Abstract
Tumour-associated fibroblasts (TAFs) mediate the differentiation of adjacent stromal cells. Berberine (BBR), a monomer of traditional Chinese herbs, exhibits a potent therapeutic effect against cancer. However, the effects of BBR on the differentiation of normal colonic epithelial cells induced by TAFs have not been determined. In the present study, we selected the TAF-like myofibroblast cell line CCD-18Co. CCD-18Co-derived conditioned medium (CM) and co-culture induced epithelial-mesenchymal transition (EMT) changes in colonic epithelial HCoEpiC cells with decreased E-cadherin and increased vimentin and α-SMA expression. In addition, CCD-18Co stimulated the expression of ZEB1 and Snail and promoted motility. We used LY364947, a TGF-β receptor kinase type I (TβRI) inhibitor, and BBR. Our results showed that LY364947 and BBR inhibited these phenomena. BBR decreased the expression of ZEB1 and Snail, and this effect was concentration dependent. BBR also downregulated the expression of TβRI, TβRII, Smad2/p-Smad2 and Smad3/p-Smad3. In addition, BBR induced apoptosis in EMT-like HCoEpiC cells in a concentration-dependent manner with upregulation of Bax and downregulation of Bcl-2. However, VX-702, an inhibitor of p38 MAPK, significantly suppressed the apoptosis rate. BBR promoted the expression of p38 MAPK and phosphorylated p38 MAPK. In conclusion, berberine inhibits EMT and promotes apoptosis in TAF-induced colonic epithelial cells through mediation of the Smad-dependent and SMAD-independent TGF-β signalling pathways.
Keywords: Tumor microenvironment, Tumor-associated fibroblasts, Epithelial-mesenchymal transition, Transforming growth factor-β, Berberine
Introduction
Substantial evidence has demonstrated that the tumour microenvironment (TME) participates in carcinogenesis. The TME is composed of various types of stromal cells, including endothelial cells, epithelial cells, fibroblasts and cancer cells. Many cytokines and inflammatory factors are also enriched in this microenvironment. These findings suggest that a complex cross-talk may exist in the niche. Although normal fibroblasts play important roles as sentinel cells to maintain epithelium homeostasis and to prevent the initiation of carcinogenesis, these cells existing in the TME, which are named cancer-associated fibroblasts (CAFs) or tumour-associated fibroblasts (TAFs) (Nissen et al. 2009), can contribute to cancer development by providing cancer cells with many cytokines and growth factors (Nissen et al. 2009; Glentis et al. 2017). Transforming growth factor-β (TGF-β) is the primary cytokine produced by TAFs (Kojima et al. 2010). In addition to the effect of TGF-β on cancer cells, TAF-derived TGF-β can also regulate the differentiation of other stromal cells (Achyut and Yang 2011). This regulation can enable the generation of an optimal microenvironment for tumour development (Neuzillet et al. 2015).
Binding of TGF-β to TGF-β receptors (TβRI and TβRII) leads to Smad2/3/4 complex formation and translocation to the nucleus (Smad pathway). These Smad complexes mediate the transcription of multiple target genes associated with EMT (Kahata et al. 2018; Loboda et al. 2016). Thus, the position of the classical Smad pathway is crucial in TGF-β-associated epithelial homeostasis and tumour progression (Yeh et al. 2018).
With the help of structural scaffolding and growth or differentiation regulatory mediators, TAFs can also communicate with other stromal cells in the TME, including epithelial cells. This communication involves the transdifferentiation of the epithelial phenotype into mesenchymal cells, which is called epithelial-mesenchymal transition (EMT) (Wang et al. 2019). Epithelial cells undergoing EMT induced by TAF-secreted TGF-β take part in the synthesis of the fibrotic matrix (Woodcock et al. 2019; Noguchi et al. 2018; Wu et al. 2015), leading to the remodelling of the TME. In this context, targeting the TGF-β pathway may be a promising strategy in abolishing the transition of epithelial cells into mesenchymal cells.
Berberine (BBR), an antibacterial agent, is an alkaloid isolated from rhizoma coptidis (Zou et al. 2017). It has been generally accepted that stromal cells and cancer cells in the TME produce various inflammatory cytokines (Huelsken and Hanahan 2018; Doldi et al. 2015; Nissinen et al. 2016), suggesting that berberine may play a potent interventional effect on these cytokines. Continuous research efforts have revealed that berberine and its derivatives have potential therapeutic activity to prevent digestive cancer (Ortiz et al. 2014; Zou et al. 2017). However, the regulation of berberine on stromal cells in the TME is vague.
In the present study, we adopted CCD-18Co cells, myofibroblasts from the colon. and extracted the supernatant (named conditioned medium). HCoEpiC, a normal colonic epithelial cell line, was treated with the medium to establish an EMT model. In addition, we also used a co-culture system to observe the influence of TAF on HCoEpiCs. The results of our study demonstrated that induction of EMT occurred in the CCD-18Co-treated HCoEpiCs, and this effect could be abolished by TGF-β1 antagonist and berberine. Our findings may suggest a novel application of BBR for anticancer treatment by mediating the differentiation of stromal cells in the TME.
Materials and methods
Cell line origin and maintenance
HCoEpiC, a human normal colonic epithelial cell line from American Type Culture Collection (ATCC), was purchased GuangZhou Jennio Biotech Co., Ltd. (China), and CCD-18Co cells, a human colonic myofibroblast line, were obtained from ATCC. HCoEpiC cells and CCD-18Co cells were recovered and cultured as previously described (Yang et al. 2018).
Primary antibodies and reagents
Berberine (BBR) and the TGFβ receptor kinase type I inhibitor LY364947 were purchased from Abcam (ab142117 and ab141890). The p38 MAPK inhibitor VX-702 was also employed (SD5960, Beyotime, China). All primary antibodies, including E-cadherin, vimentin, α-SMA, ZEB1, Snail, Smad2/p-Smad2, Smad3/p-Smad3 and Smad4, as well as secondary antibodies, were previously described (Yang et al. 2018). In addition, mouse monoclonal anti-phospho-p38 MAPK antibody (AM063, Beyotime), mouse monoclonal anti-p38 MAPK antibody (AM065, Beyotime), mouse monoclonal anti-Bax antibody (AB026, Beyotime) and rabbit monoclonal anti-Bcl-2 antibody (AB112, Beyotime) were also included.
Treatment and induction of HCoEpiC cells by a CM and co-culture model
Preparation of CCD-18Co cell-derived conditioned media (CM) was performed as previously described (Yang et al. 2018). In brief, CCD-18Co cells were treated with serum-free medium for 48 h, and the supernatants were collected (named CM). After centrifugation, the CM was mixed with serum-free medium up to 25%. Grouped HCoEpiCs were treated with CCD-18Co cell-derived CM and incubated for 24 h. Three EMT markers (E-cadherin, vimentin, and α-SMA) were detected.
The co-culture model was established. CCD-18Co and HCoEpiC cells were co-cultured based on a Transwell system with a 3:1 cell number ratio as previously described. In brief, 3 × 104 CCD-18Co cells were seeded into the ‘bottom’ chamber of a 24-mm Transwell plate. HCoEpiC cells (1 × 104) were also cultured with 1 ml serum-free RPMI 1640 onto the ‘top’ chamber of the Transwell plate. The co-cultured cells were also treated for 24 h.
Proliferation detection by cell counting kit-8 (CCK-8) assay
Cell proliferation was examined via a Cell Counting Kit-8 (C0038, Beyotime, China) according to the manufacture’s protocol. Cells were seeded into 96-well plates at a density of 5000 cells/well in a final volume of 100 μL and grown under normal conditions. The cells were treated with CM or LY364947 or BBR for 24 h; then, 10 μL CCK-8 reagent was added to each well and the cells were incubated at 37 °C for 2 h. Cell counts were calculated by measuring the absorbance at 450 nm with a microplate reader (Bio-Rad). Growth inhibition was calculated as a percentage of the normal controls (complete medium containing 10% FBS). Experiments were performed three times, and the data are presented as the mean ± standard deviation (SD) of five wells per treatment.
Cell motility detection by a wound healing assay
HCoEpiC cells grown to 90% confluency were wounded by a sterile 200-μl pipette tip and incubated with CM or CM + LY364947/BBR for 24 h, respectively. During incubation, 5 μM mitomycin C (50–07-7, Sigma) was simultaneously added. Wound healing was observed by phase contrast microscopy (10 × 10). The degree of healing was measured and quantified by an image analysis software ImageJ (National Institutes of Health, America).
Flow cytometry
The percentage of E-cadherin-labelled cells was detected by FACS flow cytometry. In brief, the cells treated with CM, LY364947 or co-culture were collected. The culture supernatant was removed, and the cells were washed with PBS. After the cells were treated by trypsinization, centrifugation was performed for 5 min (5000 r/min) to acquire cell suspension. The suspension was incubated with PE-labelled anti-E-cadherin antibody for 20 min at room temperature. Detection of these percentages of E-cadherin-marked cells was performed using BD FACSCalibur flow cytometry.
Immunofluorescence (IF) staining
Detection of immunofluorescence for E-cadherin, vimentin and α-SMA in HCoEpiC cells was performed as previously described (Yang et al. 2018). Briefly, the HCoEpiCs treated with different conditions were washed, fixed, and permeabilized. These cells were then incubated with blocking solution (PBS supplemented with 10% sheep serum) followed by incubation of primary antibodies for E-cadherin (1:200), vimentin (1:300) and α-SMA (1:300) at 4 °C overnight. These cells were incubated with goat anti-mouse AlexaFluor-488 antibodies (E-cadherin) and goat anti-rabbit FITC antibodies (vimentin and α-SMA) for 2 h at 37 °C. The cell nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (C1005, Beyotime, China). The immunofluorescence and mean intensity were observed by a fluorescence microscope (Olympus) equipped with a 960S-Fluor oil immersion lens and Image-Pro®Plusv6.0 (Media Cybernetics, America).
Apoptosis of detection by flow cytometry
All detection procedures were performed using the Annexin V/Propidium Iodide (PI) kit. In brief, suspension cells (5 × 105) were collected and centrifuged (2000 rpm for 5 min) followed by two rounds of PBS washing. Then, 500 μL binding buffer was added to the suspension followed by 5 μL Annexin V with 5 μL PI. Reaction was performed at room temperature for 10 min. The apoptosis rates were observed by flow cytometry (BD Biosciences AccuriC6, America).
Total RNA extraction and quantitative real-time polymerase chain reaction (qRT-PCR)
The cells treated by CM and BBR were washed and re-suspended in ice-cold TRIzol solution (Invitrogen, USA), and then total mRNA was extracted by an RNA Easy Kit (Invitrogen) according to the manufacturer’s instructions. The concentration of mRNA was measured by a micro-spectrophotometer K2800 (Kai’ao, Beijing, China), with the ratio of OD260/OD280 > 1.8. All reverse-transcription of total RNA into cDNA was performed using SYBR® Premix Ex Taq™ (Tli RNaseH Plus) and Reverse Transcriptase M-MLV (RNase H-) (TAKARA, Japan). The performance of real-time PCR was realized by a CFX96 PCR system (Bio-Rad, USA) using ViiA7 software (Thermo Fisher, USA), and the reaction system recommended by the manufacturer was used. Specific forward and reverse PCR primers were designed using a PRIMER5/NCBI system. TβRI: F: TCCAACTACTGGTTTACCATTGC, R: ACAGCAACTTCTTCTCCCCG, 129 bp; TβRII: F: GCAGCGCTGAGTTGAAGTTG, R: GAGGGAAGCTGCACAGGAG, 107 bp. β-actin served as an internal control in all reactions. The comparative cycle threshold (ΔCt) method was used to quantify normalized target gene expression relative to the internal β-actin. Data are shown as the relative gene expression =2-ΔΔCt.
Transfection of HCoEpiC cells with TGF-β receptor plasmids
Expression plasmids of TβRI and TβRII (the gene sequences as described previously) were purchased from Guangzhou Jennio Biotech Co., Ltd. (China). The HCoEpiCs treated with BBR were transfected by the plasmids. In brief, transient transfection of TβRI and TβRII cDNA plasmid pCMV6-XL5 (3.0 μg) into the treated HCoEpiC cells was performed using Lipofectamine (Life Technologies, Gaithersburg, MD, USA) and the manufacturer’s instructions. The cells were harvested 48 h after transfection, and 25%–30% of transfection-positive cells were identified. These transfected cells were continuously treated with BBR for 24 h, and the expression of E-cadherin, vimentin and α-SMA was detected by western blotting.
Western blotting analysis
For western blotting, protein extracts were prepared and run as previously described (Chao et al. 2016). In brief, 10 μg of each protein sample was separated by 10% SDS-PAGE gel and electrotransferred onto a PVDF membrane at 120 V for 1 h. The membranes were blocked in Tris Buffered Saline Tween (TBST) buffer (20 mM Tris–HCl, pH 7.6, 150 mM NaCl, 0.1% Tween-20) with 10% non-fat milk at 25 °C for 2 h. The incubation of membranes was performed with primary antibodies, including E-cadherin, vimentin, α-SMA, ZEB1, Snail, Smad2, Smad3, p-Smad2, p-Smad3, Smad4, p38 MAPK, Bax, Bcl-2, and β-actin antibodies, and the corresponding horseradish peroxidase (HRP)-conjugated secondary antibodies. All protein bands and intensities were measured with a Molecular Imager ® Chemi Doc™ XR+ system with Image Lab TM Software (Bio-Rad).
Statistical analysis
Analysis of all data was performed by the statistical software SPSS 20.0. Comparison between groups was performed using ANOVA, and LSD-t was used to make the post hoc multiple comparisons. Correlation analysis between effects of berberine on TGF-β receptors and p38 MAPK phosphorylation was performed by pearson (r value). Differences were considered to be significant if the P value was less than 0.05 (*P < 0.05). All data are representative of at least three different experiments.
Results
Berberine inhibited the CCD-18Co-induced EMT-like transition in colonic epithelial HCoEpiC cells
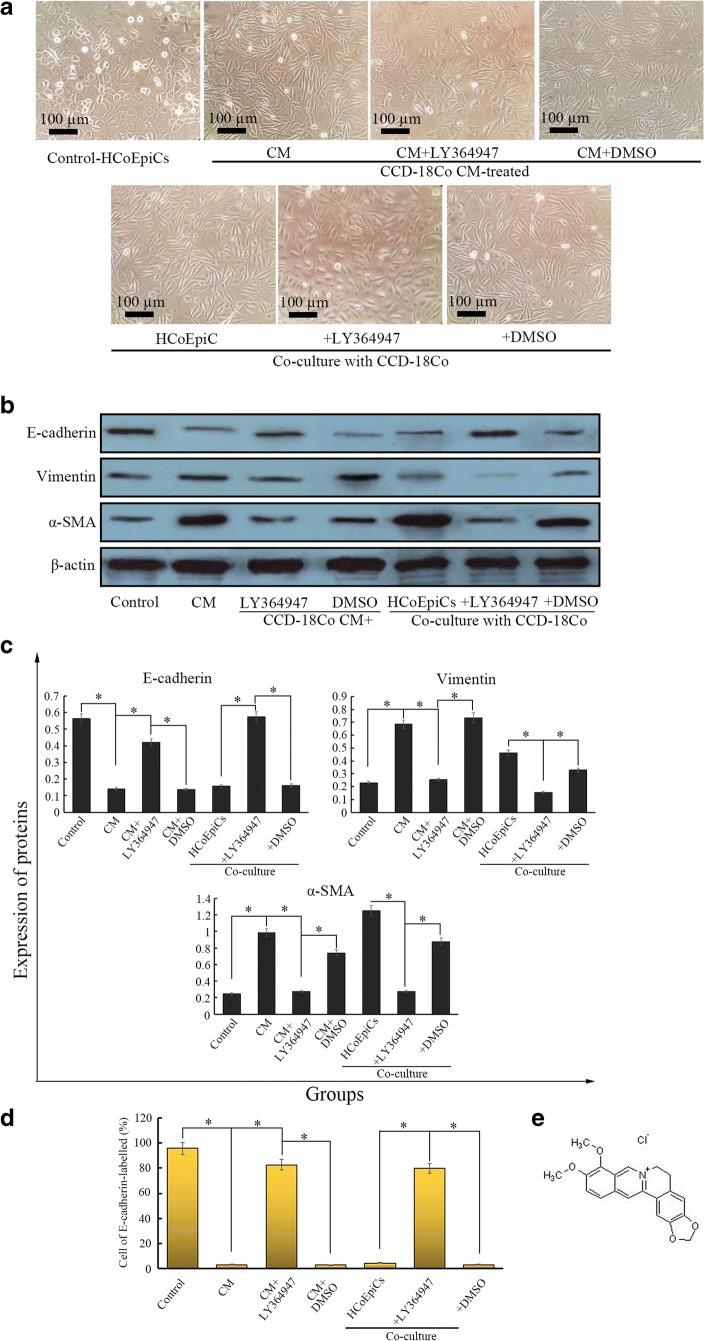
Many studies have demonstrated that TAFs participate in the transdifferentiation of other stromal cells in the TME. Our previous study found that exogenous TGF-β1 induced EMT changes in immortalized colonic epithelial NCM460 cells (Huang and Wen 2016). Considering the functions of TAFs in EMT, we anticipate that TAFs can also induce EMT transformation and motility in colonic epithelial cells. In this study, we first prepared conditioned medium (CM) derived from CCD-18Co cells. We found for the first time that preparation of 25% CM induced spindle-like morphological changes in HCoEpiCs after treatment for 24 h (Fig. 1a), compared with the control (serum-free RPMI 1640 media). We also observed a similar phenomenon in the co-cultured Transwell model (Fig. 1a). CCD-18Co decreased the expression of E-cadherin and increased the expression of vimentin and α-SMA (Fig. 1b and c) compared to the control (P < 0.05). Flow cytometry revealed that the percentage of E-cadherin-labelled cells in the CM- or co-culture-treated HCoEpiCs was 3.49% and 4.58%, respectively, which were significantly lower than that of the control (95.58%) (Fig. 1d, P < 0.05). These observations suggest that CCD-18Co induced EMT-like changes in HCoEpiCs. After treatment with the TGF-β receptor kinase I inhibitor LY364947 (5 μg/ml), the above observations were suppressed (Fig. 1a–d). LY364947 upregulated the expression of E-cadherin and the percentage of cells labelled with E-cadherin and downregulated the expression of vimentin and α-SMA compared with the CM group (P < 0.05). CCD-18Co-induced overexpression of ZEB1 and Snail was also inhibited by LY364947 (Fig. 2a–d) compared to the CM group (P < 0.05). Interestingly, these observations of LY364947 are similar to those of berberine.
Fig. 1.
HCoEpiC cells are treated by CCD-18Co-derived CM and co-cultured with the CCD-18Co cells. a In the CM model, 25% CCD-18Co CM induces HCoEpiCs for 24 h, while HCoEpiCs are co-cultured with CCD-18Co cells using a Transwell model for 24 h. The control is HCoEpiC cells without CM treatment. Morphological observation (200 x) was performed by a phase contrast microscope (Olympus). b Three EMT markers, E-cadherin, vimentin and α-SMA, were detected using western blotting in the CM-treated and co-cultured models. c Histograms of expression for the three EMT markers. d Percentage of cells remaining epithelial under these different conditions. The percentage is detected by E-cadherin-PE staining through flow cytometry. e Molecular structure of Berberine. The error bar represents the SD (n = 3). *P < 0.05
Fig. 2.
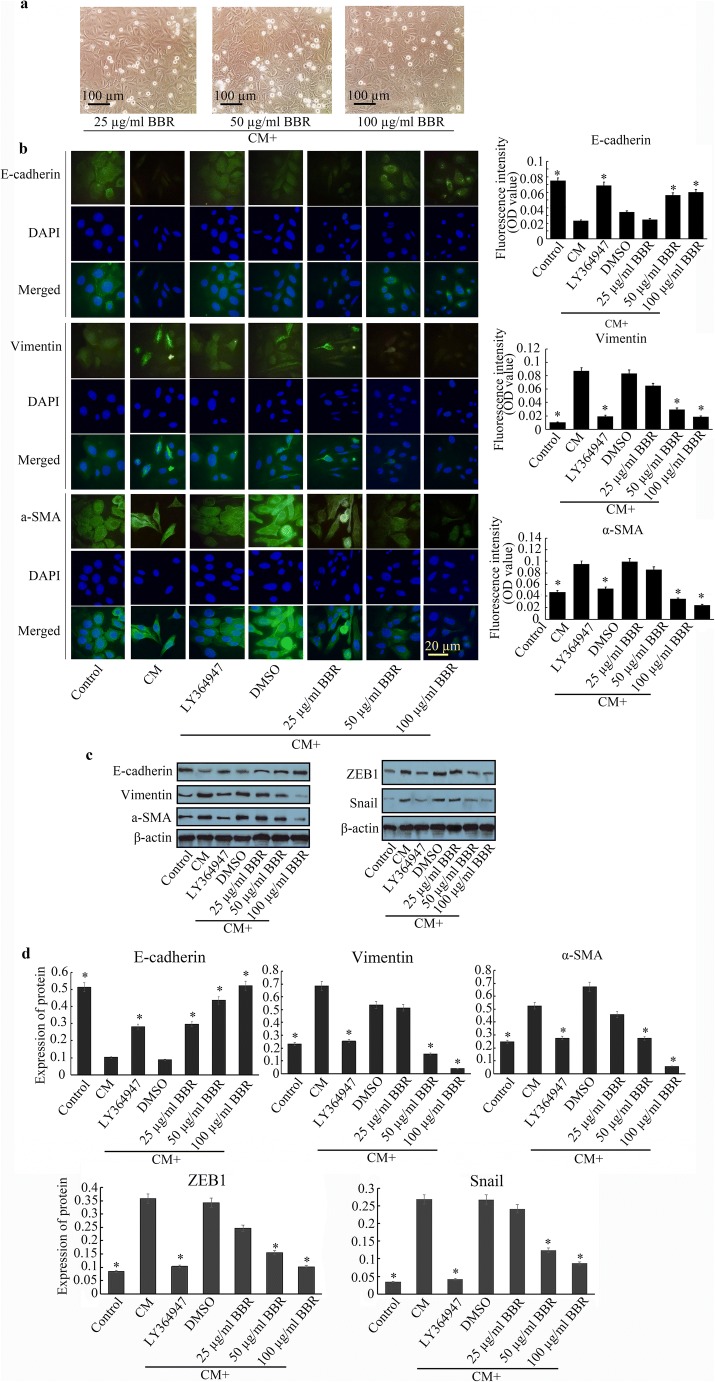
After treatment with CCD-18Co CM for 24 h, observations of the morphology and immunofluorescence of EMT markers and detection of EMT-related transcription factors ZEB1 and Snail are made. a Morphological observation of the BBR-treated cells (10 × 20). b (Left) Immunofluorescence of E-cadherin, vimentin and α-SMA is observed by a fluorescence microscope (Olympus) equipped with a 960S-Fluor oil immersion lens (10 × 100). Green fluorescence of E-cadherin is stained by AF488 (Alexa Flour 488), while stained green fluorescence of vimentin and α-SMA is performed by fluorescein isothiocyanate (FITC). Cell nucleus counterstaining was performed by DAPI. The histograms represent the fluorescence intensities of the EMT markers (right). c Detection of EMT markers and ZEB1 and Snail was performed by western blotting. d The histograms of expression for EMT markers and ZEB1 and Snail. The error bar represents the SD (n = 3). *P < 0.05 versus CM group
Berberine (BBR) is an isoquinoline quaternary alkaloid (Fig. 1e) that is extracted from several kinds of medicinal plants, such as Hydrastis canadensis, Berberis aristata, and Coptis chinensis (Remppis et al. 2010; Chen et al. 2012). Increasing research has shown that BBR plays important roles in many diseases through its antioxidant, antiapoptotic, and anticancer effects (Kulkarni and Dhir 2010; Kuo et al. 2011). BBR can not only inhibit cancer cell proliferation but also abolish the EMT of malignant cells, suggesting that the anti-EMT effect of BBR may be a key mechanism. We found that after treatment with BBR for 24 h, the above morphological changes of HCoEpiCs induced by CM were inhibited to different extents, and the inhibitory effect was the most significant at 100 μg/ml BBR followed by 50 μg/ml BBR. BBR also increased the expression of E-cadherin and decreased the expression of vimentin and α-SMA in a concentration-dependent manner (Fig. 2b–d) with a reduction of ZEB1 and Snail (Fig. 2c and d), especially 50 μg/ml BBR and 100 μg/ml BBR, compared with the CM group (P < 0.05). However, no significant difference in vimentin, α-SMA, ZEB1 and Snail was observed in the 25 μg/ml BBR group compared to the CM group.
Berberine suppressed the motility of HCoEpiCs undergoing EMT induced by CCD-18Co
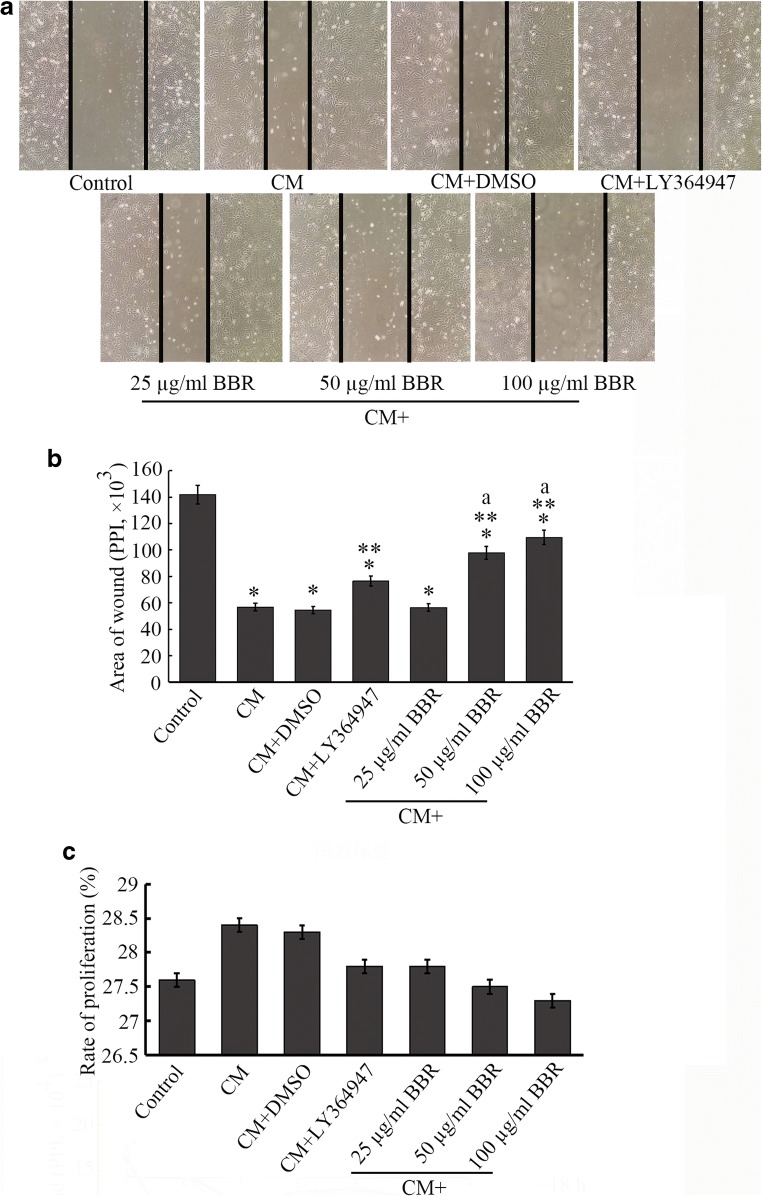
EMT can stimulate cell motility (Gok Yavuz et al. 2019), leading to enhanced invasion and migration. As demonstrated in our study (Fig. 3a and b), the motility of the HCoEpiC cells was clearly promoted by the stimulation of CCD-18Co CM (The area of wound was 56,841 ± 168 PPI), in contrast to the control cells (141,812 ± 289 PPI, P < 0.05). These results demonstrate that CCD-18Co CM stimulates the motility of HCoEpiC cells treated by mitomycin. To observe whether cell proliferation was affected by the CM, proliferation detection kit (CCK-8) was used. We found that the CM treatment mildly promoted the proliferation (28.4%), comparing to the control (serum-free medium) or LY364947 group (27.6% or 27.8%, P > 0.05, Fig. 3c).
Fig. 3.
Cultured HCoEpiC cells are wounded by a 200-μl pipette tip and observed by phase contrast microscopy (100 ×) after different treatments for 24 h (a). To measure the area of the wound, software (ImageJ) was used, and the area of wound was presented as pixels per inch (PPI), *P < 0.05 versus control, **P < 0.05 versus CM group, and aP < 0.05 versus CM + 25 μg/ml BBR group (b). The error bar represents the SD (n = 3). In addition, we conducted a CCK-8 assay to observe the effect of CM or BBR on cell proliferation. As shown in c, the percentage of proliferation of the CM or DMSO group was mildly higher than that of the control group, and there was no significant difference between the two groups. Similarly, a significant difference between the BBR groups and the CM or control group was not observed, suggesting that the effect of CM or BBR treatment on proliferation was not significant
A study by Kim observed that BBR inhibited cell motility in triple-negative breast cancers through suppression of TGF-β expression (Kim et al. 2018). After treatment with BBR, we found an inhibitory effect of BBR on motility in CCD-18Co CM-induced HCoEpiC cells (Fig. 3a and b) compared to the CM or DMSO group (P < 0.05), and this anti-motility effect was similar to that of LY364947. Although BBR decreased the proliferation percentage (Fig. 3c), there was no statistical difference between BBR group (27.8%, 27.5%, and 27.3% for 25-, 50-, 100-μg/ml BBR, respectively) and control or CM group (P > 0.05). These results suggest that BBR is likely to act through the regulation of TGF-β signalling. However, no significant difference among groups treated with 25 μg/ml, 50 μg/ml and 100 μg/ml BBR was observed at 18 h and 24 h (P > 0.05).
Berberine regulated Smad expression in TGF-β signalling during the inhibition of CCD-18Co-induced EMT in HCoEpiCs
The above results indicate that CCD-18Co induced an in vitro EMT-like transition in HCoEpiCs, which was abolished by TGF-β receptor inhibitor. These findings reflect that the TGF-β signalling pathway may be involved in the process. We observed that the mRNA levels of TβRI and TβRII were increased in CM-treated and co-cultured HCoEpiCs, which was reduced by LY364947 (Fig. 4a) compared to the CM group and co-culture group (P < 0.05). In addition, BBR also reduced the levels of both TβRI and TβRII. In the CM-treated model, the inhibitory effect of BBR on TβRI and TβRII was negatively dose-associated, but this effect was positively dose-dependent in the co-culture model. 25 μg/ml and 50 μg/ml BBR significantly downregulated the levels of TβRI and TβRII, while the effects of 50 μg/ml and 100 μg/ml BBR were obvious in the co-culture model (Fig. 4a). These results have shown that TGF-β receptor signalling may be involved in this EMT and are likely the target of the BBR. To verify this hypothesis, TβRI and TβRII plasmids were used. We found that both TβRI and TβRII reversed the upregulation of E-cadherin and the downregulation of α-SMA and vimentin by the BBR compared to the control plasmid (Fig. 4b).
Fig. 4.
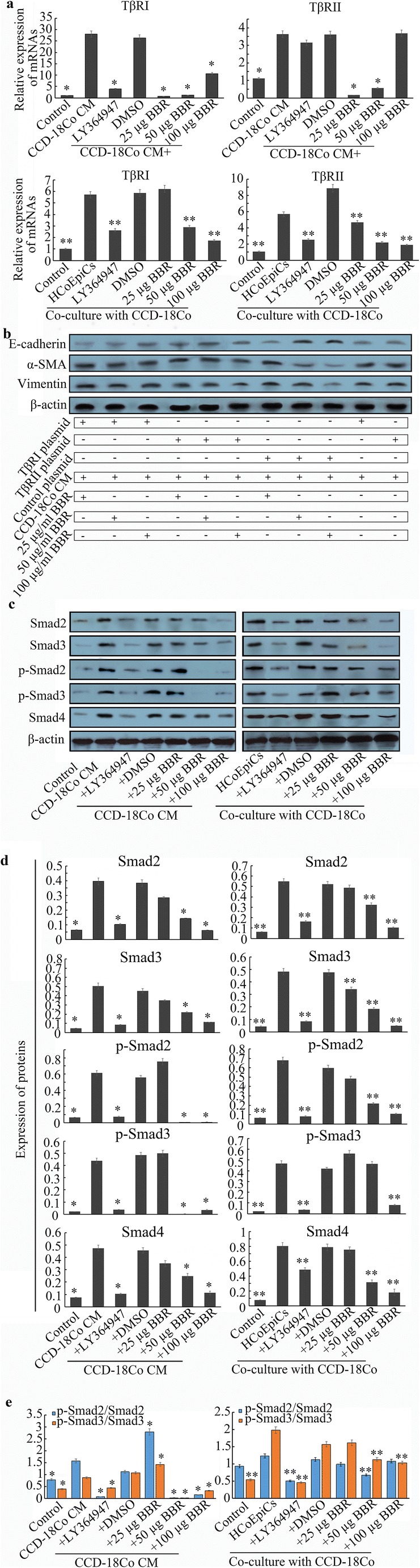
a Levels of TβRI and TβRII mRNAs are detected by qRT-PCR. CCD-18Co significantly promoted TβRI and TβRII mRNA levels in CCD-18Co CM (CM) and co-culture models compared to the control and LY364947 groups (P < 0.05). BBR downregulates the levels of TGF-β receptors in a dose-dependent manner (P < 0.05). b The effect of over-expressed TβRI and TβRII on the anti-EMT activities of BBR by establishing the expression plasmids. Overexpression of TβRI and TβRII abolishes BBR-induced E-cadherin expression to different extent. c Expression levels of Smad2, Smad3, p-Smad2, p-Smad3 and Smad4 were measured using western blotting. d The histograms of expression for the above Smads. CCD-18Co stimulates the expression of Smad2/3/4 and p-Smad2/3 compared to the control (P < 0.05). However, BBR significantly decreased the expression of Smads or p-Smads compared with the CM or co-culture model (P < 0.05), and these effects were dose dependent. e Ratios of p-Smad2/Smad2 or p-Smad3/Smad3 under different conditions. CCD-18Co promotes the ratios of p-Smads/Smads compared to the control (P < 0.05), which are reversed by BBR. These data suggest that BBR may inhibit the high activities of TGF-β/Smad signalling induced by CCD-18Co. The error bar represents the SD (n = 3). *P < 0.05 versus CM group; **P < 0.05 versus co-cultured HCoEpiCs
In addition to the influence on TβRI and TβRII, CCD-18Co also mediated the expression of Smads. As shown in Fig. 4c and d, the expression levels of Smad2, Smad3, Smad4, p-Smad2 and p-Smad3 were increased after treatment with CCD-18Co, which was blocked by LY364947. After treatment with BBR, the expression levels of Smad2 and Smad3 were reduced in the two models. In addition, BBR also downregulated the expression of p-Smad2 and p-Smad3, especially at 50 μg/ml and 100 μg/ml. Although 25 μg/ml BBR increased the ratios of p-Smad2/Smad2 and p-Smad3/Smad3 in the CM model, 50 μg/ml BBR and 100 μg/ml BBR significantly decreased this ratio (P < 0.05, Fig. 4e). Similarly, in the co-culture model, 50 μg/ml BBR decreased the ratio of p-Smad2/Smad2, and 50 μg/ml BBR and 100 μg/ml BBR significantly decreased the ratio of p-Smad3/Smad3 compared with the co-cultured HCoEpiCs (P < 0.05).
Smad4, a TGF-β signal mediator, is required for the occurrence and maintenance of EMT, and in gastrointestinal cancers, inactivation or decreased expression of Smad4 is frequently observed (David et al. 2016). Considering that TGF-β-induced EMT generally takes part in pro-tumorigenic events (David et al. 2016), Smad4 is deemed a pro-tumour factor. Hence, repressing Smad4 is a reasonable strategy. In our study, we found that CCD-18Co stimulated the upregulation of Smad4 expression in the CM and co-culture models compared to the control (P < 0.05), but BBR decreased its expression, and this effect was dose-dependent (Fig. 4c and d).
Berberine induced apoptosis and promoted phosphorylated p38 MAPK expression in CCD-18Co CM-induced HCoEpiCs
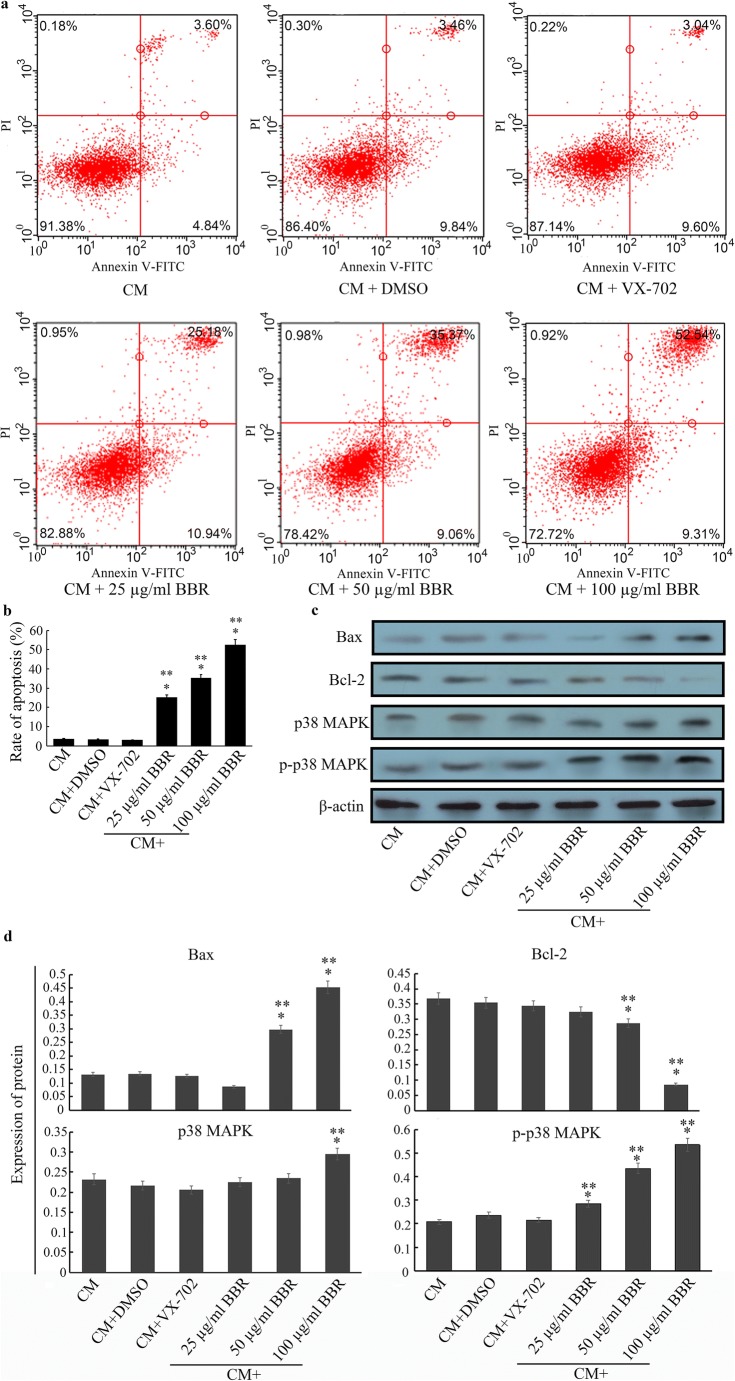
Increasing evidence has shown that BBR induces apoptosis of breast cancer cells by suppressing the HER2/PI3K/AKT pathway (Kuo et al. 2011). In our study, we found that BBR also triggered a pro-apoptotic effect on EMT-like HCoEpiC cells, as shown in Fig. 5a and b, compared with the CM group (P < 0.05). After treatment with different doses of BBR, the apoptosis rate was clearly higher than that of the CM group and DMSO group (P < 0.05), and this effect was BBR-dose-dependent. With LSD-t, the apoptosis rate was the highest in the 100 μg/ml group followed by the 50 μg/ml and 25 μg/ml groups (P < 0.05). In addition, BBR also promoted the expression of the pro-apoptotic protein Bax and inhibited the expression of the anti-apoptotic protein Bcl-2 in a dose-dependent manner (Fig. 5c and d). As a crucial component of non-Smad pathways (Wongnoppavich et al. 2017), p38 MAPK has key biological functions (He et al. 2018), including the regulation of apoptosis (Zhu et al. 2017). Previous results have revealed that BBR induced apoptosis in EMT-like HCoEpiCs and significantly increased the expression of p38 MAPK, especially at 100 μg/ml, and BBR also increased the expression of phosphorylated-p38 MAPK (p-p38 MAPK) in a dose-dependent manner (Fig. 5c and d) compared with the CM group (P < 0.05). We hypothesized that the effect of BBR was regulated by p38 MAPK. To verify this hypothesis, we used the p38 MAPK inhibitor VX-702 (0.5 mg/ml) to pre-treat CM-induced HCoEpiCs. Our results demonstrated that 50 μg/ml BBR (which we selected as a representative) did not promote the apoptosis rate in these pre-treated cells (Fig. 5a and b), and the expression of p38 MAPK and p-p38 MAPK was also inhibited in these cells (c group, Fig. 5c and d).
Fig. 5.
a Scatter diagram of apoptosis analysis by Annexin V-FITC/PI staining. b Analysis of percent apoptosis rates. The rate of apoptosis in the 100 μg/ml BBR group was 52.45%, followed by the 50 μg/ml BBR group (35.37%) and the 25 μg/ml BBR group (25.18%), which were higher than those of the CM (3.61%) or DMSO group (3.46%) (P < 0.05). A 0.5 mg/ml concentration of VX-702, a p38 MAPK inhibitor, significantly decreased the rate of apoptosis (3.04%) compared to the BBR group (P < 0.05). c Detection of apoptosis-associated proteins Bax, Bcl-2, p38 MAPK and p-p38 MAPK through western blotting. d The histograms of expression for Bax, Bcl-2, p38 MAPK and p-p38 MAPK. The 50 and 100 μg/ml concentrations of BBR significantly increased the expression of Bax and decreased the expression of Bcl-2 compared to the CM, DMSO or VX-702 groups (P < 0.05). Although only 100 μg/ml BBR upregulates the expression of p38 MAPK, p-p38 MAPK expression is increased by BBR in a dose-dependent manner compared to the CM, DMSO or VX-702 groups (P < 0.05). The error bar represents the SD (n = 3). *P < 0.05 versus CM group; **P < 0.05 versus VX-702 group
On the other hand, correlation between effects of TGF-β receptors and p38 phosphorylation was analyzed using pearson linear correlation analysis (as shown in Table 1). We found that reduced TβRI level was positively correlated with increased p38 phosphorylation in CM model (r = 0.803, P < 0.0001), while the correlation between reduced TβRII level and p-p38 MAPKwas not significant in this model (r = 0.255, P = 0.679). In transwell model, both reduced TβRI and TβRII were negatively correlated with increased p38 phosphorylation (r value was −0.982 and − 0.952, P < 0.0001).
Table 1.
Correlation analysis between effects of berberine (BBR) on TGF-β receptors and p38 phosphorylation by Pearson
| Factors affected by BBR | Increased p38 phosphorylation | |
|---|---|---|
| r value | P value | |
| Reduced TGF-β receptor I (CM model) | 0.803 | <0.0001 |
| Reduced TGF-β receptor II (CM model) | 0.255 | 0.679 |
| Reduced TGF-β receptor I (Transwell model) | −0.982 | <0.0001 |
| Reduced TGF-β receptor II (Transwell model) | −0.952 | <0.0001 |
Discussion
As fibroblasts in the normal wound healing process, fibroblasts existing in the tumour stroma present a myofibroblast-like phenotype and are referred to as myofibroblasts, tumour-associated fibroblasts (TAFs) or cancer-associated fibroblasts (CAFs). This finding emphasizes the crucial position of TAFs in carcinogenesis, supporting the hypothesis proposed by Dvorak that tumours are wounds that do not heal (Dvorak 1986). These TAFs/CAFs can produce various types of growth factors, cytokines, collagens and extracellular matrix proteins (ECM), providing scaffolds for tumour growth and development (Zhou et al. 2017; Khawar et al. 2018). These factors reinforce the cross-talk between cancer cells and stromal cells or between stromal cells.
TAFs have also been termed peritumoural (myo-)fibroblasts. CCD-18Co, a myofibroblast line (Giménez-Bastida et al. 2018), plays an important role in the remodelling of the surrounding matrix (Gok Yavuz et al. 2019; Pereira et al. 2015), which is similar to the function of TAFs in microenvironment remodelling (Kim et al. 2018). Thus, we selected CCD-18Co as a TAF model to evaluate the effect of TAFs on colonic epithelial cells. Our results have shown that CCD-18Co stimulated spindle-like changes and induced the reduction of E-cadherin and the enhancement of vimentin and α-SMA in colonic epithelial HCoEpiCs in the two models. Both models can mimic the interaction between TAF and epithelial cells, but the interaction is unilateral in the CM model and bilateral in the other model. In addition, the influence of serum on the results or the effect of BBR or LY364947 on CCD-18Co cells may exist in the co-culture model. Thus, we selected the two models to reciprocally compare the results. In addition to the morphological changes, CCD-18Co-derived CM also increased the expression of Snail and ZEB1 in a dose-dependent manner. These results suggest that CCD-18Co stimulates epithelial-mesenchymal transition (EMT)-like changes in HCoEpiCs. Interestingly, these phenomena can be abolished by the TGF-β receptor kinase type I inhibitor LY364947, indicating that TGF-β receptor signalling may participate in the induction or maintenance of EMT. Novel studies have uncovered that a great deal of TAF-secreted TGF-β can enhance the stromal reaction and induce transdifferentiation of epithelial cells. In this scenario, TGF-β pathway becomes a dominant inducer of EMT in various tumour types (Zhao et al. 2018; Wang and Huang 2019). Inhibition of the TGF-β signalling pathway may be a promising strategy for reversing EMT.
Berberine (BBR), an isoquinoline quaternary alkaloid, is extracted from varieties of medicinal plants (Akiyama et al. 2019). BBR has multiple pharmacological characteristics, including antioxidant, anti-inflammatory, and anticancer effects (Cicero and Baggioni 2016; Chen et al. 2019). Notably, BBR also has potent antimicrobial activity and cytotoxic effects on cancer cells. For example, BBR can induce growth arrest and apoptosis of cells in multiple tumour types (Du et al. 2017; Liu et al. 2018). As an inducer of EMT, TGF-β1 promoted invasion of human lung carcinoma A549 cells, which was inhibited by BBR (Chu et al. 2014). Further study has found that BBR increased the expression of E-cadherin and decreased vimentin expression (Chu et al. 2014). A previous study demonstrated that BBR inhibited the metastatic ability of prostate cancer cells by suppressing EMT-associated genes, including BMP7, NODAL and Snail (Liu et al. 2015). In a recent study by Yang (Yang et al. 2017), BBR inhibited renal tubular EMT in diabetic nephropathy model KKAy mice through suppression of the Notch/Snail pathway. These findings suggest that BBR may regulate the phenotypic transition of epithelial cells. We further found that BBR increased the expression of E-cadherin and reduced the expression of vimentin and α-SMA in a dose-dependent manner, revealing that BBR can regulate TAF-induced EMT in the colonic epithelium.
Induction of EMT is a mechanism by which cell motility is enhanced (Kryczka et al. 2019). Substantial studies have shown that BBR inhibited the migration of many types of cancer cells (Wang and Zhang 2018; Kim et al. 2018). We found that LY364947 and BBR suppressed CCD-18Co-promoted motility in HCoEpiCs, and this effect of BBR is associated with its concentrations. For this phenomenon, we consider that BBR exerts an anti-migration effect through the inhibition of matrix metalloproteinase 3 (MMP3) in a dose-independent manner (Hu et al. 2018) in addition to its inhibitory effect on EMT markers or EMT transcription factors. Therefore, the anti-motility effect of BBR is dose dependent in our study. In addition, we have observed that CM stimulation only mildly promoted the proliferation comparing with the control (P > 0.05), suggesting that growth factors promoting proliferation maybe not exist or extremely low in the CM. After treatment with BBR, the proliferation was decreased, and this effect was seemingly dose dependent, but there is no statistical difference between BBR and control or CM group (P > 0.05). We speculate that BBR indeed don’t affect the proliferation (This effect may be caused by experimental bias). Alternatively, BBR inhibits cell proliferation through mediation of antiproliferation mechanisms, such as inducing cell cycle arrest (Lin et al. 2019) and alleviating inflammatory signaling including TGF-β (Liu et al. 2018).
Type I and II TGF-β receptors (TβRI and TβRII) are key members of the TGF-β signalling pathway. TβRII binding to TGF-β ligand recruits and phosphorylates TβRI, promoting the activation and phosphorylation of the downstream mediators (Smad2 and Smad3), which contributes to combination with Smad4. Entrance of the Smad2/3/4 complex into the nucleus regulates the transcription of genes associated with proliferation, differentiation and migration (Ji et al. 2015). In the present models, CCD-18Co upregulated the expression of TβRI and TβRII mRNA and increased the expression of Smad2/p-Smad2, Smad3/p-Smad3 and Smad4. We consider that high expression of TβRI and TβRII is likely associated with increased expression at the translation level or enhanced stability at the mRNA level. In addition, because TGF-β can promote the expression of TβRI and TβRII (Bloom et al. 1996; McWhirter et al. 1994), the expression of both proteins may be mediated by the CCD-18Co-secreted TGF-β. TGF-β can contribute to the increased TGF-β1 mRNA transcription through AP-1-mediated mechanisms (Kim et al. 1990), resulting in the biological functions of TGF-β, including its pro-EMT effect, which are amplified through enhancement of TGF-β itself and its receptors. The above events were abolished by the TGF-β receptor inhibitor LY364947, which is consistent with other studies. In a study by Dong (Dong et al. 2016), TGF-β1 upregulated the expression of p-Smad3 in the process of EMT. In contrast, staining for p-Smad2/3 in colorectal cancer was dramatically reduced compared with that in adjacent stromal cells or with the epithelial compartment of pre-malignant tissue (Zhao et al. 2018). These findings have shown that p-Smad2/3 expression is different in EMT and carcinogenesis.
Existing evidence has shown that over-activated TGF-β-Smad2 signalling contributes to the establishment of EMT by epigenetic silencing of key epithelial genes, such as E-cadherin (Dong et al. 2018). In this scenario, the mediation of TGF-β/Smad signalling may be one of the mechanisms underlying the anti-EMT and anti-motility of BBR. BBR significantly decreased the levels of TβRI and TβRII mRNA in the two models. However, the relationship between BBR dose and expression of TβRI and TβRII is different in the models. This finding is likely to be associated with the experimental bias. Taken together, these findings indicate that BBR reduces the expression of TβR mRNAs. These observations imply that TGF-β receptors take part in the anti-EMT of BBR. To confirm these results, we established overexpressed TβRI and TβRII plasmids in these BBR-treated cells. Our results showed that overexpression of TβRI and TβRII relieved the upregulation of E-cadherin and the downregulation of vimentin and α-SMA induced by BBR. On the other hand, BBR also downregulated the expression of Smad2/p-Smad2 and Smad3/p-Smad3 in CCD-18Co CM and co-culture models. These results demonstrate that BBR inhibits the hyperactivity of TGF-β/Smad signalling. As shown in the comparison of the ratios of p-Smad2/Smad2 and p-Smad3/Smad3, BBR significantly downregulated the ratio of p-Smad2/Smad2 or p-Smad3/Smad3.
Smad4 is required for the activation of the TGF-β pathway. Studies have found a positive correlation of Smad4 with Snail-1, Slug and Twist-1 expression in colon tumour specimens (Ioannou et al. 2018). Thus, we consider that high-expressed Smad4 also contributes to the EMT process. In our study, CCD-18Co CM or co-culture stimulated the expression of Smad4, but it was suppressed by BBR in a dose-dependent manner. This observation suggests that BBR exerts anti-EMT effects through mediating TGF-β/Smad4 signalling.
As a crucial determinant of tumour cell sensitivity to apoptosis, TGF-β plays a crucial role in the development of cancer (Cornell et al. 2019). Non-classical Smad pathways in TGF-β signalling play crucial roles in the mediation of apoptosis (Gunaratne et al. 2015; Gaitantzi et al. 2018), including p38 MAPK [54]. Activation of p38 MAPK can further activate the phosphorylation of MAPK kinase, resulting in the expression of transcription factors, including ATF-2, Max and MEF2. p38 MAPK signalling is a key mediator of apoptosis. Thus, orchestrating the regulation of the TGF-β signalling pathway in apoptosis induction is a promising strategy. We found that BBR promoted apoptosis in CM-treated HCoEpiCs in a dose-dependent manner (Fig. 5a and b, P < 0.05). In addition, BBR also stimulated the expression of the pro-apoptotic protein Bax and repressed the expression of the anti-apoptotic protein Bcl-2 (Fig. 5c and d), which is consistent with the results of the previous study by Li, where BBR increased Bax/Bcl-2 to induce mitochondrial apoptosis in thyroid carcinoma cells (Li et al. 2017). Interestingly, BBR significantly increased the expression of p38 MAPK and phospho-p38 MAPK (p-p38 MAPK), consistent with that of Li (Li et al. 2017). Furthermore, the upregulation of the expression of p-p38 MAPK by BBR is dose dependent compared to its effect on p38 MAPK. These results suggest that BBR induces apoptosis through activation of p-p38 MAPK. After pre-treatment with the p38 MAPK inhibitor VX-702, the pro-apoptotic effect of BBR (50 μg/ml) was weak, with a reduction of Bax and an increase of Bcl-2 being observed. These results reveal that p38 MAPK signalling participates in the regulation of BBR apoptosis (Fig. 6). In addition, our results reveal that reduced TβRI level is positively correlated with increased p-p38MAPK in CM model, while both reduced TβRI and TβRII were negatively correlated with p-p38MAPK, suggesting that the link between effects of BBR on TGF-β receptors and p-p38MAPK is significant and model dependent. We consider that this dependence may be associated with different cellular environment.
Fig. 6.
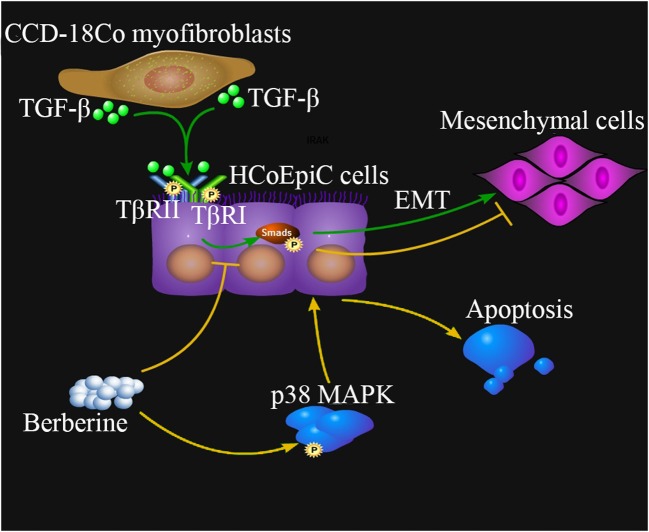
Schematic diagram for relevant mechanisms of CCD-18Co-induced EMT and berberine treatment. TGF-β, one of the cytokines produced by CCD-18Co myofibroblasts, binds TGF-β receptors (TβRII and TβRI) and induces EMT transformation of colon epithelial HCoEpiC cells through Smads signaling pathway (Green lines. Arrows represent activation or promotion). Berberine can inhibit the TGF-β/Smads signaling to suppress the CCD-18Co-stimulated EMT. Berberine also promotes apoptosis of HCoEpiCs through activation of p38 MAPK pathway (Yellow lines, Arrows represent activation or promotion, flat lines represent inhibition or down-regulation)
Taken together, our findings suggest that TGF-β may be an effector for CCD-18Co cells in communication with colonic epithelial HCoEpiC cells and that berberine inhibits the CCD-18Co-induced EMT-like changes in HCoEpiC cells through the TGF-β/Smads pathway and induces apoptosis through the p38 MAPK pathway.
Acknowledgments
This work was supported by Natural Science Foundation of Guangdong Province (2018A030310060), China Postdoctoral Foundation (2018 M643353).
Author contributions
C.H wrote the main manuscript text, F.Q and L. W prepared Figs. 1–5, and L. P examined all the manuscript. All authors reviewed the manuscript.
Compliance with ethical standards
Conflict of interest
No potential conflicts of interest were disclosed.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chao Huang, Email: huangchao06@163.com.
Zuo-liang Pang, Email: pangzuoliang@126.com.
References
- Achyut BR, Yang L. Transforming growth factor-β in the gastrointestinal and hepatic tumor microenvironment. Gastroenterology. 2011;141:1167–1178. doi: 10.1053/j.gastro.2011.07.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama H, Nose M, Takiguchi H, Sugiyama K, Tsutsui R, Hisaka S, Fuchino H, Inui T, Kawano N, Taguchi T, Kudo T, Kawahara N, Yoshimatsu K. Mutagenetic and anti-allergic studies for evaluation of extracts of Coptis Rhizome produced by an artificial hydroponic system. J Nat Med. 2019;73:608–613. doi: 10.1007/s11418-019-01288-6. [DOI] [PubMed] [Google Scholar]
- Bloom BB, Humphries DE, Kuang PP, Fine A. Goldstein RH. Structure and expression of the promoter for the R4/ALK5 human type I transforming growth factor-beta receptor: regulation by TGF-beta. Biochim Biophys Acta. 1996;1312(3):243–248. doi: 10.1016/0167-4889(96)00043-2. [DOI] [PubMed] [Google Scholar]
- Chao Huang, Bin Wen (2016) Phenotype transformation of immortalized NCM460 colon epithelial cell line by TGF-β1 is associated with chromosome instability. Mol Biol Rep 43:1069–1078 [DOI] [PubMed]
- Chen XW, Di YM, Zhang J, Zhou ZW, Li CG, Zhou SF. Interaction of herbal compounds with biological targets: a case study with berberine. Sci World J. 2012;2012:708292. doi: 10.1100/2012/708292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Shen H, Zhu L, Yang H, Ye P, Liu P, Gu Y, Chen S (2019) Berberine attenuates hypoxia-induced pulmonary arterial hypertension via bone morphogenetic protein and transforming growth factor-β signaling. J Cell Physiol. 10.1002/jcp.28370 [DOI] [PubMed]
- Chu SC, Yu CC, Hsu LS, Chen KS, Su MY, Chen PN. Berberine reverses epithelial-to- mesenchymal transition and inhibits metastasis and tumor-induced angiogenesis in human cervical cancer cells. Mol Pharmacol. 2014;86:609–623. doi: 10.1124/mol.114.094037. [DOI] [PubMed] [Google Scholar]
- Cicero AF, Baggioni A. Berberine and its role in chronic disease. Adv Exp Med Biol. 2016;928:27–45. doi: 10.1007/978-3-319-41334-1_2. [DOI] [PubMed] [Google Scholar]
- Cornell L, Wander SA, Visal T, Wagle N, Shapiro GI. MicroRNA-mediated suppression of the TGF-β pathway confers transmissible and reversible CDK4/6 inhibitor resistance. Cell Rep. 2019;26:2667–2680.e7. doi: 10.1016/j.celrep.2019.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David CJ, Huang YH, Chen M, Su J, Zou Y, Bardeesy N, Iacobuzio-Donahue CA, Massagué J. TGF-β tumor suppression through a lethal EMT. Cell. 2016;164:1015–1030. doi: 10.1016/j.cell.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doldi V, Callari M, Giannoni E, D'Aiuto F, Maffezzini M, Valdagni R, Chiarugi P, Gandellini P, Zaffaroni N. Integrated gene and miRNA expression analysis of prostate cancer associated fibroblasts supports a prominent role for interleukin-6 in fibroblast activation. Oncotarget. 2015;6:31441–31460. doi: 10.18632/oncotarget.5056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z, Tai W, Lei W, Wang Y, Li Z, Zhang T. IL-27 inhibits the TGF-β1-induced epithelial-mesenchymal transition in alveolar epithelial cells. BMC Cell Biol. 2016;17:7. doi: 10.1186/s12860-016-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong F, Liu T, Jin H, Wang W. Chimaphilin inhibits human osteosarcoma cell invasion and metastasis through suppressing the TGF-β1-induced epithelial-to-mesenchymal transition markers via PI-3K/Akt, ERK1/2, and Smad signaling pathways. Can J Physiol Pharmacol. 2018;96:1–7. doi: 10.1139/cjpp-2016-0522. [DOI] [PubMed] [Google Scholar]
- Du J, Sun Y, Lu YY, Lau E, Zhao M, Zhou QM, Su SB. Berberine and Evodiamine act synergistically against human breast Cancer MCF-7 cells by inducing cell cycle arrest and apoptosis. Anticancer Res. 2017;37:6141–6151. doi: 10.21873/anticanres.12063. [DOI] [PubMed] [Google Scholar]
- Dvorak HF. Tumors: wounds that do not heal. Similarities between tumor stroma generation and wound healing. N Engl J Med. 1986;315:1650–1659. doi: 10.1056/NEJM198612253152606. [DOI] [PubMed] [Google Scholar]
- Gaitantzi H, Meyer C, Rakoczy P, Thomas M, Wahl K, Wandrer F, Bantel H, Alborzinia H, Wölfl S, Ehnert S, Nüssler A, Bergheim I, Ciuclan L, Ebert M, Breitkopf-Heinlein K, Dooley S. Ethanol sensitizes hepatocytes for TGF-β-triggered apoptosis. Cell Death Dis. 2018;9:51. doi: 10.1038/s41419-017-0071-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giménez-Bastida JA, Laparra-Llopis JM, Baczek N, Zielinski H. Buckwheat and buckwheat enriched products exert an anti-inflammatory effect on the myofibroblasts of colon CCD-18Co. Food Funct. 2018;9:3387–3397. doi: 10.1039/c8fo00193f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glentis A, Oertle P, Mariani P, Chikina A, El Marjou F, Attieh Y, Zaccarini F, Lae M, Loew D, Dingli F, Sirven P, Schoumacher M, Gurchenkov BG, Plodinec M, Vignjevic DM. Cancer-associated fibroblasts induce metalloprotease- independent cancer cell invasion of the basement membrane. Nat Commun. 2017;8:924. doi: 10.1038/s41467-017-00985-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gok Yavuz B, Gunaydin G, Gedik ME, Kosemehmetoglu K, Karakoc D, Ozgur F, Guc D. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1+ TAMs. Sci Rep. 2019;9:3172. doi: 10.1038/s41598-019-39553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunaratne A, Chan E, El-Chabib TH, Carter D, Di Guglielmo GM. aPKC alters the TGFβ response in NSCLC cells through both Smad-dependent and Smad-independent pathways. J Cell Sci. 2015;128:487–498. doi: 10.1242/jcs.155440. [DOI] [PubMed] [Google Scholar]
- He Y, She H, Zhang T, Xu H, Cheng L, Yepes M, Zhao Y, Mao Z. p38 MAPK inhibits autophagy and promotes microglial inflammatory responses by phosphorylating ULK1. J Cell Biol. 2018;217:315–328. doi: 10.1083/jcb.201701049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu Q, Li L, Zou X, Xu L, Yi P. Berberine attenuated proliferation, invasion and migration by targeting the AMPK/HNF4α/WNT5A pathway in gastric carcinoma. Front Pharmacol. 2018;9:1150. doi: 10.3389/fphar.2018.01150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang C, Wen B. Phenotype transformation of immortalized NCM460 colon epithelial cell line by TGF-β1 is associated with chromosome instability. Mol Biol Rep. 2016;43(10):1069–1078. doi: 10.1007/s11033-016-4038-3. [DOI] [PubMed] [Google Scholar]
- Huelsken J, Hanahan D. A subset of Cancer-associated fibroblasts determines therapy resistance. Cell. 2018;172:643–644. doi: 10.1016/j.cell.2018.01.028. [DOI] [PubMed] [Google Scholar]
- Ioannou M, Kouvaras E, Papamichali R, Samara M, Chiotoglou I, Koukoulis G. Smad4 and epithelial-mesenchymal transition proteins in colorectal carcinoma: an immunohistochemical study. J Mol Histol. 2018;49:235–244. doi: 10.1007/s10735-018-9763-6. [DOI] [PubMed] [Google Scholar]
- Ji Q, Liu X, Han Z, Zhou L, Sui H, Yan L, Jiang H, Ren J, Cai J, Li Q. Resveratrol suppresses epithelial-to-mesenchymal transition in colorectal cancer through TGF-β1/Smads signaling pathway mediated snail/E-cadherin expression. BMC Cancer. 2015;15:97. doi: 10.1186/s12885-015-1119-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahata K, Dadras MS, Moustakas A (2018) TGF-β family signaling in epithelial differentiation and epithelial-mesenchymal transition. Cold Spring Harb Perspect Biol 10(1). 10.1101/cshperspect.a022194 [DOI] [PMC free article] [PubMed]
- Khawar IA, Park JK, Jung ES, Lee MA, Chang S, Kuh HJ. Three dimensional mixed-cell spheroids mimic stroma-mediated Chemoresistance and invasive migration in hepatocellular carcinoma. Neoplasia. 2018;20:800–812. doi: 10.1016/j.neo.2018.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SJ, Angel P, Lafyatis R, Hattori K, Kim KY, Sporn MB, Karin M, Roberts AB. Autoinduction of transforming growth factor beta 1 is mediated by the AP-1 complex. Mol Cell Biol. 1990;10(4):1492–1497. doi: 10.1128/mcb.10.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Lee J, You D, Jeong Y, Jeon M, Yu J, Kim SW, Nam SJ, Lee JE. Berberine suppresses cell motility through downregulation of TGF-β1 in triple negative breast cancer cells. Cell Physiol Biochem. 2018;45:795–807. doi: 10.1159/000487171. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Acar A, Eaton EN, Mellody KT, Scheel C, Ben-Porath I, Onder TT, Wang ZC, Richardson AL, Weinberg RA, Orimo A. Autocrine TGF-beta and stromal cell-derived factor-1 (SDF-1) signaling drives the evolution of tumor-promoting mammary stromal myofibroblasts. Proc Natl Acad Sci U S A. 2010;107:20009–20014. doi: 10.1073/pnas.1013805107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kryczka J, Papiewska-Pajak I, Kowalska MA, Boncela J (2019) Cathepsin B is upregulated and mediates ECM degradation in colon adenocarcinoma HT29 cells overexpressing snail. Cells 8 [DOI] [PMC free article] [PubMed]
- Kulkarni SK, Dhir A. Berberine: a plant alkaloid with therapeutic potential for central nervous system disorders. Phytother Res. 2010;24:317–324. doi: 10.1002/ptr.2968. [DOI] [PubMed] [Google Scholar]
- Kuo HP, Chuang TC, Yeh MH, Hsu SC, Way TD, Chen PY, Wang SS, Chang YH, Kao MC, Liu JY. Growth suppression of her2-overexpressing breast cancer cells by berberine via modulation of the her2/pi3k/akt signaling pathway. J Agric Food Chem. 2011;59:8216–8224. doi: 10.1021/jf2012584. [DOI] [PubMed] [Google Scholar]
- Li L, Wang X, Sharvan R, Gao J, Qu S. Berberine could inhibit thyroid carcinoma cells by inducing mitochondrial apoptosis, G0/G1 cell cycle arrest and suppressing migration via PI3K-AKT and MAPK signaling pathways. Biomed Pharmacother. 2017;95:1225–1231. doi: 10.1016/j.biopha.2017.09.010. [DOI] [PubMed] [Google Scholar]
- Lin YS, Chiu YC, Tsai YH, Tsai YF, Wang JY, Tseng LM, Chiu JH (2019) Different mechanisms involved in the berberine-induced antiproliferation effects in triple-negative breast cancer cell lines. J Cell Biochem. 10.1002/jcb.28628 [DOI] [PubMed]
- Liu CH, Tang WC, Sia P, Huang CC, Yang PM, Wu MH, Lai IL, Lee KH. Berberine inhibits the metastatic ability of prostate cancer cells by suppressing epithelial-to-mesenchymal transition (EMT)-associated genes with predictive and prognostic relevance. Int J Med Sci. 2015;12(1):63–71. doi: 10.7150/ijms.9982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Luo N, Guo J, Xie Y, Chen L, Cheng Z. Berberine inhibits growth and inflammatory invasive phenotypes of ectopic stromal cells: imply the possible treatment of adenomyosis. J Pharmacol Sci. 2018;137:5–11. doi: 10.1016/j.jphs.2017.12.001. [DOI] [PubMed] [Google Scholar]
- Loboda A, Sobczak M, Jozkowicz A, Dulak J. TGF-β1/Smads and miR-21 in renal fibrosis and inflammation. Mediat Inflamm. 2016;2016:8319283. doi: 10.1155/2016/8319283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McWhirter A, Colosetti P, Rubin K, Miyazono K, Black C. Collagen type I is not under autocrine control by transforming growth factor-beta 1 in normal and scleroderma fibroblasts. Lab Investig. 1994;71(6):885–894. [PubMed] [Google Scholar]
- Neuzillet C, Tijeras-Raballand A, Cohen R, Cros J, Faivre S, Raymond E, de Gramont A. Targeting the TGFβ pathway for cancer therapy. Pharmacol Ther. 2015;147:22–31. doi: 10.1016/j.pharmthera.2014.11.001. [DOI] [PubMed] [Google Scholar]
- Nissen NI, Karsdal M, Willumsen N. Collagens and Cancer associated fibroblasts in the reactive stroma and its relation to Cancer biology. J Exp Clin Cancer Res. 2009;38:115. doi: 10.1186/s13046-019-1110-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nissinen L, Farshchian M, Riihilä P, Kähäri VM. New perspectives on role of tumor microenvironment in progression of cutaneous squamous cell carcinoma. Cell Tissue Res. 2016;365:691–702. doi: 10.1007/s00441-016-2457-z. [DOI] [PubMed] [Google Scholar]
- Noguchi S, Saito A, Nagase T (2018) YAP/TAZ signaling as a molecular link between fibrosis and Cancer. Int J Mol Sci 19(11). 10.3390/ijms19113674 [DOI] [PMC free article] [PubMed]
- Ortiz LM, Lombardi P, Tillhon M, Scovassi AI. Berberine, an epiphany against cancer. Molecules. 2014;19:12349–12367. doi: 10.3390/molecules190812349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira C, Araújo F, Barrias CC, Granja PL, Sarmento B. Dissecting stromal-epithelial interactions in a 3D in vitro cellularized intestinal model for permeability studies. Biomaterials. 2015;56:36–45. doi: 10.1016/j.biomaterials.2015.03.054. [DOI] [PubMed] [Google Scholar]
- Remppis A, Bea F, Greten HJ, Buttler A, Wang H, Zhou Q, Preusch MR, Enk R, Ehehalt R, Katus H, Blessing E. Rhizoma coptidis inhibits lps-induced mcp-1/ccl2 production in murine macrophages via an ap-1 and nfkappab-dependent pathway. Mediat Inflamm. 2010;2010:194896. doi: 10.1155/2010/194896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang XL, Huang C (2019) difference of TGF-β/Smads signaling pathway in epithelial- mesenchymal transition of normal colonic epithelial cells induced by tumor-associated fibroblasts and colon cancer cells. Mol Biol Rep 46:2749–2759. 10.1007/s11033-019-04719-5 [DOI] [PubMed]
- Wang Y, Zhang S. Berberine suppresses growth and metastasis of endometrial cancer cells via miR-101/COX-2. Biomed Pharmacother. 2018;103:1287–1293. doi: 10.1016/j.biopha.2018.04.161. [DOI] [PubMed] [Google Scholar]
- Wang Y, Liu L, Peng W, Liu H, Liang L, Zhang X, Mao Y, Zhou X, Shi M, Xiao Y, Zhang F, Zhang Y, Liu L, Yan R, Guo B (2019) Ski-related novel protein suppresses the development of diabetic nephropathy by modulating transforming growth factor-β signaling and microRNA-21 expression. J Cell Physiol. 10.1002/jcp.28425 [DOI] [PubMed]
- Wongnoppavich A, Dukaew N, Choonate S, Chairatvit K. Upregulation of maspin expression in human cervical carcinoma cells by transforming growth factor β1 through the convergence of Smad and non-Smad signaling pathways. Oncol Lett. 2017;13:3646–3652. doi: 10.3892/ol.2017.5939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodcock HV, Eley JD, Guillotin D, Platé M, Nanthakumar CB, Martufi M, Peace S, Joberty G, Poeckel D, Good RB, Taylor AR, Zinn N, Redding M, Forty EJ, Hynds RE, Swanton C, Karsdal M, Maher TM, Bergamini G, Marshall RP, Blanchard AD, Mercer PF, Chambers RC. The mTORC1/4E-BP1 axis represents a critical signaling node during fibrogenesis. Nat Commun. 2019;10:6. doi: 10.1038/s41467-018-07858-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Lei H, Wang JY, Zhang CL, Feng H, Fu FY, Li L, Wu LL. CTRP3 attenuates post-infarct cardiac fibrosis by targeting Smad3 activation and inhibiting myofibroblast differentiation. J Mol Med (Berl) 2015;93:1311–1325. doi: 10.1007/s00109-015-1309-8. [DOI] [PubMed] [Google Scholar]
- Yang G, Zhao Z, Zhang X, Wu A, Huang Y, Miao Y, Yang M. Effect of berberine on the renal tubular epithelial-to-mesenchymal transition by inhibition of the notch/snail pathway in diabetic nephropathy model KKAy mice. Drug Des Devel Ther. 2017;11:1065–1079. doi: 10.2147/DDDT.S124971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Gong X, Wang X, Huang C (2018) A mediator of phosphorylated Smad2/3, evodiamine, in the reversion of TAF-induced EMT in normal colonic epithelial cells. Investig New Drugs. 10.1007/s10637-018-0702-x [DOI] [PubMed]
- Yeh HW, Hsu EC, Lee SS, Lang YD, Lin YC, Chang CY, Lee SY, Gu DL, Shih JH, Ho CM, Chen CF, Chen CT, Tu PH, Cheng CF, Chen RH, Yang RB, Jou YS. PSPC1 mediates TGF-β1 autocrine signalling and Smad2/3 target switching to promote EMT, stemness and metastasis. Nat Cell Biol. 2018;20:479–491. doi: 10.1038/s41556-018-0062-y. [DOI] [PubMed] [Google Scholar]
- Zhao L, Li J, Liu Y, Zhou W, Shan Y, Fan X, Zhou X, Shan B, Song Y, Zhan Q. Flotillin1 promotes EMT of human small cell lung cancer via TGF-β signaling pathway. Cancer Biol Med. 2018;15:400–414. doi: 10.20892/j.issn.2095-3941.2018.0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou P, Xiao N, Wang J, Wang Z, Zheng S, Shan S, Wang J, Du J, Wang J. SMC1A recruits tumor-associated-fibroblasts (TAFs) and promotes colorectal cancer metastasis. Cancer Lett. 2017;385:39–45. doi: 10.1016/j.canlet.2016.10.041. [DOI] [PubMed] [Google Scholar]
- Zhu J, Yu W, Liu B, Wang Y, Shao J, Wang J, Xia K, Liang C, Fang W, Zhou C, Tao H. Escin induces caspase-dependent apoptosis and autophagy through the ROS/p38 MAPK signalling pathway in human osteosarcoma cells in vitro and in vivo. Cell Death Dis. 2017;8:e3113. doi: 10.1038/cddis.2017.488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou K, Li Z, Zhang Y, Zhang HY, Li B, Zhu WL, Shi JY, Jia Q, Li YM. Advances in the study of berberine and its derivatives: a focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol Sin. 2017;38:157–167. doi: 10.1038/aps.2016.125. [DOI] [PMC free article] [PubMed] [Google Scholar]