Abstract
Volatile organic compounds (VOCs) are produced by the human metabolism, inflammation and gut microbiota and form the basis of innovative volatomics research. VOCs detected through breath and faecal analysis hence serve as attractive, non-invasive biomarkers for diagnosing and monitoring irritable bowel syndrome (IBS) and inflammatory bowel disease (IBD). This review describes the clinical applicability of volatomics in discriminating between IBS, IBD and healthy volunteers with acceptable accuracy in breath (70%-100%) and faecal (58%-85%) samples. Promising compounds are propan-1-ol for diagnosing and monitoring of IBD patients, and 1-methyl-4-propan-2-ylcyclohexa-1,4-diene as biomarker for IBS diagnosis. However, these VOCs often seem to be related to inflammation and probably will need to be used in conjunction with other clinical evidence. Furthermore, three interventional studies underlined the potential of VOCs in predicting treatment outcome and patient follow-up. This shows great promise for future use of VOCs as non-invasive breath and faecal biomarkers in personalised medicine. However, properly designed studies that correlate VOCs to IBD/IBS pathogenesis, while taking microbial influences into account, are still key before clinical implementation can be expected.
Keywords: Volatile organic compounds, VOC, Irritable bowel syndrome, IBS, inflammatory bowel disease, IBD
Abbreviations: AD, active disease; CD, Crohn's disease; HV, healthy volunteer; IBD, inflammatory bowel disease; IBS, irritable bowel syndrome; DR, disease in remission; UC, ulcerative colitis
1. Introduction
Patients presenting with chronic abdominal pain and changes in stool pattern are subjected to invasive procedures to differentiate between irritable bowel syndrome (IBS), inflammatory bowel disease (IBD), coeliac disease and colon cancer. The most prevalent of these diseases is IBS with 11% [1], followed by colon cancer (8%) [2], coeliac disease (1%) [3] and IBD (0.3%) [4]. Particularly, the differential diagnosis between IBS and IBD is challenging, since both can present similarly, especially when IBD is in remission [5,6].
IBS is characterised by abdominal pain and altered bowel habits in the absence of organic disease of the gastrointestinal tract. Its aetiology remains unknown, although patients often report an infectious, traumatic or stressful event preceding the onset of symptoms. The pathogenic mechanisms are most likely multifactorial, including increased intestinal permeability, dysmotility, intestinal dysbiosis, food hypersensitivity, visceral hypersensitivity, brain-gut axis dysregulation, inflammation, genetics, and psychological stress [7,8]. IBS has a major impact on quality of life and is responsible for high healthcare costs [9]. Four IBS subtypes are described according to the dominant stool pattern and based on the Rome criteria: diarrhoea (IBS-D), constipation (IBS-C), mixed (IBS-M) and unspecified (IBS-U) [10].
IBD encloses ulcerative colitis (UC) and Crohn's disease (CD), both having their own distinct pathological and clinical characteristics. Approximately 2⋅5–3 million people in Europe suffer from IBD [11] and they have up to 18% increased risk of developing colon cancer [12]. IBD is caused by a chronically relapsing and uncontrolled intestinal inflammation which can be difficult to manage [13]. IBD treatment focuses on immune suppression, which is associated with side effects (bone marrow suppression, infections) and a low response rate [14]. People with suspected IBD are screened for flares and inflammation by measuring faecal calprotectin, a marker for intestinal neutrophilic inflammation. However, calprotectin is a non-specific inflammatory marker (sensitivity 83–100%, specificity 60–100%,) [15], and the definitive diagnosis is made on biopsies collected through colonoscopy, which carries a risk of pain, bleeding and perforation [16].
In IBS and IBD, there is a rising interest in the role of the microbiota, which influences intestinal physiology, nutrient absorption, and activation and development of the immune system. A normal, healthy microbiota is characterised by a high individual diversity and stability over time. However, a high interindividual variability exists [17]. Several gastrointestinal diseases are associated with dysbiosis, a reduction of this diversity and stability [17,18]. Microbiota composition is closely related to stool consistency [19] and is heavily influenced by exogenous factors like antibiotics and food [17].
Non-invasive disease diagnosis and monitoring are the ‘Holy Grail’ for clinicians. Thirty-three to 57% of IBD patients in remission have motility and/or sensitivity disturbances resembling IBS [6], challenging their differential diagnosis. There is an urgent need to develop non-invasive, biomarkers that facilitate diagnosis and follow-up of IBS and IBD patients. A new development in this area is volatomics research, comprising volatile organic compounds (VOCs). VOCs have a low molecular weight (<300 Da), a high vapour pressure at room temperature [20] and are produced during physiological and pathological metabolic processes (inflammation/oxidative stress) in the human body [21], [22], [23]. Furthermore, VOCs can also originate from exogenous sources (food/drugs) and microbial metabolism [24,25] and are excreted and detected via urine, skin, blood, faeces and exhaled breath [26]. Two gasses are already used in clinical practice to evaluate carbohydrate malabsorption and bacterial overgrowth in breath analysis: methane (CH4) and hydrogen (H2) [27], [28], [29].
Since IBD and IBS are both associated with (low-grade) inflammation and microbial changes, volatomics offer a non-invasive, tool to aid in diagnosis, treatment and follow-up [21]. This review explores current knowledge about using volatomics for (differential) diagnosis of IBS and IBD, for discriminating between disease subtypes, phenotypes and active and quiescent disease, to monitor treatment response and to explore the effect of the microbiota on VOC composition.
2. Literature search
We used a systematic approach to search the literature concerning VOCs in IBS and IBD. A full description of the search methods can be found with supplementary Fig. 1. Articles studying methane or hydrogen were excluded since recent reviews showed their potential clinical use, albeit, with important limitations due to metabolic and microbial variations [27], [28], [29].
Quality of the articles was assessed with the AXIS quality appraisal tool for cross-sectional research [30,31]. Twenty questions were evaluated by two independent assessors with a score of 1 for ‘yes’ and 0 for ‘no’/‘not applicable’/‘not reported’ as answer. Every study was given a total score out of 20, with a higher score indicating a lower risk of bias and, therefore, higher study quality. Initial assessment gave an inter-assessor agreement of 97⋅1%. All studies lost approximately five points because relevant information was not mentioned, or AXIS questions were not applicable to the research protocol. Consensus was reached and the quality score per article ranged between 10 and 15 out of 20 with one study scoring 10 and one scoring 15. Most studies scored 14 (46%) or 13 (21%) out of 20, indicating average quality (supplementary Table 1).
Publication year, study (type, intervention), volatomic (source, analytical method) and population characteristics (IBS/IBD characteristics, exclusion criteria, participant numbers, gender, race, smoking, BMI), and results of interventions were catalogued. Study characteristics and technical details can be found in Table 1. A more in-depth overview of detection approaches can be found in Table 2.
Table 1.
Study characteristics.
| Author | Year | Disease criteria | Intervention | Source VOC | Analysis method | Processing and analytical information | VOC identification* | Validation† | Comparison |
|---|---|---|---|---|---|---|---|---|---|
| IBD | |||||||||
| Garner et al. [42] | 2007 | NA | No | Faeces | GC–MS | Processing: Within 1 h. Storage: −20°C. Quantity: 2–4 g. Preparation: 60°C for 1 h. Headspace: Yes, SPME. GC column: SPB®−1 Capillary GC Column (L × I.D.30 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma aldrich) and Zebron™ ZB-FFAP GC Capillary Column. | NIST | Leave-one-out CV | UC and HV and Clostridium infection |
| Kumari et al. [43] | 2013 | SCCAI | No | Faeces | GC | Processing: Within 3 h. Storage: −80°C. Quantity: 250 -mg. Preparation: Supernatans development. Headspace: No. GCcolumn: NA. | External standards | NA | UC and HV |
| Ahmed et al. [45] | 2016 | HBI, SCCAI | No | Faeces | GC–MS | Processing: Within 6 h. Storage: −20°C. Quantity: 2 g. Preparation: 60°C for 1 h. Headspace: Yes, SPME.- GCcolumn: SPB®−1 Capillary GC Column (L × I.D. 60 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma Aldrich). | NIST | NA | UC and CD and HV |
| Kokoszka et al. [39] | 1993 | Indium-labelled granulocyte nuclear imaging | No | Breath | GC | Processing: Haldane-Prestly tube -> plastic syringe. Storage: < 6 h. Quantity: 50 -ml. Preparation: NA. Headspace: NA. GCcolumn: NA. | NA | NA | IBD relapse and de novo symptoms |
| Pelli et al. [49] | 1999 | HBI, SCCAI | No | Breath | GC | Collection: 1L Tedlar bag. Storage: NA. Quantity: 100 ml. Preparation: Adsorption -> thermal desorption -> concentration. Headspace: NA.- GCcolumn: Al2O3/KCI column (25 m, 0⋅32ram, 0⋅5 mm; Chrompack). | NA | NA | UC and CD and HV |
| Dryahina et al. [48] | 2013 | NA | No | Breath | SIFT-MS | Processing: NA. Storage: No. Quantity: 3 ex- and inhalations. Preparation: NA. Headspace: NA. GC-column: NA. | Reagents | NA | UC and CD and HV |
| Bodelier et al. [53] | 2015 | HBI | No | Breath | GC–MS | Processing: Within 2 h; 5L Tedlar bag -> stainless steel adsorption tubes. Storage: Room temperature. Quantity: NA. Preparation: NA. Headspace: NA. GC-column: NA. | NIST | Training‡ | CD and HV |
| Hicks et al. [44] | 2015 | HBI, SCCAI | No | Breath | SIFT-MS | Collection: 2L Nalophan bag. Storage: < 2 h. Quantity: 2L. Preparation: 37°C for 5 min. Headspace: NA. GC-column: NA. | Reagents | Leave-one-out CV and 7-fold CV | UC and CD and HV |
| Arasaradnam et al. [41] | 2016 | HBI, SCCAI | No | Breath | FAIMS | Collection: 3L Tedlar bag. Storage: −20°C for maximum 24 h. Quantity: NA. Preparation: Room temperature for 1 h + transport time. Headspace: NA. GC-column: NA. | NA | 10-fold CV | UC and CD and HV |
| Rieder et al. [40] | 2016 | NA | No | Breath | SIFT-MS | Collection: Mylar bag. Storage: < 2 h. Quantity: NA. Preparation: 37°C for 10 min. Headspace: NA. GC-column: NA. | Reagents | NA | IBD and HV |
| Dryahina et al. [47] | 2017 | HBI, SCCAI | No | Breath | SIFT-MS, GC–MS | Collection: 3L Nalophan bag. Storage: 37°C for 5–10 min. Quantity: NA. Preparation: 37°C. Headspace: NA. GC-column: NA. | Reagents | NA | UC and CD and HV |
| Smolinska et al. [67] | 2017 | SCCAI | No | Breath | GC–MS | Processing: Within 1 h; 5L Tedlar bag -> stainless steel adsorption tube. Storage: Room temperature. Quantity: NA. Preparation: NA. Headspace: NA. GC-column: NA. | NIST | Training‡ | Active UC and UC in remission and non-IBD colitis |
| Smolinska et al. [54] | 2018 | HBI | No | Breath | GC–MS | Processing: Within 1 h; 5L Tedlar bag -> stainless steel adsorption tube. Storage: Room temperature for maximum 2 weeks. Quantity: NA. Preparation: Purged for 5 min. Headspace: NA. GC-column: Restek™ RTX-5 ms (30 m x 0⋅25 mm ID, coated with 1⋅0 mm HP-5 phase; Thermo Fisher Scientific) | NIST | NA | Active CD and CD in remission |
| Arasaradnam et al. [65] | 2011 | HBI, SSCAI | No | Urine | E-nose, MS | Processing: NA. Storage: NA. Quantity: 5–10 ml (E-nose), 1 ml (MS). Preparation: 38°C for 1 h (E-nose), 60°C for 12 min (MS). Headspace: Yes. GC-column: NA. | NA | NA | UC and CD and HV |
| Arasaradnam et al. [66] | 2013 | NA | No | Urine | E-nose, FAIMS | Processing: Within 6 h. Storage: −20°C. Quantity: 2 g. Preparation: 60°C for 1 h. Headspace: Yes, SPME. GC-column: SPB®−1 Capillary GC Column (L × I.D. 60 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma Aldrich). | NA | NA | UC and CD and HV |
| Walton et al. [46] | 2016 | HBI | Yes | Breath and faeces | GC–MS | Processing: Within 4 h (faeces), Bio-VOC sampler -> TD tube (breath). Storage: −80°C (faeces). Quantity: 5 ml (faeces). Preparation: 37°C for 10 min (faeces), purged for 2 min -> desorption (breath). Headspace: Yes, 500 ml (faeces). GC-column: Zebron™ ZB-624 GC Capillary Column (20 m x 0⋅18 mm x 1⋅00 µm; Phenomenex). | NIST | NA | CD and HV before and after treatment |
| IBS | |||||||||
| Rossi et al. [35] | 2017 | Rome III | Yes | Faeces | GC | Processing: Within 1 h; Ice -> homogenised. Storage: −80°C. Quantity: 750 mg. Preparation: 50°C for 10 min. Headspace: Yes, 2 cm3. GC-column: SPB®−1 Capillary GC Column (L × I.D.30 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma Aldrich). | NA | Bootstrapping | Predicting outcome IBS on diet or probiotic |
| Baranska et al. [24] | 2016 | Rome III | No | Breath | GC–MS | Processing: 3L Tedlar bag -> stainless steel adsorption tube. Storage: Room temperature for maximum 2–8 weeks. Quantity: NA. Preparation: No. Headspace: NA. GC-column: NA. | NIST | Bootstrapping, training‡, external validation | IBS and HV |
| Arasaradnam et al. [33] | 2014 | Rome II | No | Urine | FAIMS, GC–MS | Processing: Within 2 h. Storage: −80°C. Quantity: 5 ml. Preparation: 60°C for 5 min. Headspace: Yes, SPME (GC–MS). GC-column: NA. | NIST | Leave-one-out CV | IBS and coeliac disease |
| IBS and IBD | |||||||||
| Ahmed et al. [32] | 2013 | Manning, HBI, SCCAI | No | Faeces | GC–MS | Processing: Within 6 h. Storage: −20°C. Quantity: 2 g. Preparation: 60°C for 1 h. Headspace: Yes, SPME. GC-column: SPB®−1 Capillary GC Column (L × I.D. 60 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma Aldrich). | NIST | Leave-one-out CV, multi-step split sample CV | IBS and UC and CD and HV |
| Walton et al. [37] | 2013 | NA | Yes | Faeces | GC–MS | Processing: Within 4 h. Storage: −80°C. Quantity: 5 ml. Preparation: Nalophan bag -> 40°C for 10 min. Headspace: Yes, 500 ml. GC-column: Zebron™ ZB-624 GC Capillary Column (20 m x 0⋅18 mm x 1⋅00 µm; Phenomenex). | NIST | NA | IBS and UC and CD and HV before and after treatment |
| Shepherd et al. [34] | 2014 | Rome II, HBI, SSCAI | No | Faeces | GC | Processing: Within 6 h. Storage: −20°C. Quantity: 10 ml. Preparation: 50°C for 10 min. Headspace: Yes, 2 cm3. GC-column: SPB®−1 Capillary GC Column (L × I.D.30 m × 0⋅25 mm, df 0⋅25 μm; Supelco, Sigma Aldrich). | NA | 4-fold CV, training‡ | IBS and UC and CD and HV |
| Aggio et al. [36] | 2017 | Rome II, HBI, SCCAI | No | Faeces | GC | Processing: Within 6 h. Storage: −20°C. Quantity: 1 g. Preparation: 50°C for 10 min. Headspace: Yes, 2 cm3. GC-column: NA. | NA | Leave-one-out CV, 10-fold CV, 5-fold CV, 3-fold CV, 2-fold CV | IBS and UC and CD and HV |
| Cauchi et al. [38] | 2014 | NA | No | Breath, Faeces and urine | GC–MS | Collection: TD tube. Storage: NA. Quantity: 500 ml. Preparation: Purged for 2 min -> desorption for 5 min. Headspace: NA. GC-column: Zebron™ ZB-624, GC Capillary Column (20 m x 0⋅18 mm x 1⋅00 µm; Phenomenex). | NA | Bootstrapping, training‡ | IBS and CD and UC and HV |
CD = Crohn's disease; CV = cross validation; FAIMS = high field asymmetric waveform ion mobility spectrometry; GC = gas chromatography; HBI = Harvey–Bradshaw index; HV = healthy volunteer; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; MS = mass spectrometry; NA = not available or not applicable; NIST = National institute of Standards and Technology; SIFT = selected ion flow tube; SCCAI = Simple Clinical Colitis Activity Index; SPME = solid phase microextraction; UC = ulcerative colitis.
Methods used to identify VOCs.
Steps undertaken to mitigate against external validation in the development of models.
Use of training and validation sets in development of models.
Table 2.
Comparison analytical methods.
| Method | Description | Real-time analysis | Cost | Risk of contamination | Sample preparation | Online/offline | Storage time |
|---|---|---|---|---|---|---|---|
| Gas chromatography-mass spectrometry (GC–MS) | Separation of chemical components based on their relative affinity with a capillary column. Components elute from the GC-column with different retention times after which they are captured, ionised, accelerated, deflected and detected by the MC. | – | + | + | + | Offline | + |
| Ion mobility spectrometry (IMS) | Separation of chemical components on the basis of differences in ion mobilities within an electric field | + | – | – | – | Online | – |
| Selective ion flow tube-mass spectrometry (SIFT-MS) | Absolute quantification of trace VOC by ionisation with precursor/reagent ions | + | + | – | – | Online | – |
| Electronic nose (E-nose) | Array of sensors creating a smell “fingerprint” with pattern recognition modules resembling the olfactory system | – | – | + | + | Offline | + |
| Field asymmetric ion mobility spectrometry (FAIMS) | Separation of chemical components on the basis of differences in ion mobilities within an electric field | + | – | – | – | Online | – |
++: =applicable/high/long; -: =not applicable/low/short; Online: =immediate analysis of the sample; Offline: =preconcentration of samples and possibility of storing samples for later analysis.
Eight articles included IBS patients and defined their population by the Rome criteria (three Rome II and two Rome III), the Manning criteria (one), or did not specify the diagnostic criteria (two) (Table 1). Next to fulfilling Rome criteria, diagnosis of IBS also requires absence of organic disease, which was explicitly described by most articles. Two studies only included IBS-D patients [32,33], one all IBS subtypes [24], three multiple subtypes [34], [35], [36] and two did not specify subtypes (supplementary Table 2) [37,38]. The 17 studies including IBD patients studied CD (n = 3), UC (n = 3) or both (n = 11). Five pooled CD plus UC data in one IBD group for analysis [32,34,[39], [40], [41]]. To assess disease activity in IBD patients, the Harvey-Bradshaw index (HBI) for CD and the Simple Clinical Colitis Activity Index (SCCAI) for UC were predominantly used with the exception of one study using indium-labelled granulocyte nuclear imaging [39].
The majority of studies did not report exclusion criteria. When present, these included major comorbidities, organic disease in case of IBS, major abdominal surgery, pregnancy and breast feeding. Nine studies excluded patients with prior antibiotic use 2 to 12 weeks before sampling [35,37,38,40,[42], [43], [44], [45], [46]]. Other excluded drugs were pro/prebiotics and drugs influencing the immune system and/or microbiota.
Taking the abovementioned into account, the small number of studies and large interstudy heterogeneity undermines a thorough systematic analysis. Therefore, a narrative review is better suited to discuss the results.
3. Results
3.1. Single VOCs for diagnosis and monitoring
Differentiating patients from healthy people allows the identification of VOCs that represent a healthy volatilome. However, although healthy volunteers (HV) are rarely seen in clinical practice, and, hence, their discrimination has limited clinical utility, information about their baseline volatilome will be of interest to assess whether treatment of patients leads to normalisation of VOCs. More importantly, being able to differentiate between closely associated diseases will be key. Supplementary Table 3 summarises all VOCs that significantly differ between HV and IBS and IBD patients. Considering clinical applicability, only compounds described in more than one study are further discussed.
3.1.1. Inflammatory bowel disease
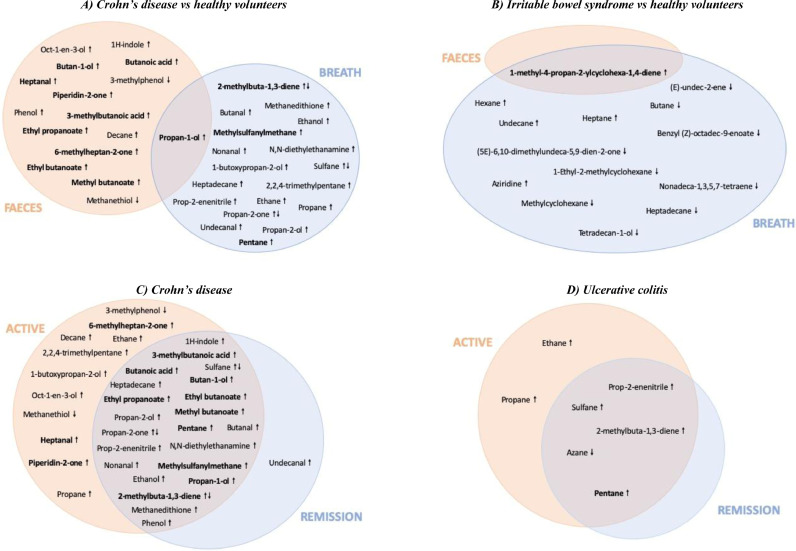
When comparing CD patients (active and remission pooled) to HV, 10 compounds were described in multiple studies (Fig. 1a). Six of these compounds were described twice by the same research group, mostly using the same or overlapping study populations [37,[46], [47], [48]]. This includes pentane [47,48], which was confirmed by Pelli et al., looking only at active CD [49]. 2-methylbuta-1,3-diene [40,47] and methylsulphanylmethane [40,44] were found increased in the breath of CD patients and contradictory results have been found for breath sulphane [44,47]. Propan-1-ol was increased in faeces and breath in four studies including CD patients [32,37,46,47]. In active CD, 6-methylheptan-2-one, heptanal and piperidin-2-one were found increased in faecal samples in two studies of the same research group [32,45]. No common compounds were found differentiating CD patients in remission from HV, suggesting a normalisation of VOCs to a baseline healthy volatilome. Two studies found an increase in breath pentane in UC patients [47,48], which was confirmed by another study in active UC patients [49].
Fig. 1.
Individual VOCs in IBD and IBS. Compounds described in more than one study are in bold. ↑: upregulated. ↓: downregulated.
3.1.2. Irritable bowel syndrome
Only one compound (1-methyl-4-propan-2-ylcyclohexa-1,4-diene) was increased in faecal and breath samples (Fig. 1b) when comparing IBS patients to HV [24,32].
3.1.3. Differential diagnosis
Regarding the use of individual VOCs for differential diagnosis, four articles (two by one group) described an increase in propan-1-ol in breath and faeces of CD patients compared to IBS-D patients, (supplementary Table 3) [32,37,46,47]. When comparing UC to IBS-D and IBS-D to general IBD patients, no compounds were identified in multiple studies. However, 1-methyl-4-propan-2-ylcyclohexa-1,4-diene was only detected in IBS patients (breath and faeces).
3.1.4. Monitoring inflammatory bowel disease
CD and UC patients have frequent flares [50,51]. Therefore, VOC analysis holds potential to monitor disease activity and assess the effect of treatment to guide personalised medicine (supplementary Table 3, Fig. 1c, d). A decrease of breath pentane in CD and UC patients in remission compared to active disease was observed [39,47]. This is not unexpected since pentane has been identified as marker for lipid peroxidation [52], making it a non-specific inflammatory biomarker. After treatment of CD patients, a significant decrease in six faecal compounds was found in two studies from the same research group: 3-methylbutanoic acid, butan-1-ol, butanoic acid, ethyl butanoate, ethyl propanoate and methyl butanoate [37,46]. A decrease in propan-1-ol in breath and faeces was described in four articles (two by the same group) [32,37,46,47].
3.2. Discriminative models combining VOCs
3.2.1. Technology and methodological issues
Identifying compounds is of great interest to explore the underlying pathological mechanisms. However, not all analytical approaches create a chemical identification of VOCs, like GC–MS, but recognise sensor responses to the bulk of VOCs as patterns or volatile ‘fingerprints’, like eNoses (Tables 1 and 2). Furthermore, no single VOC was found nor validated by multiple research groups resulting in little clinical value to be used as stand-alone biomarker for (differential) diagnosis. Therefore, combining VOCs in biomarker panels should allow to create discriminative algorithms with increased sensitivity and specificity to accurately diagnose, differentiate and monitor patients over time. Sixteen articles described 17 algorithms based upon breath, faeces and urine samples: 2 discriminating IBD patients from HV, 5 discriminating CD patients from HV, 4 discriminating UC from HV and 6 discriminating IBS from HV (Table 3). A lot of methodological variation was seen when sampling VOCs in the different matrices and even within the same sample source. Breath samples were processed between 1 and 6 h after sampling into Tedlar, Nalophan of Mylar bags or onto thermal desorption columns. Although some were stored at room temperature, one did store breath samples at −20°C (Table 1). Finally, between 50 ml and 5 L of breath samples were analysed. Faecal samples were processed between 1 and 6 h after sampling and storage at −20°C or −80°C. The volume of faecal samples ranged from 250 mg to 4 g with 2 g being the most prevalent. Also, some samples were homogenised of which 5–10 ml was sampled. The samples were heated and VOCs captured by solid phase microextraction (SPME) fibres. The small number of studies that analysed VOCs in the headspace of urine processed 1–10 ml of urine within 2–6 h after storage at −80°C and heating to 38°C or 60°C.
Table 3.
VOC models.
| ID | Year | Article | Aim | Number of compounds | Study population | Result |
|---|---|---|---|---|---|---|
| Disease versus healthy | ||||||
| Inflammatory bowel disease | ||||||
| 1 | 2014 | [34] | IBD vs HV | NA | IBD 101 HV 46 | Acc: Mean 79%; IBD 78%; HV 80% |
| 2 | 2016 | [41] | IBD vs HV | NA | IBD 54 HV 22 | Sens: 74%, Spec: 75%, AUC: 0⋅82 |
| Crohn's disease | ||||||
| 3 | 2015 | [53] | CD R vs HV | 6 | CD 191 CDR NA HV 110 | Acc: 92⋅3%, Sens: 96%, Spec: 99%, AUC: 0⋅99 |
| 4 | 2015 | [53] | CD A vs HV | 7 | CD 191 CDA NA HV 110 | Acc: 97⋅3%, Sens: 96%, Spec: 97%, AUC: 0⋅98 |
| 5 | 2015 | [44] | CD vs HV | 6 | CD 18 HV 18 | OSC-PLS-DA: Clear separation groups. Sens: 94⋅4%, Spec: 94⋅4%, AUC: 0⋅864 |
| 6 | 2016 | [41] | CD vs HV | NA | CD 25 HV 22 | Sens: 69%, Spec: 67%, AUC: 0⋅77 |
| 7 | 2014 | [38] | Faeces: CD vs HV | NA | CD 24 HV 20 | Acc: 85%, Sens: 93%, Spec: 78%, AUC: 0⋅97 |
| Ulcerative colitis | ||||||
| 8 | 2007 | [42] | UC vs HV | 32 | UC 18 HV 30 | DS: Clear separation groups. Acc: 100 (96)% |
| 9 | 2015 | [44] | UC vs HV | 6 | UC 20 HV 18 | Sens: 90⋅5%, Spec: 94⋅4%, AUC: 0⋅742 |
| 10 | 2016 | [41] | UC vs HV | NA | UC 29 HV 22 | Sens: 61%, Spec: 62%, AUC: 0⋅70 |
| 11 | 2014 | [38] | Faeces: UC vs HV | NA | UC 19 HV 20 | Acc: 58%, Sens: 43%, Spec: 69%, AUC: 0⋅54 |
| Irritable bowel syndrome | ||||||
| 12 | 2013 | [32] | IBS vs HV | 49 | IBS 30 HV 109 | Acc: IBS 70 (68)%; HV 95 (94)%, Sens: 90 (82)%, Spec: 80 (78)%, AUC: 0⋅94 (0⋅92) |
| 13 | 2014 | [34] | IBS vs HV | NA | IBS 34 HV 46 | Acc: Mean 54%; IBS 46%; HV 58% |
| 14 | 2016 | [24] | IBS vs HV | 16 | IBS 170 HV 153 | PCA: Clear separation groups. Acc: Positive 84%; Negative: 81.5%, Sens: 89⋅4%, Spec: 73⋅3%, AUC: 0⋅83 |
| 15 | 2014 | [38] | Breath: IBS vs HV | NA | IBS 28 HV 20 | Acc: 58%, Sens: 41%, Spec: 72%, AUC: 0⋅44 |
| 16 | 2014 | [38] | Faeces: IBS vs HV | NA | IBS 28 HV 20 | Acc: 61%, Sens: 51%, Spec: 71%, AUC: 0⋅63 |
| 17 | 2014 | [38] | Urine: IBS vs HV | NA | IBS 28 HV 20 | Acc: 64%, Sens: 38%, Spec: 80%, AUC: 0⋅53 |
| Active disease versus disease in remission | ||||||
| Crohn's disease | ||||||
| 18 | 2015 | [53] | CD A vs CD R | 10 | CD 191 CDA NA CDR NA | Acc: CD A 81⋅5%; CD R 86⋅4%, Sens: 81%, Spec: 80%, AUC: 0⋅88 |
| Disease versus disease | ||||||
| Inflammatory bowel disease and irritable bowel syndrome | ||||||
| 19 | 2013 | [32] | IBS vs active IBD | 60 | IBS 30 IBD 110 | Acc: IBS 80 (70)%; IBD 96 (95)%, Sens: 96 (80)%, Spec: 80 (62)%, AUC: 0⋅98 (0⋅76) |
| 20 | 2014 | [34] | IBS vs IBD | NA | IBS 34 IBD 101 | Acc: Mean 76%; IBS 68%; IBD 82%, Sens: 76%, Spec: 88% |
| Crohn's disease and ulcerative colitis | ||||||
| 21 | 2015 | [44] | CD vs UC | 6 | CD 18 UC 20 | Sens: 88⋅9%, Spec: 90⋅0%, AUC: 0⋅828 |
| 22 | 2016 | [41] | UC vs CD | NA | CD 25 UC 29 | Sens: 67%, Spec: 67%, AUC: 0⋅70 |
| Irritable bowel syndrome and Crohn's disease | ||||||
| 23 | 2013 | [32] | IBS vs CD | 44 | IBS 30 CD 62 | Acc: IBS 80 (80)%; CD 100 (97)%, Sens: 94 (90)%, Spec: 82 (80)%, AUC: 0⋅97 (0⋅93) |
| Irritable bowel syndrome and ulcerative colitis | ||||||
| 24 | 2013 | [32] | IBS vs UC | 44 | IBS 30 UC 48 | Acc: IBS 87 (83)%; UC 94 (92)%, Sens: 96 (90)%, Spec: 80 (80)%, AUC: 0⋅96 (0⋅88) |
| Irritable bowel syndrome and coeliac disease | ||||||
| 25 | 2014 | [33] | IBS vs coeliac disease | NA | IBS 20 Coeliac 27 | Heat map: Clear separation groups. Sens: 85%, Spec: 85%, AUC: 0⋅91 |
| Inflammatory bowel disease and non-inflammatory bowel disease | ||||||
| 26 | 2016 | [40] | IBD vs non-IBD | 4 | IBD 35 Non-IBD 6 | AUC: 0⋅81 |
| Combinations | ||||||
| Crohn's disease and ulcerative colitis and healthy | ||||||
| 27 | 2011 | [65] | CD vs UC vs HV | NA | CD 15 UC 4 HV 8 | PCA: Clear separation groups |
| 28 | 2013 | [66] | CD vs UC vs HV | NA | CD 24 UC 24 HV 14 | PCA: Clear separation groups. Acc: >75% |
| 29 | 2016 | [45] | CD vs UC vs HV | NA | CD 117 UC 100 HV 109 | PCA: Clear separation CD A, CD R and HV; Clear separation ileal and colon CD; Unclear separation UC A, UC R and HV |
| Crohn's disease and ulcerative colitis and irritable bowel syndrome and healthy | ||||||
| 30 | 2017 | [36] | IBS vs CD vs UC vs HV | NA | IBS 28 CD 36 UC 49 HV 41 | PCA: Clear separation CD A and IBS; Clear separation UC A and UC R; Unclear separation IBD A and IBD R Acc: Ranging between 75% and 100%†, Sens: NA, † Spec: NA, † AUC: NA, † |
| 31 | 2014 | [38] | Faeces: CD vs HV and UC and IBS | NA | IBS 28 CD 24 UC 19 HV 20 | Acc: 79%, Sens: 68%, Spec: 83% AUC: 0⋅65 |
| 32 | 2014 | [38] | Urine: CD vs HV and UC and IBS | NA | IBS 28 CD 24 UC 19 HV 20 | Acc: 72%, Sens: 48%, Spec: 81%, AUC: 0⋅59 |
| Other | ||||||
| 33 | 2015 | [53] | CD A vs CD R vs HV | 17 | CD 191 CDA NA CDR NA HV 110 | PCA: Clear separation groups. Acc: 86⋅7% |
| 34 | 2017 | [67] | UC A vs UC R vs non-IBD colitis | 11 | UC 76 UCA NA UCR NA Non-IBD 22 | PCA: Clear separation groups. Sens: 92%, Spec: 77%, AUC: 0⋅94 |
| Interventional | ||||||
| Irritable bowel syndrome | ||||||
| 35 | 2017 | [35] | Low FODMAP baseline model | 15 | Response 35 Non-response 9 | PCA: Clear separation groups. Acc: treat 97%; plac 40⋅9%, Sens: treat 100%; plac 62⋅5%, Spec: treat 88%; plac 28⋅6% |
| 36 | 2017 | [35] | Probiotic baseline model | 10 | Response 29 Non-response 16 | PCA: Clear separation groups. Acc: treat 89%; plac 45⋅5%, Sens: treat 93%; plac 75%, Spec: treat 82%; plac 28⋅6% |
| 37 | 2017 | [35] | Low FODMAP end treatment model | 9 | Response 30 Non-response 9 | PCA: Clear separation groups. Acc: 96%, Sens: 100%, Spec: 82% |
| 38 | 2017 | [35] | Probiotic end of treatment model | 11 | Response 29 Non-response 16 | PCA: Clear separation groups. Acc: 91%, Sens: 92%, Spec: 90% |
A = active disease; CD = Crohn's disease; CV = cross validation; DS = discriminant score; HV = healthy volunteer; IBD = inflammatory bowel disease; IBS = irritable bowel syndrome; NA = not available; OSC-PLS-DA = partial least squares discriminant analysis with orthogonal signal correction; PCA = principal component analysis; R = disease in remission; UC = ulcerative colitis.
Results are shown as: visual (PCA, heat map, OSC-PLS-DA, DS); Acc = accuracy% (after CV); Sens = sensitivity% (after CV); Spec = specificity% (after CV); AUC = area under the curve (after CV); treat = treatment; plac = placebo.
†This article has elaborate tables which are not included in this paper. After double cross-validation, the most clinically important findings were the accuracy of CD-A versus IBS (87%), and IBS versus HV (78%).
Furthermore, most articles combined VOCs into discriminating panels with the help of different regression models, two articles used an eNose to detect a breath pattern based upon sensor changes, analysed by pattern recognition tools like principle component analysis (PCA) (Table 3). PCA reduces a large set of variables to a small set containing most of the information of the large set by transforming a number of (possibly) correlated variables into a (smaller) number of uncorrelated variables called principal components. Although some studies reported the individual compounds that build these discriminative models (supplementary Table 4), others have not or sensor technology was used which does not identify individual compounds but compares the sensor responses by pattern recognition tools. Hence, using PCA and heat map analysis, most studies clearly discriminated healthy volunteers from patients based upon their breath pattern.
3.2.2. Inflammatory bowel disease
When discriminating CD patients from HV in breath and faeces, accuracies ranged between 85–97%, sensitivities between 69–96%, specificities between 67–99% and the area under the receiver operating characteristic curves (AUCROC) between 0⋅77–0⋅99 [38,41,44,53]. Discriminating UC patients from HV in breath and faeces yielded accuracies, sensitivities, specificities and AUCROC’s ranging between 58–96%, 43–91%, 62–94% and 0⋅54–0⋅74, respectively, with lower numbers found in faecal models [38,41,42,44]. This could be due to the larger microbial influence in the faecal VOC samples.
3.2.3. Irritable bowel syndrome
Differentiating IBS patients from healthy volunteers by breath, faeces and urine lead to accuracies, sensitivities, specificities and AUCROC’s between respectively 46–68%, 38–89%, 71–80% and 0⋅44–0⋅92 [24,32,34,38].
3.2.4. Differential diagnosis
In contrast to the higher number of studies comparing patients to HV, only eight models discriminated between patient populations in order to assess the clinical utility of VOCs for differential diagnosis. Two described the differentiation between IBD versus IBS patients (faeces) [32,34], two between CD versus UC patients (breath) [41,44], one between IBS versus CD patients (faeces) [32], one between IBS versus UC patients (faeces) [32], one between IBS versus coeliac disease patients (urine) [33] and one between IBD versus non-IBD colitis patients (breath) [40]. The models discriminating patient groups have a better performance than those comparing patients with HV, with accuracies ranging between 68–97%, sensitivities between 67–90%, specificities between 62–90% and AUCROC’s between 0⋅70–0⋅97. However, most of these models were developed in single centre studies without independent external validation by others.
3.2.5. Monitoring inflammatory bowel disease
One study discriminated patients with active CD and CD in remission, (AUCROC: 0⋅88) [53], suggesting a potential for follow-up of patients over time and to monitor disease activity. Nonetheless, the VOCs used in this algorithm remained unidentified and these results were not validated, limiting its current clinical implementation. Two studies compared patients with active IBD (CD and UC) and IBD in remission, in addition to comparing them to IBS patients and HV [36,45]. PCA analysis clearly separated patients with active CD, CD in remission and HV, but patients with active UC, UC in remission and HV could not be differentiated [45]. The second study showed a good separation between patients with active UC and UC in remission, but not between pooled patients with active IBD (CD and UC) and IBD in remission [36]. These contradictory results highlight the need for further research evaluating active disease and disease in remission before drawing definitive conclusions and implementation.
3.3. Microbial influence
In IBS and IBD, the microbiota plays an important role and is therefore a major contributor in VOC analysis [17,18]. Microbial changes influence the metabolic processes in the body (permeability, digestion) and the microbiota itself produces numerous VOCs [54]. Hence, changes in VOC composition can be expected in these patients, especially when studying faecal VOCs considering their direct contact with the gut microbiota. It remains unclear whether VOCs are produced by the microbiota or by the patient's intrinsic pathology. Only Smolinska et al. focused on this microbial relationship in CD patients. Through PCA and canonical correlation analysis, 18 VOCs significantly correlated with 19 bacterial taxa in active disease and 17 VOCs correlated with 17 bacterial taxa in patients in remission (supplementary Table 5). Three bacterial taxa and nine VOCs were present in active and inactive disease [54]. This study was the first to prove the interplay between VOCs and microbiota and highlights the need to take the microbial composition into account in future studies.
3.4. Personalised medicine
For IBS, there is currently no ‘one-fits-all’ treatment, making it a cumbersome process of trial and error, which can negatively impact patient comfort. In addition, the treatment response needs to be evaluated and adjusted accordingly. Therefore, predictive biomarkers to preselect the most suitable treatment are of great interest, fulfilling the increasing demand for personalised medicine.
Walton et al. conducted an interventional study investigating the effect of treatment on VOC composition in faeces in patients with CD, UC and IBS [37]. All patients received two weeks of treatment: CD patients (n = 8) received elemental nutrition wherein proteins are cleaved into individual amino acids, UC patients (n = 12) received oral corticosteroids and 5-aminosalicylic acid derivatives with no specific diet, and IBS patients (n = 4) were treated by an exclusion diet. Before treatment, there was a significant increase in the faecal VOC concentrations of ester and alcohol derivates of short chain fatty acids (SCFAs) and indole in CD patients compared to the other groups. In patients with UC and IBS, indole and phenol levels tended to be higher compared to HV. After treatment, faecal VOC concentrations normalised to those of HV [37].
A second interventional study (with the highest quality) by the same group evaluated the effect of enteral feeding in CD patients (n = 17) and HV (n = 7) [46]. In HV, one week of enteral feeding increased indole and phenol levels in breath together with a change in stool colour and the induction of halitosis. In CD patients, enteral feeding improved symptoms, decreased c-reactive protein (CRP) and decreased faecal concentrations of SCFAs, methyl and ethyl esters of these fatty acids, propan-1-ol and 1-butanol [46].
Rossi et al. randomised IBS patients to a low-FODMAP diet versus sham-diet and a probiotic versus placebo diet for four weeks [35]. Faecal VOCs were analysed at baseline and after treatment by an Odoreader, which has a GC front end and a gas sensor detector and detects patterns. VOC models to predict treatment response at baseline resulted in a high accuracy (low-FODMAP model: 97% and probiotic model: 89%) for the treatment groups. However, when applying the same models in the control groups (sham/placebo), a low accuracy was achieved (low-FODMAP model: 40⋅9% and probiotic model: 45⋅5%) (Table 3). This implies that these models are specific for the response to low-FODMAP and/or probiotic rather than for response to therapy in general, and emphasises the potential use of VOCs as non-invasive predictive markers to optimise personal treatment [35].
3.5. Metabolic pathways and VOCs
Table 4 describes only those compounds mentioned in two or more studies. Most of these individual compounds described in this review are found to be endogenous or exogenously present in food or produced by the microbiota. The endogenous compounds play a role in several metabolic pathways such as lipid, butanoate, ethanol, sulphur, propanoate and ketone metabolism and in the biosynthesis of tropane, piperidine, pyridine alkaloid, and terpenoid backbones (Table 1).
Table 4.
Metabolic pathways.
| Compound | CAS number† | Source | Concentration | Disease | Pathway | Origin | Articles |
|---|---|---|---|---|---|---|---|
| 2-methylbuta-1,3-diene | 78–79–5 | Breath | ↓↑ | CD | Terpenoid backbone biosynthesis. Biosynthesis of terpenoids, steroids, secondary metabolites | Endogenous, Plant, Tobacco | [47,53] |
| 3-methylbutanoic acid | 503–74–2 | Faeces | ↑ | CD | Biosynthesis of alkaloid and secondary metabolites. Protein digestion and absorption | Endogenous, Plant, Animal | [37,46] |
| 6-methylheptan-2-one | 928–68–7 | Faeces | ↑ | CD | Synthesis and degradation of ketone bodies. Cholesterol oxidation | Endogenous, Plant, Animal | [32,45] |
| Butan-1-ol | 71–36–3 | Faeces | ↑ | CD | Butanoate metabolism. Microbial metabolism. Degradation of aromatic compounds | Endogenous, Plant, Animal, Bacteria | [37,46] |
| Butanoic acid | 107–92–6 | Faeces | ↑ | CD | Butanoate metabolism. Metabolic pathways. Carbohydrate and protein digestion and absorption | Endogenous, Plant, Animal, Bacteria | [37,46] |
| Ethyl butanoate | 105–54–4 | Faeces | ↑ | CD | Lipid metabolism | Endogenous, Plant, Animal | [37,46] |
| Ethyl propanoate | 105–37–3 | Faeces | ↑ | CD | Ethanol metabolism | Endogenous, Plant | [37,46] |
| Heptanal | 111–71–7 | Faeces | ↑ | CD | Lipid metabolism | Endogenous, Plant, Animal | [32,45] |
| Methyl butanoate | 623–42–7 | Faeces | ↑ | CD | Lipid metabolism | Endogenous, Plant, Animal | [37,46] |
| Methylsulphanylmethane | 75–18–3 | Breath Faeces | ↑↓ | CD | Sulphur metabolism | Endogenous, Plant, Bacteria | [44,45] |
| Pentane | 109–66–0 | Breath Faeces | ↑↓ | CD, UC | Lipid metabolism | Endogenous, Plant | [47,48] |
| Piperidin-2-one | 675–20–7 | Faeces | ↑ | CD | Tropane, piperidine and pyridine alkaloid biosynthesis | Endogenous | [32,45] |
| Propan-1-ol | 71–23–8 | Breath Faeces | ↑ | CD | Propanoate metabolism | Endogenous, Plant, Animal, Bacteria, Fungi | [32,37,46,47] |
| Propan-2-one | 67–64–1 | Breath | ↑↓ | CD | Synthesis and degradation of ketone bodies. Propanoate metabolism. Metabolic pathways | Endogenous, Plant, Animal, Bacteria, Tobacco | [47,53] |
| Sulphane | 7783–06–4 | Breath | ↑↓ | CD | Cystine and methionine metabolism. Sulphur metabolism. Microbial metabolism. Carbon metabolism | Endogenous, Plant, Animal, Bacteria, Tobacco | [44,47] |
| 1-methyl-4-propan-2-ylcyclohexa-1,4-diene | 99–85–4 | Breath Faeces | ↑ | IBS | NA | Endogenous, Plant | [24,32] |
CAS numbers are unique numerical identifiers assigned by the Chemical Abstracts Service.
A substantial amount of the discriminative compounds are SCFAs and part of the butanoate, propanoate, and acetate metabolism. SCFAs are the main metabolic products of anaerobic bacterial fermentation, serving as fuel for intestinal epithelial cells but also modulating electrolyte and water absorption. More importantly, they have anti-inflammatory properties and mediate the effect of the microbiota on the intestinal immune function [55,56]. A second compound appearing in biomarker panels is indole. It is formed by bacterial metabolism of l-tryptophan and has anti-inflammatory properties, again stressing the importance of the microbial influence on VOCs [57].
It is currently impossible to identify the detected compounds as originating from metabolic pathways and/or from digestion of food or medication. Moreover, all these pathways are in continuous interaction with inflammatory processes. This close synergy might explain the discrepant findings in the different studies and stresses the need to clarify and explore the VOC metabolism and distribution in different body matrices. IBS is a very variable condition compared to IBD which corresponds to the inconsistent findings of VOCs in IBS patients. It appears that most of the VOCs detected in IBD patients are related to inflammation rather than being disease specific. This underlines the need for future studies to combine VOCs into panels to increase the specificity for discrimination.
4. Strengths, limitations and future perspectives
Promising VOCs for (differential) diagnosis include propan-1-ol in breath and faeces, to differentiate CD patients from HV and IBS patients. Pentane in breath differentiated patients with CD and UC from HV. Above of this, 1-methyl-4-propan-2-ylcyclohexa-1,4-diene was only detected in IBS patients, making it a promising biomarker discriminating IBS from HV. Diagnosis of IBS is currently based on the Rome IV criteria, with exclusion of organic disease. However, these criteria are not specific, emphasising the need to develop a specific diagnostic test for IBS. Using VOCs for disease monitoring demonstrated a decrease of breath pentane levels when patients with CD or UC are in remission. Remission in CD patients was also associated with a decrease in metabolites of microbial SCFAs in faeces, suggesting a correlation with microbial changes induced by treatment. However, these individual VOCs have limited specificity. They are generally related to inflammation and can also be altered in other diseases. For example, 1-propanol has been previously detected in coeliac disease [58,59] and 1-methyl-4-propan-2-ylcyclohexa-1,4-diene in colorectal cancer [60]. Pentane on the other hand has not only been detected in CD and UC but also in asthma, heart failure and non-alcoholic fatty liver disease. Breath pentane levels are an index for lipid peroxidation and therefore could serve as marker for general inflammation, ferroptosis or apoptosis. This emphasises the need to combine these VOCs in discriminative models and with other clinical/biochemical data to increase disease specificity, especially for monitoring. Furthermore, there is a need to include appropriate controls. A lot of the studies included in this review compared patients with HV. However, it would clinically be more beneficial to focus on comparing symptomatic groups of patients. Future studies should therefore focus on comparing these symptomatic groups.
Furthermore, VOC models differentiating CD patients from HV seem to be the most successful. In contrast, models discriminating IBS patients from HV showed the lowest discriminative capacity, which could be explained by the heterogeneity of the population. There is a wide variation of VOC data within the IBS population with some being clearly aberrant and others resembling HV. This emphasises the importance of studying separate IBS subtypes in the future to increase homogeneity. Subtyping patients is not only crucial in IBS, but also in IBD, since the underlying pathogenesis of CD and UC is remarkably different. Ideally, patients with active and remissive disease should also be differentiated since first results suggest a normalisation of VOC profiles in remission. Hence, pooling of these patients could cause a loss of crucial information. Most of the included studies are pilot studies having small subgroup sample sizes (supplementary Table 2). Since more VOCs are detected in breath than the number of patients included in the studies, this can lead to an overfitting of data resulting in overoptimistic results. It is therefore important for future studies to take this into account and use dimension reduction statistics and to include a sufficient number of participants in each subgroup.
The three interventional studies demonstrated the added value of VOC analysis in assessing treatment response, and in predicting response to a specific intervention and, therefore, in selecting the best treatment option. This indicates the need to develop multiple volatile models which are individually linked to a specific intervention in order to be able to accurately give the right and most effective treatment to individual patients. When looking at the VOCs in those models, limited overlap in the different models is seen (supplementary Table 4). This reflects the heterogeneity between studies and stresses the need for standardised sampling as advised by current guidelines in breath analysis [61].
By studying VOCs induced by microbiota, intrinsic VOCs originating from human pathogenic metabolism can be deducted. VOCs in this review were mostly derived from pathways with a strong synergy with inflammatory processes. This knowledge can be implemented in in vitro or in vivo experiments and in the development of novel germ-free mouse or rat models colonised with human microbiota. This will further advance IBS and IBD research by allowing validation of results. Additionally, it would be interesting to know the contribution of microbial VOCs on human physiology and symptom development in disease states. Comparing profiles of active and remissive disease will give insight into the mechanisms of different treatment options. Correlation of VOC profiles with the patient's microbiota will shed light on the role of the microbiota in symptom development and the interaction with drugs and diet. The large interindividual variability in microbial composition, even in HV, complicates defining baseline values and may impede the development of diagnostic tests. Therefore, more research looking into this interplay between VOCs and the microbiota in HV and patients is imperative.
Overall, current research is very diverse and heterogeneous, with different in- and exclusion criteria, sampling techniques, and analytical methods (Table 1). Several studies discussed the influence of sample handling, transportation, storage conditions and preparation on VOC composition [62,63]. Furthermore, different technologies will not necessarily provide the same results and even using the same equipment is no guarantee as the set-up can differ (Table 1). Most articles used breath and faeces as VOC matrix, with only four articles studying urine. Further research into the correlation between breath, faecal and urinary VOCs could give insight into the metabolic VOC processes (Fig. 2). Also, environmental confounding should be taken into account. Characteristics such as race, cultural surroundings, diet, drugs and lifestyle all have an impact on microbial composition and therefore VOCs [64]. For example, 1-methyl-4-propan-2-ylcyclohexa-1,4-diene in IBS patients can originate from exogenous (diet) and endogenous (stabilisation of cell membrane and cell signalling) sources. Recording the diet could shed light on the biochemical origin of this VOC. It is important to identify all confounding factors to gain better insight in their impact on VOCs and match patients and controls [22,62]. Therefore, we recommend taking the abovementioned confounders into account when designing future volatomic trials (Table 5).
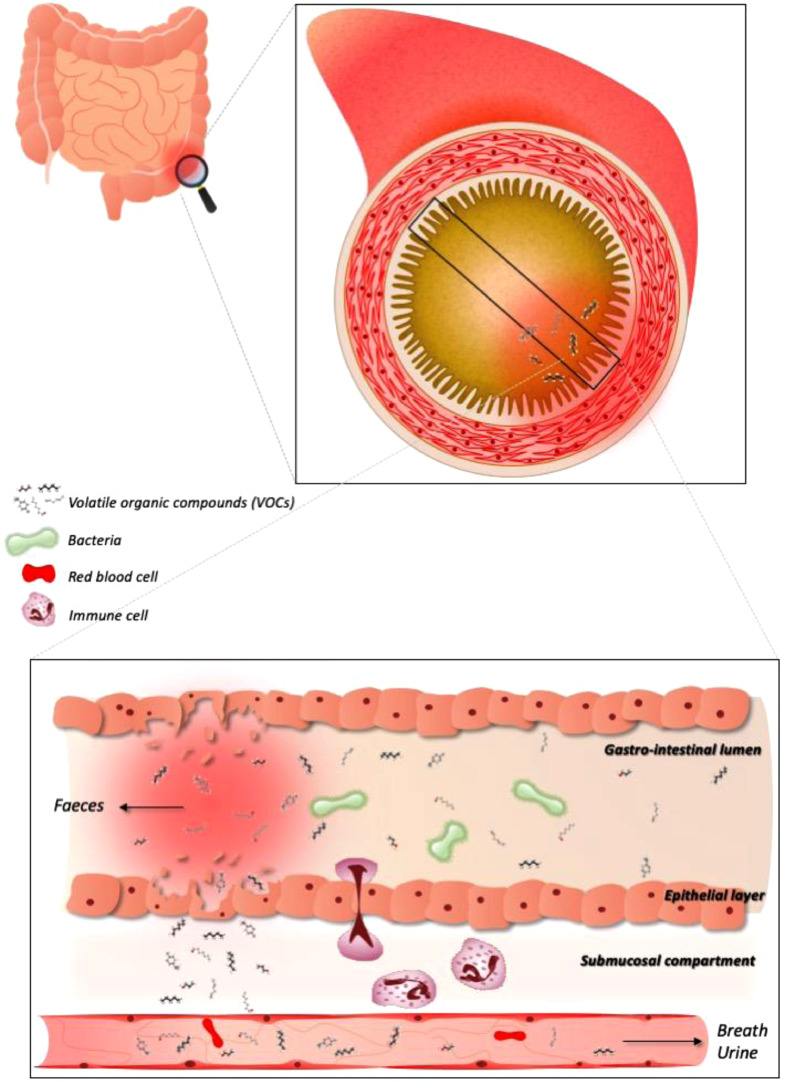
Fig. 2.
The potential of VOC analysis in the management of IBS and IBD. VOCs can originate from metabolic processes, both physiologic and pathophysiologic (inflammation or oxidative stress), and by the microbiota. Hence, a flare up or change in micobial composition can be reflected in VOC changes. However, this also stresses the potential of confounding external factors like drugs and diet that need to be accounted for. VOCs are liberated by the gastrointestinal cells and can be excreted in faeces, but are also transported through the bloodstream and can hence be detected in breath and urine, offering non-invasive alternatives for future disease management.
Table 5.
Future recommendations.
| Recommendations | |
|---|---|
| |
|
Despite good discriminative models between patients and HV, little is known about the VOC composition in HV and their natural evolution over time, the healthy human volatilome. Therefore, longitudinal prospective studies analysing VOCs in HV and patients will help determining a baseline healthy individual volatilome and map reproducibility. |
|
To be able to accurately differentiate IBS and IBD, not only from each other but also from other gastrointestinal disorders, research should ideally include a broader range of gastrointestinal disorders in a case-control design to be able to compare results and to optimise specificity. |
|
IBS and IBD patients are heterogeneous populations. Pooling data, therefore, is not advocated since it can distort results and important differences can be missed. IBS should ideally be classified according to the Rome IV criteria: diarrhoea, constipation, mixed and unspecified. The underlying pathophysiology is presumably different and different VOC patterns are thus to be expected. IBD patients should also be divided in CD and UC, and further subtyping in active disease and disease in remission could reveal interesting discriminatory characteristics. A proper sample size calculation should address the total number of patients to reveal subgroup characteristics. |
|
The quality of the research included in this review, evaluated with the AXIS tool, is reasonable, but there is room for improvement in order to pinpoint relevant specific VOCs. Hence, to be able to compare results and cluster data, it is paramount for future research to perform proper sample size calculations, and achieve a high level of quality and standardisation, in composition of the research population, used research methods and sampling conditions. The European Respiratory Society has published guidelines concerning standardisation of breath analysis and future research should take this into account [61]. Differences in used technologies should be taken into account when comparing data. Interventional trials should ideally be organised as randomised placebo-controlled trials or cross-over trials. |
|
Compounds should be described with the help of standardised international systems like the International union of pure and applied chemistry (IUPAC) nomenclature and numbers of the Chemical Abstracts Service (CAS). Compounds should ideally be verified using external standards and its concentration should be mentioned in studies for comparison. |
|
The different models show very limited similarities, making comparison difficult. We stress the need for studies to split up patients and design models in a test set, and externally validating the discriminative models in independent patient validation groups in order to assess clinical utility. When validating results in different research facilities one should try to use the same technology and setup of the equipment. Another argument to promote validation is the limited sample size of some studies, since this could lead to overfitting of the data, leading to overoptimistic results. |
|
VOCs are formed by metabolic processes and influence other pathways. Analysis of VOCs and their underlying metabolic pathways could help explain the pathophysiological mechanisms causing IBS and IBD. Pathways and VOCs of interest could then be further analysed in in vivo models and animal research, potentially leading to detection and development of novel therapeutic targets. Short chain fatty acids are the group of compounds that stand out the most across all studies. Their role in inflammatory disorders is well documented so their presence in VOC analysis is not unexpected. A VOC compound of high interest is propan-1-ol, a part of the propanoate metabolism. It is mentioned in multiple articles looking into CD, it is found to be increased in CD patients compared to HV and IBS patients and, more importantly, it decreases after effective treatment, making propan-1-ol a compound with great potential as a discriminative VOC biomarker and also in predicting treatment effects of CD patients. |
|
Future studies should compare the VOC composition in breath, urine and faeces of the same patient which could give some insights into metabolic processes playing a role in disease and to elucidate the VOC metabolism from gut to breath. |
|
|
|
The surrounding air, called exposome, can majorly influence VOC composition. Therefore, it is crucial to take background samples and correct for possible external influences. The authors should ideally describe in detail how they corrected for differences in sample collection, sample handling, storage conditions and sample preparation. |
|
Patient factors can also influence results of VOC analysis, for example diet, exercise, and drugs. For instance, the FODMAP-diet influences the microbiota [17,68] and is frequently used to treat IBS and IBD patients. FODMAP carbohydrates are poorly digested and, as a consequence, are fermented by the colon microbiota leading to increased gas production and an osmotic effect in the bowel [17]. This indicates that the therapeutic effect of dietary interventions is heavily dependent on the composition of the microbiota of the patient [68]. For example, FODMAPs cause a decrease in Clostridium coccoides, Akkermansia muciniphila, Mycoplasma hominis, Bifidobacterium and Actinobacteria; and an increase in Ruminococcus torques[68]. The microbiota on the other hand produce metabolites through digestion of nutrients, which can have a direct or indirect effect on symptoms in IBS and IBD. Possible confounding factors should, therefore, be registered and taken into account when analysing data. |
5. Conclusion
Volatomics in the diagnosis and follow-up of IBD and IBS patients show enormous potential. Promising diagnostic VOCs of interest are propan-1-ol in CD and 1-methyl-4-propan-2-ylcyclohexa-1,4-diene in IBS, in breath and faeces. However, volatomics have not yet led to a clinically useful and validated biomarker tool due to technical and quality issues. Next to this, VOCs also show clear potential for the non-invasive assessment of treatment efficacy and personalised medicine. Further development of our knowledge, uncovering underlying pathophysiological mechanisms and novel therapeutic targets, could have a major impact on patient and health care costs, since diagnosis and follow-up are often bothersome and expensive. We encourage future research to focus upon these issues and perform adequately designed, qualitative studies taking environmental factors and guidelines into account to move forward in the development of a non-invasive test for IBS and IBD. We believe VOC biomarkers will be most useful combined with other (clinical) parameters, particularly in monitoring of disease.
Declaration of Competing Interest
The authors declare no personal conflict of interests.
Acknowledgments
Acknowledgements
We would like to thank Vito Sabato (MD, PhD, Department of Immunology, Allergology, Rheumatology, Faculty of Medicine, University of Antwerp) for editorial comments and Philip Plaeke (MD, Laboratory of experimental medicine and paediatrics, Faculty of Medicine, University of Antwerp) for statistical guidance.
Funding
This study was funded by Research foundation Flanders (FWO, grant number 1144819N), the University of Antwerp (grant number 35018) and by “Kom op tegen kanker” (Stand up to cancer), the Flemish cancer society (grant ‘Emmanuel Van der Schueren’). The funders had no role in study design, data collection, data analysis, interpretation or writing of the report.
Footnotes
Supplementary material associated with this article can be found in the online version at doi:10.1016/j.ebiom.2020.102725.
Appendix. Supplementary materials
References
- 1.Canavan C., West J., Card T. The epidemiology of irritable bowel syndrome. Clin Epidemiol. 2014;6:71–80. doi: 10.2147/CLEP.S40245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Marley A.R., Nan H. Epidemiology of colorectal cancer. Int J Mol Epidemiol Genet. 2016;7(3):105–114. [PMC free article] [PubMed] [Google Scholar]
- 3.Gujral N., Freeman H.J., Thomson A.B. Celiac disease: prevalence, diagnosis, pathogenesis and treatment. World J Gastroenterol. 2012;18(42):6036–6059. doi: 10.3748/wjg.v18.i42.6036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ng S.C., Shi H.Y., Hamidi N. Worldwide incidence and prevalence of inflammatory bowel disease in the 21st century: a systematic review of population-based studies. Lancet. 2018;390(10114):2769–2778. doi: 10.1016/S0140-6736(17)32448-0. [DOI] [PubMed] [Google Scholar]
- 5.Schoepfer A.M., Trummler M., Seeholzer P., Seibold-Schmid B., Seibold F. Discriminating IBD from IBS: comparison of the test performance of fecal markers, blood leukocytes, CRP, and IBD antibodies. Inflamm Bowel Dis. 2008;14(1):32–39. doi: 10.1002/ibd.20275. [DOI] [PubMed] [Google Scholar]
- 6.De Schepper H.U., De Man J.G., Moreels T.G., Pelckmans P.A., De Winter B.Y. Review article: gastrointestinal sensory and motor disturbances in inflammatory bowel disease - clinical relevance and pathophysiological mechanisms. Aliment Pharmacol Ther. 2008;27(8):621–637. doi: 10.1111/j.1365-2036.2008.03624.x. [DOI] [PubMed] [Google Scholar]
- 7.Zhang L., Song J., Hou X. Mast cells and irritable bowel syndrome: from the bench to the bedside. J Neurogastroenterol Motil. 2016;22(2):181–192. doi: 10.5056/jnm15137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Defrees D.N., Bailey J. Irritable bowel syndrome: epidemiology, pathophysiology, diagnosis, and treatment. Prim Care. 2017;44(4):655–671. doi: 10.1016/j.pop.2017.07.009. [DOI] [PubMed] [Google Scholar]
- 9.Hungin A.P., Whorwell P.J., Tack J., Mearin F. The prevalence, patterns and impact of irritable bowel syndrome: an international survey of 40,000 subjects. Aliment Pharmacol Ther. 2003;17(5):643–650. doi: 10.1046/j.1365-2036.2003.01456.x. [DOI] [PubMed] [Google Scholar]
- 10.Lacy B.E., Patel N.K. Rome criteria and a diagnostic approach to irritable bowel syndrome. J Clin Med. 2017;6(11):99. doi: 10.3390/jcm6110099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Burisch J., Jess T., Martinato M., Lakatos P.L., EpiCom E. The burden of inflammatory bowel disease in Europe. J Crohns Colitis. 2013;7(4):322–337. doi: 10.1016/j.crohns.2013.01.010. [DOI] [PubMed] [Google Scholar]
- 12.Wang Z.H., Fang J.Y. Colorectal cancer in inflammatory bowel disease: epidemiology, pathogenesis and surveillance. Gastrointest Tumors. 2014;1(3):146–154. doi: 10.1159/000365309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kim D.H., Cheon J.H. Pathogenesis of inflammatory bowel disease and recent advances in biologic therapies. Immune Netw. 2017;17(1):25–40. doi: 10.4110/in.2017.17.1.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yanai H., Hanauer S.B. Assessing response and loss of response to biological therapies in IBD. Am J Gastroenterol. 2011;106(4):685–698. doi: 10.1038/ajg.2011.103. [DOI] [PubMed] [Google Scholar]
- 15.Waugh N., Cummins E., Royle P. Faecal calprotectin testing for differentiating amongst inflammatory and non-inflammatory bowel diseases: systematic review and economic evaluation. Health Technol Assess. 2013;17(55):xv–xix. doi: 10.3310/hta17550. 1-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang L., Mannalithara A., Singh G., Ladabaum U. Low rates of gastrointestinal and non-gastrointestinal complications for screening or surveillance colonoscopies in a population-based study. Gastroenterology. 2018;154(3) doi: 10.1053/j.gastro.2017.10.006. 540-555. [DOI] [PubMed] [Google Scholar]
- 17.Rajilic-Stojanovic M., Jonkers D.M., Salonen A. Intestinal microbiota and diet in IBS: causes, consequences, or epiphenomena. Am J Gastroenterol. 2015;110(2):278–287. doi: 10.1038/ajg.2014.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sundin J., Ohman L., Simren M. Understanding the gut microbiota in inflammatory and functional gastrointestinal diseases. Psychosom Med. 2017;79(8):857–867. doi: 10.1097/PSY.0000000000000470. [DOI] [PubMed] [Google Scholar]
- 19.Falony G., Joossens M., Vieira-Silva S. Population-level analysis of gut microbiome variation. Science. 2016;352(6285):560–564. doi: 10.1126/science.aad3503. [DOI] [PubMed] [Google Scholar]
- 20.Romano A., Capozzi V., Spano G., Biasioli F. Proton transfer reaction-mass spectrometry: online and rapid determination of volatile organic compounds of microbial origin. Appl Microbiol Biotechnol. 2015;99(9):3787–3795. doi: 10.1007/s00253-015-6528-y. [DOI] [PubMed] [Google Scholar]
- 21.Rondanelli M., Perdoni F., Infantino V. Volatile organic compounds as biomarkers of gastrointestinal diseases and nutritional status. J Anal Methods Chem. 2019;2019 doi: 10.1155/2019/7247802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brusselmans L., Arnouts L., Millevert C., Vandersnickt J., van Meerbeeck J.P., Lamote K. Breath analysis as a diagnostic and screening tool for malignant pleural mesothelioma: a systematic review. Transl Lung Cancer Res. 2018;7(5):520–536. doi: 10.21037/tlcr.2018.04.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lamote K., Nackaerts K., van Meerbeeck J.P. Strengths, weaknesses, and opportunities of diagnostic breathomics in pleural mesothelioma-a hypothesis. Cancer Epidemiol Biomarkers Prev. 2014;23(6):898–908. doi: 10.1158/1055-9965.EPI-13-0737. [DOI] [PubMed] [Google Scholar]
- 24.Baranska A., Mujagic Z., Smolinska A. Volatile organic compounds in breath as markers for irritable bowel syndrome: a metabolomic approach. Aliment Pharmacol Ther. 2016;44(1):45–56. doi: 10.1111/apt.13654. [DOI] [PubMed] [Google Scholar]
- 25.Amann A., Costello Bde L., Miekisch W. The human volatilome: volatile organic compounds (VOCs) in exhaled breath, skin emanations, urine, feces and saliva. J Breath Res. 2014;8(3) doi: 10.1088/1752-7155/8/3/034001. [DOI] [PubMed] [Google Scholar]
- 26.Haick H., Broza Y.Y., Mochalski P., Ruzsanyi V., Amann A. Assessment, origin, and implementation of breath volatile cancer markers. Chem Soc Rev. 2014;43(5):1423–1449. doi: 10.1039/c3cs60329f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rezaie A., Buresi M., Lembo A. Hydrogen and methane-based breath testing in gastrointestinal disorders: the north american consensus. Am J Gastroenterol. 2017;112(5):775–784. doi: 10.1038/ajg.2017.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siddiqui I., Ahmed S., Abid S. Update on diagnostic value of breath test in gastrointestinal and liver diseases. World J Gastrointest Pathophysiol. 2016;7(3):256–265. doi: 10.4291/wjgp.v7.i3.256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.de Lacy Costello B.P., Ledochowski M., Ratcliffe N.M. The importance of methane breath testing: a review. J Breath Res. 2013;7(2) doi: 10.1088/1752-7155/7/2/024001. [DOI] [PubMed] [Google Scholar]
- 30.Downes M.J., Brennan M.L., Williams H.C., Dean R.S. Development of a critical appraisal tool to assess the quality of cross-sectional studies (AXIS) BMJ Open. 2016;6(12) doi: 10.1136/bmjopen-2016-011458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Allen M.S., Walter E.E., Swann C. Sedentary behaviour and risk of anxiety: a systematic review and meta-analysis. J Affect Disord. 2019;242:5–13. doi: 10.1016/j.jad.2018.08.081. [DOI] [PubMed] [Google Scholar]
- 32.Ahmed I., Greenwood R., Costello Bde L., Ratcliffe N.M., Probert C.S. An investigation of fecal volatile organic metabolites in irritable bowel syndrome. PLoS ONE. 2013;8(3):e58204. doi: 10.1371/journal.pone.0058204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Arasaradnam R.P., Westenbrink E., McFarlane M.J. Differentiating coeliac disease from irritable bowel syndrome by urinary volatile organic compound analysis–a pilot study. PLoS ONE. 2014;9(10) doi: 10.1371/journal.pone.0107312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shepherd S.F., McGuire N.D., de Lacy Costello B.P. The use of a gas chromatograph coupled to a metal oxide sensor for rapid assessment of stool samples from irritable bowel syndrome and inflammatory bowel disease patients. J Breath Res. 2014;8(2) doi: 10.1088/1752-7155/8/2/026001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rossi M., Aggio R., Staudacher H.M. Volatile organic compounds in feces associate with response to dietary intervention in patients with irritable bowel syndrome. Clin Gastroenterol Hepatol. 2018;16(3) doi: 10.1016/j.cgh.2017.09.055. 385-91 e1. [DOI] [PubMed] [Google Scholar]
- 36.Aggio R.B., White P., Jayasena H., de Lacy Costello B., Ratcliffe N.M., Probert C.S. Irritable bowel syndrome and active inflammatory bowel disease diagnosed by faecal gas analysis. Aliment Pharmacol Ther. 2017;45(1):82–90. doi: 10.1111/apt.13822. [DOI] [PubMed] [Google Scholar]
- 37.Walton C., Fowler D.P., Turner C. Analysis of volatile organic compounds of bacterial origin in chronic gastrointestinal diseases. Inflamm Bowel Dis. 2013;19(10):2069–2078. doi: 10.1097/MIB.0b013e31829a91f6. [DOI] [PubMed] [Google Scholar]
- 38.Cauchi M., Fowler D.P., Walton C. Application of gas chromatography mass spectrometry (GC–MS) in conjunction with multivariate classification for the diagnosis of gastrointestinal diseases. Metabolomics. 2014;10(6):1113–1120. [Google Scholar]
- 39.Kokoszka J., Nelson R.L., Swedler W.I., Skosey J., Abcarian H. Determination of inflammatory bowel disease activity by breath pentane analysis. Dis Colon Rectum. 1993;36(6):597–601. doi: 10.1007/BF02049868. [DOI] [PubMed] [Google Scholar]
- 40.Rieder F., Kurada S., Grove D. A distinct colon-derived breath metabolome is associated with inflammatory bowel disease, but not its complications. Clin Transl Gastroenterol. 2016;7(11):e201. doi: 10.1038/ctg.2016.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Arasaradnam R.P., McFarlane M., Daulton E. Non-invasive exhaled volatile organic biomarker analysis to detect inflammatory bowel disease (IBD) Dig Liver Dis. 2016;48(2):148–153. doi: 10.1016/j.dld.2015.10.013. [DOI] [PubMed] [Google Scholar]
- 42.Garner C.E., Smith S., de Lacy Costello B. Volatile organic compounds from feces and their potential for diagnosis of gastrointestinal disease. FASEB J. 2007;21(8):1675–1688. doi: 10.1096/fj.06-6927com. [DOI] [PubMed] [Google Scholar]
- 43.Kumari R., Ahuja V., Paul J. Fluctuations in butyrate-producing bacteria in ulcerative colitis patients of North India. World J Gastroenterol. 2013;19(22):3404–3414. doi: 10.3748/wjg.v19.i22.3404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hicks L.C., Huang J., Kumar S. Analysis of exhaled breath volatile organic compounds in inflammatory bowel disease: a pilot study. J Crohns Colitis. 2015;9(9):731–737. doi: 10.1093/ecco-jcc/jjv102. [DOI] [PubMed] [Google Scholar]
- 45.Ahmed I., Greenwood R., Costello B., Ratcliffe N., Probert C.S. Investigation of faecal volatile organic metabolites as novel diagnostic biomarkers in inflammatory bowel disease. Aliment Pharmacol Ther. 2016;43(5):596–611. doi: 10.1111/apt.13522. [DOI] [PubMed] [Google Scholar]
- 46.Walton C., Montoya M.P., Fowler D.P. Enteral feeding reduces metabolic activity of the intestinal microbiome in Crohn's disease: an observational study. Eur J Clin Nutr. 2016;70(9):1052–1056. doi: 10.1038/ejcn.2016.74. [DOI] [PubMed] [Google Scholar]
- 47.Dryahina K., Smith D., Bortlik M., Machkova N., Lukas M., Spanel P. Pentane and other volatile organic compounds, including carboxylic acids, in the exhaled breath of patients with Crohn's disease and ulcerative colitis. J Breath Res. 2017;12(1) doi: 10.1088/1752-7163/aa8468. [DOI] [PubMed] [Google Scholar]
- 48.Dryahina K., Spanel P., Pospisilova V. Quantification of pentane in exhaled breath, a potential biomarker of bowel disease, using selected ion flow tube mass spectrometry. Rapid Commun Mass Spectrom. 2013;27(17):1983–1992. doi: 10.1002/rcm.6660. [DOI] [PubMed] [Google Scholar]
- 49.Pelli M.A., Trovarelli G., Capodicasa E., De Medio G.E., Bassotti G. Breath alkanes determination in ulcerative colitis and Crohn's disease. Dis Colon Rectum. 1999;42(1):71–76. doi: 10.1007/BF02235186. [DOI] [PubMed] [Google Scholar]
- 50.Hovde O., Moum B.A. Epidemiology and clinical course of Crohn's disease: results from observational studies. World J Gastroenterol. 2012;18(15):1723–1731. doi: 10.3748/wjg.v18.i15.1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bolge S.C., Waters H., Piech C.T. Self-reported frequency and severity of disease flares, disease perception, and flare treatments in patients with ulcerative colitis: results of a national internet-based survey. Clin Ther. 2010;32(2):238–245. doi: 10.1016/j.clinthera.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 52.Nycyk J.A., Drury J.A., Cooke R.W. Breath pentane as a marker for lipid peroxidation and adverse outcome in preterm infants. Arch Dis Child Fetal Neonatal Ed. 1998;79(1):F67–F69. doi: 10.1136/fn.79.1.f67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bodelier A.G., Smolinska A., Baranska A. Volatile organic compounds in exhaled air as novel marker for disease activity in Crohn's disease: a metabolomic approach. Inflamm Bowel Dis. 2015;21(8):1776–1785. doi: 10.1097/MIB.0000000000000436. [DOI] [PubMed] [Google Scholar]
- 54.Smolinska A., Tedjo D.I., Blanchet L. Volatile metabolites in breath strongly correlate with gut microbiome in CD patients. Anal Chim Acta. 2018;1025:1–11. doi: 10.1016/j.aca.2018.03.046. [DOI] [PubMed] [Google Scholar]
- 55.Tedelind S., Westberg F., Kjerrulf M., Vidal A. Anti-inflammatory properties of the short-chain fatty acids acetate and propionate: a study with relevance to inflammatory bowel disease. World J Gastroenterol. 2007;13(20):2826–2832. doi: 10.3748/wjg.v13.i20.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vinolo M.A., Rodrigues H.G., Nachbar R.T., Curi R. Regulation of inflammation by short chain fatty acids. Nutrients. 2011;3(10):858–876. doi: 10.3390/nu3100858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Whitfield-Cargile C.M., Cohen N.D., Chapkin R.S. The microbiota-derived metabolite indole decreases mucosal inflammation and injury in a murine model of NSAID enteropathy. Gut Microbes. 2016;7(3):246–261. doi: 10.1080/19490976.2016.1156827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Di Cagno R., De Angelis M., De Pasquale I. Duodenal and faecal microbiota of celiac children: molecular, phenotype and metabolome characterization. BMC Microbiol. 2011;11:219. doi: 10.1186/1471-2180-11-219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Francavilla R., Ercolini D., Piccolo M. Salivary microbiota and metabolome associated with celiac disease. Appl Environ Microbiol. 2014;80(11):3416–3425. doi: 10.1128/AEM.00362-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Silva C.L., Passos M., Camara J.S. Investigation of urinary volatile organic metabolites as potential cancer biomarkers by solid-phase microextraction in combination with gas chromatography-mass spectrometry. Br J Cancer. 2011;105(12):1894–1904. doi: 10.1038/bjc.2011.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Horvath I., Barnes P.J., Loukides S. A European Respiratory Society technical standard: exhaled biomarkers in lung disease. Eur Respir J. 2017;49(4) doi: 10.1183/13993003.00965-2016. [DOI] [PubMed] [Google Scholar]
- 62.Couch R.D., Navarro K., Sikaroodi M. The approach to sample acquisition and its impact on the derived human fecal microbiome and voc metabolome. PLoS ONE. 2013;8(11):e81163. doi: 10.1371/journal.pone.0081163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Bosch S., El Manouni El Hassani S., Covington J.A. Optimized sampling conditions for fecal volatile organic compound analysis by means of field asymmetric ion mobility spectrometry. Anal Chem. 2018;90(13):7972–7981. doi: 10.1021/acs.analchem.8b00688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gaulke C.A., Sharpton T.J. The influence of ethnicity and geography on human gut microbiome composition. Nat Med. 2018;24(10):1495–1496. doi: 10.1038/s41591-018-0210-8. [DOI] [PubMed] [Google Scholar]
- 65.Arasaradnam R.P., Quraishi N., Kyrou I. Insights into 'fermentonomics': evaluation of volatile organic compounds (VOCs) in human disease using an electronic 'e-nose'. J Med Eng Technol. 2011;35(2):87–91. doi: 10.3109/03091902.2010.539770. [DOI] [PubMed] [Google Scholar]
- 66.Arasaradnam R.P., Ouaret N., Thomas M.G. A novel tool for noninvasive diagnosis and tracking of patients with inflammatory bowel disease. Inflamm Bowel Dis. 2013;19(5):999–1003. doi: 10.1097/MIB.0b013e3182802b26. [DOI] [PubMed] [Google Scholar]
- 67.Smolinska A., Bodelier A.G., Dallinga J.W. The potential of volatile organic compounds for the detection of active disease in patients with ulcerative colitis. Aliment Pharmacol Ther. 2017;45(9):1244–1254. doi: 10.1111/apt.14004. [DOI] [PubMed] [Google Scholar]
- 68.Harris L.A., Baffy N. Modulation of the gut microbiota: a focus on treatments for irritable bowel syndrome. Postgrad Med. 2017;129(8):872–888. doi: 10.1080/00325481.2017.1383819. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.