Abstract
Chronic myeloid leukemia (CML) is a malignant blood disease with a particular chromosomal aberration that is known as a common form of leukemia. The chromene family exhibits strong anti-cancer effects. Therefore, the effects of six members of the dihydropyrano [2,3-g] chromene family on cell toxicity and apoptosis induction in K562 cancer cells were investigated and compared with those of normal peripheral blood mononuclear cells (PBMCs). The K562 cells were cultured in the presence of the aforementioned chromene derivatives at concentrations of 40 to 200 μM for 24 to 72 h. The effects of these compounds on the growth and viability of the K562 cell line and PBMCs were studied through MTT assay. Furthermore, apoptosis induction was investigated using flow cytometry. Real-time PCR was used for relative quantification of BCL2, Bax, TP53 and BCR- ABL genes after 48 h of exposing K562 cells and PBMCs to 4-Clpgc. Based on the results, these chromene derivatives inhibited the growth of K562 cells. According to the obtained data, 4-Clpgc was the strongest compound with IC50 values of 102 ± 1.6 μM and 143 ± 9.41 μM in K562 cells and PBMCs, while pgc was the weakest one with IC50 levels of 278 ± 2.7 μM and 366 ± 47 μM in K562 cells and PBMCs (after 72 h), respectively. The results demonstrated that the apoptotic cell percentage in the control group increased from 6.09% to 84.10% and 17.2% to 20.06% in K562 cells and PBMCs after 48 h of treatment, respectively. Moreover, 4-Clpgc treatment increased the expression of Bax and TP53 genes by 42.74 and 35.88 folds in K562 cells and 9.60 and 7.75 folds in PBMCs, respectively. On the other hand, the expression of BCL2 was reduced by 1.47 and 1.38 folds in K562 cells and PBMCs, respectively. These compounds were associated with less toxic effects on normal cells, compared to the cancer cells. In conclusion, these derivatives can be considered as appropriate candidates for leukemia treatment.
Keywords: Chronic myeloid leukemia; Dihydropyrano [2,3-g] chromene; Apoptosis; BCL2; Bax; BCR-ABL
Introdution
Chronic myeloid leukemia (CML) is a type of leukemia caused by excessive proliferation of myocyte cells in bone marrow (Calabretta and Perrotti 2004). The discovery of Philadelphia chromosome (Ph) in 1960, as the first chromosomal abnormality related to certain types of leukemia, has been a successful achievement in the biology of cancer (Deininger et al. 2000).
Studying Philadelphia chromosome is important in the diagnosis and treatment of this leukemia from both quantitative and qualitative perspectives. Genetic methods allow a primary diagnosis of this disease (Ye et al. 2014). Chronic myeloid leukemia is one of the malignant diseases that affect bone marrow cells. It is characterized by an increase in the size of spleen and also the number of granulocytes (especially neutrophils) and white blood cells in the blood cell count. The disease is characterized by a dislocation between components at the end of the long arm of chromosomes 9 and 22, which causes development of the Philadelphia chromosome (Cortes et al. 2012). K562 established from the pleural effusion of a 53 year-old Caucasian female with chronic myelogenous leukemia in terminal blast crises and it is one of the cell lines that have been used to investigate the advanced phase of CML. In recent years, along with the global population growth, cancer has been one of the most common causes of mortality and morbidity in the world (Sun et al. 2008).
Maintenance of tissue homeostasis depends on not only cell proliferation, but also the rate of cell death. Apoptosis is a programmed process for cell death that occurs in multicellular organisms (Taylor et al. 2008). Some biochemical events cause alterations in cell characteristics (morphology) and death. These changes include membrane blebbing, DNA fragmentation, chromatin condensation, nuclear fragmentation, cell shrinkage and apoptotic body formation (Kerr et al. 1994). Since apoptosis cannot stop once it has begun, it is a highly regulated process. It can be initiated through intrinsic or extrinsic pathways. In both mechanisms, cell death is induced by activating caspases, which are proteases or enzymes in charge of protein degradation (Domingos and Steller 2007). Several genes are involved in the apoptosis process (such as TP53, BCL2 and Bim). BCL2 gene that is located on the 18q12chromosome, is responsible for controlling the caspase enzymes, thereby playing a pivotal role in the release of cytochrome C form mitochondria. This process eventually results in the conversion of caspase 9 to caspase 3 and cell death (Elmore 2007). BCL2 is effective in both induction and prevention of apoptosis. In the case of mutation, it has a role in apoptosis prevention.
BCL2 protein, as a member of the BCL2 family, binds to the outer surface of mitochondria or sometimes endoplasmic reticulum and separates pro-apoptotic members such as Bax from mitochondria, preventing apoptotic cell death. Increased levels of BCL2 expression in cancer cells prevent apoptosis and lead to tumor progression (JC 1998). In contrast, the elevated expression of Bax induces cell death and eliminates tumor cells. Reduced expression of Bax and increased expression levels of BCL2 have been observed in many drug-resistant tumor cells. In addition, the lack of tumor suppressive genes is influential on the development of cancerous cells. The TP53 gene, which is located on the 17p13chromosome, is responsible for controlling cell division under normal circumstances. However, it can activate various genes in apoptotic pathways such as apaf1, puma, and Bax. In case TP53 undergoes mutation, all genes under TP53 control will encounter some disorders which can result in the loss of molecular cell control and consequently, these cells may turn into cancerous cells (Lebedeva et al. 2003).
Until now, various compounds have been used to cure CML. However, none of such studies have been promising, due to the drug resistance of cancer cells. Accordingly, development of new methods to facilitate preparation of pharmaceutical compounds is a pivot point in research activities in the field of biological and pharmaceutical chemistry (Mohammadi Ziarani et al. 2011). Utilization of multi-component one-pot reactions is one of the methods used for preparation of organic compounds, especially those used as the basis of many anti-cancer, anti-tumor, and anti-microbial drugs (Thomas and Zachariah 2013). Pyrans and their derivatives are a major family of oxygen heterocyclic molecules that have attracted a great deal of scientific interest, due to their biological, medicinal and reactive properties. Pyran rings have applicable interfaces, because of their activeness. These compounds exhibit medicinal properties such as anti-coagulant, anti-cancer, anti-allergy and anti-biotic characteristics (Vosooghi et al. 2010). In recent years, the cytotoxic effects of Tetrahydro-pyranochromene and Dihydro-pyranopyran derivatives have been observed in various cancer cell types (Emmadi et al. 2012). Depending on the cell type, the effects of these heterocyclic compounds may be different (Heidary Alizadeh et al. 2010). Moreover, the presence of the 4-aryl-4H-chromene family as a potent inducer of apoptosis has been reported in the T47D (breast carcinoma) and Jurkat (T cell leukemia) cell lines (Kemnitzer et al. 2008). Recently, the anti-cancer effects of [2,3-c] derivatives of benzopyran have been investigated on the K562 (CML) cell line as a laboratory model for myeloid leukemia. According to the results, these derivatives decreased the viability of K562 cells using a specific concentration and treatment duration (Mo et al. 2004). The proposed mechanism of action for this set of compounds involves binding to the binding site of Colchicine-tubulin, which leads to transformation in the structures of α and β tubulin dimers. As a result, the formation of microtubule from tubulin is prevented, leading to cell death and apoptosis (Gourdeau et al. 2004). Despite the drug resistance of cancer cells, the chromene family members have exhibited strong anti-cancer properties. Therefore in this study, the effects of some derivatives of the dihydropyrano [2,3-g] chromene family on cellular toxicity and apoptosis induction in K562 cancer cells, in comparison with normal peripheral blood mononuclear cells (PBMCs) have been investigated.
Materials and methods
Chemicals
Cell culture medium (RPMI 1640), fetal bovine serum (FBS), Phosphate-buffered saline (PBS) and penicillin–streptomycin were all purchased from Gibco BRL (Life Technologies, Paisley, Scotland). K562 cell line (code: C122) and Hela cell line (code: C115) were both provided by the National Cell Bank of Iran (NCBI, Iran). 2,7-Diamino-4,9-bis(4-chlorophenyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (4-Clpgc), 2,7-Diamino-4,9-bis(4-nitrophenyl)-5,10-dioxo-4,9-dihydropyrano[2, 3-g]chromene-3,8-dicarbonitrile (4-No2pgc), 2,7-Diamino-4,9-bis(4-bromophenyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (4-Brpgc), 2,7-Diamino-4,9-di-ortho-tolyl-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (3-Mepgc), 2,7-Diamino-4,9-di(para-tolyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (4-Mepgc), 2,7-Diamino-4,9-diphenyl-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (pgc) were synthesized in Organic Chemistry Laboratory, Faculty of Chemistry, Kashan University (Iran). Cell culture plates were obtained from SPL (Korea). Propodeum iodide (PI), acridine orange (AO), 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO), ficoll as well as Ranse and Annexing-V FITC Apoptosis kits were purchased from Sigma (Germany). The required antibodies against BCL2, Bax, TP53 and BCR- ABL were purchased from Alexis Biochemical (USA). QuantiTect Reverse Transcription kit was obtained from Qigong (USA), SYBR Green Master Mix was purchased from Applied Biosystems (USA) and RNeasy kit was provided by Gene All (Korea).
Cell culture
The K562 and Hela cell lines were cultured in RPMI-1640 medium containing penicillin (100 μg/ml), streptomycin (100 μg/ml) and 10% FBS, followed by incubation at 37 °C and 5% CO2 with 95% humidity. The culture medium was changed every 2 days.
General procedure for the synthesis of dihydropyrano[2,3-g]chromenes
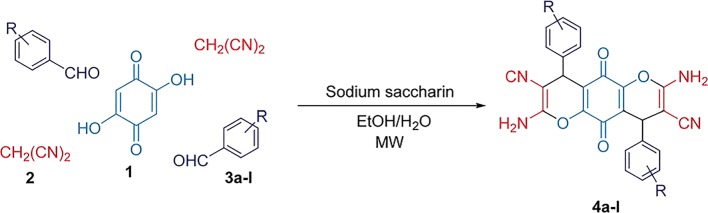
A mixture of arylaldehyde (2.0 mmol), malononitrile (0.13 g, 2.0 mmol), 2,5-dihydroxy-1,4-benzoquinone.
(0.14 g, 1.0 mmol), H2O/EtOH (1:1, 5 mL) and a catalytic amount of sodium saccharin (10 mol%) was irradiated in a microwave oven (100 W) at 30 °C for appropriate times. After completion of the reaction (monitored by TLC), the precipitated product was separated from the reaction mixture by simple filtration and then washed with EtOH to afford the products (Fig. 1) (Moradi and Sadegh 2017).
Fig. 1.
Synthesis of dihydropyrano[2,3-g]chromene derivatives catalyzed by sodium saccharin under microwave irradiation [22]
Spectral and analytical data
2,7-Diamino-4,9-bis(4-chlorophenyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile(4-Clpgc)
Brown powder (C26H14N4O4Cl2); FTIR (KBr): vmax 3317 (NH2), 3173 (=C–H aromatic), 2197 (CN), 1591 (C=C aromatic (cm−1. 1H NMR (400 MHz, DMSO-d6): δ 4.45 (s, 2H, 2CH), 7.29–7.32 (m, 12H, H–Ar, 2NH2) ppm. Anal. Calcd for C26H14N4O4Cl2: C 60.36, H 2.73, N 10.83%. Found: C 60.28, H 2.75, N 10.81%.
2,7-Diamino-4,9-bis(4-bromophenyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile(4-Brpgc)
Brown powder (C26H14N4O4Br2); FTIR (KBr): νmax 3321 (NH2), 3179 (=C–H aromatic), 2199 (CN), 1589 (C=C aromatic(cm−1. 1H NMR (400 MHz, DMSO-d6): δ4.45 (s, 2H, 2CH), 7.22–7.51 (m, 12H, H–Ar, 2NH2) ppm. Anal. Calcd for: C26H14N4O4Br2: C 51.51, H 2.33, N 9.24%. Found, C 51.60, H 2.36, N 9.26%.
2,7-Diamino-4,9-bis(4-nitrophenyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile(4-No2pgc)
Brown powder (C26H14N6O8); FTIR (KBr): νmax 3345 (NH2), 3180 (=C–H aromatic), 2196 (CN), 1592(C=C aromatic) cm−1. 1H NMR (400 MHz, DMSO-d6): δ 4.7(s, 2H, 2CH), 7.39–8.18 (m, 12H, H–Ar, 2NH2) ppm. Anal. Calcd for C26H14N6O8: C 58.00, H 2.62, N 15.61%. Found: C 58.10, H 2.61, N 15.65%.
2,7-Diamino-4,9-di-ortho-tolyl-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile(3-Mepgc)
Brown powder (C28H20N4O4); FTIR (KBr): νmax 3182 (NH2), 3053 (=C–H aromatic), 2201 (CN), 1596 (C=C aromatic(cm−1. 1H NMR (400 MHz, DMSO-d6): δ 2.48(s, 6H, 2CH3), 5.37 (s, 2H, 2CH), 6.95–7.30 (m, 12H,H–Ar, 2NH2) ppm. Anal. Calcd for C28H20N4O4: C 70.58,H 4.23, N 11.76%. Found: C 70.61, H 4.22, N 11.75%.
2,7-Diamino-4,9-di(Para-tolyl)-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (4-Mepgc)
Brown powder (C28H20N4O4); FTIR (KBr): νmax 3436(NH2), 2922 (=C–H aromatic), 2198 (CN), 1584 (C=C aromatic) cm−1. 1H NMR (400 MHz, DMSO-d6): δ 2.25(s, 6H, 2CH3), 4.45 (s, 2H, 2CH), 6.94–7.86 (m, 12H,H–Ar, 2NH2) ppm. MS m/z (%): 476 (M+), 388 (2), 313(48), 265 (11), 299 (8), 168 (21), 140 (42), 115 (34), 104(72), 91 (40), 69 (80), 42 (100). Anal. Calcd for C28H20N4O4: C 70.58, H 4.20, N 11.76%. Found: C 70.9170.70, H 4.10, N 11.91%.
2,7-Diamino-4,9-diphenyl-5,10-dioxo-4,9-dihydropyrano[2,3-g]chromene-3,8-dicarbonitrile (pgc)
Brownpowder (C26H16N4O6); FTIR (KBr): νmax 3296 (NH2),3179 (=C–H aromatic), 2202 (CN), 1595 (C=C aromatic) cm−1. 1H NMR (400 MHz, DMSO-d6): δ 4.46 (s, 2H,2CH), 7.24–7.40 (m, 12H, H–Ar, 2NH2) ppm. Anal. Calcdfor C26H16N4O6: C 69.64, H 3.60, N 12.49%. Found: C69.61, H 3.62, N 12.46% (Moradi and Sadegh 2017).
Isolation of PBMCs
PBMCs were isolated from a healthy 28-year old female donor, according to the instructions of the ethics committee of Pasteur Institute of Iran. Written informed consent for the study and analysis of biological samples was obtained from the subject under study. To isolate PBMCs, blood samples were diluted with an equal volume of PBS, carefully layered over a ficoll medium in a falcon tube and continuously centrifuged at 400–500 g for 30–40 min. Four layers were formed, each containing different cell types. The uppermost layer contained plasma, which was removed via pipetting. The second layer (which was white and cloudy) included PBMCs. These cells were gently removed using a Pasteur pipette and added to warm medium or PBS to wash off any remaining platelets. The pelleted cells were then counted and the viability percentage was estimated using trypan blue staining. Cells could be immediately used or frozen for long-term storage.
MTT assay
In order to examine cell proliferation and viability, we performed MTT test. It is a colorimetric assay based on transformation of the yellow MTT tetrazolium salt into purplish formazan crystals due to the metabolic activity of living cells by NADH and NADPH pyridine nucleotide cofactors and mitochondrial dehydrogenases. Thereafter, formazan crystals are dissolved in DMSO and the absorption of the colored solution is quantitatively inspected using an ELISA reader device. In order to perform this experiment, 15 × 103 cells were seeded on each well of 96-well plates and after 24 h, exposed to various concentrations (40, 80, 120, 160 and 200 μM) of dihydropyrano [2,3-g] chromene solution in DMSO, for 24, 48 and 72 h. Thereafter, 10 μL of MTT (5 mg/mL in PBS) was added to each well. After 4 h, 100 μL of DMSO was used to dissolve the produced formazan crystals. At last, the light absorption of each well was evaluated using an ELISA reader (Awareness technology Inc., USA) at 570 nm (Mosmann 1983).
PI staining
From a morphological point of view, necrosis and apoptosis are known as two pathways of cell death. Due to the permeability of a necrotic cell membrane, the PI dye can attach to the nucleic acid of the nucleus and its aggregation in the nucleus will be visible in the form of red spheres with diameters smaller than that of a cell (being equal to the nucleus diameter).
On the other hand, since the membranes of alive and apoptotic cells cannot be stained by PI, these cells are not detected by a fluorescent light. Therefore, after 48 h of exposure to 4-Clpgc, K562 cells in both positive (using H2O2) and negative (not treated) control groups were harvested and stained with PI and AO, for subsequent analyses using light and fluorescent microscopies.
Cell morphology examination
K562 cells were incubated with 118 μM of 4-Clpgc for 24, 48 and 72 h. After treatment, cells were collected to observe the morphological changes of apoptosis cells by light microscope (Zeiss, Germany).
Flow cytometry assessment of apoptosis in K562 cells
The annexing V/PI double staining was performed to distinguish cell death cases caused by apoptosis and necrosis. To confirm the outcomes of MTT analysis, apoptotic cells were studied using the annexing V/PI double staining method through analyzing phosphatidylserine on the outer surface of apoptotic cell membranes. The formation of phosphatidylserine (PS) residues (which are mostly hidden in the plasma membrane) on the cell surface is an initial event in apoptosis and can be used to detect and measure apoptosis.
In the apoptosis process, PS is transferred from the cytoplasmic face of the plasma membrane to the cell surface. Since annexing V exhibits a strong Ca2+ affinity to PS, it can be used as a tool to detect apoptosis.
The K562 cells (5 × 105 cells/well) were seeded on 24-well culture plates and incubated for 24 h. Thereafter, they were treated with different concentrations of the investigated 4-Clpgc and also, apoptosis (dexamethasone) and necrosis (H2O2) controlling agents. The treated and untreated K562 cells were harvested after 24 and 48 h and washed twice with PBS. The cells were then centrifuged, re-suspended in 500 μL of 1X binding buffer and added with 5 μL of annexing V-FITC and 5 μL of PI. After incubation at room temperature for 15 min in the dark, these cells were analyzed using the flow cytometry technique (Moosavi et al. 2007).
Real-time PCR
Real-time polymerase chain reaction (real-time PCR) was used for relative quantification of BCL2, Bax, TP53 and BCR- ABL genes after 48 h of exposing K562 cells to 4-Clpgc. Total RNA was extracted using RNeasy kit according to the supplier’s instructions. Furthermore, QuantiTect Reverse Transcription kit was used for cDNA synthesis from 1 mg of total RNA. Real-time PCR analysis was carried out using the primer sequences shown in Table 1 and also SYRB Green Master Mix. Quantification of gene expression was accomplished using comparative Ct method in which for each sample, Ct values of each target gene were normalized to those of GAPDH, which was used as the reference gene. Each output of the real-time PCR was reported as a fold increase with respect to the control group (Schmittgen and Livak 2008).
Table 1.
Primers used in RT-PCR Table 1. Primers used in RT-PCR
| Gene | Primer sequence |
|---|---|
| BCL2 | F: 5’-ACA GGA GCT ATA CTC CAG GAC A-3′ |
| R: 5′-GAT CAT ACC CGT CAT GGG GAT A-3′ | |
| Bax | F: 5’-CCC GAG AGG TCT TTT TCC GAG −3′ |
| R: 5’-CCA GCC CAT GAT GGT TCT GAT −3′ | |
| TP53 | F: 5′-GAT GCG GAG AAT CTT TGG AAC A-3′ |
| R: 5’-ACT TGT CGC TCT TGA AGC TAC-3′ | |
| BCR- ABL | F: 5’-ACA TCA CGC CAG TCA ACA GT-3′ |
| R: 5’-TCG GAG GAG ACG TAG AGC TT-3′ | |
| GAPDH | F: 5’-CAG AAC ATC ATC CTG CCT CT-3′ |
| R: 5’-GCT TGA CAA AGT GGT CGT TGA G-3′ |
Experimental groups
This study was performed using 5 concentrations (40, 80, 120, 160 and 200 μM) of dihydropyrano [2,3-g] chromene and 3 time intervals of 24, 48 and 72 h for two cell types (K562 cells and PBMCs) with two control groups of apoptotic (using dexamethasone) and necrotic) using H2O2) cells.
Statistical analysis
The results of MTT test for the effect of each drug on K562 cells and PBMCs at different time points were analyzed in SPSS 16.0 software, using One-Way ANOVA and the post-hoc Tukey test. In addition, due to the interference of the effects of time and concentration factors on MTT results, the Two-Way ANOVA test along with the follow-up Tukey test and also the calculation of the partial parameter 2η were used to determine the effects of time and concentration and the combination of these two factors. Also IC50 results for both cell types were compared at certain time points using Student’s t test. In all statistical analyses, P-values less than 0.05 were considered as statistically significant levels.
Results
Viability of K562 cells, Hela cells and PBMCs
Based on the results, all studied compounds reduced the growth and viability of K562 cells. Cells were treated with different concentrations of the compounds for 24, 48 and 72 h. As indicated in Tables 2, 4-Clpgc was more toxic than other compounds. Hence, in the rest of experiments, we mainly focused on this substance. Exposure of K562 cells, PBMCs and Hela cells to 118, 197 and 205 μM concentrations of 4-Clpgc, respectively, for 48 h decreased the cell viability level by approximately 50%, in comparison with the control group (Fig. 2).
Table 2.
IC50 values of investigated compounds after 48 h. The IC50 value of 4-Clpgc for K562 cells was 118 ± 8.35 μM, for PBMCs was 197 ± 12.75 μM and for Hela cells was 205.9346 ± 3.9 μM it was more toxic in comparison with other compounds
Fig. 2.
Effects of 4-Clpgc on cell viability of (A) K562 cells (B) PBMC and (C) Hela cells, subjected to indicated concentrations of 4-Clpgc for 24, 48 and 72 h (****: P < 0.0001, ***: P < 0.001, **: P < 0.01)
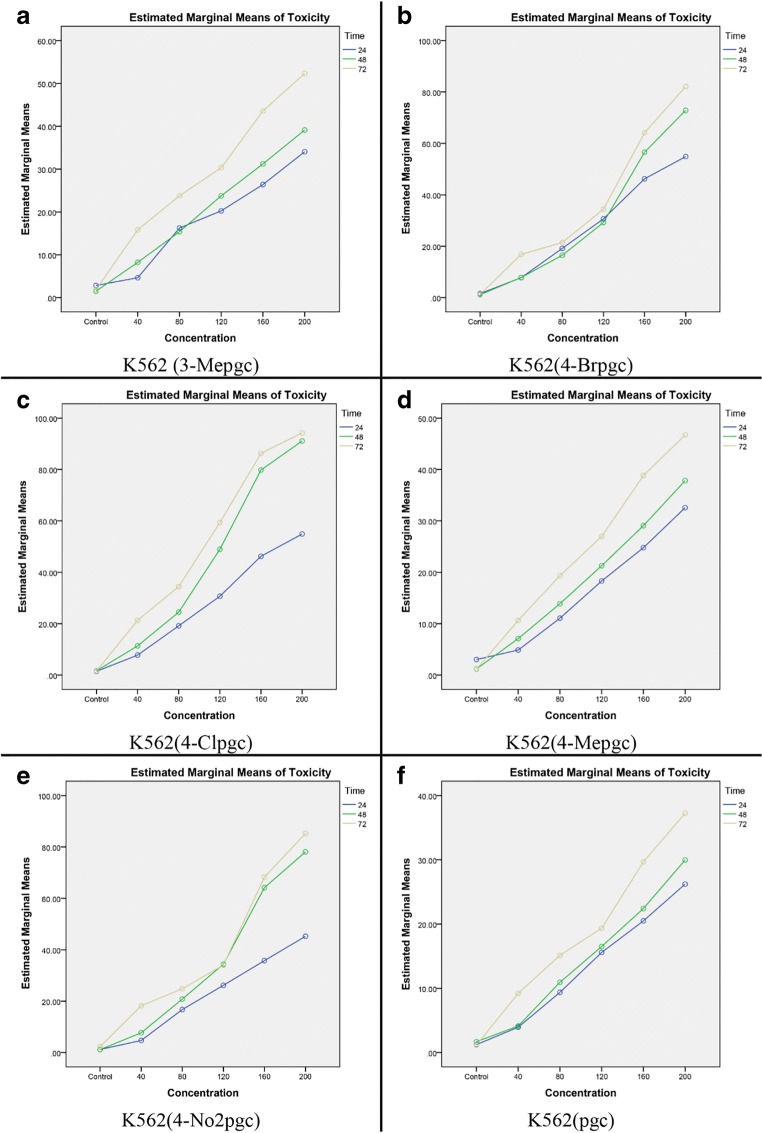
According to the results of MTT assay, induction of apoptosis in both K562 and Hela cells was strongly dependent on the concentration and time duration of treatment. The toxicity effect of the compounds increased with increasing the treatment time. In addition, these compounds were more influential on K562 cells, compared to Hela cells, which indicates the greater effect of these compounds on chronic myeloid leukemia. Therefore, subsequent complementary tests were performed on K562 cells (Fig. 2). The outcomes of Two-Way ANOVA confirmed the relationship between the simultaneous effects of time and concentration factors. The values of partial parameter η2 for the effects of time and concentration and the combination of these factors for cells and chromene derivatives have been presented in Table 3. According to this table, for both cell types and the six studied compounds, concentration was a more influential factor, compared to time, as changing the concentration for a fixed treatment time resulted in a greater change in toxicity, in comparison with the case of changing the treatment time for a fixed concentration.
Table 3.
The values of partial Parameter η2 indicating the interaction of time and concentration factors in toxicity assessment
| Partial parameter η2 | Compounds | cell | ||
|---|---|---|---|---|
| Concentration×Time | time | Concentration | ||
| 0.691 | 0.821 | 0.957 | 4-Clpgc | PBMC |
| 0.669 | 0.727 | 0.918 | 4-Brpgc | |
| 0.804 | 0.820 | 0.962 | 4-NO2pgc | |
| 0.343 | 0.666 | 0.869 | 4-MePgc | |
| 0.322 | 0.634 | 0.839 | 4-MePgc | |
| 0.288 | 0.483 | 0.866 | pgc | |
| 0.848 | 0.915 | 0.990 | 4-Clpgc | K562 |
| 0.610 | 0.596 | 0.981 | 4-Brpgc | |
| 0.810 | 0.843 | 0.983 | 4-Brpgc | |
| 0.271 | 0.461 | 0.925 | 4-MePgc | |
| 0.351 | 0.602 | 0.932 | 3-Mepgc | |
| 0.204 | 0.396 | 0.918 | pgc | |
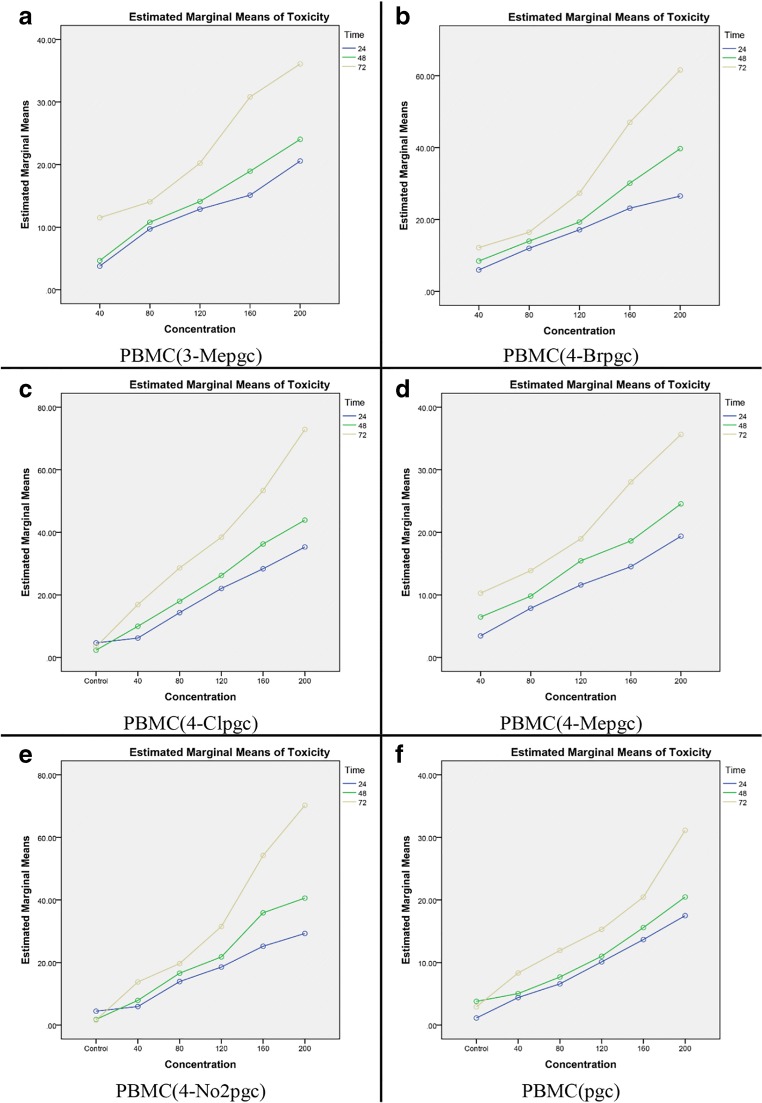
Figures 3 and 4 show the alterations in toxicity versus concentration at different time points, based on the results of Two-Way ANOVA. It is clear that the toxicity level increases over time, implying that a simultaneous increase in time and concentration would enhance the toxicity level.
Fig. 3.
Toxicity levels versus concentration at different time points in K562 for six chromene derivatives
Fig. 4.
Toxicity levels versus concentration at different time points in PBMC for six chromene derivatives
Furthermore, as illustrated in Fig. 5, the toxic effects of 4-Clpgc on normal cells were significantly lower than those on the cancer cells (with p values ˂ 0.0001 for 24 and 48 h and p value ˂ 0.001 for 72 h).
Fig. 5.
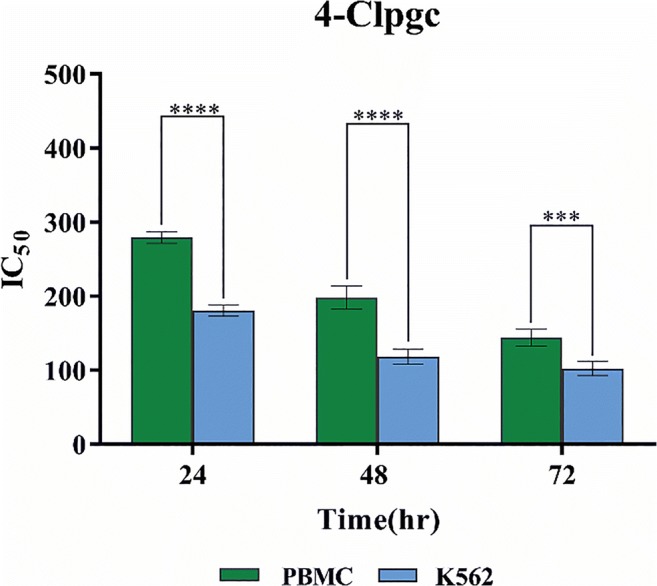
Comparing IC50 levels for 4-Clpgc on PBMCs and K562 cells after 24, 48, and 72 h (****: P < 0.0001, ***: P < 0.001)
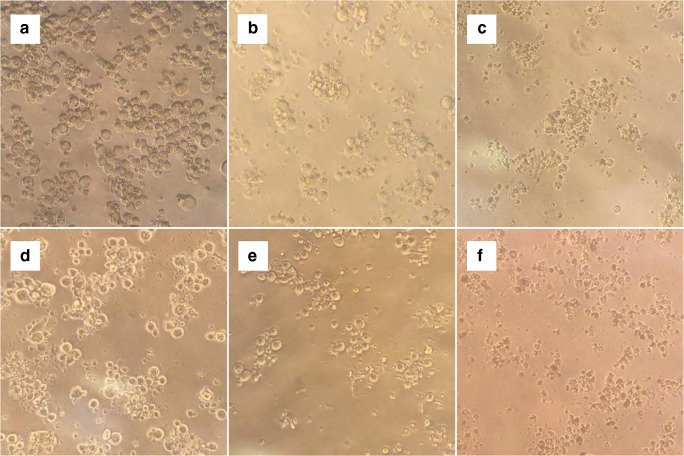
The morphologies of the K562 cells treated with 118 μM of 4-Clpgc underwent notable changes, based on observations using a light microscope. While control cells were round, the treated cells were aggregated after 24 h. Apoptotic bodies and parts of the condensed cytoplasm and nucleus were observable after 48 to 72 h (Fig. 6). These results revealed the occurrence of apoptosis in the treated cells.
Fig. 6.
Morphological changes of K562 cells treated with 118 μM of 4-Clpgc. Images of K562 cells were taken by a light microscope at 200x. a Negative control cells which were round after 24 h; b Negative control cells after 48 h; c Negative control cells after 72 h; d Aggregated cells after 24 h of exposure to 4-Clpgc; e Cells after 48 h of exposure to 4-Clpgc, having condensed cytoplasm; f Cells after 72 h of exposure to 4-Clpgc, having condensed cytoplasm and nucleus
Induction of apoptosis
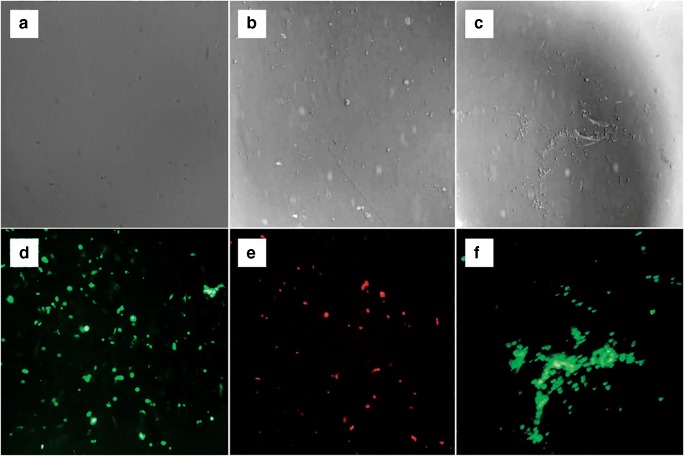
From a morphological viewpoint, necrosis and apoptosis are two distinct cell death pathways. In order to investigate the necrotic effect of chromene derivatives on the K562 cells, PI staining experiments were performed. As one can see in Fig. 7, after incubation with chromene derivatives, most of K562 cells underwent apoptosis and were not stained with PI.
Fig. 7.
After 48 h of exposure to 4-Clpgc, positive and negative control K562 cells were harvested and stained with PI for subsequent observation under light and fluorescent microscopes Light and fluorescent images (a, d) Negative control (b, e) Positive control; (c, f) After 48 h of exposures to 4-Clpgc; (100X)
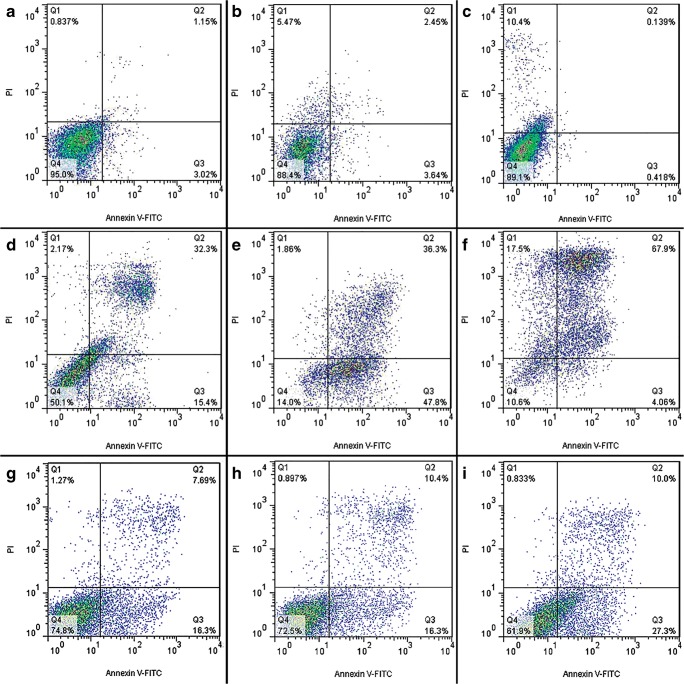
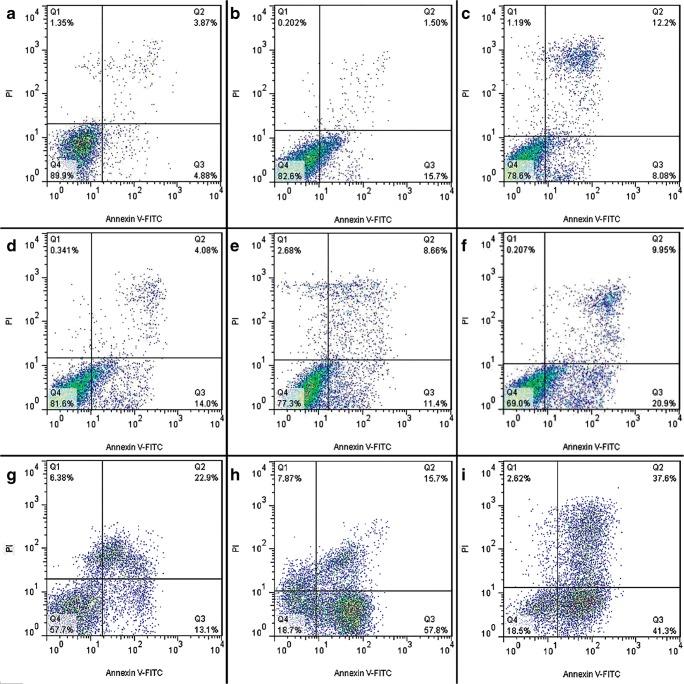
Moreover, in order to quantify the extents of apoptosis and necrosis in untreated and treated cells with chromene derivatives, flow cytometry analysis was performed using PI and Annexing-FITC markers.
In the two-dimensional graph of Annexing-FITC against PI, calculations were performed with respect to the main limits of cell population on four main areas (namely, Q1 to Q4) to determine the percentages of healthy, apoptotic and necrotic cells.
Based on the flow cytometry data, the apoptotic cell percentage in the control cells increased from 4.17% to 47.70% and 8.75% to 18.08% in K562 cells and PBMCs after 24 h, respectively. This parameter increased from 6.09% to 84.10% and 17.2% to 20.06% in K562 cells and PBMCs after 48 h of treatment, respectively. Also This parameter increased from 0.54% to 71.96% and 20.28% to 30.85% in K562 cells and PBMCs after 72 h of treatment, respectively (Figs. 8, and 9). These results indicated that apoptosis had occurred in the treated K562 cells.
Fig. 8.
Apoptosis induction by 4-Clpgc in K562. (a, b, c) Negative control after 24, 48 and 72 h, respectively; (d, e, f) in the presence of 4-Clpgc after 24, 48 and 72 h, respectively; (g, h, i) Positive control after 24, 48 and 72 h, respectively
Fig. 9.
Apoptosis induction by 4-Clpgc in PBMCs. (a, b, c) Negative control after 24, 48 and 72 h, respectively; (d, e, f) in the presence of 4-Clpgc after 24, 48 and 72 h, respectively; (g, h, i) Positive control after 24, 48 and 72 h, respectively
Expression of apoptotic genes after 4-Clpgc treatment
Bax is a pro apoptotic gene and TP53 is a tumor suppressor gene which acts to stop the formation of tumors. BCL2 is an anti-apoptotic gene and the BCR-ABL tyrosine kinase constitutively activates cytokine signal transduction pathways that stimulate growth and prevent apoptosis in hematopoietic cells.
According to Fig. 10 which indicates treating K562 cells with 4-Clpgc in IC50 values, enhanced the expression levels of Bax and TP53 genes and reduced the expressions of BCL2 and BCR-ABL in the cancer cells and normal cells.
Fig. 10.

Relative expressions of TP53, Bax, BCL2 and BCR- ABL genes after 48 h of treating K562 cells and PBMCs with 4-Clpgc (*: P < 0.05, ***: P < 0.001)
Moreover, the expressions of TP53 and Bax were increased by 42.74 and 35.88 folds, in K562 and by 9.60 and 7.75 folds in PBMC respectively. On the other hand, the expression levels of BCL2 were decreased by 1.47 and 1.38 folds in K562 and PBMC respectively. Also, the expression levels of BCR- ABL were decreased 2.50 folds, in K562 and increased by 1.68 folds, in PBMC.
Discussion
It has been reported that dihydro-pyranochromene derivatives, known as heterocyclic compounds, have remarkable effects on the biology, growth inhibition and apoptosis induction in various cancer cell types (Gourdeau et al. 2004).
In the present study, the anti-cancer effects of dihydropyrano [2,3-g] chromene derivatives on the K562 cell line (as an experimental model for CML) were investigated. We also reported an innovative series of anti-cancer agents from the dihydropyrano [2,3-g] chromene derivatives that induced apoptosis in K562 cells through TP53 activation with the down-regulation of BCL2 and up-regulation of Bax.
The 4-Clpgc compound caused a major reduction in the viability of K562 cells in a dose- and time-dependent manner. The studied chromene derivatives suppressed the growth of K562 cells. However, their effects were different and their corresponding IC50 levels were in the range of 180 to 370 μM after 24 h, 118 to 330 μM after 48 h and 102 to 278 μM after 72 h of treatment. These findings imply that the observed differences were somehow related to the adjacent R group connected to the base structure of the compounds. In this regard, in a previous study the anti-proliferative activities of some derivatives of 9-[Hydroxy(Substituted phenyl) Methyl]-2,2Dimethyl-2, 3, 8, 9-tetrahydro 4H, 10H-pyrano [2,3-f] chromene −4, 10 Dione family on breast cancer (T47D) have been evaluated. Based on the results, these compounds exhibited cytotoxic activities and inhibited cellular growth. In addition, the structure-activity relationship (SAR) of the CH3O groups on the phenyl ring of these compounds increased their activities and their IC50 levels were applied on these cells in a smaller range, in agreement with the findings of the our research (Heidary Alizadeh et al. 2010).
Among the studied compounds, 4-Clpgc with Cl position of the hexane ring was associated with the lowest IC50 value (102 μM after 72 h). On the other hand, the weakest growth inhibition effect in this group was observed in the case of pgc with the highest IC50 level (278 μM after 72 h). Indeed, pgc is the base compound of the group and does not include any R group. According to the results of a previous research on the effects of dihydropyranochromene derivatives on the K562 cell line, the impacts of these compounds were dependent on both concentration and treatment time. Functional group replacement was also influential on their toxicity. According to the results, 3-Npc exhibited the highest toxic effect. On the other hand, 4-pc resulted in the lowest toxicity level, which is consistent with the results of our study (Rahimi et al. 2015).
For each chromene derivative, the highest IC50 level was related to the normal PBMCs. This observation implies the relatively stronger effect of chromene derivatives on growth inhibition and toxicity of the K562 cancer cells, compared to the normal cell line. This observation may be due to the imbalance of cellular mechanisms and disturbance of the elimination mechanisms (metabolism and repulsion) of cells after becoming cancerous and excessive proliferation of these cells, compared to healthy cells (Dosik et al. 1981). On the other hand, the higher IC50 levels for normal cells compared to the cancer cell line may be related to the ability of these cells to excrete drugs and the desired extracts from the cell. Moreover, when being exposed to different compounds, normal cells launch counteracting pathways against the toxic effects of these compounds more quickly than the cancerous cells, which results in growth inhibition (Van Haaften et al. 2001).
Based on the findings of this study, the 4-Clpgc compound induces apoptosis in the K562 cell line. According to previous researches, the most known mechanism for the interaction of 4-Clpgc with microtubules is through 4-Clpgc attachment to β tubulin in the Colchicine site or near this location. Tubulins are vital cellular compartments that are responsible for the movement of chromosomes during interphase and mitosis. They also play a role in the initiation of filament mitosis, which leads to the transport of chromatids to the opposite poles of cell.
Therefore, inhibition of tubulin polymerization stops the cell cycle in the mitosis stage (during the anaphase/metaphase transition), thereby inducing apoptosis signaling in cells. It seems that apoptosis induction occurs through regulation of apoptotic genes such as BCL-x, BCL2 and TP53. Based on previous reports, the 4-Clpgc compound reduces the expression of BCL2 and increases the release of cytochrome C, which is an apoptosis initiating factor that results in the formation of apoptosome and increases the activity of caspases 9 and 3. Consequently, apoptosis can take place in this cell line (Fan 1999; Kasibhatla et al. 2004).
Apoptosis induction is considered as an effective strategy for cancer treatment. Different methods are used to confirm apoptosis induction in K562 cells by 4-Clpgc. Morphological changes and translocation of phosphatidic serine to the external side of cell surface are recognized as the signs of apoptosis. To confirm this hypothesis, Annexing V/PI double staining experiments were carried out. According to the results, the highest level of apoptosis could be observed 48 h after treatment, as at this time point, higher toxicity and lower necrosis levels were observed, compared to the 24-h treatment. Although the studied compounds exhibited more toxicity on cells over a period of 72 h, at this time point cells were entering a secondary apoptotic and necrotic phase and hence, it was not an appropriate time duration. These results were in agreement with the findings of a previous research by Hosadurga et al. (Keerthy et al. 2014). They have addressed the effect of 2-Amino-chromene-Nitriles on acute myeloid leukemia cells and also observed the increased secondary apoptosis after 72 h, compared to a 48-h treatment (Keerthy et al. 2014), consistent with the results of our study.
To investigate the activation of the mitochondrial pathway, the proteins of BCL2 family that play a key role in the regulation of this pathway, were studied. Several studies have shown that overexpression of the anti-apoptotic BCL2 family proteins results in cell resistance against apoptosis and reduces the efficiency of therapeutics (Jiang et al. 2007). The members of the BCL2 family are known as the main regulators of the mitochondrial (intrinsic) apoptosis (Adams and Cory 1998). BCL2 proteins regulate the release of cytochrome C from mitochondria and inhibit cell apoptosis (Plati et al. 2011). Moreover, in previous studies on drug resistant tumor cells, down-regulation of Bax and up-regulation of BCL2 have been reported (Sakamoto and Kyprianou 2010). Overexpression of BCL2 protein and aggregation of BCR-ABL enhance the rate of transfer from the chronic phase to the plastic phase in CML cells (Tzifi et al. 2012). It also leads to tumor evolution. In contrast, elevation of Bax expression level induces cellular death and removal of tumor cells (Tzifi et al. 2012). In cancer cells, BCL2 protein inhibits the formation of a pore in the mitochondrial membrane and also the release of cytochrome C through interrelating with Bax-Bak complex. Therefore, it inhibits the activation of caspase-9 and -3 in its downstream molecules (Teijido and Dejean 2010). It has been suggested that a high ratio of Bax to BCL2 causes a decline in the mitochondrial membrane potential, which leads to the release of cytochrome C and eventually, cell apoptosis (Ismail et al. 2005).
In the present study, we examined the alterations in the expression of Bax, BCL2, TP53, and BCR-ABL by cells in response to 4-Clpgc treatment. The K562 cells treated with a certain concentration (IC50 value) of 4-Clpgc, experienced an extensive and strong apoptosis induction after 48 h. TP53 and Bax expression levels increased in both cancer cells and normal cells, compared to non-treated cells. On the other hand, mRNA levels of BCL2 and BCR-ABL genes decreased in cancer cells and normal cells, in comparison with non-treated cells. These data are in agreement with the results of a previous study, in which exposing K562 cells to 4 t-QTC for 24 to 72 h has been reported to induce down-regulation of BCL2 and up-regulation of Bax (Ghasemian et al. 2015).
Our obtained data also confirmed that the observed reduction in the expression of the BCL2 protein suppresses its inhibitory effect on Bax and leads to Bax overexpression. Therefore, alterations in the ratio of pro-apoptotic and anti-apoptotic members of the BCL2 family might provide a good insight into the effects of 4-Clpgc on K562 cells.
In recent years, chemotherapy has been increasingly used to treat cancer patients. Until now, significant advances have been made in many aspects of this therapeutic strategy. In particular, the enhanced knowledge about the tumor biology has enlightened us about the mechanism of action of anti-neoplastic drugs and has been used as a basis for more rational designs of anti-cancer drugs. Regarding the drug resistance factor in the cancer types of blood cells, extensive efforts are being made for medicating the chromene derivatives.
Conclusion
In conclusion, our obtained results showed that among the studied chromene derivatives, 4-Clpgc is associated with the highest toxicity as well as desirable treatment index and apoptosis induction. Furthermore, it acted as one of the most suitable anti-cancer compounds. This compound severely induced apoptosis in the K562 cell line as a model for CML. Utilization of 4-Clpgc treatment at a concentration of 118 μM for 48 h resulted in the death of 50% of the cancer cells. It is also worth mentioning that this compound had a lower toxicity on normal cells compared to the cancer cells, leading to a relatively good treatment index. According to the results, apoptosis induction generally occurred due to the overexpression of Bax, activation of TP53 and down-regulation of BCL2 in K562 cells in response to 4-Clpgc treatment. These findings may broaden the scope for CML treatment in the future. In subsequent studies, other leukemia related cell lines should be examined, as well.
Acknowledgments
The authors would like to thank faculty of chemistry university of Kashan for synthesizing materials used in project and Pasteur Institute of Iran for providing the facilities and financial support to conduct this research project.
Footnotes
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Roya Mahinpour, Email: mahinpur@kashanu.ac.ir.
Nooshin Haghighipour, Email: haghighipour@pasteur.ac.ir.
References
- Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–1326. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- Calabretta B, Perrotti D (2004) The biology of CML blast crisis Blood 103:4010–4022 [DOI] [PubMed]
- Cortes JE et al. (2012) PACE: a pivotal phase II trial of ponatinib in patients with CML and Ph+ ALL resistant or intolerant to dasatinib or nilotinib, or with the T315I mutation. American Society of Clinical Oncology,
- Deininger MW, Goldman JM, Melo JV. The molecular biology of chronic myeloid leukemia. Blood. 2000;96:3343–3356. doi: 10.1182/blood.V96.10.3343. [DOI] [PubMed] [Google Scholar]
- Domingos PM, Steller H. Pathways regulating apoptosis during patterning and development. Curr Opin Genet Dev. 2007;17:294–299. doi: 10.1016/j.gde.2007.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosik GM, Barlogie B, Johnston D, Mellard D, Freireich EJ. Dose-dependent suppression of DNA synthesis in vitro as a predictor of clinical response in adult acute myeloblastic leukemia. Eur J Cancer. 1981;17:549–555. doi: 10.1016/0014-2964(81)90057-8. [DOI] [PubMed] [Google Scholar]
- Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emmadi NR, Atmakur K, Chityal GK, Pombala S, Nanubolu JB. Synthesis and cytotoxicity evaluation of highly functionalized pyranochromenes and pyranopyrans. Bioorg Med Chem Lett. 2012;22:7261–7264. doi: 10.1016/j.bmcl.2012.09.018. [DOI] [PubMed] [Google Scholar]
- Fan W. Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol. 1999;57:1215–1221. doi: 10.1016/S0006-2952(99)00006-4. [DOI] [PubMed] [Google Scholar]
- Ghasemian M, Mahdavi M, Zare P, Feizi MAH. Spiroquinazolinone-induced cytotoxicity and apoptosis in K562 human leukemia cells: alteration in expression levels of Bcl-2 and Bax. J Toxicol Sci. 2015;40:115–126. doi: 10.2131/jts.40.115. [DOI] [PubMed] [Google Scholar]
- Gourdeau H, et al. Antivascular and antitumor evaluation of 2-amino-4-(3-bromo-4, 5-dimethoxy-phenyl)-3-cyano-4H-chromenes, a novel series of anticancer agents. Mol Cancer Ther. 2004;3:1375–1384. [PubMed] [Google Scholar]
- Heidary Alizadeh B, et al. Synthesis and cytotoxic activity of novel 9-[hydroxy (substitutedphenyl) methyl]-2, 2-dimethyl-2, 3, 8, 9-tetrahydro-4h, 10h-pyrano [2, 3-f] chromene-4, 10-diones. Iran J Chem Chem Eng. 2010;29:189–196. [Google Scholar]
- Ismail N, Pihie AHL, Nallapan M. Xanthorrhizol induces apoptosis via the up-regulation of bax and p53 in HeLa cells. Anticancer Res. 2005;25:2221–2227. [PubMed] [Google Scholar]
- JC, R (1998) Bcl-2 family protein oncogenes 17:3225–3236 [DOI] [PubMed]
- Jiang H, Hou CH, Zhang SB, Xie HY, Zhou WY, Jin QH, Cheng XD, Qian RL, Zhang XJ. Matrine upregulates the cell cycle protein E2F-1 and triggers apoptosis via the mitochondrial pathway in K562 cells. Eur J Pharmacol. 2007;559:98–108. doi: 10.1016/j.ejphar.2006.12.017. [DOI] [PubMed] [Google Scholar]
- Kasibhatla S, et al. Discovery and mechanism of action of a novel series of apoptosis inducers with potential vascular targeting activity. Mol Cancer Ther. 2004;3:1365–1374. [PubMed] [Google Scholar]
- Keerthy HK, Garg M, Mohan CD, Madan V, Kanojia D, Shobith R, Nanjundaswamy S, Mason DJ, Bender A, Basappa, Rangappa KS, Koeffler HP. Synthesis and characterization of novel 2-amino-chromene-nitriles that target Bcl-2 in acute myeloid leukemia cell lines. PLoS One. 2014;9:e107118. doi: 10.1371/journal.pone.0107118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemnitzer W, Jiang S, Wang Y, Kasibhatla S, Crogan-Grundy C, Bubenik M, Labrecque D, Denis R, Lamothe S, Attardo G, Gourdeau H, Tseng B, Drewe J, Cai SX. Discovery of 4-aryl-4H-chromenes as a new series of apoptosis inducers using a cell-and caspase-based HTS assay. Part 5: modifications of the 2-and 3-positions. Bioorg Med Chem Lett. 2008;18:603–607. doi: 10.1016/j.bmcl.2007.11.078. [DOI] [PubMed] [Google Scholar]
- Kerr JF, Winterford CM, Harmon BV. Apoptosis. Its significance in cancer and cancer therapy. Cancer. 1994;73:2013–2026. doi: 10.1002/1097-0142(19940415)73:8<2013::AID-CNCR2820730802>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- Lebedeva IV, Su Z-Z, Sarkar D, Fisher PB (2003) Restoring apoptosis as a strategy for cancer gene therapy: focus on p53 and mda-7. In: Seminars in cancer biology, vol 2. Elsevier, pp 169–178 [DOI] [PubMed]
- Mo S, Wang S, Zhou G, Yang Y, Li Y, Chen X, Shi J. Phelligridins C− F: cytotoxic Pyrano [4, 3-c][2] benzopyran-1, 6-dione and Furo [3, 2-c] pyran-4-one derivatives from the fungus Phellinus i gniarius. J Nat Prod. 2004;67:823–828. doi: 10.1021/np030505d. [DOI] [PubMed] [Google Scholar]
- Mohammadi Ziarani G, Badiei A, Azizi M, Zarabadi P. Synthesis of 3, 4-dihydropyrano [c] chromene derivatives using sulfonic acid functionalized silica (SiO2PrSO3H) Iran J Chem Chem Eng. 2011;30:59–65. [Google Scholar]
- Moosavi MA, Yazdanparast R, Lotfi A. ERK1/2 inactivation and p38 MAPK-dependent caspase activation during guanosine 5′-triphosphate-mediated terminal erythroid differentiation of K562 cells. Int J Biochem Cell Biol. 2007;39:1685–1697. doi: 10.1016/j.biocel.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Moradi L, Sadegh MA. Sodium saccharin as an effective catalyst for rapid one-pot pseudo-five component synthesis of Dihydropyrano [2, 3-g] chromenes under microwave irradiation. Acta Chim Slov. 2017;64:506–512. doi: 10.17344/acsi.2017.3417. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Plati J, Bucur O, Khosravi-Far R. Apoptotic cell signaling in cancer progression and therapy. Integr Biol. 2011;3:279–296. doi: 10.1039/c0ib00144a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahimi R, Mahdavi M, Pejman S, Zare P, Balalaei S. Inhibition of cell proliferation and induction of apoptosis in K562 human leukemia cells by the derivative (3-NpC) from dihydro-pyranochromenes family. Acta Biochim Pol. 2015;62:83–88. doi: 10.18388/abp.2014_825. [DOI] [PubMed] [Google Scholar]
- Sakamoto S, Kyprianou N. Targeting anoikis resistance in prostate cancer metastasis. Mol Asp Med. 2010;31:205–214. doi: 10.1016/j.mam.2010.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmittgen TD, Livak KJ. Analyzing real-time PCR data by the comparative C T method. Nat Protoc. 2008;3:1101–1108. doi: 10.1038/nprot.2008.73. [DOI] [PubMed] [Google Scholar]
- Sun Y, Liu M, Yang B, Lu J, Li B. Inhibition of laryngeal cancer cell invasion and growth with lentiviral-vector delivered short hairpin RNA targeting human MMP-9 gene. Cancer Investig. 2008;26:984–989. doi: 10.1080/07357900802072897. [DOI] [PubMed] [Google Scholar]
- Taylor RC, Cullen SP, Martin SJ. Apoptosis: controlled demolition at the cellular level. Nat Rev Mol Cell Biol. 2008;9:231–241. doi: 10.1038/nrm2312. [DOI] [PubMed] [Google Scholar]
- Teijido O, Dejean L. Upregulation of Bcl2 inhibits apoptosis-driven BAX insertion but favors BAX relocalization in mitochondria. FEBS Lett. 2010;584:3305–3310. doi: 10.1016/j.febslet.2010.07.002. [DOI] [PubMed] [Google Scholar]
- Thomas N, Zachariah SM. Pharmacological activities of chromene derivatives: an overview. Asian J Pharm Clin Res. 2013;6:11–15. [Google Scholar]
- Tzifi F, Economopoulou C, Gourgiotis D, Ardavanis A, Papageorgiou S, Scorilas A (2012) The role of BCL2 family of apoptosis regulator proteins in acute and chronic leukemias advances in hematology 2012 [DOI] [PMC free article] [PubMed]
- Van Haaften RI, Evelo CT, Haenen GR, Bast A. No reduction of α-tocopherol quinone by glutathione in rat liver microsomes. Biochem Pharmacol. 2001;61:715–719. doi: 10.1016/S0006-2952(01)00545-7. [DOI] [PubMed] [Google Scholar]
- Vosooghi M, et al. Synthesis and cytotoxic activity of some 2-amino-4-aryl-3-cyano-7-(dimethylamino)-4H-chromenes. Res Pharm Sci. 2010;5:9. [PMC free article] [PubMed] [Google Scholar]
- Ye Y-X, Zhou J, Zhou YH, Zhou Y, Song XB, Wang J, Lin L, Ying BW, Lu XJ. Clinical significance of BCR-ABL fusion gene subtypes in chronic myelogenous and acute lymphoblastic leukemias. Asian Pac J Cancer Prev. 2014;15:9961–9966. doi: 10.7314/APJCP.2014.15.22.9961. [DOI] [PubMed] [Google Scholar]