Highlights
-
•
Within the TME CAFs play an essential role in tumor progression.
-
•
CAFs are implicated in the induction of radioresistance.
-
•
Crosstalk between tumor cells, immune cells and CAFs dictates radiotherapy outcome.
Keywords: Cancer Associated Fibroblasts, Tumor Microenvironment, Radiotherapy, Radioresistance, Immune response
Abstract
Tumor growth is not only dictated by events involving tumor cells, but also by the environment they reside in, the so-called tumor microenvironment (TME). In the TME, cancer-associated fibroblasts (CAFs) are often the predominant cell type. CAFs were long considered to be of limited importance in the TME, but are now recognized for their pivotal role in cancer progression. Recently, it has become evident that different subsets of CAFs exist, with certain CAF subtypes having protumorigenic properties, whereas others show more antitumorigenic characteristics. Currently, the intricate interaction between the different subsets of CAFs with tumor cells, but also with immune cells that reside in the TME, is still poorly understood. This crosstalk of CAFs with tumor and immune cells in the TME largely dictates how a tumor responds to therapy and whether the tumor will eventually be eliminated, stay dormant or will progress and metastasize. Radiotherapy (RT) is a widely used and mostly very effective local cancer treatment, but CAFs are remarkably RT resistant. Although radiation does cause persistent DNA damage, CAFs do not die upon clinically applied doses of RT, but rather become senescent. Through the secretion of cytokines and growth factors they have been implicated in the induction of tumor radioresistance and recruitment of specific immune cells to the TME, thereby affecting local immune responses. In this review we will discuss the versatile role of CAFs in the TME and their influence on RT response.
1. Introduction
For decades cancer research has mainly been focused on cell intrinsic mechanisms of carcinogenesis, especially after the discovery of tumor suppressor genes and oncogenes [1]. However, already in 1889 the “seed and soil” hypothesis was proposed by Stephen Paget suggesting that elements of the stroma were important for tumor development [2]. He suggested that metastasis is not due to random events, but rather that some tumor cells (the “seeds”) grow preferentially in selected organs (the “soil”) and that metastases only appear when the appropriate seed was implanted in its suitable soil. This hypothesis is now confirmed by an extensive body of experimental research and clinical data [3]. There has been an appreciable increase in our understanding of the crosstalk that occurs between malignant cells and their organ microenvironment on the molecular, cellular and systemic level [3]. The variety of immune cells, stromal cells such as fibroblasts and pericytes, secreted factors, extracellular matrix (ECM) proteins and the vasculature in the tumor forms the so-called tumor microenvironment (TME) [4] (Fig. 1A). The TME does not only influence tumor cell proliferation, invasion and metastasis of cancer cells, angiogenesis, regulation of immune cell infiltration and the immune response, but also has an impact on the therapy response [5]. We here discuss the cellular and molecular cross talk between cells in the TME and radiotherapy response with a special focus on cancer associated fibroblasts (CAFs).
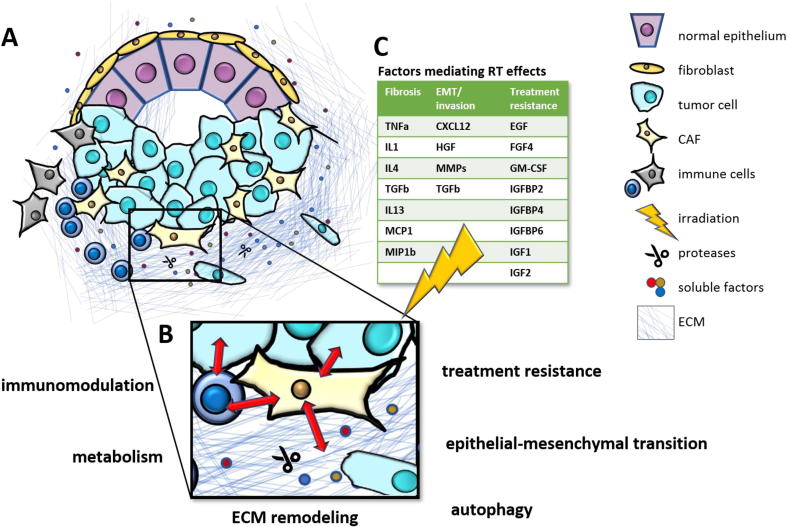
Fig. 1.
Cancer Associated Fibroblasts in the Tumor Microenvironment and therapy response A) CAFs play a central role in the TME by interacting with cancer cells and immune cells and affecting key processes such as B) immunomodulation, metabolism, ECM remodeling, autophagy, epithelial-mesenchymal transition and treatment resistance to e.g. C) radiation therapy. Different factors expressed by CAFs after RT mediate subsequent fibrosis, EMT/invasion and treatment resistance [126], [131], [132], [133].
2. Cancer-Associated Fibroblasts
Fibroblasts can be considered as the weeds of the body, as they can survive harsh conditions that usually kills other cells. Because they can survive severe stress, including chemotherapy and radiotherapy, CAFs may represent a resistant cell type that can actively contribute to tumor relapse [6]. CAFs are recognized to be of critical importance in cancer progression [7], [8], [9], and in multiple solid tumors the presence of CAFs has been associated with poor prognosis [10]. CAFs are a prominent cell type in the TME and, next to cancer cells, constitute the majority of cell populations present in many solid cancers, including head and neck squamous-cell carcinoma (HNSCC), where late-stage HNSCC for example frequently consists of up to 80% of CAFs [11], [12], [13]. CAFs can originate from various cell types including resident fibroblasts, bone marrow-derived mesenchymal cells, adipocytes, endothelial cells and stellate cells [14]. This can differ between stages of tumor initiation and progression, but also between tumor types [14], [15], [16]. In multiple solid tumors the intratumoral CAF population consists of different subsets that can respond differently to a variety of stromal stimuli, display distinctive secretory phenotypes and execute specific biological functions in the TME [17], [18], [19], [20], [21], [22], [23], [24], [25], [26]. Currently, there is not yet a universal nomenclature used for the different subsets, but ongoing efforts for this are emerging [27]. CAFs or their specific subsets are generally characterized by expression of α-smooth muscle actin (α-SMA), Fibroblast activation protein-α (FAP), S100 calcium-binding protein A4 (S100A4 or FSP1) and Platelet-derived growth factor receptor alpha/beta (PDGFRα/β). Many of these markers are, however, also present on normal (activated) fibroblasts or other cells types [6], [17], [28], [29], but together with the absence of epithelial, endothelial and immune cell markers such as Platelet endothelial cell adhesion molecule (PECAM-1 or CD31), cytokeratin and CD45 (Protein tyrosine phosphatase, receptor type, C), CAFs can be identified [13], [17], [30].
In the TME, CAFs have been demonstrated to influence many aspects of tumor biology. Most studies describe CAFs as producers of cytokines, chemokines, enzymes, metabolites and ECM (extracellular matrix) molecules [14]. Interestingly, CAFs are often found to have pro-tumorigenic properties by secreting growth factors (e.g vascular endothelial growth factor (VEGF), epidermal growth factor (EGF) and insulin-like growth factor (IGF), cytokines (e.g Interleukin (IL)-6, tumor-necrosis factor alpha (TNF-α) and Transforming Growth Factor beta (TGF-β) and chemokines (e.g. C-X-C motif chemokine (CXCL) 1, CXCL2 and CXCL12) fostering tumor growth [31], [32], [33], [34], [35], [36]. Other reports, however, have also attributed anti-tumorigenic characteristics to them; for example, they are suggested to form a physical barrier to restrict tumor cell growth and migration [37], and targeting the stroma can lead to immunosuppression, angiogenesis and worse survival [38], [39], [40].
CAFs are also known to be actively involved in the generation of desmoplasia; the growth of fibrous or connective tissue [41], [42]. In the TME, CAFs are the major cell type responsible for the synthesis of ECM proteins such as collagens, laminin, tenascin C, fibronectin, but also many matrix metallopeptidases (MMPs) [6], [43]. Interestingly, many immunosuppressive signaling molecules (e.g. VEGF and TGF-β) are matrix-bound and can be liberated from the ECM through MMPs [44], [45], thus in addition to being secreted by CAFs, also the release of immunosuppressive molecules from the ECM mediated by CAF-secreted MMPs can contribute to the immunosuppressive TME. CAFs thus play a key role in remodeling the ECM. While the matrix architecture has long been considered to simply provide the structural framework of connective tissues, it is now recognized that it plays a key role in cancer cell survival and proliferation [46] and in facilitating or suppressing antitumor immune surveillance [47]. By remodeling the ECM, CAFs can also contribute to the increased mechanical stress that is generated in the TME, which can lead to compression of blood and lymphatic vessels, reduction of the perfusion rate and thus drug delivery, but also induction of hypoxia, promoting a more aggressive cancer phenotype [27], [48].
Because CAFs can produce many different cytokines, chemokines, growth factors, ECM molecules, metabolites, miRNAs and exosomes they can influence cancer cells as well as other cells present in the TME, including immune cells (Fig. 1B).
3. CAFs and tumor cells
The interplay between cancer and stromal cells in the TME is recognized as a major driver of tumor progression and metastasis [49]. System-wide analyses have shed light on the complex tumor-fibroblast interactions that are involved in tumorigenicity [50]. This bilateral interaction of CAFs and tumor cells can oppose, but also synergize each other’s function [14], [28]. Especially the paracrine signaling between CAFs and cancer cells, where CAFs produce different growth factors, chemokines and cytokines, can lead to tumor growth, cancer invasion and metastasis [14], [33], [51], [52], [53], [54], [55], [56], [57]. The crosstalk between cancer cells and CAFs in the TME also leads to metabolic reprogramming that contributes to activation of CAFs and cancer progression [58]. Interestingly, CAFs have been shown to co-travel with tumor cells in the blood [59]. By bringing their own “soil” cancer cells facilitate their own survival and extravasation to metastatic sites [59]. After the uptake of tumor-derived exosomes also resident fibroblasts can be involved in preparing a premetastatic niche [60] and vice-versa CAF-derived exosomes are able to reprogram the metabolic machinery of tumor cells and enhance tumor growth by transferring metabolites, including amino acids, lipids and TCA-cycle intermediates to tumor cells [61].
4. CAFs and immune cells
Initially, it was believed that cancer immune evasion was dependent on cancer cells, however increasing evidence shows that also CAFs can fuel immune escape in the TME [44]. CAFs can use a wide range of mechanisms to alter the anti-tumor immune response. They can affect the anti-tumor immune response by i) influencing the recruitment of immune cells, ii) drive an immunosuppressive function in, in particular, immune cells, iii) remodel the ECM to induce an immunosuppressive microenvironment and iv) directly inhibit the killing by cytotoxic lymphocytes [62]. Hereby CAFs can attract, retain and affect multiple immune subsets into the TME, including macrophages, Dendritic cells (DCs), Myeloid Derived Suppressor cells (MDSCs), Natural Killer (NK) cells and different subsets of T cells. Emerging evidence now support the hypothesis that this is not a unidirectional effect. Tumor infiltrating immune cells can also alter the stromal compartment, emphasizing a high degree of crosstalk between stromal and immune cells [63].
4.1. Macrophages
In the TME, macrophages and especially the subset of M2 macrophages are described to be major drivers of a tumor-supporting and immunosuppressive environment in tumors, including activation of stromal cells [63], [64]. Synergistically, CAFs and tumor associated macrophages have been associated with prognostic significance in multiple types of cancer [65], [66], [67], [68]. Cell-cell interaction between these two cell types can induce the recruitment and activation of each other, and their combined activities contribute to tumor progression [68]. By secretion of CXCL12, Granulocyte Macrophage Colony-Stimulating Factor (GM-CSF) and IL-6, CAFs can actively polarize resident macrophages towards the more protumorigenic M2-phenotype [67], [69], [70]. Reciprocally, TAMs with a M2 phenotype further activate CAFs and thereby promote tumor progression [71].
It has also been postulated that via the modification of the ECM CAFs can influence the presence of macrophages in the TME. CAFs are able to synthesize ECM components such as collagen type I, II and IV, but they can also express proteases like FAP, which by cleaving type I collagen alters ECM composition. It has been demonstrated that macrophages are not able to attach to native collagen type I, but they can bind to FAP-cleaved collagen type I through the scavenger receptors they express on their surface [72]. The expression of FAP was positively correlated to the number of macrophages in the tumor stroma [72]. Thus, by modifying the ECM CAFs enhance the recruitment and retention of macrophages in the TME and probably potentiating their biological functions. Overall, the interaction of CAFs and TAMs seems to be reciprocal and triggered by cancer cells to favor the establishment of an immunosuppressive microenvironment.
4.2. Dendritic cells
DCs are the most important antigen-presenting cells that have a pivotal role in activation of T cell-mediated anti-tumor immunity. It has been reported that hepatic carcinoma derived CAFs induce Indoleamine 2,3-dioxygenase (IDO)-producing, regulatory DCs, thereby inducing higher number of T regulatory (Treg) cells, resulting in a decreased anti-tumor response [73]. CAFs are also a major producer of TGF-β [51], [74]. TGF-β can affect DC development, activity and motility and in general DCs acquire a regulatory phenotype in the presence of TGF-β [75], [76], [77]. Fibroblast-produced IL-6 is reported to favor the differentiation of monocytes into tumor associated macrophages at the expense of differentiation into DCs [78]. IL-6 however also directly influences DC function; IL-6-mediated activation of the Signal transducer and activator of transcription 3 (STAT3) pathway affects DC maturation, disabling T cell activation and subsequently inducing T cell anergy and immune tolerance [79], [80], [81]. Next to IL-6 and TGF-β, CAFs also produce VEGF [82], [83]. This may not only affect angiogenesis, but may also alter DC function as VEGF has been shown to suppress its generation and maturation [84], [85], [86].
4.3. Myeloid Derived Suppressor Cells
MDSCs form a heterogeneous population of immature myeloid cells that suppress T cell function and promote tumor angiogenesis, tumor progression and metastasis [87], [88]. By secreting factors such as CXCL12, IL-6 and chemokine (C–C motif) ligand 2 (CCL2), CAFs have been shown to drive differentiation of monocytes into MDSC as well as attract them to the TME [89], [90], [91]. Recently it has been shown that CAFs can also promote immunosuppression by inducing Reactive Oxygen Species (ROS) -generating monocytic MDSC in lung squamous cell carcinoma [92].
4.4. Natural Killer cells
NK cells are innate immune cells that have cytotoxic effector functions [93]. CAFs can modulate the activation of NK cells in the tumor stroma. CAFs derived from endometrial cancer have been shown to significantly reduce NK killing activity by direct cell-to-cell contact [94]. Compared to normal fibroblasts, CAFS have a lower expression of the Poliovirus receptor (PVR/CD155), which is a ligand for the NK activating receptor DNAX Accessory Molecule-1 (DNAM-1). By downregulating CD155, CAFs attenuate NK cell-killing activity [94]. Also, Prostaglandin E2 (PGE2) and MMPs released from CAFs significantly impairs the ability of NK cells to recognize and kill tumor cells [95], [96], [97]. CAFs can thus inhibit the activity of NK cells against tumor development directly through cell-to-cell contact and indirectly by the secretion of soluble factors [93], [98].
4.5. T Cells
The interaction between CAFs and T cells has been demonstrated by multiple studies in vivo and in vitro. Distinct subpopulations of CAFs have been shown to exhibit immunosuppressive functions through affecting attraction, survival, migration, function and differentiation of different subsets of T cells in multiple types of cancer [18], [99], [100], [101], [102]. CAFs can do this by secretion of different cytokines and chemokines such as IL-6, CXCL12 and IL-1β, by the release of nitric oxid (NO), but also by expression of molecules such as OX40-ligand (OX40L), Programmed death ligand 2 (PD-L2), Junctional adhesion molecule B (JAM2), B7H3, Dipeptidyl peptidase-4 (DPP4) and ecto-5′-nucleotidase (CD73) [18], [99], [100], [101], [102]. Interestingly, CAFs have also been demonstrated to express MHCII molecules [20], [103] a characteristic of antigen presenting cells (APCs). Via expression of MHCII molecules together with the expression of costimulatory molecules such as CD80 and CD86, professional APCs can activate T cells and initiate an antigen-specific immune response. MHCII expression in the absence of expression of costimulatory molecules leads to activation of Tregs and thus to suppression of an immune response. As CAFs typically do not express costimulatory molecules [20], [103] it suggests that CAFs induce activation of Tregs. Also normal colonic myofibroblasts have been shown to express MHCII and this expression was essential for the induction of Tregs. The authors postulate that in the normal colon the colonic myofibroblasts contribute to the suppression of active inflammation by supporting expansion of Tregs [104].
Localization and migration of T cells is dependent on the specific tissue architecture; in loose fibronectin there is active T cell motility, whereas T cells migrate poorly in dense matrix areas [105]. The composition of the ECM can thus influence antitumor immunity by controlling the positioning and migration of T cells [106]. By remodeling the ECM, also CAFs can influence T cell migration and trap T cells in the stroma by forming a physical barrier limiting access to tumor cells [44], [107], [108]. CAFs can also affect T cell function and activation by the release of the ECM protein βig-h3. The presence of βig-h3 reduces antigen-specific activation and proliferation of CD8 + T cells [109]. Interestingly, CXCL12 secreted by FAP-expressing CAFs has also been suggested to prevent effector T cells from reaching cancer cells. CXCL12 secreted by CAFs localized on cancer cells and when the ligand for CXCL12, (chemokine (C-X-C motif) receptor 4 (CXCR4)), was inhibited, T cells accumulated again to the tumor [110]. The immunosuppressive effect of CAFs may thus be partly mediated via CXCL12-mediated T cell exclusion from the tumor.
CAFs directly isolated from multiple human tumors are shown to express the PD-1 ligands PD-L1 and PD-L2 [107], [111], [112], suggesting direct suppression of T cells. Another way how CAFs can suppress T cells is via the production of metabolites such as adenosine and lactate [113], [114].
Taken together, this demonstrates the role of CAFs as important inhibitors of T effector mediated immune responses against tumors and simultaneously as potent activators of Treg functions.
5. CAFs and radiotherapy
Radiotherapy (RT) is an effective and widely used local cancer treatment, with a majority of cancer patients undergoing RT at one point during treatment. Although primarily directed at killing tumor cells, RT also affects the TME, including the immune cells, endothelial cells, vasculature and fibroblasts, with important consequences for tumor growth, -dissemination, and -control. RT is given in different doses and treatment fractions based on tumor type as well as to reduce normal tissue toxicity. How these different treatment schedules influence the TME is, however, largely unknown. Many different mechanisms have been described whereby RT via the TME, and in particular CAFs, influences tumor growth and treatment sensitivity.
CAFs are particularly radioresistant as they do not die upon RT, but rather go into senescence [115], [116]. Indeed, irradiation of CAFs has been shown to cause persistent DNA damage [116], [117], and to induce a senescence response [116], [117] and specific changes in the secretory profile (e.g. CXCL12, TGF-β1, IGF-1, IGFBP2 and NO) [117], [118], [119], [120], [121], [122], [123], [124], [125]. Notably, RT-mediated TGF-β signaling induces activation of (peri)tumoral fibroblasts to CAFs and their proliferation in the TME, which enhances the CAF mediated effects on the tumor. The altered phenotype of the CAFs subsequently induces an altered metabolic profile [121], and is associated with epithelial-to-mesenchymal transition [118] and changes in invasiveness [118], [119] of the associated tumor cells. This plethora of RT- induced changes in the interaction between tumor cells, immune cells, and the TME has particular consequences for the tumor’s sensitivity for treatment.
6. CAFs and sensitivity to RT
RT can induce antitumor immune responses, although in general this does not lead to the much-desired abscopal effect where the RT-induced immune response has systemic effects on distant metastases. The generally immunosuppressive TME may counter the RT-induced immune response. As mentioned above, RT also promotes activation of CAFs and thus modulates the behavior of the TME for the response to RT (Fig. 1C) [126]. After RT, the TME activates a number of pathways and mechanisms that may subsequently be associated with tumor radioresistance, which can be through secretion of cytokines, growth factors, exosomes, and ECM remodeling factors [127], [128]. The interactions between fibroblasts/ CAFs and cancer cells are bi-directional and promote tumor growth, cancer invasion, metastasis, and therapy resistance [129], [130].
For example, by the activation of inflammatory pathways, RT induces fibrosis by different cytokines such as TGF-β1, TNF-α, IL-1, IL-4 and IL-13; chemokines such as MCP-1 and MIP-1β; angiogenic and growth factors [131], [132], [133]. Fibrosis in turn is associated with radioresistance by supplying signals to tumor cells leading to treatment resistance, proliferation and metastasis [134]. Additionally, activated CAFs can induce epithelial-to-mesenchymal transition [118], which may promote radioresistance via loss of e-cadherin [135]. Similarly, RT induces expression of both α and β integrins within the stroma [136], [137]. This increased integrin expression induces chemoradiation resistance of tumors and induces tumor growth of multiple cancer types [138], [139]. Furthermore, radiation resistance of prostate cancer cells could be linked to the loss of the membrane protein caveolin-1 in stromal fibroblasts [140], [141], [142]. Furthermore, in vivo CAFs can induce autophagy and subsequent irradiated tumor cell recovery [128]. Autophagy is either directly involved in radioresistance by modulating DNA damage repair [143] or indirectly by enabling cells to survive hypoxia [144], thereby inducing radioresistance.
Fibroblasts have been found to secrete exosomes containing 5′-triphosphate RNA to induce an IFN-related DNA damage resistance gene signature (IRDS) by activating RIG-1. Subsequent activation of STAT1 facilitated Notch signaling in breast cancer cells inducing a RT resistant stem cell-like phenotype [145]. This IRDS and STAT1 signaling have found to be associated with radioresistant breast cancer in vitro, in vivo and in patient cohorts [146], [147]. Similarly, after multiple passages of the head and neck cancer cell line SCC-61 in irradiated mice, these became radioresistant. The resulting resistant SCC-61 cells exhibited the same IRDS in which STAT1 was demonstrated to be a key mediator of radioresistance [148].
Of note, Steer et al. tested the effect of different fibroblasts (NIH-3 T3 or L929) on several tumor cell lines in direct or indirect coculture, and found complex bi-directional direct and indirect interactions between cancer cells and fibroblasts with impact on tumor growth and therapy outcome. They found that the impact of fibroblasts on tumor cells radiation response largely depended on the fibroblast and tumor cell type, the culture conditions (direct/indirect co-culture) and the respective endpoint (short-term vs. long-term; in vitro vs. in vivo [149]. This is likely caused by the fact that tumor cells and RT influence fibroblast phenotypes, with important repercussions for the reciprocal interaction between CAFs and tumor cells, which makes interpretation of many, especially preclinical, experimental data difficult.
7. Perspectives
Although CAFs have long been ignored in cancer biology, their essential role has received more attention the last decade. CAFs affect multiple steps in cancer development, progression, metastasis and (radio)therapy response and are therefore a promising target in cancer therapy. However, general depletion of CAFs is likely not a good strategy for enhancing cancer therapy response, as pro-tumorigenic as well as anti-tumorigenic properties are ascribed to them. This is evidenced by the contradictory results found when targeting the tumor stroma [14], [32], [38], [39], [134], [150]. For CAF targeting to become a viable option, the specificity of markers to identify CAFs and their heterogeneity should be considered. Currently, several preclinical strategies are ongoing that target specific actions or subpopulations of CAFs [17], [44], [86]. Additionally, radiotherapy schedules that enhance anti-tumorigenic and/or attenuate protumorigenic functions of CAFs and immune cells may be identified, thereby enhancing radiosensitivity of solid tumors. Future studies that further decipher the complex tripartite interaction between CAFs, cancer cells and immune cells will provide a more solid base for the design of more effective (combinatorial) therapeutic strategies against cancer.
Funding
Marleen Ansems is a recipient of a long-term fellowship (BUIT 2012–5347) from the Dutch Cancer Society and a Veni-fellowship (016.156.093) from the Netherlands Organisation for Scientific Research (NWO).
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Weinberg R.A. Oncogenes and tumor suppressor genes. CA Cancer J Clin. 1994;44(3):160–170. doi: 10.3322/canjclin.44.3.160. [DOI] [PubMed] [Google Scholar]
- 2.Paget S. The distribution of secondary growths in cancer of the breast. 1889. Cancer Metastasis Rev. 1989;8(2):98–101. [PubMed] [Google Scholar]
- 3.Langley R.R., Fidler I.J. The seed and soil hypothesis revisited–the role of tumor-stroma interactions in metastasis to different organs. Int J Cancer. 2011;128(11):2527–2535. doi: 10.1002/ijc.26031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hui L., Chen Y. Tumor microenvironment: Sanctuary of the devil. Cancer Lett. 2015;368(1):7–13. doi: 10.1016/j.canlet.2015.07.039. [DOI] [PubMed] [Google Scholar]
- 5.Qi D., Wu E. Cancer prognosis: Considering tumor and its microenvironment as a whole. EBioMedicine. 2019;43:28–29. doi: 10.1016/j.ebiom.2019.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kalluri R. The biology and function of fibroblasts in cancer. Nat Rev Cancer. 2016;16(9):582. doi: 10.1038/nrc.2016.73. [DOI] [PubMed] [Google Scholar]
- 7.Goruppi S., Dotto G.P. Mesenchymal stroma: primary determinant and therapeutic target for epithelial cancer. Trends Cell Biol. 2013;23(12):593–602. doi: 10.1016/j.tcb.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fiori M.E., Di Franco S., Villanova L., Bianca P., Stassi G., De Maria R. Cancer-associated fibroblasts as abettors of tumor progression at the crossroads of EMT and therapy resistance. Mol Cancer. 2019;18(1):70. doi: 10.1186/s12943-019-0994-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Denton A.E., Roberts E.W., Fearon D.T. Stromal Cells in the Tumor Microenvironment. Adv Exp Med Biol. 2018;1060:99–114. doi: 10.1007/978-3-319-78127-3_6. [DOI] [PubMed] [Google Scholar]
- 10.Liu L., Liu L., Yao H.H., Zhu Z.Q., Ning Z.L., Huang Q. Stromal Myofibroblasts Are Associated with Poor Prognosis in Solid Cancers: A Meta-Analysis of Published Studies. PLoS ONE. 2016;11(7) doi: 10.1371/journal.pone.0159947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kumar D., New J., Vishwakarma V., Joshi R., Enders J., Lin F. Cancer-Associated Fibroblasts Drive Glycolysis in a Targetable Signaling Loop Implicated in Head and Neck Squamous Cell Carcinoma Progression. Cancer Res. 2018;78(14):3769–3782. doi: 10.1158/0008-5472.CAN-17-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Koontongkaew S. The tumor microenvironment contribution to development, growth, invasion and metastasis of head and neck squamous cell carcinomas. J Cancer. 2013;4(1):66–83. doi: 10.7150/jca.5112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.B. Peltanova M. Raudenska M. Masarik Effect of tumor microenvironment on pathogenesis of the head and neck squamous cell carcinoma: a systematic review Molecular cancer 18 1 2019 63 63 [DOI] [PMC free article] [PubMed]
- 14.LeBleu V.S., Kalluri R. A peek into cancer-associated fibroblasts: origins, functions and translational impact. Dis Model Mech. 2018:11(4). doi: 10.1242/dmm.029447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Direkze N.C., Hodivala-Dilke K., Jeffery R., Hunt T., Poulsom R., Oukrif D. Bone marrow contribution to tumor-associated myofibroblasts and fibroblasts. Cancer Res. 2004;64(23):8492–8495. doi: 10.1158/0008-5472.CAN-04-1708. [DOI] [PubMed] [Google Scholar]
- 16.Shiga K., Hara M., Nagasaki T., Sato T., Takahashi H., Takeyama H. Cancer-associated fibroblasts: their characteristics and their roles in tumor growth. Cancers. 2015;7(4):2443–2458. doi: 10.3390/cancers7040902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen X., Song E. Turning foes to friends: targeting cancer-associated fibroblasts. Nat Rev Drug Discov. 2019;18(2):99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 18.A. Costa Y. Kieffer A. Scholer-Dahirel F. Pelon B. Bourachot M. Cardon et al. C et al.: Fibroblast Heterogeneity and Immunosuppressive Environment in Human Breast Cancer Cancer Cell 33 3 2018 pp. 463–479 e410 [DOI] [PubMed]
- 19.Bartoschek M., Oskolkov N., Bocci M., Lovrot J., Larsson C., Sommarin M. Spatially and functionally distinct subclasses of breast cancer-associated fibroblasts revealed by single cell RNA sequencing. Nat Commun. 2018;9(1):5150. doi: 10.1038/s41467-018-07582-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Elyada E., Bolisetty M., Laise P., Flynn W.F., Courtois E.T., Burkhart R.A. Cross-Species Single-Cell Analysis of Pancreatic Ductal Adenocarcinoma Reveals Antigen-Presenting Cancer-Associated Fibroblasts. Cancer Discov. 2019;9(8):1102–1123. doi: 10.1158/2159-8290.CD-19-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Cully M. Tumour microenvironment: Fibroblast subtype provides niche for cancer stem cells. Nat Rev Cancer. 2018;18(3):136. doi: 10.1038/nrc.2018.18. [DOI] [PubMed] [Google Scholar]
- 22.Lambrechts D., Wauters E., Boeckx B., Aibar S., Nittner D., Burton O. Phenotype molding of stromal cells in the lung tumor microenvironment. Nat Med. 2018;24(8):1277–1289. doi: 10.1038/s41591-018-0096-5. [DOI] [PubMed] [Google Scholar]
- 23.Neuzillet C., Tijeras-Raballand A., Ragulan C., Cros J., Patil Y., Martinet M. Inter- and intra-tumoural heterogeneity in cancer-associated fibroblasts of human pancreatic ductal adenocarcinoma. J Pathol. 2019;248(1):51–65. doi: 10.1002/path.5224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ohlund D., Handly-Santana A., Biffi G., Elyada E., Almeida A.S., Ponz-Sarvise M. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J Exp Med. 2017;214(3):579–596. doi: 10.1084/jem.20162024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.S. Su J. Chen H. Yao J. Liu S. Yu L. Lao et al. F et al.: CD10(+)GPR77(+) Cancer-Associated Fibroblasts Promote Cancer Formation and Chemoresistance by Sustaining Cancer Stemness Cell 172 4 2018 pp. 841–856 e816 [DOI] [PubMed]
- 26.Patel A.K., Vipparthi K., Thatikonda V., Arun I., Bhattacharjee S., Sharan R. A subtype of cancer-associated fibroblasts with lower expression of alpha-smooth muscle actin suppresses stemness through BMP4 in oral carcinoma. Oncogenesis. 2018;7(10):78. doi: 10.1038/s41389-018-0087-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sahai E., Astsaturov I., Cukierman E., DeNardo D.G., Egeblad M., Evans R.M. A framework for advancing our understanding of cancer-associated fibroblasts. Nat Rev Cancer. 2020 doi: 10.1038/s41568-019-0238-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ishii G., Ochiai A., Neri S. Phenotypic and functional heterogeneity of cancer-associated fibroblast within the tumor microenvironment. Adv Drug Deliv Rev. 2016;99:186–196. doi: 10.1016/j.addr.2015.07.007. [DOI] [PubMed] [Google Scholar]
- 29.Nurmik M., Ullmann P., Rodriguez F., Haan S., Letellier E. In search of definitions: Cancer-associated fibroblasts and their markers. Int J Cancer. 2020;146(4):895–905. doi: 10.1002/ijc.32193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhou B., Chen W.-L., Wang Y.-Y., Lin Z.-Y., Zhang D.-M., Fan S. A role for cancer-associated fibroblasts in inducing the epithelial-to-mesenchymal transition in human tongue squamous cell carcinoma. J Oral Pathol Med. 2014;43(8):585–592. doi: 10.1111/jop.12172. [DOI] [PubMed] [Google Scholar]
- 31.Hwang R.F., Moore T., Arumugam T., Ramachandran V., Amos K.D., Rivera A. Cancer-associated stromal fibroblasts promote pancreatic tumor progression. Cancer Res. 2008;68(3):918–926. doi: 10.1158/0008-5472.CAN-07-5714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pure E., Blomberg R. Pro-tumorigenic roles of fibroblast activation protein in cancer: back to the basics. Oncogene. 2018;37(32):4343–4357. doi: 10.1038/s41388-018-0275-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Orimo A., Gupta P.B., Sgroi D.C., Arenzana-Seisdedos F., Delaunay T., Naeem R. Stromal Fibroblasts Present in Invasive Human Breast Carcinomas Promote Tumor Growth and Angiogenesis through Elevated SDF-1/CXCL12 Secretion. Cell. 2005;121(3):335–348. doi: 10.1016/j.cell.2005.02.034. [DOI] [PubMed] [Google Scholar]
- 34.Erez N., Truitt M., Olson P., Hanahan D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-κB-Dependent Manner. Cancer Cell. 2010;17(2):135–147. doi: 10.1016/j.ccr.2009.12.041. [DOI] [PubMed] [Google Scholar]
- 35.Mezawa Y., Orimo A. The roles of tumor- and metastasis-promoting carcinoma-associated fibroblasts in human carcinomas. Cell Tissue Res. 2016;365(3):675–689. doi: 10.1007/s00441-016-2471-1. [DOI] [PubMed] [Google Scholar]
- 36.Raz Y., Erez N. An inflammatory vicious cycle: Fibroblasts and immune cell recruitment in cancer. Exp Cell Res. 2013;319(11):1596–1603. doi: 10.1016/j.yexcr.2013.03.022. [DOI] [PubMed] [Google Scholar]
- 37.Barbazán J., Matic Vignjevic D. Cancer associated fibroblasts: is the force the path to the dark side? Curr Opin Cell Biol. 2019;56:71–79. doi: 10.1016/j.ceb.2018.09.002. [DOI] [PubMed] [Google Scholar]
- 38.Ozdemir B.C., Pentcheva-Hoang T., Carstens J.L., Zheng X., Wu C.C., Simpson T.R. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25(6):719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rhim A.D., Oberstein P.E., Thomas D.H., Mirek E.T., Palermo C.F., Sastra S.A. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25(6):735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Miyai Y, Esaki N, Takahashi M, Enomoto A: Cancer-associated fibroblasts that restrain cancer progression (rCAFs): hypotheses and perspectives. Cancer Science, n/a(n/a). [DOI] [PMC free article] [PubMed]
- 41.Zeltz C., Primac I., Erusappan P., Alam J., Noel A., Gullberg D. Cancer-associated fibroblasts in desmoplastic tumors: emerging role of integrins. Semin Cancer Biol. 2019 doi: 10.1016/j.semcancer.2019.08.004. [DOI] [PubMed] [Google Scholar]
- 42.DeClerck Y.A. Desmoplasia: A Response or a Niche? Cancer Discovery. 2012;2(9):772–774. doi: 10.1158/2159-8290.CD-12-0348. [DOI] [PubMed] [Google Scholar]
- 43.Erdogan B., Webb D.J. Cancer-associated fibroblasts modulate growth factor signaling and extracellular matrix remodeling to regulate tumor metastasis. Biochem Soc Trans. 2017;45(1):229–236. doi: 10.1042/BST20160387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.De Jaeghere E.A., Denys H.G., De Wever O. Fibroblasts Fuel Immune Escape in the Tumor Microenvironment. Trends Cancer. 2019;5(11):704–723. doi: 10.1016/j.trecan.2019.09.009. [DOI] [PubMed] [Google Scholar]
- 45.Horiguchi M., Ota M., Rifkin D.B. Matrix control of transforming growth factor-β function. J Biochem. 2012;152(4):321–329. doi: 10.1093/jb/mvs089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Paszek M.J., Zahir N., Johnson K.R., Lakins J.N., Rozenberg G.I., Gefen A. Tensional homeostasis and the malignant phenotype. Cancer Cell. 2005;8(3):241–254. doi: 10.1016/j.ccr.2005.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Peranzoni E., Rivas-Caicedo A., Bougherara H., Salmon H., Donnadieu E. Positive and negative influence of the matrix architecture on antitumor immune surveillance. Cell Mol Life Sci. 2013;70(23):4431–4448. doi: 10.1007/s00018-013-1339-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jain R.K., Martin J.D., Stylianopoulos T. The role of mechanical forces in tumor growth and therapy. Annu Rev Biomed Eng. 2014;16:321–346. doi: 10.1146/annurev-bioeng-071813-105259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prakash J. Cancer-Associated Fibroblasts: Perspectives in Cancer Therapy. Trends Cancer. 2016;2(6):277–279. doi: 10.1016/j.trecan.2016.04.005. [DOI] [PubMed] [Google Scholar]
- 50.Rajaram M., Li J., Egeblad M., Powers R.S. System-wide analysis reveals a complex network of tumor-fibroblast interactions involved in tumorigenicity. PLoS Genet. 2013;9(9) doi: 10.1371/journal.pgen.1003789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yu Y., Xiao C.H., Tan L.D., Wang Q.S., Li X.Q., Feng Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br J Cancer. 2014;110(3):724–732. doi: 10.1038/bjc.2013.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tauriello D.V.F., Palomo-Ponce S., Stork D., Berenguer-Llergo A., Badia-Ramentol J., Iglesias M. TGFbeta drives immune evasion in genetically reconstituted colon cancer metastasis. Nature. 2018;554(7693):538–543. doi: 10.1038/nature25492. [DOI] [PubMed] [Google Scholar]
- 53.Zhuang J., Lu Q., Shen B., Huang X., Shen L., Zheng X. TGFbeta1 secreted by cancer-associated fibroblasts induces epithelial-mesenchymal transition of bladder cancer cells through lncRNA-ZEB2NAT. Sci Rep. 2015;5:11924. doi: 10.1038/srep11924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bai Y.P., Shang K., Chen H., Ding F., Wang Z., Liang C. FGF-1/-3/FGFR4 signaling in cancer-associated fibroblasts promotes tumor progression in colon cancer through Erk and MMP-7. Cancer Sci. 2015;106(10):1278–1287. doi: 10.1111/cas.12745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Y. Sun X. Fan Q. Zhang X. Shi G. Xu C. Zou Cancer-associated fibroblasts secrete FGF-1 to promote ovarian proliferation, migration, and invasion through the activation of FGF-1/FGFR4 signaling Tumour Biol 39 7 2017 1010428317712592 [DOI] [PubMed]
- 56.De Wever O., Nguyen Q.D., Van Hoorde L., Bracke M., Bruyneel E., Gespach C. Tenascin-C and SF/HGF produced by myofibroblasts in vitro provide convergent pro-invasive signals to human colon cancer cells through RhoA and Rac. FASEB J. 2004;18(9):1016–1018. doi: 10.1096/fj.03-1110fje. [DOI] [PubMed] [Google Scholar]
- 57.Tyan S.W., Kuo W.H., Huang C.K., Pan C.C., Shew J.Y., Chang K.J. Breast cancer cells induce cancer-associated fibroblasts to secrete hepatocyte growth factor to enhance breast tumorigenesis. PLoS ONE. 2011;6(1) doi: 10.1371/journal.pone.0015313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Avagliano A., Granato G., Ruocco M.R., Romano V., Belviso I., Carfora A. Metabolic Reprogramming of Cancer Associated Fibroblasts: The Slavery of Stromal Fibroblasts. Biomed Res Int. 2018;2018:6075403. doi: 10.1155/2018/6075403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Duda D.G., Duyverman A.M., Kohno M., Snuderl M., Steller E.J., Fukumura D. Malignant cells facilitate lung metastasis by bringing their own soil. Proc Natl Acad Sci U S A. 2010;107(50):21677–21682. doi: 10.1073/pnas.1016234107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hoshino A., Costa-Silva B., Shen T.L., Rodrigues G., Hashimoto A., Tesic Mark M. Tumour exosome integrins determine organotropic metastasis. Nature. 2015;527(7578):329–335. doi: 10.1038/nature15756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zhao H., Yang L., Baddour J., Achreja A., Bernard V., Moss T. Tumor microenvironment derived exosomes pleiotropically modulate cancer cell metabolism. Elife. 2016;5 doi: 10.7554/eLife.10250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.L. Monteran N. Erez The Dark Side of Fibroblasts: Cancer-Associated Fibroblasts as Mediators of Immunosuppression in the Tumor Microenvironment Front Immunol. vol 1835, 10, 2019 [DOI] [PMC free article] [PubMed]
- 63.Turley S.J., Cremasco V., Astarita J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat Rev Immunol. 2015;15(11):669–682. doi: 10.1038/nri3902. [DOI] [PubMed] [Google Scholar]
- 64.Coussens L.M., Zitvogel L., Palucka A.K. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339(6117):286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fujii N., Shomori K., Shiomi T., Nakabayashi M., Takeda C., Ryoke K. Cancer-associated fibroblasts and CD163-positive macrophages in oral squamous cell carcinoma: their clinicopathological and prognostic significance. J Oral Pathol Med. 2012;41(6):444–451. doi: 10.1111/j.1600-0714.2012.01127.x. [DOI] [PubMed] [Google Scholar]
- 66.Herrera M., Herrera A., Dominguez G., Silva J., Garcia V., Garcia J.M. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013;104(4):437–444. doi: 10.1111/cas.12096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Comito G., Giannoni E., Segura C.P., Barcellos-de-Souza P., Raspollini M.R., Baroni G. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene. 2014;33(19):2423–2431. doi: 10.1038/onc.2013.191. [DOI] [PubMed] [Google Scholar]
- 68.Hashimoto O., Yoshida M., Koma Y., Yanai T., Hasegawa D., Kosaka Y. Collaboration of cancer-associated fibroblasts and tumour-associated macrophages for neuroblastoma development. J Pathol. 2016;240(2):211–223. doi: 10.1002/path.4769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Cho H., Seo Y., Loke K.M., Kim S.W., Oh S.M., Kim J.H. Cancer-Stimulated CAFs Enhance Monocyte Differentiation and Protumoral TAM Activation via IL6 and GM-CSF Secretion. Clin Cancer Res. 2018;24(21):5407–5421. doi: 10.1158/1078-0432.CCR-18-0125. [DOI] [PubMed] [Google Scholar]
- 70.Gok Yavuz B., Gunaydin G., Gedik M.E., Kosemehmetoglu K., Karakoc D., Ozgur F. Cancer associated fibroblasts sculpt tumour microenvironment by recruiting monocytes and inducing immunosuppressive PD-1(+) TAMs. Sci Rep. 2019;9(1):3172. doi: 10.1038/s41598-019-39553-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ueshima E., Fujimori M., Kodama H., Felsen D., Chen J., Durack J.C. Macrophage-secreted TGF-beta1 contributes to fibroblast activation and ureteral stricture after ablation injury. Am J Physiol Renal Physiol. 2019;317(7):F52–F64. doi: 10.1152/ajprenal.00260.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Mazur A., Holthoff E., Vadali S., Kelly T., Post S.R. Cleavage of Type I Collagen by Fibroblast Activation Protein-alpha Enhances Class A Scavenger Receptor Mediated Macrophage Adhesion. PLoS ONE. 2016;11(3) doi: 10.1371/journal.pone.0150287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Cheng J.T., Deng Y.N., Yi H.M., Wang G.Y., Fu B.S., Chen W.J. Hepatic carcinoma-associated fibroblasts induce IDO-producing regulatory dendritic cells through IL-6-mediated STAT3 activation. Oncogenesis. 2016;5 doi: 10.1038/oncsis.2016.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ren Y., Jia H.-h., Xu Y-q, Zhou X., Zhao X.-h., Wang Y-f. Y et al.: Paracrine and epigenetic control of CAF-induced metastasis: the role of HOTAIR stimulated by TGF-ß1 secretion. Molecular Cancer. 2018;17(1):5. doi: 10.1186/s12943-018-0758-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Seeger P., Musso T., Sozzani S. The TGF-β superfamily in dendritic cell biology. Cytokine Growth Factor Rev. 2015;26(6):647–657. doi: 10.1016/j.cytogfr.2015.06.002. [DOI] [PubMed] [Google Scholar]
- 76.Travis M.A., Sheppard D. TGF-beta activation and function in immunity. Annu Rev Immunol. 2014;32:51–82. doi: 10.1146/annurev-immunol-032713-120257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Flavell R.A., Sanjabi S., Wrzesinski S.H., Licona-Limon P. The polarization of immune cells in the tumour environment by TGFbeta. Nat Rev Immunol. 2010;10(8):554–567. doi: 10.1038/nri2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Chomarat P., Banchereau J., Davoust J., Palucka A.K. IL-6 switches the differentiation of monocytes from dendritic cells to macrophages. Nat Immunol. 2000;1(6):510–514. doi: 10.1038/82763. [DOI] [PubMed] [Google Scholar]
- 79.Kitamura H., Kamon H., Sawa S., Park S.J., Katunuma N., Ishihara K. IL-6-STAT3 controls intracellular MHC class II alphabeta dimer level through cathepsin S activity in dendritic cells. Immunity. 2005;23(5):491–502. doi: 10.1016/j.immuni.2005.09.010. [DOI] [PubMed] [Google Scholar]
- 80.Park S.J., Nakagawa T., Kitamura H., Atsumi T., Kamon H., Sawa S. IL-6 regulates in vivo dendritic cell differentiation through STAT3 activation. J Immunol. 2004;173(6):3844–3854. doi: 10.4049/jimmunol.173.6.3844. [DOI] [PubMed] [Google Scholar]
- 81.L. Ziani S. Chouaib J. Thiery Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts Front Immunol 9 2018 414 414 [DOI] [PMC free article] [PubMed]
- 82.Fukumura D., Xavier R., Sugiura T., Chen Y., Park E.-C., Lu N. Tumor Induction of VEGF Promoter Activity in Stromal Cells. Cell. 1998;94(6):715–725. doi: 10.1016/s0092-8674(00)81731-6. [DOI] [PubMed] [Google Scholar]
- 83.Sewell-Loftin M.K., Bayer S.V.H., Crist E., Hughes T., Joison S.M., Longmore G.D. Cancer-associated fibroblasts support vascular growth through mechanical force. Sci Rep. 2017;7(1):12574. doi: 10.1038/s41598-017-13006-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gabrilovich D.I., Chen H.L., Girgis K.R., Cunningham H.T., Meny G.M., Nadaf S. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat Med. 1996;2(10):1096–1103. doi: 10.1038/nm1096-1096. [DOI] [PubMed] [Google Scholar]
- 85.Dikov M.M., Ohm J.E., Ray N., Tchekneva E.E., Burlison J., Moghanaki D. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J Immunol. 2005;174(1):215–222. doi: 10.4049/jimmunol.174.1.215. [DOI] [PubMed] [Google Scholar]
- 86.Liu T., Han C., Wang S., Fang P., Ma Z., Xu L. Cancer-associated fibroblasts: an emerging target of anti-cancer immunotherapy. J Hematol Oncol. 2019;12(1):86. doi: 10.1186/s13045-019-0770-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Gabrilovich D.I., Ostrand-Rosenberg S., Bronte V. Coordinated regulation of myeloid cells by tumours. Nat Rev Immunol. 2012;12(4):253–268. doi: 10.1038/nri3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Talmadge J.E., Gabrilovich D.I. History of myeloid-derived suppressor cells. Nat Rev Cancer. 2013;13(10):739–752. doi: 10.1038/nrc3581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deng Y., Cheng J., Fu B., Liu W., Chen G., Zhang Q. Hepatic carcinoma-associated fibroblasts enhance immune suppression by facilitating the generation of myeloid-derived suppressor cells. Oncogene. 2017;36(8):1090–1101. doi: 10.1038/onc.2016.273. [DOI] [PubMed] [Google Scholar]
- 90.Kumar V, Donthireddy L, Marvel D, Condamine T, Wang F, Lavilla-Alonso S, Hashimoto A, Vonteddu P, Behera R, Goins MA et al: Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32(5):654-668 e655. [DOI] [PMC free article] [PubMed]
- 91.Yang X., Lin Y., Shi Y., Li B., Liu W., Yin W. FAP Promotes Immunosuppression by Cancer-Associated Fibroblasts in the Tumor Microenvironment via STAT3-CCL2 Signaling. Cancer Res. 2016;76(14):4124–4135. doi: 10.1158/0008-5472.CAN-15-2973. [DOI] [PubMed] [Google Scholar]
- 92.Xiang H., Ramil C.P., Hai J., Zhang C., Wang H., Watkins A.A. Song XS et al.: Cancer-associated fibroblasts promote immunosuppression by inducing ROS-generating monocytic MDSCs in lung squamous cell carcinoma. Cancer. Cancer Immunol Res. 2020;8(4):436–450. doi: 10.1158/2326-6066.CIR-19-0507. [DOI] [PubMed] [Google Scholar]
- 93.Melaiu O., Lucarini V., Cifaldi L., Fruci D. Influence of the Tumor Microenvironment on NK Cell Function in Solid Tumors. Front Immunol. 2019;10:3038. doi: 10.3389/fimmu.2019.03038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Inoue T., Adachi K., Kawana K., Taguchi A., Nagamatsu T., Fujimoto A. Cancer-associated fibroblast suppresses killing activity of natural killer cells through downregulation of poliovirus receptor (PVR/CD155), a ligand of activating NK receptor. Int J Oncol. 2016;49(4):1297–1304. doi: 10.3892/ijo.2016.3631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Balsamo M., Scordamaglia F., Pietra G., Manzini C., Cantoni C., Boitano M. Melanoma-associated fibroblasts modulate NK cell phenotype and antitumor cytotoxicity. Proc Natl Acad Sci U S A. 2009;106(49):20847–20852. doi: 10.1073/pnas.0906481106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Li T., Yi S., Liu W., Jia C., Wang G., Hua X. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med Oncol. 2013;30(3):663. doi: 10.1007/s12032-013-0663-z. [DOI] [PubMed] [Google Scholar]
- 97.Ziani L., Safta-Saadoun T.B., Gourbeix J., Cavalcanti A., Robert C., Favre G. Melanoma-associated fibroblasts decrease tumor cell susceptibility to NK cell-mediated killing through matrix-metalloproteinases secretion. Oncotarget. 2017;8(12):19780–19794. doi: 10.18632/oncotarget.15540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Rossi G.R., Trindade E.S., Souza-Fonseca-Guimaraes F. Tumor Microenvironment-Associated Extracellular Matrix Components Regulate NK Cell Function. Front Immunol. 2020;11:73. doi: 10.3389/fimmu.2020.00073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Givel A.M., Kieffer Y., Scholer-Dahirel A., Sirven P., Cardon M., Pelon F. miR200-regulated CXCL12beta promotes fibroblast heterogeneity and immunosuppression in ovarian cancers. Nat Commun. 2018;9(1):1056. doi: 10.1038/s41467-018-03348-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Cremasco V., Astarita J.L., Grauel A.L., Keerthivasan S., MacIsaac K., Woodruff M.C. FAP Delineates Heterogeneous and Functionally Divergent Stromal Cells in Immune-Excluded Breast Tumors. Cancer Immunol Res. 2018;6(12):1472–1485. doi: 10.1158/2326-6066.CIR-18-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Huang Y.H., Chang C.Y., Kuo Y.Z., Fang W.Y., Kao H.Y., Tsai S.T. Cancer-associated fibroblast-derived interleukin-1beta activates protumor C-C motif chemokine ligand 22 signaling in head and neck cancer. Cancer Sci. 2019;110(9):2783–2793. doi: 10.1111/cas.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kato T., Noma K., Ohara T., Kashima H., Katsura Y., Sato H. Cancer-Associated Fibroblasts Affect Intratumoral CD8(+) and FoxP3(+) T Cells Via IL6 in the Tumor Microenvironment. Clin Cancer Res. 2018;24(19):4820–4833. doi: 10.1158/1078-0432.CCR-18-0205. [DOI] [PubMed] [Google Scholar]
- 103.Gunaydin G., Kesikli S.A., Guc D. Cancer associated fibroblasts have phenotypic and functional characteristics similar to the fibrocytes that represent a novel MDSC subset. Oncoimmunology. 2015;4(9) doi: 10.1080/2162402X.2015.1034918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Pinchuk I.V., Beswick E.J., Saada J.I., Boya G., Schmitt D., Raju G.S. Human colonic myofibroblasts promote expansion of CD4+ CD25high Foxp3+ regulatory T cells. Gastroenterology. 2011;140(7):2019–2030. doi: 10.1053/j.gastro.2011.02.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Salmon H., Franciszkiewicz K., Damotte D., Dieu-Nosjean M.C., Validire P., Trautmann A. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J Clin Invest. 2012;122(3):899–910. doi: 10.1172/JCI45817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Sorokin L. The impact of the extracellular matrix on inflammation. Nat Rev Immunol. 2010;10(10):712–723. doi: 10.1038/nri2852. [DOI] [PubMed] [Google Scholar]
- 107.Gorchs L., Fernandez Moro C., Bankhead P., Kern K.P., Sadeak I., Meng Q. Human Pancreatic Carcinoma-Associated Fibroblasts Promote Expression of Co-inhibitory Markers on CD4(+) and CD8(+) T-Cells. Front Immunol. 2019;10:847. doi: 10.3389/fimmu.2019.00847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Hartmann N., Giese N.A., Giese T., Poschke I., Offringa R., Werner J. Prevailing role of contact guidance in intrastromal T-cell trapping in human pancreatic cancer. Clin Cancer Res. 2014;20(13):3422–3433. doi: 10.1158/1078-0432.CCR-13-2972. [DOI] [PubMed] [Google Scholar]
- 109.Goehrig D., Nigri J., Samain R., Wu Z., Cappello P., Gabiane G. Stromal protein betaig-h3 reprogrammes tumour microenvironment in pancreatic cancer. Gut. 2019;68(4):693–707. doi: 10.1136/gutjnl-2018-317570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Feig C., Jones J.O., Kraman M., Wells R.J., Deonarine A., Chan D.S. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110(50):20212–20217. doi: 10.1073/pnas.1320318110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Khalili J.S., Liu S., Rodriguez-Cruz T.G., Whittington M., Wardell S., Liu C. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin Cancer Res. 2012;18(19):5329–5340. doi: 10.1158/1078-0432.CCR-12-1632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pinchuk IV, Saada JI, Beswick EJ, Boya G, Qiu SM, Mifflin RC, Raju GS, Reyes VE, Powell DW: PD-1 ligand expression by human colonic myofibroblasts/fibroblasts regulates CD4+ T-cell activity. Gastroenterology 2008, 135(4):1228-1237, 1237 e1221-1222. [DOI] [PMC free article] [PubMed]
- 113.de Lourdes Mora-Garcia M, Garcia-Rocha R., Morales-Ramirez O., Montesinos J.J., Weiss-Steider B., Hernandez-Montes J. Mesenchymal stromal cells derived from cervical cancer produce high amounts of adenosine to suppress cytotoxic T lymphocyte functions. J Transl Med. 2016;14(1):302. doi: 10.1186/s12967-016-1057-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Comito G., Iscaro A., Bacci M., Morandi A., Ippolito L., Parri M. Lactate modulates CD4(+) T-cell polarization and induces an immunosuppressive environment, which sustains prostate carcinoma progression via TLR8/miR21 axis. Oncogene. 2019;38(19):3681–3695. doi: 10.1038/s41388-019-0688-7. [DOI] [PubMed] [Google Scholar]
- 115.Papadopoulou A., Kletsas D. Human lung fibroblasts prematurely senescent after exposure to ionizing radiation enhance the growth of malignant lung epithelial cells in vitro and in vivo. Int J Oncol. 2011;39(4):989–999. doi: 10.3892/ijo.2011.1132. [DOI] [PubMed] [Google Scholar]
- 116.Hellevik T., Pettersen I., Berg V., Winberg J.O., Moe B.T., Bartnes K. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat Oncol. 2012;7:59. doi: 10.1186/1748-717X-7-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Rodier F., Coppe J.P., Patil C.K., Hoeijmakers W.A., Munoz D.P., Raza S.R. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11(8):973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Li D., Qu C., Ning Z., Wang H., Zang K., Zhuang L. Radiation promotes epithelial-to-mesenchymal transition and invasion of pancreatic cancer cell by activating carcinoma-associated fibroblasts. Am J Cancer Res. 2016;6(10):2192–2206. [PMC free article] [PubMed] [Google Scholar]
- 119.Ohuchida K., Mizumoto K., Murakami M., Qian L.W., Sato N., Nagai E. Radiation to stromal fibroblasts increases invasiveness of pancreatic cancer cells through tumor-stromal interactions. Cancer Res. 2004;64(9):3215–3222. doi: 10.1158/0008-5472.can-03-2464. [DOI] [PubMed] [Google Scholar]
- 120.Kamochi N., Nakashima M., Aoki S., Uchihashi K., Sugihara H., Toda S. Irradiated fibroblast-induced bystander effects on invasive growth of squamous cell carcinoma under cancer–stromal cell interaction. Cancer Sci. 2008;99(12):2417–2427. doi: 10.1111/j.1349-7006.2008.00978.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Tommelein J., De Vlieghere E., Verset L., Melsens E., Leenders J., Descamps B. Radiotherapy-Activated Cancer-Associated Fibroblasts Promote Tumor Progression through Paracrine IGF1R Activation. Cancer Res. 2018;78(3):659–670. doi: 10.1158/0008-5472.CAN-17-0524. [DOI] [PubMed] [Google Scholar]
- 122.Liu D., Hornsby P.J. Senescent human fibroblasts increase the early growth of xenograft tumors via matrix metalloproteinase secretion. Cancer Res. 2007;67(7):3117–3126. doi: 10.1158/0008-5472.CAN-06-3452. [DOI] [PubMed] [Google Scholar]
- 123.Velarde M.C., Demaria M., Campisi J. Senescent cells and their secretory phenotype as targets for cancer therapy. Interdiscip Top Gerontol. 2013;38:17–27. doi: 10.1159/000343572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hellevik T., Pettersen I., Berg V., Bruun J., Bartnes K., Busund L.T. Changes in the Secretory Profile of NSCLC-Associated Fibroblasts after Ablative Radiotherapy: Potential Impact on Angiogenesis and Tumor Growth. Transl Oncol. 2013;6(1):66–74. doi: 10.1593/tlo.12349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pereira P.M.R., Edwards K.J., Mandleywala K., Carter L.M., Escorcia F.E., Campesato L.F. iNOS regulates the therapeutic response of pancreatic cancer cells to radiation therapy. Cancer Res. 2020 doi: 10.1158/0008-5472.CAN-19-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wang Z., Tang Y., Tan Y., Wei Q., Yu W. Cancer-associated fibroblasts in radiotherapy: challenges and new opportunities. Cell Commun Signal. 2019;17(1):47. doi: 10.1186/s12964-019-0362-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Zhang H., Yue J., Jiang Z., Zhou R., Xie R., Xu Y. CAF-secreted CXCL1 conferred radioresistance by regulating DNA damage response in a ROS-dependent manner in esophageal squamous cell carcinoma. Cell Death Dis. 2017;8(5) doi: 10.1038/cddis.2017.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Wang Y., Gan G., Wang B., Wu J., Cao Y., Zhu D. Cancer-associated Fibroblasts Promote Irradiated Cancer Cell Recovery Through Autophagy. EBioMedicine. 2017;17:45–56. doi: 10.1016/j.ebiom.2017.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.De Wever O., Van Bockstal M., Mareel M., Hendrix A., Bracke M. Carcinoma-associated fibroblasts provide operational flexibility in metastasis. Semin Cancer Biol. 2014;25:33–46. doi: 10.1016/j.semcancer.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 130.Alexander J., Cukierman E. Stromal dynamic reciprocity in cancer: intricacies of fibroblastic-ECM interactions. Curr Opin Cell Biol. 2016;42:80–93. doi: 10.1016/j.ceb.2016.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Johnston C.J., Piedboeuf B., Rubin P., Williams J.P., Baggs R., Finkelstein J.N. Early and persistent alterations in the expression of interleukin-1 alpha, interleukin-1 beta and tumor necrosis factor alpha mRNA levels in fibrosis-resistant and sensitive mice after thoracic irradiation. Radiat Res. 1996;145(6):762–767. [PubMed] [Google Scholar]
- 132.Buttner C., Skupin A., Reimann T., Rieber E.P., Unteregger G., Geyer P. Local production of interleukin-4 during radiation-induced pneumonitis and pulmonary fibrosis in rats: macrophages as a prominent source of interleukin-4. Am J Respir Cell Mol Biol. 1997;17(3):315–325. doi: 10.1165/ajrcmb.17.3.2279. [DOI] [PubMed] [Google Scholar]
- 133.Rubin P., Johnston C.J., Williams J.P., McDonald S., Finkelstein J.N. A perpetual cascade of cytokines postirradiation leads to pulmonary fibrosis. Int J Radiat Oncol Biol Phys. 1995;33(1):99–109. doi: 10.1016/0360-3016(95)00095-G. [DOI] [PubMed] [Google Scholar]
- 134.Valkenburg K.C., de Groot A.E., Pienta K.J. Targeting the tumour stroma to improve cancer therapy. Nat Rev Clin Oncol. 2018;15(6):366–381. doi: 10.1038/s41571-018-0007-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Theys J., Jutten B., Habets R., Paesmans K., Groot A.J., Lambin P. E-Cadherin loss associated with EMT promotes radioresistance in human tumor cells. Radiother Oncol. 2011;99(3):392–397. doi: 10.1016/j.radonc.2011.05.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Nam J.M., Onodera Y., Bissell M.J., Park C.C. Breast cancer cells in three-dimensional culture display an enhanced radioresponse after coordinate targeting of integrin alpha5beta1 and fibronectin. Cancer Res. 2010;70(13):5238–5248. doi: 10.1158/0008-5472.CAN-09-2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Goel H.L., Sayeed A., Breen M., Zarif M.J., Garlick D.S., Leav I. beta1 integrins mediate resistance to ionizing radiation in vivo by inhibiting c-Jun amino terminal kinase 1. J Cell Physiol. 2013;228(7):1601–1609. doi: 10.1002/jcp.24323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Cordes N. Integrin-mediated cell-matrix interactions for prosurvival and antiapoptotic signaling after genotoxic injury. Cancer Lett. 2006;242(1):11–19. doi: 10.1016/j.canlet.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 139.Cordes N., Seidler J., Durzok R., Geinitz H., Brakebusch C. β1-integrin-mediated signaling essentially contributes to cell survival after radiation-induced genotoxic injury. Oncogene. 2006;25(9):1378–1390. doi: 10.1038/sj.onc.1209164. [DOI] [PubMed] [Google Scholar]
- 140.Klein D., Schmitz T., Verhelst V., Panic A., Schenck M., Reis H. Endothelial Caveolin-1 regulates the radiation response of epithelial prostate tumors. Oncogenesis. 2015;4 doi: 10.1038/oncsis.2015.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Panic A., Ketteler J., Reis H., Sak A., Herskind C., Maier P. Progression-related loss of stromal Caveolin 1 levels fosters the growth of human PC3 xenografts and mediates radiation resistance. Sci Rep. 2017;7:41138. doi: 10.1038/srep41138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.J. Ketteler A. Panic H. Reis A. Wittka P. Maier C. Herskind Yague E, Jendrossek V, Klein D: Progression-Related Loss of Stromal Caveolin 1 Levels Mediates Radiation Resistance in Prostate Carcinoma via the Apoptosis Inhibitor TRIAP1 J Clin Med 2019 8(3) [DOI] [PMC free article] [PubMed]
- 143.Nagelkerke A., Bussink J., van der Kogel A.J., Sweep F.C., Span P.N. The PERK/ATF4/LAMP3-arm of the unfolded protein response affects radioresistance by interfering with the DNA damage response. Radiother Oncol. 2013;108(3):415–421. doi: 10.1016/j.radonc.2013.06.037. [DOI] [PubMed] [Google Scholar]
- 144.Rouschop K.M., van den Beucken T., Dubois L., Niessen H., Bussink J., Savelkouls K. The unfolded protein response protects human tumor cells during hypoxia through regulation of the autophagy genes MAP1LC3B and ATG5. J Clin Invest. 2010;120(1):127–141. doi: 10.1172/JCI40027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Boelens M.C., Wu T.J., Nabet B.Y., Xu B., Qiu Y., Yoon T. Exosome transfer from stromal to breast cancer cells regulates therapy resistance pathways. Cell. 2014;159(3):499–513. doi: 10.1016/j.cell.2014.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Weichselbaum R.R., Ishwaran H., Yoon T., Nuyten D.S., Baker S.W., Khodarev N. An interferon-related gene signature for DNA damage resistance is a predictive marker for chemotherapy and radiation for breast cancer. Proc Natl Acad Sci U S A. 2008;105(47):18490–18495. doi: 10.1073/pnas.0809242105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Post A.E.M., Smid M., Nagelkerke A., Martens J.W.M., Bussink J., Sweep F. Interferon-Stimulated Genes Are Involved in Cross-resistance to Radiotherapy in Tamoxifen-Resistant Breast Cancer. Clin Cancer Res. 2018;24(14):3397–3408. doi: 10.1158/1078-0432.CCR-17-2551. [DOI] [PubMed] [Google Scholar]
- 148.Khodarev N.N., Beckett M., Labay E., Darga T., Roizman B., Weichselbaum R.R. STAT1 is overexpressed in tumors selected for radioresistance and confers protection from radiation in transduced sensitive cells. Proc Natl Acad Sci U S A. 2004;101(6):1714–1719. doi: 10.1073/pnas.0308102100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Steer A., Cordes N., Jendrossek V., Klein D. Impact of Cancer-Associated Fibroblast on the Radiation-Response of Solid Xenograft Tumors. Front Mol Biosci. 2019;6:70. doi: 10.3389/fmolb.2019.00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Öhlund D., Elyada E., Tuveson D. Fibroblast heterogeneity in the cancer wound. J Exp Med. 2014;211(8):1503–1523. doi: 10.1084/jem.20140692. [DOI] [PMC free article] [PubMed] [Google Scholar]