Abstract
The non‐POU domain‐containing octamer‐binding protein NONO/p54nrb, which belongs to the Drosophila behaviour/human splicing (DBHS) family, is a multifunctional nuclear protein rarely functioning alone. Emerging solid evidences showed that NONO engages in almost every step of gene regulation, including but not limited to mRNA splicing, DNA unwinding, transcriptional regulation, nuclear retention of defective RNA and DNA repair. NONO is involved in many biological processes including cell proliferation, apoptosis, migration and DNA damage repair. Dysregulation of NONO has been found in many types of cancer. In this review, we summarize the current and fast‐growing knowledge about the regulation of NONO, its biological function and implications in tumorigenesis and cancer progression. Overall, significant findings about the roles of NONO have been made, which might make NONO to be a new biomarker or/and a possible therapeutic target for cancers.
Keywords: DBHS, NONO, splicing, tumorigenesis
1. INTRODUCTION
The NONO (non‐POU domain‐containing octamer‐binding protein) protein, also known as 54 kD nuclear RNA‐ and DNA‐binding protein (p54nrb), belongs to the multifunctional DBHS (Drosophila behaviour/human splicing) family of proteins which can bind DNA, RNA and protein.1 NONO has a nuclear localization signal (NLS) at its C‐terminal, so it is located in the nucleus of most mammalian cells and is primarily distributed in the subnuclear domain named paraspeckles.2 Emerging evidence strongly indicates new roles for NONO in tumorigenesis, including but not limited to regulating proliferation, apoptosis, cell migration and DNA damage repair. Here, we provide a comprehensive review of the NONO and its functions in tumorigenesis.
2. STRUCTURE
The human NONO gene is located on chromosome X 13p1 and encodes a 471 aa protein identified as a homolog of the Drosophila NONA/BJ6 from Hela cells.1 NONO is one of the three homologous mammalian proteins that are termed the ‘DBHS’ family, the others being SFPQ/SPF (splicing factor proline and glutamine rich) and PSPC1/PSP1 (paraspeckle component 1). The DBHS proteins share a core region ‘DBHS’ of ~300 amino acids, which is characterized by highly conserved N‐terminal RNA recognition motifs (RRMs), a NOPS (NONA/paraspeckle domain) and a C‐terminal coiled‐coil and are largely regarded as nuclear factors.1, 3 RRM domains recognize and interact with RNA and single strand DNA.4 The NOPS domain is in charge of mediating DBHS dimerization, and sometimes some surface‐exposed basic residues (R280 and R284) within the NOPS domain may serve as a single molecular interaction site to bind nucleic acids.5 The C‐terminal end facilitates dimerization and oligomerization.6 However, there are structural differences outside the ‘DBHS region’ between members of the family (Figure 1). Moreover, like other two DBHS proteins, NONO rarely functions alone, its interactions are regulated by its structure changes and largely regulated by post‐translational modifications and the interactors.6
Figure 1.
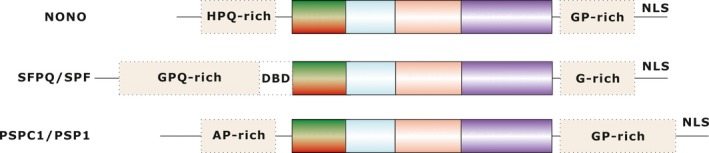
Schematic representation of human DBHS protein domain architecture. The uncharacterized DBD of SFPQ and other low complexity regions of each paralog are indicated in dashed boxes
3. REGULATION OF NONO EXPRESSION
3.1. Transcription
NONO is involved in collagen formation and fibrosis in some situations.7, 8, 9, 10 In the patients with aortic dissection (AD), there are significant correlations between NONO and collagen. NONO protein is decreased in AD tissue compared with control tissue, its mRNA expression is also decreased.10 NONO is also regulated at the transcriptional level in melanoma, because MIA (melanoma inhibitory activity) depletion can reduce significantly NONO mRNA and protein level (see Section 6.5 below).11 The detailed mechanism about transcriptional regulation of NONO still needs much more in‐depth studies.
3.2. mRNA stability
NONO can regulate the intra‐S‐phase checkpoint in response to UV radiation.12 However, UV rays could induce the expression of a microRNA, miR‐320a, which could target NONO mRNA for degradation by binding its 5′‐UTR. Interestingly, the RNA binding protein HUR (also called ELAVL1), which was also induced by UV rays, was shown to protect NONO mRNA from mir‐320a‐mediated degradation by binding an overlapping site within the 5′UTR.13 Later, it was shown that UV induce NONO protein degradation mediated by the RNF8 ubiquitin ligase and interfering with this process affects the S phase checkpoint, consistently with previous work.14 Further mechanisms of NONO mRNA regulation still need to be defined.
3.3. Post‐translational modifications
Structural and biological data suggest DBHS proteins rarely play their biological roles alone, their interactions with various proteins are regulated by post‐translational modifications.6 NONO were proved to be phosphorylated in mitosis in some independent studies.15, 16, 17, 18, 19, 20, 21, 22 CDK1 phosphorylates T412, T430 and T452 in the C‐terminal extremity of NONO, subsequently the prolyl isomerase Pin1 interacts with the phosphorylated NONO. Furthermore, Pin1 interaction with NONO depends on multisite phosphorylation.15 CDK1 also can phosphorylate T15 in the N‐terminal of NONO in vitro. Two independent studies21, 23 found that NONO could be tyrosine‐phosphorylated; however, they could not exclude that the p‐Tyr antibodies could non‐specifically bind NONO, and a p‐Tyr antibody was found having non‐specific binding affinity to NONO in another study later.22 Furthermore, crystal structure of NONO shows that the five Tyr residues of NONO are not in favourable positions to be phosphorylated because of steric hindrance.4 Even though, the tyrosine residues still regulate NONO’s multifarious nuclear functions.22
CARM1, also known as PRMT4, can methylate NONO, and R357, R365 and R378 are the major sites to be methylated.24 CARM1 knock‐down enhances the nuclear retention of mRNAs containing inverted repeated Alu elements (IRAlus), via reducing binding of NONO to target mRNAs.24 SUMOylation,25 ADP‐ribosylation26 and acetylation27 are also found in several proteomics studies, there should more in‐depth studies.
NONO half‐life is about 32 hours in Hela cell which is consistent with in silico predictions.28 Recently, there are some solid evidences proving that NONO turnover can be regulated in vivo.14, 28 NONO can be polyubiquitinated upon FBW7α28 or RNF814 interaction, and three of total 27 lysine residues of NONO are important ubiquitination sites.14
4. NONO AND GENE REGULATION
NONO engages in almost every step of gene regulation,29 including but not limited to pre‐mRNA splicing,30, 31, 32, 33 activation of transcription,32, 34 termination of transcription,35 nuclear retention of defective RNA,36, 37 DNA unwinding,29, 38 double‐stranded break repair39, 40 and maintaining correct circadian clock functions7, 41 (reviewed in Refs. 6, 29) (Figure 2).
Figure 2.
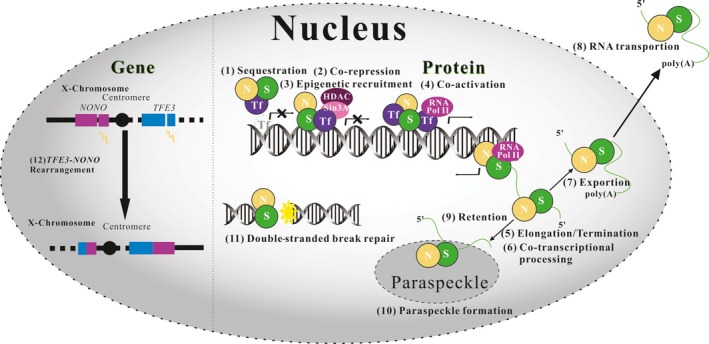
Simplified schematic representation of NONO protein function. The DBHS proteins SFPQ (S) and NONO (N) are represented as simple green and orange spheres respectively. (1) SFPQ and NONO can sequester transcription factors away from target promoters, (2) act as co‐repressors at target promoters and (3) in complex with repressors stimulate epigenetic silencing. (4) Both SFPQ and NONO are associated with co‐activation of transcription through (5) elongation up to termination. (6) SFPQ and NONO also remain associated with nascent mRNA to facilitate co‐transcriptional processing, (7) messenger ribonucleoprotein (mRNP) export and (8) cytosolic trafficking. (9,10) By virtue of their involvement in paraspeckle formation and integrity, SFPQ and NONO can facilitate nuclear RNA retention. (11) SFPQ, NONO and PSPC1 are also involved in double‐stranded break repair. (12) TFE3‐NONO rearrangement
NONO can activate the RNA transcription, most of which is nascent RNA. NONO interacts with other promoters of many transcriptionally active genes, such as rhodopsin,34 oct4,42 TORCs (transducer of regulated CREB)43 and AR (androgen receptor),44, 45 subsequently promotes transcription, which is often associated with a synergistic effect with other promoters.34 Sometimes, NONO binds to a suppressor to be prevented from transcription activation, for example, SOCS3 is a suppressor in NONO‐SOCS3 complex, after IL‐1β disrupts the interaction of NONO‐SOCS3, the downstream Mucin8 level increases in transcription level.46 Interestingly, on some other contexts, such as DBHS dimer composition, modification status, cell lines, and cellular localization, NONO co‐represses/co‐activates AR‐mediated transcription.47, 48 NONO can activate basal and cAMP‐dependent transcription of CYP17 gene,49, 50 Sin3A‐HDAC (histone deacetylases) and the binding of SF‐1(steroidogenic factor‐1)/PSF/NONO to the promoter determine the transcription activity.50 Through recruitment of epigenetic regulator HDAC, SFPQ/NONO can act on hormone receptors such as the thyroid and retinoid X receptors.51 In some cases, NONO represses transcription by sequestering activators away from target promoters. For instance, SFPQ/NONO prevents the progesterone receptor to bind to PR DNA, subsequently represses the transcription.52
mRNA splicing is a critical step in the post‐transcriptional gene regulation and expands functional proteome in eukaryotes. NONO has been identified in splicing‐related complexes in a proteomic study,31 it is homologous to another splicing‐related protein SFPQ/PSF and they associate with each other.29 NONO is not an essential component in spliceosome assembly or splicing53; however, it interacts with other spliceosomes and promotes splicing via the distal 5′ splicing site in pre‐mRNA alternative splicing.30, 53, 54 NONO also additionally interacts with the C‐terminal of RNA pol IIa and IIo.30, 55 In some conditions, NONO can also interact with 3′ end of mRNA splicing such as TNF‐a,56 the exonuclease XRN2,35 the snRNP‐free U1A57 and GLA (α‐galactosidase A).58
When it interacts with the C‐terminal of RNA pol II (RNAPII), NONO not only regulates the mRNA splicing, but also associates with transcriptional elongation and termination.30 Sometimes, NONO/SFPQ interacts with C‐terminal of both phosphorylated and unphosphorylated RNAPII and nascent RNA in the same time, because differently phosphorylated forms of RNAPII associate with the dynamic mRNA processing.59
NONO is also snRNA export stimulatory factor, accelerating the recruitment of PHAX for efficient nuclear export of snRNA, NONO and PSF form a heterodimer in this step.37
5. PHYSIOLOGICAL FUNCTIONS OF NONO
5.1. Cell proliferation
To date, it is known that NONO can induce/promote cell proliferation in a wide variety of cell types including tumour cells. In Hela,12 MCF‐760 and Mel Im11 cells, NONO silencing reduces the cell proliferation rate, and NONO‐silenced cells have a delayed G1/S transition,12 whereas the two other DBHS proteins have not significant effects on the growth of MCF‐7 cells.60 Further studies demonstrated that NONO promotes breast cancer cell proliferation through sterol regulatory element‐binding protein a (SREBP‐a).60 SREBPs are transcription factors that bind to the sterol regulatory element DNA sequence TCACNCCAC and activate the transcription of genes associated with the biosynthesis of fatty acids and cholesterol.61, 62 NONO regulates SREBP‐1a protein levels in the nucleus through a post‐transcriptional mechanism.60 Erk1/2/MAPK and PI3K/AKT activation are frequent events in oesophageal squamous cell carcinoma (ESCC), and the expression levels of phosphorylated (activated state) Erk1/2 and AKT are dramatically decreased in NONO knock‐down cells.63 Taken together, the Erk1/2 and PI3K/AKT pathways may be required for the NONO‐regulated growth of ESCC cells,63 unfortunately the exact mechanism is still not elucidated.
NONO also can inhibit cell proliferation in certain conditions. NONO was proved as a transcriptional activator of p16‐INK4A, an important checkpoint gene‐associated cell cycle. The fibroblast deficient of NONO shows increased proliferation due to low levels of p16‐INK4A. In some breast cancers, lower NONO is associated with increased proliferation.64, 65 The THP1 cell knock downed NONO shows a decrease of G0/G1 phase cells and an increase of S and G2/M phase cells compared with wild‐type or negative control cells, which indicates the knock‐down of NONO can accelerate THP1 cell cycle.66 Thus, NONO plays dual roles as either a promoter or inhibitor of cell proliferation.
5.2. Apoptosis
Similar to its effect on proliferation, NONO plays a dual effect on apoptosis. In ESCC,63 melanoma11 and NONOgt mice cells, NONO knock‐down/deficiency significantly increases cell apoptosis, including early apoptosis and late apoptosis; moreover, the apoptosis was mediated by the activation of caspase‐3 and then led to PARP binding to target DNA.63 Polypyrimidine tract‐binding protein (PTB) is critical in apoptosis.67 Remodelling of a PTB‐containing complex occurs following treatment to induce apoptosis, meanwhile, the IRES (Internal Ribosome Entry Segment)‐inhibitory protein hnRNPA1 decreases in association with PTB. NONO interferes with the association between hnRNPA1 and PTB, then stabilize hnRNPA1 resulting acceleration of apoptosis rates via changing gene expression at post‐transcriptional level.67
5.3. DNA damage repair
Homologous recombination (HR) and non‐homologous end joining (NHEJ) are two primary double‐strand break (DSB) repair pathways. The NONO/PSF complex is a principal candidate and component of the end‐joining stimulatory fraction that cooperates with other proteins known to participate in NHEJ in vivo,68 NONO knock‐down delays NHEJ kinetics in vitro.7, 69 Although NONO knock‐down has no effects on long‐term survival of cells, attenuated NONO expression can sensitize cells to ionizing irradiation, suggesting that NONO is crucial for DNA DSB repair.70 NONO knock‐down delays the resolution of γH2AX foci, increases chromosomal aberrations at the first metaphase following radiation exposure, impairs the recovery from DNA damage,39 and decreases clonogenic survival in vivo.40 Proteins involved in DSB repair via NHEJ co‐immunoprecipitate with NONO, and rapid recruitment of SFPQ·NONO to DNA damage sites are found after U2OS cells are induced by a laser microbeam,39 suggesting that the SFPQ·NONO complex is involved in the early stages of the DSB response.39 NONO is also a PAR (poly(ADP‐ribose))‐binding protein and its recruitment to DNA damage sites is PAR‐dependent.70 In the other stable reporter cell line, which can monitor HR repair pathway, knock‐down of NONO shows up‐regulation of HR by ~40%. Taken together, NONO not only facilitates NHEJ but also represses the other major DSB repair pathway (HR). Interestingly, NONO promotes NHEJ and represses HR in vivo in the same pathway as PARP‐1.70
5.4. Cell migration
NONO is strongly expressed in melanoma,11 mPCa (metastatic prostate cancer)71 and ESCC,63 and its knock‐down significantly reduces cell migration. NONO knock‐down enhances cells attachment to laminin, poly‐l‐lysine11 and the surface of culture plates,63 but interestingly, it has no effects with regard to fibronectin. These results suggest that NONO influences the ability to attach to components of the extracellular matrix.
A large number of lncRNAs are pervasively transcribed from the human genome, and aberrant expression of lncRNAs may cause abnormal cell functions, leading to various pathological conditions including cancer metastasis.72 The increased expression of GAPLINC (gastric adenocarcinoma predictive long intergenic noncoding) RNA was found to be positively correlated high metastasis, NONO protein bound to GAPLINC and reversed the cell invasion effects.73 Another lncRNA, MetaLnc9 is correlated with cell migration, NONO interacts with MetaLnc9 and reinforces a positive feedback loop for metastasis as a coactivator for the transcription factor CREB (cAMP response element‐binding protein).74 Nevertheless, the detailed molecular mechanism is still not elucidated.
6. NONO AND CANCERS
To date, all DBHS proteins have been found to be associated with cancers as either oncogenes or tumour suppressors, including NONO.6 Emerging evidence has demonstrated that NONO is overexpressed in various kinds of cancers, including bladder cancer,75 lung cancer,74, 76 prostate cancer77, 78 and ESCC.63 Furthermore, NONO protein level is an independent prognostic factor for some cancers.71, 79, 80, 81 By contrast, NONO is usually down‐regulated in ER (oestrogen receptor)‐negative breast cancer. At the gene level, renal cell carcinoma (RCC) is associated with TFE3 (transcription factor E3)‐NONO fusion.82
6.1. Chromosomal translocation/fusion involving with NONO gene and papillary renal cell carcinoma
Renal cell carcinoma is the most common cancer of the kidney. Based on the histological appearance, RCC can be divided into several subsets including papillary renal cell carcinoma which is characterized by the expression of TFE3 fusion proteins. The TFE3‐NONO gene fusion was described for the first time in 199783 (Figure 2:12). The X chromosome inversion inv(X) (p11.2; q12), which results in the fusion of the NONO gene and TFE3, is a cytogenetically defined translocation. In 2016, the TFE3‐NONO RCC morphology was described for the first time, showing subnuclear vacuoles that lead to frequent distinctive suprabasal nuclear palisading.84 Furthermore, fusion of TFE3 and NONO is associated with the loss of normal TFE3/NONO transcripts.
6.2. The CREB‐NONO axis in lung cancer
The CREB has two coactivator families, CBP/p300 and TORCs. The cAMP signal‐transduction pathway can activate transcription by stimulating interactions between CREB, CBP/p300 and TORCs.85, 86, 87 NONO is a TORC2 interactor and can act as a bridge between the CREB/TORC complex and RNA polymerase II to regulate cAMP‐mediated transcription. In A549 cells, the interaction of Gal4‐NONO and CRTC (CREB‐regulated transcription coactivator) is reduced after depletion of LINC00473, which encodes an intergenic lncRNA from the chromosome 6q27 locus. In contrast, LINC00473 overexpression promotes NONO‐CRTC interaction, suggesting that LINC00473 facilitates the interaction of NONO and CRTC and subsequently promotes the cAMP‐mediated transcription of various genes.76 Lung cancer patients with high LINC00473 expression had more aggressive pathological behaviours and shorter survival times by enhancing the interaction between NONO and CRTC.76 Another lncRNA, MetaLnc9, is overexpressed in non‐small cell lung cancer (NSCLC), subsequently causing poor prognosis and enhanced metastasis formation in patients with NSCLC. Like LINC00473, MetaLnc9 also interacts with NONO to promote the CRTC‐mediated transcription of CREB that offers a positive feedback loop for metastasis.74
6.3. Breast cancer and NONO: the dual function
Tumorigenesis is a multi‐step process from normal cells to malignancy.88 Some proteins, such as p5389 and p21,90 have dual‐ or multi‐function in a context‐dependent manner. Likewise, NONO was identified as a cancer promoter or suppressor depending on the breast cancer subtype. There are statistically significant association results between NONO expression, tumour hormonal phenotype and mean tumour size.65 In contrast to ER + human breast tumours, NONO protein expression was decreased in ER‐ breast cancers.64 Moreover, NONO expression in ER‐ breast cancers or NONO variants in ER + cancers might inform on breast tumour progression.64
6.4. NONO endowing prostate cancer with drug resistance
PCa is one of the most commonly diagnosed cancers in men worldwide and a leading cause of cancer‐related death.91 NONO is usually overexpressed in human prostate cancer,78, 92 and pathological results demonstrated that higher NONO expression correlated with poor prognosis. The relationship between NONO and AR shows a positive correlation too,92 and NONO knock‐down can effectively reduce the expression of AR/AR‐V7 at the mRNA and protein levels.71 Hormone therapy is an important method to treat prostate cancers,93, 94 and androgen deprivation therapy (ADT) is the main treatment for aggressive PCa.95 Unfortunately, like many other cancer types, resistance is a frequent event associated with PCa, such as castration‐resistant prostate cancer (CRPC).96, 97 Prostate cancer gene expression marker 1, also called PCGEM1, is a lncRNA which is often up‐regulated in prostate cancers and has been implicated in resistance to anticancer drug‐induced apoptosis.98 In CRPCs, NONO induces PCGEM1 expression and subsequently up‐regulates AR level, which promotes castration‐resistance in PCa.71, 77, 78
6.5. NONO regulating the progression of melanoma
MIA, which is secreted by melanoma cells, has been used as a tumour marker. Increased MIA serum level is related to metastatic disease or tumour recurrence99; it also likely represents a key molecule that regulates melanoma progression.100 NONO is a downstream target of MIA, and MIA knock‐down reduces NONO expression at both the mRNA and protein levels. NONO has its own downstream targets, such as Cx‐43, which allows for intercellular gap junction communication between cells to regulate cell death, proliferation and differentiation.101 NONO is highly expressed in malignant melanoma compared with melanocytes, which subsequently inhibits Cx‐43 expression. NONO not only plays an important role in the early steps of tumour formation and in the anti‐apoptosis process but also influences the migratory potential of melanoma cells; therefore, NONO may be involved in the MIA‐mediated metastasis of melanoma cells.11 Exposure to ultraviolet (UV) radiation, namely UVA (315‐400 nm) and UVB (280‐315 nm), is a major risk factor for melanoma development, as it can cause direct DNA damage.102 The NONO/PSF complex is identified as a stimulatory fraction repair system in mammalian cells that promotes DSB repair, thus confers increased radio‐sensitivity to cells,40 while NONO silencing affects the UVC‐induced DNA damage response in melanoma cells.12 Thus, its involvement in the rapid and accurate repair of DSBs makes NONO an efficient target of radiosensitizers.
6.6. Others
Colorectal cancer is one of the most commonly occurring cancers (6.1% of total diagnosed cases and 9.2% of total cancer deaths).91 YB‐1 can induce oxaliplatin resistance by interacting with NONO and RALY in colorectal cancer cells. Thus, knock‐down of NONO/RALY significantly sensitized YB‐1‐overexpressing colorectal cancer cells to oxaliplatin treatment.103 Additionally, GAPLINC binds to NONO/PSF, subsequently promoting cancer metastasis.73
As a protooncogene, Spi‐a/PU.1, an Ets‐related transcription factor, is usually overexpressed in Friend erythroleukaemia.104 Up‐regulated Spi‐1/PU.1 induces Friend erythroleukaemia via its interaction with NONO protein. Mechanistically, NONO binds Spi‐1/PU.1 via its RNA binding domain, and NONO splicing function is interfered by this binding.33 In human acute monocytic leukaemia THP1 cells, NONO is strongly expressed, while knock‐down of NONO slightly promotes cell proliferation but strongly inhibits motricity and invasion.
In primary neuroblastoma, high NONO expression level is correlated with N‐Myc expression; associated with poor patient prognoses; strongly associated with reduced overall survival; and independent of disease stage, age at the time of diagnosis and MYCN amplification.79 Differential proteomic analysis in bladder cancer demonstrated that NONO is strongly correlated with vascular invasions and appeared to be significantly (P < .0001) associated with a decreased probability of survival.75 Another computational genomic analysis demonstrated that NONO is significantly overexpressed in malignant pleural mesothelioma (MPM) and that NONO‐induced suppression of collagen biogenesis could be a nodal event in MPM.105
7. CONCLUSION AND OUTLOOK
We have summarized the evidence that NONO plays important roles in human tumorigenesis. Unlike most ‘normal’ tumour/anti‐tumour proteins, NONO has roles in tumorigenesis not only at the protein level but also at the DNA level. NONO is one of partner genes that has been identified as a fusion partner of TFE3 in RCC. Whether NONO has other gene partners or whether other cancers can occur from gene fusions involving NONO is currently unresolved. Apparently, NONO has specific roles under different contexts. The literatures examined in our review support the notion that NONO is overexpressed in most cancers, induces/promotes cell proliferation, inhibits apoptosis, impairs DNA damage repair and has other roles promoting tumorigenesis. Meanwhile, there are some cancers in which NONO is down‐regulated, and these lower NONO levels also promote cancer progression. The best example of this relationship is breast cancer, in which the loss or change in NONO expression, along with the loss of ER, results in more aggressive forms of the disease. Another interesting area is the regulation of NONO at the transcriptional and/or translational level. Not all cell lines revealed a clear correlation between NONO mRNA and protein expression levels, demonstrating that NONO could be likely post‐transcriptionally regulated.11 Recently, two E3 ubiquitin ligases were proven to mediate NONO ubiquitination,14, 28 though whether there is a deubiquitylase for NONO is still unclear. NONO is primarily distributed within the nucleus, although it is also found in the cytoplasm, and it increases gradually as breast cancer progresses.106 However, understanding the particular roles of NONO DNA, RNA, and protein in various cell processes in detail will require more studies in the future. Better understanding of the context of NONO’s functions in cells and tumorigenesis will make it therapeutically invaluable.
CONFLICT OF INTERESTS
The authors declare that no competing financial interests exist.
AUTHOR CONTRIBUTIONS
Mao Ye and Lei Zhang involved in the conception and design of the study. Peifu Feng, Ling Li, Tanggang Deng, Yan Liu, Neng Ling, Siyuan Qiu, Lin Zhang, Bo Peng, Wei Xiong, Lanqin Cao collected and assembled the data. Peifu Feng, Mao Ye, Ling Li and Lei Zhang interpreted and analysed the data.: Peifu Feng and Mao Ye involved in the writing of the manuscript. Mao Ye and Lei Zhang contributed to the administrative support. All authors involved in the final approval of the manuscript.
Feng P, Li L, Deng T, et al. NONO and tumorigenesis: More than splicing. J Cell Mol Med. 2020;24:4368–4376. 10.1111/jcmm.15141
Peifu Feng and Ling Li contributed equally to this work.
Contributor Information
Lei Zhang, Email: zhanglei_xp@163.com.
Mao Ye, Email: goldleaf@hnu.edu.cn.
REFERENCES
- 1. Dong B, Horowitz DS, Kobayashi R, Krainer AR. Purification and cDNA cloning of HeLa cell p54nrb, a nuclear protein with two RNA recognition motifs and extensive homology to human splicing factor PSF and Drosophila NONA/BJ6. Nucleic Acids Res. 1993;21:4085‐4092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Fox AH, Lamond AI. Paraspeckles. Cold Spring Harb Perspect Biol. 2010;2:a000687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Lee M, Sadowska A, Bekere I, et al. The structure of human SFPQ reveals a coiled‐coil mediated polymer essential for functional aggregation in gene regulation. Nucleic Acids Res. 2015;43:3826‐3840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Passon DM, Lee M, Rackham O, et al. Structure of the heterodimer of human NONO and paraspeckle protein component 1 and analysis of its role in subnuclear body formation. Proc Natl Acad Sci USA. 2012;109:4846‐4850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Knott GJ, Lee M, Passon DM, Fox AH, Bond CS Caenorhabditis elegans NONO‐1: insights into DBHS protein structure, architecture, and function. Protein Sci. 2015;24:2033‐2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Knott GJ, Bond CS, Fox AH. The DBHS proteins SFPQ, NONO and PSPC1: a multipurpose molecular scaffold. Nucleic Acids Res. 2016;44:3989‐4004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kowalska E, Ripperger JA, Hoegger DC, et al. NONO couples the circadian clock to the cell cycle. Proc Natl Acad Sci USA. 2013;110:1592‐1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Hata K, Nishimura R, Muramatsu S, et al. Paraspeckle protein p54nrb links Sox9‐mediated transcription with RNA processing during chondrogenesis in mice. J Clin Invest. 2008;118:3098‐3108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hui DY. A no‐no for NonO and JNK in extracellular matrix homeostasis and vascular stability. Arterioscler Thromb Vasc Biol. 2007;27:1677‐1678. [DOI] [PubMed] [Google Scholar]
- 10. Ren Z, Wang Z, Hu Z, et al. Decreased expression of P54(nrb)/NonO correlates with collagen deposition and fibrosis in human aortic dissection. Histopathology. 2014;65:570‐580. [DOI] [PubMed] [Google Scholar]
- 11. Schiffner S, Zimara N, Schmid R, Bosserhoff AK. p54nrb is a new regulator of progression of malignant melanoma. Carcinogenesis. 2011;32:1176‐1182. [DOI] [PubMed] [Google Scholar]
- 12. Alfano L, Costa C, Caporaso A, et al. NONO regulates the intra‐S‐phase checkpoint in response to UV radiation. Oncogene. 2016;35:567‐576. [DOI] [PubMed] [Google Scholar]
- 13. Alfano L, Costa C, Caporaso A, Antonini D, Giordano A, Pentimalli F. HUR protects NONO from degradation by mir320, which is induced by p53 upon UV irradiation. Oncotarget. 2016;7:78127‐78139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Deshar R, Yoo W, Cho EB, Kim S, Yoon JB. RNF8 mediates NONO degradation following UV‐induced DNA damage to properly terminate ATR‐CHK1 checkpoint signaling. Nucleic Acids Res. 2019;47:762‐778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Proteau A, Blier S, Albert AL, Lavoie SB, Traish AM, Vincent M. The multifunctional nuclear protein p54nrb is multiphosphorylated in mitosis and interacts with the mitotic regulator Pin1. J Mol Biol. 2005;346:1163‐1172. [DOI] [PubMed] [Google Scholar]
- 16. Liu L, Xie N, Rennie P, et al. Consensus PP1 binding motifs regulate transcriptional corepression and alternative RNA splicing activities of the steroid receptor coregulators, p54nrb and PSF. Mol Endocrinol. 2011;25:1197‐1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Roberts EC, Hammond K, Traish AM, Resing KA, Ahn NG. Identification of G2/M targets for the MAP kinase pathway by functional proteomics. Proteomics. 2006;6:4541‐4553. [DOI] [PubMed] [Google Scholar]
- 18. Casado P, Prado MA, Zuazua‐Villar P, et al. Microtubule interfering agents and KSP inhibitors induce the phosphorylation of the nuclear protein p54(nrb), an event linked to G2/M arrest. J Proteomics. 2009;71:592‐600. [DOI] [PubMed] [Google Scholar]
- 19. Wiktorowicz JE, Chowdhury IH, Stafford S, Choudhuri S, Dey N, Garg NJ. Integrated functional analysis of the nuclear proteome of classically and alternatively activated macrophages. Mediators Inflamm. 2019;2019:3481430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bruelle C, Bedard M, Blier S, Gauthier M, Traish AM, Vincent M. The mitotic phosphorylation of p54(nrb) modulates its RNA binding activity. Biochem Cell Biol. 2011;89:423‐433. [DOI] [PubMed] [Google Scholar]
- 21. Otto H, Dreger M, Bengtsson L, Hucho F. Identification of tyrosine‐phosphorylated proteins associated with the nuclear envelope_nouse. Eur J Biochem. 2001;268:420‐428. [DOI] [PubMed] [Google Scholar]
- 22. Lee AR, Hung W, Xie N, Liu L, He L, Dong X. Tyrosine residues regulate multiple nuclear functions of P54nrb. J Cell Physiol. 2017;232:852‐861. [DOI] [PubMed] [Google Scholar]
- 23. Kim YM, Seo J, Kim YH, et al. Systemic analysis of tyrosine phosphorylated proteins in angiopoietin‐1 induced signaling pathway of endothelial cells. J Proteome Res. 2007;6:3278‐3290. [DOI] [PubMed] [Google Scholar]
- 24. Hu SB, Xiang JF, Li X, et al. Protein arginine methyltransferase CARM1 attenuates the paraspeckle‐mediated nuclear retention of mRNAs containing IRAlus. Genes Dev. 2015;29:630‐645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Richard P, Vethantham V, Manley JL. Roles of sumoylation in mRNA processing and metabolism. Adv Exp Med Biol. 2017;963:15‐33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zhang Y, Wang J, Ding M, Yu Y. Site‐specific characterization of the Asp‐ and Glu‐ADP‐ribosylated proteome. Nat Methods. 2013;10:981‐984. [DOI] [PubMed] [Google Scholar]
- 27. Choudhary C, Kumar C, Gnad F, et al. Lysine acetylation targets protein complexes and co‐regulates major cellular functions. Science. 2009;325:834‐840. [DOI] [PubMed] [Google Scholar]
- 28. Alfano L, Caporaso A, Altieri A, et al. NONO ubiquitination is mediated by FBW7 and GSK3 beta via a degron lost upon chromosomal rearrangement in cancer. J Cell Physiol. 2018;233:4338‐4344. [DOI] [PubMed] [Google Scholar]
- 29. Shav‐Tal Y, Zipori D. PSF and p54(nrb)/NonO–multi‐functional nuclear proteins. FEBS Lett. 2002;531:109‐114. [DOI] [PubMed] [Google Scholar]
- 30. Emili A, Shales M, McCracken S, et al. Splicing and transcription‐associated proteins PSF and p54nrb/nonO bind to the RNA polymerase II CTD. RNA. 2002;8:1102‐1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Peng R, Hawkins I, Link AJ, Patton JG. The splicing factor PSF is part of a large complex that assembles in the absence of pre‐mRNA and contains all five snRNPs. RNA Biol. 2006;3:69‐76. [DOI] [PubMed] [Google Scholar]
- 32. Basu A, Dong B, Krainer AR, Howe CC. The intracisternal A‐particle proximal enhancer‐binding protein activates transcription and is identical to the RNA‐ and DNA‐binding protein p54nrb/NonO. Mol Cell Biol. 1997;17:677‐686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Hallier M, Tavitian A, Moreau‐Gachelin F. The transcription factor Spi‐1/PU.1 binds RNA and interferes with the RNA‐binding protein p54nrb. J Biol Chem. 1996;271:11177‐11181. [DOI] [PubMed] [Google Scholar]
- 34. Yadav SP, Hao H, Yang HJ, et al. The transcription‐splicing protein NonO/p54nrb and three NonO‐interacting proteins bind to distal enhancer region and augment rhodopsin expression. Hum Mol Genet. 2014;23:2132‐2144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kaneko S, Rozenblatt‐Rosen O, Meyerson M, Manley JL. The multifunctional protein p54nrb/PSF recruits the exonuclease XRN2 to facilitate pre‐mRNA 3' processing and transcription termination. Genes Dev. 2007;21:1779‐1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Peng R, Dye BT, Perez I, Barnard DC, Thompson AB, Patton JG. PSF and p54nrb bind a conserved stem in U5 snRNA. RNA. 2002;8:1334‐1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Izumi H, McCloskey A, Shinmyozu K, Ohno M. p54nrb/NonO and PSF promote U snRNA nuclear export by accelerating its export complex assembly. Nucleic Acids Res. 2014;42:3998‐4007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Straub T, Knudsen BR, Boege F. PSF/p54(nrb) stimulates "jumping" of DNA topoisomerase I between separate DNA helices. Biochemistry. 2000;39:7552‐7558. [DOI] [PubMed] [Google Scholar]
- 39. Salton M, Lerenthal Y, Wang SY, Chen DJ, Shiloh Y. Involvement of Matrin 3 and SFPQ/NONO in the DNA damage response. Cell Cycle. 2010;9:1568‐1576. [DOI] [PubMed] [Google Scholar]
- 40. Li S, Kuhne WW, Kulharya A, et al. Involvement of p54(nrb), a PSF partner protein, in DNA double‐strand break repair and radioresistance. Nucleic Acids Res. 2009;37:6746‐6753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Torres M, Kramer A. Mealtime is NONO speckled: timing hepatic adaptation to food. Cell Metab. 2018;27:268‐270. [DOI] [PubMed] [Google Scholar]
- 42. Park Y, Lee JM, Hwang MY, Son GH, Geum D. NonO binds to the CpG island of oct4 promoter and functions as a transcriptional activator of oct4 gene expression. Mol Cells. 2013;35:61‐69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Amelio AL, Miraglia LJ, Conkright JJ, et al. A coactivator trap identifies NONO (p54nrb) as a component of the cAMP‐signaling pathway. Proc Natl Acad Sci USA. 2007;104:20314‐20319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Kuwahara S, Ikei A, Taguchi Y, et al. PSPC1, NONO, and SFPQ are expressed in mouse Sertoli cells and may function as coregulators of androgen receptor‐mediated transcription. Biol Reprod. 2006;75:352‐359. [DOI] [PubMed] [Google Scholar]
- 45. Ishitani K, Yoshida T, Kitagawa H, Ohta H, Nozawa S, Kato S. p54nrb acts as a transcriptional coactivator for activation function 1 of the human androgen receptor. Biochem Biophys Res Commun. 2003;306:660‐665. [DOI] [PubMed] [Google Scholar]
- 46. Song KS, Kim K, Chung KC, Seol JH, Yoon JH. Interaction of SOCS3 with NonO attenuates IL‐1beta‐dependent MUC8 gene expression. Biochem Biophys Res Commun. 2008;377:946‐951. [DOI] [PubMed] [Google Scholar]
- 47. Adegbola O, Pasternack GR. A pp32‐retinoblastoma protein complex modulates androgen receptor‐mediated transcription and associates with components of the splicing machinery. Biochem Biophys Res Commun. 2005;334:702‐708. [DOI] [PubMed] [Google Scholar]
- 48. Ju BG, Solum D, Song EJ, et al. Activating the PARP‐1 sensor component of the groucho/ TLE1 corepressor complex mediates a CaMKinase IIdelta‐dependent neurogenic gene activation pathway. Cell. 2004;119:815‐829. [DOI] [PubMed] [Google Scholar]
- 49. Sewer MB, Waterman MR. Adrenocorticotropin/cyclic adenosine 3',5'‐monophosphate‐mediated transcription of the human CYP17 gene in the adrenal cortex is dependent on phosphatase activity. Endocrinology. 2002;143:1769‐1777. [DOI] [PubMed] [Google Scholar]
- 50. Sewer MB, Nguyen VQ, Huang CJ, Tucker PW, Kagawa N, Waterman MR. Transcriptional activation of human CYP17 in H295R adrenocortical cells depends on complex formation among p54(nrb)/NonO, protein‐associated splicing factor, and SF‐1, a complex that also participates in repression of transcription. Endocrinology. 2002;143:1280‐1290. [DOI] [PubMed] [Google Scholar]
- 51. Mathur M, Tucker PW, Samuels HH. PSF is a novel corepressor that mediates its effect through Sin3A and the DNA binding domain of nuclear hormone receptors. Mol Cell Biol. 2001;21:2298‐2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Dong X, Shylnova O, Challis JR, Lye SJ. Identification and characterization of the protein‐associated splicing factor as a negative co‐regulator of the progesterone receptor. J Biol Chem. 2005;280:13329‐13340. [DOI] [PubMed] [Google Scholar]
- 53. Zhang WJ, Wu JY. Functional properties of p54, a novel SR protein active in constitutive and alternative splicing. Mol Cell Biol. 1996;16:5400‐5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Kameoka S, Duque P, Konarska MM. p54(nrb) associates with the 5' splice site within large transcription/splicing complexes. EMBO J. 2004;23:1782‐1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Hirose Y, Manley JL. RNA polymerase II and the integration of nuclear events. Genes Dev. 2000;14:1415‐1429. [PubMed] [Google Scholar]
- 56. Buxade M, Morrice N, Krebs DL, Proud CG. The PSF.p54nrb complex is a novel Mnk substrate that binds the mRNA for tumor necrosis factor alpha. J Biol Chem. 2008;283:57‐65. [DOI] [PubMed] [Google Scholar]
- 57. Liang S, Lutz CS. p54nrb is a component of the snRNP‐free U1A (SF‐A) complex that promotes pre‐mRNA cleavage during polyadenylation. RNA. 2006;12:111‐121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Chang WH, Niu DM, Lu CY, Lin SY, Liu TC, Chang JG. Modulation the alternative splicing of GLA (IVS4+919G>A) in Fabry disease. PLoS One. 2017;12:e0175929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Lu H, Flores O, Weinmann R, Reinberg D. The nonphosphorylated form of RNA polymerase II preferentially associates with the preinitiation complex. Proc Natl Acad Sci USA. 1991;88:10004‐10008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Zhu Z, Zhao X, Zhao L, et al. p54(nrb)/NONO regulates lipid metabolism and breast cancer growth through SREBP‐1A. Oncogene. 2016;35:1399‐1410. [DOI] [PubMed] [Google Scholar]
- 61. Yokoyama C, Wang X, Briggs MR, et al. SREBP‐1, a basic‐helix‐loop‐helix‐leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell. 1993;75:187‐197. [PubMed] [Google Scholar]
- 62. Zhao X, Xiaoli , Zong H, et al. Inhibition of SREBP transcriptional activity by a boron‐containing compound improves lipid homeostasis in diet‐induced obesity. Diabetes. 2014;63:2464‐2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Cheng R, Zhu S, Guo S, et al. Downregulation of NONO induces apoptosis, suppressing growth and invasion in esophageal squamous cell carcinoma. Oncol Rep. 2018;39:2575‐2583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Pavao M, Huang YH, Hafer LJ, Moreland RB, Traish AM. Immunodetection of nmt55/p54nrb isoforms in human breast cancer. BMC Cancer. 2001;1:15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Traish AM, Huang YH, Ashba J, et al. Loss of expression of a 55 kDa nuclear protein (nmt55) in estrogen receptor‐negative human breast cancer. Diagn Mol Pathol. 1997;6:209‐221. [DOI] [PubMed] [Google Scholar]
- 66. Zhang X, Wu C, Xiong W, Chen C, Li R, Zhou G. Knockdown of p54nrb inhibits migration, invasion and TNF‐alpha release of human acute monocytic leukemia THP1 cells. Oncol Rep. 2016;35:3742‐3748. [DOI] [PubMed] [Google Scholar]
- 67. King HA, Cobbold LC, Pichon X, et al. Remodelling of a polypyrimidine tract‐binding protein complex during apoptosis activates cellular IRESs. Cell Death Differ. 2014;21:161‐171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Bladen CL, Udayakumar D, Takeda Y, Dynan WS. Identification of the polypyrimidine tract binding protein‐associated splicing factor p54(nrb) complex as a candidate DNA double‐strand break rejoining factor. J Biol Chem. 2005;280:5205‐5210. [DOI] [PubMed] [Google Scholar]
- 69. Maier B, Kramer A. A NONO‐gate times the cell cycle. Proc Natl Acad Sci USA. 2013;110:1565‐1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Krietsch J, Caron MC, Gagne JP, et al. PARP activation regulates the RNA‐binding protein NONO in the DNA damage response to DNA double‐strand breaks. Nucleic Acids Res. 2012;40:10287‐10301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Takayama KI, Suzuki T, Fujimura T, et al. Dysregulation of spliceosome gene expression in advanced prostate cancer by RNA‐binding protein PSF. Proc Natl Acad Sci USA. 2017;114:10461‐10466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Koirala P, Zou D‐H, Mo Y‐Y. Long non‐coding RNAs as key regulators of cancer metastasis. J Cancer Metastasis Treat. 2016;2:1‐10. [Google Scholar]
- 73. Yang P, Chen T, Xu Z, Zhu H, Wang J, He Z. Long noncoding RNA GAPLINC promotes invasion in colorectal cancer by targeting SNAI2 through binding with PSF and NONO. Oncotarget. 2016;7:42183‐42194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Yu T, Zhao Y, Hu Z, et al. MetaLnc9 facilitates lung cancer metastasis via a PGK1‐activated AKT/mTOR pathway. Cancer Res. 2017;77:5782‐5794. [DOI] [PubMed] [Google Scholar]
- 75. Barboro P, Rubagotti A, Orecchia P, et al. Differential proteomic analysis of nuclear matrix in muscle‐invasive bladder cancer: potential to improve diagnosis and prognosis. Cell Oncol. 2008;30:13‐26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Chen Z, Li JL, Lin S, et al. cAMP/CREB‐regulated LINC00473 marks LKB1‐inactivated lung cancer and mediates tumor growth. J Clin Invest. 2016;126:2267‐2279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Ho TT, Huang J, Zhou N, et al. Regulation of PCGEM1 by p54/nrb in prostate cancer. Sci Rep. 2016;6:34529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Yamamoto R, Osawa T, Sasaki Y, et al. Overexpression of p54(nrb)/NONO induces differential EPHA6 splicing and contributes to castration‐resistant prostate cancer growth. Oncotarget. 2018;9:10510‐10524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Liu PY, Erriquez D, Marshall GM, et al. Effects of a novel long noncoding RNA, lncUSMycN, on N‐Myc expression and neuroblastoma progression. J Natl Cancer Inst. 2014;106(7). 10.1093/jnci/dju113 [DOI] [PubMed] [Google Scholar]
- 80. Iino K, Mitobe Y, Ikeda K, et al. RNA‐binding protein NONO promotes breast cancer proliferation via posttranscriptional regulation of SKP2 and E2F8. Cancer Sci. 2020;111:148‐159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Li D, Chen Y, Mei H, et al. Ets‐1 promoter‐associated noncoding RNA regulates the NONO/ERG/Ets‐1 axis to drive gastric cancer progression. Oncogene. 2018;37:4871‐4886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Xia QY, Wang Z, Chen N, et al. Xp11.2 translocation renal cell carcinoma with NONO‐TFE3 gene fusion: morphology, prognosis, and potential pitfall in detecting TFE3 gene rearrangement. Mod Pathol. 2017;30(3):416‐426. [DOI] [PubMed] [Google Scholar]
- 83. Clark J, Lu YJ, Sidhar SK, et al. Fusion of splicing factor genes PSF and NonO (p54nrb) to the TFE3 gene in papillary renal cell carcinoma. Oncogene. 1997;15:2233‐2239. [DOI] [PubMed] [Google Scholar]
- 84. Argani P, Zhong M, Reuter VE, et al. TFE3‐fusion variant analysis defines specific clinicopathologic associations among Xp11 translocation cancers. Am J Surg Pathol. 2016;40:723‐737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Chrivia JC, Kwok RP, Lamb N, Hagiwara M, Montminy MR, Goodman RH. Phosphorylated CREB binds specifically to the nuclear protein CBP. Nature. 1993;365:855‐859. [DOI] [PubMed] [Google Scholar]
- 86. Conkright MD, Canettieri G, Screaton R, et al. TORCs: transducers of regulated CREB activity. Mol Cell. 2003;12:413‐423. [DOI] [PubMed] [Google Scholar]
- 87. Iourgenko V, Zhang W, Mickanin C, et al. Identification of a family of cAMP response element‐binding protein coactivators by genome‐scale functional analysis in mammalian cells. Proc Natl Acad Sci USA. 2003;100:12147‐12152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646‐674. [DOI] [PubMed] [Google Scholar]
- 89. Sullivan KD, Galbraith MD, Andrysik Z, Espinosa JM. Mechanisms of transcriptional regulation by p53. Cell Death Differ. 2018;25:133‐143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Rowland BD, Peeper DS. KLF4, p21 and context‐dependent opposing forces in cancer. Nat Rev Cancer. 2006;6:11‐23. [DOI] [PubMed] [Google Scholar]
- 91. Bray F, Ferlay J, Soerjomataram I, Siegel RL, Torre LA, Jemal A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin. 2018;68:394‐424. [DOI] [PubMed] [Google Scholar]
- 92. Ishiguro H, Uemura H, Fujinami K, Ikeda N, Ohta S, Kubota Y. 55 kDa nuclear matrix protein (nmt55) mRNA is expressed in human prostate cancer tissue and is associated with the androgen receptor. Int J Cancer. 2003;105:26‐32. [DOI] [PubMed] [Google Scholar]
- 93. Yeap BB, Wu FCW. Clinical practice update on testosterone therapy for male hypogonadism: contrasting perspectives to optimize care. Clin Endocrinol. 2019;90:56‐65. [DOI] [PubMed] [Google Scholar]
- 94. Michaelson MD, Cotter SE, Gargollo PC, Zietman AL, Dahl DM, Smith MR. Management of complications of prostate cancer treatment. CA Cancer J Clin. 2008;58:196‐213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Massard C, Fizazi K. Targeting continued androgen receptor signaling in prostate cancer. Clin Cancer Res. 2011;17:3876‐3883. [DOI] [PubMed] [Google Scholar]
- 96. Yap TA, Smith AD, Ferraldeschi R, Al‐Lazikani B, Workman P, de Bono JS. Drug discovery in advanced prostate cancer: translating biology into therapy. Nat Rev Drug Discov. 2016;15:699‐718. [DOI] [PubMed] [Google Scholar]
- 97. Attard G, Parker C, Eeles RA, et al. Prostate cancer. Lancet. 2016;387:70‐82. [DOI] [PubMed] [Google Scholar]
- 98. Srikantan V, Zou Z, Petrovics G, et al. PCGEM1, a prostate‐specific gene, is overexpressed in prostate cancer. Proc Natl Acad Sci USA. 2000;97:12216‐12221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Odashiro M, Hans Filho G, Pereira PR, et al. Melanoma inhibitory activity in Brazilian patients with cutaneous melanoma. An Bras Dermatol. 2015;90:327‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bosserhoff AK. Melanoma inhibitory activity (MIA): an important molecule in melanoma development and progression. Pigment Cell Res. 2005;18:411‐416. [DOI] [PubMed] [Google Scholar]
- 101. Cheng JC, Chang HM, Fang L, Sun YP, Leung PC. TGF‐beta1 up‐regulates connexin43 expression: a potential mechanism for human trophoblast cell differentiation. J Cell Physiol. 2015;230:1558‐1566. [DOI] [PubMed] [Google Scholar]
- 102. Sample A, He YY. Mechanisms and prevention of UV‐induced melanoma. Photodermatol Photoimmunol Photomed. 2018;34:13‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Tsofack SP, Garand C, Sereduk C, et al. NONO and RALY proteins are required for YB‐1 oxaliplatin induced resistance in colon adenocarcinoma cell lines. Mol Cancer. 2011;10:145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Moreau‐Gachelin F, Tavitian A, Tambourin P. Spi‐1 is a putative oncogene in virally induced murine erythroleukaemias. Nature. 1988;331:277‐280. [DOI] [PubMed] [Google Scholar]
- 105. Vavougios GD, Solenov EI, Hatzoglou C, et al. Computational genomic analysis of PARK7 interactome reveals high BBS1 gene expression as a prognostic factor favoring survival in malignant pleural mesothelioma. Am J Physiol Lung Cell Mol Physiol. 2015;309:L677‐L686. [DOI] [PubMed] [Google Scholar]
- 106. Bi Y, Tian M, Le J, et al. Study on the expression of PAK4 and P54 protein in breast cancer. World J Surg Oncol. 2016;14:160. [DOI] [PMC free article] [PubMed] [Google Scholar]


