Abstract
Clinical recognition of acute respiratory distress syndrome (ARDS) is delayed or missed entirely in a substantial proportion of patients. In the LUNG SAFE study, the largest international cohort of patients with ARDS, investigators were able to determine if ARDS was present, and at what stage the clinician made the diagnosis of ARDS. The diagnosis of ARDS was delayed or missed in two-thirds of patients, with the diagnosis missed entirely in 40% of patients, while ARDS recognition ranged from 51% in mild ARDS to 79% in severe cases. Failure to recognize ARDS in a timely fashion leads to failure to use strategies that improve survival in ARDS. Early diagnosis of ARDS may facilitate measures to abrogate progression of the lung injury, including protective mechanical ventilation, fluid restriction, and adjunctive measures proven to improve survival such as prone positioning. Information overload and a complex ‘syndrome’ diagnosis likely play key roles in ARDS under-recognition. Clinical under-recognition has important consequences particularly in terms of therapeutic options not considered. The development of approaches to enable more timely recognition has the potential to save lives.
Keywords: Acute respiratory distress syndrome, Diagnosis, Recognition, Therapy, Outcome
Take-home message
| Significant numbers of patients with ARDS are unrecognized or recognized late by clinicians; this impacts on patient management and may have important consequences for patient outcome. |
Introduction
Early recognition of acute respiratory distress syndrome (ARDS) may be important to facilitate measures to abrogate progression of the lung injury, including protective mechanical ventilation, fluid restriction, and adjunctive measures proven to improve survival such as prone positioning. ARDS diagnosis is delayed or missed entirely in a substantial proportion of patients. In the LUNG SAFE study, the largest existing international cohort of patients with ARDS, investigators were able to determine if ARDS was present, and at what stage the clinician made the diagnosis of ARDS [1]. The diagnosis of ARDS was delayed or missed in two-thirds of patients, with the diagnosis missed entirely in 40% of patients, while ARDS recognition ranged from 51% in mild ARDS to 79% in severe cases [1].
Does ARDS under-recognition matter?
Yes, several lines of evidence suggest that recognition of ARDS influences patient management. Failure of clinicians to recognize ARDS is a barrier to the use of protective lung ventilation strategies [2, 3]. The importance of early recognition and management is underscored by the finding that patients receiving higher tidal volumes shortly after the onset of ARDS onset have a higher mortality, suggesting that high tidal volume is more injurious if used earlier [4]. While in the LUNG SAFE study, patients that clinicians recognized as ARDS received only marginally lower tidal volumes, this may be more a reflection of the penetration of lower tidal volume ventilation into clinical practice. Clinician recognition of ARDS was associated with the use of higher PEEP levels and with greater use of prone positioning, neuromuscular blockade and extracorporeal membrane oxygenation, suggesting that failure to recognize ARDS in a timely fashion leads to failure to use strategies that improve survival in ARDS. Furthermore, failure of clinicians to recognise ARDS may impair broader (research funders, policy makers, general public) awareness of the impact of ARDS.
Why is ARDS under-recognized?
In the absence of a diagnostic test, patients must fulfil a set of clinical criteria within a specific time frame that have relatively high sensitivity but low specificity for ARDS (Table 1) [5]. The inter-observer reliability of the Berlin ARDS definition is moderate, mainly due to variability in chest X-ray (CXR) interpretation [6]. The oxygenation criterion, namely the ratio of arterial PO2 to inspired oxygen fraction, is not measured at standardized ventilator settings, and can vary substantially in a single patient as different FiO2 [7]. In fact, it is often not calculated at the bedside, possibly because clinicians may incorrectly assume that these patients cannot have ARDS if they receive a “safe” FiO2. The anteroposterior CXR criterion is central to the diagnosis of ARDS—yet this is a poorly reliable test with high inter-observer variability in interpretation [8] while training programs in CXR interpretation have limited efficacy [9]. Other aspects of the definition, such as the timing criterion are relatively arbitrary. These concerns may erode clinician confidence in the utility of making the diagnosis of ARDS. The Berlin definition of ARDS presents an ambiguity in how the patients non-invasively ventilated with CPAP, with a PaO2/FiO2 ratio lower that 200 mmHg should be classified as these do not, technically, fit in any of the categories [5]. The inability to proper apply the Berlin definition in these patients may contribute to ARDS under-diagnosis.
Table 1.
The Berlin definition of acute respiratory distress syndrome
Note: Reproduced from ARDS Definition Taskforce et al. [5]
| ARDS severity | Mild | Moderate | Severe |
|---|---|---|---|
| Timing | Acute onset within 1 week of a known clinical insult or new/worsening respiratory symptoms | ||
| Chest imaginga | Bilateral opacities—not fully explained by effusions, lobar/lung collapse, or nodules | ||
| Oxygenationb | PaO2/FiO2 201–300 mmHg with PEEP/CPAP ≥ 5 cm H2Oc | PaO2/FiO2 101–200 mmHg with PEEP/CPAP ≥ 5 cm H2O | PaO2/FiO2 ≤ 100 mmHg with PEEP/CPAP ≥ 5 cm H2O |
| Origin of oedema | Respiratory failure not fully explained by cardiac failure or fluid overload (objective assessment required if no ARDS risk factor present) | ||
aChest radiograph or computed tomography scan
bIf altitude is higher than 1000 m, the correction factor should be calculated as follows: [PaO2/FiO2_(barometric pressure/760)]
cThis may be delivered non-invasively in the mild acute respiratory distress syndrome group
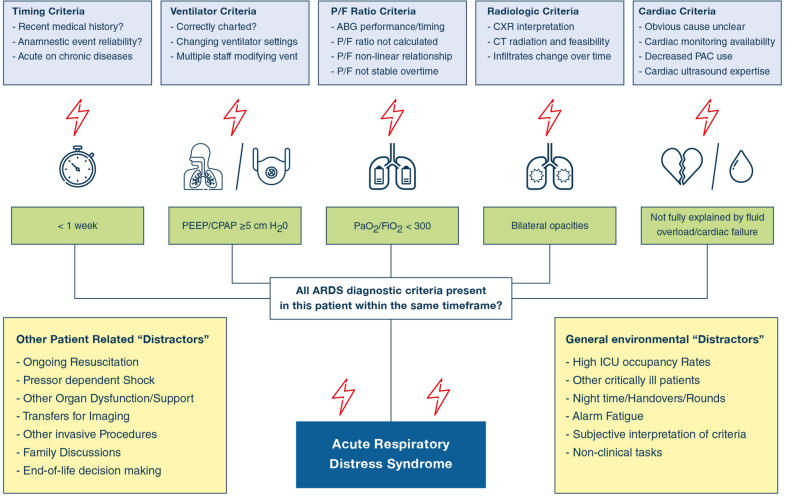
Another issue is the complexity of making a diagnosis that relies on recognition of specific criteria combining clinical, biological and radiological features (Fig. 1) in critically ill patients with multiple comorbidities and ongoing critical clinical issues. The presence of chronic underlying lung disorders may affect the ARDS radiologic or oxygenation criteria. It should not be that surprising that clinicians fail—sometimes frequently—to recognize these clinical patterns in a timely fashion. ICU clinicians are exposed to information overload from many sources, including clinical reports, flowcharts, bedside monitors, and laboratory results [10]. The ability of even experienced clinicians to consistently integrate multiple clinical variables is limited to perhaps 3–5 information chunks [11]. In LUNG SAFE, higher nurse (or physician)-to-patient ratio increased the likelihood of recognition, suggesting that clinician workload and its related information overload may promote under-recognition. Indeed, the more ‘stereotypical’ the presentation, i.e. the younger the patient, the greater the severity of hypoxemia, and the presence of pneumonia the greater the clinician recognition.
Fig. 1.
Barriers to the diagnosis of ARDS. Each item of the ARDS definition poses specific challenges that can impair ability to diagnose ARDS. In addition, other patient-specific issues and the general ICU environment may constitute further barriers to ARDS recognition. ABG arterial blood gas, CXR chest X-ray, CT computed tomography, PAC pulmonary artery catheter, PEEP positive end expiratory pressure, CPAP continuous positive airway pressure
How can we improve ARDS recognition?
Clearly understanding the importance of early diagnosis of ARDS, and the reasons underlying delayed or failed recognition is a key first step. Determining whether the clinical criteria for ARDS at standardized ventilator settings in all patients receiving mechanical ventilation with a PaO2/FiO2 ratio ≤ 300 mmHg on a standardized FiO2 (e.g. 1.0) is a reasonable option given that we know that > 20% of all ventilated patients will meet current criteria [1]. In resource constrained situations, pulse peripheral oxygen saturation to FiO2 ratio (SpO2/FiO2) might usefully replace PaO2/FiO2 ratio.
More controversially, it is time to reconsider the role of the chest radiograph in ARDS diagnosis. Low-dose computed tomography may be preferable for ARDS diagnosis [12], although it does require patient transport to the scanner, a limitation in severely ill patients. Lung ultrasound, now becoming widely available even in limited resources settings, appears to be sensitive and reproducible [13], and may have a role in ARDS diagnosis at the bedside [14]. Biomarker panels may help, but given the high sensitivity of the consensus criteria, additional markers may be superfluous for detection—but be of great use in confirmation (i.e. to reduce ‘false positives’) or to identify biologically homogenous subgroups within the ARDS population. Identifying ARDS sub-phenotype, using latent class analysis [15] or transcriptomic approaches [16] show significant promise.
Additional criteria might be applied for entry into ARDS clinical trials, particularly trials assessing biologic agents that affect specific pathways. For example, if a pathway blocker is to be tested in ARDS patients (e.g. Tocilizumab for COVID-19 ARDS), then it would be important to first demonstrate that this pathway is active (e.g. by measuring IL-6) in that patient. Such criteria would differ depending on the study, and would supplement rather than replace the clinical definition of ARDS.
ARDS recognition might be further enhanced by computer-aided pattern recognition, bypassing information overload [17]. Artificial Intelligence approaches such as machine learning may assist in identification of patients at risk of or fulfilling diagnostic criteria for ARDS, although this technology is not yet ready for clinical implementation [18].
Conclusion
ARDS continues to be under-recognized in the era of the Berlin definition. Information overload and a complex ‘syndrome’ diagnosis likely play key roles in ARDS under-recognition. Clinician under-recognition has important consequences particularly in terms of therapeutic options not considered. The development of approaches to enable more timely recognition has the potential to save lives.
Compliance with ethical standards
Conflict of interest
The authors declare that they have no conflict of interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bellani G, Laffey JG, Pham T, Fan F, Brochard L, Esteban A, Gattinoni L, van Haren F, Larsson A, McAuley DF, Ranieri M, Rubenfeld GD, Thompson BT, Wrigge H, Slutsky AS, Pesenti A, Group ObotLSIatET Epidemiology, patterns of care, and mortality for patients with acute respiratory distress syndrome in intensive care units in 50 countries. JAMA. 2016;315:788–800. doi: 10.1001/jama.2016.0291. [DOI] [PubMed] [Google Scholar]
- 2.Rubenfeld GD, Cooper C, Carter G, Thompson BT, Hudson LD. Barriers to providing lung-protective ventilation to patients with acute lung injury. Crit Care Med. 2004;32:1289–1293. doi: 10.1097/01.CCM.0000127266.39560.96. [DOI] [PubMed] [Google Scholar]
- 3.Kalhan R, Mikkelsen M, Dedhiya P, Christie J, Gaughan C, Lanken PN, Finkel B, Gallop R, Fuchs BD. Underuse of lung protective ventilation: analysis of potential factors to explain physician behavior. Crit Care Med. 2006;34:300–306. doi: 10.1097/01.CCM.0000198328.83571.4A. [DOI] [PubMed] [Google Scholar]
- 4.Needham DM, Yang T, Dinglas VD, Mendez-Tellez PA, Shanholtz C, Sevransky JE, Brower RG, Pronovost PJ, Colantuoni E. Timing of low tidal volume ventilation and intensive care unit mortality in acute respiratory distress syndrome. A prospective cohort study. Am J Respir Crit Care Med. 2015;191:177–185. doi: 10.1164/rccm.201409-1598OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ARDS Definition Task Force. Ranieri VM, Rubenfeld GD, Thompson BT, Ferguson ND, Caldwell E, Fan E, Camporota L, Slutsky AS. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307(23):2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Sjoding MW, Hofer TP, Co I, Courey A, Cooke CR, Iwashyna TJ. Interobserver reliability of the Berlin ARDS definition and strategies to improve the reliability of ARDS diagnosis. Chest. 2018;153:361–367. doi: 10.1016/j.chest.2017.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aboab J, Louis B, Jonson B, Brochard L. Relation between PaO2/FiO2 ratio and FiO2: a mathematical description. Intensive Care Med. 2006;32:1494–1497. doi: 10.1007/s00134-006-0337-9. [DOI] [PubMed] [Google Scholar]
- 8.Figueroa-Casas JB, Brunner N, Dwivedi AK, Ayyappan AP. Accuracy of the chest radiograph to identify bilateral pulmonary infiltrates consistent with the diagnosis of acute respiratory distress syndrome using computed tomography as reference standard. J Crit Care. 2013;28:352–357. doi: 10.1016/j.jcrc.2012.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Goddard SL, Rubenfeld GD, Manoharan V, Dev SP, Laffey J, Bellani G, Pham T, Fan E. The randomized educational acute respiratory distress syndrome diagnosis study: a trial to improve the radiographic diagnosis of acute respiratory distress syndrome. Crit Care Med. 2018;46:743–748. doi: 10.1097/CCM.0000000000003000. [DOI] [PubMed] [Google Scholar]
- 10.Herasevich V, Yilmaz M, Khan H, Hubmayr RD, Gajic O. Validation of an electronic surveillance system for acute lung injury. Intensive Care Med. 2009;35:1018–1023. doi: 10.1007/s00134-009-1460-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cowan N. The magical number 4 in short-term memory: a reconsideration of mental storage capacity. Behav Brain Sci. 2001;24:87–114. doi: 10.1017/S0140525X01003922. [DOI] [PubMed] [Google Scholar]
- 12.Chiumello D, Langer T, Vecchi V, Luoni S, Colombo A, Brioni M, Froio S, Cigada I, Coppola S, Protti A, Lazzerini M, Gattinoni L. Low-dose chest computed tomography for quantitative and visual anatomical analysis in patients with acute respiratory distress syndrome. Intensive Care Med. 2014;40:691–699. doi: 10.1007/s00134-014-3264-1. [DOI] [PubMed] [Google Scholar]
- 13.Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby JJ. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology. 2004;100:9–15. doi: 10.1097/00000542-200401000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Pisani L, Vercesi V, van Tongeren PSI, Lagrand WK, Leopold SJ, Huson MAM, Henwood PC, Walden A, Smit MR, Riviello ED, Pelosi P, Dondorp AM, Schultz MJ, Lung Ultrasound C. The diagnostic accuracy for ARDS of global versus regional lung ultrasound scores—a post hoc analysis of an observational study in invasively ventilated ICU patients. Intensive Care Med Exp. 2019;7:44. doi: 10.1186/s40635-019-0241-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Calfee CS, Delucchi K, Parsons PE, Thompson BT, Ware LB, Matthay MA, Network NA. Subphenotypes in acute respiratory distress syndrome: latent class analysis of data from two randomised controlled trials. Lancet Respir Med. 2014;2:611–620. doi: 10.1016/S2213-2600(14)70097-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bos LDJ, Scicluna BP, Ong DSY, Cremer O, van der Poll T, Schultz MJ. Understanding heterogeneity in biologic phenotypes of acute respiratory distress syndrome by leukocyte expression profiles. Am J Respir Crit Care Med. 2019;200:42–50. doi: 10.1164/rccm.201809-1808OC. [DOI] [PubMed] [Google Scholar]
- 17.Koenig HC, Finkel BB, Khalsa SS, Lanken PN, Prasad M, Urbani R, Fuchs BD. Performance of an automated electronic acute lung injury screening system in intensive care unit patients. Crit Care Med. 2011;39:98–104. doi: 10.1097/CCM.0b013e3181feb4a0. [DOI] [PubMed] [Google Scholar]
- 18.Zeiberg D, Prahlad T, Nallamothu BK, Iwashyna TJ, Wiens J, Sjoding MW. Machine learning for patient risk stratification for acute respiratory distress syndrome. PLoS ONE. 2019;14:e0214465. doi: 10.1371/journal.pone.0214465. [DOI] [PMC free article] [PubMed] [Google Scholar]