Abstract
The extracellular matrix (ECM) is a deformable dynamic structure that dictates the behavior, function and integrity of blood vessels. The composition, density, chemistry and architecture of major globular and fibrillar proteins of the matrisome regulate the mechanical properties of the vasculature (i.e., stiffness/compliance). ECM proteins are linked via integrins to a protein adhesome directly connected to the actin cytoskeleton and various downstream signaling pathways that enable the cells to respond to external stimuli in a coordinated manner and maintain optimal tissue stiffness. However, cardiovascular risk factors such as diabetes, dyslipidemia, hypertension, ischemia and aging compromise the mechanical balance of the vascular wall. Stiffening of large blood vessels is associated with well-known qualitative and quantitative changes of fibrillar and fibrous macromolecules of the vascular matrisome. However, the mechanical properties of the thin-walled microvasculature are essentially defined by components of the subendothelial matrix. Cellular communication network (CCN) 1 and 2 proteins (aka Cyr61 and CTGF, respectively) of the CCN protein family localize in and act on the pericellular matrix of microvessels and constitute primary candidate markers and regulators of microvascular compliance. CCN1 and CCN2 bind various integrin and non-integrin receptors and initiate signaling pathways that regulate connective tissue remodeling and response to injury, the associated mechanoresponse of vascular cells, and the subsequent inflammatory response. The CCN1 and CCN2 genes are themselves responsive to mechanical stimuli in vascular cells, wherein mechanotransduction signaling converges into the common Rho GTPase pathway, which promotes actomyosin-based contractility and cellular stiffening. However, CCN1 and CCN2 each exhibit unique functional attributes in these processes. A better understanding of their synergistic or antagonistic effects on the maintenance (or loss) of microvascular compliance in physiological and pathological situations will assist more broadly based studies of their functional properties and translational value.
Keywords: Cellular communication network; CCN1/CYR61; CCN2/CTGF, Angiogenesis, Stiffness, Extracellular matrix proteins, Rho GTPase
Introduction
The vascular system is a highly specialized compliant tubular network that invests virtually every organ system in the body. Blood vessels transport and deliver oxygen and nutrients to developing and adult tissues to ensure proper differentiation, growth, function and homeostasis. Endothelial cells (ECs), which form the inner lining of blood vessels, are tightly connected to each other providing a barrier between the blood compartment and surrounding vascular and stromal cells. ECs are held together by tight and adherens junctions, which generate a paracellular barrier while allowing highly specific transport mechanisms between the luminal and extravascular space. Smooth muscle and/or pericytes line the abluminal surface of the endothelial tubes. Mural cell-deficient vessels in genetically modified mice became leaky, hyperplastic and/or permissive for angiogenic sprouting, indicating that bidirectional signals between ECs and mural cells regulate vessel plasticity, integrity and stability (Armulik et al. 2011).
Cell-cell and cell-matrix interactions are critical for blood vessel formation, regeneration and barrier integrity (Lee et al. 2017b). During development, the patterning, anastomosis and morphogenesis of vascular sprouts are determined by the types, concentrations and density (i.e., mechanical properties) of various ECM proteins, which orchestrate cell adhesion, proliferation, motility and shape (Okamoto et al. 2019; Zanotelli and Reinhart-King 2018). Coordinated physical interactions and signaling between cells and the ECM, together with the functional crosstalk among neighboring cells, are vital for proper vascular development and response to injury.
ECM proteins are distributed in three major compartments: interstitial, basement membrane (BM) and pericellular compartments. BM and interstitial proteins provide a scaffold and physical support that define some of the functional characteristics of the cells within it. In these compartments, the collagens, laminins, large glycoproteins and proteoglycans are organized into fibers and fibrils that form a three-dimensional meshwork within which cells can migrate, proliferate, differentiate and function. In addition, large ECM proteins with their highly charged groups serve as a storage depot for growth factors, chemokines and cytokines, and as a substrate for different proteases critical for vascular tissue remodeling. Consequently, increased matrix deposition (i.e., fibrosis), cross-linking among matrix proteins and fiber realignment alter the mechanical properties of tissues (i.e., stiffness) and their constitutive cells’ behavior as well as their responses to injurious stimuli (Bezie et al. 1999; Bou-Gharios et al. 2004; Chaquour et al. 1995). These are considered major pathogenic factors in cardiovascular and eye diseases as well as aging, cancer and diabetes (Kim et al. 2019; Lacolley et al. 2009; Zhang et al. 2019).
Other ECM proteins in the vascular matrix preferentially localize pericellularly including those of the CCN family (Perbal 2004; Perbal et al. 2018). These proteins are increasingly being recognized as important in developmental angiogenesis and in pathological processes such as fibrosis, ischemia, inflammation and oncogenesis (Chintala et al. 2012; Leask 2017; Perbal 2006; Perrot et al. 2015; Todorovicc et al. 2005; Tong et al. 2009). CCN1 and CCN2 bind a variety of receptors, primarily integrins (Lau 2016), and manifest their activities at the cell-environment interface by monitoring the outside-in and inside-out flow of information allowing cells to sense, respond and adapt to internally generated forces (e.g., actomyosin cytoskeleton contractions, stiffness of the ECM) and externally applied forces (e.g., shear stress, cyclic stretch) (Chaqour 2016; Chaqour and Goppelt-Struebe 2006). Cells constantly and rather rapidly remodel their pericellular matrix (PM) proteins by adjusting their rates of synthesis and degradation. Coordinated cell-PM interactions maintain adequate ECM stress set points that ensure mechanical homeostasis i.e., optimal tissue compliance/stiffness. However, little is known about the contribution of the CCN proteins to vascular biomechanics and what role, if any, these proteins play in initiating or opposing vascular cell stiffness in physiological and pathological conditions. Here, I summarize the relationship between constitutive ECM or matricellular proteins and vascular tissue compliance and their importance in stiffness-related pathologies. I further address the state of our current knowledge of CCN1 and CCN2 contribution to the regulation of vascular stiffness. The interested reader is also referred to several other review articles that deal with different aspects of the CCN protein regulation and function in different biological settings (Holbourn et al. 2008; Krupska et al. 2015; Lau and Lam 1999; Leask 2017; Yan and Chaqour 2013).
Regulation of vascular stiffness by ECM composition and organization
Vascular stiffness is defined as reduction in vascular distensibility and compliance. It is a measure of the elastic modulus or the change of the velocity of pulse wave propagation between two neighboring points of a vascular tube (Hadjadj et al. 2019; Wuyts et al. 1995). It equally affects arterial circulation and microcirculation. Indeed, stiffening of a large blood vessel wall (e.g., aorta) increases systolic and pulsatile central hemodynamic load, leading to microcirculatory damage in peripheral tissues. Reversibly, remodeling of the microvasculature increases the systemic vascular resistance, which decreases arterial wall compliance (Yannoutsos et al. 2014). Vascular stiffness has been linked to diabetes, high blood pressure, calcification and aging, and serves as an independent predictor of human cardiovascular diseases (London et al. 2004; Palombo and Kozakova 2016). The vascular endothelium, which first experiences the effects of compliance changes, can be both responsive to and causative of vascular wall rigidity (Bar et al. 2019; Qiu et al. 2018). Various chemical and functionally linked factors regulate EC stiffness. These include (1) changes in extracellular matrix composition and density, (2) changes in cytoskeletal protein activity (e.g., contractility), (3) contraction of mural cells (e.g., smooth muscle cells, pericytes) and (4) changes in blood flow. Of these, the composition of the subendothelial matrix is a major contributing factor to vascular stiffening (Francis-Sedlak et al. 2010; Frye et al. 2018; McCurdy et al. 2010).
The variety of ECM proteins in the vascular matrix and their interactions with each other define unique biological and physical properties of the vascular wall. At least twenty ECM proteins form the subendothelial matrix and basement membrane, including the laminins, type IV collagen, nidogen, perlecan, type XV and type XVIII collagens, fibronectin and other macromolecules (Hohenester and Engel 2002; Neve et al. 2014). The relative proportion and types of ECM proteins vary among different vascular beds. Overall, stiffness of large vessels is defined by the mechanical properties of large ECM proteins such as elastin, fibrillin-1, collagens and matrix metalloproteinases (MMPs) (Ferruzzi et al. 2016; Mariko et al. 2011; Yasmin et al. 2006). Elastic fibers, which are major components of the intima and media layers of elastic arteries, are responsible for the compliance and resilience of those blood vessels. The susceptibility of the elastic fibers to mechanical fatigue and/or compositional alterations contributes to large vessel stiffness (Longobardo et al. 2018). Elastin polymorphisms or local fragmentation of elastin fibers in aging vessels were associated with increased vessel wall stiffness (Kingwell and Boutouyrie 2007). Similarly, fibrillin-1, a glycoprotein that acts as the template for elastic fibers, regulates vascular stiffness (Medley et al. 2002). Genetic mutations in fibrillin-1 cause Marfan syndrome, characterized by severe cardiovascular and ocular symptoms linked to altered vascular stiffness (Rosenbloom et al. 1993; Thomson et al. 2019). Other extracellular determinants of vascular stiffness include collagen degradation and crosslinking (Chan et al. 2014; Furber 2006). In particular, MMP polymorphisms associated with reduced gene expression and/or collagenolytic activity have been linked to increased aortic stiffness in patients with coronary diseases (Lin et al. 2012; Medley et al. 2003). Conversely, the expression of lysyl oxidase, an enzyme that cross-links and strengthens ECM fibers, increased vascular stiffness (Lopez et al. 2010; Wordinger and Clark 2014). Finally, increased deposition of other large ECM proteins such as hyaluronic acid was reported to increase arterial stiffness associated with diabetic angiopathy and atherosclerosis (Chai et al. 2005; Lorentzen et al. 2016). At the molecular level, vascular cells use integrin receptors to physically interact with different ECM proteins and detect changes in matrix rigidity and composition. The resulting intracellular signaling cascade culminates into adaptive or maladaptive responses such as proliferation, differentiation, ECM synthesis or degradation. ECM-integrin interactions in vascular smooth muscle cells may determine the stiffness of the vessel wall as a whole (Klein et al. 2009; McDaniel et al. 2007). In line with this, inhibitors of integrin signaling reduced vasoconstrictor-induced smooth muscle cell and aortic stiffness (Jiao et al. 2017; Qiu et al. 2010).
Interestingly, the subendothelial matrix of the microvasculature is poorly enriched with large ECM proteins such as fibrillar collagens, elastin and hyaluronan (Kniazeva and Putnam 2009; Worthen et al. 1989). The PM in small caliber vessels such as capillaries and precapillary venules defines the vessels’ biomechanical properties. PM proteins such as those in the CCN family are functionally designed and strategically well positioned to act as the gatekeepers of microvessel compliance (Chaqour 2013; Chaqour and Goppelt-Struebe 2006).
Role of CCN1 and CCN2 in the vascular matrix
CCNs form a six-member family of ECM proteins previously categorized as matricellular proteins (Babic et al. 1998; Bradham et al. 1991; Perbal 2001). By far, the most studied proteins of this family are the first two members, CCN1 and CCN2. Both proteins exhibit critical functions in vascular tissue development and diseases. Despite their relatively high structural homology (40% at the amino acid level), different functions have been attributed to CCN1 and CCN2 in the cardiovascular system. The CCN1 gene is particularly expressed in the developing and adult cardiovascular system (Lee et al. 2017a). Cardiac and vascular defects were the most prominent alterations observed following global deletion of CCN1, including severe atrioventricular septal defects and abnormal aorta wherein vascular cells were aberrantly distributed in the vascular wall (Mo and Lau 2006). Inducible Cre/Lox-mediated deletion of the CCN1 gene in ECs caused blood vessels to coalesce into large flat hyperplastic sinuses with loss of arteriovenous specification (Chintala et al. 2015). At the molecular level, the mere absence of CCN1 in the pericellular matrix of sprouting blood vessels altered Notch pathway guidance signals and VEGF receptor-mediated EC growth. These alterations of blood vessel wall structure, morphogenesis and integrity may be reflective of profound alterations of the vascular wall mechanics as well (Lee et al. 2017b; Yan et al. 2015).
Like CCN1, CCN2 is widely expressed in embryonic tissues that give rise to the cardiovascular system (Hall-Glenn and Lyons 2011; SM et al. 2004). Global deletion of CCN2 resulted in perinatal lethality from respiratory failure due to disruption of basic lung development and failed thoracic expansion, which was indicative of skeletal defects (Ivkovic et al. 2003). Although no obvious vascular alterations were observed during the initial formation of the primitive blood vessel, newly formed arteries in CCN2-null embryos were enlarged with localized edema. These alterations were partly attributed to reduced expression of growth factors such as angiopoietin 2, PDGF and basement membrane proteins. Whether CCN2 directly provides cues for vascular cell proliferation, differentiation and ECM protein expression and deposition is still unknown.
CCN1 and CCN2 likely play a pivotal role in integrating signals from various angiomodulatory pathways (e.g., Notch, Wnt, VEGF, angiopoietins, hippo-YAP and sonic hedgehog). This regulation is currently the subject of active investigations. Importantly, the findings that loss of either CCN1 or CCN2 produced profound alterations of blood vessel structure, architecture and integrity is suggestive of a role of these proteins in maintenance of optimal vascular stiffness. Further investigations of their contribution to the mechanical homeostasis of blood vessels are warranted.
Dysregulation of CCN1 and CCN2 gene expression in vascular pathologies
Experimental and clinical data have shown that the expression of the CCN1 and CCN2 genes was dysregulated particularly at sites of angiogenesis, inflammation, connective tissue remodeling and tissue repair (Haque et al. 2012; Jun and Lau 2018; Krupska et al. 2015; Lee et al. 2017a; Lobel et al. 2012; Yoon et al. 2010). Increased CCN1 levels were found in a number of chronic inflammatory diseases, including colitis (Choi et al. 2015), rheumatoid arthritis (Komatsu et al. 2015) and atherosclerosis (Lee et al. 2007; Schober et al. 2002). CCN1 levels were similarly elevated in several ocular vascular complications, including proliferative diabetic retinopathy and vitreopathy, glaucoma and active ophthalmopathy (Futakuchi et al. 2018; Hasan et al. 2011; Lantz et al. 2005).
Interestingly, our group and others have shown that biological fluids and tissue biopsies from human clinical specimens were enriched with degradome products of CCN1 instead of the intact CCN1 protein form, which may also be indicative of loss of CCN1 function (Choi et al. 2013; Hinton et al. 2002). Like CCN1, CCN2 undergoes in vitro degradation and proteolytic shedding of smaller peptides, some of which may convey biological activities different from the intact parent molecule (Hinton et al. 2004; Kaasboll et al. 2018). A relatively rapid turnover of these ECM proteins may reflect the absence of interactors and binding partners that hinder their accessibility to proteolytic enzymes. Both transcriptional and posttranslational alterations of CCN2 has been associated with the development of fibrotic reactions characterized by the excessive deposition of ECM proteins, recruitment of activated fibroblasts and smooth muscle cells leading to tissue contraction and scarring (Chaqour et al. 2006; Chintala et al. 2012; Hutchenreuther et al. 2017; Leask 2017). These alterations are reflective of loss of optimal stiffness of the affected tissues and organs.
RhoA GTPases as modulators of CCN1 and CCN2 and vascular stiffness
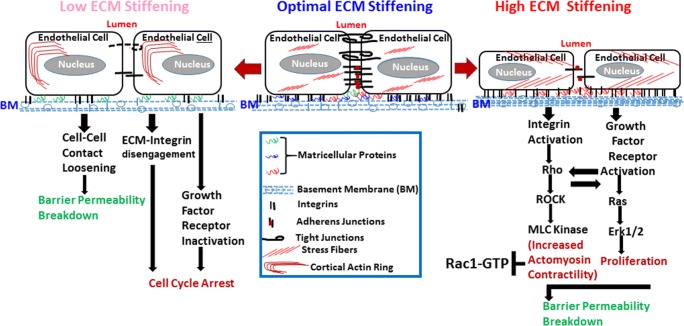
As extracellular cues, CCN1 and CCN2 initiate chemical and mechanical changes that affect cell shape, migration, motility and fate (Chaqour and Goppelt-Struebe 2006). Their abundance, or lack thereof, in the subendothelial environment influences the physical, topological and biochemical properties of the ECM. CCN1 and CCN2 activity involves multiple interactions with integrins (e.g., ανβ3, αΜβ2, ανβ1, α6β1, αIIbβ3, αΜβ2, αDβ2), non-integrin receptors (e.g., LRPs, Notch), individual ECM molecules (e.g., collagen, fibronectin, fibullin), growth factors (VEGF, BMP) and proteases (e.g., MMP-2, MMP-14, kalikrein) (Butler et al. 2008; Gao and Brigstock 2003; Guillon-Munos et al. 2011; Lau 2016). Integrins bind different ECM ligands and divalent cations via their extracellular domains, but they also interact laterally with other proteins at the cell surface, such as tetraspanins, growth factor receptors, matricellular proteins and proteases (Miranti and Brugge 2002). Integrins sense the composition and mechanical properties within the pericellular environment and transmit this information intracellularly to the cytoskeleton by recruiting cytoplasmic mediators (i.e., the adhesome) to their cytoplasmic domains at focal adhesion sites. Integrin engagement with CCN1 and CCN2 initiates a signaling cascade that culminates in Rho GTPase signaling pathway activation. The Rho GTPase family comprises at least 20 members of which Rac1, 2 and 3 and RhoA, B and C members are known to regulate lamellipodial actin polymerization and actomyosin contractility, respectively (Fig. 1). Subsequent activation of RhoA-kinase (ROCK) and inhibition of myosin phosphatase increase myosin light chain (MLC) phosphorylation, leading to clustering of actin stress fibers that promote actomyosin contractility and endogenous tension. Reversibly, activation of the Rho GTPase pathway in response to mechanical tension induces the expression of the CCN1 and CCN2 genes (Chowdhury and Chaqour 2004; Han et al. 2003). Other stimuli like thrombin and sphingosine 1-phosphate act through the G protein coupled receptors (GPCR) and RhoA signaling pathways to induce CCN1 and CCN2 gene expression (Lee et al. 2007; Walsh et al. 2008). It is these reciprocal biochemical and biophysical interactions between CCN1 and CCN2 and Rho GTPase that regulate the vascular wall stiffness (Hoon et al. 2016). RhoA GTPase activation results in binding to downstream effector proteins (e.g., Rho-associated protein kinase ROCK) and activation of signaling cascades including the phosphatidyl inositol-3 phosphate kinase, focal adhesion kinase, Src kinase, LIMK and MEK/Erk protein networks (Howe and Addison 2012; Koh et al. 2008). Activated RhoA stimulates actin polymerization via the formin protein mDia. Meanwhile, ROCK further phosphorylates and activates LIMK1, leading to the phosphorylation and inactivation of cofilin. The actin-severing activity of phosphorylated cofilin is attenuated, which increases actin polymerization and stabilization of actin filaments. In ECs, RhoA activation induces actin stress fiber formation leading to actomyosin contraction (Hoon et al. 2016). Consequently, actomyosin-based contractility promotes cellular stiffening. These alterations feed back into the organization of membrane-associated receptors, including junctional protein complexes, which eventually drives endothelial permeability and (chronic) vascular inflammation in cardiovascular diseases (Cusma-Piccione et al. 2014; Oh et al. 2016). However, RhoA GTPase output is regulated by the related GTPase Rac1. Rac1 and RhoA have opposing effects on the actomyosin cytoskeleton (Howe and Addison 2012; Koh et al. 2008). Deletion of Rac1 mimics a RhoA activation phenotype while gain of Rac1 function increased cellular deformability and pliability which protects against vascular wall stiffening and blood pressure elevation (Andre et al. 2014).
Fig. 1.
Schematic representation of the regulation of endothelial cell growth and barrier function of microvessels by ECM stiffening and the involvement of matricellular proteins in such regulation
Role of CCN1 and CCN2 in stiffness-associated pathologies
In addition to the vascular wall, CCN1 and/or CCN2 are constitutively present in many ECM-rich tissues, such as condensing mesenchyme, cartilage and cancer-associated stroma (Fukunaga et al. 2003; Ghosh et al. 2017; Kawaki et al. 2017). ECM accumulation is quite frequent in the microenvironment of these tissues, which then become highly susceptible to stiffening. Higher stiffness conditions are associated with marked increase in the amount of stress fibers as well as increased cell migration and invasion (Gkretsi and Stylianopoulos 2018). However, little is known about the contribution of CCN1 and CCN2, either individually or as ECM-associated proteins, to the mechanical properties of vascular and non-vascular tissues. CCN1 and CCN2 function in the matrix has not been associated with direct modulation of ECM organization. Instead, these proteins activate signaling cascades that may reprogram the cells’ synthetic and proliferative phenotypes that determine cell and tissue deformability.
However, the direct role of CCN1 and CCN2 in tissue stiffness is supported mainly by circumstantial evidence. In the liver, CCN1 was shown to promote resolution of fibrosis by triggering senescence in myofibroblasts, which express an anti-fibrotic gene program of matrix-degrading enzymes (Kim et al. 2015). Organs with established fibrosis are thought to be stiffer as a result of the increased quantity of ECM proteins such as fibrillar collagens (Lachowski et al. 2019; Lin et al. 2018). However, overexpression of CCN1 in hepatocytes or systemic injection of purified CCN1 protein in mice accelerates resolution of established fibrosis, which suggests anti-fibrotic and potentially anti-stiffness effects of CCN1. Using the retina as a model of vascular development and pathology, our laboratory showed that loss of CCN1 expression resulted in the uncontrolled growth of ECs and excessive enlargement of blood vessels (Chaqour 2016; Chintala et al. 2015; Lee et al. 2017b). Re-expression of CCN1 in the retinal vasculature allowed normal revascularization of the tissue through a negative feedback loop involving inactivation of the yes-associated protein (YAP), a molecular marker of tissue stiffness (Lee et al. 2019). As a negative regulator of YAP activity, CCN1 may function to also reduce microvascular stiffness.
Unlike CCN1, CCN2 was suggested to contribute to tissue stiffness. Elevated levels of CCN2 in the serum of patients with biliary atresia correlated with liver stiffness (Honsawek et al. 2013; Kobayashi et al. 2005). CCN2 is also viewed as a surrogate marker of stiffness in atherosclerosis, myocardial infarction and hypertension. The fibrosis-promoting effects of endothelin, angiotensin II, TGF-β, and mechanical stimuli have been attributed to CCN2 (Recchia et al. 2009). Inhibition of CCN2 reversed the process of fibrosis and a humanized CCN2 monoclonal antibody (FG-3019) prevented carotid artery vascular stiffness in a diabetic rat model (Lipson et al. 2012). However, the profibrotic effects of CCN2 appeared to be organ-specific because overexpression of CCN2 in the mouse heart promoted age-dependent development of cardiac hypertrophy, but did not induce cardiac fibrosis (Fontes et al. 2015; Shimo et al. 1999; Szabo et al. 2014). The overexpression of CCN2 by itself is not sufficient to induce cardiac fibrosis and the functions of CCN2 in the heart may be different from the observed CCN2 functions in the skin, lung and other tissues (Ahmed et al. 2007; Hviid et al. 2012; Mukudai et al. 2003). Of interest, a study by Overby et al. showed that Schlemm’s canal cells from glaucoma patients, which express higher levels of CCN2, were stiffer than those from matched healthy individuals corroborating the function of CCN2 in tissue stiffness (Overby et al. 2014). Altogether, these studies strongly suggest an important, if not determining, role of CCN1 and CCN2 in tissue compliance. A comprehensive analysis of CCN1 and CCN2-induced stiffness changes in vascular and non-vascular tissues and the underpinning mechanisms are warranted.
Conclusions
In essence, CCN1 and CCN2 share a multimodular structure and amino acid sequence homology. However, each protein exhibits unique functional attributes. In the vascular system, the molecular basis of their activities pertains to the cell and tissue response to mechanical and chemical injury, which involve regulation of connective tissue remodeling and induction of either reparative or destructive angiogenesis. Under physiological conditions, the presence or absence of CCN1 and CCN2 affects the topological features and biophysical cues of the cells and tissues through the organization of integrins, focal adhesion assembly and reorganization of the actin cytoskeleton. These proteins both feed off of and into the Rho GTPase signaling pathway, which, when activated, increases contractile traction forces mediated by integrin engagement with the ECM. This is one the widely documented downstream consequences of increased ECM stiffness. The biological activities of CCN1 and CCN2 suggest that these molecules are ideal candidates for the regulation of the delicate balance among different Rho GTPases (e.g., Rac and Rho) that allow the cells to maintain nearly constant physical properties in the face of growth, differentiation and ECM turnover. Understanding the contribution of the CCN1 and CCN2 proteins to the regulation of the mechanical properties of the vascular system and their potential role in mediating the long-term mechanical robustness of blood vessels are warranted investigation tasks for future studies.
Acknowledgments
We thank Sohyun Moon and Charles Karrasch for critical reading of the manuscript. This work was supported in part by grants from the National Eye Institute of the National Institutes of Health (EY022091-05A1 and EY024998).
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- Ahmed MS, Oie E, Vinge LE, von Lueder TG, Attramadal T, Attramadal H. Induction of pulmonary connective tissue growth factor in heart failure is associated with pulmonary parenchymal and vascular remodeling. Cardiovasc Res. 2007;74:323–333. doi: 10.1016/j.cardiores.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Andre G, Sandoval JE, Retailleau K, Loufrani L, Toumaniantz G, Offermanns S, Rolli-Derkinderen M, Loirand G, Sauzeau V. Smooth muscle specific Rac1 deficiency induces hypertension by preventing p116RIP3-dependent RhoA inhibition. J Am Heart Assoc. 2014;3:e000852. doi: 10.1161/JAHA.114.000852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armulik A, Genove G, Betsholtz C. Pericytes: developmental, physiological, and pathological perspectives, problems, and promises. Dev Cell. 2011;21:193–215. doi: 10.1016/j.devcel.2011.07.001. [DOI] [PubMed] [Google Scholar]
- Babic AM, Kireeva ML, Kolesnikova TV, Lau LF. CYR61, a product of a growth factor-inducible immediate early gene, promotes angiogenesis and tumor growth. Proc Natl Acad Sci U S A. 1998;95:6355–6360. doi: 10.1073/pnas.95.11.6355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bar A, Targosz-Korecka M, Suraj J, Proniewski B, Jasztal A, Marczyk B, Sternak M, Przybylo M, Kurpinska A, Walczak M, Kostogrys RB, Szymonski M, Chlopicki S. Degradation of Glycocalyx and multiple manifestations of endothelial dysfunction coincide in the early phase of endothelial dysfunction before atherosclerotic plaque development in apolipoprotein E/Low-density lipoprotein receptor-deficient mice. J Am Heart Assoc. 2019;8:e011171. doi: 10.1161/JAHA.118.011171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bezie Y, Daniel-Lamaziere JM, Gabella G, Koffi I, Laurent S, Lacolley P. Molecular and cellular determinants of arterial stiffness: role of cell-matrix connections. Pathol Biol (Paris) 1999;47:669–676. [PubMed] [Google Scholar]
- Bou-Gharios G, Ponticos M, Rajkumar V, Abraham D. Extra-cellular matrix in vascular networks. Cell Prolif. 2004;37:207–220. doi: 10.1111/j.1365-2184.2004.00306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradham DM, Igarashi A, Potter RL, Grotendorst GR. Connective tissue growth factor: a cysteine-rich mitogen secreted by human vascular endothelial cells is related to the SRC-induced immediate early gene product CEF-10. J Cell Biol. 1991;114:1285–1294. doi: 10.1083/jcb.114.6.1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler GS, Dean RA, Tam EM, Overall CM. Pharmacoproteomics of a metalloproteinase hydroxamate inhibitor in breast cancer cells: dynamics of membrane type 1 matrix metalloproteinase-mediated membrane protein shedding. Mol Cell Biol. 2008;28:4896–4914. doi: 10.1128/MCB.01775-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai S, Chai Q, Danielsen CC, Hjorth P, Nyengaard JR, Ledet T, Yamaguchi Y, Rasmussen LM, Wogensen L. Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res. 2005;96:583–591. doi: 10.1161/01.RES.0000158963.37132.8b. [DOI] [PubMed] [Google Scholar]
- Chan KLS, Khankhel AH, Thompson RL, Coisman BJ, Wong KHK, Truslow JG, Tien J. Crosslinking of collagen scaffolds promotes blood and lymphatic vascular stability. J Biomed Mater Res A. 2014;102:3186–3195. doi: 10.1002/jbm.a.34990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaqour B. Molecular control of vascular development by the matricellular proteins (Cyr61/CCN1) and (CTGF/CCN2) Trends Dev Biol. 2013;7:59–72. [PMC free article] [PubMed] [Google Scholar]
- Chaqour B. Regulating the regulators of angiogenesis by CCN1 and taking it up a notch. J Cell Commun Signal. 2016;10:259–261. doi: 10.1007/s12079-016-0328-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaqour B, Goppelt-Struebe M. Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. FEBS J. 2006;273:3639–3649. doi: 10.1111/j.1742-4658.2006.05360.x. [DOI] [PubMed] [Google Scholar]
- Chaqour B, Yang R, Sha Q. Mechanical stretch modulates the promoter activity of the profibrotic factor CCN2 through increased actin polymerization and NF-kappaB activation. J Biol Chem. 2006;281:20608–20622. doi: 10.1074/jbc.M600214200. [DOI] [PubMed] [Google Scholar]
- Chaquour B, Seite S, Coutant K, Fourtanier A, Borel JP, Bellon G. Chronic UVB- and all-trans retinoic-acid-induced qualitative and quantitative changes in hairless mouse skin. J Photochem Photobiol B. 1995;28:125–135. doi: 10.1016/1011-1344(94)07080-8. [DOI] [PubMed] [Google Scholar]
- Chintala H, Liu H, Parmar R, Kamalska M, Kim YJ, Lovett D, Grant MB, Chaqour B. Connective tissue growth factor regulates retinal neovascularization through p53 protein-dependent transactivation of the matrix metalloproteinase (MMP)-2 gene. J Biol Chem. 2012;287:40570–40585. doi: 10.1074/jbc.M112.386565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chintala H, Krupska I, Yan L, Lau L, Grant M, Chaqour B. The matricellular protein CCN1 controls retinal angiogenesis by targeting VEGF, Src homology 2 domain phosphatase-1 and notch signaling. Development. 2015;142:2364–2374. doi: 10.1242/dev.121913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J, Lin A, Shrier E, Lau LF, Grant MB, Chaqour B. Degradome products of the matricellular protein CCN1 as modulators of pathological angiogenesis in the retina. J Biol Chem. 2013;288:23075–23089. doi: 10.1074/jbc.M113.475418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi JS, Kim KH, Lau LF. The matricellular protein CCN1 promotes mucosal healing in murine colitis through IL-6. Immunol: Mucosal; 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury I, Chaqour B. Regulation of connective tissue growth factor (CTGF/CCN2) gene transcription and mRNA stability in smooth muscle cells. Involvement of RhoA GTPase and p38 MAP kinase and sensitivity to actin dynamics. Eur J Biochem. 2004;271:4436–4450. doi: 10.1111/j.1432-1033.2004.04382.x. [DOI] [PubMed] [Google Scholar]
- Cusma-Piccione M, Zito C, Khandheria BK, Pizzino F, Di Bella G, Antonini-Canterin F, Vriz O, Bello VA, Zimbalatti C, La Carrubba S, Oreto G, Carerj S. How arterial stiffness may affect coronary blood flow: a challenging pathophysiological link. J Cardiovasc Med (Hagerstown) 2014;15:797–802. doi: 10.2459/JCM.0000000000000185. [DOI] [PubMed] [Google Scholar]
- Ferruzzi J, Bersi MR, Mecham RP, Ramirez F, Yanagisawa H, Tellides G, Humphrey JD. Loss of elastic Fiber integrity compromises common carotid artery function: implications for vascular aging. Artery Res. 2016;14:41–52. doi: 10.1016/j.artres.2016.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontes MS, Kessler EL, van Stuijvenberg L, Brans MA, Falke LL, Kok B, Leask A, van Rijen HV, Vos MA, Goldschmeding R, van Veen TA. CTGF knockout does not affect cardiac hypertrophy and fibrosis formation upon chronic pressure overload. J Mol Cell Cardiol. 2015;88:82–90. doi: 10.1016/j.yjmcc.2015.09.015. [DOI] [PubMed] [Google Scholar]
- Francis-Sedlak ME, Moya ML, Huang JJ, Lucas SA, Chandrasekharan N, Larson JC, Cheng MH, Brey EM. Collagen glycation alters neovascularization in vitro and in vivo. Microvasc Res. 2010;80:3–9. doi: 10.1016/j.mvr.2009.12.005. [DOI] [PubMed] [Google Scholar]
- Frye M, Taddei A, Dierkes C, Martinez-Corral I, Fielden M, Ortsater H, Kazenwadel J, Calado DP, Ostergaard P, Salminen M, He L, Harvey NL, Kiefer F, Makinen T. Matrix stiffness controls lymphatic vessel formation through regulation of a GATA2-dependent transcriptional program. Nat Commun. 2018;9:1511. doi: 10.1038/s41467-018-03959-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukunaga T, Yamashiro T, Oya S, Takeshita N, Takigawa M, Takano-Yamamoto T. Connective tissue growth factor mRNA expression pattern in cartilages is associated with their type I collagen expression. Bone. 2003;33:911–918. doi: 10.1016/j.bone.2003.07.010. [DOI] [PubMed] [Google Scholar]
- Furber JD. Extracellular glycation crosslinks: prospects for removal. Rejuvenation Res. 2006;9:274–278. doi: 10.1089/rej.2006.9.274. [DOI] [PubMed] [Google Scholar]
- Futakuchi A, Inoue T, Wei FY, Inoue-Mochita M, Fujimoto T, Tomizawa K, Tanihara H. YAP/TAZ are essential for TGF-beta2-mediated conjunctival fibrosis. Invest Ophthalmol Vis Sci. 2018;59:3069–3078. doi: 10.1167/iovs.18-24258. [DOI] [PubMed] [Google Scholar]
- Gao R, Brigstock DR. Low density lipoprotein receptor-related protein (LRP) is a heparin-dependent adhesion receptor for connective tissue growth factor (CTGF) in rat activated hepatic stellate cells. Hepatol Res. 2003;27:214–220. doi: 10.1016/s1386-6346(03)00241-9. [DOI] [PubMed] [Google Scholar]
- Ghosh P, Banerjee S, Maity G, De A, Banerjee SK. Detection of CCN1 and CCN5 mRNA in human Cancer samples using a modified in situ hybridization technique. Methods Mol Biol. 2017;1489:495–504. doi: 10.1007/978-1-4939-6430-7_41. [DOI] [PubMed] [Google Scholar]
- Gkretsi V, Stylianopoulos T. Cell adhesion and matrix stiffness: coordinating Cancer cell invasion and metastasis. Front Oncol. 2018;8:145. doi: 10.3389/fonc.2018.00145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon-Munos A, Oikonomopoulou K, Michel N, Smith CR, Petit-Court S, Canepa P, Reverdiau N, Heuze-Vourc'h EPD, Courty Y. Kallikrein-related peptidase 12 hydrolyzes matricellular proteins of the CCN family and modifies interactions of CCN1 and CCN5 with growth factors. J Biol Chem. 2011;286:25505–25518. doi: 10.1074/jbc.M110.213231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hadjadj L, Monori-Kiss A, Horvath EM, Heinzlmann A, Magyar A, Sziva RE, Miklos Z, Pal E, Gal J, Szabo I, Benyo Z, Nadasy GL, Varbiro S. Geometric, elastic and contractile-relaxation changes in coronary arterioles induced by vitamin D deficiency in normal and hyperandrogenic female rats. Microvasc Res. 2019;122:78–84. doi: 10.1016/j.mvr.2018.11.011. [DOI] [PubMed] [Google Scholar]
- Hall-Glenn F, Lyons KM. Roles for CCN2 in normal physiological processes. Cell Mol Life Sci. 2011;68:3209–3217. doi: 10.1007/s00018-011-0782-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han JS, Macarak E, Rosenbloom J, Chung KC, Chaqour B. Regulation of Cyr61/CCN1 gene expression through RhoA GTPase and p38MAPK signaling pathways. Eur J Biochem. 2003;270:3408–3421. doi: 10.1046/j.1432-1033.2003.03723.x. [DOI] [PubMed] [Google Scholar]
- Haque I, De A, Majumder M, Mehta S, McGregor D, Banerjee SK, Van VP, Banerjee S. The matricellular protein CCN1/Cyr61 is a critical regulator of sonic hedgehog in pancreatic carcinogenesis. J Biol Chem. 2012;287:38569–38579. doi: 10.1074/jbc.M112.389064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan A, Pokeza N, Shaw L, Lee HS, Lazzaro D, Chintala H, Rosenbaum D, Grant MB, Chaqour B. The matricellular protein cysteine-rich protein 61 (CCN1/Cyr61) enhances physiological adaptation of retinal vessels and reduces pathological neovascularization associated with ischemic retinopathy. J Biol Chem. 2011;286:9542–9554. doi: 10.1074/jbc.M110.198689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinton DR, He S, Jin ML, Barron E, Ryan SJ. Novel growth factors involved in the pathogenesis of proliferative vitreoretinopathy. Eye. 2002;16:422–428. doi: 10.1038/sj.eye.6700190. [DOI] [PubMed] [Google Scholar]
- Hinton DR, Spee C, He S, Weitz S, Usinger W, LaBree L, Oliver N, Lim JI. Accumulation of NH2-terminal fragment of connective tissue growth factor in the vitreous of patients with proliferative diabetic retinopathy. Diabetes Care. 2004;27:758–764. doi: 10.2337/diacare.27.3.758. [DOI] [PubMed] [Google Scholar]
- Hohenester E, Engel J. Domain structure and organisation in extracellular matrix proteins. Matrix Biol. 2002;21:115–128. doi: 10.1016/s0945-053x(01)00191-3. [DOI] [PubMed] [Google Scholar]
- Holbourn KP, Acharya KR, Perbal B. The CCN family of proteins: structure-function relationships. Trends Biochem Sci. 2008;33:461–473. doi: 10.1016/j.tibs.2008.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honsawek S, Udomsinprasert W, Chirathaworn C, Anomasiri W, Vejchapipat P, Poovorawan Y. Correlation of connective tissue growth factor with liver stiffness measured by transient elastography in biliary atresia. Hepatol Res. 2013;43:795–800. doi: 10.1111/hepr.12015. [DOI] [PubMed] [Google Scholar]
- Hoon JL, Tan MH, Koh CG (2016) The regulation of cellular responses to mechanical cues by rho GTPases. Cells 5:1–20 [DOI] [PMC free article] [PubMed]
- Howe GA, Addison CL. RhoB controls endothelial cell morphogenesis in part via negative regulation of RhoA. Vasc Cell. 2012;4:1. doi: 10.1186/2045-824X-4-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchenreuther J, Leask A, Thompson K. Studying the CCN proteins in fibrosis. Methods Mol Biol. 2017;1489:423–429. doi: 10.1007/978-1-4939-6430-7_35. [DOI] [PubMed] [Google Scholar]
- Hviid CV, Erdem JS, Kunke D, Ahmed SM, Kjeldsen SF, Wang YY, Attramadal H, Aasen AO. The matri-cellular proteins cysteinerich, angiogenic-inducer, 61 and connective tissue growth factor are regulated in experimentally-induced sepsis with multiple organ dysfunction. Innate Immun. 2012;18:717–726. doi: 10.1177/1753425912436764. [DOI] [PubMed] [Google Scholar]
- Ivkovic S, Yoon BS, Popoff SN, Safadi FF, Libuda DE, Stephenson RC, Daluiski A, Lyons KM. Connective tissue growth factor coordinates chondrogenesis and angiogenesis during skeletal development. Development. 2003;130:2779–2791. doi: 10.1242/dev.00505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiao Y, Li G, Li Q, Ali R, Qin L, Li W, Qyang Y, Greif DM, Geirsson A, Humphrey JD, Tellides G. mTOR (mechanistic target of rapamycin) inhibition decreases Mechanosignaling, collagen accumulation, and stiffening of the thoracic aorta in elastin-deficient mice. Arterioscler Thromb Vasc Biol. 2017;37:1657–1666. doi: 10.1161/ATVBAHA.117.309653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jun JI, Lau LF. Resolution of organ fibrosis. J Clin Invest. 2018;128:97–107. doi: 10.1172/JCI93563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaasboll OJ, Gadicherla AK, Wang JH, Monsen VT, Hagelin EMV, Dong MQ, Attramadal H. Connective tissue growth factor (CCN2) is a matricellular preproprotein controlled by proteolytic activation. J Biol Chem. 2018;293:17953–17970. doi: 10.1074/jbc.RA118.004559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaki H, Kubota S, Takigawa M. Immunohistochemical analysis of CCN proteins in calcified tissues. Methods Mol Biol. 2017;1489:53–62. doi: 10.1007/978-1-4939-6430-7_6. [DOI] [PubMed] [Google Scholar]
- Kim KH, Chen CC, Alpini G, Lau LF. CCN1 induces hepatic ductular reaction through integrin alphavbeta(5)-mediated activation of NF-kappaB. J Clin Invest. 2015;125:1886–1900. doi: 10.1172/JCI79327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim M, Kim RY, Kim JY, Park YH. Correlation of systemic arterial stiffness with changes in retinal and choroidal microvasculature in type 2 diabetes. Sci Rep. 2019;9:1401. doi: 10.1038/s41598-018-37969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingwell B, Boutouyrie P. Genetic influences on the arterial wall. Clin Exp Pharmacol Physiol. 2007;34:652–657. doi: 10.1111/j.1440-1681.2007.04655.x. [DOI] [PubMed] [Google Scholar]
- Klein EA, Yin L, Kothapalli D, Castagnino P, Byfield FJ, Xu T, Levental I, Hawthorne E, Janmey PA, Assoian RK. Cell-cycle control by physiological matrix elasticity and in vivo tissue stiffening. Curr Biol. 2009;19:1511–1518. doi: 10.1016/j.cub.2009.07.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kniazeva E, Putnam AJ. Endothelial cell traction and ECM density influence both capillary morphogenesis and maintenance in 3-D. Am J Phys Cell Phys. 2009;297:C179–C187. doi: 10.1152/ajpcell.00018.2009. [DOI] [PubMed] [Google Scholar]
- Kobayashi H, Hayashi N, Hayashi K, Yamataka A, Lane GJ, Miyano T. Connective tissue growth factor and progressive fibrosis in biliary atresia. Pediatr Surg Int. 2005;21:12–16. doi: 10.1007/s00383-004-1254-z. [DOI] [PubMed] [Google Scholar]
- Koh W, Mahan RD, Davis GE. Cdc42- and Rac1-mediated endothelial lumen formation requires Pak2, Pak4 and Par3, and PKC-dependent signaling. J Cell Sci. 2008;121:989–1001. doi: 10.1242/jcs.020693. [DOI] [PubMed] [Google Scholar]
- Komatsu M, Nakamura Y, Maruyama M, Abe K, Watanapokasin R, and Kato H (2015). Expression profiles of human CCN genes in patients with osteoarthritis or rheumatoid arthritis. J Orthop Sci [DOI] [PubMed]
- Krupska I, Bruford EA, Chaqour B. Eyeing the Cyr61/CTGF/NOV (CCN) group of genes in development and diseases: highlights of their structural likenesses and functional dissimilarities. Hum Genomics. 2015;9:1–13. doi: 10.1186/s40246-015-0046-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachowski D, Cortes E, Rice A, Pinato D, Rombouts K, Del Rio Hernandez A. Matrix stiffness modulates the activity of MMP-9 and TIMP-1 in hepatic stellate cells to perpetuate fibrosis. Sci Rep. 2019;9:7299. doi: 10.1038/s41598-019-43759-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacolley P, Challande P, Osborne-Pellegrin M, Regnault V. Genetics and pathophysiology of arterial stiffness. Cardiovasc Res. 2009;81:637–648. doi: 10.1093/cvr/cvn353. [DOI] [PubMed] [Google Scholar]
- Lantz M, Vondrichova T, Parikh H, Frenander C, Ridderstrale M, Asman P, Aberg M, Groop L, Hallengren B. Overexpression of immediate early genes in active Graves' ophthalmopathy. J Clin Endocrinol Metab. 2005;90:4784–4791. doi: 10.1210/jc.2004-2275. [DOI] [PubMed] [Google Scholar]
- Lau LF. Cell surface receptors for CCN proteins. J Cell Commun Signal. 2016;10:121–127. doi: 10.1007/s12079-016-0324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau LF, Lam SC. The CCN family of angiogenic regulators: the integrin connection. Exp Cell Res. 1999;248:44–57. doi: 10.1006/excr.1999.4456. [DOI] [PubMed] [Google Scholar]
- Leask A. CCN2 in Skin Fibrosis. Methods Mol Biol. 2017;1489:417–421. doi: 10.1007/978-1-4939-6430-7_34. [DOI] [PubMed] [Google Scholar]
- Lee HY, Chung JW, Youn SW, Kim JY, Park KW, Koo BK, Oh BH, Park YB, Chaqour B, Walsh K, Kim HS. Forkhead transcription factor FOXO3a is a negative regulator of angiogenic immediate early gene CYR61, leading to inhibition of vascular smooth muscle cell proliferation and neointimal hyperplasia. Circ Res. 2007;100:372–380. doi: 10.1161/01.RES.0000257945.97958.77. [DOI] [PubMed] [Google Scholar]
- Lee S, Elaskandrany M, Ahad A, Chaqour B. Analysis of CCN protein expression and activities in Vasoproliferative retinopathies. Methods Mol Biol. 2017;1489:543–556. doi: 10.1007/978-1-4939-6430-7_46. [DOI] [PubMed] [Google Scholar]
- Lee S, Elaskandrany M, Lau LF, Lazzaro D, Grant MB, Chaqour B. Interplay between CCN1 and Wnt5a in endothelial cells and pericytes determines the angiogenic outcome in a model of ischemic retinopathy. Sci Rep. 2017;7:1405. doi: 10.1038/s41598-017-01585-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Ahad A, Luu M, Moon S, Caesar J, Cardoso WV, Grant M, Chaqour B (2019) CCN1-YAP feedback loop regulates physiological and pathological angiogenesis. Mol Cell Biol [DOI] [PMC free article] [PubMed]
- Lin RT, Chen CH, Tsai PC, Ho BL, Juo SH, Lin HF. Sex-specific effect of matrix metalloproteinase-9 functional promoter polymorphism on carotid artery stiffness. Atherosclerosis. 2012;223:416–420. doi: 10.1016/j.atherosclerosis.2012.05.031. [DOI] [PubMed] [Google Scholar]
- Lin Z, Liang J, Zhu J, Hu C, Gu Y, Lai J, Zheng Y, Gao Z. Diverse correlations between fibrosis-related factors and liver stiffness measurement by transient elastography in chronic hepatitis B. Eur J Gastroenterol Hepatol. 2018;30:217–225. doi: 10.1097/MEG.0000000000001023. [DOI] [PubMed] [Google Scholar]
- Lipson, K.E., C. Wong, Y. Teng, and S. Spong (2012) CTGF is a central mediator of tissue remodeling and fibrosis and its inhibition can reverse the process of fibrosis. Fibrogenesis Tissue Repair 5:S24. [DOI] [PMC free article] [PubMed]
- Lobel M, Bauer S, Meisel C, Eisenreich A, Kudernatsch R, Tank J, Rauch U, Kuhl U, Schultheiss HP, Volk HD, Poller W, Scheibenbogen C. CCN1: a novel inflammation-regulated biphasic immune cell migration modulator. Cell Mol Life Sci. 2012;69:3101–3113. doi: 10.1007/s00018-012-0981-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- London GM, Marchais SJ, Guerin AP, Pannier B. Arterial stiffness: pathophysiology and clinical impact. Clin Exp Hypertens. 2004;26:689–699. doi: 10.1081/ceh-200031982. [DOI] [PubMed] [Google Scholar]
- Longobardo L, Carerj ML, Pizzino G, Bitto A, Piccione MC, Zucco M, Oreto L, Todaro MC, Calabro MP, Squadrito F, Di Bella G, Oreto G, Khandheria BK, Carerj S, Zito C. Impairment of elastic properties of the aorta in bicuspid aortic valve: relationship between biomolecular and aortic strain patterns. Eur Heart J Cardiovasc Imaging. 2018;19:879–887. doi: 10.1093/ehjci/jex224. [DOI] [PubMed] [Google Scholar]
- Lopez B, Gonzalez A, Hermida N, Valencia F, de Teresa E, Diez J. Role of lysyl oxidase in myocardial fibrosis: from basic science to clinical aspects. Am J Physiol Heart Circ Physiol. 2010;299:H1–H9. doi: 10.1152/ajpheart.00335.2010. [DOI] [PubMed] [Google Scholar]
- Lorentzen KA, Chai S, Chen H, Danielsen CC, Simonsen U, Wogensen L. Mechanisms involved in extracellular matrix remodeling and arterial stiffness induced by hyaluronan accumulation. Atherosclerosis. 2016;244:195–203. doi: 10.1016/j.atherosclerosis.2015.11.016. [DOI] [PubMed] [Google Scholar]
- Mariko B, Pezet M, Escoubet B, Bouillot S, Andrieu JP, Starcher B, Quaglino D, Jacob MP, Huber P, Ramirez F, Faury G. Fibrillin-1 genetic deficiency leads to pathological ageing of arteries in mice. J Pathol. 2011;224:33–44. doi: 10.1002/path.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCurdy S, Baicu CF, Heymans S, Bradshaw AD. Cardiac extracellular matrix remodeling: fibrillar collagens and secreted protein acidic and rich in cysteine (SPARC) J Mol Cell Cardiol. 2010;48:544–549. doi: 10.1016/j.yjmcc.2009.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaniel DP, Shaw GA, Elliott JT, Bhadriraju K, Meuse C, Chung KH, Plant AL. The stiffness of collagen fibrils influences vascular smooth muscle cell phenotype. Biophys J. 2007;92:1759–1769. doi: 10.1529/biophysj.106.089003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medley TL, Cole TJ, Gatzka CD, Wang WY, Dart AM, Kingwell BA. Fibrillin-1 genotype is associated with aortic stiffness and disease severity in patients with coronary artery disease. Circulation. 2002;105:810–815. doi: 10.1161/hc0702.104129. [DOI] [PubMed] [Google Scholar]
- Medley TL, Kingwell BA, Gatzka CD, Pillay P, Cole TJ. Matrix metalloproteinase-3 genotype contributes to age-related aortic stiffening through modulation of gene and protein expression. Circ Res. 2003;92:1254–1261. doi: 10.1161/01.RES.0000076891.24317.CA. [DOI] [PubMed] [Google Scholar]
- Miranti CK, Brugge JS. Sensing the environment: a historical perspective on integrin signal transduction. Nat Cell Biol. 2002;4:E83–E90. doi: 10.1038/ncb0402-e83. [DOI] [PubMed] [Google Scholar]
- Mo FE, Lau LF. The matricellular protein CCN1 is essential for cardiac development. Circ Res. 2006;99:961–969. doi: 10.1161/01.RES.0000248426.35019.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukudai Y, Kubota S, Takigawa M. Conserved repressive regulation of connective tissue growth factor/hypertrophic chondrocyte-specific gene 24 (ctgf/hcs24) enabled by different elements and factors among vertebrate species. Biol Chem. 2003;384:1–9. doi: 10.1515/BC.2003.001. [DOI] [PubMed] [Google Scholar]
- Neve A, Cantatore FP, Maruotti N, Corrado A, Ribatti D. Extracellular matrix modulates angiogenesis in physiological and pathological conditions. Biomed Res Int. 2014;2014:756078. doi: 10.1155/2014/756078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh MJ, Zhang C, LeMaster E, Adamos C, Berdyshev E, Bogachkov Y, Kohler EE, Baruah J, Fang Y, Schraufnagel DE, Wary KK, Levitan I. Oxidized LDL signals through rho-GTPase to induce endothelial cell stiffening and promote capillary formation. J Lipid Res. 2016;57:791–808. doi: 10.1194/jlr.M062539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okamoto T, Usuda H, Tanaka T, Wada K, Shimaoka M (2019) The functional implications of endothelial gap junctions and cellular mechanics in vascular angiogenesis. Cancers (Basel) 11 [DOI] [PMC free article] [PubMed]
- Overby DR, Zhou EH, Vargas-Pinto R, Pedrigi RM, Fuchshofer R, Braakman ST, Gupta R, Perkumas KM, Sherwood JM, Vahabikashi A, Dang Q, Kim JH, Ethier CR, Stamer WD, Fredberg JJ, Johnson M. Altered mechanobiology of Schlemm's canal endothelial cells in glaucoma. Proc Natl Acad Sci U S A. 2014;111:13876–13881. doi: 10.1073/pnas.1410602111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palombo C, Kozakova M. Arterial stiffness, atherosclerosis and cardiovascular risk: pathophysiologic mechanisms and emerging clinical indications. Vasc Pharmacol. 2016;77:1–7. doi: 10.1016/j.vph.2015.11.083. [DOI] [PubMed] [Google Scholar]
- Perbal B. NOV (nephroblastoma overexpressed) and the CCN family of genes: structural and functional issues. Mol Pathol. 2001;54:57–79. doi: 10.1136/mp.54.2.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perbal B. CCN proteins: multifunctional signalling regulators. Lancet. 2004;363:62–64. doi: 10.1016/S0140-6736(03)15172-0. [DOI] [PubMed] [Google Scholar]
- Perbal B. The CCN3 protein and cancer. Adv Exp Med Biol. 2006;587:23–40. doi: 10.1007/978-1-4020-5133-3_3. [DOI] [PubMed] [Google Scholar]
- Perbal B, Tweedie S, Bruford E. The official unified nomenclature adopted by the HGNC calls for the use of the acronyms, CCN1-6, and discontinuation in the use of CYR61, CTGF, NOV and WISP 1-3 respectively. J Cell Commun Signal. 2018;12:625–629. doi: 10.1007/s12079-018-0491-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrot A, Schmitt KR, Roth EM, Stiller B, Posch MG, Browne EN, Timmann C, Horstmann RD, Berger F, Ozcelik C. CCN1 mutation is associated with atrial septal defect. Pediatr Cardiol. 2015;36:295–299. doi: 10.1007/s00246-014-1001-8. [DOI] [PubMed] [Google Scholar]
- Qiu H, Zhu Y, Sun Z, Trzeciakowski JP, Gansner M, Depre C, Resuello RR, Natividad FF, Hunter WC, Genin GM, Elson EL, Vatner DE, Meininger GA, Vatner SF. Short communication: vascular smooth muscle cell stiffness as a mechanism for increased aortic stiffness with aging. Circ Res. 2010;107:615–619. doi: 10.1161/CIRCRESAHA.110.221846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Y, Ahn B, Sakurai Y, Hansen CE, Tran R, Mimche PN, Mannino RG, Ciciliano JC, Lamb TJ, Joiner CH, Ofori-Acquah SF, Lam WA. Microvasculature-on-a-chip for the long-term study of endothelial barrier dysfunction and microvascular obstruction in disease. Nat Biomed Eng. 2018;2:453–463. doi: 10.1038/s41551-018-0224-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Recchia AG, Filice E, Pellegrino D, Dobrina A, Cerra MC and Maggiolini M (2009) Endothelin-1 induces connective tissue growth factor expression in cardiomyocytes. J Mol Cell Cardiol. 46:352–359. [DOI] [PubMed]
- Rosenbloom J, Abrams WR, Mecham R. Extracellular matrix 4: the elastic fiber. FASEB J. 1993;7:1208–1218. [PubMed] [Google Scholar]
- Schober JM, Chen N, Grzeszkiewicz TM, Jovanovic I, Emeson EE, Ugarova TP, Ye RD, Lau LF, Lam SC. Identification of integrin alpha(M)beta(2) as an adhesion receptor on peripheral blood monocytes for Cyr61 (CCN1) and connective tissue growth factor (CCN2): immediate-early gene products expressed in atherosclerotic lesions. Blood. 2002;99:4457–4465. doi: 10.1182/blood.v99.12.4457. [DOI] [PubMed] [Google Scholar]
- Shimo T, Nakanishi T, Nishida T, Asano M, Kanyama M, Kuboki T, Tamatani T, Tezuka K, Takemura M, Matsumura T, Takigawa M. Connective tissue growth factor induces the proliferation, migration, and tube formation of vascular endothelial cells in vitro, and angiogenesis in vivo. J Biochem. 1999;126:137–145. doi: 10.1093/oxfordjournals.jbchem.a022414. [DOI] [PubMed] [Google Scholar]
- SM CdSL, Feijen A, Korving J, Korchynskyi O, Larsson J, Karlsson S, Ten DP, Lyons KM, Goldschmeding R, Doevendans P, Mummery CL. Connective tissue growth factor expression and Smad signaling during mouse heart development and myocardial infarction. Dev Dyn. 2004;231:542–550. doi: 10.1002/dvdy.20162. [DOI] [PubMed] [Google Scholar]
- Szabo Z, Magga J, Alakoski T, Ulvila J, Piuhola J, Vainio L, Kivirikko KI, Vuolteenaho O, Ruskoaho H, Lipson KE, Signore P, Kerkela R. Connective tissue growth factor inhibition attenuates left ventricular remodeling and dysfunction in pressure overload-induced heart failure. Hypertension. 2014;63:1235–1240. doi: 10.1161/HYPERTENSIONAHA.114.03279. [DOI] [PubMed] [Google Scholar]
- Thomson J, Singh M, Eckersley A, Cain SA, Sherratt MJ, Baldock C. Fibrillin microfibrils and elastic fibre proteins: functional interactions and extracellular regulation of growth factors. Semin Cell Dev Biol. 2019;89:109–117. doi: 10.1016/j.semcdb.2018.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todorovicc V, Chen CC, Hay N, Lau LF. The matrix protein CCN1 (CYR61) induces apoptosis in fibroblasts. J Cell Biol. 2005;171:559–568. doi: 10.1083/jcb.200504015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong Z, Chen R, Alt DS, Kemper S, Perbal B, Brigstock DR. Susceptibility to liver fibrosis in mice expressing a connective tissue growth factor transgene in hepatocytes. Hepatology. 2009;50:939–947. doi: 10.1002/hep.23102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh CT, Radeff-Huang J, Matteo R, Hsiao A, Subramaniam S, Stupack D, Brown JH. Thrombin receptor and RhoA mediate cell proliferation through integrins and cysteine-rich protein 61. FASEB J. 2008;22:4011–4021. doi: 10.1096/fj.08-113266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wordinger RJ, Clark AF. Lysyl oxidases in the trabecular meshwork. J Glaucoma. 2014;23:S55–S58. doi: 10.1097/IJG.0000000000000127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worthen GS, Schwab B, 3rd, Elson EL, Downey GP. Mechanics of stimulated neutrophils: cell stiffening induces retention in capillaries. Science. 1989;245:183–186. doi: 10.1126/science.2749255. [DOI] [PubMed] [Google Scholar]
- Wuyts FL, Vanhuyse VJ, Langewouters GJ, Decraemer WF, Raman ER, Buyle S. Elastic properties of human aortas in relation to age and atherosclerosis: a structural model. Phys Med Biol. 1995;40:1577–1597. doi: 10.1088/0031-9155/40/10/002. [DOI] [PubMed] [Google Scholar]
- Yan L, Chaqour B. Cysteine-rich protein 61 (CCN1) and connective tissue growth factor (CCN2) at the crosshairs of ocular neovascular and fibrovascular disease therapy. J Cell Commun Signal. 2013;7:253–263. doi: 10.1007/s12079-013-0206-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan L, Lee S, Lazzaro DR, Aranda J, Grant MB, Chaqour B. Single and compound Knock-outs of MicroRNA (miRNA)-155 and its Angiogenic gene target CCN1 in mice Alter vascular and Neovascular growth in the retina via resident microglia. J Biol Chem. 2015;290:23264–23281. doi: 10.1074/jbc.M115.646950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yannoutsos A, Levy BI, Safar ME, Slama G, Blacher J. Pathophysiology of hypertension: interactions between macro and microvascular alterations through endothelial dysfunction. J Hypertens. 2014;32:216–224. doi: 10.1097/HJH.0000000000000021. [DOI] [PubMed] [Google Scholar]
- Yasmin CM, McEniery KM, O'Shaughnessy P, Harnett A, Arshad S, Wallace K, Maki-Petaja B, McDonnell MJ, Ashby J, Brown JRC, Wilkinson IB. Variation in the human matrix metalloproteinase-9 gene is associated with arterial stiffness in healthy individuals. Arterioscler Thromb Vasc Biol. 2006;26:1799–1805. doi: 10.1161/01.ATV.0000227717.46157.32. [DOI] [PubMed] [Google Scholar]
- Yoon PO, Lee MA, Cha H, Jeong MH, Kim J, Jang SP, Choi BY, Jeong D, Yang DK, Hajjar RJ, Park WJ. The opposing effects of CCN2 and CCN5 on the development of cardiac hypertrophy and fibrosis. J Mol Cell Cardiol. 2010;49:294–303. doi: 10.1016/j.yjmcc.2010.04.010. [DOI] [PubMed] [Google Scholar]
- Zanotelli MR, Reinhart-King CA. Mechanical forces in tumor angiogenesis. Adv Exp Med Biol. 2018;1092:91–112. doi: 10.1007/978-3-319-95294-9_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang, X., S.C. Lim, S. Tavintharan, L.Y. Yeoh, C.F. Sum, K. Ang, D. Yeo, S. Low, and N. Kumari. 2019. Association of central arterial stiffness with the presence and severity of diabetic retinopathy in Asians with type 2 diabetes. Diab Vasc Dis Res:1479164119845904 [DOI] [PubMed]