Abstract
Abstract
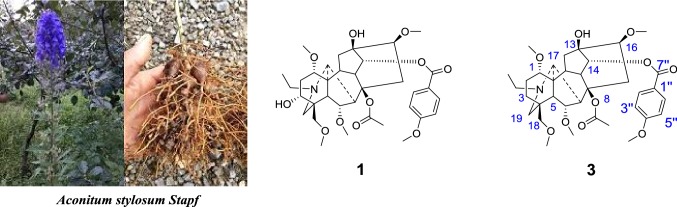
Both Aconitum hemsleyanum and Aconitum geniculatun have abundant contents of yunaconitine (1). Yunaconitine (1) has similar skeleton to crassicauline A (3); the only difference between them is that 1 contains a α-hydroxyl group at C-3. Our team attempts to convert 1 into 3 because 3 owns pharmacological activity. There are two steps to achieve the transformation above: firstly, use dehydration reaction to transform yunaconitine (1) into dehydroyunaconitine (2); secondly, use hydrogen reduction to acquire crassicauline A (3). Compared with other methods, this one below is more suitable for production application and more concise; moreover, the cost is lower with higher yield.
Graphic Abstract
Keywords: Diterpenoid alkaloids, Yunaconitine, Crassicauline A
Introduction
Diterpenoid alkaloids are the main pharmacological constituents of Aconitum. Diterpenoid alkaloids have anti-inflammatory, analgesic, sedative, antipyretic, and antineoplastic effection; the weight of them is accounting for about 7%–10% of Aconitum. Yunaconitine (1), as a C-19 diester diterpene alkaloid, distributes in almost the whole Yunnan Province; Aconitum hemsleyanum and A. geniculatum contains yunaconitine (1) [1–3] abundantly. However, 1 has very strong toxicity (LD50 for mice is 585 μg/kg (i.p.), for rats is 50 μg/kg (i.v.), and for dogs is 30 μg/kg (i.v.) [4].). Additionally, although crassicauline A (3) has high treatment index, low toxicity, strong analgesic activity and no pain tolerance, it is lack of contents in Aconitum [5–9]. Moreover 3 [10] exists in a tiny minority of Acontium species that produced in Li Jiang, Yunnan, China. Hence, there is a high cost in the application of 3. At present, 3 has been widely used in clinical treatment for more than 30 years in China [4] including the treatment of rheumatoid arthritis, osteoarthritis, neuropathic and chronic pain. The main available dosage forms of crassicauline A (3) on the market include tablets, capsules, injections. Some studies have shown the subtle difference between 1 and 3: Yunaconitine (1) contains a α-hydroxyl group at C-3; we believe that partial synthesis of 3 from 1 deserves to be mentioned.
A patent published by Zhang et al. [3] has reported four partial synthesis methods to afford crassicauline A (3) but the methods are complicated and difficult to purify the products with low yield. Therefore, we try to obtain semi synthetic 3 on the basis of increasing yield and getting a concise route.
Results, Discussion and Conclusion
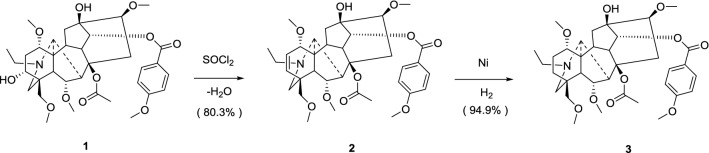
At first, yunaconitine (1) was treated with thionyl chloride to acquire a dehydration product. Then the product was treated with silica gel column chromatography. After that, dehydroyunaconitine (2) (Fig. 1) was obtained. The 1H NMR spectra (δH 6.01, 5.77) and 13C NMR spectra (δC 137.6, 125.3) of 2 illustrated the presence of a double bond at C-2. Besides, in HRESIMS+, a quasi-molecular ion peak appeared at m/z 642 [M+H]+; we could totally confirmed the double bond again. Lastly, under hydrogen reduction with Raney Ni catalysis, dehydroyunaconitine (2) converted to another product 3 (Fig. 1) which showed the same data (1H NMR, 13C NMR, ESIMS) as standard sample of crassicauline A. In this way, semi synthesis of crassicauline A (3) from yunaconitine (1) had been completed.
Fig. 1.
Chemical transformation of 1 to 3
We reported a new method to acquire crassicauline A (3), which had not been reported in the literature before. In our research, the semi synthesis of 3 from dehydroyunaconitine (2) was one of the most difficult steps. After a series of unsuccessful experiments, we finally found a practicable method. Compared with other methods, this one below was more suitable for production application and more concise; moreover, the cost was lower with higher yield.
In summary, we would continue to study the nature of yunaconitine (1) further. Previous studies have shown that (C-3, C-8, C-14) bonds of yunaconitine (1) have high pharmacological activity. Hence, we would tentatively synthesize a series of derivatives and establish some drug models in mice firstly; then, we expect to find better analgesic effect and fewer side effects with lower toxic compounds. Generally, this subject would be very useful for studies of structure–function relationship regard to diterpenoid alkaloids.
Experimental Sections
UV spectra were measured on a UV 210A Shimadzu spectrometer. IR spectra were recorded on a Vector 22 spectrometer with KBr pellets. Optical rotations were measured on Rudolph Autopol VI polarimeter. (Rudolph Research Analytical, Hacketstown, NJ, USA).One-dimensional NMR spectra were recorded with Avance spectrometer operating at 400 MHz for 1H and at 100 MHz for 13C. The chemical shifts (δ) were measured in CDCl3 (solvent signals: δH 7.24, δC 76.90) with TMS as an internal standard. ESI mass spectra were recorded on VG Auto Spec-3000 spectrometer.
Separation of Yunaconitine (1)
In a normal method [11], firstly, we crushed the roots of A. hemsleyanum or A. geniculatum into powders. Secondly, above-mentioned powders (1.5 kg) were soaked by 10% sodium carbonate and extracted with chloroform. Thirdly, the concentrated extracts were diluted with water and acidified with 2% hydrochloric acid. Fourthly, the liquor from last step was filtered; the filter liquor was alkalized with ammonium hydroxide and extracted with ether. Lastly, the ether solvent was dried over anhydrous sodium sulfate and concentrated under vacuum to acquire yunaconitine (1) (15.6 g, yield: 1.04%). After the final step, 1 was recrystallized several times by using ether to form crystals.
Preparation of Dehydroyunaconitine (2)
At first, yunaconitine (1) (1.07 g) was dissolved in thionyl chloride (15 ml) and the resulting mixture in a round bottom flask was refluxed at 80 ℃ for 11 h. In the second step, the mixture above was filtered and the filter liquor was evaporated to dryness under reduced pressure to give a residue (1.45 g). Thirdly, the residue was dissolved in H2O and alkalized to pH 8 with saturated sodium carbonate. After that, the liquor from last step was extracted with dichloromethane and the dichloromethane solvent was dried over anhydrous sodium sulfate. Finally, the above-mentioned solvent was concentrated under vacuum to obtain dehydroyunaconitine (2) and the compound was purified with silica gel column chromatography (eluted with acetone: petroleum ether 3:7; chloroform: methanol 9.5:0.5, respectively) to afford the pure product 2. (836 mg, yield: 80.3%). 2 could be recrystallized by using acetone: n-hexane mixed in specific proportion to form crystals.
Dehydroyunaconitine (2): cubic crystals, mp 271.5–274.0 °C; + 42.65 (c 0.068, CHCl3); UV (MeOH) λmax (log ε): 201.5 (3.63), 207.5 (3.73), 259 (3.80) nm. One of the IR (KBr) νmax 1635 cm−1. The 13C NMR spectra (CDCl3, 100 MHz) see Table 1. ESIMS m/z 642 [M+H]+; positive ion HRESIMS m/z 642.3200 (calcd for C35H47NO11 [M+H]+, 642.3199).
Table 1.
13C NMR Data of compounds 1, 2, 3 in CDCl3
| NO | 1 | 2 | 3 |
|---|---|---|---|
| C(1) | 83.1 | 83.5 | 85.0 |
| C(2) | 33.5 | 125.3 | 26.3 |
| C(3) | 71.4 | 137.6 | 34.9 |
| C(4) | 43.1 | 40.8 | 39.1 |
| C(5) | 47.4 | 47.8 | 50.2 |
| C(6) | 82.2 | 80.9 | 83.8 |
| C(7) | 44.7 | 44.5 | 45.1 |
| C(8) | 85.6 | 85.8 | 85.6 |
| C(9) | 48.7 | 46.4 | 50.0 |
| C(10) | 40.8 | 41.0 | 41.0 |
| C(11) | 50.2 | 48.8 | 49.1 |
| C(12) | 35.1 | 33.8 | 35.8 |
| C(13) | 74.6 | 74.8 | 74.8 |
| C(14) | 78.5 | 78.5 | 78.6 |
| C(15) | 39.5 | 40.1 | 39.3 |
| C(16) | 83.5 | 83.5 | 83.1 |
| C(17) | 61.6 | 59.2 | 61.7 |
| C(18) | 76.7 | 78.2 | 80.4 |
| C(19) | 48.7 | 52.6 | 53.6 |
| N-CH2-CH3 | 47.4 | 47.8 | 49.6 |
| N-CH2-CH3 | 13.1 | 12.4 | 13.4 |
| 1-OCH3 | 55.7 | 56.1 | 56.0 |
| 6-OCH3 | 58.7 | 58.8 | 58.7 |
| 16-OCH3 | 57.7 | 57.8 | 57.7 |
| 18-OCH3 | 59.0 | 59.2 | 59.0 |
| O = C-CH3 | 169.9 | 169.8 | 169.7 |
| O = C-CH3 | 21.5 | 21.5 | 21.6 |
| C(7″) | 166.1 | 165.8 | 166.0 |
| C(1″) | 122.5 | 122.6 | 122.8 |
| C(2″) or C(6″) | 131.6 | 131.6 | 131.7 |
| C(3″) or C(5″) | 113.7 | 113.8 | 113.7 |
| C(4″) | 163.5 | 163.5 | 163.5 |
| 4″-OCH3 | 55.3 | 55.4 | 55.4 |
Chemical shifts (δ) in ppm relative to TMS in CDCl3
Preparation of Crassicauline A (3)
Firstly, dehydroyunaconitine (2) (250 mg) was dissolved in 95% ethanol (5 mL) and under hydrogen reduction with Raney Ni catalysis (1.5 g), the resulting mixture in a round bottom flask was stirred at room temperature for 8 h. Next, after reaction was completed, the Raney Ni was removed by filtration. Lastly, the filter liquor was evaporated to dryness under reduced pressure to acquire a product 3 as white powders (238 mg, yield: 94.9%).
3 had the same data (1H NMR, 13C NMR, ESIMS) as standard sample of crassicauline A; the 13C NMR spectra of 3 see Table 1. At the same time, 3 also showed the same spot as crassicauline A in thin-layer chromatography. Therefore, the product 3 could be proved to be crassicauline A.
Acknowledgements
The authors appreciate the members at Kunming Institute of Botany, Chinese Academy of Science for measuring the spectroscopic data and the members of Yunnan toxic medicinal plants research team for picking and treatment of pharmaceutical raw materials. This work was supported by "YunLing Scholars" project (2020HC008).
Compliance with Ethical Standards
Conflict of interest:
The authors declare no conflict of interest.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Footnotes
Rong-Ping Zhang and Yan-Jun Lin have contributed equally to this work.
References
- 1.Chen SY. Acta Chem. Sin. 1979;37:15–20. [Google Scholar]
- 2.Wu C. J. Xinjiang Med. Univ. 2011;34:1153–1157. [Google Scholar]
- 3.Zhang HB, Hu WY, Li L, Dai XT, Zhang DZ. China. Patent. 1991;1054976:2. [Google Scholar]
- 4.Zhang RP, Chen SY, Zhou J. Acta Bot. Yunnanica Plant Divers. 1998;20:474–478. [Google Scholar]
- 5.Tang XC, Liu XJ, Lu WH. Acta Pharm. Sin. 1986;21:886. [PubMed] [Google Scholar]
- 6.Liu YQ, Ding XN, Zhang YD. Chin. J. Pain Med. 2011;17:314–315. [Google Scholar]
- 7.Zhen XH. Clin. Med. 2005;25:161. [Google Scholar]
- 8.Chodoeva A, Bosc J-J, Robert J. Aconitum Alkaloids and Biological Activities. Natural Products. Heidelberg: Springer; 2013. pp. 1503–1523. [Google Scholar]
- 9.Tang XC. Chin. J. N. Drugs Clin. Remedies. 1986;5:120–121. [Google Scholar]
- 10.The Pharmaceutical Standard, the Healthy Department of Yunnan Province, 1985
- 11.Gilman RE, Marion L. Can. J. Chem. 1962;40:1713–1716. doi: 10.1139/v62-259. [DOI] [Google Scholar]