Abstract
Meningiomas are primary intracranial tumors derived from arachnoid cap cells or meningothelial cells and usually display dural attachment. However, a small proportion of meningiomas that arise from the Sylvian fissure do not manifest dural attachment. Sylvian fissure meningiomas are relatively rare and have differential characteristics as compared with typical meningiomas. Herein, we reported a special case of atypical meningioma in the Sylvian fissure, that showed non-enhancement after contract management. The patient was a 30-year-old woman with a 2-year history of seizures. Preoperative computerized tomography and magnetic resonance imaging scans showed a calcific, non-enhancing lesion in the right insula lobe. The lesion was excised surgically for seizure control. Intraoperatively, the tumor was observed to be closely adhered to the middle cerebral artery (MCA), resulting in mild arterial damage. A case of Sylvian fissure meningioma was ultimately diagnosed by histopathological examination of the resected specimens. Sylvian fissure meningiomas are closely associated with the MCA and exhibit unusual imaging characteristics. Preoperative misdiagnosis may have serious adverse consequences and may result in incorrect surgery. To improve awareness of Sylvian fissure meningiomas on the differential diagnosis of Sylvian fissure lesions among clinicians, we present this report and briefly summarize previously reported cases to describe the clinical, pathological, radiological, and surgical features.
Keywords: meningiomas, Sylvian fissure, case report, atypical, neurosurgery
Introduction
Generally, meningiomas originate from the dura mater; however, some cases originate from the Sylvian fissure and show non-dural attachment. These cases can be easily missed preoperatively or may be misdiagnosed. Atypical meningioma is a distinct meningioma subtype and accounts for a small proportion of meningiomas with poor prognosis (1). At present, 38 cases of Sylvian fissure meningiomas have been reported (2–32), and four cases presented an atypical type (19, 22, 25, 30), but none were non-enhanced lesions (30). In this report, we describe a patient who presented with an intracranial mass in the right insula lobe, which was clinically diagnosed as a low-grade glioma based on the results of preoperative neuroimaging, with a subsequent revised diagnosis of meningioma confirmed by postoperative histopathology. In contrast with previous reported cases, our case presented characteristic non-enhancement of the meningioma lesion on contrast-enhanced magnetic resonance imaging (MRI), which is unique. In addition, we have reviewed the relevant literature and summarized the major findings herein to improve the awareness of Sylvian fissure meningiomas among clinicians for the differential diagnosis of Sylvian fissure lesions.
Case Presentation
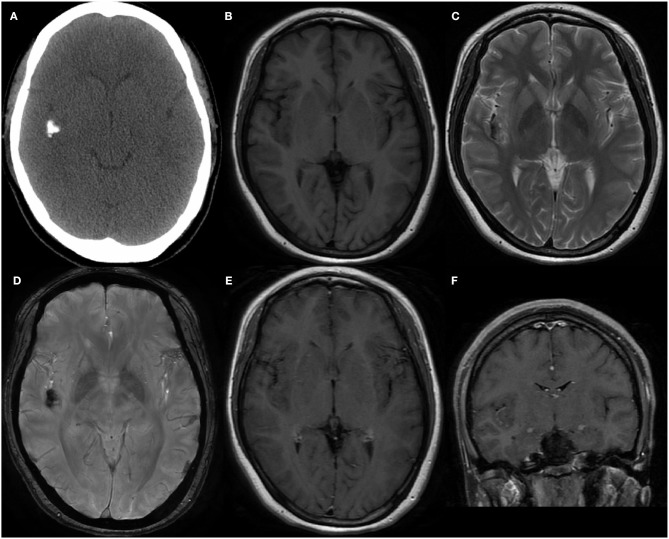
The patient was a 30-year-old homemaker with a 2-year history of seizures before admission. She had previously been treated with a routine antiepileptic (sodium valproate; 1,000 mg per day) for 3 months; however, the drug was weaned off because of its side effect (weight gain). Subsequently, she was prescribed lamotrigine (200 mg per day) and topiramate (75 mg per day) for seizure control, but continued to have occasional seizures, once or twice a month. She had no other neurological deficit or past medical history, and results of systemic examinations were normal. She had no family history of hereditary diseases. Electroencephalography performed after admission detected epileptiform activity in the right cerebral hemisphere. Computerized tomography (CT) demonstrated a calcific lesion in the right Sylvian fissure and posterior part of the insula (Figure 1A). An MRI scan revealed a lesion in the right insular lobe without any dural attachment, which was primarily hypointense on both T1- and T2-weighted MRI (Figures 1B,C). Additionally, the lesion appeared hypointense on susceptibility-weighted imaging (Figure 1D), with no obvious enhancement despite administration of gadolinium contrast (Figures 1E, F). The results of all preoperative laboratory tests were within normal ranges. Preoperatively, low-grade glioma, including oligodendroglioma, and diffuse astrocytoma, was suspected.
Figure 1.
(A) The Computed tomography demonstrated the calcification in right Sylvian fissure and posterior part of insula. (B,C) MRI scans revealed a lesion without dural attachment located in right insular lobe which was mainly hypointense on both T1-weighted and T2-weighted. (D) Lesion showed hypointense on susceptibility weighted imaging. Enhanced MRI showed no obvious enhancement after gadolinium administration in axial (E) and coronal (F) position.
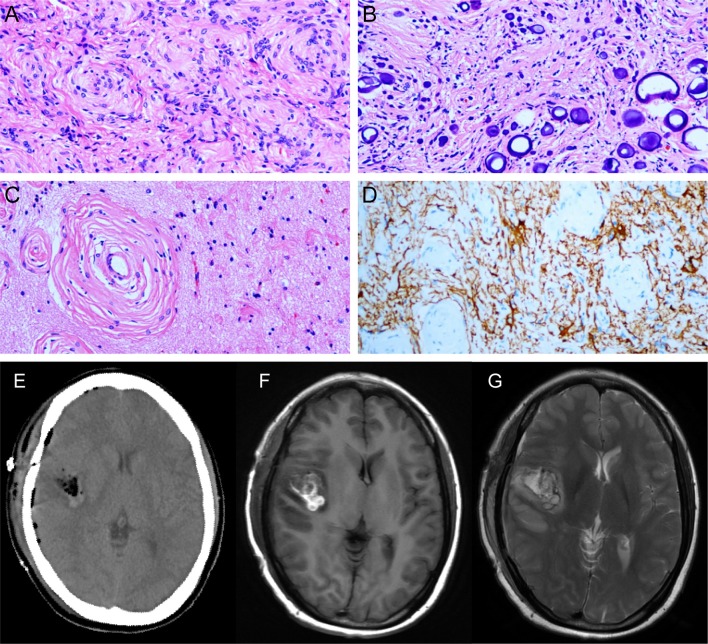
With this provisional diagnosis, the patient was referred for surgical management and underwent a right temporal craniotomy for gross total resection of the tumor and for seizure control. Upon opening the dura and separating the Sylvian fissure, a gray-white lesion (diameter, about 25*20 mm) was observed. It had a relatively elastic consistency and the mass partially invading the brain parenchyma of the insula, was densely adhered to the branches of the medial cerebral artery (MCA), and had partial calcification. Intraoperatively, despite careful micro-dissection, the MCA perforators were mildly damaged. Ultimately, several surgical specimens of the excised tumor were sent for histopathological analysis. Postoperatively, the results of histopathological examinations revealed a World Health Organization (WHO) grade II meningioma of atypical type (Figures 2A–D). Immunohistochemical examinations revealed negative immunoreactivity for Ki-67, progesterone receptor, p53, epithelial membrane antigen, and positive immunoreactivity for vimentin, and somatostatin receptor 2.
Figure 2.
(A) The tumor tissue arranged in swirl structure to form the meningeal corpuscle. (B) Psammoma bodies. (C) The meningeal corpuscles invade normal brain tissue. (D) GFAP staining show brain tissue (+) and meningeal corpuscles (-). Postoperative CT (E) and MRI (F,G) showed tumor gross total resection.
Postoperatively, the patient regained consciousness with a mild clinical symptom of hemiplegia in her left limbs, which lasted for nearly 2 weeks but improved gradually with rehabilitation therapy. A follow-up gadolinium-enhanced MRI (postoperative 2 weeks) showed complete total excision of the tumor (Figures 2E–G). The patient presented no other postoperative neurological deficit or seizure recurrence for approximately a year and a half. An MRI scan will be performed annually to continuously monitor for any evidence of tumor recurrence.
Discussion
Meningiomas are the second most common primary central nervous system tumors (CNST), accounting for approximately one-third of the primary CNST cases (33, 34). They are postulated to originate from the arachnoid cap and meningothelial cells, which are present in the arachnoid layer of the meninges or Pacchionian granulation, and typically display dural attachment. Occasionally, the arachnoid cap cells can appear in the pia mater of the Sylvian fissure or Virchow-Robin space along the MCA or its branches (23, 31) (Figure 3). Our patient presents an additional case of Sylvian fissure meningioma with unusual imaging characteristics and a rare atypical subtype.
Figure 3.
Schematic diagram of the origin of Sylvian fissure meningiomas.
The mean onset age of typical meningiomas is about 55 years, and the incidence increases with age, with women having a higher morbidity than men (34). However, the clinical characteristics of Sylvian fissure meningiomas are different. A retrospective analysis of 38 [25 men (65.8%) and 13 female (34.2%)] patients with Sylvian fissure meningiomas reported from 1938 to 2019 (Table 1) revealed that the average onset age is about 22.5 ± 17.4 years. Seizure was the major symptom of Sylvian fissure meningiomas (65.8%, 25 patients). Other reported symptoms included headache (39.5%, 15 patients) and hemiparesis (18.4%, 7 patients). Rare instance of initial symptom included visual impairment (2.6%, 1 patient), incidental (2.6%, 1 patient), and dizziness (2.6%, 1 patient). The incidence of seizures in patients with Sylvian fissure meningiomas (65.8%) was reported to be higher than those with common supratentorial meningiomas (29.2%) (35). Generally, seizure frequency depends on tumor location, and the limbic and temporal lobe had the lowest threshold for producing a seizure. Also, hypoxia and metabolic imbalances caused by tumor invasion and extrusion were the potential etiological mechanisms (36).
Table 1.
Summary of Sylvian fissure meningiomas up to October 31, 2019.
| References | Age/Sex | Clinical features | Imaging features | Removal | Histopathology | Follow-up |
|---|---|---|---|---|---|---|
| Cushing et al. (2) | 8/M | Epilepsy | N/A | Subtotal | Psammomatous | 5y: died |
| 48/F | Epilepsy | N/A | Subtotal | Psammomatous | 1d: died | |
| Barcia-Goyanes et al. (3) | 20/F | Epilepsy | N/A | N/A | Psammomatous | N/A |
| Mori et al. (4) | 23/M | Epilepsy | N/A | Subtotal | Transitional | N/A |
| Saito et al. (5) | 31/F | Epilepsy | Hyperdense lesion in CT | Gross total | Psammomatous | N/A |
| Tsuchida et al. (6) | 46/M | Headache | N/A | Gross total | Psammomatous | 4y: relapse-free |
| Awa et al. (7) | 16/M | Headache | N/A | Gross total | Meningothelial | 2y: relapse-free |
| Okamoto et al. (9) | 27/F | Headache | N/A | Gross total | Fibroblastic | 5y: died |
| 35/F | Headache and visual impaired | Severe edema, hyperdense, and homogeneous lesion in CT | Gross total | Fibroblastic | N/A | |
| Drake et al. (8) | 3/F | Headache | N/A | Gross total | Malignant | N/A |
| Hirao et al. (10) | 34/F | Epilepsy | Homogeneous enhancement in CT | Gross total | Fibroblastic | N/A |
| Silbergeld et al. (11) | 4/F | Epilepsy | Homogeneous enhancement in CT | Subtotal, radiation therapy | Meningothelial | N/A |
| Cho et al. (12) | 2/M | Epilepsy and hemiparesis | Severe edema, hyperdense with homogeneous lesion in CT | Gross total | Transitional | 2y: relapse-free |
| Graziani et al. (13) | 19/M | Headache and hemiparesis | Moderate edema, calcifications, Hypointense in T1 and T2 | Gross total | Psammomatous | N/A |
| Mori et al. (15) | 12/M | Headache | Slight edema, well-enhanced tumor in CT and MRI | Gross total | Transitional | 1y: relapse-free |
| Chiocca et al. (14) | 26/F | Epilepsy | Slight edema, hypointense and homogeneous in T1 and T2 | Gross total | Fibroblastic | N/A |
| Matsumoto et al. (16) | 62/F | Epilepsy | Hypointense in T1 and T2 | Gross total | Psammomatous | N/A |
| Cooper et al. (17) | 4/M | Headache | Severe edema, heterogeneous intensity in T1 and T2 | Gross total | Transitional | 1y: relapse-free |
| Mitsuyama et al. (18) | 1/M | Epilepsy | Well-enhanced tumor in CT and MRI | Gross total | Fibroblastic | N/A |
| Kaplan et al. (19) | 11/M | Epilepsy | Isointense in T1 and heterogeneous intensity in T2 | Gross total | Atypical | N/A |
| Chang et al. (20) | 35/M | Epilepsy | Severe edema, isointense, and homogeneous in T1 and T2 | Subtotal, gamma-knife | Transitional | N/A |
| Mclver et al. (21) | 23/M | Epilepsy | Heterogeneous intensity in T1 and T2 | Subtotal | Chordoid | 17m: stable residual tumor |
| Samson et al. (23) | 6/M | Epilepsy | Calcification, edema, heterogeneous enhancement, hypointense on T1 and hyperintense on T2 | Gross total | N/A | 4y: relapse-free |
| Cecchi et al. (22) | 23/M | Headache and hemiparesis | Moderate edema, heterogeneous intensity in T1 and T2 | Subtotal, radiation therapy | Atypical | 2y: stable residual tumor |
| Ma et al. (25) | 53/M | Epilepsy | Homogeneous enhancement, edema | Subtotal, gamma-knife | Atypical | 2y: stable residual tumor |
| Chae et al. (24) | 69/M | Incidental | Calcification, edema, heterogeneous intensity in T2, heterogenous enhancement | Subtotal | Psammomatous | N/A |
| Aras et al. (26) | 15/M | Epilepsy | Homogeneous enhancement, hypointense on T1 and iso-hypointense on T2, mild edema | 1st surgery: subtotal 2nd surgery: gross total | Fibroblastic | 3y: relapse-free |
| 28/M | Epilepsy and hemiparesis | Heterogenous enhancement, hypointense on T1 and heterogeneous intensity on T2, edema | 1st surgery: subtotal 2nd surgery: gross total | Meningothelial | 5y: relapse-free | |
| Kim et al. (27) | 43/M | Epilepsy | Minimal calcification, Isointense in T1 and T2, ring like enhancement, edema | Subtotal | Lymphoplasmacyte-rich | 4y: stable residual tumor |
| Fukushima et al. (28) | 10/M | Epilepsy | Heterogeneous enhancement | Subtotal | Sclerosing | 1y: stable residual tumor |
| Donovan et al. (29) | 11/M | Epilepsy | Calcification, homogenous enhancement | Gross total | Transitional | 10y: relapse-free |
| 7/M | Epilepsy and headache | Calcification, homogenous enhancement | 1st surgery: subtotal 2nd surgery: subtotal | Fibroblastic | 2y: stable residual tumor | |
| 16/F | Epilepsy | Partial calcification, minimal enhancement | Subtotal | Meningothelial | 5y: stable residual tumor | |
| Brogna et al. (30) | 32/M | Headache and dizziness | Isointense on T1 and T2, homogenous enhancement | Gross total | Atypical | 3y: relapse-free |
| Yamagishi et al. (32) | 32/M | Headache | Isointense on T1 and T2, homogenous enhancement | Gross total | Transitional | 6m: relapse-free |
| Amirjamshidi et al. (31) | 7/F | Headache and hemiparesis | Calcification, edema, isointense on T1 and T2, vivid enhancement | Gross total | Meningothelial | 13y: relapse-free |
| 5/F | Headache, epilepsy, and hemiparesis | Isointense on T1 and T2, homogenous enhancement | Gross total | Meningothelial | 5y: relapse-free | |
| 7/M | Headache, epilepsy, and hemiparesis | Isointense on T1, homogenous enhancement | Gross total | Meningothelial | 2y: relapse-free |
The majority of common meningiomas are WHO grade I, with approximately 16.9% cases being atypical (WHO grade II) (37). However, the proportion is expected to rise according to the newly recommended WHO 2016 criteria for the classification of atypical meningioma (38). Sylvian fissure meningiomas comprise three types of WHO grades, and previously reported cases were histologically diagnosed as psammomatous (8 cases, 21.1%), transitional (7 cases, 18.4%), meningothelial (7 cases, 18.4%), fibroblastic (7 cases, 18.4%), atypical (4 cases, 10.5%), chordoid (1 case, 2.6%), sclerosing (1 case, 2.6%), lymphoplasmacyte-rich (1 case, 2.6%), and malignant (1 case, 2.6%). The arachnoid cells in all of these cases can manifest divergent differentiation. Presence of brain invasion, which was added to the histological criteria, alone can aid the diagnosis of atypical meningiomas according to the 2016 WHO classification of CNSTs (38). Invasion of the brain parenchyma in our case confirmed a final diagnosis of atypical type meningioma with WHO II grade, which is the fifth case reported till date.
The main radiological characteristics of meningiomas generally include extra-axial occupation and dura mater attachment (dural tail sign). Contrarily, radiological imaging features of Sylvian fissure meningiomas have differential characteristics, such as intra-axial mass without dural attachment. It is extremely difficult to differentiate Sylvian fissure meningioma from other intracranial masses, such as low-grade glioma, teratoma, metastasis, cavernous hemangioma, tuberculous granuloma, etc. The previously reported 38 cases of Sylvian fissure meningiomas frequently showed hypointense or isointense lesions on T1- and T2-weighted MRI, with homogeneous or heterogeneous enhancement after contrast administration. Some cases also showed calcification or edema on CT and MRI scans, but these features were non-specific. Interestingly, the tumor in our case presented obvious calcification with negligible enhancement on the preoperative contrast-enhanced MRI scan, which significantly complicated the preoperative diagnosis. Meningiomas without enhancement have rarely been reported previously. Kubota et al. (39) and Zhang et al. (40) reported two cases of non-enhancement meningioma, due to the cystic or necrotic changes and distinctive pathological features. We considered that the tumor calcification, as a large number of psammoma bodies are found in pathology, result in non-enhancement in our case.
It is well-acknowledged that the progressive growth and enlargement of typical meningiomas can oppress or enclose the arteries. However, owing to the layer of arachnoid membrane between the blood vessels and tumors, it is relatively easy to intraoperatively discern and separate the meningiomas from the adjacent arteries. Because of the close relationship between the Sylvian fissure meningiomas with the MCA and its branches, blood vessels are more likely to be damaged during resection surgeries. In our case, a branch of the MCA was injured intraoperatively despite careful micro-dissection, and the patient had a mild cerebral infarction postoperatively. Fortunately, the patient recovered without any obvious neurological impairment after an adequate recovery period and remained seizure free for about a year and a half postoperatively. According to the previously reported cases, 22 (57.9%) patients achieved gross total resection in the first attempt and showed favorable outcomes with a relapse-free status (92.3%, 12/13 patients, some patients had no follow-up data) at follow-up even after several years. Of the 15 patients who underwent subtotal resection of tumor, three patients underwent a secondary surgery, and four patients accepted subsequent radiation or gamma-knife therapy. However, the residual tumors in all 15 patients showed no sign of further progression. Patients at risk of serious postoperative complications with gross total excision surgery can be recommended for radiation or gamma-knife therapy (11, 20, 22, 25). Atypical meningiomas are intermediate-grade tumor with a relatively greater risk of recurrence, requiring longitudinal monitoring by sequential radiological imaging. Optionally, radiation therapy can be used if required. The patient was satisfied with the overall treatment course, operation, and intensive nursing; moreover, her seizures are now well under control.
Conclusion
Sylvian fissure meningiomas are rare, and a preoperative diagnosis is difficult without adequate knowledge of the case. Due to the special origin, Sylvian fissure meningiomas generally present non-dural attachment and have a close relationship with the MCA (30). Tumor onset usually occurs at a young age and seizures are the most common initial symptom. Sylvian fissure meningiomas have multiple pathological types and effective treatment can ensure a favorable prognosis. However, the tumor should be carefully resected in cases with arterial adherence to avoid collateral artery injuries and postoperative infarction. This study is the fifth report of a rare case of an atypical Sylvian fissure meningioma with unusual presentations on preoperative radiological examination. The case report adds new knowledge to the existing literature and will help to remind clinicians of the rare presentations of atypical meningioma.
Ethics Statement
The studies involving human participants were reviewed and approved by Institutional Review Board of the Second Affiliated Hospital of Zhejiang University School of Medicine [Hangzhou]. Written informed consent was obtained from the patient for the publication of this case report.
Author Contributions
CC and ZZhu: drafted the manuscript. XinG and XiaG: acquisition of data. HJ, ZZhe, and AS: analysis or interpretation of data. JZhu and JZha: study concept and design.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
I would like to show my deepest gratitude to my supervisor, JZhu, who has provided me with valuable guidance.
References
- 1.Garzon-Muvdi T, Yang W, Lim M, Brem H, Huang J. Atypical and anaplastic meningioma: outcomes in a population based study. J Neuro Oncol. (2017) 133:321–30. 10.1007/s11060-017-2436-6 [DOI] [PubMed] [Google Scholar]
- 2.Cushing HJSCCT. Meningiomas: their classification, regional behavior, life history, and surgical end result. Am J Surg. (1938) 111:735. [Google Scholar]
- 3.Barcia-Goyanes J, Calvo-Garra WJ. Meningiomas without arachnoid attachment. Acta Neurochir. (1953) 3:241–7. 10.1007/BF01407054 [DOI] [PubMed] [Google Scholar]
- 4.Mori S, Ishihara H, Sogabe T, Kodama Y, Hibino H. A case of deep sylvian meningioma (author's transl). No Shinkei geka. (1977) 5:385–92. [PubMed] [Google Scholar]
- 5.Saito A, Mizuno Y, Adachi Y, Itoh T. Deep Sylvian psammomeningioma, report of a case (author's transl). No to shinkei. (1979) 31:79–83. [PubMed] [Google Scholar]
- 6.Tsuchida T, Ito J, Sekiguchi K, Honda H, Ueki K. A case of deep sylvian meningioma with intracerebral hematoma (author's transl). No Shinkei geka. (1981) 9:395–400. [PubMed] [Google Scholar]
- 7.Awa H, Asakura T, Kasamo S, Nakamura KJAMUK. Cystic sylvian meningioma of young adolescence: a case report. Acta Med Univ Kagoshima. (1982) 24:49–59. [Google Scholar]
- 8.Drake JM, Hendrick B, Becker LE, Chuang SH, Hoffman HJ, Humphreys RP. Intracranial meningiomas in children. Pediatr Neurosurg. (1985) 12:134–9. 10.1159/000120235 [DOI] [PubMed] [Google Scholar]
- 9.Okamoto S, Handa H, Yamashita J, Tokuriki YJ. Deep sylvian meningiomas. Surg Neurol. (1985) 23:303–8. 10.1016/0090-3019(85)90101-6 [DOI] [PubMed] [Google Scholar]
- 10.Hirao M, Oka N, Hirashima Y, Horie Y, Takaku A. Deep sylvian meningioma: case report and review of the literature. No Shinkei geka. (1986) 14:1471–8. [PubMed] [Google Scholar]
- 11.Silbergeld D, Berger M, Griffin B. Sylvian fissure meningioma in a child: case report and review of the literature. Pediatr Neurosci. (1988) 14:50–3. 10.1159/000120362 [DOI] [PubMed] [Google Scholar]
- 12.Cho BK, Wang K, Chang K, Chi JG. Deep sylvian meningioma in a child. Childs Nerv Syst. (1990) 6:228–30. 10.1007/BF01850980 [DOI] [PubMed] [Google Scholar]
- 13.Graziani N, Donnet A, Vincentelli F, Dechambenoit G, Grisoli F. [Deep sylvian meningioma. Apropos of a case. Review of the literature]. Neuro Chirurgie. (1992) 38:179–82. [PubMed] [Google Scholar]
- 14.Chiocca EA, Boviatsis EJ, Westmark RM, Short MP, Richardson EP, Zervas NT. Deep sylvian fissure meningioma without dural attachment in an adult: case report. Neurosurgery. (1994) 35:944–6. 10.1227/00006123-199411000-00021 [DOI] [PubMed] [Google Scholar]
- 15.Mori Y, Shibuya M, Sugita K, Nagasaka T. Deep sylvian meningioma: a case report of a child. No Shinkei geka. (1994) 22:1147–51. [PubMed] [Google Scholar]
- 16.Matsumoto S, Yamamoto T, Ban S, Sato S, Shingu T, Yoshida S, et al. A case of deep sylvian meningioma presenting temporal lobe epilepsy. No to shinkei. (1995) 47:503–8. [PubMed] [Google Scholar]
- 17.Cooper JR, Marshman LA, Smith CM, Powell T. Case report: Sylvian fissure meningioma without dural attachment in a 4-year-old child. Clin Radiol. (1997) 52:874–6. 10.1016/S0009-9260(97)80088-8 [DOI] [PubMed] [Google Scholar]
- 18.Mitsuyama T, Kasuya H, Kubo O, Hirasawa K, Hori T. [Left Sylvian fissure meningioma in a one-year-eight-month old child]. No Shinkei Geka. (2000) 28:459–64. [PubMed] [Google Scholar]
- 19.Kaplan SS, Ojemann JG, Park TS. Pediatric sylvian fissure meningioma. Pediatr Neurosurg. (2002) 36:275–6. 10.1159/000058433 [DOI] [PubMed] [Google Scholar]
- 20.Chang JH, Kim JA, Chang JW, Park YG, Kim TS. Sylvian meningioma without dural attachment in an adult. J Neuro Oncol. (2005) 74:43–5. 10.1007/s11060-005-2896-y [DOI] [PubMed] [Google Scholar]
- 21.McIver JI, Scheithauer BW, Atkinson JL. Deep Sylvian fissure chordoid meningioma: case report. Neurosurgery. (2005) 57:E1064; discussion E1064. 10.1227/01.NEU.0000180038.12168.ca [DOI] [PubMed] [Google Scholar]
- 22.Cecchi PC, Campello M, Rizzo P, Mair K, Schwarz A. Atypical meningioma of the sylvian fissure. J Clin Neurosci. (2009) 16:1234–9. 10.1016/j.jocn.2008.10.027 [DOI] [PubMed] [Google Scholar]
- 23.Samson Sujit Kumar G, Rajshekhar V. Deep sylvian meningioma: a case report and review of literature. Child's Nerv Syst. (2009) 25:129–32. 10.1007/s00381-008-0686-5 [DOI] [PubMed] [Google Scholar]
- 24.Chae MP, Song SW, Park SH, Park CK. Experience with 5-aminolevulinic Acid in fluorescence-guided resection of a deep sylvian meningioma. J Korean Neurosurg Soc. (2012) 52:558–60. 10.3340/jkns.2012.52.6.558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ma L, Xiao SY, Zhang YK. Atypical meningioma of sylvian fissure with a 20-year history: a rare case report. Neurol Sci. (2012) 33:143–5. 10.1007/s10072-011-0637-x [DOI] [PubMed] [Google Scholar]
- 26.Aras Y, Akcakaya MO, Aydoseli A, Izgi N. Staged surgery for sylvian fissure meningiomas without dural attachment: report of two cases. Clin Neurol Neurosurg. (2013) 115:1527–9. 10.1016/j.clineuro.2012.12.010 [DOI] [PubMed] [Google Scholar]
- 27.Kim JY, Lee EJ, Chang HW, Jung HR, Kim EM, Kim SP. Deep Sylvian Meningioma in a 43-year-old man: a case report. J Korean Soc Magn Reson Med. (2013) 17:308–11. 10.13104/jksmrm.2013.17.4.308 [DOI] [Google Scholar]
- 28.Fukushima S, Narita Y, Yonezawa M, Ohno M, Arita H, Miyakita Y, et al. Short communication: sclerosing meningioma in the deep sylvian fissure. Brain Tumor Pathol. (2014) 31:289–92. 10.1007/s10014-013-0167-8 [DOI] [PubMed] [Google Scholar]
- 29.Donovan DJ, Thavapalan V. Pediatric meningeal tumors of the sylvian fissure region without dural attachment: a series of three patients and review of the literature. Surg J (N Y). (2016) 2:e31–6. 10.1055/s-0036-1584166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brogna C, Lavrador JP, Patel S, Ribas EC, Aizpurua M, Vergani F, et al. Grade II Sylvian fissure meningiomas without dural attachment: case report and review of the literature. CNS Oncol. (2018) 7:Cns20. 10.2217/cns-2018-0004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Amirjamshidi A, Khalatbari MR, Abbasioun K, Ghasemi B. Sylvian fissure meningioma in childhood: report of 3 cases and review of the literature. Pediatr Neurosurg. (2019) 54:270–6. 10.1159/000500765 [DOI] [PubMed] [Google Scholar]
- 32.Yamagishi M, Bohara M, Komasaku S, Yamada M, Kawahara D, Sadamura Y, et al. Deep sylvian meningioma without dural attachment - a case report. NMC Case Rep J. (2019) 6:51–5. 10.2176/nmccrj.cr.2018-0215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Dolecek TA, Propp JM, Stroup NE, Kruchko C. CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the United States in 2005-2009. Neuro Oncol. (2012) 14(Suppl. 5):v1–49. 10.1093/neuonc/nos218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wiemels J, Wrensch M, Claus EB. Epidemiology and etiology of meningioma. J Neuro Oncol. (2010) 99:307–14. 10.1007/s11060-010-0386-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Englot DJ, Magill ST, Han SJ, Chang EF, Berger MS, McDermott MW. Seizures in supratentorial meningioma: a systematic review and meta-analysis. J Neurosurg. (2016) 124:1552–61. 10.3171/2015.4.JNS142742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Beaumont A, Whittle IR. The pathogenesis of tumour associated epilepsy. Acta Neurochir. (2000) 142:1–15. 10.1007/s007010050001 [DOI] [PubMed] [Google Scholar]
- 37.Ostrom QT, Gittleman H, Xu J, Kromer C, Wolinsky Y, Kruchko C, et al. CBTRUS statistical report: primary brain and other central nervous system tumors diagnosed in the United States in 2009-2013. Neuro Oncol. (2016) 18:v1–75. 10.1093/neuonc/now207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Louis DN, Perry A, Reifenberger G, von Deimling A, Figarella-Branger D, Cavenee WK, et al. The 2016 World Health Organization Classification of tumors of the central nervous system: a summary. Acta Neuropathol. (2016) 131:803–20. 10.1007/s00401-016-1545-1 [DOI] [PubMed] [Google Scholar]
- 39.Kubota Y, Ueda T, Kagawa Y, Sakai N, Hara A. Microcystic meningioma without enhancement on neuroimaging–case report. Neurologia Med Chir. (1997) 37:407–10. 10.2176/nmc.37.407 [DOI] [PubMed] [Google Scholar]
- 40.Zhang Q, Wang X. A Distinctive pathologic meningioma completely without enhancement and dural tail sign on imaging findings. World Neurosurg. (2017) 101:815.e9–12. 10.1016/j.wneu.2017.02.099 [DOI] [PubMed] [Google Scholar]