Summary
Many animals perceive features of higher-order visual motion that are beyond the spatiotemporal correlations of luminance defined in first-order motion. Although the neural mechanisms of first-order motion detection have become understood in recent years, those underlying higher-order motion perception remain unclear. Here, we established a paradigm to assess the detection of theta motion—a type of higher-order motion—in freely walking Drosophila. Behavioral screening using this paradigm identified two clusters of neurons in the central brain, designated as R18C12, which were required for perception of theta motion but not for first-order motion. Furthermore, theta motion-activated R18C12 neurons were structurally and functionally located downstream of visual projection neurons in lobula, lobula columnar cells LC16, which activated R18C12 neurons via interactions of acetylcholine (ACh) and muscarinic acetylcholine receptors (mAChRs). The current study provides new insights into LC neurons and the neuronal mechanisms underlying visual information processing in complex natural scenes.
Subject Areas: Biological Sciences, Neuroscience, Molecular Neuroscience, Sensory Neuroscience
Graphical Abstract

Highlights
-
•
Perception of theta motion requires LC16 and R18C12 neurons
-
•
R18C12 neurons are activated by theta motion
-
•
R18C12 neurons form synaptic connections with LC16 neurons
-
•
LC16 neurons activate R18C12 neurons through ACh acting on mAChR
Biological Sciences; Neuroscience; Molecular Neuroscience; Sensory Neuroscience
Introduction
Motion perception is crucial for survival and reproduction in many animals. First-order motion, such as that produced by a brightly colored bird flying through the sky, is defined by spatiotemporal correlations in luminance that obey the theoretical basis of elementary motion detectors (EMDs) (Hassenstein and Reichardt, 1956, Borst and Egelhaaf, 1989). However, the motion of a bird can still be perceived if it flies under the shadow of a leafy tree, even though the coherent space-time correlations in luminance are broken. This type of motion is called higher-order motion, or non-Fourier motion (Chubb and Sperling, 1988, Burr and Thompson, 2011). Widespread in nature, higher-order motion refers to motion containing paradoxical motion cues (Quenzer and Zanker, 1991) or movement of visual objects that have no net motion energy (Chubb and Sperling, 1988, Lee and Nordstrom, 2012). Generally, it is defined by spatiotemporal correlations in signals, including contrast, texture, or various cues other than luminance (Badcock and Derrington, 1985, Cavanagh and Mather, 1989). One type of higher-order motion is theta motion (Zanker, 1990), which contains moving objects with moving textures. Notably, the textures of the objects move coherently in the direction opposite the object movement (Lee and Nordstrom, 2012). The perception of higher-order motion contributes to the discrimination of complex moving features in detailed natural scenes (Aptekar and Frye, 2013). Several studies have reported that, in addition to humans (Lu and Sperling, 1995), higher-order motion can be perceived by fish (Orger et al., 2000) and even flies (Theobald et al., 2008), suggesting convergent evolution for this type of visual perception.
The theoretical model for biological computation of first-order motion was first proposed by Werner Reichardt and Bernard Hassenstein (Hassenstein-Reichardt model of elementary motion) over half a century ago. It has received extensive support from experimental studies of visual systems, including that of fruit flies. However, our understanding of higher-order motion perception is still relatively primitive, without verifiable theoretical models and far fewer publications. Thus, the neural mechanisms underlying higher-order motion perception is an appealing topic.
Ample evidence suggests that first-order and higher-order motion are processed separately, at least in part (Nishida, 2011, Vaina and Cowey, 1996, Vaina et al., 2000). Therefore, how these processes differ and which brain regions are involved need to be further studied. Identifying the responsible neurons in the visual processing pathways is an essential first step. In cats, Y ganglion cells have been found to respond to higher-order motion, implementing demodulating nonlinearity of complex visual features, which indicates a distinct pathway for responses to non-Fourier visual features (Demb et al., 2001, Rosenberg et al., 2010, Rosenberg and Issa, 2011). However, aside from Y ganglion cells, no specific neuronal circuits underlying the perception of higher-order motion have been identified in mammals.
In the visual systems of insects, scientists have identified several types of neurons that respond to higher-order motion, such as T5 neurons and lobula plate tangential cells (Quenzer and Zanker, 1991, Lee and Nordstrom, 2012, Aptekar et al., 2015). The lobular tangential cells LT10 and LT11 are thought to participate in the perception of motion-defined higher-order motion in Drosophila (Zhang et al., 2012). Additional behavioral and physiological evidence from studies of flies has also suggested separate processing pathways for first-order elementary motion (EM) and higher-order figure motion (FM) (Aptekar et al., 2012, Lee and Nordstrom, 2012, Theobald et al., 2010, Bahl et al., 2013, Schnell et al., 2012).
Since the discovery that Drosophila is able to detect higher-order motion, which had been commonly believed to require a more powerful nervous system, the underlying neural mechanism has been a compelling research subject. One reason is that neither the circuity nor the computational algorithm is clear in fruit flies or in more complex organisms. The hope is that success with the relatively simple nervous system of the fruit fly will lead to a rapid breakthrough that provides clues as to how other animals detect higher-order motion. In recent years, rapid development of experimental methods and techniques for genetic manipulation in the fruit fly has led to substantial progress in identifying the neural circuits underlying first-order motion (Borst and Helmstaedter, 2015, Mauss et al., 2017). The progress and technical advances would also help to understand how an animal, such as a fruit fly, processes complex natural visual scenes beyond relatively simple first-order motion.
The studies on lobula plate have revealed its important roles in first-order motion perception (Borst and Helmstaedter, 2015, Mauss et al., 2017). However, the functions of lobula need to be further investigated. Recently, 22 kinds of visual projection neurons (VPNs) in the lobula have been identified (Wu et al., 2016). These neurons carry retinotopic visual signals from upstream neuropils and project to the central brain where the axon terminals from different VPNs form separate non-retinotopic optic glomeruli. Moreover, previous research has shown that specific VPNs are responsible for specific behaviors. For example, lobula columnar (LC) 6 and LC16 cells sense looming objects (Wu et al., 2016). Additionally, LC16 cells trigger retreat behavior through the moonwalker descending neurons (Sen et al., 2017). A growing body of evidence suggests that the optic glomeruli encode visual information in the same way as olfactory glomeruli (Keleş and Frye, 2017). Activation patterns formed by different combinations of optic glomeruli might be responsible for particular features of visual objects. To answer this question, the specific function of LC neurons and those downstream of them should be investigated. Knowing the function of LC neurons might help to explain the features that are processed in the visual pathway. Except for the giant fiber, other neurons directly downstream of LCs remain unknown (Ache et al., 2019). Searching for neurons directly downstream of LCs and investigating the signal transduction from LCs to the downstream neurons might help clarify the mechanisms underlying the perception of higher-order motion.
In the current study, we developed a paradigm that allowed us to discern first-order and higher-order motion-processing ability in freely walking flies. Using this paradigm, we identified a group of critical neurons in the central brain, namely, R18C12, which were specifically involved in the perception of theta motion. This was accomplished through signals from the lobular visual projection neurons LC16 via mAChRs. Our results indicate a specific neuronal pathway for perceiving higher-order motion and provide support for the idea that first-order and higher-order motion are processed separately.
Results
Tracking Theta Motion in Freely Walking Flies
We developed a simple paradigm that was suitable for large-scale behavior-based screening (Figure 1A). In our paradigm, one single wing-clipped fly was put on a round platform at the bottom of the setup and presented with different visual motion stimuli via a cylindrical LED screen that surrounded them. When flies tracked apparent visual motion, it resulted in circular trajectories that were quantifiable using image analysis (Figures 1B and 1C). We measured the portion of the time a fly spent tracking the motion and designated it as the performance index (PI, Figure 1B, for details see Transparent Methods). The overall tracking performance was reflected in the average PI toward clockwise-rotating (PIclc) and anticlockwise-rotating (PIanticlc, Figures S1A–S1C) stimuli over the test periods (90 s each). In addition to tracking behavior itself, we also calculated locomotion activity, speed, and distance from the flies' movement trajectories (Figures S1D–S1F).
Figure 1.
A Paradigm for Evaluating Visual Motion Perception in Freely Walking Flies
(A) A schematic diagram of the experimental apparatus. Red dots represent the wing-clipped flies.
(B) A schematic diagram of trajectory analysis. The black dashed circle indicates the border of the platform. The gray dashed circle indicates the LED screen. The curved blue arrow outside the circle represents the direction of the moving bars in the visual motion stimuli. When a fly walked from A to B to C (or C′) in two sequential frames, projection of its trajectory on the outer circle form point B1 and C1 (or C1’). The direction of the movement from B1 to C1 (or C1’) represented the turning response of the fly to the visual motion. When presented with a clockwise stimulus (blue arrow), we calculated the proportion of time spent walking with the visual motion (A-B-C) or walking against it (A-B-C′). Their difference was used as an indicator of behavioral performance (see details in Transparent Methods).
(C) Space-time plots of theta and Fourier motion stimuli (upper panels). Only a single row of the stimuli is shown here. Changes over time are displayed from top to bottom. For theta motion, the bars moved rightward (the window motion) but the dots inside the bars moved leftward (the contrast motion) at the same speed. Lower panels show the walking trajectories of WTCS flies presented with theta and Fourier motion stimuli. Blue and red lines represented the trajectories corresponding to the clockwise and anticlockwise motion stimuli, respectively.
(D) The PI scores of WTCS flies when presented with theta and Fourier motion stimuli at various speeds (mean + SEM; n = 32 each; n.s., not significant, p > 0.05; ∗∗∗p < 0.001; Mann-Whitney test).
See also Figures S1, S2 and S5.
After pilot tests, we used two rotating bars spaced 180° apart on the LED display as the visual stimuli. Wild-type Canton-S (WTCS) flies were able to track both higher-order theta motion and first-order Fourier motion (Figure 1C and Video S1). Over a broad range of speeds for both types of visual motion stimuli, the wild-type flies exhibited strong and consistent tracking responses (Figure 1D). Notably, flies showed weaker responses toward theta motion than to Fourier motion in all tested conditions (Figure 1D). Unlike Fourier motion, in theta motion, the direction that the dotted patterns moved (contrast motion) was opposite that of the visual bar (window motion). Flies exhibited turning behavior when exposed to visual motion stimuli, including turning with and turning against the motion. To estimate the relative contributions of the two kinds of turns to overall performance, we separated the PIs in Figure 1D into responses for turning with and turning against the visual motion (Figure S1G). When exposed to theta motion stimuli, turning with the motion was turning with the moving bars (window motion), whereas turning against the motion was turning with the patterns inside the bars (first-order motion). As the difference in PI values under Fourier motion and theta motion could also be caused by a difference in the overall time spent walking or in the proportion of time spent turning, we further quantified the amount of time WTCS flies spent walking and the time they walked straight when presented with the visual motion stimuli. As shown in Figures S1H and S1I, the walking activities (the fraction of walking time in a 90-s observation period) and the proportion of straight walks (the fraction of time spent walking relatively straight in the 90 s) were similar for Fourier motion and theta motion and were not influenced by the moving speed of either visual motion.
Upper panels show theta motion and Fourier motion stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
Because the composition, angular velocity, and angular position of visual motion might affect the visual response, we used high-resolution videos to further analyze the instantaneous response (walking distance and turning angle) of WTCS flies in relation to the movement of visual bars. The main parameters, including the direction of walking (heading) and angle of turning (changes of heading), were calculated every 0.1 s (Figure S2A). The direction and speed of visual motion were derived from a video and used to generate parameters such as the angular position and angular velocity of the motion bars from the point view of the fly. Importantly, we dynamically assigned the two motion bars (180° apart) as the current anterior motion bar and the current posterior motion bar. For this set of analyses, the motion bars always rotated clockwise, and a clockwise rotation or turn defined a positive angle (Figure S2A).
The distribution of heading angles indicated that heading frequencies were similar across all directions (Figures S2B and S2C), confirming the isotropic nature of our setup. At all walking directions, flies turned with the visual motion (turning angle >0°) more than they turned against the visual motion (turning angle <0°). Notably, the tendency to turn with the motion was higher for the Fourier motion condition than for the theta motion condition (Figures S2B and S2C), indicating that flies exhibited weaker tracking responses toward theta motion. This was consistent with the quantification results using PI values in Figure 1D.
The distribution of view angles for the motion bars indicated that, when presented with Fourier motion, flies had a high tendency to keep one moving bar in front of it (Figures S2D–S2F) and the other behind it (Figure S2G). Interestingly, instead of centering at 0°, turning-with-motion events were biased toward the right-front visual field of the flies, centered at about 18° (median: 17.8, the 50% population was between 5.4° and 31.0°) (Figures S2D and S2E). This suggests a strong tracking response toward the anterior motion bar moving clockwise from 0° to 40° (regressive). Furthermore, the distribution of view angles for the posterior motion bar in Figure S2G was broader than that of the anterior bar in Figure S2D, suggesting that the anterior motion bar was more effective, although both bars induced the flies to turn with the visual motion. Together, the PI values toward Fourier motion largely reflect the response of flies to the regressive bar in front of them.
Similar distributions of the view angles and turning angles were observed in WTCS flies when presented with theta motion (Figures S2H–S2K). However, comparing the response to Fourier motion (Figures S2D–S2F) and theta motion (Figures S2H–S2J) revealed a lower response toward theta motion. This was driven by fewer turn-with-motion events in the right-front quadrant (corresponding to frontal regressive motion) and more turn-against-motion events in the left-front quadrant (corresponding to frontal progressive motion) (Figures S2E and S2I). This might have been due to a negative effect of the first-order motion component (the dots rotated anticlockwise to induce an opposite tracking response) of theta motion (the bar rotating clockwise). Other mechanisms unrelated to first-order motion, but inherently related to high-order motion processing, also likely influence the visual behavior.
Like tethered flight in a flight simulator or fixed walking on a suspended ball, our paradigm provides an effective alternative for investigating the neural mechanisms underlying higher-order motion perception.
Identifying Neurons Critical for Perception of Theta Motion but Not Fourier Motion
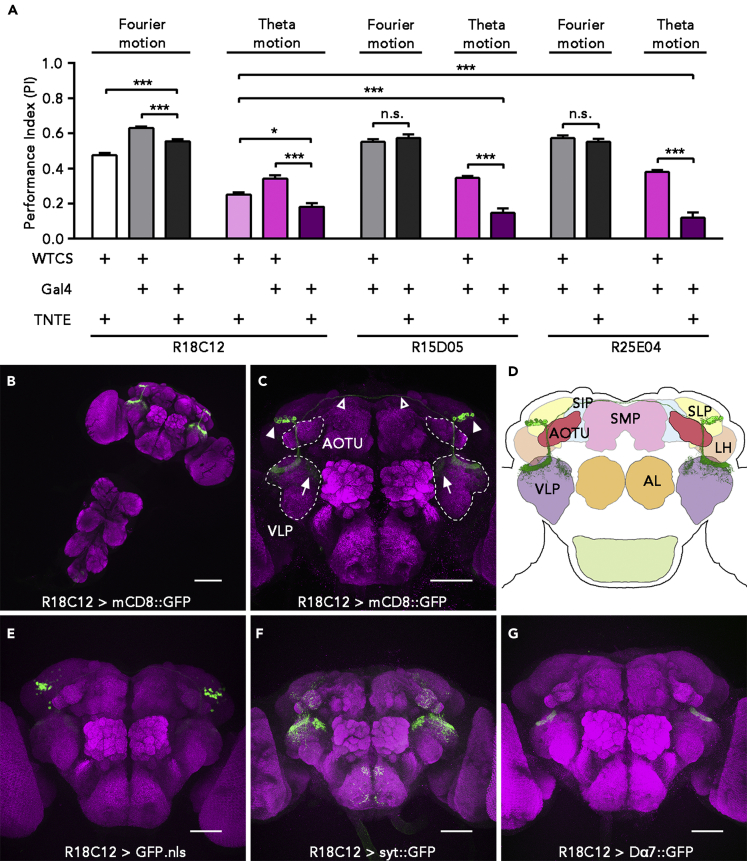
To identify neurons involved in the perception of theta motion, we performed a behavioral screen using our paradigm. We screened approximately 420 Gal4 lines that were crossed with UAS-TNTE flies to block synaptic transmission in the corresponding Gal4 labeled neurons (Brand and Perrimon, 1993, Sweeney et al., 1995). Flies with candidate neurons silenced were evaluated for their response to theta motion. We identified three Gal4 lines with defective responses, R18C12-, R15D05-, and R25E04-Gal4. When Gal4-labeled neurons in these lines were blocked by tetanus toxin light chain, flies exhibited significantly decreased responses toward theta motion but normal responses to Fourier motion (Figure 2A). Among the three silenced lines, R18C12>TNTE and R15D05>TNTE flies did not show any signs of locomotion difficulty, including moving activity, speed, and distance (Figure S3). Blocking a majority of Gal4-labeled neurons did not cause significant deficits in perceiving theta motion. Four examples are shown in Figure S4A, highlighting the specific roles of R18C12 neurons in theta motion detection.
Figure 2.
R18C12 Neurons Were Specifically Involved in Perception of Theta Motion
(A) The performance indexes (PIs) of R18C12>TNTE, R15D05>TNTE, R25E04>TNTE flies and their genetic controls when presented with Fourier motion and theta motion stimuli at 60 pps. Black and gray bars indicate the responses to Fourier motion, whereas light and dark purple bars indicate the responses to theta motion (mean + SEM; n = 20–32; n.s., not significant, p > 0.05; ∗p < 0.05; ∗∗∗p < 0.001; Mann-Whitney test).
(B) Expression pattern of mCD8::GFP under the control of R18C12-GAL4 in the central brain and VNC. Scale bar, 100 μm.
(C) Expression pattern of R18C12>mCD8::GFP in the central brain.
(D) A schematic diagram referring to the morphology and location of R18C12 neurons (green).
(E) Cell bodies of R18C12 neurons labeled by nuclear GFP.
(F) Presynaptic region of R18C12 neurons labeled by UAS-syt::GFP.
(G) Postsynaptic region of R18C12 neurons marked by UAS-Dα7::GFP.
Scale bar applied to (C) and (E–G), 50 μm. SMP, superior medial protocerebrum; SIP, superior intermediate protocerebrum; SLP, superior lateral protocerebrum; AOTU, anterior optic tubercle; VLP, ventrolateral protocerebrum; LH, lateral horn; AL, antennal lobe; GFP signals are shown in green, and nc82 signals (as counterstain) are shown in magenta. See also Figures S3–S5.
To confirm that silencing the neurons labeled by the three Gal4s results in defective perception of theta motion, we silenced these neurons with Kir2.1, an inwardly rectifying potassium channel that causes membrane hyperpolarization (Baines et al., 2001). Both R18C12>Kir2.1 flies and R15D05>Kir2.1 flies showed significantly decreased responses to theta motion but responded normally to Fourier motion (Figures S4B and S4C). The R25E04>Kir2.1 flies were embryonic lethal.
To identify which neurons were involved in theta motion perception circuits, we examined the expression pattern of the three Gal4s using UAS-mCD8::GFP. Confocal imaging revealed that these Gal4s contained the same two clusters of neurons (Figures S4D–S4Fii). Considering the expression pattern of the Gal4s and the motor abilities in these blocked flies, we chose R18C12-Gal4 for the subsequent experiments that investigated the cellular mechanisms underlying theta motion perception. The R18C12-Gal4-labeled neurons were named R18C12 neurons.
To determine whether R18C12 neurons are also involved in the perception of other types of higher-order motion, we exposed flies to theta-like (TL) motion and drift-balanced (DB) motion and their corresponding first-order motions, Fourier motion TL and Fourier motion DB (Figures S5A and S5B, Videos S2 and S3). WTCS flies were able to track theta-like motion, drift-balanced motion, and the corresponding first-order motions (Figures S5A and S5B). Perception of theta-like motion in the R18C12>TNTE or R18C12>Kir2.1 flies was significantly decreased, compared with the genetic controls (Figures S5C and S5E). Notably, flies with silenced R18C12 neurons responded normally to drift-balanced motion and Fourier motion DB, suggesting that R18C12 neurons are not required for the perception of drift-balanced motion or that redundant sensing pathways might exist (Figures S5D and S5F).
Upper panels show theta-like and Fourier motion TL stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
Upper panels show drift-balanced and Fourier motion DB stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
The results showed that inactivating the R18C12 neurons led to a significant reduction in perception of theta and theta-like motion, without compromising the ability to perceive first-order motion. Therefore, R18C12 neurons are required for the perception of at least two types of high-order motion.
R18C12 Neurons Are Located in an Important Visual Processing Center
R18C12-Gal4 mainly labeled two clusters of neurons, one on each side of the brain (Figures 2B and 2C). These neurons innervated the ventrolateral protocerebrum (VLP), which is considered to be involved in visual processing (Ito et al., 2014). The two clusters of neurons were symmetrical and possibly interconnected with each other through the nerve fibers above the superior medial protocerebrum (SMP) (Figures 2C and 2D, hollow triangle). The cell bodies of R18C12 neurons were located between the superior intermediate protocerebrum (SIP) and the lateral horn (LH), in front of the anterior superior lateral protocerebrum (SLP) and above the anterior optic tubercle (AOTU) (Figures 2C and 2D, arrowhead). Counting the number of nuclei in R18C12>nlsGFP flies revealed a total of 16.96 ± 2.293 (mean ± SD, n = 24) labeled cells in one cluster (Figure 2E). We found no significant differences in the number of labeled cells between male and female flies, or between the left and right hemisphere.
Most nerve fibers of R18C12 neurons were bundled together and projected to the dorsal posterior VLP where they formed a dense structure with widespread tree-like dendrites that innervated the dorsal VLP. In addition, several fibers projected to the medial dorsal VLP to form a relatively low-density structure compared with the structure described above (Figure 2C, arrow). In addition to the VLP, R18C12-Gal4 also weakly labeled the AOTU. Notably, R18C12-Gal4 did not label any visual neurons in the optic lobe, suggesting that the impaired perception of higher-order motion in flies when blocking R18C12 neurons was unlikely to be caused by dysfunctions of the optic lobes (Figures S4D–S4Fii).
To analyze the input and output sites of R18C12 neurons, we expressed green fluorescent protein (GFP)-fused synaptotagmin (Syt) (Zhang et al., 2002, Christiansen et al., 2011) and nAChR-Dα7 (Dα7) (Leiss et al., 2009) in R18C12 neurons to visualize the pre- and post-synaptic regions, respectively. The distribution of Syt::GFP signals was similar to mCD8::GFP, except for strong signals in the VLP branches and faint signals in regions of cell bodies (Figure 2F). R18C12>Dα7::GFP primarily labeled a restricted region inside of the dense structure at the top of the posterior VLP (Figure 2G). These results indicate that R18C12 neurons receive most information from the dorsal posterior VLP and send information out over a broader range of sites, including the dorsal VLP and AOTU.
In summary, R18C12-Gal4 labeled two symmetrical clusters of neurons that innervated the VLP, suggesting that R18C12 neurons are directly involved in visual information processing of VLP on signals from the optic lobes.
R18C12 Neurons Are Activated Specifically by Theta Motion
To examine how R18C12 neurons respond to theta motion stimuli, we performed patch-clamp recordings on GFP-labeled R18C12 neurons while presenting the visual motion to the flies (Figure 3A). When presented with a theta motion stimulus, R18C12 neurons showed strong depolarization (Figure 3B, right). In contrast, applying a Fourier motion stimulus resulted in a weak and delayed response, which might have been an under-threshold response that could not elicit spikes (Figure 3B, left). To quantify the R18C12 neuronal responses to both types of visual motion stimuli, we calculated the maximum action potential frequencies using Spikes 2.0 software (Figure 3C) and found that theta motion stimuli induced significantly stronger responses than Fourier motion stimuli, which suggested that R18C12 neurons were specifically responsible for processing theta motion.
Figure 3.
R18C12 Neurons Were Activated by Theta Motion
(A) Schematic diagram of electrophysiological experiments showing the fly holder, the recording electrode, and the display for presenting stimuli.
(B) Representative results of in vivo patch-clamp recordings from R18C12 neurons stimulated with clockwise Fourier motion (left) and theta motion (right). The recording trace showed a phasic-tonic response to the theta motion stimulus. Detailed recording traces are shown at expanded time scales below.
(C) Maximum firing rates for the Fourier and theta motion groups (n = 8; ∗∗p < 0.01; paired t test). The maximum spike rate (Max Frequency) was the largest response rate for the neuron and was calculated by Spikes 2.0 software.
(D and E) Visualization of active neurons in response to visual motion using CaMPARI (the arrows indicate the position of R18C12 cell bodies). (D–Dii) The green CaMPARI (D) in the R18C12 neurons photoconverted to red (Di) in some R18C12 neurons when flies were presented with theta motion stimuli. (Dii) The merged image of (D) and (Di). The dotted boxes on the bottom show the magnified region of cell bodies pointed to by the arrows. (E–Eii) Photo-conversion of the R18C12 neurons did not occur when static dots were used instead of theta motion as a control.
(F) Quantitation of the relative red/green CaMPARI fluorescence in R18C12 neurons stimulated by either theta motion or static dots (control) (n = 42; ∗∗p < 0.01; Wilcoxon matched-pairs signed rank test).
(G–I) R18C12 neurons were not activated by Fourier motion. (G and H) The green CaMPARI in the R18C12 neurons was not photoconverted to red (the arrow heads indicate the position of R18C12 cell bodies) when flies were presented with Fourier motion stimuli (Gi and Gii) or static dots (Hi and Hii). Scale bar, 50 μm. (I) Quantitation of the relative red/green CaMPARI fluorescence in R18C12 neurons stimulated by either Fourier motion or static dots (n = 19; n.s., not significant, p > 0.05; paired t test). Boxplots indicate the median and middle 50% of the data, while Tukey whiskers are used to indicate variability outside the upper and lower quartiles.
We next used the calcium sensor calcium-modulated photoactivatable ratiometric integrator (CaMPARI) to visualize the response of R18C12 neurons to theta motion at the population level (Fosque et al., 2015). As shown in Figures 3D–3Dii, when flies were presented with a theta motion stimulus, the CaMPARI signals in R18C12 neurons were photo-converted from green to red in the presence of 405-nm light. In contrast, no color changes occurred in the flies presented with static dots (control) (Figures 3E–3Eii). Importantly, photo-conversion of the R18C12 neurons did not occur when flies were presented with Fourier motion (Figures 3G–3Hii). To quantify the activity of R18C12 neurons, we calculated the relative ratio of red versus green fluorescent signals in the cell bodies of R18C12 neurons and found that these ratios also indicated a significantly stronger response to theta motion than to Fourier motion (Figures 3F and 3I).
Together, these results strongly support the hypothesis that R18C12 neurons are responsible for processing theta motion but not Fourier motion.
R18C12 Neurons Form Synaptic Connections with LC16 Visual Projection Neurons
The pre- and post-synaptic sites of the R18C12 neurons indicated that potential upstream and downstream neurons should innervate the top region of the VLP. We analyzed the known expression patterns of different Gal4s and found that LC16 visual projection neurons had targeting sites in optic glomeruli that potentially overlapped with the post-synaptic sites of R18C12 neurons (Figures 4A, S6A and S6B). Moreover, analyzing the pre- and post-synaptic regions of LC16 neurons suggested that they might exchange information with R18C12 neurons through this overlapping region (Figures 4B and 4C).
Figure 4.
LC16 Neurons Act Upstream in Theta Motion Perception, Releasing ACh onto R18C12 Neurons through mAChRs
(A) Expression of mCD8::GFP under the control of LC16-GAL4 (OL0092C) in the central brain.
(B) Postsynaptic region of LC16 neurons marked by UAS-Dα7::GFP.
(C) Presynaptic region of LC16 neurons labeled by UAS-syt::GFP.
(D) The t-GRASP signal (green, indicated by arrows) reconstituted by post-t-GRASP (GFP1-10) under the control of R18C12-LexA and pre-t-GRASP (GFP11) under the control of LC16-Gal4 (OL0092C). Nc82 (gray) served as counterstaining. Scale bar applied to (A)–(D), 30 μm.
(E) The performance index values for LC16>TNTE flies and their genetic controls when presented with Fourier motion and theta motion stimuli at 60 pps (mean + SEM; n = 20 each; n.s., not significant, p > 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001; unpaired t test).
(F) Schematic diagram of the experimental design for testing the connection between LC16 and R18C12 neurons via functional imaging.
(G–I) Changes in activity in R18C12 neurons after stimulating LC16 neurons. (G) Experimental groups (Exp) showed increased activity after treatment with 1 mM ATP or 20 mM KCl. (H) The control groups (CTL) showed no ATP-related response. (I) Significant differences of ΔF/F in LC16 neurons of Exp and CTL groups (n = 15; ∗∗∗p < 0.001; Kolmogorov-Smirnov test).
(J) Immunostaining of R18C12 neurons with cholinergic markers. (Top) GFP-labeled R18C12 neurons (green), (middle) anti-ChAT4B1 signals (magenta), (bottom) colocalization of both. Scale bar, 2 μm.
(K and L) Activity changes in R18C12 neurons when simultaneously applying different ACh antagonists and activating LC16 neurons with ATP. (K) In calcium imaging experiments, ATP responses were blocked via pre-incubation (5 min) with the muscarinic ACh receptor antagonist scopolamine (Scop, 100 μM) but not with the nicotinic ACh receptor antagonist mecamylamine (MCA, 100 μM). Solid bars at X axis indicated the duration of incubation with antagonists. (L) Increased ΔF/F in R18C12 neurons in response to ATP-activated LC16 neurons was significantly blocked after Scop treatment (n = 19; ∗∗∗p < 0.001; Wilcoxon matched-pairs signed rank test) but not affected by MCA treatment (n = 19; n.s., not significant, p > 0.05; paired t test). Boxplots indicate the median and middle 50% of the data, while Tukey whiskers are used to indicate variability outside the upper and lower quartiles.
See also Figures S6 and S7.
To determine whether LC16 neurons form direct connections with R18C12 neurons, we performed GFP reconstitution across synaptic partners (GRASP) (Gordon and Scott, 2009) and target GRASP (t-GRASP) (Shearin et al., 2018) experiments. The GRASP signals of reconstituted GFP were distributed in the top VLP region, suggesting the dendrites of R18C12 neurons and LC16 neurons were located in close proximity to each other (Figure S6C). Importantly, we observed t-GRASP signals when testing whether the LC16 neurons were upstream of the R18C12 neurons (Figure 4D) but not when testing if they were downstream (Figure S6D). To determine whether LC16 neurons participate in theta motion perception, we blocked LC16 neurons using OL0092C-Gal4 as the driver and presented the flies with theta and Fourier motion. Both OL0092C>TNTE and OL0092C>Kir2.1 flies exhibited severely reduced perception of theta motion but relatively normal perception of Fourier motion (Figures 4E and S6E). Therefore, LC16 and R18C12 neurons might act as upstream-downstream partners in processing theta motion information.
We next investigated whether these neurons formed functional synapses by measuring changes in R18C12 activity while artificially stimulating the upstream LC16 neurons (Figure 4F). For this purpose, we generated a fly strain that expressed P2X2 channels (ATP-gated cation channels; Lima and Miesenböck, 2005) in the LC16 neurons and GCaMP6s (a calcium indicator) in the R18C12 neurons. In the experimental group, R18C12 neuronal activity increased significantly after stimulating LC16 neurons with 1 mM ATP (Figure 4G). In contrast, R18C12 neurons in the control flies were only activated by the application of 20 mM KCl, suggesting that these neurons were viable but did not directly respond to ATP (Figures 4H and 4I). Additionally, ATP increased intracellular Ca2+ levels in R18C12 neurons in a dose-dependent manner (500 μM–5 mM), with desensitization occurring at 10 mM (Figure S7A).
Taken together, the structural proximity, synaptic connectivity, and functional causality between the LC16 and R18C12 neurons strongly suggested that R18C12 neurons are a postsynaptic target for LC16 neurons in processing theta motion signals.
Muscarinic Acetylcholine Receptors Mediate Communication at the LC16 to R18C12 Synapses
Because LC16 neurons act as upstream partners of R18C12 neurons, we next surveyed the neurotransmitters involved in the LC16 to R18C12 neuronal signaling. Application of 1 mM ACh effectively elevated intracellular Ca2+ levels in R18C12 neurons (Figure S7B), whereas application of other neurotransmitters at 1 mM, including GABA, glutamate, dopamine, octopamine, serotonin, and histamine, had no effect of calcium levels (Figure S7B). To further confirm that the LC16 neurons are indeed cholinergic neurons, we used immunostaining to locate choline acetyltransferase, an enzyme critical for ACh synthesis. As shown in Figure 4J, anti-ChAT4B1 signals colocalized with the GFP-labeled LC16 neurons, suggesting that LC16 neurons could use ACh as neurotransmitter for the signal transduction.
To identify which type of ACh receptor is expressed by R18C12 neurons (muscarinic or nicotinic), we tested different antagonists for their abilities to block the LC16-induced activation of R18C12 neurons. We applied scopolamine (Scop, an mAChR antagonist) or mecamylamine (MCA, a nicotinic AChR antagonist) to the bath 5 min before ATP application. Pre-incubation with scopolamine blocked the ATP-induced Ca2+ increase in R18C12 neurons, whereas mecamylamine did not (Figures 4K and 4L). Additionally, the ACh response was not blocked by pre-incubation with 1 μM tetrodotoxin (TTX), ruling out the involvement of other neurons in this process (Figure S7A). Together, these results suggested that R18C12 neurons were activated by LC16 neurons via mAChRs.
Discussion
In the natural environment, animals need to process a large amount of visual information, including higher-order motion. In the current study, we established a paradigm to effectively evaluate the ability of freely walking flies to perceive Fourier (first-order) and theta (higher-order) motion. Through behavioral screening, we identified R18C12 neurons in the central brain as a pivotal component of the neural circuit underlying the perception of theta motion. Furthermore, structural and functional analysis revealed that R18C12 neurons are connected with upstream LC16 visual projection neurons, which likewise play an essential role in theta motion perception. Our study provides the neuronal evidence for critical players in higher-order motion perception both in the optic lobe and in the central brain.
Most optomotor investigations in Drosophila have used two classes of behavioral paradigms: tethered flights in a flight simulator (Götz, 1964, Heisenberg and Wolf, 1984, Wolf and Heisenberg, 1980) or fixed walking on an air-suspended ball (Götz and Wenking, 1973). Although freely walking flies have been found to track gratings presented on a cylindrical LED screen in an inherent closed-loop fashion, only a few experiments have investigated responses to visual motion in freely walking flies (Strauss et al., 1997, Katsov and Clandinin, 2008). The amount of work needed to quantitatively evaluate the behavior of hundreds of lines in a reasonable period of time is daunting if one uses the common flight simulator. Instead, we developed a walking paradigm, which enabled us to conduct an effective screen in a reasonable amount of time. Importantly, testing walking flies bypass the requirement for the ability or motivation to fly, overcoming the challenge posed by flies failing to fly after genetic manipulations, which we have commonly faced. Furthermore, in a walking paradigm, the pretreatments and experimental procedures are much simpler, making it easier to carry out large-scale behavior-based screens. Compared with a flight simulator system, our tracking system is able to calculate the turning direction of the flies at every recording time point. Therefore, it exhibits sufficient resolving power to accurately assess the visual ability of the flies. From the screen, we identified R18C12 cells, which provides a long-sought-after handle for us to peek into the neural circuit and mechanisms of theta motion detection.
What are the roles of LC16 and R18C12 neurons in the visual pathways of Drosophila? Our results showed that R18C12 acted as a downstream partner of LC16 to further process theta motion signals. However, blocking LC16 neurons elicited more severe defects in theta motion perception than did blocking R18C12 neurons. This indicated that LC16 plays a crucial role in perceiving theta motion and might relay theta motion signals to neurons other than R18C12 neurons. Moreover, R18C12 neurons might also receive signals from other upstream neurons. When analyzing the morphology of R18C12 neurons and other visual projection neurons, we found that LT10/11 and other LC neurons, such as LC6, LC9, and LC25, might form synapses with R18C12 neurons. The optic glomeruli innervated by visual projection neurons have been suggested to provide parallel pathways to different classes of information (Wu et al., 2016, Keleş and Frye, 2017). Thus, R18C12 neurons might innervate several optic glomeruli that responded to theta motion and other higher-order motion. This was supported by our results that R18C12 neurons were also involved in the perception of theta-like motion (Figure S5). Notably, nerve fibers of R18C12 neurons projected across the midline and connected the two symmetrical clusters of neurons on each side, suggesting communication between the two hemispheres during motion processing. We hypothesize that R18C12 neurons do not simply relay the upstream signals from LC16, but that they also receive visual information from other visual projection neurons in the ipsilateral or contralateral hemispheres. This would allow further processing of theta motion or even other kinds of higher-order motions.
Aside from the pathways involving R18C12 neurons, additional pathways and neurons might also contribute to the perception of theta motion. Indeed, when silencing the R18C12 neurons, the optomotor response to theta motion decreased but was not completely abolished. Although this could be due to incomplete blocking, five types of visual projection neurons, LT10, LT11, LC9, LC10a, and LC12, have also been reported to respond to theta motion (Aptekar et al., 2015, Zhang et al., 2012). These neurons project to different regions of the VLP or the optic tubercle. Our results regarding the LC16-R18C12 pathway do not exclude the possibility that other neurons downstream of LC16 also receive theta motion signals from LC16 or other visual projection neurons. Our silencing screen with TNT also revealed the involvement of neurons other than R18C12 in the perception of higher-order motion. In this screen, 17 of 420 Gal4 lines exhibited reduced tracking ability in response to theta motion. Among these, 13 silenced lines (including R18C12) showed decreased PIs that were significantly higher than zero, three silenced lines showed PIs close to zero, and one silenced line had a negative PI score. Further characterization of those candidates could help build a complete picture of how theta motion is extracted and relayed into the central brain.
To transmit neuronal signals, ACh interacts with two classes of acetylcholine receptors: nicotinic acetylcholine receptors (nAChRs) that are coupled with cation permeable ion channels and muscarinic acetylcholine receptors (mAChRs) that are coupled with G-proteins. In Drosophila, nAChRs mediate fast synaptic transmission in the central nervous system. The nAChRs in Tm2 and lobula plate tangential cells (LPTCs) are crucial for motion detection (Brotz and Borst, 1996, Takemura et al., 2011). Recently, an inhibitory mAChR was found to contribute to OFF selectivity in the Drosophila larval visual system (Qin et al., 2019). However, little is known about the physiological function of mAChRs in the visual processing of adult flies. Our result that excitatory mAChRs mediated the LC16-R18C12 circuit is intriguing, as nAChRs are more common in the Drosophila visual system. We propose that mAChRs play essential roles in high-order motion processing in Drosophila, similar to their counterparts in the vertebrate visual system. Vertebrate mAChRs in the primary visual cortex (V1) improve visual perception by regulating neuronal sensitivity via altering membrane conductance, synaptic strength, or connectivity with other neurons (Groleau et al., 2015). Similarly, the post-synaptic mAChRs in R18C12 neurons might broadly influence the connections between R18C12 and other neurons. Another function of mAChRs in V1 is to modulate neuronal synchronization to form macroscopic oscillations that change cortical activity (Groleau et al., 2015, Kang et al., 2014). If this is applicable to flies, the mAChRs in R18C12 might be involved in synchronizing the activities of different neurons related to higher-order motion. Moreover, mAChRs in V1 also contribute to attentional mechanisms (Herrero et al., 2008, Klinkenberg and Blokland, 2010), which seems to be in accord with the need for theta motion perception in flies. Taken together, mAChRs in R18C12 neurons might not only transmit signals related to theta motion but might also modulate the status of R18C12 neurons and even the local neural network.
Being a functional component involved in theta motion detection, R18C12 cells in the central brain also put the relationship between first-order motion detection and higher-order motion detection into perspective. Photoreceptors serve as the common input for perception of both types of motion. However, although blocking LC16 and R18C12 neurons strongly decreased the perception of theta motion, effects on the perception of first-order motion were minimal, suggesting the neural pathways for processing these types of motion are segregated into distinct streams early in the lobular lobes. Interestingly, other known neurons potentially related to R18C12 in functions located in the peripheral or the optic lobes, including the Y-ganglion cells in cats (Demb et al., 2001, Rosenberg et al., 2010, Rosenberg and Issa, 2011) and the figure-ground discriminating neurons in Drosophila (Aptekar et al., 2015). Identifying the R18C12 neurons promise new insights into the signal processing further in the central. Our findings should serve as a reference for further research on higher-order motion both in fruit flies and more complex animals. The next stage of investigation would be to retrogradely survey the upstream neurons to understand where the segregation first occurs.
Limitations of the Study
We demonstrated that R18C12 neurons in the central brain function downstream of LC16 neurons in the pathway that processes theta motion signals. However, the lack of direct recordings from LC16 makes it difficult to distinguish the roles of R18C12 and LC16 neurons in the perception to theta motion. Electrophysiological recordings from these neurons would be ideal for exploring their dynamic properties, but we were unable to do so for two technical reasons. First, unlike R18C12 neurons, LC16 neurons reside deep in the brain. Moreover, operations to prepare the brain for electrophysiological recordings, particularly removing tissue above the LC16 neurons, would likely damage their connections. Second, LC16 neurons represent a large population with small cell bodies, which are unsuitable for direct recording. Because it was challenging to survey all R18C12 neurons, we cannot exclude the possibility that they are functionally homogeneous. Nevertheless, we achieved patch-clamp recordings from several R18C12 neurons, which revealed explicit responses to different visual motions and shed light on neuronal pathways.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
This work was supported by grants from the National Natural Sciences Foundation of China (31871046, 91632107, 91232720, 91632112 and 31800867), the Chinese Academy of Sciences (XDB02040002, QYZDY-SSW-SMC015), and the Ministry of Science and Technology of China (2012CB825504). We thank Dr. Michael Reiser and Dr. Yijin Wang for providing the flies. We thank Haiyun Gong, Yanqiong Zhou, Yifei Du, Shan Gao, and Xudong Zhao of the IBP core facility center for technical assistance. We also thank the Janelia Fly Light Project Team, Bloomington Drosophila Stock Centers, and Drosophila Genetic Resource Center at Kyoto Institute of Technology.
Author Contributions
X.J., H.W., L.L., and Y.Z. designed the study. X.J., D.Y., and Y.C. performed behavioral experiments. X.J. performed CaMPARI and immunostaining experiments. H.W., X.W., J.Y.G., J.Y., and P.H. performed calcium imaging and electrophysiological experiments. X.J., H.W., L.L., and Y.Z. analyzed data and wrote the paper with the help of all authors.
Declaration of Interests
The authors declare no competing interests.
Published: April 24, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.101041.
Contributor Information
Li Liu, Email: liuli@ibp.ac.cn.
Yan Zhu, Email: zhuyan@ibp.ac.cn.
Data and Code Availability
The data that support the findings of this study are available from the authors upon reasonable request.
Supplemental Information
References
- Ache J.M., Polsky J., Alghailani S., Parekh R., Breads P., Peek M.Y., Bock D.D., von Reyn C.R., Card G.M. Neural basis for looming size and velocity encoding in the Drosophila giant fiber escape pathway. Curr. Biol. 2019;29:1073–1081. doi: 10.1016/j.cub.2019.01.079. [DOI] [PubMed] [Google Scholar]
- Aptekar J.W., Frye M.A. Higher-order figure discrimination in fly and human vision. Curr. Biol. 2013;23:R694–R700. doi: 10.1016/j.cub.2013.07.022. [DOI] [PubMed] [Google Scholar]
- Aptekar J.W., Keles M.F., Lu P.M., Zolotova N.M., Frye M.A. Neurons forming optic glomeruli compute figure-ground discriminations in Drosophila. J. Neurosci. 2015;35:7587–7599. doi: 10.1523/JNEUROSCI.0652-15.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aptekar J.W., Shoemaker P.A., Frye M.A. Figure tracking by flies is supported by parallel visual streams. Curr. Biol. 2012;22:482–487. doi: 10.1016/j.cub.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Badcock D.R., Derrington A.M. Detecting the displacement of periodic patterns. Vision Res. 1985;25:1253–1258. doi: 10.1016/0042-6989(85)90040-9. [DOI] [PubMed] [Google Scholar]
- Bahl A., Ammer G., Schilling T., Borst A. Object tracking in motion-blind flies. Nat. Neurosci. 2013;16:730–738. doi: 10.1038/nn.3386. [DOI] [PubMed] [Google Scholar]
- Baines R.A., Uhler J.P., Thompson A., Sweeney S.T., Bate M. Altered electrical properties in Drosophila neurons developing without synaptic transmission. J. Neurosci. 2001;21:1523–1531. doi: 10.1523/JNEUROSCI.21-05-01523.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borst A., Egelhaaf M. Principles of visual motion detection. Trends Neurosci. 1989;12:297–306. doi: 10.1016/0166-2236(89)90010-6. [DOI] [PubMed] [Google Scholar]
- Borst A., Helmstaedter M. Common circuit design in fly and mammalian motion vision. Nat. Neurosci. 2015;18:1067–1076. doi: 10.1038/nn.4050. [DOI] [PubMed] [Google Scholar]
- Brand A.H., Perrimon N. Targeted gene-expression as a means of altering cell fates and generating dominant phenotypes. Development. 1993;118:401–415. doi: 10.1242/dev.118.2.401. [DOI] [PubMed] [Google Scholar]
- Brotz T.M., Borst A. Cholinergic and GABAergic receptors on fly tangential cells and their role in visual motion detection. J. Neurophysiol. 1996;76:1786–1799. doi: 10.1152/jn.1996.76.3.1786. [DOI] [PubMed] [Google Scholar]
- Burr D., Thompson P. Motion psychophysics: 1985-2010. Vision Res. 2011;51:1431–1456. doi: 10.1016/j.visres.2011.02.008. [DOI] [PubMed] [Google Scholar]
- Cavanagh P., Mather G. Motion: the long and short of it. Spat. Vis. 1989;4:103–129. doi: 10.1163/156856889x00077. [DOI] [PubMed] [Google Scholar]
- Christiansen F., Zube C., Andlauer T.F., Wichmann C., Fouquet W., Owald D., Mertel S., Leiss F., Tavosanis G., Luna A.J. Presynapses in Kenyon cell dendrites in the mushroom body calyx of Drosophila. J. Neurosci. 2011;31:9696–9707. doi: 10.1523/JNEUROSCI.6542-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chubb C., Sperling G. Drift-balanced random stimuli: a general basis for studying non-Fourier motion perception. J. Opt. Soc. Am. A. 1988;5:1986–2007. doi: 10.1364/josaa.5.001986. [DOI] [PubMed] [Google Scholar]
- Demb J.B., Zaghloul K., Sterling P. Cellular basis for the response to second-order motion cues in Y retinal ganglion cells. Neuron. 2001;32:711–721. doi: 10.1016/s0896-6273(01)00484-6. [DOI] [PubMed] [Google Scholar]
- Fosque B.F., Sun Y., Dana H., Yang C.T., Ohyama T., Tadross M.R., Patel R., Zlatic M., Kim D.S., Ahrens M.B. Neural circuits. Labeling of active neural circuits in vivo with designed calcium integrators. Science. 2015;347:755–760. doi: 10.1126/science.1260922. [DOI] [PubMed] [Google Scholar]
- Götz K.G. Optomoter studies of the visual system of several eye mutants of the fruit fly Drosophila. Kybernetik. 1964;2:77–92. doi: 10.1007/BF00288561. [DOI] [PubMed] [Google Scholar]
- Götz K.G., Wenking H. Visual control of locomotion in the walking fruitfly Drosophila. J. Comp. Physiol. 1973;85:235–266. [Google Scholar]
- Gordon M.D., Scott K. Motor control in a Drosophila taste circuit. Neuron. 2009;61:373–384. doi: 10.1016/j.neuron.2008.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groleau M., Kang J.I., Huppé-Gourgues F., Vaucher E. Distribution and effects of the muscarinic receptor subtypes in the primary visual cortex. Front. Synaptic Neurosci. 2015;7:10. doi: 10.3389/fnsyn.2015.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassenstein B., Reichardt W. Systemtheoretische analyse der zeit, reihenfolgen und vorzeichenauswertung bei der bewegungsperzeption des russelkafers chlorophanus. Z. Naturforsch. B. 1956;11:513–524. [Google Scholar]
- Heisenberg M., Wolf R. Springer-Verlag Berlin Heidelberg; 1984. Vision in Drosophila. Genetics of Microbehavior. [Google Scholar]
- Herrero J.L., Roberts M.J., Delicato L.S., Gieselmann M.A., Dayan P., Thiele A. Acetylcholine contributes through muscarinic receptors to attentional modulation in V1. Nature. 2008;454:1110–1114. doi: 10.1038/nature07141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K., Shinomiya K., Ito M., Armstrong J.D., Boyan G., Hartenstein V., Harzsch S., Heisenberg M., Homberg U., Jenett A. A systematic nomenclature for the insect brain. Neuron. 2014;81:755–765. doi: 10.1016/j.neuron.2013.12.017. [DOI] [PubMed] [Google Scholar]
- Kang J.I., Huppé-Gourgues F., Vaucher E. Boosting visual cortex function and plasticity with acetylcholine to enhance visual perception. Front. Syst. Neurosci. 2014;8:172. doi: 10.3389/fnsys.2014.00172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katsov A.Y., Clandinin T.R. Motion processing streams in Drosophila are behaviorally specialized. Neuron. 2008;59:322–335. doi: 10.1016/j.neuron.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keleş M., Frye M.A. The eyes have it. Elife. 2017;6:e24896. doi: 10.7554/eLife.24896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klinkenberg I., Blokland A. The validity of scopolamine as a pharmacological model for cognitive impairment: a review of animal behavioral studies. Neurosci. Biobehav. Rev. 2010;34:1307–1350. doi: 10.1016/j.neubiorev.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Lee Y.J., Nordstrom K. Higher-order motion sensitivity in fly visual circuits. Proc. Natl. Acad. Sci. U S A. 2012;109:8758–8763. doi: 10.1073/pnas.1203081109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leiss F., Koper E., Hein I., Fouquet W., Lindner J., Sigrist S., Tavosanis G. Characterization of dendritic spines in the Drosophila central nervous system. Dev. Neurobiol. 2009;69:221–234. doi: 10.1002/dneu.20699. [DOI] [PubMed] [Google Scholar]
- Lima S.Q., Miesenböck G. Remote control of behavior through genetically targeted photostimulation of neurons. Cell. 2005;121:141–152. doi: 10.1016/j.cell.2005.02.004. [DOI] [PubMed] [Google Scholar]
- Lu Z.L., Sperling G. The functional architecture of human visual motion perception. Vision Res. 1995;35:2697–2722. doi: 10.1016/0042-6989(95)00025-u. [DOI] [PubMed] [Google Scholar]
- Mauss A.S., Vlasits A., Borst A., Feller M. Visual circuits for direction selectivity. Annu. Rev. Neurosci. 2017;40:211–230. doi: 10.1146/annurev-neuro-072116-031335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishida S. Advancement of motion psychophysics: review 2001-2010. J. Vis. 2011;11:11. doi: 10.1167/11.5.11. [DOI] [PubMed] [Google Scholar]
- Orger M.B., Smear M.C., Anstis S.M., Baier H. Perception of Fourier and non-Fourier motion by larval zebrafish. Nat. Neurosci. 2000;3:1128–1133. doi: 10.1038/80649. [DOI] [PubMed] [Google Scholar]
- Qin B., Humberg T.H., Kim A., Kim H.S., Short J., Diao F., White B.H., Sprecher S.G., Yuan Q. Muscarinic acetylcholine receptor signaling generates OFF selectivity in a simple visual circuit. Nat. Commun. 2019;10:4093. doi: 10.1038/s41467-019-12104-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quenzer T., Zanker J. Visual detection of paradoxical motion in flies. J. Comp. Physiol. A. 1991;169:331–340. [Google Scholar]
- Rosenberg A., Issa N.P. The Y cell visual pathway implements a demodulating nonlinearity. Neuron. 2011;71:348–361. doi: 10.1016/j.neuron.2011.05.044. [DOI] [PubMed] [Google Scholar]
- Rosenberg A., Husson T.R., Issa N.P. Subcortical representation of Non-Fourier image features. J. Neurosci. 2010;30:1985–1993. doi: 10.1523/JNEUROSCI.3258-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell B., Raghu S.V., Nern A., Borst A. Columnar cells necessary for motion responses of wide-field visual interneurons in Drosophila. J. Comp. Physiol. 2012;198:389–395. doi: 10.1007/s00359-012-0716-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen R., Wu M., Branson K., Robie A., Rubin G.M., Dickson B.J. Moonwalker descending neurons mediate visually evoked retreat in Drosophila. Curr. Biol. 2017;27:766–771. doi: 10.1016/j.cub.2017.02.008. [DOI] [PubMed] [Google Scholar]
- Shearin H.K., Quinn C.D., Mackin R.D., Macdonald I.S., Stowers R.S. t-GRASP, a targeted GRASP for assessing neuronal connectivity. J. Neurosci. Methods. 2018;306:94–102. doi: 10.1016/j.jneumeth.2018.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss R., Schuster S., Götz K.G. Processing of artificial visual feedback in the walking fruit fly Drosophila melanogaster. J. Exp. Biol. 1997;200:1281–1296. doi: 10.1242/jeb.200.9.1281. [DOI] [PubMed] [Google Scholar]
- Sweeney S.T., Broadie K., Keane J., Niemann H., O'Kane C.J. Targeted expression of tetanus toxin light chain in Drosophila specifically eliminates synaptic transmission and causes behavioral defects. Neuron. 1995;14:341–351. doi: 10.1016/0896-6273(95)90290-2. [DOI] [PubMed] [Google Scholar]
- Takemura S.Y., Karuppudurai T., Ting C.Y., Lu Z., Lee C.H., Meinertzhagen I.A. Cholinergic circuits integrate neighboring visual signals in a Drosophila motion detection pathway. Curr. Biol. 2011;21:2077–2084. doi: 10.1016/j.cub.2011.10.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theobald J.C., Duistermars B.J., Ringach D.L., Frye M.A. Flies see second-order motion. Curr. Biol. 2008;18:R464–R465. doi: 10.1016/j.cub.2008.03.050. [DOI] [PubMed] [Google Scholar]
- Theobald J.C., Shoemaker P.A., Ringach D.L., Frye M.A. Theta motion processing in fruit flies. Front. Behav. Neurosci. 2010;4:35. doi: 10.3389/fnbeh.2010.00035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaina L.M., Cowey A. Impairment of the perception of second order motion but not first order motion in a patient with unilateral focal brain damage. Proc. Biol. Sci. 1996;263:1225–1232. doi: 10.1098/rspb.1996.0180. [DOI] [PubMed] [Google Scholar]
- Vaina L.M., Soloviev S., Bienfang D.C., Cowey A. A lesion of cortical area V2 selectively impairs the perception of the direction of first-order visual motion. Neuroreport. 2000;11:1039–1044. doi: 10.1097/00001756-200004070-00028. [DOI] [PubMed] [Google Scholar]
- Wolf R., Heisenberg M. On the fine structure of yaw torque in visual flight orientation of Drosophila melanogaster. J. Comp. Physiol. 1980;140:69–80. [Google Scholar]
- Wu M., Nern A., Williamson W.R., M orimoto M.M., Reiser M.B., Card G.M., Rubin G.M. Visual projection neurons in the Drosophila lobula link feature detection to distinct behavioral programs. eLife. 2016;5:e21022. doi: 10.7554/eLife.21022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanker J.M. Theta motion: a new psychophysical paradigm indicating two levels of visual motion perception. Naturwissenschaften. 1990;77:243–246. doi: 10.1007/BF01138495. [DOI] [PubMed] [Google Scholar]
- Zhang X., Liu H., Lei Z., Wu Z., Guo A. Lobula-specific visual projection neurons are involved in perception of motion-defined second-order motion in Drosophila. J. Exp. Biol. 2012;16:524–534. doi: 10.1242/jeb.079095. [DOI] [PubMed] [Google Scholar]
- Zhang Y.Q., Rodesch C.K., Broadie K. Living synaptic vesicle marker: synaptotagmin-GFP. Genesis. 2002;34:142–145. doi: 10.1002/gene.10144. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Upper panels show theta motion and Fourier motion stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
Upper panels show theta-like and Fourier motion TL stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
Upper panels show drift-balanced and Fourier motion DB stimuli for about 3 s. Lower panels show the space-time plots of the rows indicated by red arrowheads in the corresponding upper panels. The changes over time are displayed from top to bottom.
Data Availability Statement
The data that support the findings of this study are available from the authors upon reasonable request.