Reports from countries struck by the coronavirus disease-2019 (COVID-19) pandemic have consistently highlighted physician shortages and the utilization of physicians not specifically trained in critical care to care for patients with COVID-19. Given the significant overlap between cardiology and critical care, cardiologists may be among the first physicians asked to step in to fill this shortage. If and when this occurs, a basic framework for recognition of acute respiratory failure, acute respiratory distress syndrome (ARDS), and initial ventilator management is imperative. The following is a brief review of ARDS and an overview of ventilator management designed to help ensure physician comfort and patient safety.
Data from China suggest respiratory findings are common in patients who are positive for COVID-19. Pneumonia was present in 91.1% of cases, and 3.4% of all patients developed ARDS. Oxygen therapy was used in 41.3% of patients, and infection required mechanical ventilation in 6.1% of those infected (1). The incidence has been even higher in the Italian series, with up to 10% of infected patients in Lombardy developing ARDS (2). It is likely that many American physicians will be called on to treat pneumonia, hypoxemic respiratory failure, and ARDS, regardless of their specialty.
Acute Respiratory Distress Syndrome
ARDS is a life-threatening form of lung injury. This lung injury can be the result of primary pulmonary parenchymal injury such as pneumonia or aspiration or from a systemic process such as sepsis or trauma. Increased capillary permeability leading to inflammation is the inciting factor for ARDS. Damage to the capillary endothelium and alveolar epithelium results in protein accumulation within the alveoli, activation of proinflammatory cytokines, and then pulmonary fibrosis. This cascade leads to loss of functional lung tissue. Chest radiography demonstrates bilateral opacities. As ARDS progresses, lung compliance decreases, hypoxemia ensues, and patients can progress to ventilator dependence (3,4).
In practice, ARDS is defined by the Berlin definition. This requires that patients have acute onset of lung injury, bilateral opacities on chest radiography, and nonhydrostatic pulmonary edema, in other words, respiratory failure may not be the result of left-sided heart failure. The Berlin definition of ARDS stratifies the severity of lung injury using a ratio of the arterial partial pressure of oxygen (PaO2) to the fraction of inhaled oxygen (FiO2), measured at a pulmonary end-expiratory pressure (PEEP) or continuous positive airway pressure of ≥5 cm H2O. Mild ARDS, moderate ARDS, and severe ARDS are defined as a PaO2/FiO2 of 200 to 300, 100 to 200, and <100, respectively (5).
At present, there are no studies examining specific ventilatory strategies in patients with COVID-19, however, there is a large body of experience with patients with ARDS. Until more COVID-19–specific evidence-based medicine is available, expert recommendations support adherence to these ventilatory strategies in patients with COVID-19 (6). Mechanical ventilation is recommended for patients with moderate or severe ARDS who remain hypoxemic or profoundly symptomatic despite supplemental oxygen. In mechanically ventilated patients, a lung-protective ventilation strategy should be employed to decrease ventilator-induced lung injury. When treating ARDS patients with mechanical ventilation, the target tidal volume is typically 6 ml/kg of predicted body weight with goal plateau pressure <30 cm H2O. One interesting finding in COVID-19 ARDS is that some patients are found to be severely hypoxemic, but with surprisingly good lung compliance. In these patients, more liberal tidal volume settings may be considered. PEEP should be adjusted based on FiO2, with consideration of higher PEEP (>10 cm H2O) in moderate and severe ARDS. Notably, lung protective ventilation (low tidal volume) allows permissive respiratory acidosis with maintenance of a pH ≥7.25. Relative hypoxemia with a PaO2 of 55 to 80 mm Hg or SpO2 of 88% to 95% may also be considered to limit high oxygen requirements (3). In patients with ARDS with PaO2/FiO2 ≤150 mm Hg, consideration should be given to initiation of prone positioning. If significant ventilator dyssynchrony is present, neuromuscular blockade may also be considered (7).
It should be noted that mild ARDS may be managed with noninvasive forms of ventilation. However, during the present pandemic, modifications to usual critical care may be necessary. Given concern for viral transmission, current recommendations advise “caution when using high-flow nasal oxygen or noninvasive ventilation due to risk of dispersion of aerosolized virus in the healthcare environment with poorly fitting masks” (8). Institution-specific policies should guide the decision for use of noninvasive ventilation versus early endotracheal intubation in this population.
Invasive Mechanical Ventilation
Invasive mechanical ventilation ensures oxygenation and ventilation via positive pressure delivered through a secure airway. In the intensive care unit, most patients undergoing mechanical ventilation will have an endotracheal tube (ETT) in place. This is a polyvinylchloride tube that is placed between the vocal cords and into the trachea. ETTs have various internal diameters. Most adults will be intubated with a 7.0- to 8.0-mm ETT. The smaller the internal diameter, the higher the resistance to airflow, and the more the patient and/or the ventilator will have to work to overcome this resistance. ETTs have inflated cuffs (balloons) that occupy the space of the trachea, ensuring all gas exchange occurs via the ETT, while offering some protection to the lung from gastric and oral secretions. On chest radiography, the distal end of the ETT should be 4 ± 2 cm above the level of the carina to ensure correct placement. After placement of the ETT, auscultation for bilateral breath sounds is imperative. A chest radiograph should be ordered immediately to further confirm correct tube location.
In recent years, various taxonomies have been developed to describe the different types of ventilator breaths. In this taxonomy scheme, each ventilator mode is first named by its control variable, or the variable manipulated by you, the operator. This is most often either pressure or volume. The second variable in this scheme is breath sequence. This is based on whether the patient or the machine trigger (initiates) and cycle (terminates) the breath. When all breaths are triggered and/or cycled by the machine, this is continuous mandatory ventilation (CMV), whereas synchronized intermittent mandatory ventilation allows the patient to take spontaneous breaths between mandatory breaths (9).
Assist-control (AC) ventilation, a form of CMV, is among the most common ventilator modes in the modern intensive care unit. In AC ventilation, the physician chooses to control either the pressure or volume delivered by the ventilator. In pressure-control (PC) ventilation, a set pressure is delivered to the patient with each breath, whereas a volume-control mode delivers a specific volume. In AC modes, the ventilator will then deliver this pre-specified volume or pressure for all breaths, regardless of whether the breath is triggered by the machine or the patient. These modes are most frequently referred to as volume control (VC), PC, VC-CMV or PC-CMV.
In all AC ventilation, the operator additionally chooses the FiO2, the PEEP, and the minimum respiratory rate. PEEP provides a continuous end-expiratory pressure to maintain alveolar recruitment. Taken together, PEEP and the FiO2 are variables that directly affect oxygenation. Carbon dioxide clearance (i.e., ventilation) is maintained by the minute ventilation, which is a product of the respiratory rate and tidal volume.
No compelling data exist to support the use of one mode over another, suggesting that nonintensivist physicians should use whichever mode they are most familiar with. AC is more typically used at the onset of respiratory failure, as it is more likely to relieve the patient from respiratory work.
Positive-pressure ventilation relies on an understanding of respiratory system compliance. Pulmonary compliance is defined as the change in volume over inspiration (tidal volume delivered) divided by the change in pressure from end-inspiration to end-expiration (plateau pressure – total PEEP). When using a PC mode of ventilation, a given amount of positive pressure is introduced into the lungs and the delivered tidal volume then depends on the lung compliance. Conversely, in a VC mode, a given tidal volume is administered by the ventilator and the resultant pressures are dictated by the patient’s underlying respiratory mechanics. Both modes are effective methods of ventilating patients with ARDS.
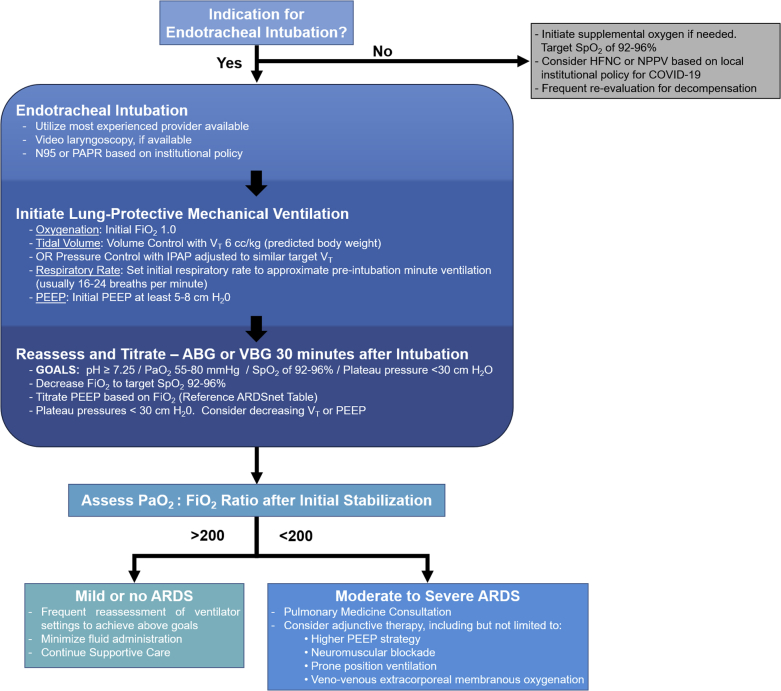
When faced with patients with COVID-19 with hypoxemic respiratory failure secondary to ARDS, it is appropriate to initiate a lung-protective ventilation strategy (6). Consider VC as the initial mode of mechanical ventilation. This ensures adequate tidal volume and minimizes the risk of volutrauma. In accordance with the ARDSnet (ARDS Network) study protocol, it is reasonable to choose an initial tidal volume of 6 ml/kg (predicted body weight). Begin with an initial FiO2 of 1.0, which may then be gradually decreased as tolerated, while monitoring pulse oximetry. It is advisable to reference the widely published ARDSnet protocol for guidance in determining appropriate PEEP for a given FiO2. Decisions regarding a lower versus higher PEEP strategy may be tailored based on underlying cardiac physiology. Initially, it is reasonable to consider a higher PEEP strategy. Initial respiratory rate should be set to approximate the patient’s pre-intubation minute ventilation (usually 20 to 28 breaths/min); this may then be adjusted based on the patient’s arterial pCO2 (Figure 1).
Figure 1.
Proposed Framework for Initial Ventilatory Strategy for the Care of Patients With COVID-19
Proposed framework for initial ventilatory strategy for the care of patients with coronavirus disease-2019 (COVID-19). As knowledge of the pulmonary pathophysiology of this syndrome evolves, ventilator management should be adapted to target our understanding of the underlying process. ABG = arterial blood gas; ARDS = acute respiratory distress syndrome; ARDSnet = ARDS Network; FiO2 = fraction of inspired air; HFNC = high-flow nasal cannula; IPAP = inspiratory positive airway pressure; NPPV = noninvasive positive pressure ventilation; PaO2 = partial pressure of oxygen in blood; PAPR = powered air-purifying respirator; PEEP = positive end-expiratory pressure; SpO2 = peripheral capillary oxygen saturation; VBG = venous blood gas; VT = tidal volume.
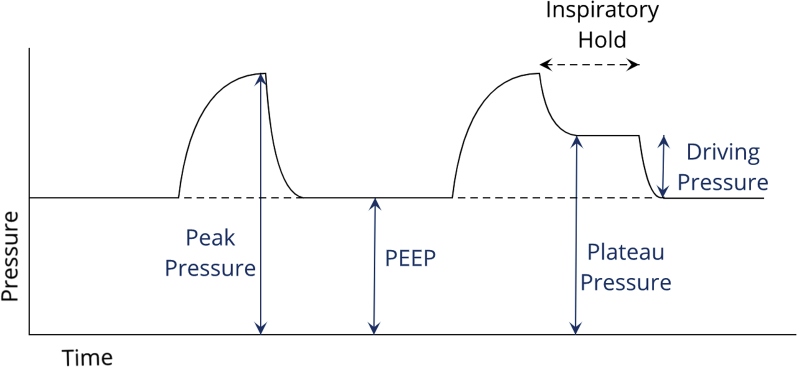
After initial stabilization, it is critical to appropriately titrate settings to minimize ventilator-induced lung injury. One of the most common methods for doing this is careful monitoring of the plateau pressure (Figure 2). Although the peak inspiratory pressure represents the pressure to which the proximal large airways are exposed, the plateau pressure is representative of the pressure present in the alveoli at end inspiration, and thus is an indicator of transpulmonary pressure, lung overdistention, and ventilator-induced lung injury. Plateau pressure is measured after a 0.5- to 1.0-s inspiratory pause maneuver. If the plateau pressure is >30 cm H2O, consider further reducing the delivered tidal volume. It is also important to monitor the patient’s driving pressure, or difference between the PEEP and plateau pressure, as increased driving pressures have been associated with higher mortality in ARDS (10).
Figure 2.
Most Commonly Assessed Airway Pressures
A representation of the most commonly assessed airway pressures. PEEP = positive end-expiratory pressure.
Conclusions
With a basic understanding of these fundamentals, it is possible for all cardiologists to provide safe and effective care for our patients with COVID-19. As many of us prepare to use skill sets long forgotten, it will be important to remember to ask for help when needed. One of the few bright spots in this pandemic has been the resurgence of interdisciplinary teamwork—we are all in this together.
Acknowledgment
The authors acknowledge Dr. R. Phillip Dellinger, Professor of Medicine at Cooper Medical School of Rowan University (Cooper University Hospital), for his assistance in preparation of this manuscript.
Footnotes
The authors have reported that they have no relationships relevant to the contents of this paper to disclose.
The authors attest they are in compliance with human studies committees and animal welfare regulations of the authors’ institutions and Food and Drug Administration guidelines, or patient consent where appropriate. For more information, visit the JACC: Case Reportsauthor instructions page.
References
- 1.Guan W., Ni Z., Hu Y. Clinical characteristics of coronavirus disease 2019 in China. N Engl J Med. 2020;382:1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Remuzzi A., Remuzzi G. COVID-19 and Italy: what next? Lancet. 2020 Mar doi: 10.1016/S0140-6736(20)30627-9. [E-pub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fan E., Brodie D., Slutsky A.S. Acute respiratory distress syndrome: advances in diagnosis and treatment. JAMA. 2018;319:698–710. doi: 10.1001/jama.2017.21907. [DOI] [PubMed] [Google Scholar]
- 4.Pierrakos C., Karanikolas M., Scolletta S., Karamouzos V., Velissaris D. Acute respiratory distress syndrome: pathophysiology and therapeutic options. J Clin Med Res. 2012;4:7–16. doi: 10.4021/jocmr761w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.ARDS Definition Task Force. Ranieri V.M., Rubenfeld G.D. Acute respiratory distress syndrome: the Berlin definition. JAMA. 2012;307:2526–2533. doi: 10.1001/jama.2012.5669. [DOI] [PubMed] [Google Scholar]
- 6.Alhazzani W., Møller M.H., Arabi Y.M. Surviving sepsis campaign: guidelines on the management of critically ill adults with coronavirus disease 2019 (COVID-19) Intensive Care Med. 2020;46:854–887. doi: 10.1007/s00134-020-06022-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beitler J.R., Shaefi S., Montesi S.B. Prone positioning reduces mortality from acute respiratory distress syndrome in the low tidal volume era: a meta-analysis. Intensive Care Med. 2014;40:332–341. doi: 10.1007/s00134-013-3194-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murthy S., Gomersall C.D., Fowler R.A. Care for critically ill patients with COVID-19. JAMA. 2020 Mar 11 doi: 10.1001/jama.2020.3633. [E-pub ahead of print] [DOI] [PubMed] [Google Scholar]
- 9.Alviar C.L., Miller P.E., McAreavey D., for the ACC Critical Care Cardiology Working Group Positive pressure ventilation in the cardiac intensive care unit. J Am Coll Cardiol. 2018;72:1532–1553. doi: 10.1016/j.jacc.2018.06.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Amato M.B.P., Meade M.O., Slutsky A.S. Driving pressure and survival in the acute respiratory distress syndrome. N Engl J Med. 2015;372:747–755. doi: 10.1056/NEJMsa1410639. [DOI] [PubMed] [Google Scholar]