Abstract
Background
Neurocognitive impairments robustly predict functional outcome. However, heterogeneity in neurocognition is common within diagnostic groups, and data-driven analyses reveal homogeneous neurocognitive subgroups cutting across diagnostic boundaries.
Aims
To determine whether data-driven neurocognitive subgroups of young people with emerging mental disorders are associated with 3-year functional course.
Method
Model-based cluster analysis was applied to neurocognitive test scores across nine domains from 629 young people accessing mental health clinics. Cluster groups were compared on demographic, clinical and substance-use measures. Mixed-effects models explored associations between cluster-group membership and socio-occupational functioning (using the Social and Occupational Functioning Assessment Scale) over 3 years, adjusted for gender, premorbid IQ, level of education, depressive, positive, negative and manic symptoms, and diagnosis of a primary psychotic disorder.
Results
Cluster analysis of neurocognitive test scores derived three subgroups described as ‘normal range’ (n = 243, 38.6%), ‘intermediate impairment’ (n = 252, 40.1%), and ‘global impairment’ (n = 134, 21.3%). The major mental disorder categories (depressive, anxiety, bipolar, psychotic and other) were represented in each neurocognitive subgroup. The global impairment subgroup had lower functioning for 3 years of follow-up; however, neither the global impairment (B = 0.26, 95% CI −0.67 to 1.20; P = 0.581) or intermediate impairment (B = 0.46, 95% CI −0.26 to 1.19; P = 0.211) subgroups differed from the normal range subgroup in their rate of change in functioning over time.
Conclusions
Neurocognitive impairment may follow a continuum of severity across the major syndrome-based mental disorders, with data-driven neurocognitive subgroups predictive of functional course. Of note, the global impairment subgroup had longstanding functional impairment despite continuing engagement with clinical services.
Keywords: Social functioning, outcome studies, psychotic disorders, anxiety disorders, depressive disorders
Mental disorders are a leading cause of functional disability worldwide.1 Although the adverse impacts of these disorders on work, study and relationships are experienced across the lifespan, their significance is especially negative during the formative years of adolescence and young adulthood.1 Birth cohort studies show that a mental disorder such as anxiety or depression during adolescence is prognostic of a range of adverse life outcomes including reduced workforce participation, academic underachievement and welfare dependence.2–4 As early social and economic disengagement can have long-term scarring effects on later social and health outcomes,5 it is vital that we improve our understanding of the barriers to social and occupational functioning in young people in the early phases of mental disorders.
Neurocognition in mental disorders
One of the strongest predictors of social and occupational functioning in mental disorders is neurocognition. This relationship has high face validity – skills related to work, study and social interaction require an ability to learn and remember new information and flexibly shift processing across changing tasks and environments. Meta-analyses demonstrate that many individuals with depressive, bipolar and psychotic disorders have impairments of small-to-large magnitude across most measured neurocognitive domains,6–8 and mounting evidence shows that neurocognitive impairments limit adaptive functioning across these disorders.9–12 Importantly however, heterogeneity in neurocognition is common within the major mental disorders,13 and diagnosis-level analysis may obscure neurocognition–functioning relationships.
Data-driven neurocognitive subgroups in mental disorders
One potential way to reframe neurocognition in mental disorders is to search for subgroups with greater homogeneity than is found in the major diagnostic groupings. To this end, data-driven statistical techniques such as cluster analysis have been used to derive neurocognitive subgroups within samples of people with schizophrenia for three decades.14 Data-driven studies in schizophrenia and more broadly defined psychotic disorders have typically separated patients into subgroups of global neurocognitive impairment, normal range ability and mixed or intermediate profiles.14–17 Recently, evidence of similar subgroups have been shown within samples of participants with depressive18 and bipolar disorders,19 and notably, across broader samples comprised of people with multiple major diagnostic groups.20–23 Taken together, these findings of homogeneous subgroups within diagnostic groups suggest that neurocognitive impairment may follow a continuum of severity distributed across mental disorders, with data-driven subgroups potentially representing a more useful level of analysis as regards neurocognition and associated factors.
The current study
To date, the predictive utility of data-driven neurocognitive subgroups has not been robustly evaluated. Several studies have shown that neurocognitive subgroups within psychotic disorders have different levels of social and occupational functioning cross-sectionally,15,17 and one study has reported distinct courses of functioning over 6 months among neurocognitive subgroups with first-episode psychosis.16 Two questions with potential clinical implications remain unanswered. First: are neurocognitive subgroups associated with functional course for a greater duration than 6 months? And second: does the relationship between neurocognitive subgroups and functional course extend to broader transdiagnostic samples? Accordingly, this study aimed to determine whether data-driven neurocognitive subgroups of adolescents and young adults with emerging mental disorders are associated with distinct courses of social and occupational functioning over 3 years of contact with clinical services. Secondarily, we aimed to determine whether these subgroups differ in clinical or sociodemographic factors that may be modifiable or explain neurocognitive differences. Based on previous work,16 we expected that the subgroup with the greatest neurocognitive impairment would have the poorest course of functioning for at least 6 months.
Method
Participants
Participants were drawn from a cohort of 6743 consecutive referrals to youth mental health clinics at the Brain and Mind Centre in Sydney, Australia, who were recruited to a case register of adolescents and young adults with mood, psychotic, developmental and other mental disorders between 2004 and 2018 (‘Brain and Mind Research Institute Patient Research Register’).24 These clinics (for example headspace) provide youth-friendly and highly accessible early-intervention services for young people with emerging substance use and/or mental disorders, and primarily attracts young people with a range of subthreshold and threshold mental health problems (typically anxiety and mood syndromes).24 headspace consists of an integrated mix of primary-level services and more specialised services (for example psychiatry, drug and alcohol, occupational support), and all participants were receiving clinician-based case management and relevant social, psychological and/or medical treatments as part of standard care, which may have involved contact with a psychiatrist, psychologist, occupational therapist, support worker or admission to hospital for those whose need exceeded the capacity of the services.
Eligibility criteria
Eligibility criteria for this study were:
a neurocognitive assessment with no missing data across nine predetermined domains;
aged 12 to 30 years at the time of neurocognitive assessment;
a proforma assessment (see below) within 3 months of the neurocognitive assessment (see Iorfino et al25 for more detail); and
willing and able to give informed consent (and/or parental consent was obtained).
Exclusion criteria were:
history of neurological disease;
medical illness known to affect neurocognitive/brain function (for example cancer, epilepsy);
received electroconvulsive therapy in the 3 months prior to assessment;
clinically evident intellectual disability; and/or
insufficient understanding of the English language to allow participation in verbal assessments or testing.
Ethics approval and informed consent
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008. All procedures involving patients were approved by the University of Sydney Human Research Ethics Committee (project numbers: 2012/1626, 2012/1631). Written informed consent was obtained from participants aged 16 and older, and parental/guardian consent was obtained for participants younger than 16 years.
Outcome variable (longitudinal)
A standardised clinical proforma was used to gather retrospective demographic, clinical, and functioning data from clinical case files across up to eight predetermined time points (baseline, 3 months, 6 months, 1 year, 2 years, 3 years, 4 years and 5 years). The proforma collects standardised information25 regarding: (a) basic demographics; (b) mental health diagnoses (based on DSM-5 criteria26); (c) clinical course (for example admission to hospital); (d) comorbidities (such as physical health diagnoses); and (e) functioning. Phase I of data extraction of the Optymise cohort concluded in 2018 and comprised 2767 individuals who were initially recruited to the Brain and Mind Research Institute Patient Research Register (n = 6743).
The outcome variable for this study was social and occupational functioning, as measured by the Social and Occupational Functioning Assessment Scale (SOFAS).27 The SOFAS is a 100-point scale (higher scores denoting better functioning), with instructions that the rater avoid confounding the rating of functioning with symptoms. The SOFAS is widely used and has good construct validity,28 interrater reliability28 and predictive validity.29
Predictor variables (baseline)
A subset of the wider cohort participated in clinical and neurocognitive assessments between 2008 and 2015 as part of a neurobiological study. A board-certified neuropsychologist, research psychologist or supervised doctoral student administered the neurocognitive battery assessing the following domains: processing speed (Trail Making Test, part-A),30 cognitive flexibility (Trail Making Test, part-B),30 verbal learning (sum of trials 1–5 of the Rey Auditory Verbal Learning Test; RAVLT),31 verbal memory (20-minute delayed recall of the RAVLT),31 sustained attention (A’ Prime subtest of the Rapid Visual Information Processing Test),32 set-shifting (Intra-Extra Dimensional Set Shift),32 visuospatial memory (Paired Associates Learning Task),32 working memory (Spatial Span Task),32 and verbal fluency (Controlled Oral Word Association Test, letters).33 Premorbid intellectual functioning (premorbid IQ) was estimated using word-reading tests; the Wide Range Achievement Test (fourth edition)34 was used for participants younger than 16 years and the Wechsler Test of Adult Reading35 was used for participants older than 16 years. Neurocognitive test scores were standardised to age- and gender-matched norms (z-scores) using established criteria, as described previously.36,37 To avoid derivation of small, independent subgroups influenced by extreme scores,23 z-scores beyond 5.0 or −5.0 were winsorised to 5.0 or −5.0, depending on the direction.
The 24-item Brief Psychiatric Rating Scale (BPRS)38 measured symptom type and severity, with four dimensions derived (depressive, positive, negative and manic). The 10-item Kessler Psychological Distress scale (K10)39 measured perceived severity of psychological distress. Age at onset of psychiatric symptoms was self-reported, and duration of illness was estimated by subtracting age at onset from age at baseline assessment. The World Health Organization's Alcohol, Substance, and Smoking Involvement Screening Test version 2.0 (WHO-ASSIST 2.0)40 measured lifetime and recent (past 3 months) substance use. We added a question to item one (lifetime use) to estimate age of first use: ‘If yes, at what age did you first use?’. The 10-item Alcohol Use Disorders Identification Test (AUDIT)41 assessed severity of alcohol use.
As neurocognition was the key baseline predictor in this study, the nearest proforma assessment within 3 months of the neurocognitive assessment was selected as the participants’ baseline proforma time point (T1), and subsequent proforma time points were accordingly recoded. As we allowed a 3-month interval between the neurocognitive and proforma assessments, the 3-month proforma time point was excluded from analysis. The 4- and 5-year time points were also excluded from analysis as sample attrition exceeded 80%.
Statistical analysis
Analyses were performed using R statistical software, version 3.4.2 (R Foundation).42
Model-based cluster analysis
The mclust package,43 version 5.4.1, was used to derive subgroups of participants based on neurocognitive z-scores across nine domains. The ‘Mclust’ function uses mixture modelling via expectation-maximisation algorithms to iteratively fit a variety of covariance structures to the data, comparing Bayesian Information Criterion (BIC) values for each model to select the optimal data structure (a larger BIC indicates stronger evidence for a corresponding model). The ‘Mclust’ function fits 14 covariance structures across nine components (clusters) as default. Once an optimal solution was selected (based on model fit and parsimony), cluster groups were compared on sociodemographic and clinical factors using one-way analysis of variance for continuous variables and χ2-tests for categorical variables.
Mixed-effects modelling
Linear mixed-effects models were built using the nlme package, version 3.1-137,44 with missing follow-up data handled using maximum-likelihood estimation. The outcome variable was participants’ SOFAS scores at each time point. SOFAS scores for all available time points were used, and participants could contribute one or multiple scores over time.
Analyses were conducted sequentially. First, we built an unconditional model with random intercepts and no predictor variables, positing a linear trajectory in SOFAS over time across the sample. Next, we determined whether the model fit could be improved by fitting random slopes, with goodness-of-fit compared using the likelihood ratio test (LRT) statistic, which expresses how many times more likely the data are under one model relative to another. We then built a conditional model, testing interindividual differences in functioning at baseline and the rate of change in functioning over time as a function of several predetermined factors. A ‘time’ variable represented the time point of each SOFAS score and was coded numerically. To avoid listwise deletion of participants with missing predictors, we imputed missing predictor data with the sample mean before modelling (no more than 8% of data were missing for any predictor; see supplementary Table 1 available at https://doi.org/10.1192/bjo.2020.12). Normality of residuals was visually inspected using Q–Q plots, with an approximate normal distribution evident. Multicollinearity was evaluated using the variation inflation factor (VIF), with no predictor observed to have a VIF over 2. Model coefficients (B) are presenting alongside 95% confidence intervals, test statistic and parameter-specific P-values.
Results
Participant characteristics
A total of 2767 participants from the wider Optymise cohort had an available proforma assessment. Of these, 629 participants met all eligibility criteria (see supplementary Fig. 1 for participant flow). At baseline, there were 629 participants and of these 350 were female (55.6%) and 279 were male (44.4%), with a median age of 20 (interquartile range 6). More than 90% of the sample were aged 12–25 years.
The majority of participants presented with a primary mood or anxiety disorder (428/629, 68.0%). Numbers and proportions of each primary diagnostic group were: depressive disorders (244/629, 38.8%), anxiety disorders (96/629, 15.3%), bipolar and related disorders (88/629, 14.0%), schizophrenia spectrum and other psychotic disorders (82/629, 13.0%), neurodevelopmental disorders (37/629, 5.9%) disruptive, impulse-control and conduct disorders (20/629, 3.2%), substance-use and addictive disorders (12/629, 1.9%), trauma- and stressor-related disorders (12/629, 1.9%), obsessive–compulsive and related disorders (10/629, 1.6%), personality disorders (6/629, 1.0%) and feeding and eating disorders (4/629, 0.6%). There was a total of 18 participants who had no diagnosis or an uncertain diagnosis (2.9%).
Cluster solution
The results of the cluster analysis across the nine neurocognitive tests indicated that the optimal model was a seven-cluster solution with an ellipsoidal, equal orientation covariance structure and the second-best model was an eight-cluster solution with the same covariance structure (BICs for all solutions are presented in supplementary Table 2). However, the third best model was a three-cluster solution with the same covariance structure and a similar BIC (best: seven-cluster BIC = −16 147.2; second best: eight-cluster BIC = −16 233.29; third best: three-cluster BIC = −161 241.5). As the three-cluster solution was more parsimonious and largely capitulated the largest cluster groups from the other solutions, we selected the three-cluster solution.
Demographic and clinical characteristics of the three cluster groups
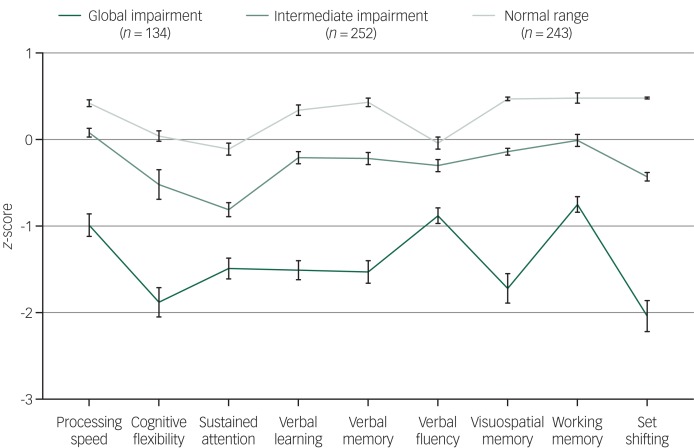
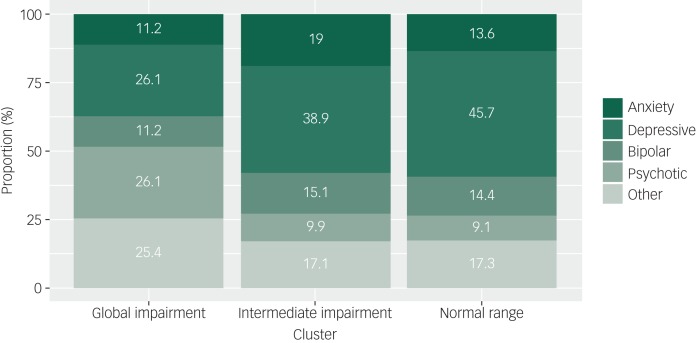
The three neurocognitive cluster groups were best described as ‘global impairment’ (n = 134; 21% total sample), ‘intermediate impairment’ (n = 252; 40% total sample), and ‘normal range’ (n = 243; 39% total sample) (Fig. 1). As shown in Table 1, cluster-group differences were observed for gender, level of education, premorbid IQ, baseline SOFAS, level of negative and positive symptom severity, and daily tobacco use (pairwise comparisons correcting for multiple comparisons are in Table 1). No significant differences were observed for age, level of depressive or manic symptom severity, psychological distress, self-reported age of psychiatric symptoms onset, estimated duration of illness, or any other substance use parameter. Of note, primary diagnostic groups were distributed across the three cluster groups, albeit unevenly (Table 2 and Fig. 2).
Fig. 1.
Baseline neurocognitive profiles (z-scores and s.e.) for the three cluster groups of 629 adolescents and young adults with emerging mental disorders.
Table 1.
Demographic, clinical, and substance use characteristics of three neurocognitive cluster groups
| Characteristic | Cluster 1, global impairment (n = 134) |
Cluster 2, intermediate impairment (n = 252) |
Cluster 3, normal range (n = 243) | Statistics | Effect size (Cohen's d) | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| d.f. | F or χ2 | P | 1 v. 2 | 2 v. 3 | 1 v. 3 | |||||||
| Demographic | ||||||||||||
| Age, years: mean (s.d.) | 19.9 | (3.7) | 19.8 | (4.2) | 20.1 | (4.1) | 2, 626 | 0.27 | 0.765 | – | – | – |
| Gender (female), n (%) | 51/134 | (38) | 145/252 | (58) | 154/243 | (63) | 2 | 23.04 | <0.001 | – | – | – |
| Education, years: mean (s.d.) | 11.4 | (2.4) | 11.6 | (2.5) | 12.2 | (2.5) | 2, 612 | 4.96 | 0.007 | 0.08 | 0.24* | 0.33* |
| Premorbid IQ, mean (s.d.) | 97.5 | (10.6) | 101.7 | (9.2) | 105.5 | (8.9) | 2, 589 | 30.50 | <0.001 | 0.42*** | 0.42*** | 0.82*** |
| SOFAS, mean (s.d.) | 55.9 | (9.8) | 59.8 | (9.6) | 62.8 | (9.4) | 2, 626 | 22.96 | <0.001 | 0.40*** | 0.32** | 0.72*** |
| Clinical status | ||||||||||||
| BPRS depressive, mean (s.d.) | 13.3 | (4.9) | 13.5 | (5.0) | 13.8 | (5.4) | 2, 576 | 0.53 | 0.588 | – | – | – |
| BPRS negative, mean (s.d.) | 8.3 | (3.3) | 6.9 | (2.6) | 7.0 | (2.7) | 2, 575 | 10.68 | <0.001 | 0.47*** | 0.04 | 0.43*** |
| BPRS positive, mean (s.d.) | 11.7 | (4.7) | 10.8 | (3.8) | 10.0 | (2.9) | 2, 577 | 8.24 | <0.001 | 0.21 | 0.24* | 0.44*** |
| BPRS mania, mean (s.d.) | 9.6 | (2.9) | 9.6 | (3.4) | 9.3 | (3.1) | 2, 583 | 0.68 | 0.507 | – | – | – |
| K10, mean (s.d.) | 28.0 | (9.6) | 27.6 | (8.4) | 27.3 | (8.1) | 2, 551 | 0.27 | 0.765 | – | – | – |
| Age at onset, mean (s.d.) | 15.8 | (4.5) | 14.8 | (4.3) | 15.0 | (4.2) | 2, 462 | 1.72 | 0.180 | – | – | – |
| Illness duration, mean (s.d.) | 4.4 | (3.7) | 5.3 | (4.2) | 4.9 | (3.8) | 2, 462 | 1.51 | 0.222 | |||
| Substance use | ||||||||||||
| Alcohol, AFU: mean (s.d.) | 14.9 | (2.2) | 14.8 | (2.6) | 14.8 | (2.7) | 1, 434 | 0.09 | 0.916 | – | – | – |
| Tobacco, AFU: mean (s.d.) | 15.1 | (2.8) | 15.2 | (2.5) | 15.3 | (2.9) | 2, 325 | 0.12 | 0.883 | – | – | – |
| Cannabis, AFU: mean (s.d.) | 15.7 | (2.6) | 15.7 | (3.2) | 15.8 | (2.9) | 2, 304 | 0.05 | 0.947 | – | – | – |
| ATS, AFU: mean (s.d.) | 17.4 | (2.7) | 16.9 | (4.0) | 16.3 | (4.8) | 2, 190 | 0.85 | 0.431 | – | – | – |
| AUDIT (total), mean (s.d.) | 6.4 | (8.7) | 7.1 | (8.2) | 6.1 | (6.8) | 2, 540 | 0.85 | 0.427 | – | – | – |
| Daily tobacco, n (%) | 34/97 | (35) | 41/194 | (21) | 39/194 | (20) | 2 | 9.05 | 0.011 | – | – | – |
| Weekly + cannabis, n (%) | 13/96 | (14) | 21/192 | (11) | 23/196 | (12) | 2 | 0.42 | 0.811 | – | – | – |
| Monthly + ATS,a n (%) | 8/97 | (8) | 9/194 | (5) | 8/197 | (4) | – | 2.43 | 0.304 | – | – | – |
SOFAS, Social and Occupational Functioning Assessment Scale; BPRS, Brief Psychiatric Rating Scale; K10, Kessler Psychological Distress Scale (10-item); AFU, age first use; ATS, Amphetamine-type stimulant; AUDIT, Alcohol Use Disorders Identification Test.
a. Fisher's exact test.
***P < 0.001; **P < 0.01; *P < 0.05 (Tukey's honest significant difference post hoc comparisons).
Table 2.
Numbers and proportions (%) of each major diagnostic group within each cluster group
| Diagnostic group | n (%) | ||
|---|---|---|---|
| Global impairment (n = 134) | Intermediate impairment (n = 252) | Normal range (n = 243) | |
| Anxiety disorder | 15 (11.2) | 48 (19.0) | 33 (13.6) |
| Depressive disorder | 35 (26.1) | 98 (38.9) | 111 (45.7) |
| Bipolar disorder | 15 (11.2) | 38 (15.1) | 35 (14.4) |
| Psychotic disorder | 35 (26.1) | 25 (9.9) | 22 (9.1) |
| Other disorders | 34 (25.4)a | 43 (17.1)b | 42 (17.3)c |
Post-traumatic stress disorder (n = 3); adjustment disorder (n = 2); substance-use disorder (n = 4); obsessive-compulsive disorder (n = 2); autism spectrum disorder (n = 4); attention-deficit hyperactivity disorder (n = 3); unspecified neurodevelopmental disorder (n = 1); borderline personality disorder (n = 1); unspecified personality disorder (n = 1); conduct disorder (n = 1); oppositional defiant disorder (n = 5); unspecified disruptive, impulse-control, and conduct disorder (n = 3); uncertain/no diagnosis (n = 4)
Post-traumatic stress disorder (n = 1); acute stress disorder (n = 1); unspecified trauma- and stress-related disorder (n = 2); substance-use disorder (n = 4); obsessive-compulsive disorder (n = 4); autism spectrum disorder (n = 6); attention-deficit hyperactivity disorder (n = 9); unspecified neurodevelopmental disorder (n = 1); borderline personality disorder (n = 3); conduct disorder (n = 1); oppositional defiant disorder (n = 2); unspecified disruptive, impulse-control, and conduct disorder (n = 4); bulimia (n = 2); uncertain/no diagnosis (n = 3).
Post-traumatic stress disorder (n = 2); unspecified trauma- and stress-related disorder (n = 1) substance-use disorder (n = 4); obsessive-compulsive disorder (n = 4); autism spectrum disorder (n = 4); attention-deficit hyperactivity disorder (n = 9); borderline personality disorder (n = 1); conduct disorder (n = 1); oppositional defiant disorder (n = 2); unspecified disruptive, impulse-control, and conduct disorder (n = 1); bulimia (n = 1); unspecified eating disorder (n = 1); uncertain/no diagnosis (n = 11).
Fig. 2.
Proportions of major diagnostic groups allocated to each neurocognitive cluster group.
Neurocognitive profiles of the three cluster groups are presented in Fig. 1. As expected, cluster-group differences were observed for all neurocognitive domains. Cluster one (n = 134) had global impairment across all domains, with z-scores 1–2 s.d. below the norm for cognitive flexibility (−1.88), sustained attention (−1.49), verbal learning (−1.51), verbal memory (−1.53), visuospatial memory (−1.72) and set-shifting (−2.04), and z-scores 0.5–1 s.d. below the norm for processing speed (−0.99), verbal fluency (−0.88) and working memory (−0.75).
Cluster two (n = 252) had intermediate impairment, with all domains falling within normal limits (i.e. −1.0 to 1.0 s.d.), with only cognitive flexibility (−0.52) and sustained attention (−0.81) falling below −0.5 s.d. Cluster three (n = 243) had a normal range profile, with performance 0–0.5 s.d. above the norm for all domains except sustained attention (−0.11) and verbal fluency (−0.04). Importantly, cluster-group differences in neurocognitive scores remained statistically significant when adjusting for estimated premorbid IQ (supplementary Table 3).
Associations between neurocognitive subgroups and functioning at baseline and over time
Unconditional model
We first constructed an unconditional model (i.e. no predictors) with random intercepts. We next included the fixed relationship between SOFAS and ‘time’ with a linear term, which was significant and indicated that SOFAS scores increased over time across all participants (B = 0.55, 95% CI 0.29–0.81, P < 0.001). Next, slopes were randomly varied across participants. This random slopes and random intercepts model fit the data substantially better than the random intercepts and fixed slopes model (LRT = 74.64, P < 0.001).
Modelling functioning at baseline and over time: unadjusted associations
Next, we modelled relationships between all predictor variables and variation in baseline SOFAS, and between cluster-group membership and the rate of change in SOFAS over time (with the normal range cluster group serving as the reference). As shown in Table 3, the unadjusted models showed significant associations between all predictor variables and baseline SOFAS. Lower baseline functioning was associated with membership in the global impairment cluster group (B = −6.59, 95% CI −8.50 to −4.68, P < 0.001), membership in the intermediate cluster group (B = −2.49, 95% CI −4.07 to −0.90, P = 0.002), male gender (B = −3.24, 95% CI −4.70 to −1.77, P < 0.001), lower premorbid IQ (B = 0.20, 95% CI 0.13 to 0.28, P < 0.001), fewer years of education (B = 0.80, 95% CI 0.50 to 1.09, P < 0.001) and greater level of depressive (B = −0.38, 95% CI −0.53 to −0.23, P < 0.001), positive (B = −0.74, 95% CI −0.94 to −0.55, P < 0.001), negative (B = −1.01, 95% CI −1.27 to −0.75, P < 0.001) and manic symptom severity (B = −0.36, 95% CI −0.59 to −0.12, P = 0.003). There was no significant difference in the rate of change in SOFAS over time for the intermediate impairment cluster group compared with the normal range cluster group (B = 0.27, 95% CI −0.18 to 0.71, P = 0.236); however, members of the global impairment cluster group had a lesser rate of SOFAS improvement over time compared with the normal range cluster group (B = −0.85, 95% CI −1.50 to −0.21, P = 0.010).
Table 3.
Unadjusted and adjusted linear mixed-effects models (n = 629) examining associations between Social and Occupational Functioning Assessment Scale; intercept (i.e. baseline) and slope (i.e. longitudinal change) and neurocognitive clusters, sociodemographics, and symptom typology and severity
| Unadjusted | Adjusted | |||||||
|---|---|---|---|---|---|---|---|---|
| Coefficient | 95% CI | t | P | Coefficient | 95% CI | t | P | |
| Intercept | 60.53 | 59.79 to 61.26 | 161.54 | <0.001 | 56.73 | 48.51 to 64.96 | 13.48 | <0.001 |
| Time | 0.53 | 0.20 to 0.86 | 3.13 | 0.002 | 0.27 | −0.25 to 0.79 | 1.01 | 0.311 |
| Clusters | ||||||||
| Global impairment | −6.59a | −8.50 to −4.68 | −6.77 | <0.001 | −4.18a | −6.57 to −1.80 | −3.43 | <0.001 |
| Intermediate impairment | −2.49a | −4.07 to −0.90 | −3.07 | 0.002 | −2.31a | −4.21 to −0.41 | −2.38 | 0.018 |
| Sociodemographics | ||||||||
| Gender (male) | −3.24 | −4.70 to −1.77 | −4.33 | <0.001 | −1.96 | −3.35 to −0.56 | −2.73 | 0.006 |
| Premorbid IQ | 0.20 | 0.13 to 0.28 | 5.33 | <0.001 | 0.11 | 0.04 to 0.19 | 2.98 | 0.003 |
| Education (years) | 0.80 | 0.50 to 1.09 | 5.30 | <0.001 | 0.53 | 0.25 to 0.81 | 3.67 | <0.001 |
| Symptom severity | ||||||||
| BPRS depressive | −0.38 | −0.53 to −0.23 | −5.08 | <0.001 | −0.27 | −0.42 to −0.12 | −3.45 | <0.001 |
| BPRS positive | −0.74 | −0.94 to −0.55 | −7.49 | <0.001 | −0.34 | −0.56 to −0.13 | −3.16 | 0.002 |
| BPRS negative | −1.01 | −1.27 to −0.75 | −7.63 | <0.001 | −0.60 | −0.86 to −0.33 | −4.43 | <0.001 |
| BPRS manic | −0.36 | −0.59 to −0.12 | −3.00 | 0.003 | −0.07 | −0.29 to 0.16 | −0.59 | 0.555 |
| Associations with rate of change | ||||||||
| Time × global impairment | −0.85 | −1.50 to −0.21 | −2.59 | 0.010 | 0.26a | −0.67 to 1.20 | 0.55 | 0.581 |
| Time × intermediate impairment | 0.27 | −0.18 to 0.71 | 1.19 | 0.236 | 0.46a | −0.26 to 1.19 | 1.25 | 0.211 |
BPRS, Brief Psychiatric Rating Scale.
Normal range cluster group represents the reference category.
Modelling functioning at baseline and over time: adjusted associations
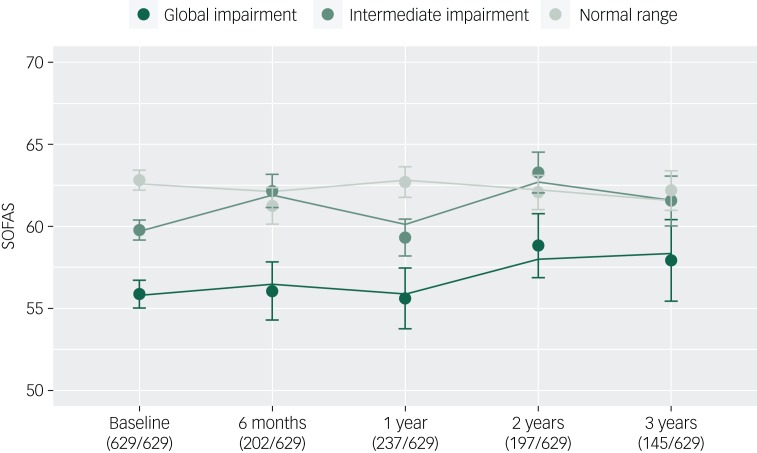
Finally, we examined whether the neurocognitive cluster groups would be associated with variation in baseline SOFAS and SOFAS change over time after statistical adjustment for sociodemographic and symptom variables. As shown in Table 3, lower baseline functioning was again associated with membership in the global impairment cluster group (B = −4.18, 95% CI −6.57 to −1.80, P < 0.001) and the intermediate cluster group (B = −2.31, 95% CI −4.21 to −0.41, P = 0.018) (relative to the normal range cluster), as well as male gender (B = −1.96, 95% CI −3.35 to −0.56, P < 0.006), lower premorbid IQ (B = 0.11, 95% CI 0.04 to 0.19, P = 0.003), fewer years of education (B = 0.53, 95% CI 0.25 to 0.81, P < 0.001), and greater level of depressive (B = −0.27, 95% CI −0.42 to −0.12, P < 0.001), positive (B = −0.34, 95% CI −0.56 to −0.13, P = 0.002) and negative symptom severity (B = −0.60, 95% CI −0.86 to −0.33, P < 0.001). Neither the global impairment (B = 0.26, 95% CI −0.67 to 1.20, P = 0.581) nor the intermediate cluster groups (B = 0.46, 95% CI −0.26 to 1.19, P = 0.211) differed from the normal range cluster group in their rate of change in SOFAS over time. The trajectories of functioning of the three cluster groups are presented in Fig. 3.
Fig. 3.
Functional trajectories of three neurocognitive clusters of young people (n = 629) with emerging mental disorders over 3 years of contact with clinical services (lines, fitted model; filled circles, observed data; bars, s.e.).
Proportions of participants with an assessment at each time point are presented. SOFAS, Social and Occupational Functioning Assessment Scale.
Sensitivity analysis
To evaluate whether associations between the global impairment cluster group and functioning were in part driven by a greater proportion of psychotic disorders in this subgroup, we included a dichotomous variable representing the presence or absence of a primary psychotic disorder at baseline. Although there was a significant relationship between functioning and having a psychotic disorder (B = −4.08, 95% CI −5.95 to −2.21, P < 0.001), all other associations remained statistically significant (supplementary Table 4).
Discussion
Principal findings
This study reports the longer-term course of social and occupational functioning of a large clinical cohort of adolescents and young adults accessing youth mental health services. We demonstrate for the first time that data-driven neurocognitive subgroups are predictive of functional course for up to 3 years, with a global impairment subgroup following the poorest course of functioning independent of gender, premorbid IQ, level of education, level of symptom severity and presence of a primary psychotic disorder. Notably, all major diagnostic groups were represented in each subgroup (Fig. 2). Taken together, these findings suggest neurocognitive impairment may be distributed along a continuum of severity across syndrome-based major mental disorders and is a robust and transdiagnostic predictor of functional course.
Neurocognitive subgroups cut across major syndrome-based diagnostic groups
Our observation that primary diagnostic groups were distributed across the three cluster groups is consistent with previous work in schizophrenia and bipolar disorder20 and in a transdiagnostic in-patient sample.21 In the current study, around one-quarter of the global impairment cluster had a primary depressive disorder and another quarter had a primary bipolar or anxiety disorder. Notably, more than half of the participants with a primary psychotic disorder were allocated to the normal range or intermediate subgroups (supplementary Table 5), highlighting that within-diagnosis neurocognitive heterogeneity may be obscured by diagnosis-level comparisons, which tend to report a gradient of worst impairment in psychotic disorders, followed by bipolar and depressive disorders.13,20 However, consistent with other transdiagnostic – or cross-diagnostic – studies,17,20,21 participants with psychotic disorders were overrepresented in the global impairment subgroup. Biological factors such as brain abnormalities or genetic risk for neurocognitive impairment may be important factors for such individuals with global impairment, as reported in several studies of neurocognitively impaired subgroups with schizophrenia.45–48 These factors may also be relevant for bipolar and other non-psychotic disorders, especially given the degree of shared genetic risk across the major mental disorders.49–52
Sociodemographic and clinical differences between neurocognitive subgroups
Several important factors differed between the neurocognitive subgroups. First, the global impairment subgroup had lower premorbid IQ and an overrepresentation of males, providing some evidence in support of a neurodevelopmental component in this group. Second, there were group differences in positive and negative symptoms, which might be explained by the greater proportion of psychotic disorders in the global impairment subgroup relative to the normal range subgroup (26.1% v. 9.1%), or alternatively, by shared precursors to neurocognitive impairments and positive and negative symptoms. Finally, tobacco use was more common in the global impairment subgroup, which might be explained by higher rates of tobacco use in individuals with psychosis53 or acute self-medication of neurocognitive impairments, although support for the latter is equivocal.54
Strengths of the study
Several strengths of this study are worth mentioning. The cohort was a large group of young people accessing transdiagnostic youth mental health services, and the naturalistic design gives insight into real-world patterns of functioning over time, which may be generalisable to similar transdiagnostic youth mental health services that are emerging around the world in Australia, the UK, Ireland, Canada, Denmark, Asia and the USA.55 Second, multiple ratings of functioning allowed us to model the rate of change in functioning over time, building on many previous reports examining only one or two follow-up time points. Third, this is one of the largest studies of its kind, with most cross-sectional neurocognitive cluster studies totalling fewer than 200 participants. Fourth, we extend previous findings of an association between neurocognitive cluster group and functional course from 6 months16 to 3 years, and show broader implications across mood, anxiety, psychotic and other disorders.
Limitations
Several limitations are worth mentioning. First, studies in schizophrenia consistently report mediation of the path from neurocognition to functional outcome by several factors that were unmeasured here, including social cognition and intrinsic motivation56; they are likely relevant beyond schizophrenia. Second, we relied on a single neurocognitive assessment and cannot evaluate the stability of our neurocognitive subgroups over time. Third, sample attrition (Fig. 3) may have biased model estimates; however, differences between participants lost to follow-up and retained were small (supplementary Table 6). Fourth, there were differences in neurocognitive test scores across the major diagnostic groups (supplementary Table 7), and it is possible that a subgroup of participants with severe psychotic disorders may have influenced our findings; however we also adjusted our models for the presence of a psychotic disorder (supplementary Table 4). Fifth, there was some evidence of bimodal distributions for cognitive flexibility and set-shifting in the global impairment cluster (supplementary Fig. 2a–i), which may have influenced the mean severity of this cluster group. However, model residuals were approximately normally distributed, meeting a key assumption of the mixed-effects framework. Sixth, the wide age-range of the participants may mean that age-related neurocognitive test heterogeneity may have influenced our results. However, there were no significant differences between cluster groups in age (P = 0.765) and differences in neurocognitive test scores between participants below 18 years (n = 191) versus those aged 18 years and over (n = 438) were small (supplementary Table 8). Finally, individuals in the global impairment subgroup were more likely to be using antipsychotic medication, which is likely related to the overrepresentation of psychotic disorders in this group (supplementary Table 9). However, rates of missing medication data did not allow us to model medication as a covariate.
Implications and future directions
Taken together, our results support the strong association between neurocognitive ability and social and occupational functioning among young people with emerging mental disorders, with novel transdiagnostic and longitudinal implications. Longitudinal studies before and after illness onset are needed to identify unique and/or shared genetic or neurodevelopmental pathways to neurocognitive impairment, that may speculatively evolve independently of later syndrome-based diagnostic group. These studies will be important to determine whether observed subgroups represent biologically meaningful, ‘natural kinds’ of groupings,57 or instead represent segments of a neurocognitive continuum distributed throughout the population. Moreover, future studies should utilise machine-learning approaches to better select variables to be used in clustering algorithms (for example Dwyer et al58), and to broaden outcome variables to model relationships between data-driven subgroups and other clinical and functional outcomes (for example clinical stage transition, admission to hospital), which may assist in planning of personalised interventions.59
Acknowledgements
We thank the wonderful young people who have participated in our research over the years.
Funding
This study was supported by grants from the Australian Government (Research Training Program Scholarship) and the National Health & Medical Research Council (NHMRC) including: Centre of Research Excellence (No. 1061043), NHMRC Fellowship (No. 1046899) and Clinical Research Fellowship (No. 402864). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Data availability
De-identified data may be made available from the corresponding author upon reasonable request.
Author contributions
J.J.C., K.M.C., D.F.H., and I.B.H. were involved in formulating research questions, study design, data analysis and interpretation and writing the article. F.I., J.S.C., D.W., A.N., N.Z., A.M.T., R.S.C.L., S.L.N., and E.M.S. were involved in data acquisition and interpretation. J.S. was involved in data analysis and interpretation. All authors critically revised the manuscript for intellectual content and approved the final version.
Declaration of interest
E.M.S. reports personal fees from St Vincent's Private Hospital, grants from Servier, personal fees from Servier, personal fees from Eli- Lilly, personal fees from Pfizer, outside the submitted work. She has received honoraria for educational seminars related to the clinical management of depressive disorders supported by Servier and Eli-Lilly pharmaceuticals. She has participated in a national advisory board for the antidepressant compound Pristiq, manufactured by Pfizer. She was the National Coordinator of an antidepressant trial sponsored by Servier. I.B.H reports personal fees from National Mental Health Commission, personal fees from Medibank Clinical Reference Group, non-financial support from Psychosis Australia Trust, grants from NHMRC, grants from Innowell Pty LTD, grants from NHMRC, grants from NHMRC, outside the submitted work. I.B.H. was an inaugural Commissioner on Australia's National Mental Health Commission (2012–2018). The BMC operates an early-intervention youth services at Camperdown under contract to headspace. I.B.H. has previously led community-based and pharmaceutical industry-supported (Wyeth, Eli Lily, Servier, Pfizer, AstraZeneca) projects focused on the identification and better management of anxiety and depression. He was a member of the Medical Advisory Panel for Medibank Private until October 2017, a Board Member of Psychosis Australia Trust and a member of Veterans Mental Health Clinical Reference group. He is the Chief Scientific Advisor to, and an equity shareholder in, Innowell. Innowell has been formed by the University of Sydney and PwC to deliver the $30m Australian Government-funded ‘Project Synergy’. Project Synergy is a 3-year programme for the transformation of mental health services through the use of innovative technologies. J.J.C., K.M.C., F.I., J.S.C., D.W., A.N., N.Z., A.M.T., R.S.C.L., S.L.N., J.S., D.F.H. report no competing interests.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/bjo.2020.12.
click here to view supplementary material
References
- 1.Whiteford HA, Ferrari AJ, Degenhardt L, Feigin V, Vos T. The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLOS One 2015; 10: e0116820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Woodward LJ, Fergusson DM. Life course outcomes of young people with anxiety disorders in adolescence. J Am Acad Child Adolesc Psychiatry 2001; 40: 1086–93. [DOI] [PubMed] [Google Scholar]
- 3.Fergusson DM, Woodward LJ. Mental health, educational, and social role outcomes of adolescents with depression. Arch Gen Psychiatry 2002; 59: 225–31. [DOI] [PubMed] [Google Scholar]
- 4.Fergusson DM, Boden JM, Horwood LJ. Recurrence of major depression in adolescence and early adulthood, and later mental health, educational and economic outcomes. Br J Psychiatry 2007; 191: 335–42. [DOI] [PubMed] [Google Scholar]
- 5.Wadsworth ME, Montgomery SM, Bartley MJ. The persisting effect of unemployment on health and social well-being in men early in working life. Soc Sci Med 1999; 48: 1491–9. [DOI] [PubMed] [Google Scholar]
- 6.Mesholam-Gately RI, Giuliano AJ, Goff KP, Faraone SV, Seidman LJ. Neurocognition in first-episode schizophrenia: a meta-analytic review. Neuropsychology 2009; 23: 315–36. [DOI] [PubMed] [Google Scholar]
- 7.Lee RS, Hermens DF, Scott J, Redoblado-Hodge MA, Naismith SL, Lagopoulos J, et al. A meta-analysis of neuropsychological functioning in first-episode bipolar disorders. J Psychiatr Res 2014; 57: 1–11. [DOI] [PubMed] [Google Scholar]
- 8.Lee RS, Hermens DF, Porter MA, Redoblado-Hodge MA. A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord 2012; 140: 113–24. [DOI] [PubMed] [Google Scholar]
- 9.Green MF, Horan WP, Lee J. Nonsocial and social cognition in schizophrenia: current evidence and future directions. World Psychiatry 2019; 18: 146–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mora E, Portella MJ, Forcada I, Vieta E, Mur M. Persistence of cognitive impairment and its negative impact on psychosocial functioning in lithium-treated, euthymic bipolar patients: a 6-year follow-up study. Psychol Med 2013; 43: 1187–96. [DOI] [PubMed] [Google Scholar]
- 11.Withall A, Harris LM, Cumming SR. The relationship between cognitive function and clinical and functional outcomes in major depressive disorder. Psychol Med 2009; 39: 393–402. [DOI] [PubMed] [Google Scholar]
- 12.Crouse JJ, Chitty KM, Iorfino F, Carpenter JS, White D, Nichles A, et al. Modelling associations between neurocognition and functional course in young people with emerging mental disorders: a longitudinal cohort study. Transl Psychiatry 2020; 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bora E. Differences in cognitive impairment between schizophrenia and bipolar disorder: considering the role of heterogeneity. Psychiatry Clin Neurosci 2016; 70: 424–33. [DOI] [PubMed] [Google Scholar]
- 14.Goldstein G. Neuropsychological heterogeneity in schizophrenia: a consideration of abstraction and problem-solving abilities. Arch Clin Neuropsychol 1990; 5: 251–64. [PubMed] [Google Scholar]
- 15.Crouse JJ, Moustafa AA, Bogaty SER, Hickie IB, Hermens DF. Parcellating cognitive heterogeneity in early psychosis-spectrum illnesses: a cluster analysis. Schizophr Res 2018; 202: 91–8. [DOI] [PubMed] [Google Scholar]
- 16.Uren J, Cotton SM, Killackey E, Saling MM, Allott K. Cognitive clusters in first episode psychosis: Overlap with healthy controls and relationship to concurrent and prospective symptoms and functioning. Neuropsychology 2017; 31: 787–97. [DOI] [PubMed] [Google Scholar]
- 17.Lewandowski KE, Sperry SH, Cohen BM, Ongur D. Cognitive variability in psychotic disorders: a cross-diagnostic cluster analysis. Psychol Med 2014; 44: 3239–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pu S, Noda T, Setoyama S, Nakagome K. Empirical evidence for discrete neurocognitive subgroups in patients with non-psychotic major depressive disorder: clinical implications. Psychol Med 2018; 48: 2717–29. [DOI] [PubMed] [Google Scholar]
- 19.Burdick KE, Russo M, Frangou S, Mahon K, Braga RJ, Shanahan M, et al. Empirical evidence for discrete neurocognitive subgroups in bipolar disorder: clinical implications. Psychol Med 2014; 44: 3083–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Rheenen TE, Lewandowski KE, Tan EJ, Ospina LH, Ongur D, Neill E, et al. Characterizing cognitive heterogeneity on the schizophrenia-bipolar disorder spectrum. Psychol Med 2017; 47: 1848–64. [DOI] [PubMed] [Google Scholar]
- 21.Ambrosini F, Benassi M, Sant'Angelo RP, Raggini R, Mandolesi L, Piraccini G. Two step cluster analysis application to a sample of psychiatric inpatients at psychiatric service of diagnosis and care. Eur Psychiatry 2017; 41: S226–S7. [Google Scholar]
- 22.Cotrena C, Damiani Branco L, Ponsoni A, Milman Shansis F, Paz Fonseca R. Neuropsychological clustering in bipolar and major depressive disorder. J Int Neuropsychol Soc 2017; 23: 584–93. [DOI] [PubMed] [Google Scholar]
- 23.Hermens DF, Redoblado Hodge MA, Naismith SL, Kaur M, Scott E, Hickie IB. Neuropsychological clustering highlights cognitive differences in young people presenting with depressive symptoms. J Int Neuropsychol Soc 2011; 17: 267–76. [DOI] [PubMed] [Google Scholar]
- 24.Scott EM, Hermens DF, Glozier N, Naismith SL, Guastella AJ, Hickie IB. Targeted primary care-based mental health services for young Australians. Med J Aust 2012; 196: 136–40. [DOI] [PubMed] [Google Scholar]
- 25.Iorfino F, Scott EM, Carpenter JS, Cross SP, Hermens DF, Killedar M, et al. Clinical stage transitions in persons aged 12 to 25 years presenting to early intervention mental health services with anxiety, mood, and psychotic disorders. JAMA Psychiatry 2019; 76: 1167–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders (5th edn). American Psychiatric Association, 2013. [Google Scholar]
- 27.Goldman HH, Skodol AE, Lave TR. Revising axis V for DSM-IV: a review of measures of social functioning. Am J Psychiatry 1992; 149: 1148–56. [DOI] [PubMed] [Google Scholar]
- 28.Hilsenroth MJ, Ackerman SJ, Blagys MD, Baumann BD, Baity MR, Smith SR, et al. Reliability and validity of DSM-IV axis V. Am J Psychiatry 2000; 157: 1858–63. [DOI] [PubMed] [Google Scholar]
- 29.Hay P, Katsikitis M, Begg J, Da Costa J, Blumenfeld N. A two-year follow-up study and prospective evaluation of the DSM-IV axis V. Psychiatr Serv 2003; 54: 1028–30. [DOI] [PubMed] [Google Scholar]
- 30.Tombaugh TN. Trail Making Test A and B: Normative data stratified by age and education. Arch Clin Neuropsychol 2004; 19: 203–14. [DOI] [PubMed] [Google Scholar]
- 31.Taylor EM. Psychological Appraisal of Children with Cerebral Deficits. Harvard University Press, 1959. [Google Scholar]
- 32.Sahakian BJ, Owen AM. Computerized assessment in neuropsychiatry using CANTAB: Discussion paper. J R Soc Med 1992; 85: 399–402. [PMC free article] [PubMed] [Google Scholar]
- 33.Tombaugh TN, Kozak J, Rees L. Normative data stratified by age and education for two measures of verbal fluency: FAS and animal naming. Arch Clin Neuropsychol 1999; 14: 167–77. [PubMed] [Google Scholar]
- 34.Wilkinson G, Robertson G. Wide Range Achievement Test 4th edn. Psychological Assessment Resources, 2006. [Google Scholar]
- 35.Wechsler D. Wechsler Test of Adult Reading (WTAR). The Psychological Corporation, 2001. [Google Scholar]
- 36.Tombaugh T. Normative data for the trail making test In A Compendium of Neuropsychological Tests: Administration, Norms and Commentary (2nd edn) (eds Spreen O, Strauss) E. Oxford University Press, 1998. [Google Scholar]
- 37.Lee RSC, Hermens DF, Naismith SL, Lagopoulos J, Jones A, Scott J, et al. Neuropsychological and functional outcomes in recent-onset major depression, bipolar disorder and schizophrenia-spectrum disorders: a longitudinal cohort study. Transl Psychiatry 2015; 5; e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dingemans PM, Linszen DH, Lenior ME, Strauss RM. Component structure of the expanded Brief Psychiatric Rating Scale (BPRS-E). Psychopharmacology 1995; 122: 263–7. [DOI] [PubMed] [Google Scholar]
- 39.Kessler RC, Barker PR, Colpe LJ, Epstein JF, Gfroerer JC, Hiripi E, et al. Screening for serious mental illness in the general population. Arch Gen Psychiatry 2003; 60: 184–9. [DOI] [PubMed] [Google Scholar]
- 40.WHO ASSIST Working Group. The Alcohol, Smoking and Substance Involvement Screening Test (ASSIST): development, reliability and feasibility. Addiction 2002; 97: 1183–94. [DOI] [PubMed] [Google Scholar]
- 41.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption: II. Addiction 1993; 88: 791–804. [DOI] [PubMed] [Google Scholar]
- 42.R Core Team. R: A Language and Environment for Statistical Computing. R Core Team, 2014 (http://www.R-project.org/). [Google Scholar]
- 43.Scrucca L, Fop M, Murphy TB. Raftery AE. mclust 5: clustering, classification and density estimation using gaussian finite mixture models. The R Journal 2016; 8: 289–317. [PMC free article] [PubMed] [Google Scholar]
- 44.Pinheiro J, Bates D, DebRoy S, Sarkar D, R Core Team. nlme: Linear and Nonlinear Mixed Effects Models. R package version 3.1-137. R Core Team, 2018. (https://CRAN.R-project.org/package=nlme). [Google Scholar]
- 45.Green MJ, Cairns MJ, Wu J, Dragovic M, Jablensky A, Tooney PA, et al. Genome wide supported variant MIR137 and severe negative symptoms predict membership of an impaired cognitive subtype of schizophrenia. Mol Psychiatry 2013; 18: 774–80. [DOI] [PubMed] [Google Scholar]
- 46.Hallmayer JF, Kalaydjieva L, Badcock J, Dragovic M, Howell S, Michie PT, et al. Genetic evidence for a distinct subtype of schizophrenia characterized by pervasive cognitive deficit. Am J Hum Gen 2005; 77: 468–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wexler BE, Zhu H, Bell MD, Nicholls SS, Fulbright RK, Gore JC, et al. Neuropsychological near normality and brain structure abnormality in schizophrenia. Am J Psychiatry 2009; 166: 189–95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cobia DJ, Csernansky JG, Wang L. Cortical thickness in neuropsychologically near normal schizophrenia. Schizophr Res 2011; 133: 68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anttila V, Bulik-Sullivan B, Finucane HK, Walters RK, Bras J, Duncan L, et al. Analysis of shared heritability in common disorders of the brain. Science 2018; 360: eaap8757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Gandal MJ, Haney JR, Parikshak NN, Leppa V, Ramaswami G, Hartl C, et al. Shared molecular neuropathology across major psychiatric disorders parallels polygenic overlap. Science 2018; 359: 693–7.29439242 [Google Scholar]
- 51.O'Donovan MC, Owen MJ. The implications of the shared genetics of psychiatric disorders. Nat Med 2016; 22: 1214. [DOI] [PubMed] [Google Scholar]
- 52.Lee SH, Ripke S, Neale BM, Faraone SV, Purcell SM, Perlis RH, et al. Genetic relationship between five psychiatric disorders estimated from genome-wide SNPs. Nat Genet 2013; 45: 984–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.de Leon J, Diaz FJ. A meta-analysis of worldwide studies demonstrates an association between schizophrenia and tobacco smoking behaviors. Schizophr Res 2005; 76: 135–57. [DOI] [PubMed] [Google Scholar]
- 54.Hahn B, Harvey AN, Concheiro-Guisan M, Huestis MA, Holcomb HH, Gold JM. A test of the cognitive self-medication hypothesis of tobacco smoking in schizophrenia. Biol Psychiatry 2013; 74: 436–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McGorry PD, Goldstone SD, Parker AG, Rickwood DJ, Hickie IB. Cultures for mental health care of young people: an Australian blueprint for reform. Lancet Psychiatry 2014; 1: 559–68. [DOI] [PubMed] [Google Scholar]
- 56.Galderisi S, Rossi A, Rocca P, Bertolino A, Mucci A, Bucci P, et al. The influence of illness-related variables, personal resources and context-related factors on real-life functioning of people with schizophrenia. World Psychiatry 2014; 13: 275–87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Kendler KS, Zachar P, Craver C. What kinds of things are psychiatric disorders? Psychol Med 2011; 41: 1143–50. [DOI] [PubMed] [Google Scholar]
- 58.Dwyer DB, Kalman JL, Budde M, Kambeitz J, Ruef A, Antonucci LA, et al. An investigation of psychosis subgroups with prognostic validation and exploration of genetic underpinnings: the PsyCourse Study. JAMA Psychiatry 2020. [Epub ahead of print] 12 Feb 2020. Available from: https://doi:10.1001/jamapsychiatry.2019.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hickie IB, Scott EM, Cross SP, Iorfino F, Davenport TA, Guastella AJ, et al. Right care, first time: a highly personalised and measurement-based care model to manage youth mental health. Med J Aust 2019; 211 (suppl 9): S3–s46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
For supplementary material accompanying this paper visit http://dx.doi.org/10.1192/bjo.2020.12.
click here to view supplementary material
Data Availability Statement
De-identified data may be made available from the corresponding author upon reasonable request.