Abstract
PURPOSE:
Acute hemorrhagic rectal ulcer syndrome is characterized by sudden onset, painless, and massive hemorrhage from rectal ulcer(s) in patients with serious underlying illnesses. It is a matter of controversy whether acute hemorrhagic rectal ulcer syndrome is a distinct clinical entity. This is the first Asian report on acute hemorrhagic rectal ulcer syndrome to be made outside Japan.
METHODS:
From January 1989 to December 1999, 8,085 patients underwent total colonoscopy at our institution. We retrospectively analyzed the medical records and colonoscopic files. The diagnosis of acute hemorrhagic rectal ulcer syndrome was made by means of the clinical, histologic, and colonoscopic findings.
RESULTS:
Among the 8,085 patients, 19 patients (11 males; mean age, 71.2 ± 10.1 years) were diagnosed with acute hemorrhagic rectal ulcer syndrome, which accounted for 2.8 percent of the patients with massive lower gastrointestinal bleeding. The duration from hospitalization to the onset of massive bleeding ranged from 3 to 14 (mean, 9 ± 3.3) days. Characteristics of colonoscopic appearance were solitary or multiple rectal ulcer(s), with round, circumferential, geographical, or Dieulafoy-like lesions located within a mean of 4.7 cm ± 1.5 cm from the dentate line. Histopathologically, the lesions appeared as necrosis with denudation of covering epithelium, hemorrhage, and multiple thrombi in the vessels of the mucosa and underlying stroma, which is considered to be similar to stress-related mucosa injury. Successful hemostasis was obtained in 74 percent (14/19) of patients with direct therapeutic maneuvers. Prognosis was largely dependent on accurate diagnosis and management of the underlying disorders.
CONCLUSIONS:
We assert that acute hemorrhagic rectal ulcer syndrome is a rare but important entity and stress that awareness of this clinical entity should lead to a high index of suspicion resulting in early detection, diagnosis, and appropriate therapy.
Key words: Acute hemorrhagic rectal ulcer syndrome, Rectal bleeding, Rectum, Critical illness, Rectal ulcer
In 1974, Delancy and Hitch1 first described three cases of acute, asymptomatic, and life- threatening hemorrhage from solitary ulcers of the rectum. In 1981, Soeno et al.2 used the term “acute hemorrhagic rectal ulcer” (AHRU) to describe such cases. At approximately the same time, Duff and Wright3 reported seven cases of “acute bleeding rectal ulcer” with similar presentations. In 1993, Fujimaki et al.4 considered AHRU as a syndrome. Clinically, such lesions were characterized by sudden onset, painless, massive hemorrhage from solitary or multiple rectal ulcer(s) in patients with serious underlying illnesses. The lesions usually lie 3 to 10 cm above the dentate line, and their pathogenesis is largely unknown.
With the increase of aging population and the improvement of survival of critically ill patients, the incidence of “acute hemorrhagic rectal ulcer syndrome” (AHRUS) recently has been increasing in Japan. However, AHRUS is still a controversial disease entity and is rarely cited in gastroenterology or endoscopy textbooks. We present a retrospective study of 19 cases of AHRUS to stress the existence of this uncommon disease entity as a potential etiology of massive rectal bleeding in critically ill patients.
METHODS
Between January 1989 and December 1999, 8,085 patients underwent total colonoscopy at our institution, a tertiary referral center in Southern Taiwan. Medical records and colonoscopic files were then carefully reviewed. Those patients who fulfilled all of the following criteria were considered to have AHRUS and included into this study: 1) sudden onset of painless, massive rectal bleeding, which was defined as: hemoglobin < 10 g/dl with shock (systolic blood pressure < 90 mmHg and/or pulse rate > 110/min) or hemoglobin < 7 g/dl; 2) serious underlying disorders; 3) presence of ulcerations with ongoing bleeding or stigmata of recent bleeding in the rectum, as confirmed by colonoscopy; 4) sources of bleeding from the upper gastrointestinal tract were excluded by esophagogastroduodenoscopy; 5) stool cultures and cultures of biopsy specimens from the margins of these ulcers were negative; and 6) no history of nonsteroidal anti-inflammatory drug (NSAID) administration within one month before the bleeding episode. We analyzed clinical features, colonoscopic findings, treatment, and outcome of the patients. The histologic slides of biopsy specimens were reviewed by one of the authors (K-BT), an independent and experienced pathologist.
RESULTS
Clinical Features
Of 8,085 patients, 691 patients underwent colonoscopy under a tentative diagnosis of massive lower gastrointestinal (LGI) bleeding. Of 691 patients, 19 patients (11 males; age range, 47–82 years; mean age, 71.2 ± 10.1 years) were diagnosed with AHRUS. The clinical features of the 19 patients are summarized in Table 1. Time elapsed from hospitalization for underlying disorders to the onset of massive bleeding ranged from 3 to 14 (mean, 9 ± 3.3) days. All patients were admitted to the intensive care unit and required massive amounts of blood transfusion to maintain hemodynamic stabilization. The mean amount of blood transfusion was 3,723 ml ± 2,094 ml (range, 1,200–8,000 ml). These patients had normal bowel habits (≦ 3 times/day and ≧ 3 times/week) or diarrhea (>3 times/day and stool weight >200 g/day).
Table 1.
Clinical Characteristics of 19 Acute Hemorrhagic Rectal Ulcer Syndrome Patients
| Patient No. | Age (yr) | Gender | Year | Underlying Disorder | Shock Episode Before Hematochezia | Duration (days)a | Blood Transfusion (ml) | Constipation | Treatment | Hemostasis | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 76 | M | 1989 | Cerebral infarction, PN | (-) | 14 | 4,500 | (-) | GT+SL | (-) | Died |
| 2 | 60 | M | 1991 | Cerebral infarction, BTI | (-) | 10 | 6,500 | (-) | PEI | (+) | Survived |
| 3 | 68 | M | 1993 | Renal failure | (+) | 14 | 8,000 | (-) | SL | (+) | Died |
| 4 | 64 | F | 1993 | DKA, sepsis | (-) | 7 | 6,250 | (-) | GT+SL | (-) | Died |
| 5 | 76 | F | 1994 | DKA, sepsis, ARDS | (-) | 7 | 4,500 | (-) | SL | (+) | Died |
| 6 | 81 | F | 1995 | HCC, LC, CNS infection | (-) | 12 | 4,250 | (-) | GT+SL | (-) | Died |
| 7 | 50 | M | 1995 | Oral cancer, sepsis | (-) | 7 | 1,250 | (-) | GT+SL | (+) | Survived |
| 8 | 47 | F | 1996 | Cholangiocarcinoma, sepsis | (+) | 7 | 6,000 | (-) | GT+SL | (+) | Died |
| 9 | 75 | M | 1996 | Cerebral infarction | (-) | 10 | 6,000 | (-) | GT+SL | (+) | Survived |
| 10 | 82 | M | 1997 | Renal failure | (-) | 14 | 1,000 | (-) | HP | (+) | Survived |
| 11 | 74 | F | 1997 | Cerebral hemorrhage | (-) | 3 | 3,000 | (-) | GT | (+) | Survived |
| 12 | 81 | M | 1997 | Cerebral infarction | (-) | 7 | 2,500 | (-) | SL | (+) | Survived |
| 13 | 82 | M | 1998 | Cerebral infarction | (-) | 5 | 4,000 | (-) | HP | (+) | Survived |
| 14 | 76 | F | 1998 | Lung cancer, sepsis | (-) | 7 | 3,250 | (-) | GT+SL | (-) | Died |
| 15 | 78 | F | 1998 | Colon cancer, sepsis | (-) | 12 | 1,250 | (-) | GT | (+) | Died |
| 16 | 68 | F | 1999 | Cerebral hemorrhage | (-) | 10 | 2,500 | (-) | HP | (+) | Died |
| 17 | 78 | M | 1999 | Cerebral infarction, PN | (-) | 8 | 3,250 | (-) | GT+SL | (-) | Died |
| 18 | 74 | M | 1999 | Renal failure | (-) | 5 | 1,250 | (-) | GT | (+) | Survived |
| 19 | 64 | M | 1999 | Liver failure | (-) | 12 | 1,500 | (-) | HP | (+) | Survived |
BTI = biliary tract infection; DKA = diabetic ketoacidosis; ARDS = acute respiratory distress syndrome; HCC = hepatocellular carcinoma; LC = liver cirrhosis; PN = pneumonia; GT = gauze tamponade; SL = suture ligation; PEI = pure ethanol injection; HP = heater probe.
a Duration from hospitalization for underlying disorders to hematochezia.
Endoscopic Findings
Colonoscopy
All patients underwent colonoscopic examination within 24 to 48 hours after onset of rectal bleeding (Table 2; Fig. 1). The distance from ulcer(s) to the dentate line ranged from 1 to 7 cm, with a mean of 4.7 cm ± 1.5 cm. Eight patients had a solitary ulcer (Patients 2, 3, 7, 10, 11, 14, 15, and 17), and 11 patients had multiple ulcers (Patients 1, 4–6, 8, 9, 12, 13, 16, 18, and 19). Among those patients with solitary ulcers, four patients (Patients 2, 3, 7, and 10) had a round type, one patient (Patient 14) had a geographic type, one patient (Patient 17) had a circumferential type, and two patients (Patients 11 and 15) had Dieulafoy-like type lesions (a minute submucosal arteriole that bleeds through an erosion in otherwise normal surrounding mucosa). Among those patients with multiple ulcers, one patient (Patient 8) had round types, five patients (Patients 4, 6, 13, 14, and 19) had geographical types, and five patients (Patients 1, 5, 9, 12, and 18) had circumferential types. Exposed vessels at the base of the ulcers were found in five patients (Patients 2, 10, 13, 16, and 19).
Table 2.
Endoscopic Appearance and Histologic Findings of Acute Hemorrhagic Rectal Ulcer
| Patient No. | Colonoscopy | Histologic Findings | ||
|---|---|---|---|---|
| Distance from Dentate Line (cm) | No. of Ulcers | Features of Ulcer(s) | ||
| 1 | 7 | Multiple | Circumferential | ND |
| 2 | 7 | Solitary | Round | Focal denudation of epithelium, hemorrhage, and multiple thrombi in the vessels of mucosa and the underlying stroma |
| 3 | 6 | Solitary | Round | Focal denudation of epithelium, multiple thrombi in the vessels of lamina propria beneath the surface epithelium with focal erosion |
| 4 | 5 | Multiple | Geographic | ND |
| 5 | 5 | Multiple | Circumferential | Complete denudation of epithelium and multiple thrombi in the vessels of the underlying stroma |
| 6 | 3 | Multiple | Geographic | ND |
| 7 | 3 | Solitary | Round | Focal denudation of epithelium, and multiple thrombi in the vessels of mucosa and the underlying stroma |
| 8 | 1 | Multiple | Round | Complete denudation of epithelium, and multiple thrombi in the vessels of the underlying stroma |
| 9 | 6 | Multiple | Circumferential | Complete denudation of epithelium, and multiple thrombi in the vessels of the underlying stroma |
| 10 | 5 | Solitary | Round | Focal denudation of epithelium, hemorrhage and multiple thrombi in the vessels of the underlying stroma |
| 11 | 2 | Solitary | Dieulafoy-like | Focal denudation of epithelium, multiple thrombi in the vessels of lamina propria beneath the surface epithelium with focal erosion |
| 12 | 6 | Multiple | Circumferential | ND |
| 13 | 4 | Multiple | Geographic | Complete denudation of epithelium, and multiple thrombi in the vessels of the underlying stroma |
| 14 | 6 | Solitary | Geographic | Focal denudation of epithelium, hemorrhage and multiple thrombi in the vessels of the underlying stroma |
| 15 | 4 | Solitary | Dieulafoy-like | Focal denudation of epithelium, multiple thrombi in the vessels of lamina propria beneath the surface epithelium with focal erosion |
| 16 | 6 | Multiple | Geographic | Focal denudation of epithelium, and multiple thrombi in the vessels of the underlying stroma |
| 17 | 5 | Solitary | Circumferential | ND |
| 18 | 4 | Multiple | Circumferential | Focal denudation of epithelium, multiple thrombi in the vessels of lamina propria beneath the surface epithelium with focal erosion |
| 19 | 4 | Multiple | Geographic | Complete denudation of epithelium, and multiple thrombi in the vessels of the underlying stroma |
EGD = esophagogastroduodenoscopy; AGML = acute gastric mucosa lesion; DU = duodenal ulcer; GU = gastric ulcer; EV = esophageal varice; ND = not done.
Fig. 1.
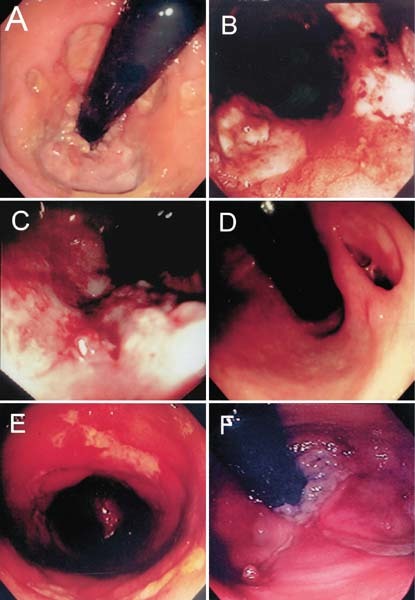
Colonoscopic findings of acute hemorrhagic rectal ulcer syndrome. A. Patient 8. Multiple round ulcers, adjacent to the dentate line. B. Patient 13. Multiple geographical ulcers, 4 cm proximal to the dentate line. C. Patient 14. Solitary geographical ulcer, 6 cm proximal to the dentate line. D. Patient 10. Solitary round ulcer, 5 cm proximal to the dentate line. E. Patient 18. Multiple circumferential ulcers, 4 cm proximal to the dentate line. F. Patient 15. Solitary Dieulafoy-like ulcer, 4 cm proximal to the dentate line.
Esophagogastroduodenoscopy
Esophagogastroduodenoscopy findings included gastroduodenal mucosa lesions in 14 of 19 patients and esophageal varices in 1 (Patient 6). For the absence of stigmata of recent hemorrhage, none of the lesions was considered the bleeding source in these patients.
Histopathology
The biopsy specimens were obtained from margin of rectal ulcer(s). Five of 19 patients (Patients 1, 4, 6, 12, and 17) did not undergo an endoscopic biopsy because of active bleeding of the lesions. The findings of all patients were uniform and demonstrated necrosis with denudation of covering epithelium, hemorrhage, and multiple thrombi in the vessels of the mucosa and underlying stroma (Table 2; Fig. 2).
Fig. 2.
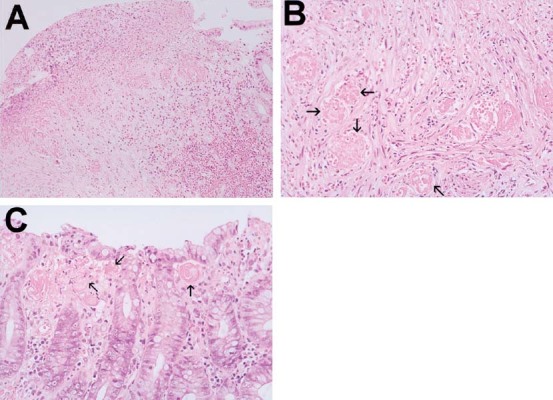
Histologic findings of acute hemorrhagic rectal ulcer syndrome.(Patient 2). A. Necrosis with focal denudation of covering epithelium, hemorrhage and multiple thrombi in the vessels of the underlying stroma (hematoxylin and eosin (H & E); ×20). B. Multiple thrombi (arrow) in the vessels within mildly inflammatory stroma (H & E; ×40). C. Multiple thrombi (arrow) in the vessels of lamina propria beneath the surface epithelium with focal erosion (H & E; ×40).
Obliteration of the lamina propria by fibrosis and smooth muscle fibers extending from a thickened muscularis mucosa, characteristic findings of solitary rectal ulcer syndrome (SRUS), were not identified in every case.
Blood and Stool Cultures
Blood cultures were performed in ten patients with evidence of infection. The results revealed Pseudomonas in four patients (Patients 1, 5, 7, 14, and 17), Escherichia coli in two patients (Patients 2 and 15), Enterobacter in one patient (Patient 4), Klebsiella in one patient (Patient 8), and no bacterial growth in one patient.
Stool samples of all patients and biopsy specimens from 14 patients were sent for bacterial cultures, including Salmonella, Shigella, and Campylobacter, and ova and parasites examination. The results were negative.
Treatment and Clinical Course
The bleeding of sources with exposed vessels was stopped by cauterization in four patients (Patients 10, 13, 16, and 19) and injection of pure ethanol in one patient (Patient 2). Other patients without identified bleeders were treated with transanal suture ligation (Patients 3, 5, and 12) or gauze tamponade (Patients 1, 4, 6–9, 11, 14, 15, 17, and 18). Among those patients treated with gauze tamponade, eight patients (Patients 1, 4, 6–9, 14, and 17) suffered recurrent bleeding (occurrence of new hematochezia after ≧ 24 hours of stable vital signs) and were treated with transanal suture ligation later. Fourteen of 19 patients (Patients 2, 3, 5, 7–13, 15, 16, 18, and 19) successfully achieved hemostasis. Unfortunately, five of these 14 patients (Patients 3, 5, 8, 15, and 16) with successful hemostasis later died of multiple organ failure.
DISCUSSION
We reviewed 19 cases of AHRUS, which account for 2.8 percent of patients with massive LGI bleeding in a tertiary referral medical center in Southern Taiwan during an 11-year period. The diagnosis of AHRUS was made in patients presenting with characteristic, painless, massive, rectal bleeding from acute rectal ulceration and serious underlying medical illness for whom other possible etiologies for rectal bleeding and rectal ulcers had been carefully excluded by history, physical checkup, endoscopic findings, and histopathology (if available), as did a previous article.1, 3, 5, 6 Gender distribution of AHRUS was roughly equal between males and females. The mean age of patients was aged 71.2 years; 90 percent (17/19) of patients were older than aged 60 years. The median interval between hospitalization for underlying medical illness and the onset of AHRUS was 7.5 (range,3–14) days.
The colonofiberscopic features of rectal ulcers in our patients appeared as round, geographical, circumferential, or Dieulafoy-like ulcer without significant surrounding inflammation, exudates, or ecchymosis. These lesions were located within a mean of 4.7 (range, 1–7) cm from the dentate line. For the acute nature of the ulceration, without significant changes on surrounding mucosa, and their close to the anal verge in location, the rectal ulcers of AHRUS can be easily overlooked during the emergent or elective anoscopy or sigmoidoscopy by physicians who are not familiar with this special disease entity, which happened to five patients (Patients 1, 5, 7, 8, and 10). Therefore, high index of suspicion with retroflex inspection of distal end of rectal ampulla during colonofiberscopic examination (Fig. 1) are invariably required for the identification of these distal rectal ulcers. After the diagnosis of first case in 1989, only three additional cases were identified in the following five years (1989–1993). With the recognition and familiarization of this disease entity, the diagnosed case number abruptly increased to 15 in the next six-year period (1994–1999).
Histopathologic examination of rectal ulcer(s) from all 14 biopsied patients showed uniform features consisting of necrosis with denudation of covering epithelium, hemorrhage, and multiple thrombi in the vessels of the epithelium and underlying stroma. These findings are identical to those of hemorrhagic necrosis of gastrointestinal tract in patients with cardiovascular dysfunction, shock, or sepsis at autopsy.7 Therefore, we concur that the pathogenesis of AHRUS may lie in a stress-induced disturbance of the circulation in the small intramural vessels, caused by secretion of catecholamines or vasoconstrictive gastrointestinal polypeptides as suggested by Ming.7 It has been well documented that severe physiologic stress (e.g., caused by major trauma, sepsis, head injury, burns, acute renal failure, cirrhosis, and multiple organ failure) often is associated with mucosa injury of the upper gastrointestinal (UGI) tract8, 9, 10; however, compared with the higher prevalence of mucosa injury of the UGI tract, stress-related mucosa disease of the colon and rectum is relatively uncommon. For patients with stress-related gastrointestinal bleeding, the source of bleeding was found to originate from rectal lesion in 1.2 to 6.7 percent at autopsy.7, 11
In the past decade, traditional interventions for patients with unidentified sources of rectal bleeding have been urgent visceral angiography or emergent surgery.12 However, AHRUS patients treated using these approaches have had a high rate of mortality and renewed bleeding.13, 14 Because angiography may not be useful but may be hazardous because of delay in controlling the bleeding and surgery with colectomy, as performed for angiodysplasia or diverticular disease, is useless in this situation. On the other hand, hemostasis was obtained in 74 percent (14/19) of patients, by means of direct therapeutic maneuvers such as cauterization, local injection, or suture ligation. Therefore, prognosis of AHRUS was primarily dependent on accurate diagnosis and management of the underlying disorders.
The differential diagnoses of AHRUS include solitary rectal ulcer syndrome (SRUS),15, 16, 17, 18 stercoral ulcer,19, 20 infectious rectal ulcers,21, 22, 23 ischemic proctitis,24, 25 trauma-induced rectal ulcer, and drug-associated or radiation-associated proctopathy.26, 27, 28 SRUS often occurs in young adults with a previous history of constipation, self-digitation, or rectal prolapse.29 The histologic features of SRUS are not found in AHRUS.17, 18, 30 In stercoral ulcer, chronic constipation always precedes the occurrence of the disease, and sometimes residual solid fecal mass can be found adhering to the margin of the ulcer,20, 31, 32, 33, 34 whereas none of our patients had a history of constipation. Patients with acute ischemic procitis often have clearly identifiable precipitating factors such as aortoiliac operations or shock, and suffer from abdominal pains.35, 36 Lesions are most commonly involved with segmental erythema and edema, with or without ulceration. In AHRUS, however, the surrounding mucosa of the rectal ulcers was either normal or only slightly hyperemic. Radiation, NSAID-induced, or trauma-induced rectal ulcers can be readily distinguished from AHRUS by a negative history of pelvic radiotherapy, NSAID use (oral or suppository form), and trauma (enema, catharsis use, and foreign objects insertion). It may be difficult to make a differential diagnosis between infectious rectal ulcers and AHRUS; however, the culture results of stool and biopsy specimens of our patients were all negative, and no typical nuclear inclusion bodies of biopsy specimens could be found, as is true in cases of rectal ulcers induced by herpes simplex virus and cytomegalovirus.23, 37, 38, 39
CONCLUSIONS
Our experience suggests that AHRUS is a rare but extremely important entity when diagnosing a bleeding rectal ulcer in patients with serious underlying disorders who present with sudden, massive, life-threatening LGI bleeding. AHRUS diagnosis is based on clinical characteristics, endoscopic findings, and exclusion of other causes. Awareness of this clinical entity should lead to a higher index of suspicion, resulting in early diagnosis and appropriate therapy.
Invited Commentary
To the Editor—Acute lower gastrointestinal bleeding is relatively uncommon and accounts for 1.5 percent of all surgical emergencies.1 Bleeding is usually self-limiting rather than ongoing. In western countries, the commonest causes are angiodysplasia, diverticular disease, neoplasms, and internal hemorrhoids. There are many other less frequent and rare causes, including solitary rectal ulcer syndrome2 and Dieulafoy-like lesions.3, 4 Solitary rectal ulcer syndrome has characteristic histologic criteria: namely, the presence of fibrous obliteration of the lamina propria with disorientation of the lamina propria with disorientation of the muscularis mucosae and extension of smooth muscle fibers into the lamina propria.5 Solitary rectal ulcer syndrome causes massive lower gastrointestinal hemorrhage generally in seriously ill patients.6, 7
In this issue of the Journal, Tseng and colleagues from Taiwan reported 19 cases of acute hemorrhagic rectal ulcer syndrome that were histologically distinct from solitary rectal ulcer syndrome. The term “acute hemorrhagic rectal ulcer” was first introduced by our Japanese colleagues in Japanese literature8, 9 but a clear clinicopathologic distinction from solitary rectal ulcer syndrome has not been made. In the study by Tseng et al., histology was retrospectively reviewed from margins of the rectal ulcers from 14 of 19 patients. The authors described necrosis with denudation of covering epithelium, hemorrhage, and multiple thrombi in the vessels of the mucosa and underlying stroma, without any of the characteristic features of solitary rectal ulcer syndrome. To add to the confusion, the authors described Dieulafoy-like lesions through erosion in otherwise normal surrounding mucosa in two patients. Of interest, both patients had biopsies taken that revealed identical histologic features as patients with clear-cut ulcers. Histologic diagnosis of solitary rectal ulcer syndrome can be difficult.
In a review from the Cleveland Clinic by a dedicated colorectal pathologist, of 23 patients reviewed, inadequate specimens and failure to recognize diagnostic features of solitary rectal ulcer syndrome contributed to delayed diagnosis in 13 and 10 patients, respectively.5 Further assessment of this rare but important condition is necessary, before a new syndrome is accepted, which will further confuse a previously confusing entity. Indeed, the work by Delancy and Hitch,6 quoted by the authors, is traditionally thought to represent patients with solitary rectal ulcer syndrome.
The clinical features are similar between the western and eastern entities. The endoscopic appearances and locations within the rectum are similar and the condition tends to occur in patients with serious comorbidity, which has a high mortality. Patients tend to succumb from underlying comorbid illness with a bleeding rectal ulcer rather than from an uncontrolled bleeding rectal ulcer. The treatment in this group of morbidly unwell patients calls for common sense and entails the simplest measure to provide hemostasis: transanal suture ligation, cauterization, local injection with hemostatic agents, or local tamponade.
The pathogenesis of solitary rectal ulcer syndrome is diverse. Although straining, pelvic floor incoordination, and prolapse are common associations, ischemia and trauma could induce similar histologic features. Severe physiologic stress associated with a serious illness could induce or exacerbate similar changes within the rectum.
Reports like this emphasized the importance of a rectal examination and proctosigmoidoscopy in patients with major rectal bleeding, which will lead to early diagnosis and appropriate therapy. Further coordinated prospective reviews by clinicians and pathologists will help clarify whether acute hemorrhagic rectal ulcer is indeed distinctly different from the better-known solitary rectal ulcer syndrome.
—Joe J. Tjandra, M.D., F.R.A.C.S., Melbourne, Australia
The Authors Reply
To the Editor—Benign ulcerative diseases of the rectum are unusual disorders with diverse causes and varied clinical behavior. According to the literature, these benign lesions typically behave as a chronic inflammatory disease with variable endoscopic features, including local proctitis, frank ulceration, and polypoid mass; however, characteristic histologic changes are as follows: fibromuscular obliteration of the lamina propria, hypertrophy of muscularis mucosae, displacement of glands and small cysts into the submucosa, erosion of the mucosa.1 To our knowledge, most of the information on this disease entity comes from studies in western countries. Researchers in western countries termed these benign rectal ulcerations “solitary rectal ulcers” and the corresponding disease entity “solitary rectal ulcers.” Most commonly, these ulcers affected young adults who had chronic constipation and straining defecation. The major problems are long-standing bowel disturbance and rectal bleeding. The intensity of bleeding is usually mild, and rectal bleeding requiring transfusion has rarely been reported. Cases of severe bleeding from a solitary rectal ulcer has been respectively reported by Delancy and Hitch2 and Haycock et al.3; however, no typical histologic appearance was seen. We suggest that the case reported by Delancy and Hitch were clinically similar to our presenting cases of acute hemorrhagic rectal ulcers, whereas the case reported by Hancock et al. was an ulcer produced by pressure necrosis from a pelvic tumor and was pathophysiologically similar to the stercoral ulcer of rectum.
The pathogenesis of the solitary rectal ulcer syndrome is unknown. Suggested causes include chronic constipation, excessive straining at defecation, spastic pelvic floor syndrome, rectal prolapse, and psychologic factors. It seems likely these benign chronic inflammatory diseases were produced by the repeated traumatic injury on the prolapsing rectal mucosa during the digital evacuation and straining at defecation.4
It is noteworthy that in certain conditions such as severe sepsis, acute cerebral vascular diseases, end-stage renal disease requiring hemodialysis, respiratory failure requiring mechanical ventilation, advanced liver cirrhosis, and advanced malignancy, etc., these benign lesions can be associated with an acute inflammatory disease, endoscopically acute bleeding ulceration, and pathologically nonspecific inflammatory change. Most information on this clinical entity comes from the work of Japanese investigators who termed these benign lesions “acute hemorrhagic rectal ulcer ” and the corresponding disease entity “acute hemorrhagic rectal ulcers syndrome.” In western countries, there have been no reports of acute hemorrhagic rectal ulcer; however, there have been two case-series reports on diseases similar to acute hemorrhagic rectal ulcer.5, 6
In contrast to their chronic counterparts, these benign acute bleeding rectal ulcers mainly affected aged patients who had significant comorbidity conditions. The major problem is life-threatening hemorrhage, which can pose an added risk of fatal sequelae to previously severely ill victims.
Although the histologic data in these studies were limited, they provided no evidence of solitary rectal ulcer syndrome. The etiopathogenesis of benign acute bleeding rectal ulcer was unknown until now. Duff and Wright5 reported that benign bleeding acute rectal ulcers were associated with serious illness or injury, similar to the illnesses or injuries thought to cause stress ulcers of the stomach and duodenum. Kanwai et al. 6 reported that rectal trauma, fecal impaction, ischemia, rectal prolapse, and idiopathy are the possible causes of benign bleeding rectal ulcers. We presumed that the 7 cases of acute bleeding rectal ulcers reported by Duff et al. and the 11 cases of idiopathic cause of bleeding rectal ulcers reported by Kanwai et al. were clinically similar to our reported series. From the viewpoint of demography, clinical characteristics, and colonoscopic findings, we suppose that both acute hemorrhagic rectal ulcer and benign acute bleeding rectal ulcer were essentially a similar clinical entity and quite different from solitary rectal ulcer. We advocate the thesis of Fujimaki et al. 7 that acute hemorrhagic rectal ulcer should be regarded as a syndrome caused by various factors. We also agree with Tjandra et al. 1 that further close examination on the colonoscopy, histology, and clinical characteristics of each patient is necessary to identify the real entity of acute hemorrhagic rectal ulcer.
—Chang-An Tseng, M.D.1
Li-Tzong Chen, M.D.2,3
Kun-Bow Tsai, M.D.2
Yu-Chung Su, M.D.2
Deng-Chyang Wu, M.D.2
Chang-Ming Jan, M.D.2
Wen-Ming Wang, M.D.2
Yong-Sang Pan, M.D.2
1 Dalin, Taiwan
2 Kaohsiung, Taiwan
3 Taipei, Taiwan
ACKNOWLEDGMENTS
The authors thank Miss Pei-Lin Wu for her assistance in conducting this study.
REFERENCES
To the article
- 1.Delancy H, Hitch WS. Solitary rectal ulcer a cause of life-threatening hemorrhage. Surgery. 1974;76:830–2. [PubMed] [Google Scholar]
- 2.Soeno T, Shoji S, Sakuraba K, et al. Acute hemorrhagic rectal ulcer accompanied with the brain disease. Akita J Med. 1981;8:207–13. [Google Scholar]
- 3.Duff JH, Wright FF. Acute and chronic benign ulcers of the rectum. Surg Gynecol Obstet. 1981;153:398–400. [PubMed] [Google Scholar]
- 4.Fujimaki E, Sugawara M, Inoue Y, et al. Endoscopical findings and clinical features of acute rectal ulcers. Gastroenterol Endosc. 1993;35:2421–4. [Google Scholar]
- 5.Peterman A, Harrison SK, Naylor AR, Donnelly PK. Benign rectal ulcer: a rare cause of life threatening haemorrhage. Scott Med J. 1993;38:48–9. doi: 10.1177/003693309303800205. [DOI] [PubMed] [Google Scholar]
- 6.Binderow SR, Mayer R, Freed JS. Massive hemorrhage from solitary rectal ulcer: toward a definitive treatment. Mt Sinai J Med. 1995;62:308–11. [PubMed] [Google Scholar]
- 7.Ming SC. Hemorrhagic necrosis of the gastrointestinal tract and its relation to cardiovascular status. Circulation. 1965;32:332–41. doi: 10.1161/01.cir.32.3.332. [DOI] [PubMed] [Google Scholar]
- 8.Haglund U. Stress ulcers. Scand J Gastroenterol Suppl. 1990;175:27–33. doi: 10.3109/00365529009093124. [DOI] [PubMed] [Google Scholar]
- 9.Tryba M, Cook D. Current guidelines on stress ulcer prophylaxis. Drugs. 1997;54:581–96. doi: 10.2165/00003495-199754040-00005. [DOI] [PubMed] [Google Scholar]
- 10.Soderstrom CA, Ducker TB. Increased susceptibility of patients with cervical cord lesions to peptic gastrointestinal complications. J Trauma. 1985;25:1030–8. [PubMed] [Google Scholar]
- 11.Prasad JK, Thomson PD, Feller I. Gastrointestinal haemorrhage in burn patients. Burns Incl Therm Inj. 1987;13:194–7. doi: 10.1016/0305-4179(87)90165-3. [DOI] [PubMed] [Google Scholar]
- 12.Colacchio TA, Forde KA, Patsos TJ, Nunez D. Impact of modern diagnostic methods on the management of active rectal bleeding. Ten year experience. Am J Surg. 1982;143:607–10. doi: 10.1016/0002-9610(82)90175-1. [DOI] [PubMed] [Google Scholar]
- 13.Wright HK. Massive colonic hemorrhage. Surg Clin North Am. 1980;60:1297–304. doi: 10.1016/s0039-6109(16)42252-8. [DOI] [PubMed] [Google Scholar]
- 14.McGuire HH, Jr, Haynes BW., Jr Massive hemorrhage for diverticulosis of the colon: guidelines for therapy based on bleeding patterns observed in fifty cases. Ann Surg. 1972;175:847–55. doi: 10.1097/00000658-197206010-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niv Y, Bat L. Solitary rectal ulcer syndrome—clinical, endoscopic, and histological spectrum. Am J Gastroenterol. 1986;81:486–91. [PubMed] [Google Scholar]
- 16.Haycock CE, Suryanarayan G, Spillert CR, Lazaro EJ. Massive hemorrhage from benign solitary ulcer of the rectum. Am J Gastroenterol. 1983;78:83–5. [PubMed] [Google Scholar]
- 17.Alberti-Flor JJ, Halter S, Dunn GD. Solitary rectal ulcer as a cause of massive lower gastrointestinal bleeding. Gastrointest Endosc. 1985;31:53–4. doi: 10.1016/s0016-5107(85)71978-5. [DOI] [PubMed] [Google Scholar]
- 18.Tjandra JJ, Fazio VW, Church JM, Lavery IC, Oakley JR, Milsom JW. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum. 1992;35:227–34. doi: 10.1007/BF02051012. [DOI] [PubMed] [Google Scholar]
- 19.Sutton R, Blake JR. Massive rectal bleeding following faecal impaction. Br J Surg. 1984;71:631. doi: 10.1002/bjs.1800710826. [DOI] [PubMed] [Google Scholar]
- 20.Knigge KL, Katon RM. Massive hematochezia from a visible vessel within a stercoral ulcer: effective endoscopic therapy. Gastrointest Endosc. 1997;46:369–70. doi: 10.1016/s0016-5107(97)70130-5. [DOI] [PubMed] [Google Scholar]
- 21.Arnold C, Moradpour D, Blum HE. Tuberculous colitis mimicking Crohn’s disease. Am J Gastroenterol. 1998;93:2294–6. doi: 10.1111/j.1572-0241.1998.00644.x. [DOI] [PubMed] [Google Scholar]
- 22.Klotz SA, Drutz DJ, Tam MR, Reed KH. Hemorrhagic proctitis due to lymphogranuloma venereum serogroup L2. Diagnosis by fluorescent monoclonal antibody. N Engl J Med. 1983;308:1563–5. doi: 10.1056/NEJM198306303082604. [DOI] [PubMed] [Google Scholar]
- 23.Ng JW, Chan AY. Severe hemorrhage from cytomegalovirus rectal ulcers in a burned adult. Am J Gastroenterol. 1987;82:695–8. [PubMed] [Google Scholar]
- 24.Nelson RL, Briley S, Schuler JJ, Abcarian H. Acute ischemic proctitis: report of six cases. Dis Colon Rectum. 1992;35:375–80. doi: 10.1007/BF02048118. [DOI] [PubMed] [Google Scholar]
- 25.Scowcroft CW, Sanowski RA, Kozarek RA. Colonoscopy in ischemic colitis. Gastrointest Endosc. 1981;27:156–61. doi: 10.1016/s0016-5107(81)73182-1. [DOI] [PubMed] [Google Scholar]
- 26.Gizzi G, Villani V, Brandi G, Paganelli GM, Di Febo G, Biasco G. Ano-rectal lesions in patients taking suppositories containing non-steroidal anti-inflammatory drugs (NSAID) Endoscopy. 1990;22:146–8. doi: 10.1055/s-2007-1012822. [DOI] [PubMed] [Google Scholar]
- 27.Crowe J, Stellato TA. Radiation-induced solitary rectal ulcer. Dis Colon Rectum. 1985;28:610–2. doi: 10.1007/BF02554159. [DOI] [PubMed] [Google Scholar]
- 28.Lanthier P, Detry R, Debongnie JC, Mahieu P, Vanheuverzwyn R. Solitary lesions of the rectum caused by suppositories combining acetylsalicylic acid and paracetamol. Gastroenterol Clin Biol. 1987;11:250–3. [PubMed] [Google Scholar]
- 29.Keighley MR, Shouler P. Clinical and manometric features of the solitary rectal ulcer syndrome. Dis Colon Rectum. 1984;27:507–12. doi: 10.1007/BF02555506. [DOI] [PubMed] [Google Scholar]
- 30.Madigan MR, Morson BC. Solitary ulcer of the rectum. Gut. 1969;10:871–81. doi: 10.1136/gut.10.11.871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Maull KI, Kinning WK, Kay S. Stercoral ulceration. Am Surg. 1982;48:20–4. [PubMed] [Google Scholar]
- 32.Matsushita M, Hajiro K, Takakuwa H, Nishio A, Tominaga M. Bleeding stercoral ulcer with visible vessels: effective endoscopic injection therapy without electrocoagulation. Gastrointest Endosc. 1998;48:559. doi: 10.1016/s0016-5107(98)70115-4. [DOI] [PubMed] [Google Scholar]
- 33.Gekas P, Schuster MM. Stercoral perforation of the colon: case report and review of the literature. Gastroenterology. 1981;80:1054–8. [PubMed] [Google Scholar]
- 34.Serpell JW, Nicholls RJ. Stercoral perforation of the colon. Br J Surg. 1990;77:1325–9. doi: 10.1002/bjs.1800771204. [DOI] [PubMed] [Google Scholar]
- 35.Travis S, Davies DR, Creamer B. Acute colorectal ischaemia after anaphylactoid shock. Gut. 1991;32:443–6. doi: 10.1136/gut.32.4.443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bharucha AE, Tremaine WJ, Johnson CD, Batts KP. Ischemic proctosigmoiditis. Am J Gastroenterol. 1996;91:2305–9. [PubMed] [Google Scholar]
- 37.Goodell SE, Quinn TC, Mkrtichian E, Schuffler MD, Holmes KK, Corey L. Herpes simplex virus proctitis in homosexual men. Clinical, sigmoidoscopic, and histopathological features. N Engl J Med. 1983;308:868–71. doi: 10.1056/NEJM198304143081503. [DOI] [PubMed] [Google Scholar]
- 38.Blackman E, Vimadalal S, Nash G. Significance of gastrointestinal cytomegalovirus infection in homosexual males. Am J Gastroenterol. 1984;79:935–40. [PubMed] [Google Scholar]
- 39.McMillan A, Smith IW. Painful anal ulceration in homosexual men. Br J Surg. 1984;71:215–6. doi: 10.1002/bjs.1800710318. [DOI] [PubMed] [Google Scholar]
To the Invited Commentary
- 1.Stower MJ, Hardcastle JD, Bourke JB. Surgical emergencies and manpower. Ann R Coll Surg Engl. 1984;66:117–9. [PMC free article] [PubMed] [Google Scholar]
- 2.Tjandra JJ, Fazio VW, Church JM, Lavery IC, Oakley JR, Milsom JW. Clinical conundrum of solitary rectal ulcer. Dis Colon Rectum. 1992;35:227–34. doi: 10.1007/BF02051012. [DOI] [PubMed] [Google Scholar]
- 3.Abdulian JD, Santoro MJ, Chen YK, Collen MJ. Dieulafoy-like lesion of the rectum presenting with exsanguinating haemorrhage: successful endoscopic sclerotherapy. Am J Gastroenterol. 1993;88:1939–41. [PubMed] [Google Scholar]
- 4.Franko E, Chardovoyne R, Wise L. Massive rectal bleeding from a Dieulafoy’s type ulcer in the rectum: a review of this unusual disease. Am J Gastroenterol. 1991;86:1545–7. [PubMed] [Google Scholar]
- 5.Tjandra JJ, Fazio VW, Petras RE, et al. Clinical and pathologic factors associated with delayed diagnosis in solitary rectal ulcer syndrome. Dis Colon Rectum. 1993;36:146–53. doi: 10.1007/BF02051170. [DOI] [PubMed] [Google Scholar]
- 6.Delancey H, Hitch WS. Solitary rectal ulcer: a cause of life-threatening hemorrhage. Surgery. 1974;76:830–2. [PubMed] [Google Scholar]
- 7.Haycock CE, Suryanarayan G, Spiller CR, et al. Massive hemorrhage from benign solitary rectal ulcer of the rectum. Am J Gastroenterol. 1983;78:83–5. [PubMed] [Google Scholar]
- 8.Soeno T, Shoji S. Acute hemorrhagic rectal ulcer accompanied with the brain disease (in Japanese with English abstract) Akita J Med. 1981;8:207–13. [Google Scholar]
- 9.Fujimaki E, Sugawara M, Inoue Y, et al. Endoscopic findings and clinical features of acute rectal ulcers (in Japanese with English abstract) Gastroenterol Endosc. 1993;35:2421–4. [Google Scholar]
To The Authors Reply
- 1.Tjandra JJ, Fazio VW, Church JM, et al. Clinical conundrum of solitary rectal ulcers. Dis Colon Rectum. 1992;35:227–34. doi: 10.1007/BF02051012. [DOI] [PubMed] [Google Scholar]
- 2.Delancy H, Hitch WS. Solitary rectal ulcer: a cause of life-threatening hemorrhage. Surgery. 1974;76:830–2. [PubMed] [Google Scholar]
- 3.Haycock CE, Suryanarayan G, Spiller CR, et al. Massive hemorrhage from benign solitary rectal ulcer of the rectum. Am J Gastroenterol. 1983;78:83–5. [PubMed] [Google Scholar]
- 4.Stein E. Solitary rectal ulcer. In: Stein E, editor. Anorectal and colon diseases. Textbook and color atlas of proctology. Springer-Verlag: Berlin; 2003. pp. 383–6. [Google Scholar]
- 5.Duff JH, Wright FF. Acute and chronic benign ulcers of the rectum. Surg Gynecol Obstet. 1981;153:398–400. [PubMed] [Google Scholar]
- 6.Kanwai F, Dulai G, Jensen DM, et al. Major stigmata of recent hemorrhage on rectal ulcers in patients with severe hematochezia: endoscopic diagnosis, treatment, and outcomes. Gastrointest Endosc. 2003;57:462–8. doi: 10.1067/mge.2003.147. [DOI] [PubMed] [Google Scholar]
- 7.Fujimaki E, Sugawara M, Inoue Y, et al. Endoscopic findings and clinical features of acute rectal ulcers (in Japanese with English abstract) Gastroenterol Endosc. 1993;35:2421–4. [Google Scholar]


