Abstract
Combat-related gunshot and blast injuries of the craniomaxillofacial region present a unique and challenging situation for the maxillofacial and reconstructive surgeon. The devastating cosmetic deformities and severe functional debility ensuing as a result of extensive hard and soft tissue disruption caused by these highly complex injuries, can have disastrous consequences, unless managed in a swift and efficient manner, by a multidisciplinary team approach. Large calvarial defects and deformities are frequent sequelae of these injuries and could result from shattering of the cranial vault by the force of an exploding shell, mine or improvised explosive device, or due to penetration of the skull by the projectile, such as a bullet, flying splinters or shrapnel. It could also result from the decompressive craniectomy carried out in these patients as a neurosurgical procedure to deal with the traumatic brain injury sustained. Management of such injuries is significantly different from that of other craniomaxillofacial injuries, owing to the quantum and severity of hard and soft tissue destruction encountered in the former and also the need to deal with aspects such as splinters from the projectile deeply embedded within vital structures such as the delicate brain tissue and meninges. Further, restoration of the lost structural and functional integrity of the cranial vault using the most suitable cranioplasty material, is imperative to provide protection to the vulnerable and vital cranial contents. Correction of the cranial deformity is also essential from an esthetic and psychosocial standpoint, to restore the morale of the patient. The present study elaborates the immediate/primary management as well as the secondary/definitive management of blast and ballistic head injury patients. Comprehensive treatment and rehabilitation of these patients, including reconstruction of extensive calvarial defects and deformities, resulting either directly or indirectly from combat injuries, have been described in detail. This study also aims to analyze, review and reassess the currently accepted management perspectives and treatment protocols of combat-related cranial injuries and proposes a useful algorithm to best manage them.
Keywords: Gunshot wound (GSW), Improvised explosive device (IED) blast, Craniomaxillofacial (CMF) injury, Traumatic brain injury (TBI), Decompressive craniectomy (DC), Splinter and shrapnel injury, Cranioplasty, Fronto–temporo–parietal craniectomy, Autologous calvarial bone flap, 3-D Dynamic Titanium mesh implant
Introduction
Gunshot and blast injuries of the craniomaxillofacial region present a unique and challenging situation for the maxillofacial and reconstructive surgeon. The essential difference between combat-related craniomaxillofacial injuries (, e.g., gunshot wounds, splinter, shrapnel and blast injuries) and those due to other causes (such as road traffic accidents, falls, sports related injuries, etc.), is the greater extent, severity and complexity of injuries sustained in the former.
Blast and ballistic injuries are usually associated with extensive and composite tissue disruption and loss [1], with discernment and identification of anatomic planes practically impossible within bleeding, macerated and destroyed soft and hard tissues. These injuries can have devastating esthetic and functional consequences and require a carefully phased and staged management protocol, and a multidisciplinary team approach to deal with them adequately [2]. Managing such injuries is vastly different from other maxillofacial injuries, especially while dealing with aspects peculiar to them, such as shattering and disintegration of the cranial vault brought on by the force of the impacting projectiles, issues such as splinters from the projectile deeply embedded within vital structures such as the delicate brain tissue and as also the profound potential for wound contamination and infection.
As compared to routine maxillofacial injuries, the literature on the management of blast and ballistic cranial injuries is relatively scarce. The current principles and pillars in the protocols for management of blast (improvised explosive devices (IEDs), shell and shrapnel) injuries and ballistic (firearm related GSWs) craniomaxillofacial injuries have largely been the result of an exhaustive experimental approach spanning centuries of warfare [3, 4]. While medical professionals strive endlessly to improve and revolutionize healthcare in combat trauma, there have been simultaneous efforts directed toward creation of newer and more destructive arms and ammunition, with current weaponry possessing disastrous biodynamic wounding effects [5]. Consequently, healthcare doctrines have continued to evolve and have undergone significant modifications and transformations keeping pace with an increasing understanding of contemporary ballistics, as well as with the development of newer surgical techniques and equipment [6].
The prime goal in the management of craniofacial gunshot wounds is to rehabilitate the patient to pre-injury function and aesthetics [6]. While there has been a gradual shift from conservative, multistaged, delayed definitive repair, to an early, aggressive, one- or two-stage management approach, the controversies surrounding the timing and extent of intervention have not yet ceased to exist. Such an ongoing debate is largely due to the fact that the majority of treatment outcomes of these types of injuries continue to be unsatisfactory regardless of the treatment methodology adopted [7, 8].
It has been our experience that it is not always easy to get a flawless postoperative outcome with one particular treatment modality or a single surgical technique. Hence, the treatment needs to be timed and tailored depending upon the presentation, type and severity of the injury, amount of composite tissue loss and damage, general health and neurological status of the patient, surgeon’s expertise and availability of resources. All these factors play an important role in determining the final aesthetic and functional outcome. A useful algorithm has been proposed to approach and effectively deal with these injuries.
Head injuries sustained in combat are diverse and varied in type (closed or open), presentation, severity and extent, and hence need to be managed on a case-to-case basis [4]. The goals of surgery are debridement of devitalized tissue, removal of accessible mass lesions, removal of bone and bullet fragments, hemostasis, watertight dural closure and adequate scalp closure [4, 9]. Often, splinters and shrapnel from the projectiles and even fragments of the shattered cranium penetrate the brain substance and need to be dealt with appropriately. There is significant controversy in existing literature as to whether or not to remove deeply embedded splinters and bone fragments within vital structures such as the brain. The cases presented propose the best way to deal with them.
Decompressive craniectomy/craniotomy (DC) is a potentially lifesaving neurosurgical intervention [10], often performed in these patients either to debride disintegrated pieces of the bony calvarium shattered by the projectile, to evacuate intracerebral hemorrhage or hematoma, or to manage intractable intracranial hypertension in patients with traumatic brain injury (TBI) by restoring the barometric pressure, cerebrospinal fluid (CSF) flow and the cerebral blood flow (CBF) [11, 12]. However, numerous complications may arise as a result of the pathophysiological events that accompany removal of a large piece of skull bone [13]. The forces of atmospheric pressure and gravity overwhelm intracranial pressures, leading to the depression of the scalp flap and compression of the intracranial contents with serious derangements in the cerebral hemodynamics like cerebrospinal fluid (CSF) pressure, cerebral blood flow (CBF) and cerebral perfusion (CP) [14, 15]. Subsequently, there exist new cognitive, sensorimotor, neurological and psychological deficits, which constitute a well-known syndrome complex called the motor trephine syndrome (MTS) [16, 17].
Restoration of the calvarial bone defect with either the excised bone flap or an alloplastic implant or autogenous split calvarial bone grafting, essentially not only restores esthetics, but also replaces the lost cranial shield to provide protection to the vulnerable cerebral contents and to reverse the neurological deterioration and sensorimotor deficits resulting from MTS [18, 19]. In this way, cranioplasty not only has a cosmetic and protective role, but also a definite therapeutic and functional role as well, by reversing the neurological deterioration secondary to large cranial defects by [20] restoring the cranial shield or cover, allowing the intracranial contents to expand to their original volume, dimensions and position, and normalizing the cerebral hemodynamics [21–23].
Four cases of combat-related head injuries are presented, in whom emergency craniectomy had been carried out in an immediate post-injury scenario by the neurosurgeon to manage the intracranial injuries. Subsequently, when the patient’s condition stabilized, their cosmetic and functional rehabilitation was planned and executed in a carefully staged and timed manner so as to achieve the best possible outcome.
Case Reports
Case 1
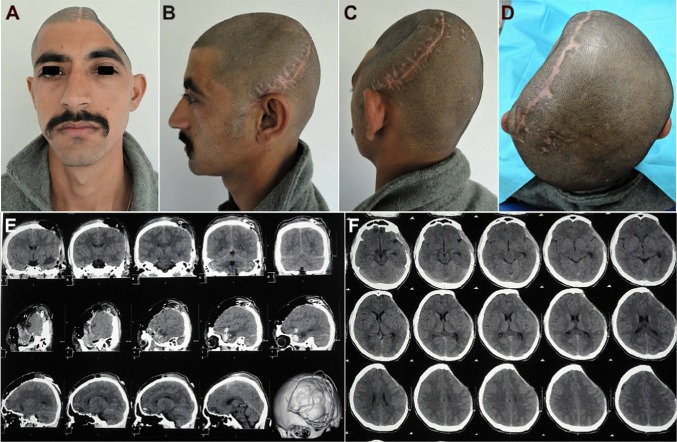
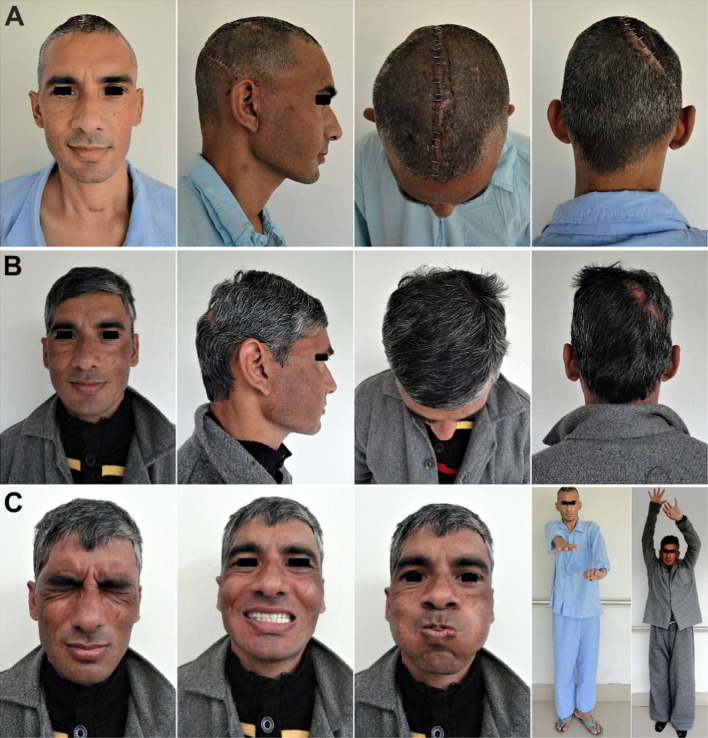
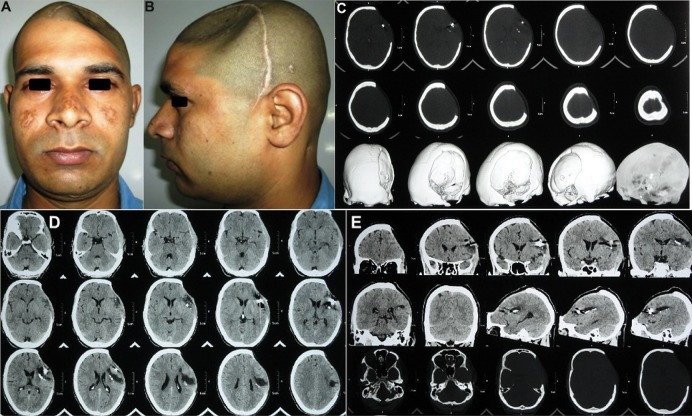
A 21-year-old soldier was referred for cranioplasty repair of a large (18 × 12 cm) left fronto–temporo–parietal (FTP) cranial defect (Fig. 1a–d). History revealed that he had sustained a combat-related blunt head injury from being struck on the head with a large fragment of an exploding mine 3 months ago. The soldier was at a distance from the blast, so the fragment had not penetrated the skull, but the impact sustained was severe enough to fracture the parietal bone on the left and cause an acute subdural hematoma (SDH) of the left frontal region. The neurosurgeon had performed an emergency left fronto–temporo–parietal (FTP) decompressive craniectomy and evacuated the hematoma (Fig. 1e, f). The patient recovered well from the surgery, but 3 months later, he started developing acute depression, anxiety attacks, mood swings, memory loss, headache, dizziness and seizures, all familiar objective and subjective symptoms of the motor trephine syndrome. The patient was referred for an early cranioplasty, to reverse the neurological deterioration and sensorimotor deficits.
Fig. 1.
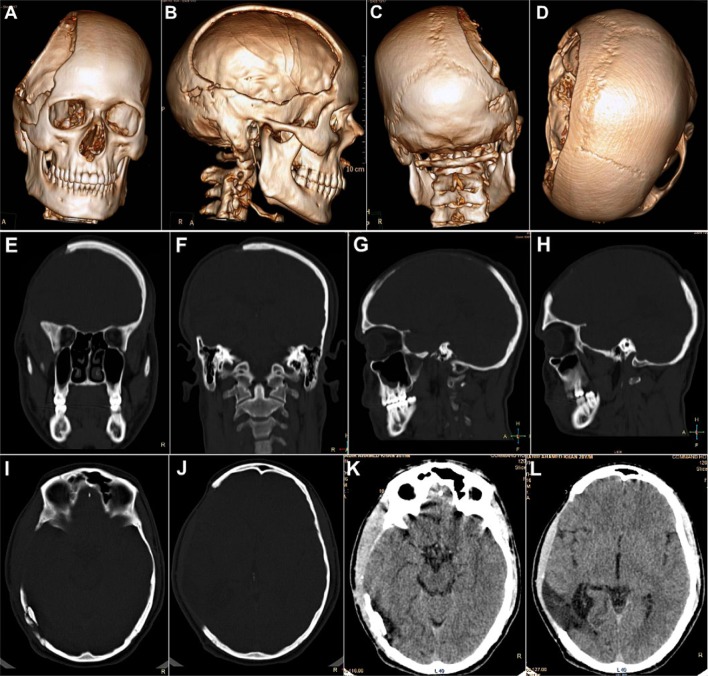
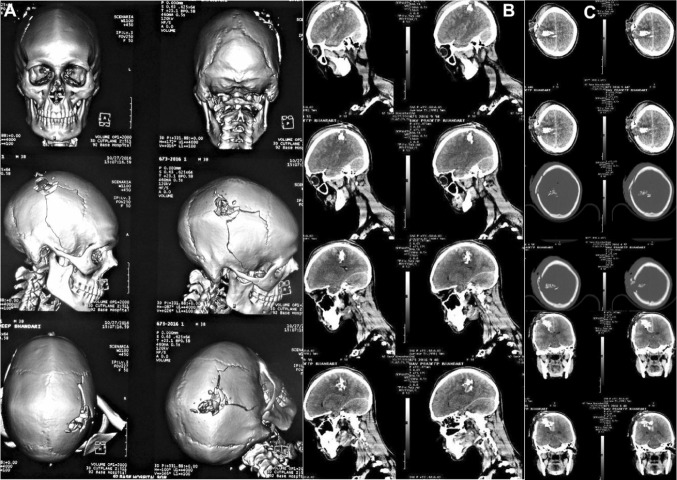
Case No. 1: a–d a 21-year-old soldier, who reported for cranioplasty repair of a large (18 × 12 cm) post-craniectomy calvarial defect. A severe cosmetic deformity caused by the large cranial defect, with evidence of features of the ‘sinking skin flap syndrome’ (SSFS). e, f Non-contrast computed tomography (NCCT) scans carried out following the first surgery, revealed an emergency left fronto–temporo–parietal (FTP) decompressive craniectomy (DC) that had been carried out post-injury, for evacuation of the subdural hematoma in the left frontal region, sustained in the mine blast
Non-contrast computed tomography (NCCT) of the craniomaxillofacial region was carried out (Fig. 2a–l), which gave a clear picture of the morphology and dimensions of the large cranial defect, in addition to corroborating the diagnosis of the motor trephine syndrome (MTS)/sinking skin flap syndrome (SSFS) by the distinctly visualized concavo–convex, ‘kidney bean’ appearance of the cranial contents (Fig. 2e–h).
Fig. 2.
Case No. 1: a–d NCCT 3-D reformatted images of craniomaxillofacial skeleton, revealing the large left FTP post-craniectomy defect. e–h NCCT axial sections revealing the concavo-convex, ‘kidney bean’ shape of the cranial contents, typical of the SSFS secondary to the large calvarial defect, resulting in an absence of the cranial shield or barrier, thus allowing the atmospheric pressure to bear down directly upon the unprotected cranial contents in the region. i–l NCCT coronal and sagittal sections
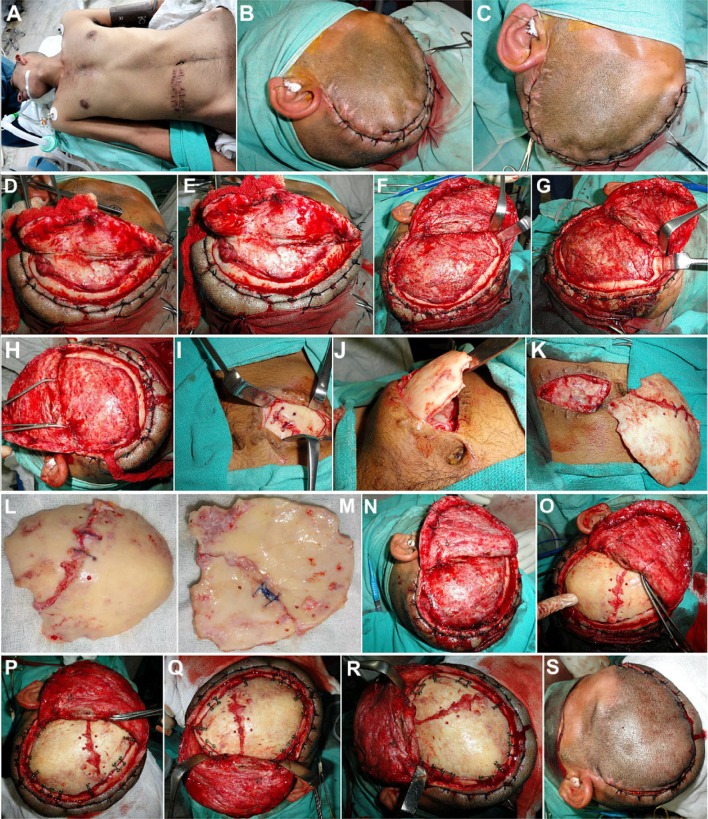
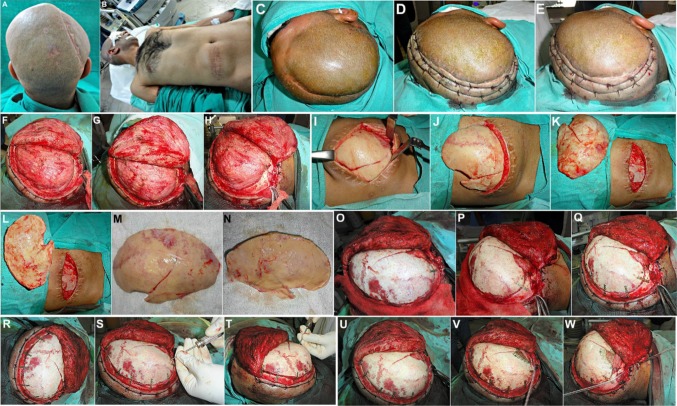
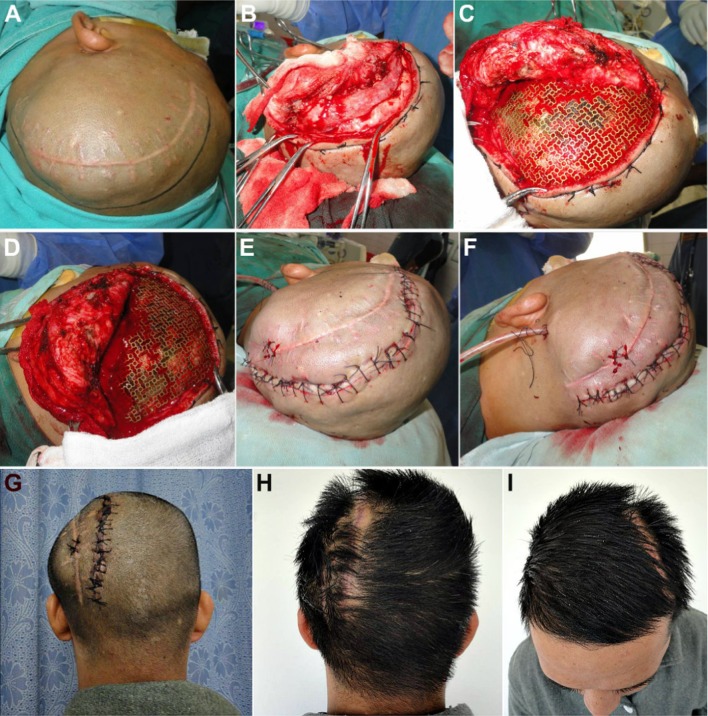
As the excised bone flap had been preserved in his abdominal wall, it was retrieved, the scalp flap overlying the defect was carefully raised, and the bone flap replaced, realigned and fixed in place using titanium microscrews, thus restoring the cranial shield for the vulnerable cerebral contents (Fig. 3a–s).
Fig. 3.
a Case No. 1: Patient taken up for autologous bone flap cranioplasty under general anesthesia. b, c Hemostatic sutures placed on either side of the proposed incision line, in order to reduce bleeding from the scalp flap. d–h Scalp flap raised carefully by dissecting along the subgaleal plane, exposing the entire defect. i–m The excised fractured calvarial bone flap preserved intracorporeally within the subcutaneous fat picket of the patient’s abdominal wall, was carefully retrieved. n, o The bone flap was repositioned over the calvarial defect, and carefully realigned to ensure a snug fit. p–r Fixation of the bone flap to the adjacent cranial bone was carried out using titanium microplates and screws. s Scalp flap was replaced and closed in layers after placement of a vacuum-assisted closed suction drain
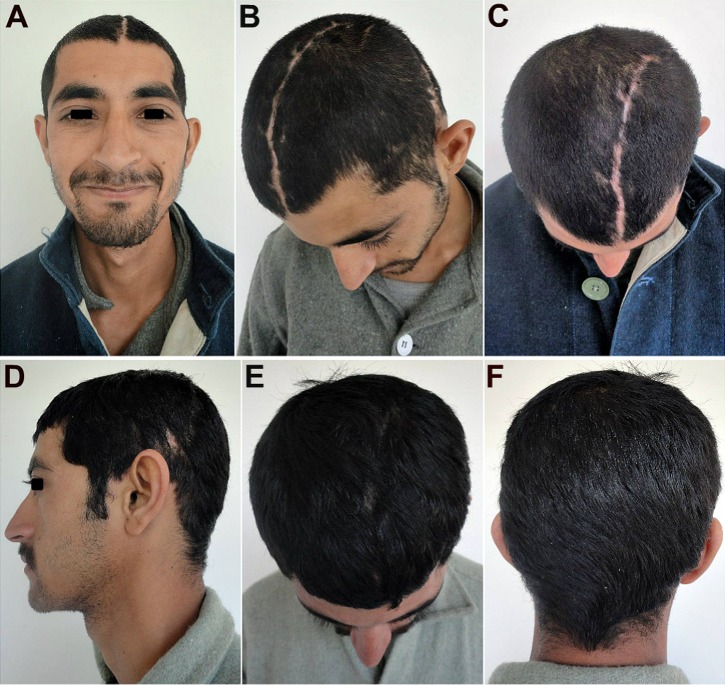
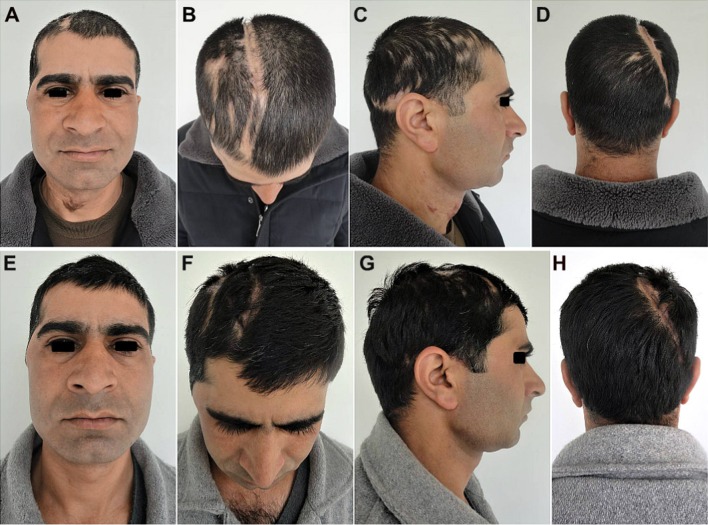
The post-cranioplasty CT scans (Fig. 4) showed the snug fit of the large autologous calvarial bone flap, and a good esthetic as well as functional recovery of the patient with satisfactory restoration of the calvarial contour and continuity (Fig. 5). There was also seen an immediate reversal of his objective and subjective symptoms of MTS following cranioplasty, which thus served not only as a cosmetic and functional reconstructive procedure, but also as a definite therapeutic procedure as well.
Fig. 4.
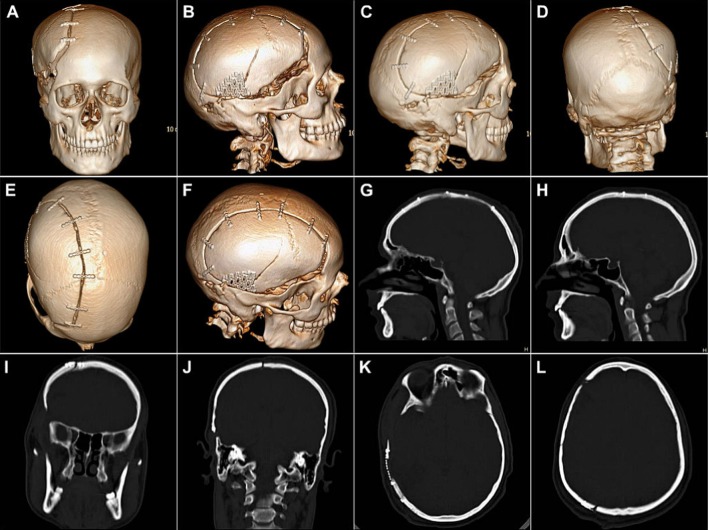
Case No. 1: Postoperative NCCT of the craniofacial region. a Coronal sections, b sagittal sections, c axial sections and d 3-D reformatted images, showing the well-adapted snug fit of the replaced autologous bone flap, fixed to the adjacent cranial bone with the help of titanium microplates and screws. A good restoration of the calvarial contour, continuity and cerebral protection could be achieved by the reconstructed cranial shield
Fig. 5.
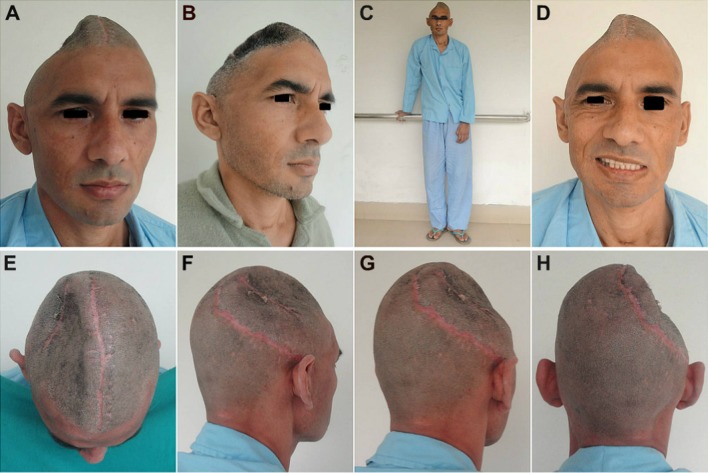
Case No. 1: a–c An excellent esthetic outcome with restoration of calvarial shape, contour and continuity. There was a steady reversal of the subjective and objective symptoms of the MTS and other neurological deficits. d–f A good hair growth 3 months post-cranioplasty, further helped to camouflage the incision line
Case 2
A 37-year-old soldier, an old case of GSW head sustained 3 months ago, was referred for autologous bone flap cranioplasty repair of a large post-craniectomy cranial defect (Fig. 6a, b). The cranial contents appeared to be herniating and bulging out through the large cranial defect (Fig. 6c–g). The autologous calvarial bone flap that had been preserved intracorporeally in the abdominal wall of the patient could be clearly visualized on the radiograph (Fig. 6h).
Fig. 6.
Case No. 2: a, b A 37-year-old soldier, an old case of GSW to the head with a large right FTP post-craniectomy defect referred for autologous bone flap cranioplasty. c–g Cranial contents could be seen to be bulging and herniating out through the large cranial defect. h Excised calvarial bone flap, preserved intracorporeally in the abdominal wall post-craniectomy, could be visualized on the radiograph of the abdomen
History revealed that the projectile had not penetrated the skull but only grazed it, fracturing the right parietal bone and lacerating the scalp. The intracranial hemorrhage and cerebral contusion (Fig. 7a–d) detected on NCCT were managed by the neurosurgeon, who performed an immediate right fronto–temporo–parietal (FTP) craniectomy (Fig. 7e). The large excised calvarial bone flap was preserved in the subcutaneous fat pocket of the patient’s abdominal wall. After adequate time was allowed for the intracranial hematomas and contusions to resolve, the patient was referred for the cranioplasty repair of the large cranial defect 3 months later (Fig. 6).
Fig. 7.
Case No. 2: a, b NCCT showing the immediate post-injury status of the patient with fracture of the right parietal bone caused by the grazing impact of the projectile. c, d Intracranial hemorrhage and cerebral contusions are evident in the parietal lobe on the coronal and axial sections. e Immediate post-craniectomy status showing successful evacuation of the intracranial hematoma. Cerebral contusions are persisting, with pneumocephalus
NCCT just prior to cranioplasty (Fig. 8) showed evidence of the resolving cerebral contusions and the herniating cranial contents bulging outward through the large cranial defect. The extent and severity of the cranial defect and deformity were evident on the 3-D reformatted images of the NCCT scans (Fig. 8a–d) in the coronal (Fig. 8e, f), sagittal (Fig. 8g, h) and axial sections (Fig. 8i–l).
Fig. 8.
Case No. 2: NCCT scans taken 3 months post-craniectomy. a–d 3-D Reformatted images showing the morphology of the large cranial defect. e–h Coronal and sagittal sections showing the extent of defect in the cranial shield increasing vulnerability of the cranial contents to further injury. i–l Cerebral contusions can be observed to be resolving. Cranial contents appear to be herniating and bulging out through the large defect in the calvarial vault
The soldier was taken up for cranioplasty (Fig. 9). The cranial defect was exposed (Fig. 9a–h), by carefully raising a full-thickness scalp flap over it by subgaleal dissection, taking care not to damage the dura stretched over the tense intracranial contents. The intracorporeally preserved calvarial bone flap was carefully retrieved from the abdominal wall (Fig. 9i–n). The fracture line running across it, caused by the impact of the projectile, was evident, and there was also a small 2 × 2.5 cm bone deficiency at the region where the cranial bone had shattered and disintegrated by its impact (Fig. 9l–n).
Fig. 9.
Case No. 2: Intraoperative photographs of the cranioplasty procedure. a–e Intracranial contents appear to be tense and bulging, pushing outward through the large cranial defect. Cutaneous hemostatic sutures placed on either side of the proposed incision line, to reduce intraoperative bleeding from the scalp flap. f–h Full-thickness scalp flap raised from over the defect by careful electrodissection along the subgaleal plane. i–n Intracorporeally preserved calvarial bone flap retrieved from the patient’s abdominal wall. The fracture through the bone flap, caused by the impact from the projectile, is evident. o–t The autologous bone flap was replaced over the cranial defect, properly aligned in place, and fixed with the adjacent cranial bone, using titanium microplates and screws. u–w Area of defect in the bone flap was reconstructed by using a patch of 3-D dynamic titanium mesh implant, cut into the proper size and shape. Vacuum-assisted closed suction drain placed prior to layerwise closure of the scalp flap
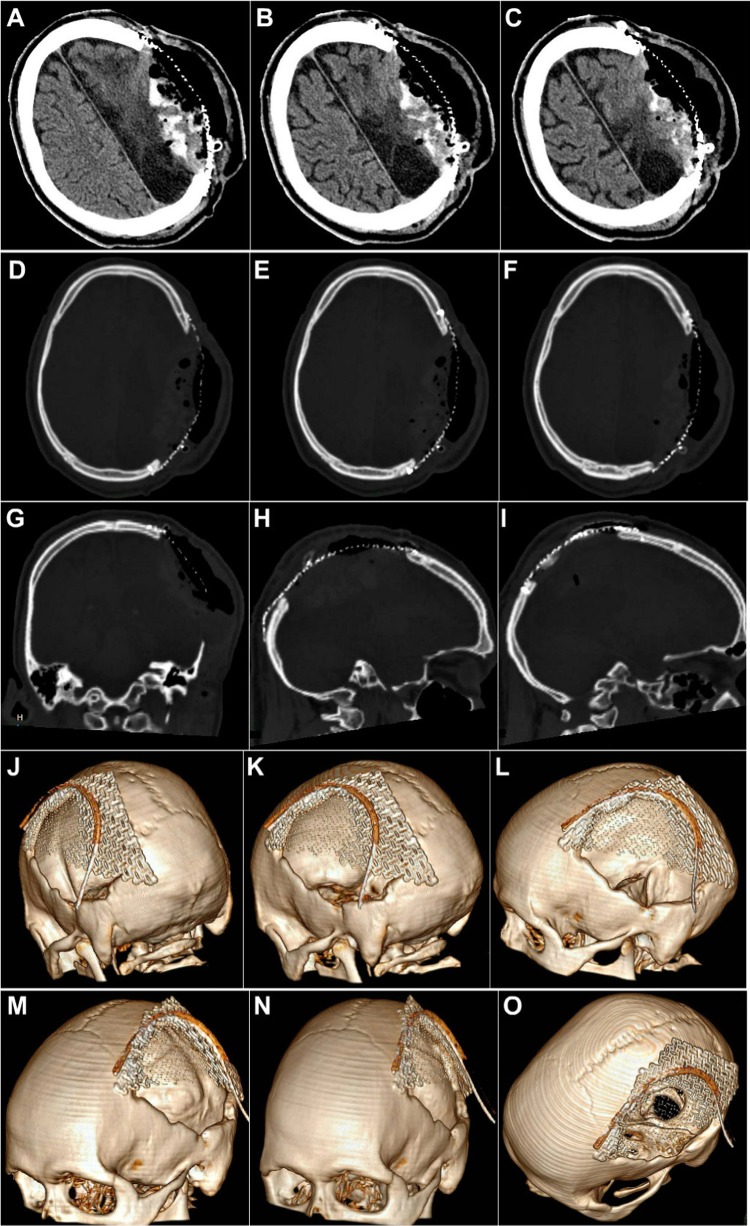
The bone flap was replaced over the cranial defect and fixed with microplates and screws (Fig. 9o–t), after intraoperative administration of mannitol to reduce the intracranial pressure and tenseness of the brain. The area of bone deficiency was reconstructed using a patch of 3-D dynamic (0.7 mm profile) titanium mesh implant (Fig. 9u–w). The postoperative recovery of the patient was smooth with a successful, with restoration of the herniating cranial contents back to their proper dimensions and position and a good esthetic and functional rehabilitation of the patient (Fig. 10). NCCT post-cranioplasty (Fig. 11) showed a snug fit of the replaced autologous calvarial bone flap, along with all the fixation implants in situ, and a successful restoration of the integrity of cranial vault. The titanium mesh implant used to patch the area of bone defect could be clearly visualized on the CT scan.
Fig. 10.
Case No. 2: Postoperative appearance at 02 months following cranioplasty (a–d) and at 04 months post-cranioplasty (e, h), showing an excellent esthetic outcome with successful restoration of form and continuity of the calvarium. Areas of alopecia can be noted where the projectile had torn and lacerated the scalp
Fig. 11.
Case No. 2: NCCT post-cranioplasty, showing a snug fit of the replaced autologous calvarial bone flap, along with all the fixation implants in situ, and a successful restoration of the integrity of cranial vault. The titanium mesh implant used to patch the area of bone defect can be clearly visualized
Case 3
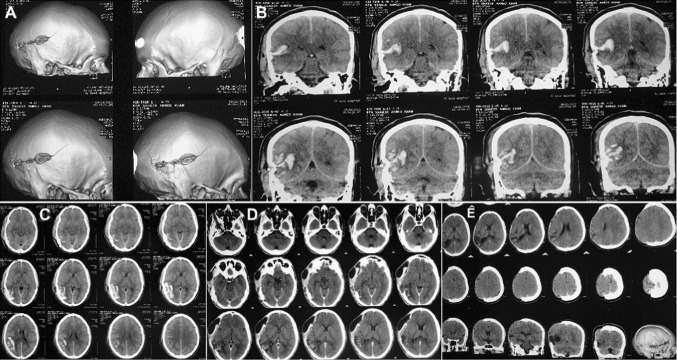
A 37-year-old soldier had sustained a close range GSW to the head. Non-contrast computed tomography (NCCT) scan of the head with 5-mm slice contiguous axial sections from skull base to vertex (Fig. 12), revealed comminuted fracture of the right parietal bone, extending to the temporal bone. Multiple shells as well as bone fragments were seen within the right parietal lobe reaching the midline. There were associated intraparenchymal hemorrhage, cerebral contusion, pneumocephalus and subarachnoid hemorrhage (SAH). There was no significant midline shift.
Fig. 12.
Case No. 3: Coronal, axial and sagittal sections of non-contrast computed tomography scans (NCCT) with 3-D reformatting of a 36-year-old soldier, carried out post-gunshot injury, showing comminuted fracture of the right parieto–temporal bone, with intraparenchymal hemorrhage, cerebral contusion, pneumocephalus and subarachnoid hemorrhage. Multiple bone fragments and metallic splinters from the projectile were seen within the right parietal lobe reaching till the midline
An emergency right fronto–temporo–parietal (FTP) craniotomy, with debridement, lax duraplasty and placement of the shattered pieces of the calvarial bone flap within the subcutaneous pocket of the abdominal wall, was planned by a team comprising of a neurosurgeon and a maxillofacial surgeon.
Intraoperatively, there was a 12 × 6 × 1 cm gap of scalp through which brain matter could be seen to be protruding out. A hemicoronal incision was placed, and a full-thickness right fronto–temporo–parietal (FTP) scalp flap was raised. A central bone defect measuring 3 × 2 × 1 cm was observed through which multiple fragments and shrapnel from the projectile as well as shattered cranial bone fragments had pierced and entered into the brain substance. Pericranium was harvested from the inner aspect of the raised flap for its subsequent use in duraplasty. After elevation of the bone flap, the dura was opened, SDH was evacuated, and the brain was found to be tense. Debridement was carried out, and all accessible fragments of the projectile and shattered bits of bone were meticulously removed. After careful deliberation intraoperatively, it was decided that removal of deeply embedded shrapnel and bone splinters would not offer much clinical benefit and may in fact increase the risk of further damage to vital structures such as the brain and cranial nerves, so they were left in situ. Hemostasis was achieved, lax duraplasty was carried out, and a subgaleal drain was placed before closure of the scalp flap. The shattered pieces of the excised calvarial bone were preserved in the abdominal wall.
Immediate post-craniectomy status revealed diffuse cerebral edema of the cerebral hemispheres with shell and bone fragments embedded in the right parietal region with pockets of air/gas around them (Fig. 13). The sulcal spaces were effaced and the basal cisterns were open. Extra axial pockets of air were seen in the right temporal and frontal regions, with drainage tubes in situ. No significant intracranial collection or midline shift was seen.
Fig. 13.
Case No. 3: NCCT head of the immediate post-craniectomy status showing resolution of the midline shift and of the intracranial hemorrhage and hematoma. No significant intracranial collections were evident, although the cerebral hemispheres showed diffuse cerebral edema with metallic and bone fragments deeply embedded in the right parietal region with pockets of air/gas along with them
Such cases of GSWs to the head are usually fatal, but thanks to the speedy evacuation of the soldier from the site of the skirmish to the nearest hospital, and an immediate intervention by the team of surgeons, his life could be saved.
Tracheostomy which had been carried out intraoperatively was maintained for prolonged ventilatory support. An intercostal chest tube and drain were placed for right-sided pneumothorax. The postoperative recovery of the patient was slow but steady. He was hemodynamically stable and his GCS returned to normal by the second postoperative day. However, he had severe motor deficits which included a left side hemiplegia and an upper motor neuron-type paresis of the facial nerve on the left.
Three months later, once the cerebral edema had subsided, an early cranioplasty was planned, to reconstruct the large residual calvarial defect so as to reverse the sensorimotor deficits and to prevent further possible neurological deterioration secondary to the motor trephine syndrome effect (Fig. 14). The patient demonstrated various neurological deficits, such as partial hemiparesis of the left side with inability to walk or stand without support and drooping and weakness of the left shoulder with inability to move the arm or hand on that side. He also had an upper motor neuron (UMN)-type facial paresis of the right facial nerve, with weakness of facial muscles on the left. An indrawn, tense, sunken-in appearance of the scalp overlying the cranial defect was further evident of the ‘sinking skin flap syndrome (SSFS) (Fig. 14).
Fig. 14.
Case No. 3: a, b 37-year-old soldier with head injury resulting from a GSW, who reported with a large post-craniectomy fronto–temporo–parietal (FTP) calvarial defect, which had resulted in a severe craniofacial deformity. The absence of the cranial shield on the right affected the calvarial shape, contour and continuity, resulting in not only a gross esthetic abnormality, but also severe functional debility by leaving the intracranial contents like the brain and meninges vulnerable to further injury. c Neurological deficits developing secondary to the large cranial defect. Partial hemiparesis of the left side with inability to walk or stand without support and drooping and weakness of the left shoulder with inability to move the arm or hand on that side. d Upper motor neuron (UMN)-type facial paresis of the right facial nerve, with weakness of facial muscles on the left. e–h Indrawn, tense, sunken-in appearance of the scalp overlying the cranial defect, evident of the ‘sinking skin flap syndrome (SSFS).’ Hemicoronal incision scar from the previous craniectomy procedure and a second large scar resulting from the penetrating projectile, evident across the scalp over the center of the defect
NCCT of the present status (Fig. 15) revealed the concavo–convex, ‘kidney bean’ appearance of the intracranial contents typical of the ‘sinking skin flap syndrome,’ secondary to the large cranial defect on the right side. Also visible were the deeply embedded metallic splinters and fragments of bone within the right parietal lobe. NCCT of the abdomen (Fig. 16) revealed images of the excised, shattered calvarial bone flap which had been intracorporeally preserved within the abdominal wall of the patient.
Fig. 15.
Case No. 3: a, b NCCT axial sections, revealing the concavo-convex, ‘kidney bean’ appearance of the intracranial contents typical of the ‘sinking skin flap syndrome,’ secondary to the large cranial defect n the right side. Also visible are the deeply embedded metallic splinters and fragments of bone within the right parietal lobe. c Reformatted CT images revealing the indrawn, sunken-in appearance of the surface and the large dimensions of the fronto–temporo–parietal calvarial defect. d 3-Dimensional reformatted CT scan images showing the extent of the defect and revealing the radiopaque shrapnel and bone fragments embedded within the brain
Fig. 16.
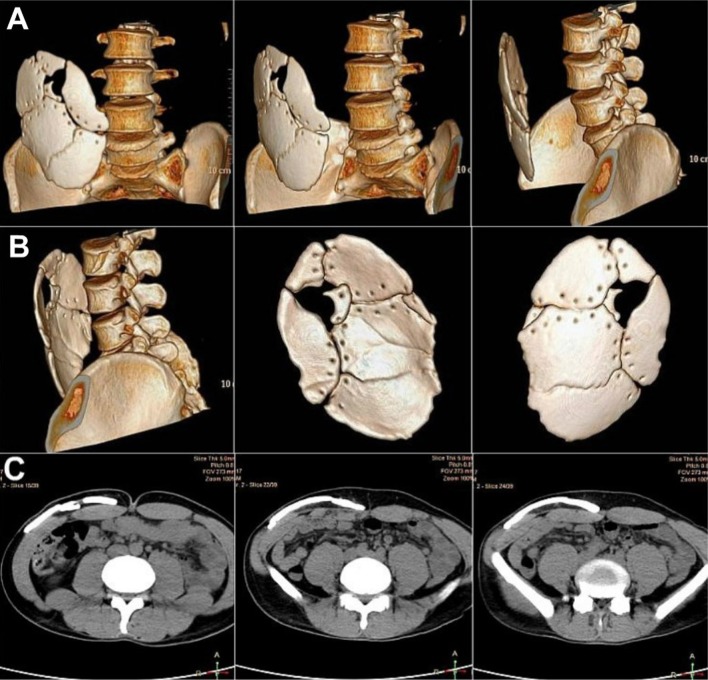
Case No. 3: a, b 3-D Reformatted NCCT images of the excised, shattered calvarial bone flap preserved within the abdominal wall of the patient. c Transverse sections of NCCT abdomen showing the fragmented bone flap stores intracorporeally within the subcutaneous fat pocket of the abdominal wall on the right side
The patient was taken up for cranioplasty (Fig. 17a). The hemicoronal incision was placed along the existing scar of the incision line of the previous surgery (Fig. 17b–e). The scalp flap was raised by careful electrodissection between the undersurface of the scalp flap and the reconstructed dura overlying the meninges and brain (Fig. 17f–h). The shattered pieces of the previously excised calvarial bone flap were retrieved from the abdominal wall (Fig. 17i–m), very carefully pieced together, repositioned over the cranial defect (Fig. 17n, o), aligned with the surrounding bone and fixed in place using microplates and screws (Fig. 17p–s).
Fig. 17.
Case No. 3: a The patient was scrubbed, draped and prepared for cranioplasty. b, c The old scar of the entry site of the bullet could be visualized where it had torn through scalp, penetrating the skull. d, e Hemostatic sutures were placed on either side of the existing scar of the previous hemicoronal incision. f–h Incision was placed along the same line and scalp flap raised in a subgaleal plane, exposing the entire cranial defect. i–m Preserved bone flap was retrieved from the abdominal wall of the patient. It was found to be shattered and in several pieces, with multiple areas of missing bone. n, o The fragments of the bone flap were carefully positioned over the cranial defect. p–s Fixation with the adjacent cranial bone borders was carried out using titanium microplates and screws. t–z Multiple areas of missing bone were reconstructed using patches of 0.7-mm profile 3-D dynamic titanium mesh implants. aa, ab Closure of the scalp flap
There were multiple areas of missing bone (Fig. 17r, s). The central bone defect which was probably the site of entry of the shrapnel from the projectile, and lateral defects, where the bone had shattered and disintegrated from the force of the impact, were reconstructed using patches of 0.7-mm-thick three-dimensional dynamic titanium mesh implants (Fig. 17t–z). These implants were cut to the desired size and shape, molded to the desired contour used to bridge and repair the central large and the linear lateral cranial defects.
The postoperative recovery of the patient was smooth and uneventful. There was an excellent esthetic outcome with restoration of the pre-injury contour and shape of the head (Fig. 18a), which was further enhanced by his thick hair growth (Fig. 18b, c). In addition, there was a very good neurological and functional recovery as well, with reversal of the left-sided hemiparesis and facial nerve paresis, that the patient had prior to cranioplasty (Fig. 18c). He regained strength in the left limbs and by the second week post-cranioplasty, he regained full use of his left arm and hand and was even able to walk using a crutch, and the cranioplasty was a success from both an esthetic and functional point of view. There was an excellent restoration of calvarial form, shape, contour and continuity with a reconstitution of the cranial shield affording protection to the vital and vulnerable intracranial contents. There was reversal of the sensorimotor deficits as well as a steady reversal of the neurological deterioration of the patient who gradually regained strength in his left limbs and was soon able to walk using a crutch.
Fig. 18.
Case No. 3: a 10 days post-cranioplasty, showing a good restoration of the calvarial contour and shape and a healthy operated site, with skin staples in situ. b 25 days post-cranioplasty showing good hair growth at the operated site, which helped to hide the scars of injury as well as surgery, aiding in an excellent esthetic and cosmetic outcome. c A rapid neurological improvement with reversal of the sensorimotor deficits that had existed before the calvarial repair
On the postoperative CT scans, there was seen a good restoration of the cranial contour and continuity and we can also appreciate some of the deeply embedded shrapnel within the brain substance (Fig. 19).
Fig. 19.
Case No. 3: NCCT Post-cranioplasty. a–c Coronal and axial sections showing a good restoration of the calvarial contour. Radio-dense splinters and bone fragments seen embedded in the cerebrum at the site of the gunshot wound. Hypodensity was seen in the right fronto–temporo–parietal region involving the gray and white matter. d The large 18 × 13 cm cranial defect successfully reconstructed using the fragmented pieces of calvarial bone that had been preserved in the abdominal wall of the patient. The areas of missing bone were successfully restored by 3-D dynamic titanium mesh implants, thus bridging the entire calvarial defect
Case 4
A previously operated 34-year-old soldier who had sustained GSW to the head 18 months ago, was referred for cranioplasty of a left-sided fronto–temporo–parietal (FTP) post-craniectomy defect measuring 15 × 9 cm (Fig. 20a, b). The excised bone flap had not been preserved as it had been shattered into multiple small pieces. The patient had weakness in his right limbs, with difficulty in walking and inability to hold a pen and write. He also suffered from seizures, anxiety attacks, mood swings and episodes of acute depression, features suggestive of the motor trephine syndrome (MTS) secondary to the long-standing large cranial defect.
Fig. 20.
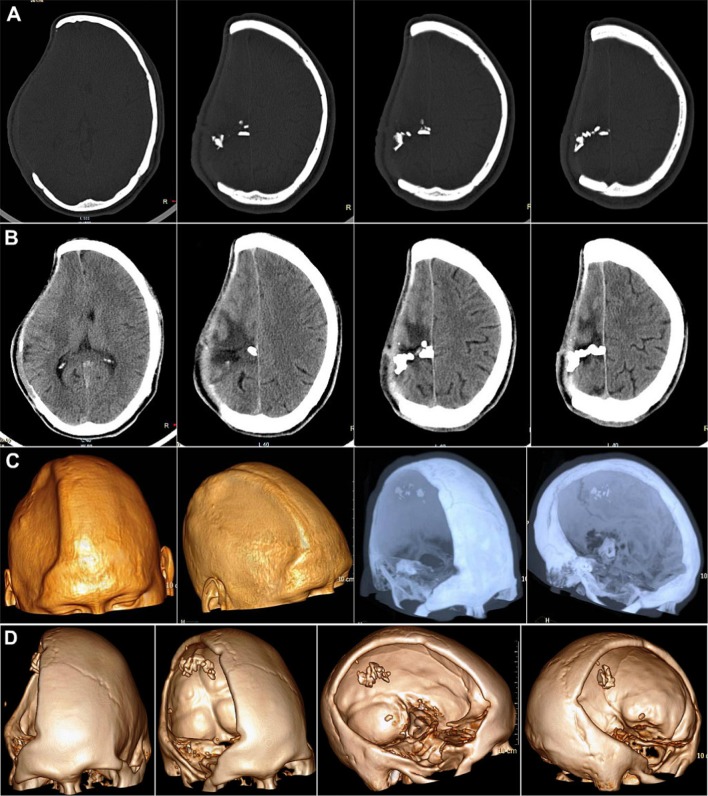
Case No. 4: a, b A 34-year-old patient operated 18 months ago for GSW to the head, referred presently for cranioplasty of the residual cranial defect. c–e NCCT Head showed the post-craniectomy status with encephalomalactic changes in the left frontal lobe
NCCT head (Fig. 20c–e) revealed the post-craniectomy status with encephalomalactic changes on the left frontal lobe. There was no evidence of subdural (SDH) or extradural hematoma (EDH), normal morphology and density of the cerebral hemispheres, basal ganglia, internal capsules and thalami. The ventricular system, posterior fossa structures, cisterns and sulcal spaces appeared normal. Multiple metallic density splinters were seen in the left frontal region. An area of encephalomalacia was seen on the left frontal lobe measuring 27 × 58 × 16 mm (TR × AP × CC) (transverse × anteroposterior × cephalo-caudal).
Due to severe damage to the dura by the penetrating splinters from the projectile, synthetic dural substitute had been used by the neurosurgeon for duraplasty at the time of the initial craniectomy. However, when the patient was taken up for cranioplasty, and the scalp flap was raised, there was seen extensive dural graft loss with exposure of brain tissue and meninges intraoperatively, with considerable hemorrhage (Fig. 21a, b). Also, the brain tissue and meninges appeared to be closely adherent to the undersurface of the scalp flap, making dissection and exposure of the cranial defect extremely difficult. A team approach, with neurosurgical intervention to stop the intraoperative intracranial bleed, followed immediately by reconstruction of the defect by the maxillofacial surgeon using a 3-D (1.4 mm profile) dynamic titanium mesh implant was effective in attaining an ideal outcome (Fig. 21c–f).
Fig. 21.
Case No. 4: Intraoperative photographs of the titanium mesh cranioplasty procedure. a, b Upon raising the scalp flap, there was noted dural graft loss with exposure of brain tissue and meninges. As the brain tissue and meninges were closely adherent to the undersurface of the scalp flap, there was considerable intraoperative hemorrhage. c–f A team approach, with neurosurgical intervention to stop the intracranial bleed, and reconstruction of the defect by the maxillofacial surgeon using a 3-D titanium mesh implant was effective in attaining an ideal outcome. g–i Successful reconstruction of the cranial defect with restoration of calvarial contour and continuity
In such cases where the situation is already compromised, and there is no bone available for reconstruction, titanium mesh cranioplasty is the safest, quickest and most effective treatment option. The patient recovered uneventfully with nil neurological deficits or complications with successful restoration of the cranial vault integrity (Fig. 21g–i).
Further, its mesh structure also permits aspiration of subdural or epidural fluid collections and hematomas if they develop in the immediate postoperative period without the need to have to reopen the operated site and remove the entire graft or implant. Post-cranioplasty, the intracranial clots took some time to resolve (Fig. 22a–c); however, there was no adverse outcome, and an excellent esthetic as well as functional outcome was achieved with restoration of the calvarial integrity achieved using the 3-D dynamic titanium mesh implant (Fig. 22d–o).
Fig. 22.
Case No. 4: NCCT taken immediately after cranioplasty. a–c The intracranial clots are evident, which took some time to resolve postoperatively. d–o A successful reconstruction of the large calvarial defect by the 3-D dynamic titanium mesh implant
Discussion
Cranioplasty is the surgical repair, reconstruction and replacement of a missing part of the cranial vault, thus restoring calvarial shape and continuity and bringing about its morphological and functional rehabilitation [24].
A wide array of cranioplasty materials have been used over the years, ranging from alloplastic implants to autogenous flaps and grafts.
The simplest way to perform a mechanically sound cranioplasty is to replace the same bone flap which was excised at the time of craniectomy as it has the most perfect fit [25]. The flap is usually stored in the subcutaneous fat pocket of the patient’s abdominal wall, to preserve its viability (intracorporeal preservation) [26]. At the time of cranioplasty, it is retrieved from there and replaced at the site of the cranial defect and fixed in place, and quite undoubtedly has the most perfect fit. This was the technique and protocol employed in the first two patients described, with large post-craniectomy calvarial defects.
Alternatively, apart from the re-implantation of the excised calvarial bone flap, there are a few other options for successful reconstruction of large cranial defects. Reconstruction using autogenous split thickness cortico-cancellous calvarial bone grafts, although a physiologically ideal and inexpensive option, has been contraindicated in patients with severe head injuries, owing to the risk of further jeopardizing the brain that is already traumatized [25]. Alloplastic three-dimensional titanium mesh implants, as was used in the fourth case, is a safe efficient and reliable cranioplasty material, when there is no autologous bone flap available. The mesh can be easily cut and shaped intraoperatively to the desired size and contour and can be effectively employed to bridge even large cranial defects [27]. It is versatile in its use and can even be effectively used to supplement and bridge defects in the excised autologous bone flap shattered by the projectiles, as was done in the second and third cases. Its mesh structure permits easy aspiration of subdural or epidural collections if they develop in the postoperative period without having to remove the implant altogether [28]. Titanium mesh is CT and MRI safe and has excellent biocompatibility with no cases of implant rejection or infection reported in any of our cases till date.
Although custom-made polymethyl methacrylate implants (PMMA) are also a viable and inexpensive option for restoring cranial defects, it has been our experience that leaching of monomer in the postoperative period often contributes to delayed resolution of neurological deficits. Also incidence of implant rejection is quite high, which is a risk better not taken with the already compromised and complicated cases of blast and ballistic head injuries as these patients are particularly prone to wound contamination and infection.
Till date, there has been no unanimous opinion as to the ideal timing for cranioplasty [26]. Earlier studies have advocated a delay of at least 6 months to a year, for the brain to recover from the initial traumatic episode and for adequate healing of the dura and revascularization of the overlying scalp flap to take place, prior to cranioplasty [25, 28]. However, recent publications claim that shortening the time between craniectomy and cranioplasty to less than 3 months [27], improves the overall functional and neurological outcome and reduces complications [29], such as bone flap resorption and infection, and that 3 months is the maximum allowable time after DC, 6 weeks being ideal time for cranioplasty [25, 30]. In our cases too, the best esthetic and functional outcome was achieved in those cases in whom early cranioplasty was performed.
Another dilemma frequently faced is how best to deal with the deeply embedded fragments from the projectile [31]. One school of thought proposes that bullets that enter the body are almost sterile due to heat generated by discharge/explosion of the propellant and friction between the bullet and the rifle barrel [32]. Another school of thought considers the heat so generated insufficient to sterilize the bullet [33]. Also, the bullet gathers dust and numerous microflora as it traverses the air, in addition to carrying skin flora into the wound. Hence, as far as possible, they must be retrieved from the wound, no matter how deeply embedded they are, to prevent possible wound infection [34]. Also, broad spectrum antibiotics must be started within the first 3 to 6 h, to provide cover against Staphylococci, Clostridium perfringens and Acinetobacter [35].
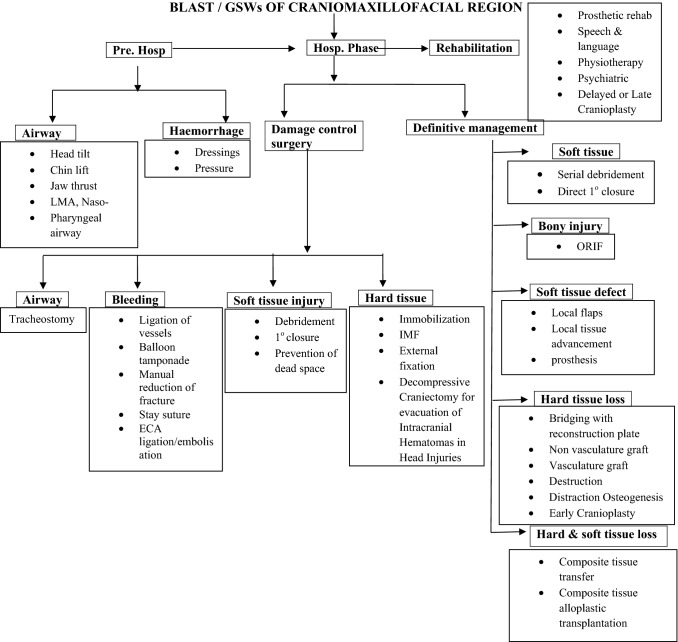
As far as ballistic head injuries are concerned, it is now accepted that removal of deeply embedded shrapnel or splinters may not offer much clinical benefit and may in fact increase the risk of damage to vital structures such as the brain and cranial nerves and hence, they are best left in situ [36]. In the third case presented, the deeply embedded fragments were left in situ and there was no adverse outcome noted due to this, in the follow-up period. The decision, however, needs to be judiciously made on an individual case-to-case basis carefully taking into consideration all the above factors. All possible care must be taken to avoid the risk of wound contamination and infection, while at the same time to also avoid jeopardizing the vital structures. A useful algorithm is proposed for the management of Combat-related Blast and Gunshot Wounds of the Craniomaxillofacial region (Fig. 23).
Fig. 23.
Suggested protocol for management of combat-related blast and gunshot wounds of the craniomaxillofacial region
Conclusion
Management of head injuries and calvarial defects resulting from blasts and GSWs sustained in combat and conflict scenarios, can be extremely challenging to the surgical team. A multidisciplinary team approach involving concerted and specialized efforts of the maxillofacial surgeon, neurosurgeon, trauma surgeon, intensivist and plastic and reconstructive surgeon together in a carefully and systematically staged and phased manner, meticulously dealing with the various aspects and facets of the injuries sustained, ultimately helps in bringing about the most ideal aesthetic as well as functional repair and rehabilitation of the patient.
In combat victims with large post-craniectomy calvarial defects, both timing of the cranioplasty and the choice of the ideal cranioplasty material to be used need to be judiciously decided on an individual case-to-case basis. Autogenous grafts such as the excised bone flap (preserved intracorporeally) are the more physiologic and less expensive option. Titanium mesh cranioplasty is the safer, more reliable and often preferred option, particularly when the defects are large and when there is no autologous excised bone flap available for repair and reconstruction. Combination cranioplasties, using 3-D dynamic titanium mesh implants to supplement defects in the autologous bone flap, are an effective means to reconstruct the badly shattered cranial vault.
Keeping in mind the patient’s health and neurological status, early cranioplasty, carried out within 1–3 months of craniectomy, offers the best esthetic as well as functional and neurological outcome, as the cases presented would elucidate.
Compliance with Ethical Standards
Conflict of interest
The author of this article has not received any research grant, remuneration, or speaker honorarium from any company or committee whatsoever and neither owns any stock in any company. The author declares that she does not have any conflict of interest.
Human and Animals Rights
All procedures performed on the patients (human participants) involved were in accordance with the ethical standards of the institution and/or national research committee, as well as with the 1964 Helsinki Declaration and its later amendments and comparable ethical standards.
Ethical Approval
This article does not contain any new studies with human participants or animals performed by the author.
Informed Consent
Informed consent was obtained from all the individual participants in this study.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Dolin J, Scalea T, Mannor L, et al. The management of gunshot wounds to the face. J Trauma. 1992;33:508–514. doi: 10.1097/00005373-199210000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Cole RD, Browne JD, Phipps CD. Gunshot wounds to the mandible and midface: evaluation, treatment, and avoidance of complications. Otolaryngol Head Neck Surg. 1994;111:739–745. doi: 10.1177/019459989411100607. [DOI] [PubMed] [Google Scholar]
- 3.Powers DB, Delo RI. Characteristics of ballistic and blast injuries. Atlas Oral Maxillofac Surg Clin N Am. 2013;21(21):15–24. doi: 10.1016/j.cxom.2012.12.001. [DOI] [PubMed] [Google Scholar]
- 4.Motamedi MH. Primary management of maxillofacial hard and soft tissue gunshot and shrapnel injuries. J Oral Maxillofac Surg. 2003;61:1390–1398. doi: 10.1016/j.joms.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 5.Kaufman Y, Cole P, Hollier LH. Facial gunshot wounds: trends in management. Craniomaxillofac Trauma Reconstr. 2009;2:85–90. doi: 10.1055/s-0029-1202595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vayvada H, Menderes A. Management of close range, high energy shotgun and rifle wounds to the face. J Craniofac Surg. 2005;16:794–804. doi: 10.1097/01.scs.0000180014.06352.65. [DOI] [PubMed] [Google Scholar]
- 7.Anmole O, Osunde O, Akhiwu B. A 14-year review of Craniomaxillofacial Gunshot wounds in a resource-limited setting. Craniomaxillofac Trauma Reconstr. 2017;10:130–137. doi: 10.1055/s-0037-1601341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Breeze J, Bryant D. Current concepts in the epidemiology and management of battlefield head, face and neck trauma. J R Army Med Corps. 2009;155:274–278. doi: 10.1136/jramc-155-04-07. [DOI] [PubMed] [Google Scholar]
- 9.Grahm TW, Williams FC, Jr, Harrington T, et al. Civilian gunshot wounds to the head: a prospective study. Neurosurgery. 1990;27:696–700. doi: 10.1227/00006123-199011000-00005. [DOI] [PubMed] [Google Scholar]
- 10.Huang AP, Tu YK, Tsai YH, Chen YS, Hong WC, Yang CC, Kuo LT, Su IC, Huang SH, Huang SJ. Decompressive craniectomy as the primary surgical intervention for hemorrhagic contusion. J Neurotrauma. 2008;25:1347–1354. doi: 10.1089/neu.2008.0625. [DOI] [PubMed] [Google Scholar]
- 11.Cooper DJ, Rosenfeld JV, Murray L, Arabi YM, Davies AR, D’Urso P, et al. Decompressive craniectomy in diffuse traumatic brain injury. N Engl J Med. 2011;364:1493–1502. doi: 10.1056/NEJMoa1102077. [DOI] [PubMed] [Google Scholar]
- 12.Jiang JY, Xu W, Li WP, Xu WH, Zhang J, Bao YH, et al. Efficacy of standard trauma craniectomy for refractory intracranial hypertension with severe traumatic brain injury: a multicenter, prospective, randomized controlled study. J Neurotrauma. 2005;22:623–628. doi: 10.1089/neu.2005.22.623. [DOI] [PubMed] [Google Scholar]
- 13.Chang V, Hartzfeld P, Langlois M, Mahmood A, Seyfried D. Outcomes of cranial repair after craniectomy. J Neurosurg. 2010;112:1120–1124. doi: 10.3171/2009.6.JNS09133. [DOI] [PubMed] [Google Scholar]
- 14.Dujovny M, Agner C, Aviles A. Syndrome of the trephined: theory and facts. Crit Rev Neurosurg. 1999;9:271–278. doi: 10.1007/s003290050143. [DOI] [PubMed] [Google Scholar]
- 15.Joseph V, Reilly P. Syndrome of the trephined. J Neurosurg. 2009;111:650–652. doi: 10.3171/2009.3.JNS0984. [DOI] [PubMed] [Google Scholar]
- 16.Albanese J, Leone M, Alliez JR, Kaya JM, Antonini F, Alliez B, et al. Decompressive craniectomy for severe traumatic brain injury: evaluation of the effects at one year. Crit Care Med. 2003;31:2535–2538. doi: 10.1097/01.CCM.0000089927.67396.F3. [DOI] [PubMed] [Google Scholar]
- 17.Honeybul S. Neurological susceptibility to a skull defect. Surg Neurol Int. 2014;4:83–84. doi: 10.4103/2152-7806.133886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kuo JR, Wang CC, Chio C, Cheng TJ. Neurological improvement after cranioplasty: analysis by transcranial Doppler ultrasonography. J Clin Neurosci. 2004;11:486–489. doi: 10.1016/j.jocn.2003.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Honeybul S, Janzen C, Kruger K, Ho KM. The impact of cranioplasty on neurological function. Br J Neurosurg. 2013;27:636–641. doi: 10.3109/02688697.2013.817532. [DOI] [PubMed] [Google Scholar]
- 20.Jeyaraj P. Importance of early cranioplasty in reversing the “Syndrome of the trephine/motor trephine syndrome/sinking skin flap syndrome”. J Maxillofac Oral Surg. 2015;14:666–673. doi: 10.1007/s12663-014-0673-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Isago T, Nozaki M, Kikuchi Y, Honda T, Nakazawa H. Sinking skin flap syndrome: a case of improved cerebral blood flow after cranioplasty. Ann Plast Surg. 2004;53:288–292. doi: 10.1097/01.sap.0000106433.89983.72. [DOI] [PubMed] [Google Scholar]
- 22.Segal DH, Oppenheim JS, Murovic JA. Neurological recovery after cranioplasty. Neurosurgery. 1994;34:729–731. doi: 10.1227/00006123-199404000-00024. [DOI] [PubMed] [Google Scholar]
- 23.Winkler PA, Stummer W, Linke R, Krishnan KG, Tatsch K. Influence of cranioplasty on postural blood flow regulation, cerebro-vascular reserve capacity, and cerebral glucose metabolism. J Neurosurg. 2000;93:53–61. doi: 10.3171/jns.2000.93.1.0053. [DOI] [PubMed] [Google Scholar]
- 24.Kwon SM, Cheong JH, Kim JM, Kim CH. Reperfusion injury after autologous cranioplasty in a patient with sinking skin flap syndrome. J Korean Neurosurg Soc. 2012;51:117–119. doi: 10.3340/jkns.2012.51.2.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuss P, Vatter H, Marquardt G, Imohl L, Ulrich CT, Seifert V, et al. Cranioplasty after Decompressive craniectomy: the effect of timing on postoperative complications. J Neurotrauma. 2012;29:1090–1095. doi: 10.1089/neu.2011.2176. [DOI] [PubMed] [Google Scholar]
- 26.Beauchamp KM, Kashuk J, Moore EE, Bolles G, Rabb C, Seinfeld J, et al. Cranioplasty after postinjury Decompressive craniectomy: is timing of the essence? J Trauma. 2010;69:270–274. doi: 10.1097/TA.0b013e3181e491c2. [DOI] [PubMed] [Google Scholar]
- 27.Chun HJ, Yi HJ. Efficacy and safety of early cranioplasty, at least within 1 month. J Craniofac Surg. 2011;22:203–207. doi: 10.1097/SCS.0b013e3181f753bd. [DOI] [PubMed] [Google Scholar]
- 28.Archavlis E, Carvi Y, Nievas M. The impact of timing of cranioplasty in patients with large cranial defects after decompressive hemicraniectomy. Acta Neurochir (Wien) 2012;154:1055–1062. doi: 10.1007/s00701-012-1333-1. [DOI] [PubMed] [Google Scholar]
- 29.Chibbaro S, Di Rocco F, Mirone G, Fricia M, Makiese O, Di Emidio P, et al. Decompressive craniectomy and early cranioplasty for the management of severe head injury: a prospective multicenter study on 147 patients. World Neurosurg. 2011;75:558–562. doi: 10.1016/j.wneu.2010.10.020. [DOI] [PubMed] [Google Scholar]
- 30.Thavarajah D, De Lacy P, Hussien A, Sugar A. The minimum time for cranioplasty insertion from craniectomy is six months to reduce risk of infection: a case series of 82 patients. Br J Neurosurg. 2012;26:78–80. doi: 10.3109/02688697.2011.603850. [DOI] [PubMed] [Google Scholar]
- 31.Kummoona R, Muna AM. Evaluation of immediate phase of managements of missile injuries affecting maxillofacial region in Iraq. J Craniofacial Surg. 2006;17:217–223. doi: 10.1097/00001665-200603000-00003. [DOI] [PubMed] [Google Scholar]
- 32.Thoresby FP, Darlow HM. The mechanisms of primary infection of bullet wounds. Br J Surg. 1967;54:359–361. doi: 10.1002/bjs.1800540509. [DOI] [PubMed] [Google Scholar]
- 33.Kummoona R. Posttraumatic missile injuries of the orofacial region. J Craniofac Surg. 2008;19:300–305. doi: 10.1097/SCS.0b013e3181577b97. [DOI] [PubMed] [Google Scholar]
- 34.Clasper JC, Hill PF, Watkins PE. Contamination of ballistic fractures: an in vitro model. Injury. 2002;33:157–160. doi: 10.1016/S0020-1383(01)00136-X. [DOI] [PubMed] [Google Scholar]
- 35.Yetiser S, Kahramanyol M. High-velocity gunshot wounds to the head and neck: a review of wound ballistics. Mil Med. 1998;163:346–351. doi: 10.1093/milmed/163.5.346. [DOI] [PubMed] [Google Scholar]
- 36.Stuehmer C, Blums KS. Influence of different types of guns, projectiles and propellants on patterns of injury to the viscerocranium. J Oral Maxillofac Surg. 2009;67:775–781. doi: 10.1016/j.joms.2008.08.036. [DOI] [PubMed] [Google Scholar]