Abstract
Autophagy occurs at basal levels for cellular homeostasis under normal conditions and is increased in response to nutrient starvation or stress to ensure cell survival. However, excessive autophagy can be deleterious to cardiomyocytes. CCN1/Cyr61, a matricellular protein, is expressed in the stressed heart to induce cardiomyopathy. The role of autophagy in CCN1-associated cardiotoxicity was not clear. Here, we found that autophagy was induced in the myocardium of the isoproterenol (ISO; 100 mg/kg/day for 5 days; s.c.) treated mice, where CCN1 expression is colocalized. The knock-in mice carrying an integrin α6β1-binding-defective mutant allele Ccn1-dm were resistant to the ISO-induced cardiac injury and autophagy. Our in vitro studies demonstrated that CCN1 dose- and time-dependently induced GFP-LC3-labeled autophagosome formation in rat cardiomyoblast H9c2 cells. The formation of autolysosomes in response to CCN1 (5 μg/ml; 3 h) treatment was identified by the acridine orange staining. The autophagy induction was confirmed by the elevated protein levels of Beclin 1, Atg5, and LC3-II, and the decrease of p62. Inhibition of autophagy by 3-methyladenine or by silencing Atg5 gene enabled CCN1-induced apoptosis in H9c2 cells, suggesting a protective role of autophagy. CCN1 binds to integrin α6β1 to induce autophagy through reactive oxygen species, and the activation of ERK and JNK. Furthermore, mitophagy was observed after CCN1 treatment for the clearance of depolarized mitochondria. Together, these results demonstrated that autophagy is induced in response to CCN1/α6β1 signaling in cardiomyocytes to alleviate CCN1-associated cardiotoxicity.
Electronic supplementary material
The online version of this article (10.1007/s12079-019-00534-6) contains supplementary material, which is available to authorized users.
Keywords: CCN1, Integrin α6β1, Autophagy, Mitophagy, Apoptosis
Introduction
Autophagy is an adaptive process for cells to respond to different forms of stress, such as nutrient starvation, growth factor depletion, and hypoxia. The function of autophagy is to provide nutrients for vital cellular structure and activities, and also to selectively remove harmful organelles and aggregated proteins generated in the stressed conditions (Dikic and Elazar 2018). Despite the beneficial role of autophagy, excessive or insufficient autophagic activities have been associated with pathological conditions. Autophagy was found to be the most prominent cause of cardiomyocyte death in patients with end-stage heart failure (Kostin et al. 2003). Nonetheless, autophagy can be adaptive during ischemia or ischemia/reperfusion involving Beclin 1 activation independent of the AMPK/mTOR pathway (Matsui et al. 2007). Conventional apoptotic stimuli, such as reactive oxygen species (ROS) accumulation or the release of mitochondrial apoptotic factors, can trigger the autophagic machinery to counteract cell apoptosis through clearance of free radicals and damaged mitochondria (Kubli and Gustafsson 2012). The role of autophagy in cardiovascular disease is intricate and needs to be determined in consideration of the context.
The matricellular protein CCN1 is induced in the cardiomyocytes of a stressed heart to promote cardiomyocyte apoptosis (Hsu et al. 2013). CCN1, a member of CCN protein family, binds to at least 5 different integrins (αvβ3, αvβ5, α6β1, αIIbβ3, and αMβ2) to regulate diverse cellular activities including cell adhesion, migration, differentiation, proliferation, and survival/apoptosis/senescence (Lau 2016). CCN1 expression tapers off after birth and is redeployed in ischemic cardiomyopathy, doxorubicin-induced cardiomyopathy, and pressure overload-induced cardiac injury, and atherosclerosis (Hilfiker-Kleiner et al. 2004; Hsu and Mo 2016; Hsu et al. 2013). CCN1 promotes cardiomyocyte death not by itself, but through potentiating other apoptotic stimuli (Su and Mo 2014). CCN1 triggers ROS accumulation and mitochondrial outer membrane permeabilization (MOMP) through binding to integrin α6β1 to enhance FasL-induced apoptosis in H9c2 cardiomyoblasts (Su and Mo 2014). Because both ROS and MOMP trigger autophagic response (Kubli and Gustafsson 2012), we intended to assess the regulation of autophagy in cardiomyocytes upon CCN1 stimulation.
Materials and methods
Reagents
Recombinant CCN1 and CCN1-DM proteins are gifts from Dr. Lester. F. Lau (University of Illinois at Chicago) (Kireeva et al. 1996). 3-Methyladenine (3-MA, M9281), N-acetylcysteine (NAC, A9165), U0126 (U120) and SP600125 (S5567) were purchased from Sigma. MitoTracker (M7510) was from Invitrogen. β-tubulin antibody (05–661) was from Millipore. Antibodies used for p62 (PM045) and LC3 (PM046) were from MBL international. Beclin 1 antibody (11306–1-AP) was from Proteintech. Atg5 antibody (NB110–53818) was from Novus. ERK (9102), phospho-ERK (9101), JNK (9252), phospho-JNK (9251) antibodies were from Cell Signaling.
Animal
All experiments were performed in conformity with the Guide for the Care and Use of Laboratory Animals published by the United States National Institutes of Health and the animal use protocols have been reviewed and approved by the Institutional Animal Care and Use Committee of National Cheng Kung University (approval number 101134). The cardiac work-overload mouse model was induced by isoproterenol (ISO) as described previously (Hsu et al. 2013). Briefly, C57BL/6 male wild type and Ccn1dm/dm mice were subcutaneously injected with ISO (100 mg/kg/day; Sigma) for 5 days. The heart was collected 2 days after the completion of ISO treatment, and processed for further histological analyses.
Cell culture and knockdown
Cardiomyoblast H9c2 cells were cultured in Dulbecco’s Modified Eagle’s Medium (Hyclone, SH30003.02) supplemented with 10% fetal bovine serum (FBS; Hyclone, SH30071.03). For knockdown experiments, cells were transfected with Atg5-siRNA or non-targeting control (NTG) (Invitrogen) for 48 h, and followed by CCN1 treatment (5 μg/ml, 3 h).
Transient transfection of GFP-LC3
H9c2 cells were transfected with pEGFP-LC3 (GFP-LC3; a gift from Dr. Tamotsu Yoshimori, Osaka University, Japan) using TurboFect (Fermantas) according to the manufacturer’s instructions and cultured for 2 days. Transfected cells were treated with CCN1 or CCN1-DM protein as indicated. Autophagic cells displaying GFP-LC3 puncta were countered in 10 random high power fields by using fluorescent microscope.
Acridine orange (AO) staining
AO (Sigma) generates green fluorescence in cytoplasm and turns into red fluorescence in the acidic vesicle organelles, and can be used to identify autolysosomes. Cells were stimulated with CCN1 (5 μg/ml) for 3 h and subsequently incubated with AO 1 μg/ml for 15 min. Normal cells displayed diffuse green fluorescence in cytoplasm. AO+ autophagic cells displaying red fluorescent puncta were scored in 10 random high power fields by using fluorescent microscope.
Western blotting
Total cell lysates from treated cells were immunoblotted for LC3, Atg5, Beclin 1, p62, ERK, phospho-ERK, JNK, phospho-JNK, and β-tubulin. The intensity of protein bands was quantified using the NIH ImageJ program.
Immunocytochemistry and immunofluorescent staining
After cells were incubated with CCN1 (5 μg/ml, 3 h), cells were stained with MitoTracker (500 nM) for 15 min, and then fixed with 3.7% paraformaldehyde and 90% methanol, blocked with 3% FBS in PBS for 30 min before incubated with LC3 antibody (1:300) for 1 h, and counterstained with 4′,6-diamidino-2-phenylindole (DAPI) for nuclei. Images were taken and processed by the Nikon microscope (Nikon ECLIPSE TS-100) and the Nikon Element software (Nikon Elements D 3.2). For tissue Immunofluorescent staining, cardiac tissue sections were incubated with LC3 antibody (1:300), and counterstained with DAPI. For TUNEL staining, cardiac tissue sections were stained with TUNEL according to the manufacturer’s protocol (Millipore, S7165).
Apoptotic assay
Treated cells were fixed with formaldehyde. Apoptosis was identified by chromatin condensation after DAPI staining as previously described (Su and Mo 2014). Apoptotic cells with condensed nuclei were scored in 10 random high power views by using a Nikon fluorescence microscope (Nikon ECLIPSE TS-100).
Statistical analysis
Quantitative results were analyzed by one- or two-way ANOVA and post-hoc Tukey’s tests, and p < 0.05 was statistically significant. Significance is indicated as *p < 0.05; **p < 0.01; ***p < 0.001.
Results
CCN1 induces autophagy in cardiomyocytes
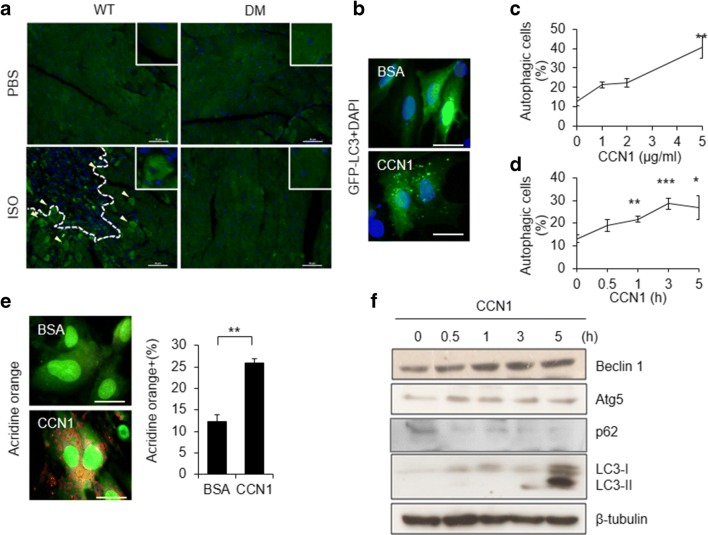
Previously, we demonstrated the induction of CCN1 by cardiac work overload after chronic treatments of the β-adrenergic agonist ISO, which lead to functional ischemia and coronary insufficiency, thereby myocardial injury in mice (Hsu et al. 2013). Ccn1dm/dm mice carrying an integrin α6β1 binding-defective Ccn1 mutant allele (Ccn1-dm) are resistant to ISO-induced cardiomyocyte apoptosis and myocardial injury (Hsu et al. 2013). The regulation of autophagy in CCN1-associated heart injuries was not clear. Here, we examined autophagy in the myocardial injury model induced by ISO (100 mg/kg/day for 5 days; s.c.) in mice. We found that autophagy identified by punctate LC3 immunostaining (green; Fig. 1a) was abundant in the myocardium surrounding the injured areas (dashed line in Fig. 1a), where sparse apoptosis (pink nuclei after TUNEL staining; supplemental Figure 1) was also detected in WT mice. Whereas no heart injury or apoptosis was observed in Ccn1dm/dm mice, ISO-induced autophagy was abrogated as well (Fig. 1a), suggesting that CCN1 and its receptor integrin α6β1 are essential for the induction of autophagy. To test whether CCN1 directly acts on cardiomyocytes to induce autophagy, cardiomyoblast H9c2 cells transfected with GFP-LC3 were used. Cells were treated with CCN1 before autophagy was evaluated based on autophagosome formation determined by the punctate GFP-LC3 fluorescence (Fig. 1b). We found that CCN1 protein dose- and time-dependently increased the percentages of cells displaying punctate fluorescence (Figs. 1c, d). The progression of autophagy was confirmed by using a lysotropic dye AO to stain autolysosomes. AO exhibits green fluorescence in cytoplasm and becomes red within acidic vesicles (Fig. 1e). CCN1 (5 μg/ml, 3 h) increased AO+ cells to >25% compared to 12% in control cells (Fig. 1e). Accordingly, CCN1 (5 μg/ml) elevated the levels of autophagy related gene products such as Atg5, Beclin 1, LC3, and LC3-I/II conversion measured by immunoblotting the total cell lysates of H9c2 cells after the time indicated (Fig. 1f, the quantitative analysis of the Western-blot bands shown in supplemental Figure 2). Autophagy is accompanied by the downregulation of the adaptor protein p62 (Mizushima 2007). Indeed, p62 was reduced after 3-h CCN1 treatment (Fig. 1f, the quantitative results shown in supplemental Figure 2). Together, these findings demonstrate that CCN1 directly promotes autophagy in H9c2 cells.
Fig. 1.
CCN1 triggered autophagy in cardiomyocytes in vivo and in vitro. a Wild type (WT) and Ccn1dm/dm (DM) mice were subcutaneously injected with isoproterenol (ISO, 100 mg/kg/day) or PBS for 5 days. Heart tissue was collected 2 days after the completion of ISO treatment. Cardiac tissue sections were immunostained for LC3 (green) and 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) counterstaining for nuclei (blue). Yellow arrow heads indicate LC3+ autophagic cells. The injured area was marked by a dashed line. Bars: 50 μm. b, c, d Autophagy was determined by the formation of LC3+ vacuoles in H9c2 cells transfected with GFP-LC3 plasmids (b). The percentages of cells displaying punctate green LC3-labeled fluorescence after CCN1 at increasing doses for 3 h (c), or CCN1 (5 μg/ml) for the time indicated (d). BSA (bovine serum albumin): vehicle control. Bar: 25 μm. e H9c2 cells were treated with CCN1 (5 μg/ml) for 3 h and subsequently incubated with acridine orange (AO; 1 μg/ml) for additional 15 min. AO exhibits green fluorescence in cytoplasm and becomes orange within acidic vesicles. Bar: 25 μm. f Total cell lysates from H9c2 cells treated with CCN1 (5 μg/ml) for the time indicated were subjected to Western blotting for Beclin 1, Atg5, p62, LC3, and β-tubulin. Statistical significance between controls and the treatment was calculated using one-way ANOVA and post-hoc Tukey’s tests, *P < 0.05, **P < 0.01, ***P < 0.001
Autophagy is an adaptive mechanism to suppress CCN1-induced apoptosis in cardiomyocytes
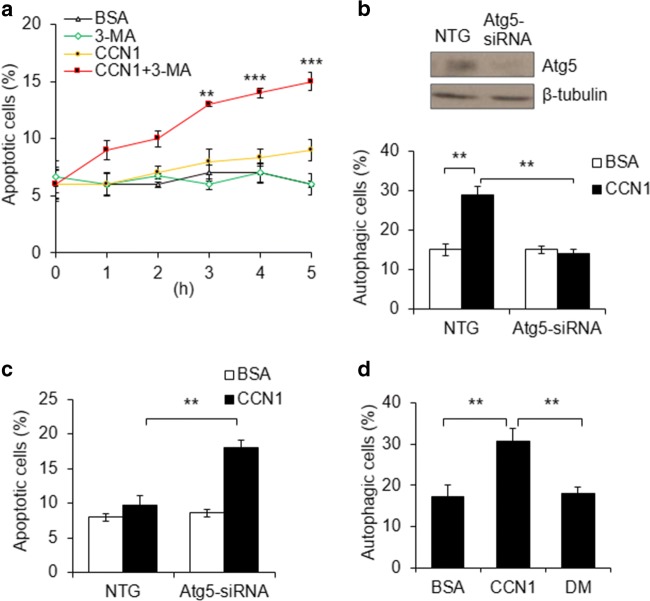
CCN1 by itself does not induce apoptosis, but facilitates FasL-induced apoptosis through disrupting XIAP (X-linked inhibitor of apoptosis protein) in cardiomyocytes (Su and Mo 2014). We intended to assess the intricate interplay between the autophagy and apoptosis induced by CCN1 in H9c2 cells. First, we blocked autophagy by pretreatment with 3-MA for 30 min and measured apoptosis after CCN1 treatment (5 μg/ml) for additional 3 h. We found that CCN1 alone did not affect cell apoptosis compared with BSA or 3-MA controls (Fig. 2a). Interestingly, CCN1 + 3-MA time-dependently increase apoptotic cells from 6% to 15% (Fig. 2a). Second, autophagy was blocked by knocking down Atg5 gene with Atg5-siRNA. The silencing efficiency of Atg5-siRNA was demonstrated by immunoblotting Atg5 protein (Fig. 2b). CCN1-induced autophagy observed in NTG controls was abrogated by Atg5-siRNA (Fig. 2b). Autophagy inhibition by Atg5-silencing predisposed H9c2 cells to CCN1-induced apoptosis (Fig. 2c). Taken together, these results indicate that autophagy is an adaptive mechanism in cardiomyocytes upon CCN1 stimulation to prevent apoptosis.
Fig. 2.
CCN1/α6β1-induced autophagy suppressed the apoptotic effects of CCN1 in H9c2 cells. a H9c2 cells were pretreated with 3-Methyladenine (3-MA; 5 mM; 30 min) before incubated with CCN1 (5 μg/ml) for the indicated time. Apoptosis was identified by chromatin condensation after DAPI staining. Statistical significance between CCN1 and CCN1 + 3-MA at the same time point was calculated using two-way ANOVA and post-hoc Tukey’s tests, **P < 0.01, ***P < 0.001. b, c Cells were transfected with Atg5-siRNA or non-targeting (NTG) control for 48 h. Atg5 knockdown efficiency was confirmed by Western blotting (upper panel). Transfected cells were treated with CCN1 (5 μg/ml, 3 h) before autophagy (b) or apoptosis (c) was measured. Autophagy was determined by the formation of GFP-LC3 puncta. Apoptosis was identified by chromatin condensation after DAPI staining. Statistical significance was calculated using two-way ANOVA and post-hoc Tukey’s tests, **P < 0.01. d Cells were treated with CCN1 (5 μg/ml), CCN1-DM (DM), or BSA control for 3 h before autophagy was scored by the formation of LC3-labeled puncta. Statistical significance was calculated using one-way ANOVA and post-hoc Tukey’s tests, **P < 0.01
CCN1 induces autophagy via integrin α6β1
The resistance to autophagy observed in Ccn1dm/dm mice suggests that CCN1 may induce autophagy through binding to integrin α6β1. To test the integrin requirement for CCN1, H9c2 cells were treated with recombinant CCN1 or CCN1-DM (DM) protein (5 μg/ml, 3 h). α6β1-binding-defective DM protein failed to trigger autophagy (Fig. 2d), indicating that integrin α6β1 is required for CCN1-induced autophagy.
Redox, ERK, and JNK are essential for CCN1-induced autophagy
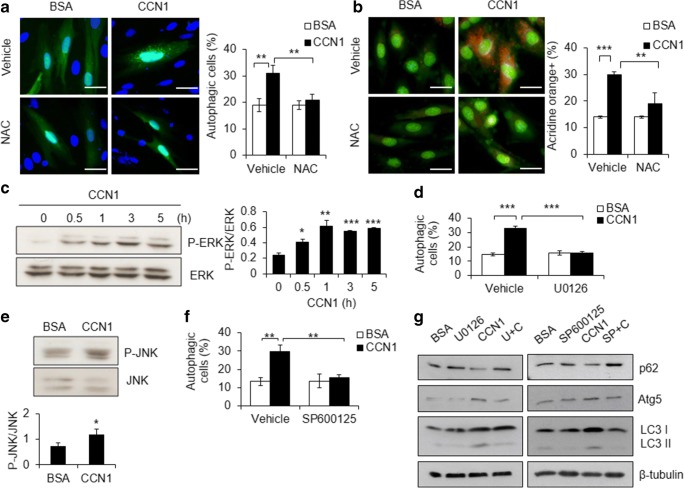
CCN1/α6β1 signaling increases cellular ROS to mediate its cytotoxicity in cardiomyocytes (Su and Mo 2014). Here, we tested the role of ROS in autophagy by pretreating H9c2 cells with the ROS scavenger NAC (10 mM; 30 min) before treatment with CCN1 (5 μg/ml) for additional 3 h. We found that CCN1-induced autophagosome formation (green punctate GFP-LC3 staining in Fig. 3a) and autolysosome accumulation (orange punctate AO staining in Fig. 3b) in control cells were completely blocked by NAC (Fig. 3a, b), indicating an essential role of ROS in CCN1-induced autophagy. ROS can promote autophagy through non-canonical pathways by activating ERK or JNK (Kim et al. 2017; Zhong et al. 2017). Indeed, we found that ERK phosphorylation (activation) was increased by CCN1 (5 μg/ml) within 0.5 h after treatment measured by Western blotting for phospho (P)-ERK1/2 (Fig. 3c). Inhibition of ERK by the specific inhibitor U0126 (10 μM, 0.5-h pretreatment) abrogated CCN1-induced autophagy (Fig. 3d). Similarly, phosphorylation (activation) of JNK was induced by CCN1 (Fig. 3e), and inhibition of JNK by the specific inhibitor SP600125 (10 μM, 0.5-h pretreatment) blocked CCN1-induced autophagy (Fig. 3f). The regulation of autophagy-related proteins, including the reduction of p62, the increase of Atg5 and LC3-II, by CCN1 were reversed by U0126 or SP600125 treatments measured by immunoblotting the total cell lysates (Fig. 3g, the quantitative analysis of the Western-blot bands shown in supplemental Figure 3), confirming the essential roles of ERK and JNK in CCN1 signaling. These results suggest that both ERK and JNK are activated by CCN1 and required for CCN1-induced autophagy. Blocking either ERK or JNK is sufficient to abolish CCN1-induced autophagy.
Fig. 3.
Redox, ERK, and JNK are essential for CCN1-induced autophagya, b H9c2 cells were pretreated with N-acetylcysteine (NAC; 10 mM; 30 min) before incubated with CCN1 (5 μg/ml) for additional 3 h. Autophagy was determined by GFP-LC3 labeled autophagosome formation (a) or AO staining (b) as described in Fig. 1. Statistical significance was calculated using two-way ANOVA and post-hoc Tukey’s tests, **P < 0.01, ***P < 0.001. Bars: 50 μm. c Total cell lysates from cells treated with CCN1 (5 μg/ml) for the time indicated were immunoblotted for phospho (P)-ERK and ERK. The intensity of protein bands was quantified using the ImageJ program. Statistical significance of p-ERK/ERK between controls and the treatment was calculated using one-way ANOVA and post-hoc Tukey’s tests, *P < 0.05, **P < 0.01, ***P < 0.001. d Cells were preincubated with U0126 (10 μM) for 0.5 h before CCN1 treatment (5 μg/ml, 3 h). Autophagy was scored as in a. Statistical significance was calculated using two-way ANOVA and post-hoc Tukey’s tests, ***P < 0.001. e Total cell lysates from cells treated with CCN1 (5 μg/ml, 3 h) were immunoblotted for phospho (P)-JNK and JNK. f Cells were preincubated with SP600125 (10 μM) for 0.5 h before CCN1 treatment (5 μg/ml, 3 h). Autophagy was scored as a. Statistical significance was calculated using two-way ANOVA and post-hoc Tukey’s tests, **P < 0.01. g Cells were preincubated with SP600125 (10 μM) for 0.5 h before CCN1 treatment (5 μg/ml, 5 h). Total cell lysates from treated cells were immunoblotted for p62, Atg5, LC3, and β-tubulin
CCN1 activates mitophagy in H9c2 cells
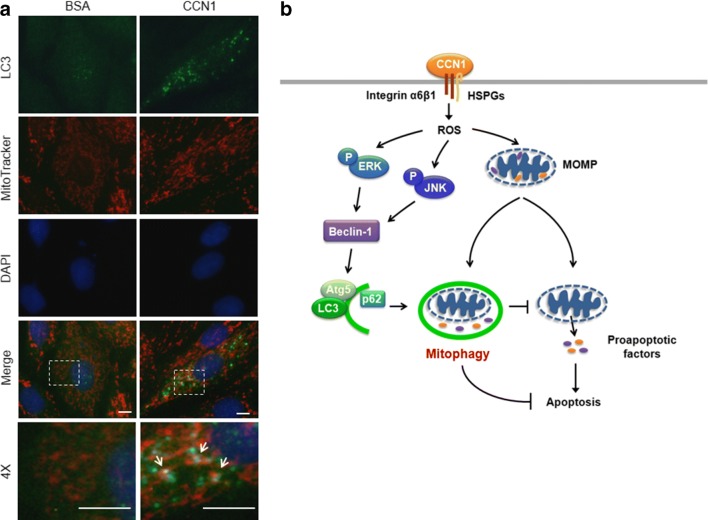
CCN1 increases MOMP leading to the release of mitochondrial HtrA2 and Smac to facilitate apoptosis in H9c2 cells (Su and Mo 2014). Mitochondrial autophagy (mitophagy) functions as a cardioprotective process by selective degradation of dysfunctional mitochondria (Kubli and Gustafsson 2012). Here, we assessed the involvement of mitophagy by labeling mitochondria with MitoTracker in the GFP-LC3 transfected H9c2 cells. We found that extensive mitophagy was induced by CCN1 (5 μg/ml, 3 h) evidenced by the colocalized green GFP-LC3 and red MitoTracker fluorescence (yellow after merge as pointed by arrows in Fig. 4a), suggesting that mitophagy is triggered to remove dysfunctional mitochondria after CCN1 treatment and prevent apoptosis.
Fig. 4.
Mitophagy was triggered by CCN1 in H9c2 cells. a H9c2 cells were treated with CCN1 (5 μg/ml, 3 h), and subsequently stained with MitoTracker (red), immunostained for LC3 (green), and counterstained with DAPI. Mitophagy was identified by colocalization of mitochondria and autophagosome (yellow after merge, arrows). 4X are the higher magnification of dashed boxes in the merged images. Bars: 10 μm. b A diagram summarizes the mechanism by which autophagy is triggered in response to CCN1 stimulation to protect cardiomyocytes from apoptosis. HSPGs: heparan sulfate proteoglycans; MOMP: mitochondrial outer membrane permeabilization; P: phospho-; ROS: reactive oxygen species
Discussion
Cardiac myocytes possess very limited regenerative capacity (Bergmann et al. 2015). It is critical to prevent cardiomyocyte loss under stress or pathological conditions to preserve cardiac function. We previously demonstrated that CCN1 is induced in the cardiomyocytes in the stressed heart and critically mediates the work-overload-induced myocardial injury in mice (Hsu et al. 2013). Though CCN1 promotes cell death by priming cardiomyocytes to FasL-induced apoptosis, CCN1 by itself does not induce apoptosis (Hsu et al. 2013; Su and Mo 2014). Here, we assessed the role of autophagy in the regulation of cell apoptosis/survival by CCN1. We found that extensive autophagy was induced in the myocardium of a stressed heart in mice. Ccn1dm/dm mice were resistant to the stress-associated cardiac injury, and did not exhibit detectable autophagic response. Inhibition of autophagy by the inhibitor 3-MA or Atg5-siRNA enabled CCN1-induced apoptosis in cultured cardiomyoblasts, suggesting a protective role of autophagy in response to CCN1 stimulation. The engagement of α6β1 with the coreceptor heparan sulfate proteoglycans (HSPGs) by CCN1 increases ROS levels and the activation of ERK and JNK leading to the induction of autophagy and mitophagy (Fig. 4b).
Autophagy has historically been considered as a nonselective process. More recent studies demonstrate that autophagy can also selectively eliminate harmful cytosolic materials, such as damaged mitochondria (Dikic and Elazar 2018). CCN1/α6β1 signaling induces MOMP in cardiomyocytes (Su and Mo 2014). MOMP leads to the release of mitochondrial ROS and the recruitment of PINK1 (PTEN-induced putative kinase 1) and PRKN (Parkin) to the depolarized mitochondria to promote ubiquitination (the ‘eat-me’ signal for autophagy) on mitochondrial membrane (Xian and Liou 2019). Additionally, both MOMP (Choe et al. 2015) and ERK-CREB pathways (Li et al. 2018) promote Bnip3-mediated mitophagy. The activation of ERK also contributes to the induction of LC3 expression (Kim et al. 2017), and Beclin 1 expression (Wang et al. 2009) and activation (Elgendy et al. 2011). CCN1 can activate the autophagic machinery through ERK activation to target the ubiquitinated mitochondria. Blocking ERK abrogated CCN1-induced autophagy in our study (Fig. 3d).
Interestingly, blocking JNK activation effectively abolished CCN1-induced autophagy as well (Fig. 3f). JNK is activated by hypoxia-induced ROS to promote Bnip3-induced autophagy in keratinocytes (Zhang et al. 2019). Furthermore, both ERK and JNK are activated by UVB-generated ROS to activate Bnip3-mediated autophagy in keratinocytes (Moriyama et al. 2017). The ROS-induced ERK and JNK signaling converges at Bnip-3 to activate autophagy. In addition, JNK also induces the expression of Beclin 1 (Li et al. 2009). JNK phosphorylates inhibitory BCL-2 to prevent its interaction with Beclin 1, thereby freeing Beclin 1 from BCL-2 to induce autophagy (Orogo and Gustafsson 2015). The downstream targets of ERK and JNK signaling seem to be similar, and blocking either ERK or JNK is sufficient to abrogate CCN1-induced autophagy, suggesting that ERK and JNK may regulate the same pathway. The interaction between ERK and JNK merits further investigation. Our results do not exclude the involvement of other receptors in addition to α6β1 mediating the activation of ERK and JNK by CCN1, though no α6β1-independent CCN1 signaling pathways have been reported in cardiomyocytes.
In regard of the levels of CCN1 in pathophysiological situations, because CCN1 is a secreted protein and is stabilized after being deposited in the extracellular matrix, the induction of CCN1 in our cardiac work overload mouse model can be accumulated after sustained stimuli (Hsu et al. 2013). Additional studies are warranted to address how changes of local CCN1 concentrations in cardiomyopathy impact on CCN1-mediated injury and apoptosis. A limitation of the current study is that H9c2 cells derived from rat embryonic hearts, though maintain many cardiac myocyte features, are not functional mature cardiomyocytes. Primary culture cardiomyocytes can be used to confirm the findings here.
Autophagy is downregulated in the failing hearts induced by pressure overload (Wang et al. 2018), in the aging hearts (Ren and Zhang 2018), and in diabetic cardiomyopathy (Kenny and Abel 2019). Deficiency in autophagy may exacerbate CCN1-induced cardiotoxicity. In addition to blocking CCN1/α6β1 engagement, the treatments to enhance autophagic response can be used to prevent CCN1-associated cardiac injury.
Electronic supplementary material
(PDF 167 kb)
Acknowledgements
We thank Dr. Lester Lau and Dr. Tamotsu Yoshimori for gifts of reagents.
Abbreviations
- AO
Acridine orange
- Atg5
Autophagy gene 5
- BSA
Bovine serum albumin
- FasL
Fas ligand
- HSPGs
Heparan sulfate proteoglycans
- ISO
Isoproterenol
- LC3
Microtubule-associated protein light chain 3
- MOMP
Mitochondrial outer membrane permeabilization
- 3-MA
3-Methyladenine
- NAC
N-acetylcysteine
- ROS
Reactive oxygen species
Funding information
This work was supported by the Ministry of Science and Technology of Taiwan [grant 1062320B006043MY3].
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Bergmann O, Zdunek S, Felker A, Salehpour M, Alkass K, Bernard S, Sjostrom SL, Szewczykowska M, Jackowska T, Dos Remedios C, et al. Dynamics of cell generation and turnover in the human heart. Cell. 2015;161:1566–1575. doi: 10.1016/j.cell.2015.05.026. [DOI] [PubMed] [Google Scholar]
- Choe SC, Hamacher-Brady A, Brady NR. Autophagy capacity and sub-mitochondrial heterogeneity shape Bnip3-induced mitophagy regulation of apoptosis. Cell Commun Signal. 2015;13:37. doi: 10.1186/s12964-015-0115-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dikic I, Elazar Z. Mechanism and medical implications of mammalian autophagy. Nat Rev Mol Cell Biol. 2018;19:349–364. doi: 10.1038/s41580-018-0003-4. [DOI] [PubMed] [Google Scholar]
- Elgendy M, Sheridan C, Brumatti G, Martin SJ. Oncogenic Ras-induced expression of Noxa and Beclin-1 promotes autophagic cell death and limits clonogenic survival. Mol Cell. 2011;42:23–35. doi: 10.1016/j.molcel.2011.02.009. [DOI] [PubMed] [Google Scholar]
- Hilfiker-Kleiner D, Kaminski K, Kaminska A, Fuchs M, Klein G, Podewski E, Grote K, Kiian I, Wollert KC, Hilfiker A, et al. Regulation of proangiogenic factor CCN1 in cardiac muscle: impact of ischemia, pressure overload, and neurohumoral activation. Circulation. 2004;109:2227–2233. doi: 10.1161/01.CIR.0000127952.90508.9D. [DOI] [PubMed] [Google Scholar]
- Hsu PL, Mo FE. Matricellular protein CCN1 mediates doxorubicin-induced cardiomyopathy in mice. Oncotarget. 2016;7:36698–36710. doi: 10.18632/oncotarget.9162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu PL, Su BC, Kuok QY, Mo FE. Extracellular matrix protein CCN1 regulates cardiomyocyte apoptosis in mice with stress-induced cardiac injury. Cardiovasc Res. 2013;98:64–72. doi: 10.1093/cvr/cvt001. [DOI] [PubMed] [Google Scholar]
- Kenny HC, Abel ED. Heart failure in type 2 diabetes mellitus. Circ Res. 2019;124:121–141. doi: 10.1161/CIRCRESAHA.118.311371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim SH, Kim KY, Park SG, Yu SN, Kim YW, Nam HW, An HH, Kim YW, Ahn SC. Mitochondrial ROS activates ERK/autophagy pathway as a protected mechanism against deoxypodophyllotoxin-induced apoptosis. Oncotarget. 2017;8:111581–111596. doi: 10.18632/oncotarget.22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kireeva ML, Mo FE, Yang GP, Lau LF. Cyr61, a product of a growth factor-inducible immediate-early gene, promotes cell proliferation, migration, and adhesion. Mol Cell Biol. 1996;16:1326–1334. doi: 10.1128/MCB.16.4.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kostin S, Pool L, Elsasser A, Hein S, Drexler HC, Arnon E, Hayakawa Y, Zimmermann R, Bauer E, Klovekorn WP, et al. Myocytes die by multiple mechanisms in failing human hearts. Circ Res. 2003;92:715–724. doi: 10.1161/01.RES.0000067471.95890.5C. [DOI] [PubMed] [Google Scholar]
- Kubli DA, Gustafsson AB. Mitochondria and mitophagy: the yin and yang of cell death control. Circ Res. 2012;111:1208–1221. doi: 10.1161/CIRCRESAHA.112.265819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10:121–127. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DD, Wang LL, Deng R, Tang J, Shen Y, Guo JF, Wang Y, Xia LP, Feng GK, Liu QQ, et al. The pivotal role of c-Jun NH2-terminal kinase-mediated Beclin 1 expression during anticancer agents-induced autophagy in cancer cells. Oncogene. 2009;28:886–898. doi: 10.1038/onc.2008.441. [DOI] [PubMed] [Google Scholar]
- Li R, Xin T, Li D, Wang C, Zhu H, Zhou H. Therapeutic effect of Sirtuin 3 on ameliorating nonalcoholic fatty liver disease: the role of the ERK-CREB pathway and Bnip3-mediated mitophagy. Redox Biol. 2018;18:229–243. doi: 10.1016/j.redox.2018.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsui Y, Takagi H, Qu X, Abdellatif M, Sakoda H, Asano T, Levine B, Sadoshima J. Distinct roles of autophagy in the heart during ischemia and reperfusion: roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ Res. 2007;100:914–922. doi: 10.1161/01.RES.0000261924.76669.36. [DOI] [PubMed] [Google Scholar]
- Mizushima N. Autophagy: process and function. Genes Dev. 2007;21:2861–2873. doi: 10.1101/gad.1599207. [DOI] [PubMed] [Google Scholar]
- Moriyama M, Moriyama H, Uda J, Kubo H, Nakajima Y, Goto A, Morita T, Hayakawa T. BNIP3 upregulation via stimulation of ERK and JNK activity is required for the protection of keratinocytes from UVB-induced apoptosis. Cell Death Dis. 2017;8:e2576. doi: 10.1038/cddis.2017.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orogo AM, Gustafsson AB. Therapeutic targeting of autophagy: potential and concerns in treating cardiovascular disease. Circ Res. 2015;116:489–503. doi: 10.1161/CIRCRESAHA.116.303791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren J, Zhang Y. Targeting autophagy in aging and aging-related cardiovascular diseases. Trends Pharmacol Sci. 2018;39:1064–1076. doi: 10.1016/j.tips.2018.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su BC, Mo FE. CCN1 enables Fas ligand-induced apoptosis in cardiomyoblast H9c2 cells by disrupting caspase inhibitor XIAP. Cell Signal. 2014;26:1326–1334. doi: 10.1016/j.cellsig.2014.02.019. [DOI] [PubMed] [Google Scholar]
- Wang J, Whiteman MW, Lian H, Wang G, Singh A, Huang D, Denmark T. A non-canonical MEK/ERK signaling pathway regulates autophagy via regulating Beclin 1. J Biol Chem. 2009;284:21412–21424. doi: 10.1074/jbc.M109.026013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Nie J, Wu L, Hu Y, Wen Z, Dong L, Zou MH, Chen C, Wang DW. AMPKalpha2 protects against the development of heart failure by enhancing Mitophagy via PINK1 phosphorylation. Circ Res. 2018;122:712–729. doi: 10.1161/CIRCRESAHA.117.312317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian H, Liou YC. Loss of MIEF1/MiD51 confers susceptibility to BAX-mediated cell death and PINK1-PRKN-dependent mitophagy. Autophagy. 2019;20:1–19. doi: 10.1080/15548627.2019.1596494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Zhang C, Jiang X, Li L, Zhang D, Tang D, Yan T, Zhang Q, Yuan H, Jia J, et al. Involvement of autophagy in hypoxia-BNIP3 signaling to promote epidermal keratinocyte migration. Cell Death Dis. 2019;10(3):234. doi: 10.1038/s41419-019-1473-9. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Zhong L, Shu W, Dai W, Gao B, Xiong S. Reactive oxygen species-mediated c-Jun NH2-terminal kinase activation contributes to hepatitis B virus X protein-induced autophagy via regulation of the Beclin-1/Bcl-2 interaction. J Virol. 2017;91(15):JVI.00001–JVI.00017. doi: 10.1128/JVI.00001-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(PDF 167 kb)