin, resistin, adipsin, visfatin, hsCRP, alpha1-AGP, and IL-6 (all p < 0.02). The CCN4 concentration correlated positively with percentage of fat mass in central abdominal area, as well as with leptin, resistin and visfatin levels; negative correlation was found between CCN4 and mean adipocyte diameter. In multiple regression analysis fat mass in central abdominal area was independent predictor for CCN4 concentration.
Conclusion
In subjects with type 2 diabetes serum levels of CCN4 are associated with central abdominal fat mass and adipose tissue dysfunction.
Keywords: WISP-1/CCN4, Type 2 diabetes, Cytokines, Obesity, Adipose tissue
Introduction
Adipose tissue dysfunction is considered to play an important role in the development of metabolic disorders in obesity and type 2 diabetes (Oh et al. 2016; Tanaka et al. 2018). Specifically, the imbalance in production of adipokines and other regulatory molecules in adipose tissue can contribute to insulin resistance and chronic low-grade inflammation (Guzik et al. 2017; Unamuno et al. 2018). Understanding the molecular mechanisms underlying adipose tissue dysfunction may lead to the identification of novel therapeutic strategies to prevent or treat obesity-induced metabolic abnormalities.
Recently Wnt1-inducible signaling pathway protein 1, or cellular communication network factor 4 (CCN4, formerly known as WISP-1), was validated as a novel adipose tissue derived cytokine that released by differentiated human adipocytes (Murahovschi et al. 2015). This protein is a member of the CCN family of secreted, extracellular matrix associated signaling proteins and a target gene of the Wingless-type (WNT) signaling pathway. The signal transduction pathways of CCN4 are broad and involve phosphoinositide 3-kinase, protein kinase B (Akt), mitogen activated protein kinase, c-Jun N-terminal kinase, caspases, forkhead transcription factors, sirtuins, c-myc, glycogen synthase kinase 3β, β-catenin, miRNAs, and the mechanistic target of rapamycin (mTOR) (Maiese 2014). Accordingly, CCN4 participates in many cellular processes, including proliferation, differentiation, apoptosis, autophagy and adhesion. The role of CCN4 in pathological processes, including the inflammation, fibrosis, traumatic injury, neurodegeneration, musculoskeletal disorders, cardiovascular disease, pulmonary compromise, and cancer, has also been postulated (Maiese 2014; Gurbuz and Chiquet-Ehrismann 2015; Feng and Jia 2016).
A growing body of evidences indicates to CCN4 as an active player in pathophysiology of obesity and type 2 diabetes. In human the expression of CCN4 was revealed in both visceral and subcutaneous adipose tissue. In glucose tolerant subjects CCN4 expression in subcutaneous and visceral adipocytes was associated with markers of insulin resistance and inflammation (Murahovschi et al. 2015). In human skeletal muscle cells and murine hepatocytes CCN4 impaired insulin action by inhibiting phosphorylation of insulin receptor, Akt and its substrates glycogen synthase kinase 3β, FOXO1 and p70S6 kinase, and inhibiting insulin-stimulated glycogen synthesis and suppression of gluconeogenic genes (Hörbelt et al. 2018). In mice CCN4 knockdown mitigated insulin resistance caused by high-fat diet in skeletal muscle cells and reduced proinflammatory factors, including NF-κB nuclear translocation and IκB phosphorylation in the liver (Jung et al. 2018).
Recent studies revealed increased plasma/serum CCN4 levels in obese subjects without diabetes (Wang et al. 2018; Tacke et al. 2018). The data of the changes in circulating CCN4 levels in subjects with type 2 diabetes and their relationships with clinical covariates are limited. In two preceding studies (Tacke et al. 2018; Barchetta et al. 2017) no differences in plasma CCN4 concentrations were found between individuals with and without type 2 diabetes; nevertheless, the positive association between CCN4 and obesity was observed.
In this study we aimed to assess the relationships between circulating CCN4, adipose tissue distribution and function, and chronic low-grade inflammation in subjects with type 2 diabetes.
Materials and methods
Design
Observational cross-sectional study.
Ethical issues
The study was conducted in accordance with the principles of the Declaration of Helsinki and the International Conference on Harmonization Good Clinical Practice guidelines. The protocol of the study was approved by institutional ethic committee of Research Institute of Clinical and Experimental Lymphology. Written informed consents were obtained from all patients prior to the study procedures.
Patients
One hundred and fifty six patients with type 2 diabetes, 45 men and 111 women, aged from 43 to 80 years (median 61 years), were recruited in accordance with the set of inclusion and exclusion criteria. The inclusion criteria were: 1) men and women of Caucasian origin; 2) age 40–80 years; 3) type 2 diabetes, diagnosed according to WHO criteria; 4) diabetes duration >1 year. Treatment with thiazolidinediones or anorectic agents, bariatric procedures in medical history, chronic heart failure class III-IV by NYHA, chronic kidney disease (CKD) with estimated glomerular filtration rate (eGFR) <30 ml/min/1.73 m2, malignancy and autoimmune diseases were acted as exclusion criteria.
Diabetes duration varied from 1 to 36 years (median 13 years). Most of the subjects received insulin therapy (n = 132), in combination with oral antihyperglycemic agents, including metformin (n = 104), sulfonylurea (n = 59) or dipeptidyl peptidase-4 inhibitors (n = 13). Sixteen patients were treated by combination of metformin and sulfonylurea and eight ones were on metformin therapy only. The levels of glycated hemoglobin A1c (HbA1c) varied from 5.3 to 11.7% (median 8.2%). The list of complications and accompanying conditions included arterial hypertension (n = 149), diabetic neuropathy (n = 141), CKD stage 1–3 (n = 133), diabetic retinopathy (n = 119), peripheral artery disease (n = 75), and coronary artery disease (n = 61).
Fourteen patients had normal body mass index (BMI), 40 ones were overweight (BMI 25–29.9 kg/m2), and 102 subjects had obesity (BMI >30 kg/m2). Patients with BMI <30 kg/m2 were considered as one group and compared with obese diabetic subjects in statistical analysis.
Laboratory investigations
It was shown that serum samples are preferred over plasma EDTA or citrate samples for optimal detection of circulating CCN4 (Tacke et al. 2018). Accordingly, we measured the concentrations of CCN4, as well as those of adipokines and inflammatory markers, in blood serum. The serum from 24 non-obese non-diabetic subjects, 7 men and 17 women, from 41 to 70 years of age, were acted as control. Blood samples were collected from cubital vein after 8-h fasting. Serum was frozen after separation immediately and stored at −70 °C until the analysis.
We used human WISP-1/CCN4 DuoSet® ELISA kits (R&D Systems, USA), that were validated to ensure reliable results in the analysis of circulating CCN4 in clinical studies (Tacke et al. 2018). The high-sensitivity C-reactive protein (hsCRP), α1-acid glycoprotein (α1-AGP) and macrophage inflammatory protein-1α (MIP-1α) were measured by ELISA with commercially available kits (Biomerica, USA, for hsCRP; AssayPro, USA, for α1-AGP). The concentrations of leptin, resistin, visfatin, adipsin, adiponectin, interleukin-6, −8, −18 (IL-6, IL-8, IL-18), and tumor necrosis factor α (TNF-α) were determined by Multiplex analysis, or Bio-Plex Pro™ human diabetes immunoassays using the commercially available kits (Bio-Rad, USA). Analyses were performed blinded and in accordance with instructions from manufacturers.
Body composition assessment
The body composition parameters were assesses in 47 patients, including 22 non-obese ones and 25 subjects with obesity, by dual-energy X-ray absorptiometry (DEXA) with the use of Lunar Prodigy densitometer and the Total Body Composition program (GE, USA). The total and truncal fat mass, as well as lean mass, were determined. Fat distribution patterns were differentiated based on the ratio of fat mass in the central abdominal and hip areas (“android” and “gynoid” fat respectively). The central abdominal fat was measured in the area from the top of the iliac crest to 20% of the distance from the iliac crest to the neck; from the sides, this area is limited to the cut of the arms. Upper boundary of gynoid region is below the pelvis line by the distance of 1.5 times of the android region area. Laterally, this area is bounded by leg sections (Fig. 1).
Fig. 1.
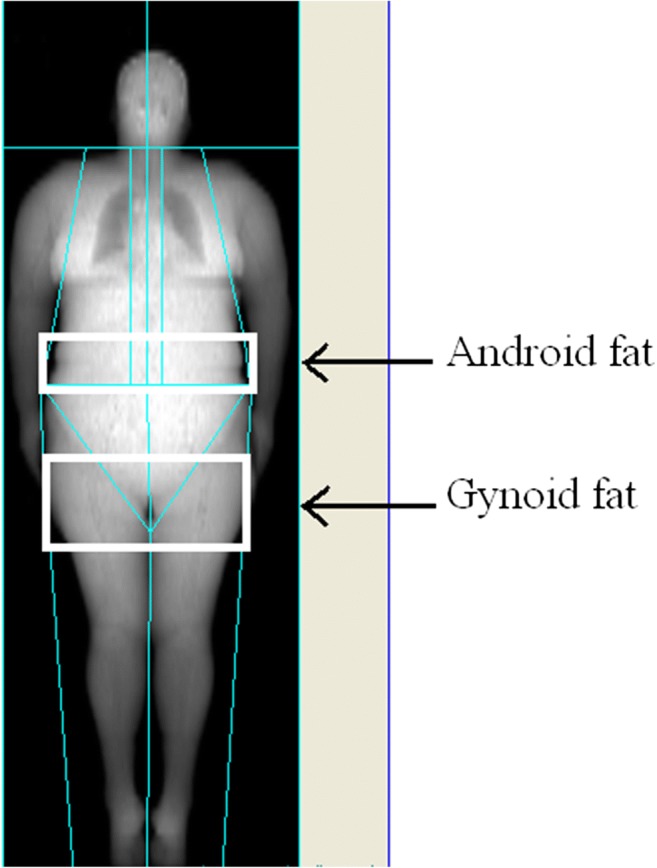
Andoid and gynoid fat mass assessment with Total Body Composition option (GE, USA)
Mean adipocyte diameter estimation
The samples of subcutaneous adipose tissue were obtained in 21 diabetic subjects, 7 men and 14 women. Among them, 10 individuals had obesity, 3 patients had normal BMI, and 8 subjects were overweight. A knife biopsy of the subcutaneous adipose tissue from paraumbilical area was performed in the supine position with local anesthesia (lidocaine 2%). The samples of paraumbilical adipose tissue from 10 non-obese non-diabetic individuals died in car accidents, 4 men and 6 women, from 43 to 65 years of age, were acted as control. Sampling was carried out according to the same method as in patients with type 2 diabetes in the first 4 h after death. The obtained samples were fixed in 4% formaldehyde solution, processed by standard histological method and embedded in paraffin. The mean adipocyte diameter was estimated by semi-automated method of quantitatively analyzing, proposed by Parlee S.D. et al. (2014) with the use of Image J, an open-access platform for scientific image analysis (https://imagej.net).
Analysis of glucose fluctuations
Blinded continuous glucose monitoring (CGM) recordings were performed in 65 patients, including 42 individuals with obesity, using Medtronic MiniMed iPro2 system (iPro2™ digital recorder, MMT-7741) with an Enlite™ sensor (MMT-7008). All CGM data were uploaded into the online system (CareLink iPro™ Therapy Management Software for Diabetes, MMT-7340), through the iPro2™ Docking Station (MMT-7742 or Dock). After the uploading, the glucose meter readings and any other recorded events were manually entered into CareLink iPro to calibrate the sensor data. All the patients’ reports were viewed individually to find and eliminate calibration errors. Then all CGM data were exported from CareLink iPro online system as character-separated value files. These files were manually processed for further glucose variability estimation. Recordings that included data from at least two consecutive nights were used for analysis; tracings that were interrupted were discarded from the dataset. The data of initial 2 h of monitoring, which is considered to be an unstable calibration period, were excluded. No medical procedures, except injections of regular agents, were performed during CGM.
We determined glucose variability by calculating the Standard Deviation (SD), Mean Amplitude of Glucose Excursions (MAGE), Lability Index (LI), 2-h Continuous Overlapping Net Glycemic Action (2-h CONGA), Low Blood Glucose Index (LBGI), High Blood Glucose Index (HBGI), and Mean Absolute Glucose (MAG). The diagnostic and prognostic value of these indices had been reviewed recently (Inchiostro et al. 2013, Suh and Kim 2015). In brief, SD, LI and MAGE refer mostly the amplitude of glucose fluctuations; MAG reflects the rate of glucose concentration changes; HBGI and CONGA2 associated with hyperglycemic excursions predominantly, while LBGI is most sensitive to hypoglycemia. Mean glucose and GV indices were computed by EasyGV calculator (v. 9.0) proposed by Hill et al. (2011).
Statistical analysis
The data were preceded statistically with STATISTICA 64 v. 10 (StatSoft.Inc, USA). The significance of differences between groups was assessed by ANOVA Kruskal–Wallis and Mann–Whitney test when appropriate. Spearmen rank correlation analysis and stepwise multiple regression analysis was applied for assessment of the relationships between studied parameters. A P value of <0.05 was considered to be statistically significant. Data are presented as medians, 25th and 75th percentiles, unless stated otherwise.
Results
Clinical characteristics of patient groups are summarized in Table 1. Patients with obesity, as compared to those without, demonstrated elevated levels of HbA1c, uric acid, and lower concentrations of HDL-cholesterol. As expected, the total, truncal, android and gynoid fat mass was increased significantly in patients with obesity as compared to non-obese diabetic subjects (Table 2). In patients with obesity CGM-derived mean glucose and 2-h CONGA was higher; meanwhile other CGM-derived parameters demonstrated no significant differences between two groups (Table 3).
Table 1.
Clinical characteristics of type 2 diabetic subjects with and without obesity
| Parameter | BMI <30 kg/m2 (n = 54) | BMI >30 kg/m2 (n = 102) | P |
|---|---|---|---|
| Age, years | 63 (57; 66) | 61 (58; 65) | 0.95 |
| Diabetes duration, years | 14 (9; 19) | 13 (9; 18) | 0.46 |
| BMI, kg/m2 | 27.3 (26; 29.1) | 33.8 (32; 37.5) | <0.0001 |
| Waist-to-hip ratio | 0.93 (0.89; 1.02) | 0.97 (0.93; 1) | 0.4 |
| HbA1c, % | 7.9 (7; 9.4) | 8.5 (7.4; 9.9) | 0.04 |
| Cholesterol, mmol/l | 5.5 (4.5; 6.5) | 5.1 (4.3; 6.2) | 0.34 |
| LDL-cholesterol, mmol/l | 3.23 (2.46; 3.8) | 3.2 (2.4; 4) | 0.9 |
| HDL-cholesterol, mmol/l | 1.36 (1.12; 1.63) | 1.12 (0.98; 1.34) | 0.01 |
| Triglycerides, mmol/l | 1.55 (1; 2.7) | 1.9 (1.4; 2.7) | 0.14 |
| Uric acid, μmol/l | 295 (233.1; 341.5) | 315 (258.5; 395.7) | 0.008 |
| eGFR, (Ahmed et al. 2017) ml/min/1.73 m2 | 72.5 (55; 85) | 66 (57; 80) | 0.56 |
| UACR, mg/mmol | 1.72 (0.45; 3.7) | 0.88 (0.46; 1.8) | 0.58 |
Data are shown as medians (25th; 75th percentiles). BMI, body mass index; HbA1c, glycated hemoglobin A1c; LDL, low-density lipoproteins; HDL, high-density lipoproteins; hsCRP, high-sensitivity C-reactive protein; eGFR, estimated glomerular filtration rate (асcording to CKD-EPI formula, 2009); UACR, urinary albumin/creatinine ratio
Table 2.
Parameters of total body composition in type 2 diabetic subjects with and without obesity
| Parameter | BMI <30 kg/m2(n = 22) | BMI >30 kg/m2(n = 25) | P |
|---|---|---|---|
| Total fat mass, kg | 26 (21.2; 31.3) | 35.9 (32; 40.3) | <0.0001 |
| Total fat mass, % | 35.9 (32; 40.3) | 43.2 (40; 46.4) | 0.0004 |
| Truncal fat mass, kg | 16.1 (14; 19.2) | 21.4 (19.4; 23.4) | 0.0001 |
| Android fat mass, kg | 2.8 (2.6; 3.3) | 4.1 (3.7; 4.5) | <0.0001 |
| Gynoid fat mass, kg | 3.7 (2.5; 4.8) | 5.0 (4.3; 6.6) | 0.001 |
| Lean mass, kg | 44.1 (39.2; 53.9) | 46.2 (42; 48.8) | 0.48 |
Data are shown as medians (25th; 75th percentiles)
Table 3.
CGM-derived mean glucose and glucose variability parameters in subjects with type 2 diabetes
| Parameter | BMI <30 kg/m2 (n = 23) |
BMI >30 kg/m2 (n = 42) |
P |
|---|---|---|---|
| Mean glucose, mmol/l | 7.3 (6.8; 9.3) | 8.6 (7.1; 10.2) | 0.04 |
| SD, mmol/l | 2.1 (1.8; 2.6) | 2.1 (1.7; 2.4) | 0.61 |
| MAGE, mmol/l | 3.9 (3.4; 5.1) | 4.1 (3.3; 5.0) | 0.51 |
| LI, (mmol/l) (Barchetta et al. 2017)/h | 2.4 (1.6; 3.5) | 1.8 (1.4; 2.7) | 0.11 |
| 2-h CONGA, mmol/l | 6.0 (5.6; 7.0) | 7.6 (5.9; 9.1) | 0.02 |
| LBGI | 1.6 (0.7; 5.0) | 1.1 (0; 2.5) | 0.07 |
| HBGI | 3.9 (2.7; 7.6) | 6.5 (2.9; 11.4) | 0.26 |
| MAG, mmol/L/h | 2.1 (1.8; 2.7) | 2.0 (1.6; 2.6) | 0.33 |
Data are shown as medians (25th; 75th percentiles).CGM, continuous glucose monitoring; 2-h CONGA, 2-h Continuous Overlapping Net Glycemic Action; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, Mean Absolute Glucose rate of change; MAGE, Mean Amplitude of Glucose Excursions; SD, Standard Deviation
The mean CCN4 level was higher in patients with diabetes, as compared to control (155.7, 134.1–180.5 and 140.0, 113.5–158.6 pg/ml respectively, p = 0.02). Men and women with diabetes had similar CCN4 concentrations (155.7, 132.3–177.3 and 1557, 138.6–186.3 pg/ml respectively, p = 0.46). When compared to control, patients with obesity demonstrated increased concentrations of CCN4 (156, 138–172 pg/ml, p = 0.04), meanwhile patients without obesity showed a tendency to increase (158, 129–188 pg/ml, p = 0.06). Nevertheless, we found no significant differences in CCN4 levels between diabetic subjects with and without obesity (p = 0.75). Besides, we failed to find significant correlation between CCN4 concentration and BMI (r = 0.05, p = 0.58).
When compared to total body composition parameters, serum CCN4 levels demonstrated positive correlation with percentage of central abdominal or android fat mass (r = 0.46, p = 0.001). At the same time, no association with total fat mass, truncal fat mass, gynoid fat mass and lean mass was estimated.
As expected, the mean diameter of subcutaneous adipocytes was increased significantly in patients with diabetes as compared to control (230, 180–316 and 54, 18–86 μm respectively, p = 0.0001). In diabetic group, a negative correlation between concentration of CCN4 and mean adipocyte diameter was found (r = −0.39, p = 0.03).
Subjects with diabetes, as compared to control, demonstrated elevated levels of leptin (p = 0.005), resistin (p < 0.0001), adipsin (p < 0.0001), and visfatin (p = 0.0003). Contrary, the concentration of adiponectin did not show any difference between the groups (p = 0.16). The levels of leptin, resistin and visfatin were higher in patients with obesity as compared to those without (Table 4). The concentration of CCN4 demonstrated weak positive correlations with that of resistin (r = 0.36, p < 0.0001), visfatin (r = 0.28, p = 0.002) and leptin (r = 0.19, p = 0.03). No significant correlations with adipsin and adiponectin levels were found.
Table 4.
Serum concentrations of adipokines in control and type 2 diabetic subjects with and without obesity
| Parameter | Control (n = 24) |
Type 2 diabetes | |
|---|---|---|---|
| BMI <30 kg/m2 (n = 54) | BMI >30 kg/m2 (n = 102) | ||
| Leptin, pg/ml |
7.0 (3.9; 14.8) |
8.3 (5.0; 24.6) |
16.7 (9.7; 33.3)*# |
| Resistin, pg/ml |
2.8 (2.3; 3.1) |
3.5 (2.9; 7.6)* |
9.1 (3.5; 11.3)*# |
| Visfatin, pg/ml |
1.7 (1.3; 2.1) |
2.4 (1.2; 4.2) |
3.6 (1.8; 4.6)*# |
| Adipsin, ng/ml |
1.1 (0.9; 1.3) |
1.6 (1.3; 2.0)* |
1.8 (1.2; 2.4)*# |
| Adiponectin, ng/ml |
9.8 (5.7; 11.5) |
8.1 (4.6; 11.6) |
6.6 (5.1; 10.4) |
Data are shown as medians (25; 75 percentiles)
*p < 0.05 vs. control # p < 0.05 vs. non-obese diabetic subjects
Patients with diabetes had increased serum levels of hsCRP (p < 0.0001), AGP (p < 0.0001), MIP-1α (р = 0.006) and IL-6 (p = 0.006). Other inflammatory mediators (IL-8, IL-18 and TNF-α) did not show significant differences. The levels of IL-6, IL-8 and TNF-α were higher in diabetic subjects with obesity as compared to those without (Table 5). The concentration of CCN4 demonstrated weak positive correlations with hsCRP and MIP-1α levels (r = 0.19, p = 0.03 and r = 0.47, p < 0.0001 respectively), but it did not correlated with other inflammatory markers.
Table 5.
Serum levels of inflammatory markers in control and type 2 diabetic subjects with and without obesity
| Parameter | Control (n = 24) |
Type 2 diabetes | |
|---|---|---|---|
| BMI <30 kg/m2 (n = 54) | BMI >30 kg/m2 (n = 102) | ||
| hsCRP, mg/l |
1.5 (1.2; 1.8) |
2.7 (1.9; 3.3)* |
3.1 (2.1; 5.2)* |
| α1-AGP, mg/ml |
1.3 (1.1; 1.6) |
2.3 (1.6; 3.1)* |
2.2 (1.6; 2.7)* |
| IL-6, pg/ml | 21.7 (14.3; 43.4) |
36.3 (22.3; 88.9) |
74.4 (34; 161.9)*# |
| IL-8, pg/ml |
12.4 (7.3; 55.0) |
19.8 (15.2; 52.9) |
39.8 (18.2; 95.7)*# |
| IL-18, pg/ml |
155 (101; 677) |
242 (121; 434) |
368 (208; 654)* |
| TNF-α, pg/ml |
18.1 (11.8; 30.6) |
19.4 (15.1; 44.3) |
40.4 (20.5; 93.2)*# |
| MIP-1α, pg/ml |
11.0 (9.2; 18.0) |
22.7 (14.3; 27.5) |
19.4 (13.6; 40.7)* |
Data are shown as medians (25; 75 percentiles)
*p < 0.05 vs. control # p < 0.05 vs. non-obese diabetic subjects
α1-AGP, α1-acid glycoprotein; BMI, body mass index; hsCRP, high-sensitivity C-reactive protein; IL-6, interleukin-6; IL-8, interleukin-8; IL-18, interleukin-18; MIP-1α, macrophage inflammatory protein-1α (MIP-1α); TNF-α, tumor necrosis factor α
There were no correlations between CCN4 levels and parameters of glycemic control: HbA1c and glucose variability indices (Table 6).
Table 6.
The correlations between CCN4 serum levels and parameters of glycemic control
| Parameter | R | P |
|---|---|---|
| HbA1c | −0.08 | 0.32 |
| Mean glucose | 0.2 | 0.18 |
| SD | −0.01 | 0.95 |
| MAGE | −0.17 | 0.25 |
| LI | −0.07 | 0.64 |
| 2-h CONGA | 0.25 | 0.09 |
| MAG | 0.02 | 0.89 |
Spearmen correlation coefficients are shown. 2-h CONGA, 2-h Continuous Overlapping Net Glycemic Action; HBGI, High Blood Glucose Index; LBGI, Low Blood Glucose Index; LI, Lability Index; MAG, Mean Absolute Glucose rate of change; MAGE, Mean Amplitude of Glucose Excursions; SD, Standard Deviation
We found no differences in CCN4 concentrations in dependence on metformin, sulfonylurea or insulin treatment. Among insulin-treated subjects, no correlation with daily insulin dose was observed. The CCN4 levels did not correlate with сholesterol, triglycerides, and eGFR values. The same way, no correlation was found between CCN4 and diabetes duration.
In a multiple stepwise regression analysis android fat mass was the only reliable predictor of serum CCN4 concentration independent from age, BMI, HbA1c, and other body composition parameters (beta = 0.458, R2 = 0.12, p = 0.005).
Discussion
The results of the study indicate the relationships between increased serum levels of CCN4, adiposity and adipose tissue dysfunction in subjects with type 2 diabetes. In our cohort of diabetic subjects, the levels of CCN4 were increased significantly in obese individuals and the fat mass in central abdominal area (so called android fat mass) was independent predictor of CCN4. These data is in agreement with results of Murahovschi et al. (2015) demonstrated that circulating CCN4 concentration strongly correlates with CCN4 expression in subcutaneous and visceral adipose tissue, which therefore represents a major source of this adipokine in humans (Murahovschi et al. 2015). The increase in CCN4 gene expression in adipose tissue was observed in mice with both high-fat diet induced obesity (Murahovschi et al. 2015; Ferrand et al. 2017) and in leptin-deficient ob/ob obese mice (Ferrand et al. 2017). In females, CCN4 mRNA in subcutaneous adipose tissue was reduced markedly after weight loss (Murahovschi et al. 2015).
The role of CCN4 in pathogenesis of obesity and related disorders is emerging topic of research. It was demonstrated that Wnt-1 signaling pathway is involved in adipogenesis and adipocyte differentiation. It was revealed that expression of CCN4 in mouse adipogenesis is dynamic with the lowest expression in fully differentiated cells. Wherein, CCN4 inhibits adipocyte differentiation through downregulation of peroxisome proliferator activated receptor gamma (PPARγ) activity (Ferrand et al. 2017). Specifically, in mice Wnt signaling maintains preadipocytes in an undifferentiated state through inhibition of the adipogenic transcription factors CCAAT/enhancer binding protein alpha and PPARγ (Ross et al. 2000). In contrast, human adipocyte differentiation is accompanied by substantial increase in CCN4 mRNA and protein levels (Murahovschi et al. 2015). In humans, the relationships between CCN4 production and adipocyte morphology in obesity have not been studied yet. In our study CCN4 level demonstrated weak negative correlation with mean diameter of subcutaneous adipocytes. Previously it was shown that CCN4 expression correlates weakly with adipocyte size in subcutaneous, but not visceral adipose tissue (Murahovschi et al. 2015). Probably, these data reflect the differences in CCN4 secretion by adipocytes at different stages of differentiation and hypertrophy.
The increased plasma or serum CCN4 levels in obesity could be considered as a marker of adipose tissue dysfunction. In favor to this point of view, the observed relationships between shifts in concentration of circulating CCN4 and other adipokines. In this study we found positive correlations between CCN4 concentrations and the levels of other adipokines, including leptin, resistin and visfatin. These relationships could be explained by the impact of fat mass as confounding factor. Whether CCN4 or other molecules of Wnt-1 signaling pathway have a direct effect on the adipokine production and secretion is not studied yet.
As it was already mentioned, CCN4 impairs insulin signaling in muscle cells and hepatocytes (Hörbelt et al. 2018; Jung et al. 2018). Accordingly, CCN4 overproduction might contribute to insulin resistance. It was demonstrated that CCN4 mRNA levels in subcutaneous adipose tissue from non-diabetic subjects are related to fasting insulin levels (Murahovschi et al. 2015). The association between CCN4 levels and insulin resistance were recently reported in women with gestational diabetes (Sahin Ersoy et al. 2017) and in type 2 diabetic subjects (Barchetta et al. 2017). In this study we did not assess the relationships between CCN4 and insulin resistance as most of our patients were treated by combined antihyperglycemic therapy, including the insulin.
The chronic low-grade inflammation could be causally related with enhanced CCN4 production. An association between CCN4 levels and MRI-assessed visceral adipose tissue inhomogeneity, considered as indirect sign of adipose tissue inflammation, has been reported recently in patients with type 2 diabetes (Barchetta et al. 2017). Immune cell infiltration is a prominent feature of the dysfunctional adipose tissue. These immune cells include M1 and M2 macrophages, effector and memory T cells, IL-10 producing FoxP3 + T regulatory cells, natural killer, NKT cells and granulocytes (Guzik et al. 2017). Adipocyte-macrophage interaction is considered as a cornerstone in obesity-related adipose tissue remodeling and dysfunction (Engin 2017). Previously some of us shown that in subjects with different state of glucose tolerance the expression of CCN4 mRNA in subcutaneous and visceral adipose tissue is correlated with macrophagal infiltration. Recombinant CCN4 stimulates expression of cytokines (IL-6, TNF-α, IL-1β and IL-10) in human macrophages. Moreover, CCN4 induces an increase in expression of inflammatory M1 markers of macrophage polarization (Murahovschi et al. 2015). In this study we assessed the association between CCN4 serum levels and the panel of circulating inflammatory markers. Among those, hsCRP and MIP-1α demonstrated positive correlations with CCN4. It was revealed that hsCRP serum levels are associated with total, truncal and android fat mass in type 2 diabetic subjects (Klimontov et al. 2016a). Thus, the correlation between hsCRP and CCN4 could be attributed to adipose tissue enlargement. Chemokine MIP-1α (CCL3), expressed by lymphocytes and monocytes/macrophages, realize its effect in inflammation by enhancing the chemotaxis and release of proinflammatory cytokines from macrophages (Maurer and von Stebut 2004). The explants of human obese omental adipose tissue, obtained during bariatric surgery, released higher levels of MIP-1α when compared to explants of lean subcutaneous adipose tissue (Renovato-Martins et al. 2017). The data could elucidate the relationships between CCN4 and MIP-1α concentrations.
Proinflammatory effect of hyperglycemia in diabetes is discussed widely. Recent data indicate that high glucose enhance stimulated secretion of proinflammatory cytokines from macrophages in vitro (Grosick et al. 2018). Hyperglycemia could induce epigenetic changes that promote an inflammatory macrophage phenotype (Ahmed et al. 2017). It was shown that both hypoglycemia and hyperglycemia enhance plasma concentrations of inflammatory markers in healthy subjects and patients with type 1 diabetes (Ceriello et al. 2012). The associations between levels of acute phase proteins and glucose variability parameters in type 2 diabetic subjects were also reported (Chang et al. 2012; Klimontov et al. 2016a, b). Accordingly, one could anticipate that hyperglycemia or enhanced glucose fluctuations can contribute to elevation of serum CCN4 levels in diabetes. However, in this study we did not find any relationships of CCN4 levels with HbA1c and CGM-derived glucose variability parameters. It was revealed recently that in healthy subjects CCN4 serum levels do not change significantly neither in liquid meal challenge test nor in hyperinsulinemic euglycemic clamp (Tacke et al. 2018). Therefore, it looks most likely that obesity rather the diabetes per se contribute to changes in circulating CCN4 levels in subjects with type 2 diabetes.
Our study has some limitations. The cross-sectional design does not prove causality. All included subjects received antihyperglycemic therapy, which could inverse or mask some relationships between variances. We did not differentiate subcutaneous and visceral adipose tissue, using DEXA for the fat distribution estimation. The CGM and total body composition assessment, as well as adipose tissue biopsy, were performed in limited proportions of subjects. Nevertheless, this is the first study demonstrating the association of increased serum CCN4 levels with fat distribution and adipose tissue dysfunction in individuals with mostly long-term, insulin-treated type 2 diabetes.
In conclusion, patients with type 2 diabetes and obesity have increased serum levels of CCN4 as compared to non-obese non-diabetic subjects. In patients with type 2 diabetes circulating CCN4 is associated with adipose tissue dysfunction. Central abdominal fat mass, but not total fat mass or glycemic control parameters, is predictor of serum CCN4 concentration. The data provide further support to notion that CCN4 is a new marker of adiposity in patients with type 2 diabetes.
Acknowledgments
This work was financially supported by a grant to N.R. from European Foundation for Study of Diabetes (EFSD/AZ Cellular Plasticity “Unravelling the role of WISP1 on metabolic and cellular plasticity in white adipose tissue”) and by a grant to O. P.-R. from the German Center for Diabetes Research (DZD) (“Role of Wnt-inducible signaling pathway protein-1 (WISP-1) in adipose tissue and liver fibrosis in mice and men”).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Olga Pivovarova-Ramich and Natalia Rudovich contributed equally to this work.
References
- Ahmed M, de Winther MPJ, Van den Bossche J. Epigenetic mechanisms of macrophage activation in type 2 diabetes. Immunobiology. 2017;222:937–943. doi: 10.1016/j.imbio.2016.08.011. [DOI] [PubMed] [Google Scholar]
- Barchetta I, Cimini FA, Capoccia D, De Gioannis R, Porzia A, Mainiero F, et al. CCN4 is a marker of systemic and adipose tissue inflammation in dysmetabolic subjects with or without type 2 diabetes. J Endocr Soc. 2017;103:660–670. doi: 10.1210/js.2017-00108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceriello A, Novials A, Ortega E, La Sala L, Pujadas G, Testa R, Bonfigli AR, Esposito K, Giugliano D. Evidence that hyperglycemia after recovery from hypoglycemia worsens endothelial function and increases oxidative stress and inflammation in healthy control subjects and subjects with type 1 diabetes. Diabetes. 2012;61:2993–2997. doi: 10.2337/db12-0224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang CM, Hsieh CJ, Huang JC, Huang IC. Acute and chronic fluctuations in blood glucose levels can increase oxidative stress in type 2 diabetes mellitus. Acta Diabetol. 2012;49:171–177. doi: 10.1007/s00592-012-0398-x. [DOI] [PubMed] [Google Scholar]
- Engin AB. Adipocyte-macrophage cross-talk in obesity. Adv Exp Med Biol. 2017;960:327–343. doi: 10.1007/978-3-319-48382-5_14. [DOI] [PubMed] [Google Scholar]
- Feng M, Jia S. Dual effect of WISP-1 in diverse pathological processes. Chin J Cancer Res. 2016;28:553–560. doi: 10.21147/j.issn.1000-9604.2016.06.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrand N, Béreziat V, Moldes M, Zaoui M, Larsen AK, Sabbah M. CCN4/CCN4 inhibits adipocyte differentiation through repression of PPARγ activity. Sci Rep. 2017;7:1749. doi: 10.1038/s41598-017-01866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grosick R, Alvarado-Vazquez PA, Messersmith AR, Romero-Sandoval EA. High glucose induces a priming effect in macrophages and exacerbates the production of pro-inflammatory cytokines after a challenge. J Pain Res. 2018;11:1769–1778. doi: 10.2147/JPR.S164493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurbuz I, Chiquet-Ehrismann R. CCN4/CCN4 (WNT1 inducible signaling pathway protein 1): a focus on its role in cancer. Int J Biochem Cell Biol. 2015;62:142–146. doi: 10.1016/j.biocel.2015.03.007. [DOI] [PubMed] [Google Scholar]
- Guzik TJ, Skiba DS, Touyz RM, Harrison DG. The role of infiltrating immune cells in dysfunctional adipose tissue. Cardiovasc Res. 2017;113:1009–1023. doi: 10.1093/cvr/cvx108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill NR, Nick SO, Choudhary P, Levy JC, Hindmarsh P, Matthews DR. Normal reference range for mean tissue glucose and glycemic variability derived from continuous glucose monitoring for subjects without diabetes in different ethnic groups. Diabetes Technol Ther. 2011;13:921–928. doi: 10.1089/dia.2010.0247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörbelt T, Tacke C, Markova M, Herzfeld de Wiza D, Van de Velde F, Bekaert M, et al. The novel adipokine CCN4 associates with insulin resistance and impairs insulin action in human myotubes and mouse hepatocytes. Diabetologia. 2018;61:2054–2065. doi: 10.1007/s00125-018-4636-9. [DOI] [PubMed] [Google Scholar]
- Inchiostro S, Candido R, Cavalot F. How can we monitor glycaemic variability in the clinical setting? Diabetes Obes Metab. 2013;15:13–16. doi: 10.1111/dom.12142. [DOI] [PubMed] [Google Scholar]
- Jung TW, Kang C, Goh J, Chae SI, Kim HC, Lee TJ, Abd El-Aty AM, Jeong JH. CCN4 promotes non-alcoholic fatty liver disease and skeletal muscle insulin resistance via TLR4/JNK signaling. J Cell Physiol. 2018;233:6077–6087. doi: 10.1002/jcp.26449. [DOI] [PubMed] [Google Scholar]
- Klimontov VV, Tyan NV, Fazullina ON, Myakina NE, Lykov AP, Konenkov VI. Clinical and metabolic factors associated with chronic low-grade inflammation in type 2 diabetic patients. Diabetes Mellitus. 2016;19:295–302. doi: 10.14341/DM7928. [DOI] [Google Scholar]
- Klimontov VV, Tyan NV, Fazullina ON, Myakina NE, Orlov NB, Konenkov VI. Acute-phase serum proteins and adipocytokines in women with type 2 diabetes mellitus: relationships with body composition and blood glucose fluctuations. Ter Arkh. 2016;88:35–41. doi: 10.17116/terarkh2016881035-41. [DOI] [PubMed] [Google Scholar]
- Maiese K. CCN4: clinical insights for a proliferative and restorative member of the CCN family. Curr Neurovasc Res. 2014;11:378–389. doi: 10.2174/1567202611666140912115107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maurer M, von Stebut E. Macrophage inflammatory protein-1. Int J Biochem Cell Biol. 2004;36:1882–1886. doi: 10.1016/j.biocel.2003.10.019. [DOI] [PubMed] [Google Scholar]
- Murahovschi V, Pivovarova O, Ilkavets I, Dmitrieva RM, Döcke S, Keyhani-Nejad F. CCN4 is a novel adipokine linked to inflammation in obesity. Diabetes. 2015;64:856–866. doi: 10.2337/db14-0444. [DOI] [PubMed] [Google Scholar]
- Oh KJ, Lee DS, Kim WK, Han BS, Lee SC, Bae KH. Metabolic adaptation in obesity and type II diabetes: myokines, adipokines and hepatokines. Int J Mol Sci. 2016;18:E8. doi: 10.3390/ijms18010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parlee SD, Lentz SI, Mori H, MacDougald OA. Quantifying size and number of adipocytes in adipose tissue. Methods Enzymol. 2014;537:93–122. doi: 10.1016/B978-0-12-411619-1.00006-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renovato-Martins M, Matheus ME, de Andrade IR, Moraes JA, da Silva SV, Citelli Dos Reis M, de Souza AA, da Silva CC, Bouskela E, Barja-Fidalgo C. Microparticles derived from obese adipose tissue elicit a pro-inflammatory phenotype of CD16+, CCR5+ and TLR8+ monocytes. Biochim Biophys Acta Mol Basis Dis. 2017;1863:139–151. doi: 10.1016/j.bbadis.2016.09.016. [DOI] [PubMed] [Google Scholar]
- Ross SE, Hemati N, Longo KA, Bennett CN, Lucas PC, Erickson RL, MacDougald OA. Inhibition of adipogenesis by Wnt signaling. Science. 2000;289:950–953. doi: 10.1126/science.289.5481.950. [DOI] [PubMed] [Google Scholar]
- Sahin Ersoy G, Altun Ensari T, Subas S, Giray B, Simsek EE, Cevik O. CCN4 is a novel adipokine linked to metabolic parameters in gestational diabetes mellitus. J Matern Fetal Neonatal Med. 2017;30:942–946. doi: 10.1080/14767058.2016.1192118. [DOI] [PubMed] [Google Scholar]
- Suh S, Kim JH. Glycemic variability: how do we measure it and why is it important? Diabetes Metab J. 2015;39:273–282. doi: 10.4093/dmj.2015.39.4.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacke C, Aleksandrova K, Rehfeldt M, Murahovschi V, Markova M, Kemper M, Hornemann S, Kaiser U, Honig C, Gerbracht C, Kabisch S, Hörbelt T, Ouwens DM, Weickert MO, Boeing H, Pfeiffer AFH, Pivovarova O, Rudovich N. Assessment of circulating Wnt1 inducible signalling pathway protein 1 (WISP-1)/CCN4 as a novel biomarker of obesity. J Cell Commun Signal. 2018;12:539–548. doi: 10.1007/s12079-017-0427-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka M, Itoh M, Ogawa Y, Suganami T. Molecular mechanism of obesity-induced ‘metabolic’ tissue remodeling. J Diabetes Investig. 2018;9:256–261. doi: 10.1111/jdi.12769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unamuno X, Gómez-Ambrosi J, Rodríguez A, Becerril S, Frühbeck G, Catalán V. Adipokine dysregulation and adipose tissue inflammation in human obesity. Eur J Clin Investig. 2018;48:12997. doi: 10.1111/eci.12997. [DOI] [PubMed] [Google Scholar]
- Wang AR, Yan XQ, Zhang C, Du CQ, Long WJ, Zhan D, Ren J, Luo XP. Characterization of Wnt1-inducible signaling pathway protein-1 in obese children and adolescents. Curr Med Sci. 2018;38:868–874. doi: 10.1007/s11596-018-1955-5. [DOI] [PubMed] [Google Scholar]


